Abstract
The UL3 open reading frame (ORF) has the coding capacity of 235 codons. The proteins reacting with the anti-UL3 antibody form in denaturing polyacrylamide gel bands with apparent Mrs of 34,000, 35,000, 38,000, 40,000, 41,000, and 42,000 and designated 1 to 6, respectively. Studies on their origins revealed the following. (i) The UL3 proteins forming all six bands were present in lysates of cells infected with wild-type virus and treated with tunicamycin or monensin or in cells infected with the mutant lacking the gene encoding the US3 protein kinase. (ii) The proteins contained in the slower-migrating bands were absent from cells infected with the mutant lacking the UL13 protein kinase. Bands 1 and 3, however were phosphorylated in cells infected with this mutant. (iii) Band 2 protein was absent from cells transfected with a plasmid carrying a substitution of the predicted first methionine codon of the UL3 ORF and superinfected with the UL3− mutant. (iv) Band 1 and 3 proteins were absent from lysates of cells transfected with a plasmid carrying a substitution of the second (M12) methionine codon of the UL3 ORF and superinfected with the UL3− mutant. (v) Cells superinfected with mutants lacking both methionine codons did not accumulate any of the proteins contained in the six bands. (vi) In vitro transcription-translation studies indicated that the translation of band 1 protein was initiated from the second (M12) methionine codon and that band 3 protein represented a UL13-independent, posttranslationally processed form of these proteins. The results indicate that the UL3 protein of herpes simplex virus 1 is translated predominantly from the second in-frame methionine codon and is subject to at least two posttranslational modifications.
The proteins encoded by herpes simplex virus 1 (HSV-1) possess two interesting characteristics. First, their electrophoretic mobility in denaturing gels is usually slower than that predicted by their molecular weight. This property may be related to the relatively high proline content of most HSV-1 proteins. The second property of many protein products of viral genes is that they form multiple bands on electrophoresis through denaturing polyacrylamide gels (28). This property is particularly prevalent among regulatory proteins (e.g., infected-cell protein 0 [ICP0], ICP4, ICP22, ICP27, etc.) (1, 2). The mechanisms underlying the formation of these isoforms is generally unknown. To investigate one case in some detail, we chose the products of the UL3 gene.
The HSV-1 UL3 gene is predicted to encode a protein of 235 amino acids reported to contain a signal sequence, a potential hydrophobic domain, and a probable N-glycosylation site (21). The UL3 open reading frame (ORF) contains six in-frame methionine codons; the first two are 10 codons apart. Ghiasi et al. (12) reported that in baculovirus, UL3 was unmodified by tunicamycin, that it was present in both cytoplasm and nuclei, and that it formed two bands with Mrs of 27,000 and 33,000 corresponding to nonphosphorylated and phosphorylated forms of UL3, respectively. It has been also reported that a single mRNA of 2.4 kb spans the UL3 ORF (29). The attraction of UL3 for the studies that we had in mind was twofold. First, UL3 is dispensable for growth in cells in culture (3), and therefore genetic manipulations of the gene would not cause significant selective pressure for emergence of second-site, compensatory mutants. Second, preliminary studies showed that the protein products of UL3 form at least six bands differing in electrophoretic mobility in denaturing gels.
In this report, we show that (i) UL3 protein forms at least six bands with apparent Mrs ranging from 34,000 to 42,000, (ii) neither monensin nor tunicamycin affects the electrophoretic mobility of the UL3 protein isoforms contained in lysates of infected mammalian cells, (iii) the isoforms arise predominantly by translation of the mRNA from the second methionine codon, and (iv) the translation product initiated from the second methionine codon underwent at least two posttranslational modifications. Phosphorylation of the UL3 protein mediated by the viral UL13 protein kinase accounted for the slower-migrating protein bands inasmuch as they were absent from lysates of cells infected with UL13-deficient (UL13−) virus.
MATERIALS AND METHODS
Cells and viruses.
Vero and HEp-2 cell lines were obtained from the American Type Culture Collection. Virus stocks were grown in HEp-2 cells [HSV-1 strain F {HSV-1(F)}] or Vero cells (mutants), and their titers were determined on Vero cells. All transfections were done in rabbit skin cells originally obtained from J. McLaren. The human 143 thymidine kinase-deficient (143TK−) cells, originally obtained from Carlo Croce, were used for selection of tk− recombinant viruses. Cell lines were grown in Dulbecco’s modified Eagle medium supplemented with either 5% newborn calf serum (Vero and rabbit skin cells) or 5% fetal calf serum (HEp-2 and 143TK− cells) supplemented with 40 μg of bromodeoxyuridine per ml of medium for 143TK− cells. Infected cells were maintained in medium 199V, consisting of mixture 199 supplemented with 1% calf serum.
HSV-1(F) is the prototype HSV-1 used in this laboratory (9). Recombinant virus HSV-1(F)Δ305 lacks the 500-bp SacI-BglII fragment from the domain of the tk gene and is therefore tk− (22, 23). The recombinant virus R7205 was derived from HSV-1(F)Δ305 and contains the coding sequence of the tk gene fused to the promoter of the α27 gene inserted into the BamHI site between the UL3 and UL4 ORFs (Fig. 1, line 6). The polyadenylation signal for UL5, separated from the UL5 coding sequence by this insertion, was functionally replaced by the insertion of the polyadenylation signal from the surface antigen of the hepatitis B virus (3). The recombinant virus R7211 (3) contains a deletion in the UL3 gene downstream of the EcoRV site (Fig. 1, line 12) approximately 43 codons from the start site of the UL3 ORF predicted by McGeoch et al. (21). The recombinant viruses R7041, R7356, and R7353 contain deletions in the gene encoding the viral kinases US3 and UL13 individually and both US3 and UL13, respectively (24–26). In the recombinant R7358, the UL13 coding sequence deleted in R7356 has been restored (26).
FIG. 1.
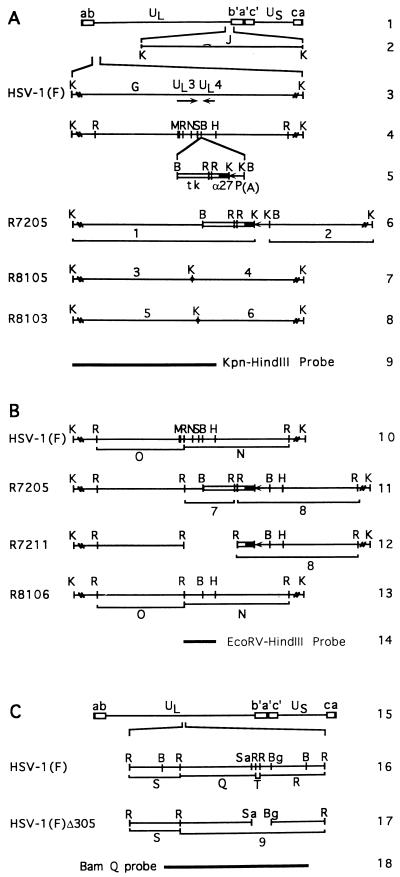
Schematic diagram of the sequence arrangement of the genomes of HSV-1(F) and relevant domains of the recombinant viruses. (A) Construction of recombinant viruses containing a CMV epitope. Line 1, HSV-1 genome. The lines represent unique long (UL) and short (US) sequences, whereas the rectangles represent the inverted repeats ab b′a′, a′c′, and ca, as marked. Line 2, representation of the Kpn J fragment, which shares portions of the b sequence with the KpnI G fragment. Line 3, positions of UL3 and UL4 ORFs in the KpnI G fragment. Line 4, relevant restriction endonuclease cleavage sites in the KpnI G fragment. Line 5, representation of the α27-tk gene (open rectangle, tk gene; filled rectangle, α27 promoter) and the poly(A) signal (line) inserted into the BamHI site located between UL3 and UL4 to create R7205. Line 6, restriction sites within the R7205 virus and newly created KpnI fragments 1 and 2 detected by a KpnI-HindIII probe shown in line 9. Lines 7 and 8, location of the oligonucleotide encoding the CMV epitope containing a diagnostic KpnI restriction site inserted within the predicted UL3 ORF at the NarI (R8105) and SfiI (R8103) restriction sites. The insertion of the oligonucleotide containing the KpnI site divides the KpnI G fragment into two roughly equivalent fragments predicted in this figure and identified in Fig. 2 as bands 3-4 and 5-6. (B) Construction of the UL3− virus R7211 and its repair virus R8106. Line 10, restriction endonuclease cleavage sites in the KpnI G fragment of HSV-1(F). Cleavage at three EcoRV restriction endonuclease sites within the KpnI G fragment is predicted to result in two EcoRV, fragments O and N, which contain portions of the UL3 ORF. Line 11, chimeric α27-tk gene and the polyadenylation signal inserted into the BamHI site between UL3 and UL4 ORFs in R7205, creating new EcoRV fragments, 7 and 8, detected by an EcoRV-HindIII probe (line 14). Line 12, sequence arrangement of R7211 recombinant virus after the deletion of the UL3 ORF downstream of the EcoRV site and the accompanying tk sequence (fragment 7 in line 11). Line 13, sequence arrangement of R8106 recombinant in which the natural sequences in the UL3 and UL4 regions have been restored to that of HSV-1(F). Line 14, location of the EcoRV-HindIII fragment used as a radiolabeled probe. (C) Line 15, sequence arrangement of HSV-1(F) DNA; line 16, relevant restriction endonuclease sites and EcoRV fragments detected by the BamHI-Q probe shown in line 18; line 17, the EcoRV fragment 9 created in HSV-1(F)Δ305 DNA. Abbreviations: B, BamHI; Bg, BglII; H, HindIII; K, KpnI; M, MseI; N, NarI; R, EcoRV; S, SfiI, Sa, SacII.
Reagents and antisera.
Restriction endonucleases were from New England Biolabs; T4 DNA ligase was from United States Biochemicals; the Klenow enzyme and calf intestinal alkaline phosphatase were obtained from Boehringer Mannheim; tunicamycin and monensin were obtained from Sigma Chemical Co. (St. Louis, Mo.)
Monoclonal antibody CH-28-2, which reacts with an epitope embedded in a 20-amino-acid sequence of human cytomegalovirus (CMV) glycoprotein B (gB) (19), was purchased from the Goodwin Institute, Plantation, Fla. The antibody to UL10 (gM) was described elsewhere (4). The antibody to HSV-2 UL3 protein used in the immunoprecipitation experiment was a gift from S. Caradonna (31).
Construction of plasmids.
Plasmid pRB442 contains a 6.3-kbp fragment encoding the UL1 to UL4 genes and portions of the UL5 gene of HSV-1(F) DNA. pRB447, a subclone of pRB442, contains only UL3 and UL4 in their entirety, specifically from the XbaI site at nucleotide (nt) 10637 pairs to the HindIII site at nt 12717 in the vector pGEM3Z. Both plasmids are described elsewhere (3).
Plasmids pRB4657 and pRB4722 were constructed to express the epitope of CH-28-2. The epitope was inserted in the middle (pRB4657) or near the carboxyl terminus (pRB4722) of UL3 (Fig. 1, lines 7 and 8). The EcoNI-BamHI fragment containing the UL3 ORF was excised from pRB447 and inserted into the SmaI-BamHI sites of pGEM3Zf(+) to create pRB4634. Plasmid pRB4637 was constructed by the in-frame insertion of the oligonucleotide 5′-cgAAGGGACAGAAGCCCAACCTGCTAGACCGACTGCGACACCGCAAAAACAGGTACC GACACg-3′ annealed with its complement strand 5′-cgcGTGTCGGTACCTGTTTTTGCGGTGTCGCAGTCGGTCTAGCAGGTTGGGCTTCTGTCCCT T-3′ into the NarI site within UL3 of plasmid pRB4634. The lowercase letters identify linker sequences for the epitope or HSV-1 DNA. Plasmid pRB4624 contained the EcoNI fragment (nt 10799 to 12678) in the SmaI site of pGEM3Zf(+) oriented such that a BamHI digest of this plasmid released a fragment which contained the UL4 ORF. The final plasmid, pRB4657, was made by inserting this BamHI fragment into the BamHI site of plasmid pRB4637, thereby reintroducing the UL4 ORF in its native orientation with respect to the epitope-tagged UL3.
pRB4662 was constructed by cleavage of plasmid pRB4637 with XhoI followed by ligation, thereby removing one of two SfiI sites, followed by the in-frame insertion of the oligonucleotide 5′-ggAAGGGACAGAAGCCCAACCTGCTAGACCGACTGCGACACCGCAAAAACAGGTACCGACACgtgtcgg-3′ annealed with its complement strand 5′-acacGTGT C GGTACCTGTTTTTGCGGTGTCGCAGTCGGTCTAGCAGGTTGGGCTTCTGTCCCTTccccg-3′ into the remaining SfiI site at the 3′ end of the UL3 ORF. pRB4666 was constructed by reintroducing the BamHI fragment of pRB4624 into the BamHI site of pRB4662, thereby restoring UL4 in its native orientation. To restore the majority of UL3 excised earlier by the XhoI collapse, the final plasmid, pRB4722, was made by reintroducing the EcoRV-XhoI fragment of pRB4634 into the blunted SacI and XhoI sites of pRB4666.
Plasmid pRB5260 contained the α27 promoter and a bidirectional polyadenylation signal. It was generated in two steps. In the first step, a 970-bp PCR fragment (nt 112699 to 113669) containing the α27 promoter flanked by NotI and SpeI restriction sites was inserted into these sites in the vector pBluescript II KS+. The PCR fragment was generated by amplification with the PCR primers 5′-cgcgaaggaagcggccgcCTGCAGTACCCCTACACGAA AATTAC-3′ and 5′-accggactagTAGCGAGCGACCGGGCCCGAATCGGGGA. In the second step, a 270-bp XhoI-KpnI fragment containing the bidirectional polyadenylation signal from pRB5160 (15) was inserted into the plasmid from step 1.
pRB5255 contains the entire coding region of the UL3 ORF inserted between the α27 promoter and the bidirectional polyadenylation signal in plasmid pRB5260. It was generated by the insertion of an EcoRI fragment containing the UL3 coding region (nt 10959 to 11666) into the EcoRI-digested and alkaline phosphatase-treated vector pRB5260. The following primers were used to amplify the UL3 ORF with flanking EcoRI restriction sites: 5′-ggtcgagaattcATGGTTAAACCTCTGGTCTCATACG-3′ and tatttagaattcgtcgacCGGT TACTCGGCCCCCGAGG-3′.
Plasmids pRB5253 and pRB5254 are variants of pRB5255 in which the first and second ATG codons of the UL3 sequence, respectively, are mutated. They were generated by using a Quickchange site-directed mutagenesis kit (Stratagene) as recommended by the manufacturer along with the following primers: 5′-CCCCCGGGCTGCAGGAATTCGAAGTTAAACCTCTGG-3′ and its complementary strand; and 5′-GGTCTCATACGGGTCGGTTAACTCGGGCGTCGGGGG-3′ and its complementary strand. Deviations of the oligonucleotide sequence from that of the HSV-1(F) sequence in pRB5255 are underlined. The mutations created the restriction sites BstBI and HpaI, respectively, allowing verification by restriction digest analysis that the plasmids used for transfection experiments contained the desired mutations. Plasmids containing mutations in both the first and the second in-frame ATG codons were generated from plasmids pRB5253 and pRB5254 by using the primers described above and were designated pRB5256 and pRB5257, respectively. These two plasmids were made to preclude the possibility of adventitious mutations during the second round of site-directed mutagenesis. Inasmuch as both pRB5256 and pRB5257 yielded the same results, only the results for pRB5256 are presented.
Plasmids pRB5253 and pRB5254, containing mutations in a single ATG codon, were repaired back to the original sequence by using the primers 5′-CCCCCGGGCTCGAGGAATTCATGGTTAAACCTCTGG-3′ plus its complementary strand and 5′-GGTCTCATACGGGTCGGTGATGTCGGGCGTCGGGGG-3′ plus its complementary strand. The repaired plasmids were designated pRB5258 and pRB5259 for repairs of the first (pRB5253) and second (pRB5254) ATG codons, respectively.
Production of the GST-UL3 fusion protein and rabbit polyclonal antiserum.
Plasmid pRB5250 was constructed by the in-frame insertion of the EcoRV-ApoI fragment from pRB447 encoding the last 192 codons of the UL3 ORF into the SmaI-EcoRI sites of the glutathione S-transferase (GST) protein expression vector pGEX2T (Pharmacia Biotech). The junction between the pGEX2T and the UL3 sequences was sequenced to verify that the coding sequences of the two proteins are in frame (data not shown). Escherichia coli BL21 cells transformed with this vector were grown at 30°C to an optical density of between 0.7 and 1.2 and induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside for 2 h, and the expressed fusion protein was affinity purified as instructed by the manufacturer (Pharmacia Biotech). Antibody to the eluted chimeric protein was made in rabbits at Josman Laboratories (Napa, Calif.) according to their standard protocol.
Multiple sequence alignment.
The amino acid sequence alignment was compiled initially by using the Wisconsin Package (Genetics Computer Group, Madison, Wis.) and then optimized by hand. The UL3 homologs were from HSV-1 (21), HSV-2 (20), equine herpesvirus (EHV) (30), canine herpesvirus (CHV) (27), bovine herpesvirus (BHV) (16), varicella-zoster virus (VZV) (7), pseudorabies virus (PRV) (8), Marek’s disease virus (MDV) (32), and infectious laryngotracheitis virus (10). The consensus line shows residues shared among at least four of the nine UL3 homologs.
Construction of recombinant viruses.
The plasmids and viral DNAs used in the construction of the recombinant viruses described in this report are listed in Table 1. Viral DNAs were prepared from potassium acetate gradients as described elsewhere (13). Recombinant viruses R8103 and R8105 were constructed by cotransfection of R7205 DNA with plasmids pRB4722 and pRB4657, respectively, into rabbit skin cells by the DEAE-dextran method as described elsewhere (23). R8106 was constructed by cotransfection of R7211 DNA with pRB442 plasmid DNA into rabbit skin cells. R8108 was constructed by cotransfection of R8105 DNA with pRB165 plasmid DNA containing the entire BamHI Q fragment. tk− progeny were selected on 143TK− cells in the presence of bromouracil deoxyriboside. tk+ viruses were selected on 143TK− cells overlayed with HAT medium, consisting of Dulbecco’s modified Eagle’s medium supplemented with hypoxanthine, aminopterin, thymidine, and 5% fetal bovine serum, as described elsewhere (22, 23). Viral isolates were plaque purified at least three times on Vero cells, and repair of the tk gene was verified by hybridization.
TABLE 1.
Genotypes of HSV-1 viral recombinants and plasmids used or made in these studies
| Virus | Genotypea | Plasmid and virus used for construction | Reference |
|---|---|---|---|
| HSV-1(F) | Wild type | ||
| R7041 | ΔUS3, βtk− | 9 | |
| R7353 | ΔUL13, βtk | 24 | |
| R7356 | ΔUS3/ΔUL13, βtk | 25 | |
| R7358 | UL13R/βtk− | 26 | |
| HSV-1(F)Δ305 | βtk− | 22 | |
| R7205 | βtk−/α27-tk | pRB3975 and HSV-1(F)Δ305 | 3 |
| R7208 | ΔUL3/βtk− | pRB4034 and R7205 | 3 |
| R7211 | ΔUL3/βtk | pRB1028 and R7208 | 3 |
| R8103 | UL3+/βtk−; iCMV (SfiI) | pRB4722 and R7205 | |
| R8105 | UL3+/βtk−; iCMV (NarI) | pRB4657 and R7205 | |
| R8106 | UL3R/βtk | pRB442 and R7211 | |
| R8108 | UL3+/βtk, iCMV (NarI) | pRB165 and R8105 |
βtk, tk gene located in its natural locus; α27-tk, tk gene driven by the α27 promoter and located in the BamHI site between UL3 and UL4; R, deleted gene repaired; CMV, CMV epitope inserted into site in parentheses.
Southern analyses of viral DNA.
Viral DNA was prepared from cytoplasmic extracts of infected Vero cells and purified by phenol-chloroform extraction (EcoRV digests) or potassium acetate gradients (KpnI digests) was digested with EcoRV or KpnI, electrophoretically separated in agarose gels, and transferred to Zeta-Probe blotting membranes (Bio-Rad Laboratories, Hercules, Calif.) overnight by the alkaline blotting method. The DNAs bound to the membrane were hybridized with the nick-translated probe prepared as instructed by the manufacturer (Promega).
Polyacrylamide gel electrophoresis and immunoblotting.
Rabbit skin cells in 25- or 150-cm2 flasks were mock infected or exposed to 10 PFU of the virus per cell. The cells were harvested, rinsed once with phosphate-buffered saline, solubilized in disruption buffer containing sodium dodecyl sulfate (SDS) and β-mercaptoethanol, subjected to electrophoresis on SDS-polyacrylamide gels, and transferred to a nitrocellulose sheet. The electrophoretically separated proteins were blocked in 5% milk in phosphate-buffered saline for 1 h, exposed to antibody for 2 h, rinsed three times for 10 min each in buffered 5% milk, exposed to the secondary antibody for 1 h with alkaline phosphatase-conjugated goat anti-rabbit or anti-mouse serum (Bio-Rad), washed again with buffered saline, rinsed in alkaline phosphatase buffer, and reacted with alkaline phosphatase substrates nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Bio-Rad).
Immunoprecipitation.
Replicate 25-cm2 cultures of rabbit skin cells were infected with either HSV-1(F) or R7353 (UL13−/US3−) in medium 199V and then incubated in phosphate-free Eagle minimal essential medium. After 1 h, the cells were incubated in the same medium but supplemented with [32P]orthophosphate (100 μCi per 25-cm2 culture). The cells were harvested at 16 h after infection. UL3 was precipitated from cell lysates with the HSV-2 UL3 antibody as described elsewhere (31).
Transfection-superinfection assay.
Plasmid DNA from bacterial clones pRB5253 through pRB5259 prepared by the Wizard maxiprep DNA purification system (Promega) were transfected into 25-cm2 cultures of rabbit skin cells with the aid of the Lipofectamine Plus system (Life Technologies). Subconfluent cultures were transfected with 3 μg of plasmid DNA and 21 h later infected with the ΔUL3 virus, R7211. Replicate untransfected cultures infected with HSV-1(F) or R7211 served as positive and negative controls. Cells were harvested 20 h after infection.
In vitro transcription-translation assay.
Two different PCR products were generated to link a T7 promoter upstream of the UL3 ORF. The first PCR product encoding the entire UL3 ORF was generated by standard techniques and primers 5′-TAATACGACTCACTATAGGGAGACCACATCGAATTCATGGTTAAACC-3′ and 5′-GTTTTTCAGTATCTACGATTCATAGATCTCTGCAGGTCGACGGATCC-3′ for the 5′ and 3′ ends, respectively. The second PCR product was generated in the same way except that the sequence for the 5′ primer contained a GAG codon instead of the ATG codon underlined above. The resulting PCR products were transcribed and translated by using the TNT coupled reticulocyte lysate system (Promega) and the T7 polymerase according to manufacturer’s directions.
RESULTS
Construction of recombinant viruses R8105 and R8103, containing an epitope-tagged UL3 ORF.
To identify the coding sequence of the UL3 ORF and characterize its product, we inserted into the UL3 ORF oligonucleotide sequences encoding the CMV epitope recognized by monoclonal antibody CH-28-2. To construct the viruses containing the epitope tags inserted into the middle or at the 3′ terminus of the UL3 ORF, rabbit skin cells were cotransfected with R7205 DNA and plasmid pRB4657 or pRB4722 (Table 1). The recombinant virus R7205, which served as a parent for the construction of the epitope-tagged viruses, contained the chimeric α27-tk gene inserted into the BamHI site between UL3 and UL4 (Fig. 1, line 6). In recombinant progeny, the α27-tk gene was replaced with the UL3 gene carrying the tag. Positions of the tags in the sequence of the UL3 protein are indicated by the central KpnI site in Fig. 1 (lines 7 and 8).
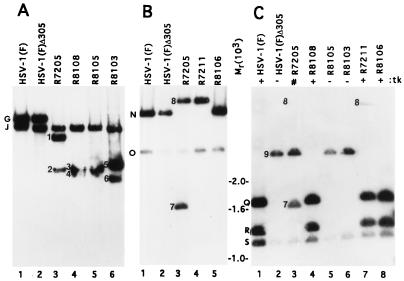
To verify the structure of the epitope-tagged viruses, KpnI digests of epitope-tagged UL3 or parent virus DNAs were electrophoretically separated on agarose gels, transferred to Zeta-Probe blotting membranes, and hybridized with a nick-translated KpnI-HindIII subfragment of the KpnI G fragment, containing ORFs UL1 through UL4 and portions of the b sequence repeat (Fig. 1, line 9). The KpnI J fragment, which shares the b sequence with KpnI G fragment, is also detected by this probe and was present in all recombinant viruses (Fig. 1, line 2; Fig. 2A). The 9.6-kbp KpnI G fragment which contains UL3 (Fig. 1, line 3) was readily detected as the larger of two bands illuminated by the radiolabeled probe in the electrophoretically separated digests of parent viruses HSV-1(F) and HSV-1(F)Δ305 (Fig. 2A, lanes 1 and 2). The α27-tk poly(A) construct inserted into the BamHI site between UL3 and UL4 in R7205 contained KpnI sites, and therefore KpnI digests of R7205 divided the KpnI G fragment into two fragments designated bands 1 and 2 (Fig. 1, line 6; Fig. 2A, lane 3). Similarly, the insertion of the oligonucleotide encoding the CMV epitope containing a KpnI site into the UL3 sequence divided the KpnI G fragment into two fragments designated bands 3-4 (for viruses R8108 and R8105) and 5-6 (for virus R8103) (Fig. 1, lines 7 and 8; Fig. 2A, lanes 4 to 6). The KpnI G fragments in digests of DNA of R8105 and its βtk repair R8108 (Fig. 2A, lanes 4 and 5) were very close in size and barely differentiated in this blot.
FIG. 2.
Autoradiographic images of electrophoretically separated restriction endonuclease digests of DNAs from HSV-1(F) and recombinant viruses transferred to Zeta-Probe membranes and probed with radiolabeled fragments of the relevant HSV-1(F) domain. The letters and numbers beside the lanes in panels A to C denote the DNA fragments generated by restriction endonuclease cleavages indicated in Fig. 1A to C, respectively. (A) Viral DNA was cleaved with KpnI and probed with a radiolabeled KpnI-HindIII fragment (Fig. 1A, line 9). (B and C) Viral DNA was cleaved with EcoRV and probed with a radiolabeled EcoRV-HindIII fragment (Fig. 1B, line 14) or BamHI-Q fragment (Fig. 1C, line 18). Markers for fragment sizes are indicated for and between panels B and C.
R7211 (ΔUL3 [3]) was made in two steps. In the first, cotransfection of R7205 viral DNA with pRB4034 yielded the recombinant virus R7208 (UL3−/βtk−). In the second step, the native tk gene (βtk) was restored by cotransfection of R7208 viral DNA with pRB1028. Plasmid pRB4034 and therefore also the R7211 recombinant virus lack the EcoRV-BamHI sequence containing UL3 and noncoding DNA, downstream of the UL3 ORF, and most of the inserted α27-tk gene (Fig. 1, line 12). To construct a virus in which the deleted UL3 sequences had been restored, R7211 viral DNA was cotransfected with plasmid pRB442, containing an intact UL3. The progeny viruses were screened for the presence of the UL3 gene. To verify that the UL3 ORF sequences deleted in R7211 were restored in R8106, electrophoretically separated EcoRV digests of R8106 viral DNA and those of the parent viruses were hybridized with a radiolabeled EcoRV-HindIII probe containing UL3 and UL4 coding sequences (Fig. 1, line 14). EcoRV digests of HSV-1(F) or HSV-1(F)Δ305 generated the adjacent DNA fragments EcoRV-O and EcoRV-N, each of which contained portions of the UL3 gene (Fig. 1, line 10). The radiolabeled probe readily detected the EcoRV N fragment and reacted with a lower intensity to the EcoRV O fragment (Fig. 2B, lanes 1 and 2). An additional EcoRV fragment present in the sequence containing α27-tk and poly(A) signal that was inserted into R7205 caused cleavage of EcoRV-N into fragments designated 7 and 8 (Fig. 1, line 11: Fig. 2B, lane 3). Only band 8 was detected in EcoRV restriction digests of R7211 (ΔUL3) since the fragment corresponding to band 7 had been deleted (Fig. 1, line 12; Fig. 2B, lane 4). Since band 8 contains only a small portion of the tk gene and thus would weakly hybridize to the BamHI-Q probe, band 8 is only weakly detected in R7205 and R7211 (Fig. 2C, lanes 3 and 7 [3]). Restoration of the UL3 sequences in the recombinant virus R8106 was evident from the absence of fragments represented by bands 7 and 8 and the reappearance of the EcoRV N fragment identical in mobility to that of the HSV-1(F) virus (Fig. 1, line 13; Fig. 2B, lane 5).
Rabbit skin cells were cotransfected with R8105 viral DNA and plasmid pRB165, containing the BamHI Q fragment to repair the tk gene at its natural location. To verify the presence of the natural tk gene in the repaired virus (R8108), electrophoretically separated EcoRV digests of viral DNAs were probed with a radiolabeled BamHI Q fragment containing the tk gene (Fig. 1, line 18). The BamHI-Q probe hybridized with EcoRV fragments S, Q, T, and R (Fig. 1, line 16). In the blot shown the smallest band, T, was lost from the electrophoretically separated digests of viral DNA containing an intact tk gene [HSV-1(F) (Fig. 2C, lane 1), R8108 (lane 4), R7211 (lane 7), or R8106 (lane 8)].
The EcoRV T fragment and two EcoRV restriction sites are contained in the 500-bp sequence deleted from BamHI-Q of HSV-1(F)Δ305. In consequence, EcoRV fragments Q and R form a chimeric fragment designated band 9 (Fig. 1, line 17). This fragment migrates more slowly than the Q, R, or S fragment (Fig. 2C, lane 2). In as much as R7205 lacks the 500-bp sequence from the BamHI Q fragment, its EcoRV restriction pattern (Fig. 2C, lane 3) showed the characteristic band 9 and S fragment. In addition, because it also contained the α27-tk gene inserted elsewhere (Fig. 1, line 11), the BamHI-Q probe also detected fragments 7 and 8 (Fig. 2C, lane 3; band 8 was not seen in the exposure shown). Although the tk gene was restored at its natural locus, R7211 retained a portion of the α27-tk gene inserted in the BamHI site, and therefore band 8 was also present (Fig. 1, line 12; Fig. 2C, lane 7). This band was absent from R8106, in which the UL3 sequence was restored and vestiges of the α27-tk gene were removed (Fig. 2C, lane 8). As expected, the CMV epitope-tagged viruses R8105 and R8103 lacked the tk gene and showed the restriction pattern characteristic of parent virus HSV-1(F)Δ305 (Fig. 2C, lanes 2, 5, and 6).
UL3 protein forms numerous isoforms in denaturing polyacrylamide gels.
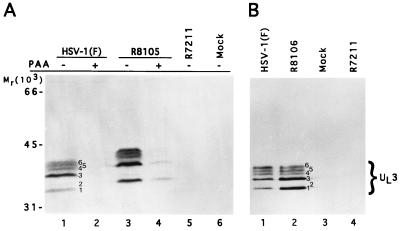
Electrophoretically separated lysates of rabbit skin cells mock infected or infected with HSV-1(F), R8105 (epitope tagged), or R7211 (ΔUL3) were reacted with either the monoclonal antibody directed against the CMV tag or the polyclonal antibody made against the GST-UL3 fusion protein. The products of the UL3 ORF detected by the anti-UL3 polyclonal rabbit antibody (Fig. 3) formed at least six bands designated 1 to 6 and characterized by apparent Mrs of 34,000, 35,000, 38,000, 40,000, 41,000, and 42,000, respectively. These bands were absent from lysates of mock-infected cells (Fig. 3A, lane 6; Fig. 3B, lane 3) or of cells infected with the R7211 mutant (Fig. 3A, lane 5; Fig. 3B, lane 4). The UL3 proteins from lysates of cells infected with the R8105 recombinant (Fig. 3A, lane 3) and detected by the anti-UL3 antibody migrated more slowly than the wild-type proteins, reflecting the increase in the size of the proteins due to the insertion of the epitope. All of these bands were also detected by the antibody to the CMV epitope (data not shown).
FIG. 3.
Photographs of immunoblots of electrophoretically separated lysates of mock-infected or infected rabbit skin cells reacted with UL3 polyclonal antibody. (A) Cells were infected in the presence (lanes 2 and 4) or absence (lanes 1, 3, 5, and 6) of PAA. (B) Cells were mock infected (lane 3) or infected with HSV-1(F) (lane 1), R8106 (UL3 repair; lane 2), or R7211 (ΔUL3; lane 4).
UL3 isoforms are γ2 proteins.
Replicate cultures of rabbit skin cells infected with HSV-1(F) or R8105 were exposed at 2 h after infection to phosphonoacetate (PAA; 300 μg/ml of medium; gift from Abbott Laboratories). This concentration of the drug is sufficient to totally block viral DNA synthesis (14). UL3 was absent from HSV-1(F)-infected cells treated with PAA (Fig. 3A, lane 2), indicating that UL3 was regulated as a γ2 gene. The epitope-tagged proteins were also expressed as γ2 proteins (Fig. 3A, lane 4).
UL3 isoforms are not glycosylated.
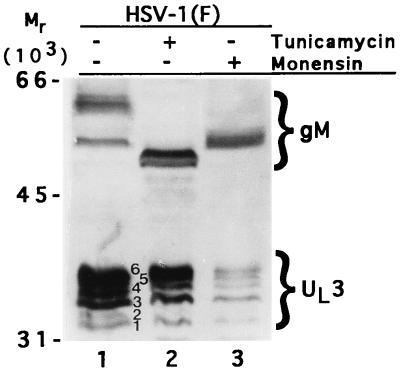
The presence of the putative N-linked glycosylation site NKS at codon 162 of the predicted sequence of the UL3 protein prompted us to determine whether this protein is glycosylated in mammalian cells. Earlier studies (12) on UL3 protein in baculovirus were not compelling inasmuch as they were not done in the context of infected cells and it could be argued that UL3 protein must associate with another viral protein to undergo glycosylation. In these experiments, replicate cultures of rabbit skin cells exposed to HSV-1(F) for 2 h were either mock treated or overlaid with medium 199V containing 10 μM monensin or tunicamycin (5 μg/ml). The cells harvested 19 h after infection were solubilized, electrophoretically separated on a denaturing gel, transferred to a nitrocellulose sheet, and reacted with polyclonal antibodies to both UL3 and gM (UL10). The drugs had no effect on the mobility of UL3 proteins, whereas as expected the electrophoretic mobility of gM encoded by the UL10 gene was affected by both tunicamycin and monensin (Fig. 4, lanes 2 and 3). These results do not support the hypothesis that the UL3 is N glycosylated.
FIG. 4.
Photograph of an immunoblot of HSV-1(F)-infected cell lysates probed with antibodies to UL3 and to gM. Lane 1, untreated infected cells; lane 2, cells treated with tunicamycin; lane 3, cells treated with monensin.
UL13 mediates posttranslational modification of UL3.
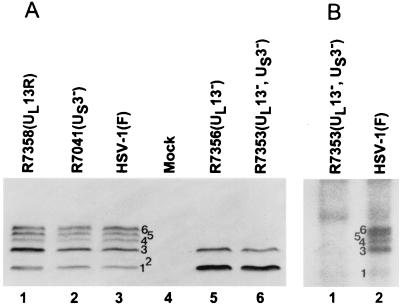
The ladder-like appearance of the UL3 protein bands in denaturing polyacrylamide gels prompted us to test the hypothesis that they represent differential posttranslational modifications mediated by one or both HSV-1 protein kinases (UL13 and US3). In these experiments, electrophoretically separated lysates of rabbit skin cells mock infected or infected with HSV-1(F), R7356 (ΔUL13), R7041 (ΔUS3), R7353 (ΔUL13/ΔUS3), or R7358 (UL13R) were transferred to a nitrocellulose sheet and reacted with the UL3 antibody. The electrophoretic profiles of UL3 proteins in Fig. 5A indicate that the slowest-migrating three isoforms represent posttranslational modifications mediated by the UL13 protein kinase. Specifically, isoforms 4 to 6 were absent from the lysates of ΔUL13 virus-infected cells but were present in cells infected with wild-type virus, ΔUS3, or the virus in which the deleted UL13 sequences had been restored.
FIG. 5.
(A) Photograph of an immunoblot of electrophoretically separated lysates of cells mock infected or infected with HSV-1(F) or recombinant viruses reacted with the antibody to UL3. (B) Photograph of an autoradiogram of electrophoretically separated UL3 protein isoforms immunoprecipitated from [32P]orthophosphate-labeled HSV-1(F)- or R7353-infected cells with the UL3 HSV-2 antibody.
Earlier studies have shown that two isoforms of the HSV-2 UL3 protein are posttranslationally phosphorylated (31). To verify that HSV-1 UL3 is also a phosphoprotein and to identify the phosphorylated isoforms of HSV-1 UL3, UL3 was immunoprecipitated from 32P-labeled HSV-1(F)- or R7353 (UL13−/US3−)-infected cells. The immunoprecipitated proteins were electrophoretically separated in denaturing polyacrylamide gels, transferred to nitrocellulose, and exposed to film. The five predominant isoforms of UL3 from HSV-1 infected cells identified in Fig. 5B (lane 2) are 32P labeled. In contrast, only isoforms 1 and 3 were 32P labeled in the lysates of cells infected with R7353. Inasmuch as isoform 2 most frequently accumulated in very small amounts, it is unclear whether it is also phosphorylated or whether the phosphoprotein was present in amounts too small to be detected. These results support the finding that the HSV-1 protein kinase UL13 was responsible for the phosphorylation of the UL3 isoforms 4 to 6 and suggest that isoforms 1 and 3 are phosphorylated by another kinase.
Analyses of isoforms of UL3 by tagging the gene at middle or 3′ terminus of the ORF.
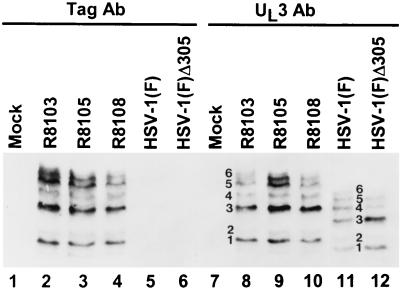
To determine whether the rapidly migrating UL3 isoforms represent proteolytic cleavage of the translation product, we inserted in frame at two sites in the UL3 ORF a sequence encoding the epitope of a known monoclonal antibody (Table 1; Fig. 1A, lines 7 and 8). In R8105 and R8108 (repair of tk at its natural locus), the epitope was inserted in frame into the NarI restriction site approximately in the middle of the UL3 ORF, whereas in R8103 the epitope was inserted in frame into the SfiI restriction site two codons from the 3′ terminus of the UL3 ORF. The electrophoretically separated lysates of cells infected with HSV-1(F), HSV-1(F)Δ305, or the tagged viruses were reacted with the monoclonal antibody to the CMV epitope (Fig. 6, lanes 1 to 6) or the rabbit polyclonal antibody to the HSV-1 UL3 protein (lanes 7 to 12). The protein products of the UL3 ORF present in lysates of cells infected with R8103, R8105, or R8108 visualized with the antibody to the epitope tag were identical to each other and to those visualized with the antibody to UL3. The electrophoretic profiles in lanes 7 to 12 show, however, the expected decrease in electrophoretic mobility caused by the insertion of the epitope tag compared to the untagged proteins (compare lanes 2 to 4 and 8 to 10 with lanes 11 and 12). Inasmuch as the pattern of protein products of the lysates from cells infected with the virus containing the carboxyl-terminal tag (R8103) were identical to those of cells infected with the tag in the center of the UL3 ORF (R8105 and R8108), these results do not support the hypothesis that the two fastest-migrating forms of UL3 were products of proteolytic cleavage at or near the carboxyl terminus.
FIG. 6.
Photograph of immunoblots of electrophoretically separated lysates of cells mock infected or infected with HSV-1(F) or recombinant viruses reacted with the antibody (Ab) to UL3 or to the epitope tag, as indicated. In addition to the six isoforms of UL3 typically observed in these studies, in this immunoblot a protein migrating faster than isoform 1 was faintly detected by both antibodies.
UL3 protein is translated largely from the second in-frame methionine codon of the ORF.
To identify the initiator methionine, we constructed a series of plasmids containing the UL3 ORF driven by the α27 promoter. Included in this series were plasmids carrying the substitutions M1E (pRB5253) or M12N (pRB5254) or both M1E and M12N (pRB5256). In addition, plasmids containing single methionine codon substitutions were repaired, to recreate the E1M and N12M codons (pRB5258 and pRB5259), respectively, to ensure that the phenotype observed with these plasmids was a result of the substitution of the ATG codon rather than to an adventitious mutation elsewhere in the plasmid.
Replicate cultures of rabbit skin cells were mock treated or transfected with DNA from individual plasmids and then infected 24 h later with the deletion virus R7211 (ΔUL3). Nontransfected rabbit skin cells were infected simultaneously with HSV-1(F) or the recombinant R7211. The cells were harvested 21 h after infection, solubilized, electrophoretically separated in a polyacrylamide gel, transferred to a nitrocellulose sheet, and probed with the anti-UL3 antibody (Fig. 7A). The results were as follows.
FIG. 7.
Photograph of immunoblots probed with antibody to UL3 protein. (A) Cells were infected with HSV-1(F) (lane 3) or R7211 (ΔUL3; lane 1) or first transfected with the indicated plasmid and then infected with R7211 (lanes 2 and 4 to 8). The letters and numbers assigned to individual protein bands are described in the text. (B) Immunoblots of UL3 proteins obtained by in vitro translation of UL3 transcripts of plasmids containing an intact M1 ATG (lane 2) or a M1E substitution (lane 3). Lysates of cells transfected and superinfected with pRB5258 and R7211 or infected with HSV-1(F) alone are shown in lanes 1 and 4, respectively.
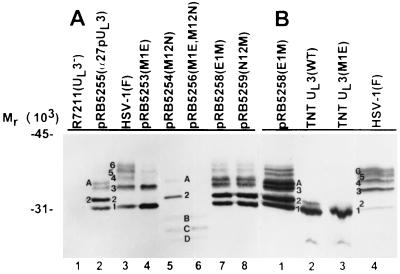
(i) The lysates of cells transfected with the plasmid containing the wild-type UL3 ORF (pRB5255) yielded two prominent bands in addition to bands 1 and 3. The more rapidly migrating band comigrated with band 2 but was more abundant than that formed by lysates of wild-type virus-infected cells. The slower-migrating band A migrated at a position intermediate between bands 3 and 4 (Fig. 7A; compare lanes 2 and 3).
(ii) The lysates of cells transfected with the plasmid carrying the UL3 ORF substitution M1E exhibited prominent bands 1 and 3 but lacked band 2 (pRB5253 [lane 4]).
(iii) The lysates of cells transfected with the plasmid containing the M12N substitution contained abundant band 2 and less abundant band A described above but lacked the UL3 bands 1 and 3 (pRB5254 [lane 5]).
(iv) The proteins contained in bands 1 to 6 and band A could not be detected in lysates of cells transfected with the plasmid containing substitutions of both the first and second methionine codons (pRB5256 [lane 6]).
(v) The lysates of cells transfected with plasmids containing a substitution of the second methionine codon alone or together with a substitution of the first methionine codon (pRB5254 and pRB5256 [lanes 5 and 6]) contained additional faster-migrating protein products (bands B, C, and D).
(vi) The electrophoretic mobility of the UL3 protein products in lysates of cells transfected with plasmids in which the original methionine codon had been restored (pRB5258 and pRB5259 [lanes 7 and 8]) corresponded to those observed in lysates of cells transfected with plasmids containing the wild-type UL3 ORF sequence (compare lane 2 [pRB5255] with lanes 7 and 8). Our impression is that expression of the UL3 protein products encoded by pRB5258 and pRB5259 was higher than expression of those encoded by pRB5255. In consequence, the more slowly migrating UL3 protein products were more readily detected in lysates of cells transfected with pRB5258 and pRB5259 compared to those transfected with pRB5255.
(vii) As shown previously, lysates of cells infected with the virus R7211 do not express UL3 protein products (lane 1).
These results indicate the following. (i) Bands 1 and 3 were the products of translation initiated at the second methionine codon, whereas bands 2 and A were the products of translation initiated at the first methionine codon; (ii) the bulk of the UL3 protein was initiated from the second methionine in cells infected with wild-type virus; (iii) the fast-migrating bands in cells transfected with plasmids lacking both the first and the second methionines may have been initiated from downstream methionine codons.
UL3 isoforms 1 and 2 result from initiation at the second and first ATG codons of UL3, respectively.
Although the transfection-superinfection assay indicated that the second ATG codon was the preferred initiator codon for the UL3 protein products, it was not clear whether isoform 3 was a modification of isoform 1 or whether isoform 1 was a cleavage product of form 3, as they were always present in pairs. To determine the answer to this question, the UL3 ORF was translated in a rabbit reticulocyte lysate system. Two sets of PCR primer pairs were designed so as to allow amplification of the entire UL3 ORF attached to a T7 promoter. In one set of primers, the first ATG was left intact (PCR product WT [wild type]) whereas in the second set it was substituted (M1E). The protein products of this assay were electrophoretically separated on an SDS-polyacrylamide gel alongside lysates from HSV-1(F)-infected cells, and cells transfected with pRB5258 and infected with R7211. The electrophoretically separated proteins from cell lysates were transferred to nitrocellulose and probed with the antibody to UL3. The results were as follows.
(i) The UL3 protein products translated from PCR product WT contained two closely migrating bands (Fig. 7B, lane 2) which comigrate with bands 1 and 2 of cells transfected with pRB5258 (first ATG of UL3 repaired) and superinfected with R7211 and thus with bands 1 and 2 of HSV-1(F)-infected cells (lanes 1 and 4).
(ii) Only one protein product was present in the rabbit reticulocyte lysate in which the PCR product carrying the M1E substitution was translated (lane 3). This product comigrated with the fastest-migrating product of HSV-1(F)-infected cell lysates (lane 4) and thus represents the unmodified form of UL3 isoform translated from the second ATG codon.
Inasmuch as bands 3 and A were not translated in the rabbit reticulocyte lysate system, our results suggest that the isoforms contained in these bands are posttranslational modifications of proteins contained in bands 1 and 2, respectively.
DISCUSSION
The ORF designated UL3 by McGeoch et al. (21) could be expected to yield a protein of 235 amino acid residues with an expected molecular weight of 26,500. The striking feature of the protein products of the UL3 ORF were the number of isoforms. We have identified at least six isoforms designated 1 to 6 and characterized as having apparent Mrs of 34,000, 35,000, 38,000, 40,000, 41,000, and 42,000, respectively. Worrad and Carradonna (31) demonstrated multiple forms of UL3 in HSV-2 with apparent Mrs of 28,000, 30,500, and 33,000. HSV proteins frequently migrate in denaturing gels as proteins of higher apparent molecular weights, and moreover, they commonly form multiple isoforms (28). This is especially true of regulatory proteins exemplified by ICP0, ICP4, ICP22, ICP27, etc. (1, 2). The presence of isoforms raise two questions: are the various isoforms the products of posttranslational modification of the product of a single coding domain, and does each isoform perform a unique function not shared with other isoforms? UL3 appeared to be an ideal target of an initial study largely because the gene is dispensable for viral replication in cells in culture. Attempts to unravel the origin of the isoforms led to the following observations.
(i) The UL3 ORF was expressed as a γ2 gene in that the gene products were not detected in infected cells overlaid with medium containing sufficient PAA to block DNA synthesis. This finding is in accord with the report of Singh and Wagner (29) that the domain of the UL3 gene is transcribed late in infection. This observation makes it highly unlikely that the synthesis of the isoforms is differentially regulated.
(ii) Exposure of infected cells to tunicamycin or monensin in concentrations sufficient to block N-linked glycosylation of glycoprotein M had no effect on the electrophoretic mobility of UL3 protein products.
(iii) UL3 proteins are posttranslationally modified by UL13 protein kinase but not by the US3 protein kinase. Thus, the slow-migrating isoforms of UL3 proteins are missing from lysates of cells infected with ΔUL13 recombinant virus.
(iv) Isoforms 1 and 3 appear to be the products of translation initiated from the second methionine (M12) in the UL3 ORF. Thus cells transfected with a plasmid carrying the substitution of M12N did not contain either product. We have excluded the possibility that band 1 contains the cleavage products of proteins present in band 3 inasmuch as band 1 was the only product of in vitro transcription-translation of the UL3 ORF lacking the first methionine (Fig. 7B, lane 3). On the basis of this finding and other data present in this report, we conclude that band 3 contains a posttranslational modification of band 1 protein that is not mediated by the UL13 protein kinase.
(v) The UL3 band 2 appears to contain the protein product initiated at the first methionine. This conclusion is based on the observation that it was absent from lysates of cells transfected with the plasmid containing the substitution M1E. It is of interest that band 2 was overexpressed in lysates of cells transfected with wild-type UL3 ORF driven by the α27 promoter but present in much smaller amounts in cells infected with wild-type parent virus. We cannot exclude the possibility that the products of the first methionine were processed more rapidly than those initiated at the second methionine. We should note, however, that band A was present at significant levels only in lysates containing large amounts of band 2 (e.g., lysates of cells transfected with plasmids containing an intact M12 [Fig. 7A, lanes 2, 5, 7, and 8]). Band A therefore may contain a posttranslationally modified band 2 protein. The necessary conclusion is that in cells infected with wild-type virus, the proteins initiated at the second methionine (M12) accumulate in larger amounts than those initiated at the first methionine.
(vi) None of the UL3 protein products accumulating in cells infected with wild-type virus are products of proteolytic cleavage at or near the carboxyl-terminal domain of the translated product of the UL3 ORF. This conclusion is based on the observation that all isoforms of UL3 protein contained the epitope tag inserted two codons from the 3′ terminus of the ORF.
(vii) We have observed several fast-migrating forms of the UL3 protein in lysates of cells transfected with plasmids lacking the second in-frame methionine codon of the UL3 ORF. One hypothesis to explain their presence is that they represent products initiated at methionine codons located 3′ to M12.
In infected cells, the UL3 mRNA appears to be preferentially translated beginning with the second methionine codon. The apparent preference for the second codon may be due to the presence of a small upstream ORF (uORF) of five codons (uORF3), located just upstream of the first methionine codon of the UL3 ORF. uORF3 is in a different reading frame than the UL3 ORF; moreover, the uORF3 stop codon, TAA, shares a nucleotide with the first methionine start codon, ATG. Numerous examples have been identified in which the reading frame of a small uORF that overlaps another ORF can prevent efficient usage of the first methionine codon of the downstream ORF (reviewed in references 11 and 18). In support of the hypothesis that uORF3 interferes with the utilization of the first methionine codon in the UL3 ORF is the observation that the first ORF was used very efficiently in cells transfected with plasmids lacking uORF3. Thus, the band corresponding to band 2 of infected cells was the predominant product of the UL3 ORF contained in pRB5255. It is noteworthy that the DNA sequence of UL3 in HSV-2 also contains a uORF (predicted to be 17 amino acids in size) whose stop codon TGA overlaps the ATG of the first methionine of HSV-2 UL3.
In addition to uORF3, other factors may promote the strong usage of the second methionine codon for initiation of protein synthesis. In the context of the viral genome sequence, the first and second methionine codons each have one optimal base in one of the two critical bases −3 and +4 with A of ATG as +1 (reviewed in reference 18). In the environment of the first UL3 ATG, TTAATGGTT, the −3 nucleotide is a T (poor) and the +4 is a G (good), whereas in the environment of the second UL3 ATG, GTGATGTCG, the −3 nucleotide is G (good) and the +1 is T (adequate). These environments were retained in the plasmids used for the in vitro transcription-translation or transfection-superinfection assays. Thus, although the exact sequence 5′ to the first ATG was altered to GAATTCATGGTT, the −3 nucleotide is still a T (poor) and the +1 remains a G (good). The sequence 3′ of the M12 codon, however, does contain an interesting pattern AGAGGGAGTTCCCTCT. Using the underlined nucleotides as unpaired components of a loop, this sequence could form a hairpin 14 nt downstream of the M12 codon. Hairpins in this position, 12 to 15 nt downstream of the AUG codon, have been reported to allow the 40S subunit of the ribosome to stall long enough to recognize and initiate at the second methionine (17). In contrast, the sequence 12 to 15 nt downstream of the first AUG do not contain a sequence consistent with the formation of a hairpin; rather, the sequence containing MseI sites flanking the M1, TTAATGGTTAA, forms a complementary sequence which could form a weak hairpin hiding the first ATG.
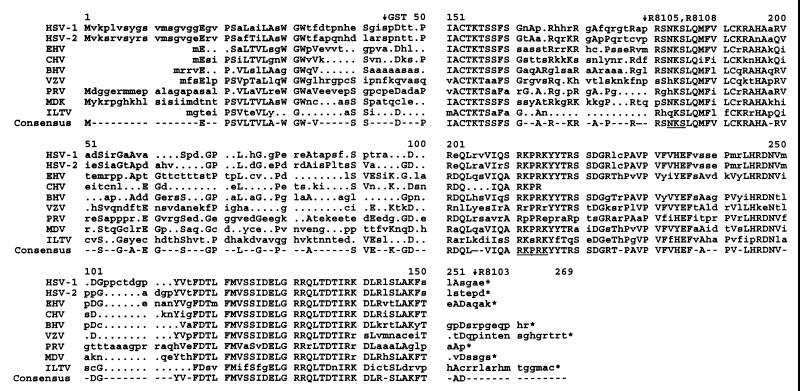
Examination of the DNA sequence and predicted amino acid sequences of the UL3 protein homologs in other members of the Herpesviridae family is consistent with the hypothesis that the first 11 codons of UL3 may not play a significant coding role. A multiple sequence alignment of UL3 homologs of members of the Alphaherpesvirinae is shown in Fig. 8. The alignment shows that in both HSV-1 and HSV-2 UL3 ORFs, the first two methionines are separated by only 10 codons, of which 7 are identical. There is no identity with those of PRV and little (1 of 10) with those of MDV. Rather, a high degree of identity among the homologs of UL3 begins to be apparent at the sixth amino acid from the second methionine of HSV-1 and HSV-2 (Fig. 8). Furthermore, both MDV and PRV contain ATG codons downstream of the first predicted initiation codon, and these are located prior to the first region in which all viruses show a strong homology. In addition, the sequences encoding the UL3 homologs for EHV, CHV, BHV, and VZV all begin downstream of the second HSV-1 ATG codon.
FIG. 8.
Multiple sequence alignment of the HSV-1- and HSV-2-encoded UL3 ORF and of UL3 homologs of other members of the Alphaherpesvirinae subfamily (EHV [ORF60], CHV [ORF7], BHV, VZV [ORF58], PRV, MDV, and infectious laryngotracheitis virus [ILTV]). The bottom line shows the amino acids shared by at least four of the nine UL3 homologs. Capital letters in other lines indicate that the amino acid is identical to the consensus amino acid for that position; the amino acid position indicated is for the consensus alignment and does not intentionally correspond to that of any individual viral sequence. A highly conserved nuclear localization motif is located at amino acids 211 to 215 (underlined). The highly conserved N-linked glycosylation motif NKS is located at amino acids 183 to 185 (underlined); the CMV tag in R8103 is located near the end of the C terminus (arrow); the CMV tag in R8105 is located just upstream of the N-linked glycosylation site (arrow). Note that the DNA sequence encoding the amino-terminal portion of the UL3 protein homolog in CHV was not available.
In this report, we have not specifically addressed the functions of the various isoforms of UL3 protein products. We should note, however, that the virus has evolved what appears to be an elaborate mechanism to use predominantly, but not exclusively, M12 as the initiator methionine codon. Moreover, there appear to be at least two independent posttranslational modifications, those mediated by UL13 protein kinase (bands 4 to 6) and those independent of protein kinase UL13 (band 3). For heuristic reasons, it is convenient to consider two hypotheses to explain the accumulation of isoforms of the M12 protein products. The first is that posttranslational modifications are sequential and cumulative, leading from band 1 (nascent protein) to band 6 (presumably the most extensively posttranslationally modified). Given that UL13 protein kinase is responsible for protein accumulating in bands 4 to 6, the amounts of UL3 proteins in band 3 to 5 would be expected to fluctuate in time and from one experiment to the next. The alternative hypothesis is that the modifications are mutually exclusive and totally dependent on the interactive protein partners. Implicit in the interactive-partner hypothesis is the notion that each isoform has a unique function defined by both the partner and the posttranslational modification it bears. We prefer the second hypothesis based largely on other HSV examples. ICP22, for example, also forms multiple isoforms dependent on both UL13 and US3 protein kinases (26). ICP22 has been shown to interact with several proteins (5, 6). Of particular interest is the observation that only the posttranslationally underprocessed form of ICP22 interacts with a host protein designated p60 (5).
ACKNOWLEDGMENTS
We thank A. P. W. Poon for careful reading of the manuscript.
These studies were aided by Public Health Service grants CA47451, CA71933, and CA78766 from the National Cancer Institute.
REFERENCES
- 1.Ackermann M, Braun D K, Pereira L, Roizman B. Characterization of herpes simplex virus 1 α proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984;52:108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M, Sarmiento M, Roizman B. Application of antibody to synthetic peptides for the characterization of the intact and truncated α22 protein specified by herpes simplex virus 1 and the R325 α22− deletion mutant. J Virol. 1985;56:207–215. doi: 10.1128/jvi.56.1.207-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines J D, Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J Virol. 1991;65:938–944. doi: 10.1128/jvi.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines J D, Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993;67:1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruni R, Fineschi B, Ogle M R, Roizman B. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J Virol. 1999;73:3810–3817. doi: 10.1128/jvi.73.5.3810-3817.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruni R, Roizman B. The herpes simplex virus 1 regulatory protein ICP22 interacts with a new cell cycle-regulated factor and accumulates in a cell cycle-dependent fashion in infected cells. J Virol. 1998;72:8525–8531. doi: 10.1128/jvi.72.11.8525-8531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 8.Dean H J, Cheung A K. A 3′ coterminal gene cluster in pseudorabies virus contains herpes simplex virus UL1, UL2, and UL3 gene homologs and a unique UL3.5 open reading frame. J Virol. 1993;67:5955–5961. doi: 10.1128/jvi.67.10.5955-5961.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ejercito P M, Kieff E D, Roizman B. Characteristics of herpes simplex virus strains differing in their effect on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs W, Mettenleiter T C. DNA sequence and transcriptional analysis of the UL1 to UL5 gene cluster of infectious laryngotracheitis virus. J Gen Virol. 1996;77:2221–2229. doi: 10.1099/0022-1317-77-9-2221. [DOI] [PubMed] [Google Scholar]
- 11.Geballe A P. Translational control mediated by upstream AUG codons. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 173–197. [Google Scholar]
- 12.Ghiasi H, Perng G-C, Cai S, Nesburn A B, Wechsler S L. The UL3 open reading frame of herpes simplex virus type 1 codes for a phosphoprotein. Virus Res. 1996;44:137–142. doi: 10.1016/0168-1702(96)01330-5. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi K, Fawl R, Roller R J, Roizman B. Construction and properties of a recombinant herpes simplex virus 1 lacking both S component origins of DNA synthesis. J Virol. 1993;67:2123–2132. doi: 10.1128/jvi.67.4.2123-2132.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones P C, Roizman B. Regulation of herpesvirus macromolecular synthesis. VIII. The transcription program consists of three phases during which both extent of transcription and accumulation of mRNA in the cytoplasm are regulated. J Virol. 1979;31:299–314. doi: 10.1128/jvi.31.2.299-314.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khattar S K, van Drunen Littel-van den Hurk S, Babiuk L A, Tikoo S K. Identification and transcriptional analysis of a 3′-coterminal gene cluster containing UL1, UL2, UL3, and UL3.5 open reading frames of bovine herpesvirus-1. Virology. 1995;213:28–37. doi: 10.1006/viro.1995.1543. [DOI] [PubMed] [Google Scholar]
- 17.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 18.Kozak M. Interpreting cDNA sequences: some insights from studies on translation. Mamm Genome. 1996;7:563–574. doi: 10.1007/s003359900171. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Roizman B. The promoter, transcriptional unit, and coding sequence of herpes simplex virus 1 family 35 proteins are contained within and in frame with the UL26 open reading frame. J Virol. 1991;65:206–212. doi: 10.1128/jvi.65.1.206-212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGeoch D J, Cunningham C, McIntyre G, Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J Gen Virol. 1991;72:3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- 21.McGeoch D J, Dalrymple M A, Davidson A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of the herpes simplex virus type I. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 22.Post L E, Roizman B. A generalized technique for deletion of specific genes in large genomes: α gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 23.Post E, Makem S, Roizman B. Regulation of α genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with α gene promoters. Cell. 1981;24:5555–5565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- 24.Purves F C, Longnecker R M, Leader D P, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein α22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remond M, Sheldrick P, Lebreton F, Nardeus P, Foulon T. Gene organization in the UL region and inverted repeats of the canine herpesvirus genome. J Gen Virol. 1996;77:37–48. doi: 10.1099/0022-1317-77-1-37. [DOI] [PubMed] [Google Scholar]
- 28.Roizman B, Sears A E. The replication of herpes simplex viruses. In: Fields B N, Knipe D M, Howley P, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. In Fields’ Virology. 3rd ed. New York, N.Y: Lippincott-Raven Press; 1996. pp. 2231–2295. [Google Scholar]
- 29.Singh J, Wagner E K. Transcriptional analysis of the herpes simplex virus type 1 region containing the TRL/UL junction. Virology. 1993;196:220–231. doi: 10.1006/viro.1993.1470. [DOI] [PubMed] [Google Scholar]
- 30.Telford E A, Watson M S, McBride K, Davidson A. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 31.Worrad D M, Caradonna S. The herpes simplex virus type 2 UL3 open reading frame encodes a nuclear localizing phosphoprotein. Virology. 1993;195:364–376. doi: 10.1006/viro.1993.1386. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida S, Lee L F, Yanagida N, Nazerian K. Identification and characterization of a Marek’s disease virus gene homologous to glycoprotein L of herpes simplex virus. Virology. 1994;204:414–419. doi: 10.1006/viro.1994.1546. [DOI] [PubMed] [Google Scholar]