Abstract
Chinese hamster ovary (CHO) cells have recently been used for identification of receptors for several alphaherpesviruses, including pseudorabies virus (PrV) (R. J. Geraghty, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear, Science 280:1618–1620, 1998). The experiments were based on the fact that CHO cells are inefficient target cells for PrV. However, a detailed analysis of the interaction between PrV and CHO wild-type and recombinant PrV-receptor bearing cells has not been performed. We show here that PrV has a growth defect on CHO cells which leads to a ca. 100-fold reduction in plating efficiency, strongly delayed penetration kinetics, and a 104-fold reduction in one-step growth. Entry of PrV into CHO cells is significantly delayed but is not affected by inhibitors of endocytosis, suggesting that the mechanism of penetration resembles that on permissive cells. The defects in plating efficiency and penetration could be corrected by expression of herpesvirus entry mediators B (HveB), HveC, or HveD, with HveC being the most effective. However, the defects in one-step growth and plaque formation were not corrected by expression of PrV receptors, indicating an additional restriction in viral replication after entry. Surprisingly, PrV infection of CHO cells was sensitive to neutralization by a gB-specific monoclonal antibody, which does not inhibit PrV infection of other host cells. Moreover, the same monoclonal antibody neutralized PrV infectivity on cells displaying the interference phenomenon by overexpression of gD and subsequent intracellular sequestration of gD receptors. Thus, absence of gD receptors on two different host cells leads to an increased sensitivity of PrV toward gB neutralization. We hypothesize that this is due to the increased requirement for interaction of gB with a cellular surface protein in the absence of the gD-gD receptor interaction. As expected, CHO cells are as susceptible as other host cells to infection by PrV gD− Pass, an infectious gD-negative PrV mutant. However, PrV gD− Pass was also not able to form plaques on CHO cells.
Infectious entry of herpesviruses into target cells involves several virion envelope glycoproteins which interact with cellular surface components functioning as virus receptors (36). For the alphaherpesviruses pseudorabies virus (PrV), herpes simplex virus (HSV), and bovine herpesvirus 1 (BHV-1) primary attachment of free virions to target cells is mediated by interaction between glycoprotein C (gC) and heparan sulfate proteoglycans in the cytoplasmic membrane (9, 27, 29). This initial binding is relatively labile and sensitive to competition by exogenous heparin, a structural analogon of heparan sulfate. A secondary interaction involves gD and results in a more stable and, presumably, closer binding (14, 22). Following attachment, fusion between the virion envelope and the cellular cytoplasmic membrane occurs. This penetration step requires presence of glycoproteins B, D, H, and L (21, 24, 36).
Early studies indicated that HSV and PrV may use a set of common, overlapping receptors, although distinct differences were also noted (20, 37, 38). Both viruses exhibit a wide host range in vitro, and numerous cell lines from a variety of animal species are infectable. The host range in vivo is, however, different in that the natural host of PrV is the pig whereas the primary host species of HSV is the human. Moreover, PrV naturally infects a wide range of animals with fatal consequences, and only horses and higher primates including humans are resistant to infection (25). In contrast, HSV normally does not naturally infect other species, although a number of species can be experimentally infected. Thus, it is expected that there may be receptors used by both viruses and others exclusive for only one of these viruses.
An interaction between alphaherpesvirus gD and a cellular receptor was deduced from studies with gD-deficient HSV and with soluble gD (10, 11). Additional evidence was derived from studies on the infectibility of cell lines constitutively expressing HSV, PrV, or BHV-1 gD (2–4, 12, 30). It has been noted that these cells are partially resistant to infection by the homologous and sometimes also the heterologous virus. This phenomenon had been explained by the possibility that intracellularly expressed gD sequesters receptors, which are therefore not available for the infecting virion (2).
To identify virus receptors, a successful approach has involved expression cloning in cells which are resistant to infection by the respective virus due to absence of the receptor. As indicated, PrV and HSV are able to infect a wide range of host cells, and it has proven difficult to identify target cells with a specific defect in initiation of infection. Chinese hamster ovary (CHO) cells are one of the few cell types with a significant resistance to infection by PrV and HSV. These cells express the primary receptor heparan sulfate, so that initial binding of virions can occur (35). However, virion-cell fusion does not or only inefficiently ensues, due to the absence or strongly decreased levels of secondary receptors. Whereas fusion between the virion envelope and the cellular cytoplasmic membrane, which leads to release of the nucleocapsid into the cytoplasm, occurs at neutral pH at the cell surface in herpesviruses, electron microscopic observations indicated that uptake of PrV into CHO cells occurs by endocytosis followed by degradation of virions (32).
Expression cloning in CHO cells led to the identification of coreceptors for HSV and PrV, which have been designated herpesvirus entry mediator B (HveB), HveC, and HveD (5, 6, 39). The last is identical to poliovirus receptor, whereas the others are also known as poliovirus receptor-related proteins 2 (HveB) and 1 (HveC). In addition, HveA, a member of the tumor necrosis factor receptor family, can mediate infection of CHO cells by HSV but not PrV (28). Subsequently, it has been demonstrated that these proteins interact with glycoprotein D, thereby constituting the long-sought gD receptors (19, 40).
As an easy means of testing infectability, virus mutants were used that, upon successful infection of target cells, expressed β-galactosidase, which can easily be quantitated by enzymatic batch assays (6, 39). However, infection by PrV of either wild-type or receptor-expressing CHO cells has not been analyzed in greater detail, especially in comparison with normally susceptible host cells. Thus, we initiated studies to compare the infectious entry of PrV into CHO, gD-receptor expressing CHO, and normally susceptible host cells.
Although interaction of gD with receptors appears to be required for infection of target cells by wild-type HSV-1, PrV, and BHV-1, variants have been isolated from the last two viruses which are infectious in the absence of gD (33, 34). Thus, gD-gD-receptor binding appears not to be absolutely required for infection or can be compensated for by other virion-cell interactions. Therefore, we also analyzed the infection of wild-type and gD receptor-expressing CHO cells by infectious gD-negative PrV.
MATERIALS AND METHODS
Cells and viruses.
All virus mutants are based on PrV strain Ka (PrV-Ka) (13). PrV-1112 carries a lacZ insertion at the nonessential gG locus (26). So far, in a multitude of in vitro and in vivo tests, this virus behaved like wild-type PrV. PrV-8411 is a gC-negative derivative of PrV-1112 (15). PrV gD− Pass is derived from PrV-gD− by passaging in cell culture. It is infectious in the absence of gD (33). PrV gCD− Pass is a gC-negative derivative of PrV gD− Pass (16). The PrV strains were propagated on rabbit kidney (RK13) cells and tested on CHO cells. Vesicular stomatitis virus (VSV; kindly provided by Horst Schirrmeier, Federal Research Centre for Virus Diseases of Animals, Insel Riems, Germany) was propagated on bovine kidney (MDBK) cells.
CHO cells expressing herpesvirus receptors HveB, HveC, and HveD (6, 39) were kindly provided by P. G. Spear, Northwestern University, Chicago, Ill. RK13-gD and CHO-gD cells were isolated after transfection of plasmid gDgI-CMV carrying the gDgI expression unit under control of the human cytomegalovirus immediate-early promoter/enhancer (7). MT50-5 cells are bovine kidney (MDBK) cells carrying the gDgI expression unit under control of the mouse metallothionein promoter (31).
The amount of gD present at the surface of the cells was determined by fluorescence-activated cell sorter analysis with gD-specific monoclonal antibody (MAb) c14-c27 (18).
Plaque assays.
Cells were infected in six-well tissue culture dishes with serial dilutions of the respective virus for 1 h at 37°C. Thereafter, the inoculum was removed and the cells were overlaid with semisolid methylcellulose medium. Two days after infection, the monolayers were fixed and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Plaques, foci, or single infected cells were counted (see Fig. 1).
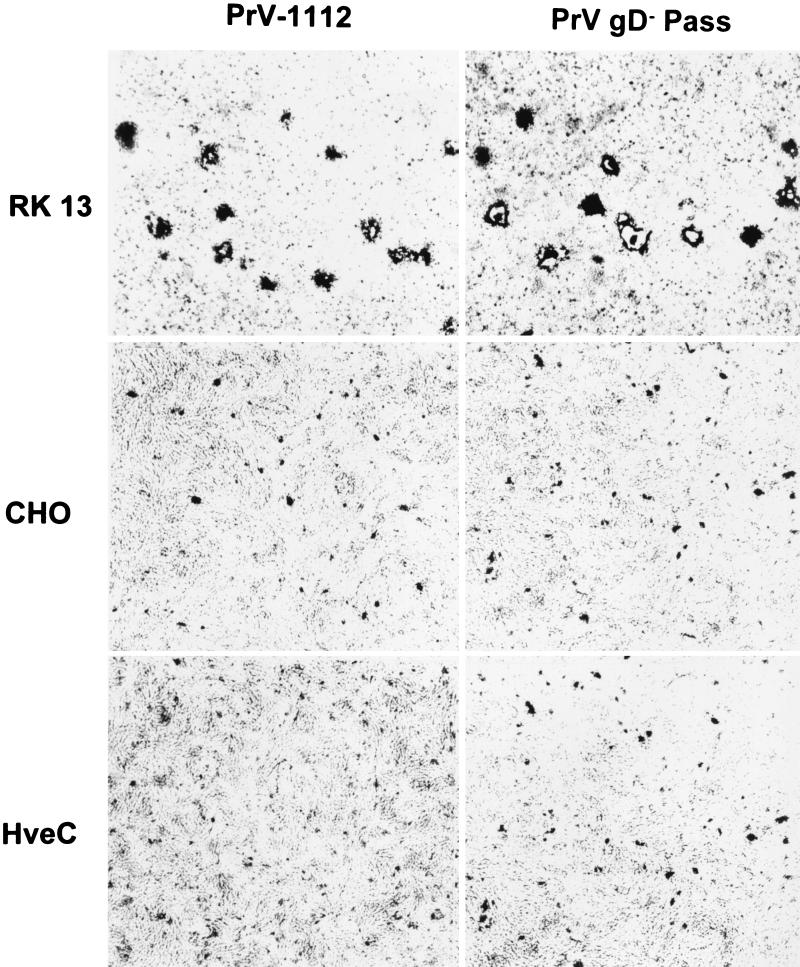
FIG. 1.
Infection of CHO cells by PrV. RK13, CHO, and HveC-expressing CHO cells were infected with wild-type-like PrV-1112 or PrV gD− Pass and stained with X-Gal 2 days after infection. Input virus was adjusted to yield similar numbers of infectious units in each assay. Whereas plaques developed on RK13 cells, only single infected cells or small foci of infection were observed on CHO and HveC-expressing CHO cells.
Penetration and one-step growth assays.
One-step growth analysis was performed essentially as described previously (17). Assay of penetration kinetics by low-pH inactivation of extracellular virus has been described previously (23). The input virus amount was 500 PFU per well of a six-well tissue culture plate.
Inhibition of endocytosis.
RK13 or CHO cells in 24-well culture dishes were treated for 1 h at 37°C with medium containing 100 μM chloroquine, 0.1% sodium azide, or 10 mM ammonium chloride (1). Thereafter, the medium was removed and cells were inoculated with (per well) approximately 250 PFU of PrV or VSV diluted in medium containing endocytosis inhibitors. After 2 h at 37°C, the inoculum was removed and extracellular virus was inactivated by treatment with citrate buffer (pH 3.0) for 2 min. After repeated washing with phosphate-buffered saline (PBS), the cells were overlaid with semisolid methylcellulose medium. Two days later, the cells were fixed and stained with X-Gal (for PrV) or crystal violet (for VSV), and plaques, foci, or infected cells were counted microscopically.
Neutralization assays.
Approximately 200 PFU of PrV-1112 was incubated with serial dilutions of hybridoma b43-b5 supernatant in a 200-μl volume for 1 h at 37°C. MAb b43-b5 recognizes the larger, amino-terminal subunit of the cleaved PrV gB (18). Thereafter, cells were inoculated with the assay solution for 1 h at 37°C, the inoculum was removed, and the cells were overlaid with semisolid methylcellulose medium. After 2 days, the monolayers were fixed and stained with X-Gal, and plaques or infected cells were counted.
RESULTS
Infection of CHO cells by PrV.
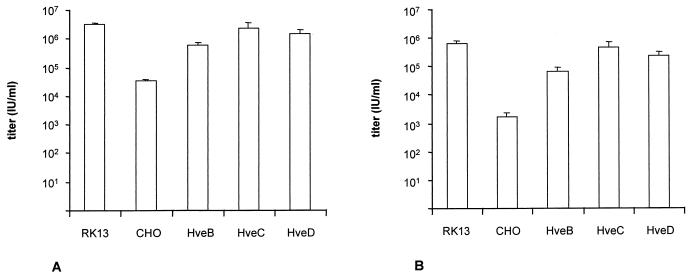
CHO cells have been described as resistant to infection by PrV (32), but an exact quantitation has never been reported. Thus, CHO monolayer cells were infected with serial dilutions of PrV-1112. Two days p.i. the cells were stained with X-Gal. As shown in Fig. 1, PrV-1112 was unable to form plaques on CHO cells as opposed to susceptible RK13 cells. Only single infected CHO cells or small foci of up to five infected cells were observed. Counting of plaques on RK13 and infected cells on CHO cells revealed that the efficiency of plating on CHO cells was approximately 100-fold lower than on RK13 cells. Titers decreased from ca. 5 × 106 PFU/ml on RK13 cells to 5 × 104 infectious units per ml on CHO cells (Fig. 2A). Thus, although CHO cells are ca. 100-fold less infectible by PrV than are RK13 cells, a significant proportion of virions were still able to infect these cells. A 103-fold reduction in plating efficiency was observed after infection of CHO cells by gC-negative PrV-8411 (Fig. 2B).
FIG. 2.
PrV titration on CHO cells. PrV-1112 (A) and gC− PrV-8411 (B) were titrated on RK13 and CHO cells as well as recombinant CHO cells expressing HveB, HveC, and HveD. Titers are indicated as infectious units (IU) per milliliter of virus suspension as analyzed after X-Gal staining of the monolayer.
The infectivity of PrV-1112 and PrV-8411 on CHO cells was significantly increased by expression of HveB, HveC, or HveD. Levels of infectivity similar to those seen on susceptible RK13 cells were observed in particular in HveC-expressing CHO cells (Fig. 2). Thus, HveB, HveC, and HveD expression rescued the defect in infection of CHO cells, with HveC-expressing cells being the most susceptible. However, expression of HveC (Fig. 1), HveB, or HveD (data not shown) did not result in plaque formation of PrV on CHO cells. Thus, although entry was enhanced by expression of the receptor proteins, direct cell-to-cell spread did not ensue.
Entry of PrV into CHO cells.
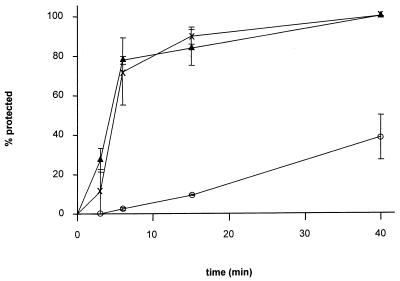
CHO cells are supposed to be deficient in entry of PrV due to endocytosis of adsorbed virions and subsequent destruction in endosomal vesicles (32). Since these conclusions are based on electron microscopic observations only, we wanted to analyze the entry of PrV into CHO cells in more detail. Therefore, penetration kinetics were established by using low-pH inactivation of extracellular virions. As shown in Fig. 3, entry of PrV into CHO cells was significantly delayed, with a half time of penetration of approximately 50 min. In contrast, entry into RK13 cells proceeded with a half time of approximately 5 min. A similar rate of entry was observed in CHO cells expressing HveC. Obviously, expression of HveC increased the kinetics of penetration to levels seen in fully susceptible cells.
FIG. 3.
Penetration kinetics. The rates of entry of PrV-1112 into RK13 (▴), CHO (○), and HveC-expressing CHO cells (X) were determined by low-pH inactivation of extracellular virus. The percentage of infectious events (plaques, foci, or infected single cells [Fig. 1]) at a given time point calculated in comparison with PBS-treated control plates is shown. Data are averages of three independent experiments. Vertical lines indicate standard deviations.
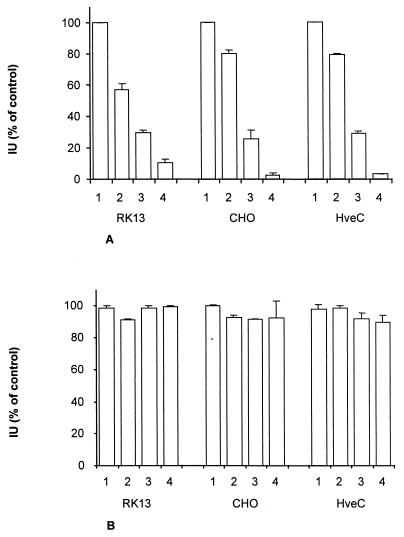
To analyze whether the delay in penetration of PrV into CHO cells is due to aberrant entry by endocytosis, endocytosis was blocked by treatment with azide, or endosomal pH was raised by treatment with ammonium chloride or chloroquine. Neither of these regimens had any effect on infection by PrV (Fig. 4B) on any cell line tested, whereas infection by VSV was strongly inhibited (Fig. 4A).
FIG. 4.
Infectious entry of PrV into CHO cells does not occur by endocytosis. RK13, CHO, and HveC-expressing CHO cells were infected with VSV (A) or PrV (B) and treated with PBS (bars 1), sodium azide (bars 2), ammonium chloride (bars 3), or chloroquine (bars 4). Indicated is the percentage of infectious events (plaques, foci, or infected single cells [Fig. 1]) compared to untreated control cells. Data are averages of three independent experiments. Vertical lines indicate standard deviations.
Replication of PrV in CHO cells.
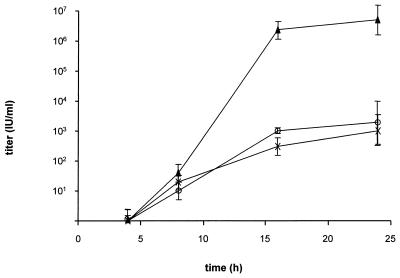
To analyze whether CHO cells are able to productively replicate PrV and produce infectious progeny, one-step growth kinetics were assayed. To this end, RK13, CHO, and recombinant HveC-expressing CHO cells were infected at a multiplicity of infection (MOI) of 5 as determined by titration of input virus stocks on each cell line separately. After attachment and a 2-h penetration period, extracellular virions were inactivated by low pH. At different times after infection, supernatants were harvested and titrated on RK13 cells. As shown in Fig. 5, early kinetics of virus replication were similar in RK13, CHO, and HveC-expressing CHO cells, with infectious extracellular virions first appearing at 8 h postinfection. However, the final titers on RK13 cells were approximately 4 log10 units higher than on CHO cells. Surprisingly, expression of HveC did not increase the production of infectious PrV in CHO cells.
FIG. 5.
One-step growth. One-step growth kinetics of PrV-1112 in RK13, CHO, and HveC-expressing CHO cells were established after infection at an MOI of 5. Titers are indicated in infectious units (IU) per milliliter. Data are averages of two independent experiments. Vertical lines indicate standard deviations.
Lack of gD-mediated interference in infection of CHO cells by PrV.
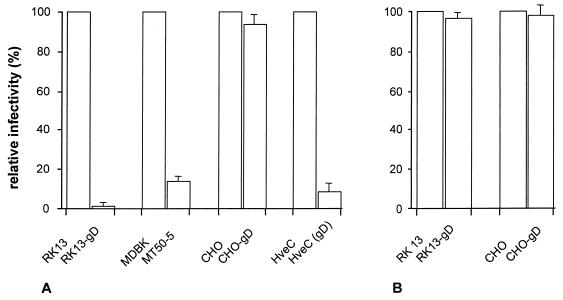
Cell lines expressing gD exhibit a distinct resistance to infection by homologous and sometimes heterologous alphaherpesviruses (2–4, 12). This phenomenon has been explained by sequestration of gD receptors by the intracellularly expressed gD, which, consequently, are not available for the infecting virus. If CHO cells are devoid of gD receptors, expression of gD in recombinant CHO cells should not lead to interference. To test this hypothesis, stable CHO and RK13 cell lines were established with a plasmid from which the expression of gD occurred under the control of the human cytomegalovirus major immediate-early promoter/enhancer. As shown in Fig. 6A, CHO cells were similarly susceptible to infection by PrV irrespective of the intracellular expression of gD. In contrast, RK13 cells transfected with the same plasmid exhibited a significant resistance to infection by gD-positive PrV, with a reduction in titer of approximately 100-fold. As expected, infectious PrV gD− Pass did not exhibit interference on either cell (Fig. 6B) due to the use of a gD-independent entry pathway. Since RK13-gD cells expressed approximately 5-fold more gD at the surface than CHO-gD cells did, parallel titrations were performed on MT50-5 cells (31), whose surface gD expression was found to be similar to that of CHO-gD cells (data not shown). As shown in Fig. 6A, an approximately 10-fold reduction in plating efficiency was observed on MT50-5 cells compared to parental MDBK cells. Transient expression of gD in HveC-expressing CHO cells after transfection resulted in a 10-fold decrease in plating efficiency of gD-positive PrV, indicating restoration of interference by coexpression of gD and HveC (Fig. 6A).
FIG. 6.
Lack of gD-mediated interference in PrV infection of CHO cells. PrV-1112 (A) and PrV gD− Pass (B) were titrated on CHO and gD-expressing CHO cells, RK13 and gD-expressing RK13 (RK13-gD) cells, and MDBK and gD-expressing MDBK (MT50-5) cells. Relative titers compared to parental RK13, MDBK, and CHO cells are indicated. In addition, parallel titrations were performed on HveC-expressing CHO cells after transfection with either a control plasmid (HveC) or a gD-expression plasmid [HveC (gD)]. Data are averages of three independent experiments. Vertical lines indicate standard deviations.
Increased sensitivity of PrV to gB neutralization in the absence of gD receptors.
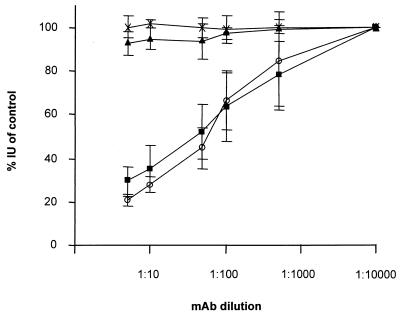
CHO cells appear to be deficient in gD receptor(s). However, since infection of CHO cells by PrV still can proceed, albeit significantly less efficiently than on gD receptor-expressing cells, we analyzed whether one of the other glycoproteins involved in entry, i.e., gB and the gH/L complex, did exhibit an altered biological function. To this end, neutralization tests involving MAbs directed against gB, gC, gD, and gH and a polyclonal gL-specific serum were performed. Virions were plated onto RK13 and CHO cells, and plaques or infected cells or foci were counted. For most antibodies, there was no difference in the result on RK13 or CHO cells (data not shown). However, as shown in Fig. 7, anti-gB MAb b43-b5 efficiently neutralized PrV infectivity only on CHO cells and not on RK13 cells, although the input amount of virus to obtain a similar MOI on both cell lines was significantly higher for CHO than for RK13 cells. Interestingly, on HveC-expressing CHO cells, neutralization was not observed. This indicates that absence of gD receptors renders virions sensitive to neutralization by this anti-gB MAb. To obtain independent confirmation of this correlation, virus was incubated with the MAb and plated onto gD-expressing RK13 cells. These cells presumably lack gD receptors due to intracellular sequestration. As also demonstrated in Fig. 7, infectivity of PrV on RK13-gD cells was inhibited by MAb b43-b5 whereas infection of parental RK13 cells was not impaired. Thus, in the absence of gD receptors, the biological role of gB appears to be altered, with an increased sensitivity to neutralization with MAb b43-b5. This MAb only marginally reduces infectivity of PrV gD− Pass on any cell line tested (data not shown).
FIG. 7.
Neutralization assay. PrV-1112 was incubated with the indicated dilutions of anti-gB MAb b43-b5 and subjected to titer determination on RK13 (▴), gD-expressing RK13 (■), CHO (○), or HveC-expressing CHO (X) cells. Indicated are percentages of infectious units (IU) compared to an untreated control. Data are averages of three independent experiments. Vertical lines indicate standard deviations.
Normal and gD-receptor expressing CHO cells are similarly susceptible to infection by infectious gD− PrV.
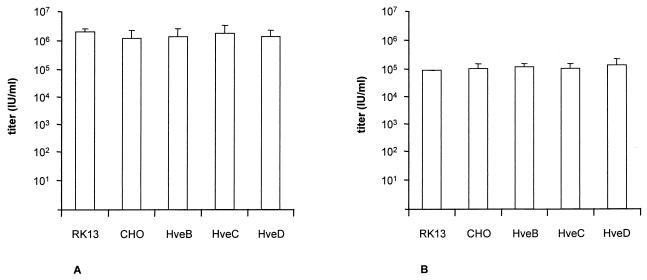
We recently isolated an infectious gD-negative PrV mutant, PrV gD− Pass, and hypothesized that this virus enters cells by a gD- and gD receptor-independent pathway (33). Formal proof of this assumption would be provided by finding a similar sensitivity of isogenic cells either bearing or lacking gD receptors. To test for this, RK13, CHO, and HveB-, HveC-, or HveD-expressing CHO cells were infected with PrV gD− Pass (Fig. 8A) or PrV gCD− Pass (Fig. 8B), and plaques, foci, or single infected cells were counted. As shown in Fig. 8, both infectious gD-negative PrV mutants infected all cells with equal efficiency irrespective of the presence or absence of gD receptors. However, PrV gD− Pass, like wild-type PrV, was unable to form plaques on either normal or gD receptor-expressing CHO cells (Fig. 1). Thus, although PrV gD− Pass virions were capable of efficiently entering CHO cells, they were unable to spread, a prerequisite for plaque formation.
FIG. 8.
Titration of infectious gD− PrV on CHO cells. PrV gD− Pass (A) and PrV gCD− Pass (B) were titrated on RK13, CHO, and recombinant CHO cells expressing HveB, HveC, or HveD. Titers are indicated in infectious units (IU) per milliliter. Data are averages of three independent experiments. Vertical lines indicate standard deviations.
DISCUSSION
CHO cells exhibit a resistance to infection by HSV-1 and PrV which, at least in part, can be attributed to a defect in viral entry. We show here that expression of the herpesvirus gD receptors HveB, HveC, or HveD increases the infectivity of PrV on CHO cells by approximately 100-fold, resulting in plating efficiencies similar to those found on normally susceptible rabbit kidney (this report), porcine, or bovine kidney cells (data not shown). Thus, expression of gD receptors rescues the entry defect of PrV into CHO cells (6, 39) (see above). However, wild-type PrV is still able to infect CHO cells with titers of approximately 105 infectious units per ml. Therefore, there have to be other, so far unknown, receptors on CHO cells which can mediate PrV entry. Whether these receptors also interact with gD or mediate infection by binding to other virion glycoproteins is unclear at present. Our data on the increased sensitivity of PrV infection of cells deficient in gD receptors (CHO and RK13-gD) to neutralization by an anti-gB MAb indicates differences in biological function of gB on these cells compared to gD receptor-expressing cells. This could be indicative of a receptor-binding function of gB which is demonstrable only when the gD–gD receptor interaction is missing.
However, there is a distinct quantitative difference between the infectivity of PrV-gD− on gD receptor-expressing cells and the infectivity of wild-type PrV on CHO cells deficient in gD receptors. Infectivity of PrV-gD− on normal host cells is decreased by approximately 105-fold compared to that of wild-type PrV (31), whereas titers of wild-type PrV on CHO cells are decreased by only approximately 102-fold (see above). Thus, there could be additional gD receptors present on CHO cells. Alternatively, gD could execute functions beyond receptor binding, e.g., in initiation of membrane fusion. Our data on the lack of interference by expression of gD in CHO cells, as well as similar sensitivity of PrV to anti-gB neutralization on CHO and RK13-gD cells, favors the latter alternative.
We also demonstrated that infectious entry of PrV into CHO cells does not occur by endocytosis, which is in contrast to electron microscopic observations by others (32). Presumably, the decrease in plating efficiency of PrV on CHO cells of ca. 2 log10 units can be attributed to a defect in entry which results in extracellularly remaining virus being endocytosed at later times. Endocytosis of adsorbed PrV virions in wild-type virus infection of normally susceptible cells has been demonstrated (8). Interestingly, penetration of PrV into CHO cells is strongly delayed, a defect which can also be rescued by expression of gD receptor. Thus, interaction of gD with its receptor appears to be required for rapid membrane fusion. Whether this is solely due to the stronger and presumably closer binding of virions to cells via gD-gD receptor interaction or whether the reaction of gD with its receptor triggers conformational changes required for membrane fusion is unclear.
Whereas the defect in entry of PrV into CHO cells is overcome by expression of HveB, HveC, or HveD (6, 39) (see above), viral propagation in these cells as assayed by one-step growth kinetics did not differ in wild-type or HveC-expressing CHO cells, which may be distinct from the situation in HSV-1 (28). Thus, besides the defect at entry, there must be restriction later in the infectious cycle, leading to inefficient production of infectious virus, which is not affected by the expression of gD receptors. Since infectivity was assayed by visualization of β-galactosidase activity in infected cells, and since in our recombinants lacZ is under control of the “early” glycoprotein G promoter, this additional defect appears to restrict virus replication after early gene expression. The defect seen in one-step growth correlates with an inability of wild-type PrV to form plaques on either normal or gD receptor-expressing cells. Thus, expression of receptors rescued the entry defect but did not result in plaque formation.
Isolation of an infectious gD-negative PrV mutant, PrV gD− Pass, by serial passaging in cell culture indicated that compensatory mutations can render gD nonessential for viral replication (33). So far, it was unclear whether other (glyco)proteins would acquire the receptor-binding function of gD and substitute for gD in interaction with the gD receptor or whether a gD receptor-independent entry pathway is being used by PrV gD− Pass (16). Data presented here show that PrV gD− Pass infects cells with defects in gD receptors just as well as it infects cells expressing gD receptors. This indicates that PrV gD− Pass indeed uses an entry pathway independent of HveB, HveC, or HveD. Titers of PrV gD− Pass on CHO cells were approximately 10-fold higher than that of wild-type PrV, indicating that PrV gD− Pass is well adapted to infect gD receptor-deficient cells. Interestingly, PrV gD− Pass, like wild-type PrV, was also not able to form plaques on normal or gD receptor-expressing CHO cells. Therefore, the restriction in cell-to-cell spread affected both viruses independent of the mode of entry. So far, it is unclear at which step in viral replication this intracellular restriction takes place.
In summary, we show that expression of gD receptors in CHO cells increases the infectivity of PrV by approximately 100-fold and enhances the kinetics of penetration to levels seen in other susceptible cells. However, viral yields from CHO cells and plaque formation were not affected by expression of gD receptors, indicating the presence of other restrictions beyond the entry defect. Our data also show that PrV is able to infect CHO cells, which is accompanied by an increased sensitivity to anti-gB neutralization. Thus, there must be other PrV receptors on these cells besides the identified gD-interacting molecules. Lastly, efficient infection of CHO cells by PrV gD− Pass indicates that this virus mutant relies on an entry pathway which does not involve any of the known gD receptors.
ACKNOWLEDGMENTS
This study was supported by the Deutsche Forschungsgemeinschaft (Me 854/4-1).
We thank P. G. Spear for the generous gift of recombinant CHO cells expressing HveB, HveC, or HveD.
REFERENCES
- 1.Blumenthal R, Bali-Puri A, Walter A, Covell D, Eidelman O. pH-dependent fusion of vesicular stomatitis virus with Vero cells. J Biol Chem. 1987;262:13614–13619. [PubMed] [Google Scholar]
- 2.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chase C C, Lohff C, Letchworth G J D. Resistance and susceptibility of bovine cells expressing herpesviral glycoprotein D homologs to herpesviral infections. Virology. 1993;194:365–369. doi: 10.1006/viro.1993.1269. [DOI] [PubMed] [Google Scholar]
- 4.Chase C C, Carter-Allen K, Lohff C, Letchworth G. Bovine cells expressing bovine herpesvirus 1 (BHV1) glycoprotein IV resist infection by BHV1, herpes simplex virus, and pseudorabies virus. J Virol. 1990;64:4866–4872. doi: 10.1128/jvi.64.10.4866-4872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 7.Gerdts V, Jöns A, Makoschey B, Visser N, Mettenleiter T C. Protection of pigs against Aujeszky’s disease by DNA vaccination. J Gen Virol. 1997;78:2139–2146. doi: 10.1099/0022-1317-78-9-2139. [DOI] [PubMed] [Google Scholar]
- 8.Granzow H, Weiland F, Jöns A, Klupp B, Karger A, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson D C, Ligas M W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson D C, Burke R L, Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990;64:2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson R M, Spear P G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989;63:819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan A S, Vatter A. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959;13:78–92. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 14.Karger A, Mettenleiter T C. Glycoproteins gIII and gp50 play dominant roles in the biphasic attachment of pseudorabies virus. Virology. 1993;194:654–664. doi: 10.1006/viro.1993.1305. [DOI] [PubMed] [Google Scholar]
- 15.Karger A, Saalmüller A, Tufaro F, Banfield B W, Mettenleiter T C. Cell surface proteoglycans are not essential for infection by pseudorabies virus. J Virol. 1995;69:3482–3489. doi: 10.1128/jvi.69.6.3482-3489.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karger A, Schmidt J, Mettenleiter T C. Infectivity of a pseudorabies virus mutant lacking attachment glycoproteins C and D. J Virol. 1998;72:7341–7348. doi: 10.1128/jvi.72.9.7341-7348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klupp B G, Fuchs W, Weiland E, Mettenleiter T C. Pseudorabies virus glycoprotein gL is necessary for virus infectivity but dispensable for virion localization of glycoprotein H. J Virol. 1997;71:7687–7695. doi: 10.1128/jvi.71.10.7687-7695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klupp, B. G., and E. Weiland. Unpublished results.
- 19.Krummenacher C, Nicola A, Whitbeck J C, Lou H, Hou W, Lambris J, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpes virus entry mediator, two structurally unrelated mediators of viral entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee W-C, Fuller A O. Herpes simplex virus type 1 and pseudorabies virus bind to a common saturable receptor on Vero cells that is not heparan sulfate. J Virol. 1993;67:5088–5097. doi: 10.1128/jvi.67.9.5088-5097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, van Drunen Littel-van den Hurk S, Babiuk L A, Liang X. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol. 1995;69:4758–4768. doi: 10.1128/jvi.69.8.4758-4768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClain D S, Fuller A O. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology. 1994;198:690–702. doi: 10.1006/viro.1994.1081. [DOI] [PubMed] [Google Scholar]
- 23.Mettenleiter T C. Glycoprotein gIII deletion mutants of pseudorabies virus are impaired in virus entry. Virology. 1989;171:623–625. doi: 10.1016/0042-6822(89)90635-1. [DOI] [PubMed] [Google Scholar]
- 24.Mettenleiter T C. Initiation and spread of α-herpesvirus infections. Trends Microbiol. 1994;2:2–4. doi: 10.1016/0966-842x(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 25.Mettenleiter T C. Pseudorabies (Aujeszky’s disease) virus: state of the art. Acta Vet Hung. 1994;42:153–177. [PubMed] [Google Scholar]
- 26.Mettenleiter T C, Rauh I. A glycoprotein gX–β-galactosidase fusion gene as insertional marker for rapid identification of pseudorabies virus mutants. J Virol Methods. 1990;30:55–66. doi: 10.1016/0166-0934(90)90043-f. [DOI] [PubMed] [Google Scholar]
- 27.Mettenleiter T C, Zsak L, Zuckermann F, Sugg N, Kern H, Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparin-like substance mediates adsorption of pseudorabies virus. J Virol. 1990;64:278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 29.Okazaki K, Matsuzaki T, Sugahara Y, Okadad J, Hasebe M, Iwamura Y, Ohnishi M, Kanno T, Shimizu M, Honda E, Kono Y. BHV-1 adsorption is mediated by the interaction of glycoprotein gIII with heparin-like moiety on the cell surface. Virology. 1991;181:666–670. doi: 10.1016/0042-6822(91)90900-v. [DOI] [PubMed] [Google Scholar]
- 30.Petrovskis E A, Meyer A L, Post L E. Reduced yield of infectious pseudorabies virus and herpes simplex virus from cell lines producing viral glycoprotein gp50. J Virol. 1988;62:2196–2199. doi: 10.1128/jvi.62.6.2196-2199.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawitzky D, Hampl H, Habermehl K-O. Entry of pseudorabies virus into CHO cells is blocked at the level of penetration. Arch Virol. 1990;115:309–316. doi: 10.1007/BF01310539. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt J, Klupp B G, Karger A, Mettenleiter T C. Adaptability in herpesviruses: glycoprotein D-independent infectivity of pseudorabies virus. J Virol. 1997;71:17–24. doi: 10.1128/jvi.71.1.17-24.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schröder C, Linde G, Fehler F, Keil G M. From essential to beneficial: glycoprotein D loses importance for replication of bovine herpesvirus 1 in cell culture. J Virol. 1997;71:25–33. doi: 10.1128/jvi.71.1.25-33.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shieh M-T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 37.Subramanian G, McClain D S, Perez A, Fuller A O. Swine testis cells contain functional heparan sulfate but are defective in entry of herpes simplex virus. J Virol. 1994;68:5667–5676. doi: 10.1128/jvi.68.9.5667-5676.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian G, LeBlanc R, Wardley R C, Fuller A O. Defective entry of herpes simplex virus types 1 and 2 into porcine cells and lack of infection in infant pigs indicate species tropism. J Gen Virol. 1995;76:2375–2379. doi: 10.1099/0022-1317-76-9-2375. [DOI] [PubMed] [Google Scholar]
- 39.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 40.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]