Video
XXX.
The usefulness of endoscopic submucosal dissection (ESD) for superficial esophageal carcinoma above esophageal varices associated with liver cirrhosis (LC) has been reported.1,2 However, the treatment of gastric varices is more complicated than that of esophagogastric varices,3 and reports of ESD for early gastric cancer (EGC) above gastric varices (GVs) associated with LC are lacking. In this study, we report a case of EGC above GVs that was safely treated with ESD after percutaneous transhepatic obliteration (PTO) and partial splenic embolization (PSE) of GVs (Video 1, available online at www.videogie.org).
The patient was a 69-year-old woman with a history of pyloric gastrectomy for gastric cancer and ESD for EGC located on the anterior wall of the lesser curvature of the residual stomach. She had been diagnosed with Child–Pugh grade A cirrhosis and esophagogastric varices due to nonalcoholic steatohepatitis. She had been visiting the hospital for follow-up of her gastric cancer and LC. A follow-up EGD revealed a new EGC, prompting another visit to our hospital.
A type 0–IIc lesion with a size of 30 mm was located at the lesser curvature of the cardia; an EV with F1 formed on the oral side of the lesion, and GVs with F2 formed just below the lesion. Additionally, the lesion on the anterior wall side was adjacent to a scar due to a previous ESD (Fig. 1). EUS revealed large-diameter vessels in the submucosa (Fig. 2). Due to the potential risk of significant bleeding during the ESD procedure, the treatment of GVs was prioritized. Endoscopic injection sclerotherapy was challenging due to the narrow diameter of the esophageal varices. Moreover, in cases of extravascular injection, ESD following endoscopic injection sclerotherapy could be more difficult to perform due to submucosal fibrosis, which can cause submucosal inflammation. Additionally, a 3-dimensional CT scan showed GVs extending to the splenic vein, without spleno-renal shunting; therefore, performing balloon-occluded retrograde transvenous obliteration was considered difficult. We decided that the GVs under the EGC should be treated with PTO before ESD. Following the placement of a contrast catheter in the splenic vein, 2 branches entering the GVs were identified and subsequently embolized (Fig. 3) (Supplementary Fig. 1, available online at www.videogie.org). Additionally, PSE was performed to reduce portal pressure. EGD revealed a reduction of GVs at 7 days after embolization (Fig. 4). Therefore, ESD for EGC was performed at 14 days after embolization (Fig. 5). On occurrence of bleeding during ESD, performing endoscopic hemostasis using hemoclips or hemostatic forceps was planned. Furthermore, on persistent bleeding despite endoscopic hemostasis, we were prepared to perform surgical gastrectomy. Therefore, ESD was performed with the patient under general anesthesia with the surgeon on call. Submucosal injection of 10% glycerin solution (Glyceol; Chugai Pharmaceutical Co, Ltd, Tokyo, Japan) was administered using a 23-gauge sharp needle (ICHIBANYARI; KANEKA, Tokyo, Japan). The energy device used was a high-frequency electrosurgical machine (VIO 3; ERBE Elektromedizin, Tübingen, Germany). A 2-mm needle knife with an injection function (Dual Knife J; Olympus, Tokyo, Japan) was used for mucosal incision (Endocut function, 1-3-1) and submucosal dissection (PreciseSECT, efficacy 3.0; Swift Coagulation, efficacy 3.0; Spray Coagulation, efficacy 2.0). A pair of 5-mm hemostatic forceps (Coagrasper; Olympus) was used to cauterize the vessel (Soft Coagulation, efficacy 7.4). First, a glycerin solution with a small amount of indigocarmine and epinephrine was carefully injected, and a shallow mucosal incision was made on the anterior wall, anal, and scar sides of the lesion to avoid bleeding from the vessels. The deep mucosal incision was made after the exposed vessels were coagulated. Precoagulation of vessels using hemostatic forceps was easy because the varicose vein had been sufficiently reduced by PTO + PSE. Second, we made a shallow mucosal incision on the esophageal side, and then a full circumferential incision. The lesion was dissected using the clip-with-line traction technique. Submucosal dissection was performed by dissecting the entire varicose vein at a layer deeper than the varicose vein, leading to less bleeding during ESD.
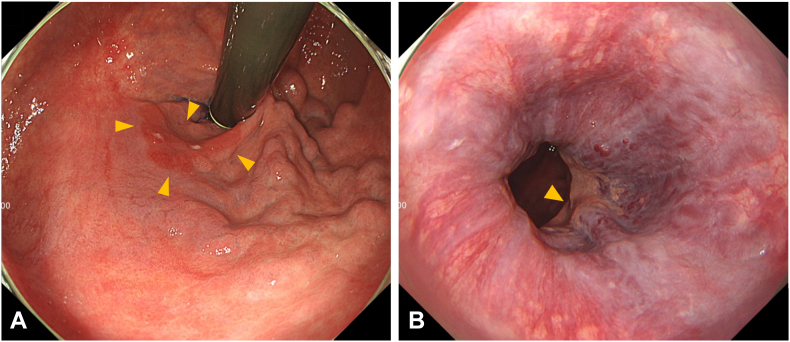
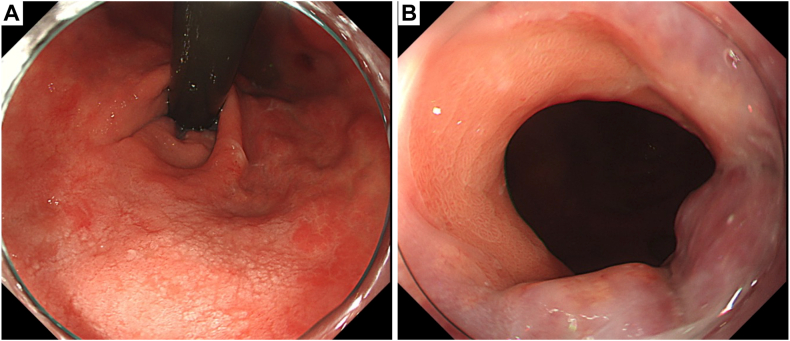
Figure 1.
The white-light image of the lesion reveals a 30-mm 0–IIc lesion located near the posterior wall of the lesser curvature of the cardia (red arrow). A, F2 gastric varices directly below the lesion. B, F1 esophagogastric varices on the lesion’s oral side.
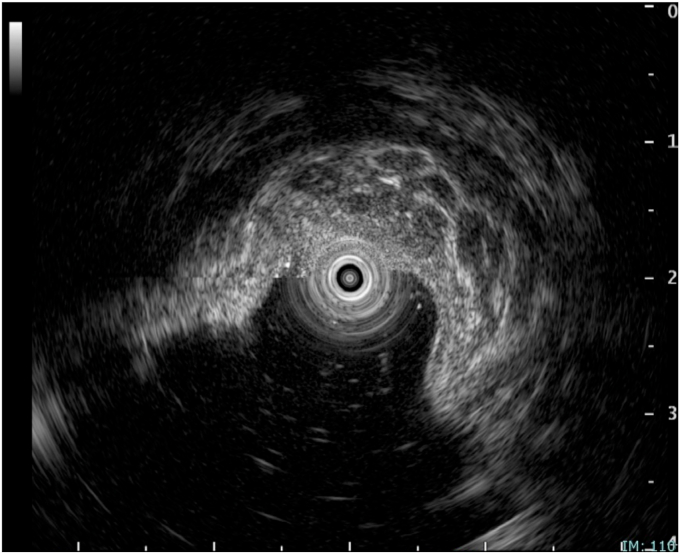
Figure 2.
EUS shows large-diameter vessels within the submucosa.
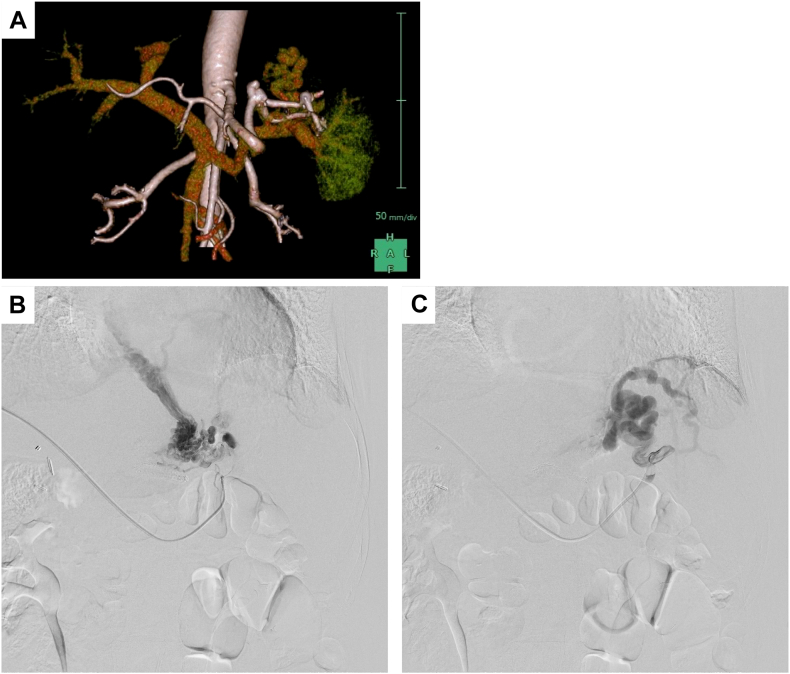
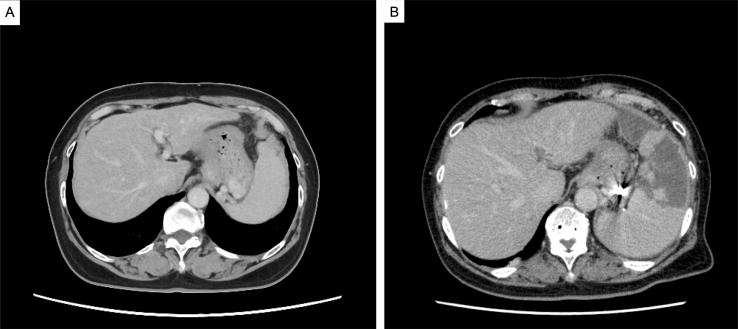
Figure 3.
Esophagogastric varices. A, A 3-dimensional CT scan shows gastric varices leading to the splenic vein, and no spleno-renal shunting was observed. B and C, Two branches entering the gastric varices were identified and subsequently treated with percutaneous transhepatic obliteration. Additionally, partial splenic embolization was performed to alleviate portal pressure.
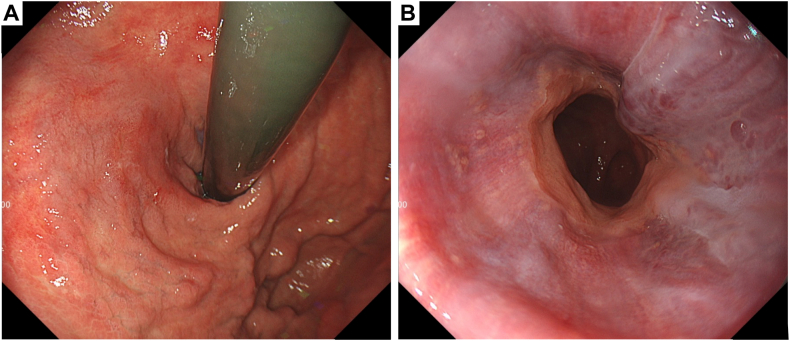
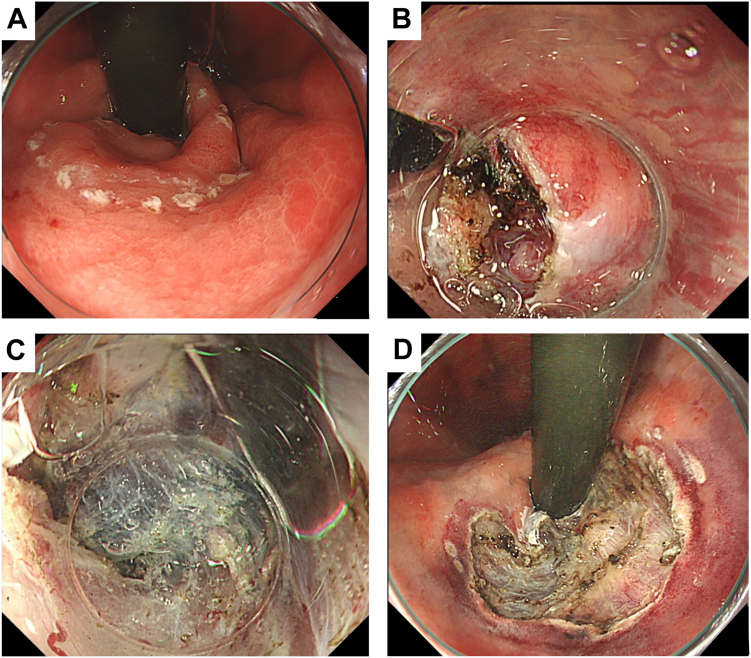
Figure 4.
At 7 days after percutaneous transhepatic obliteration and partial splenic embolization, the gastric varices showed a tendency to shrink (A), and the esophageal varices did not worsen (B).
Figure 5.
At 14 days after percutaneous transhepatic obliteration and partial splenic embolization, endoscopic submucosal dissection was performed. A, The gastric varices show a further shrinkage trend. B, The esophageal varices did not worsen.
En bloc resection was achieved in 80 minutes without any adverse events (Fig. 6). After ESD, the patient was hospitalized for 5 days as originally planned in Japan. The final pathology result was well-differentiated intramucosal adenocarcinoma with negative horizontal and vertical margins (Fig. 7). In conclusion, by performing PTO and PSE for GVs before ESD, the procedure could safely be performed without severe bleeding from GVs.
Figure 6.
Endoscopic submucosal dissection. A, Markings were placed around the lesion. B and C, A shallow mucosal incision was made, the blood vessels were exposed, and the deeper incision were made while the vessels were cauterized. D, White-light image of the post–endoscopic submucosal dissection ulcer. En bloc resection was achieved without any adverse events.
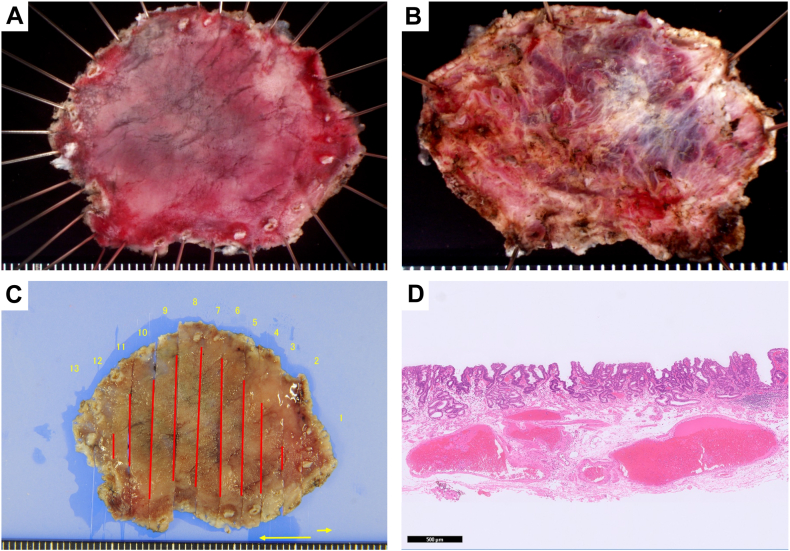
Figure 7.
Image of the resected specimen and pathological findings. A, En bloc resection was achieved. B, Dissection, including the gastric varices, was performed (the back of the resected specimen). C, Mapping image shows a negative horizontal margin. D, The final pathology result was a well-differentiated intramucosal adenocarcinoma (H&E, orig. mag. ×20).
Disclosure
All authors disclose no financial relationships.
Supplementary data
ESD for early gastric cancer with esophagogastric varices safely treated after PTO and PSE.
Supplementary Fig 1.
References
- 1.Sawaguchi M., Jin M., Matsuhashi T., et al. The feasibility of endoscopic submucosal dissection for superficial esophageal cancer in patients with cirrhosis (with video) Gastrointest Endosc. 2014;79:681–685. doi: 10.1016/j.gie.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Robbins G., Kantsevoy S., Raina A. Variceal ligation in decompensated cirrhosis before esophageal endoscopic submucosal dissection. VideoGIE. 2022;7:432–435. doi: 10.1016/j.vgie.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry Z., Patel K., Patton H., Saad W. AGA clinical practice update on management of bleeding gastric varices: expert review. Clin Gastroenterol Hepatol. 2021;19:1098–1107. doi: 10.1016/j.cgh.2021.01.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
XXX.
ESD for early gastric cancer with esophagogastric varices safely treated after PTO and PSE.