Abstract
Background/Aims
Vasomotor symptoms (VMS) adversely affect postmenopausal quality of life. However, their association with bone health has not been elucidated. This study aimed to systematically review and meta-analyze the evidence regarding the association of VMS with fracture risk and bone mineral density (BMD) in peri- and postmenopausal women.
Methods
A literature search was conducted in PubMed, Scopus and Cochrane databases until 31 August 2023. Fracture, low BMD (osteoporosis/osteopenia) and mean change in lumbar spine (LS) and femoral neck (FN) BMD were assessed. The results are presented as odds ratio (OR) and mean difference (MD), respectively, with a 95% confidence interval (95% CI). The I2 index quantified heterogeneity.
Results
Twenty studies were included in the qualitative and 12 in the quantitative analysis (n=49,659). No difference in fractures between women with and without VMS was found (n=5, OR 1.04, 95% CI 0.93–1.16, I2 16%). However, VMS were associated with low BMD (n=5, OR 1.54, 95% CI 1.42–1.67, I2 0%). This difference was evident for LS (MD -0.019 g/cm2, 95% CI -0.03 to -0.008, I2 85.2%), but not for FN BMD (MD -0.010 g/cm2, 95% CI -0.021 to 0.001, I2 78.2%). These results were independent of VMS severity, age and study design. When the analysis was confined to studies that excluded menopausal hormone therapy use, the association with BMD remained significant.
Conclusions
The presence of VMS is associated with low BMD in postmenopausal women, although it does not seem to increase fracture risk.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-024-07075-8.
Keywords: Osteoporosis, Fractures, hot flushes, Night sweats, Vasomotor symptoms, Postmenopausal women
Introduction
Vasomotor symptoms (VMS), mainly hot flushes and night sweats, are quite common in women entering menopause and adversely affect their quality of life [1, 2]. An abrupt increase in VMS occurs during the first two years before the final menstrual period (FMP) (i.e., during the late perimenopausal phase) in 60-80% of women, presenting a peak one year after the FMP. VMS persist in 50% of cases during the first four years after the FMP [1, 2]. Interestingly, 10% of postmenopausal women may experience VMS even 12 years after their FMP [1]. The prevalence of VMS varies across different geographic regions and is estimated at 22-63% in Asia, 36-50% in North America and 45-74% in Europe [3].
VMS reflect the fluctuations in the hormonal milieu during the transition to menopause [1, 2]. Except for their impact on quality of life, severe VMS have been associated with increased prevalence of cardiovascular risk factors, such as type 2 diabetes mellitus (T2DM), smoking and obesity [4], predisposing to increased risk of cardiovascular disease (CVD), especially in women younger than 60 years [5]. Furthermore, the peak VMS prevalence coincides with accelerated bone loss during the menopausal transition [6]. Therefore, the severity of VMS has been proposed as a marker of climacteric bone loss [6]. The first report of a potentially adverse effect of VMS on peri- and postmenopausal bone health was made in 1994 by Lee et al., who showed that the prevalence of osteoporosis in postmenopausal women who recalled severe and persistent VMS was higher than those who had no VMS [7]. After that, several studies of different designs had contradictory results regarding the impact of VMS both on fracture risk and low BMD in peri- and postmenopausal women [8–13].
The primary aim of this study was to systematically review and meta-analyze the available evidence regarding the association between VMS and the risk of fractures or low BMD (osteopenia/osteoporosis) in peri- and postmenopausal women. The secondary endpoint was to investigate the difference in BMD between women with and without VMS.
Materials and Methods
Guidelines followed
The present study followed the MOOSE (Meta-analyses Of Observational Studies in Epidemiology) guidelines [14] and has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) system (CRD42023387161).
Search strategy
Six reviewers (G.A, K.B, D.D, M.Z, A.P, E.F.) conducted a literature search in the electronic databases MEDLINE (PubMed), Scopus and Cochrane (CENTRAL) until 31 August 2023. A representative example of search strategy in MEDLINE was : (“hot flush*”[tiab] OR “hot flash*”[tiab] OR “night sweat*”[tiab] OR “vasomotor symptom*”[tiab] OR “menopausal symptom*”[tiab] OR “postmenopausal symptom*”[tiab] OR “post-menopausal symptom*”[tiab] OR climacteric[tiab]) AND (fracture[MeSH] OR fracture*[tiab] OR “bone density”[MeSH] OR “bone mineral density”[tiab] OR BMD[tiab] OR “bone mass”[tiab]). Two senior authors resolved discrepancies. Furthermore, conference abstracts, grey literature (using Opengrey.eu) and reference sections of the included studies were screened manually. E-mails were sent to the authors of the eligible studies to obtain missing data.
Study selection
The following PICO (Population, Intervention, Comparison and Outcome) elements were set as inclusion criteria: (i) Population: peri- or postmenopausal women, (ii) Intervention: VMS (hot flushes and/or night sweats), (iii) Comparison: peri- or postmenopausal women without VMS, (iv) Outcome: fractures (vertebral, non-vertebral, hip), low BMD (defined as osteopenia or osteoporosis) and mean difference in BMD between groups. Observational studies (cohort, cross-sectional and case-control) and randomized-controlled trials were included in the analysis. Exclusion criteria were: (i) studies that involved solely premenopausal women, (ii) studies that did not provide information on fracture incidence or BMD, (iii) non-English papers and (iv) animal studies.
Menopause was defined as the absence of menstruation for ≥12 months (natural menopause) or the period of time starting immediately after bilateral oophorectomy (surgical menopause). Women were classified as “premenopausal” if they had no change in menstrual regularity during the past year, or as “perimenopausal”, if they reported menstruation during the past three months, but with decreased irregularity (early perimenopausal) or no menses during the past 3-11 months (late perimenopausal).
Data extraction
Two independent researchers extracted data from eligible studies that met the predefined criteria. A standardized form was used to record: (i) first author, (ii) year of publication, (iii) country in which the study was conducted, (iv) study design, (v) total sample size, (vi) mean participants’ age, (vii) number of patients with and without VMS, (viii) type and severity of VMS, (ix) number of patients with fractures (hip, vertebral, non-vertebral), (x) number of patients with low BMD, (xi) BMD at lumbar spine (LS), femoral neck (FN) and total hip (TH) BMD in VMS and non-VMS groups.
Risk of bias and study quality assessment
The Newcastle-Ottawa scale (NOS) was used to evaluate the quality of the selected studies. This scale consists of the following domains: (i) selection of participants, with a maximum rating of four stars, (ii) comparability of study groups, with a maximum of two stars, and (iii) assessment of outcome or exposure, with the highest rating reaching three stars. According to NOS, a study is characterized as “good quality” if it gets 3-4 stars in the selection domain, 1-2 stars in the comparability domain, and 2-3 stars in the outcome/exposure domain. “Fair quality” is considered when the study gets two, 1-2 and 2-3 stars in the selection, comparability and outcome/exposure domain, respectively. Finally, a study is characterized as “poor quality” in the case of 0-1, 0 and 0-1 stars in the selection, comparability and outcome/exposure domain, respectively [15].
Statistical analysis
The dichotomous data results regarding the association between VMS status and the risk of fracture or low BMD were expressed as odds ratio (OR), with 95% confidence interval (CI), whereas the difference in BMD between women with and without VMS was expressed as mean difference (MD) with 95% CI. Heterogeneity among studies was quantified by the I2 index, with values 30% being considered as “low”, 30- 60% as “moderate” and >60% as “high heterogeneity” [16]. The fixed effect model was used for low heterogeneity, whereas the random effects model was used for cases with moderate or high heterogeneity.
Subgroup and sensitivity analysis was implicated to eliminate possible sources of heterogeneity. Also, in order to investigate the effect of possible modifiers (i.e., baseline patients’ characteristics, such as age) on the outcome, meta-regression analysis (based on the random effects model) was conducted. The risk of publication bias was examined with the Harbord-Egger’s test [16]. All statistical tests were two-sided, with a p-value <0.05 considered significant. All analyses were conducted using the Review Manager (RevMan 5.4.1 software) computer program (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, September 2020).
Results
Descriptive analysis
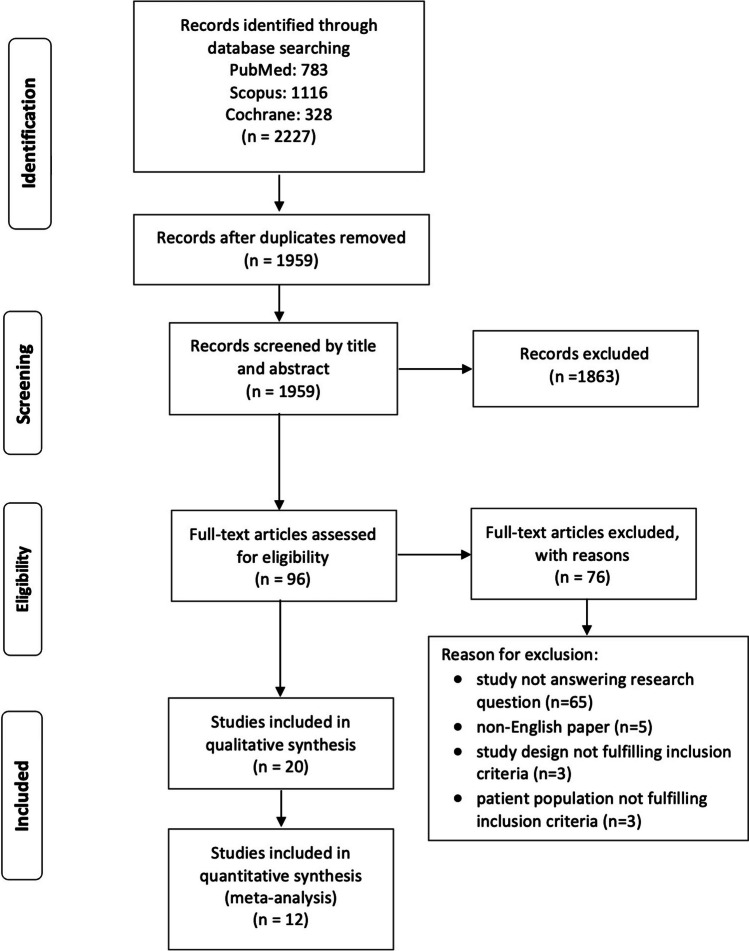
The initial search provided 1,959 results after excluding duplicates, 96 of which were reviewed for full-text eligibility. Of those, 76 articles were excluded with reasons (Supplementary Table 1). Finally, 20 studies were selected for qualitative and 12 for quantitative analysis [7–10, 12, 13, 17–22] (eight studies [11, 23–29] were excluded from the meta-analysis due to a lack of extractable data for comparisons). Notably, the study by Nudy et al. [11], and Hippisley-Cox et al. [29], were excluded since they did not provide the exact number of patients with VMS, either as hot flushes or night sweats. A flow chart diagram is illustrated in Fig. 1.
Fig. 1.
Flow chart diagram
Five [7, 8, 12, 19, 22] studies investigated the association between VMS and fracture risk, whereas five [7, 10, 12, 13, 21] addressed the occurrence of low BMD (osteopenia or osteoporosis). Furthermore, eight [8, 9, 12, 17–20, 22], seven [8, 9, 12, 18–20, 22] and four [9, 18, 20, 22] studies provided data regarding the difference in LS, FN, TH BMD, respectively, between patients with and without VMS. Overall, 49,659 patients were included in the analysis.
VMS and risk of fracture
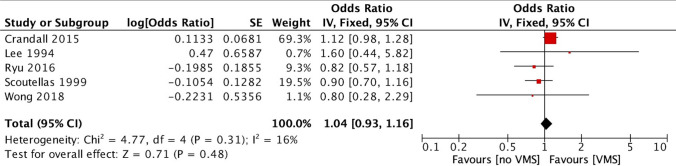
The five studies [7, 8, 12, 19, 22] assessing the association between VMS and fractures, found no increased risk in women with VMS compared with those without VMS (OR 1.04, 95% 0.93–1.16, I2 16%) (Fig. 2). Regarding the type of fracture, three studies provided data on vertebral fractures showing again no association with VMS (OR 0.95, 95% CI 0.77–1.19, I2 0%) [7, 8, 19]. Only the study by Crandall et al., published in 2015 [8], provided data on hip fractures, showing increased risk in women with moderate/severe VMS (compared with no VMS) [hazard ratio (HR) 1.78, 95% CI 1.20–2.64].
Fig. 2.
Forest plot of the fracture risk between women with and without vasomotor symptoms (VMS)
VMS and risk of low BMD (osteopenia or osteoporosis)
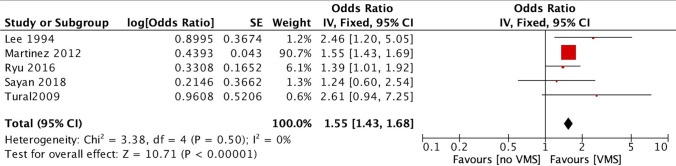
Since only three studies provided data for the diagnosis of osteoporosis and two studies for osteopenia, these were combined into one category termed “low BMD” [7, 10, 12, 13, 21]. The presence of VMS was associated with an increased risk of low BMD compared with the absence of VMS (OR 1.55, 95% CI 1.43–1.68, I2 0%) (Fig. 3).
Fig. 3.
Forest plot of the low BMD risk (osteopenia or osteoporosis) between women with and without vasomotor symptoms (VMS)
VMS and mean difference in BMD
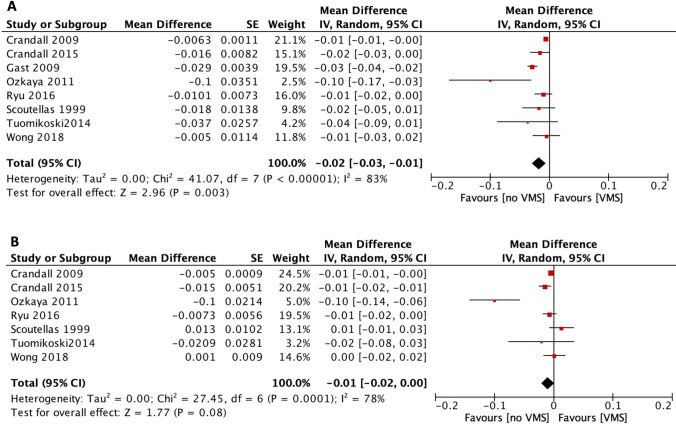
Regarding LS BMD, patients with VMS had lower values compared with those without VMS (n=8, MD -0.019 g/cm2, 95% CI -0.03 to -0.008, I2 85.2%) [8, 9, 12, 17–20, 22]. In contrast, there was no difference in FN (n=7) [8, 9, 12, 18–20, 22] or TH BMD (n=4) [9, 18, 20, 22] between women with and without VMS [MD -0.010 g/cm2 (95% CI -0.021 to 0.001, I2 78.2%) and -0.040 g/cm2 (95% CI -0.0 to 0.008, I2 94.2%, respectively)]. The difference in LS and FN BMD between women with and without VMS is presented in Fig. 4.
Fig. 4.
Forest plot of the difference in A) lumbar spine (LS) and B) femoral neck (FN) BMD between women with and without vasomotor symptoms (VMS)
Subgroup analysis
The effect of VMS severity on fracture risk and BMD
Three studies [7, 8, 12] were eligible for comparing moderate/severe VMS and no VMS, while two studies [8, 12] provided data on mild VMS. No association with the risk of fracture was found when the analysis was confined to these subgroups [OR 1.08 (95% CI 0.79–1.46, I2 40%) and 1.08 (95% CI 0.60–1.94, I2 64%) for moderate/severe and mild VMS, respectively, compared with no VMS]. However, both moderate/severe (n=4) [7, 10, 12, 13] and mild VMS (n=2) [10, 12] were associated with an increased risk of low BMD compared with no VMS [OR 1.79 (95% CI 1.63–1.96, I2 0%) and 1.28 (95% CI 1.15–1.41, I2 0%), respectively].
Moreover, women with moderate/severe VMS demonstrated lower LS (n=4, MD -0.025 g/cm2, 95% CI -0.042 to -0.008, I2 72.9%) [8, 12, 17, 20] and FN BMD (n=3, MD -0.011 g/cm2, 95% CI -0.02 to -0.004, I2 0%), respectively) than those without VMS [8, 12, 20]. Mild VMS were also associated with lower BMD in LS, but not FN, compared with no VMS. There were no available data regarding the effect of VMS severity on TH BMD.
The effect of study design on the risk of fracture and BMD
Regarding fracture risk, there was still no association with VMS, when the analysis was limited to cohort studies (n=2, OR 1.11, 95% CI 0.98–1.28) [8, 22] or case-control studies (n=2, OR 0.86, 95% CI 0.61–1.22) [7, 12]. Furthermore, when data from case-control studies [7, 12, 13] were analyzed separately, the risk of low BMD still was increased in women with a history of VMS (OR 1.39, 95% CI 1.06–1.82) similarly to cross-sectional studies (OR 1.56, 95% CI 1.43–1.69) [10, 21]. Regarding BMD, cohort and cross-sectional studies showed similar differences for LS and FN between groups.
The effect of age on fracture risk and BMD
Only one study provided sufficient data for different age groups regarding the fracture risk [8], showing an increased risk of hip fracture in patients with VMS only for the older age group (70-79 years old; HR 1.93, 95% CI 1.11–3.53). No such risk was demonstrated for vertebral and non-vertebral fractures [8].
Notably, meta-regression analysis showed that age was not a predictor neither for fracture risk (p=0.11) nor for low BMD (p=0.707). Age also did not affect MD in LS (p=0.807) and FN BMD (p=0.498) between groups.
Sensitivity analysis
A sensitivity analysis was performed according to study quality and MHT use. Classification of studies according to NOS is presented in Supplementary Table 2. After excluding two fair-quality studies [12, 18], the risk estimation for fractures, low BMD and LS or FN BMD remained unaltered.
Concerning MHT use, there were four studies [13, 17, 19, 22] in which MHT was used by a variable proportion of patients (although MHT was included in the multivariable-adjusted models). However, the association between VMS and low BMD and MD in LS BMD between groups remained significant after restricting the analysis to studies where MHT was an exclusion criterion, but presence of VMS and fracture risk remained not significant (OR 1.12, 95% CI 0.98–1.28).
Publication bias
Publication bias was not evident regarding the risk of fracture (p=0.546) or low BMD (p=0.435), and TH BMD (p>0.20). However, Egger’s test showed significance for MD in LS (p=0.09) and FN BMD (p=0.06), possibly due to the effect of small sample size on study outcomes (Supplementary Figure 1).
Discussion
This study shows that the presence of VMS in peri- and postmenopausal women is associated with a higher degree of bone loss, since these women are more prone to osteopenia or osteoporosis than those without VMS. This effect is independent of age, VMS severity, study quality and MHT use, although moderate/severe VMS affect LS and FN BMD to a greater extent than mild VMS. However, no association between VMS and fracture risk was found.
This meta-analysis is the first considering the effect of VMS on bone health in postmenopausal women. Recent studies have underscored the impact of the severity of VMS on the cardiovascular system, including risk factors, such as diabetes, smoking, obesity [4], and CVD events, especially in women younger than 60 years [5]. Therefore, the presence and severity of VMS appear as key players in the accelerated bone loss in peri- and postmenopausal women, necessitating the administration of MHT. Another clinical implication is that women who experience moderate/severe VMS constitute a distinct postmenopausal population at higher risk of osteoporosis and, potentially, fractures and should be screened by dual-energy X-ray absorptiometry (DXA) earlier than the generally recommended threshold of 65 years [30].
Estrogens are essential for acquiring and maintaining peak bone mass by acting at the osteoclast and osteoblast levels [31]. In particular, estrogen receptors α upregulate the expression of Wnt co-receptor lipoprotein receptor-related protein (LRP)-5, which is essential for osteoblast differentiation. They also inhibit osteoblast apoptosis by decreasing the production of Fas ligand (FasL) [31]. At the osteoclast level, they prevent osteoclast differentiation and proliferation indirectly through the osteoprotegerin (OPG)/receptor activator of NF-κB (RANK)/RANK ligand (RANKL) axis [31]. They also induce osteoclast apoptosis (through the FasL) and inhibit osteocyte apoptosis by increasing the expression of semaphorin 3A [31]. During the menopausal transition (i.e., from the first year before and three years after the FMP), a 9-10% bone loss occurs [32]. This fact is of clinical relevance in women with early menopause (<45 years), a category that is predisposed to increased fracture risk [33].
The exact pathogenetic mechanisms linking VMS with bone loss are not fully understood. Most [34, 35], but not all studies [36], have shown that the decline in estradiol (E2) concentrations is associated with VMS. However, the individual’s sensitivity to the fluctuations rather than the absolute decrease in E2 concentrations seem to account for the association of VMS with accelerated bone loss [17]. Furthermore, increased sympathetic activity has been reported in women with VMS [37], which fosters bone loss through increased bone resorption and decreased bone formation. Indeed, β2- and α-adrenergic receptors have been identified in human osteoblasts [38, 39]. Cortisol concentrations also increase after hot flushes, contributing to bone loss [40]. Another potential mechanism could be endothelial dysfunction [41] and increased concentrations of inflammatory markers, such as interleukin-8 and tumor necrosis factor α; these molecules have been identified in the presence of VMS and may impair bone quality [42]. Finally, VMS are associated with increased oxidative stress, which inhibits the Wnt pathway and, subsequently, bone formation [43].
Some limitations should be acknowledged, such as the heterogeneity regarding the definition of VMS and study design, and the increased risk of recall or selection bias. However, the effect of these limitations on the present study’s validity was mitigated by applying meta-regression, subgroup and sensitivity analysis, which did not significantly modify the study’s outcomes (except for the effect of MHT on fracture risk). The heterogeneity in the DXA device and the number of vertebrae assessed constitutes another limitation. Finally, no subgroup analysis for peri- and postmenopausal women could be performed by available data.
In conclusion, this study shows that the history of VMS (hot flushes and/or night sweats), especially in their moderate/severe form, is associated with greater bone loss, compared with no VMS, in peri- and postmenopausal women. This association involves both LS and FN BMD, potentially predisposing the woman to an increased risk of fracture, although this was not proven by the present study. This aspect of postmenopausal health, in combination with CVD, should be considered in future studies, designating the potentially beneficial role of MHT in this population.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file3 Funnel plots assessing publication bias for each outcome and p-value from Egger’s test: a) risk of fracture (p=0.425); b) low BMD (p=0.470); c) LS BMD (p=0.06); d) FN BMD (p=0.11); e) TH BMD, p=0.08 (TIFF 29512 KB)
Funding
Open access funding provided by HEAL-Link Greece.
Declarations
Competing interest
Panagiotis Anagnostis, Konstantinos Lallas, Anna Pappa, Georgios Avgeris, Kristina Beta, Dimitrios Damakis, Eirini Fountoukidou, Maria Zidrou, Irene Lambrinoudaki and Dimitrios G. Goulis declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Politi MC, Schleinitz MD, Col NF (2008) Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med 23:1507–1513 10.1007/s11606-008-0655-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Granger AL, Fehnel SE, Levine KB, Jordan J, Clark RV (2008) Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric 11:32–43 10.1080/13697130701744696 [DOI] [PubMed] [Google Scholar]
- 3.Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P (2010) Age of menopause and impact of climacteric symptoms by geographical region. Climacteric 13:419–428 10.3109/13697137.2010.507886 [DOI] [PubMed] [Google Scholar]
- 4.Armeni E, Kopanos S, Verykouki E, et al. (2023) The severity of menopausal symptoms is associated with diabetes, and cardiometabolic risk factors in middle-aged women. Minerva Endocrinol (Torino) [DOI] [PubMed]
- 5.Armeni A, Anagnostis P, Armeni E, Mili N, Goulis D, Lambrinoudaki I (2023) Vasomotor symptoms and risk of cardiovascular disease in peri- and postmenopausal women: A systematic review and meta-analysis. Maturitas 171:13–20 10.1016/j.maturitas.2023.02.004 [DOI] [PubMed] [Google Scholar]
- 6.Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B (2006) Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab 91:1261–1267 10.1210/jc.2005-1836 [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Kanis JA (1994) An association between osteoporosis and premenstrual symptoms and postmenopausal symptoms. Bone Miner 24:127–134 10.1016/S0169-6009(08)80150-X [DOI] [PubMed] [Google Scholar]
- 8.Crandall CJ, Aragaki A, Cauley JA et al (2015) Associations of menopausal vasomotor symptoms with fracture incidence. J Clin Endocrinol Metab 100:524–534 10.1210/jc.2014-3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crandall CJ, Zheng Y, Crawford SL, Thurston RC, Gold EB, Johnston JM, Greendale GA (2009) Presence of vasomotor symptoms is associated with lower bone mineral density: a longitudinal analysis. Menopause 16:239–246 10.1097/gme.0b013e3181857964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez Perez JA, Palacios S, Chavida F, Perez M (2013) Severity of menopausal symptoms and cardiovascular and osteoporosis risk factors. Climacteric 16:226–234 10.3109/13697137.2012.688077 [DOI] [PubMed] [Google Scholar]
- 11.Nudy M, Jiang X, Aragaki AK et al (2020) The severity of vasomotor symptoms and number of menopausal symptoms in postmenopausal women and select clinical health outcomes in the Women’s Health Initiative Calcium and Vitamin D randomized clinical trial. Menopause 27:1265–1273 10.1097/GME.0000000000001667 [DOI] [PubMed] [Google Scholar]
- 12.Ryu KJ, Park HT, Kim YJ, Yi KW, Shin JH, Hur JY, Kim T (2016) Vasomotor symptoms and osteoporosis in Korean postmenopausal women. Maturitas 87:27–32 10.1016/j.maturitas.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 13.Sayan S, Pekin T, Yildizhan B (2018) Relationship between vasomotor symptoms and metabolic syndrome in postmenopausal women. J Int Med Res 46:4157–4166 10.1177/0300060518790709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 15.Wells GA SB, O’Connell D, Peterson J, Welch V, Tugwell P (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. In: 3rd symposium on systematic reviews: beyond the basics. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 16.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VAe (2023) Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane
- 17.Gast GC, Grobbee DE, Pop VJ, Keyzer JJ, Wijnands-van Gent CJ, Samsioe GN, Nilsson PM, van der Schouw YT (2009) Vasomotor symptoms are associated with a lower bone mineral density. Menopause 16:231–238 10.1097/gme.0b013e318185e25b [DOI] [PubMed] [Google Scholar]
- 18.Ozkaya E, Cakir E, Kara F, Okuyan E, Cakir C, Ustun G, Kucukozkan T (2011) Impact of hot flashes and night sweats on carotid intima-media thickness and bone mineral density among postmenopausal women. Int J Gynaecol Obstet 113:235–238 10.1016/j.ijgo.2010.12.020 [DOI] [PubMed] [Google Scholar]
- 19.Scoutellas V, O’Neill TW, Lunt M, Reeve J, Silman AJ (1999) Does the presence of postmenopausal symptoms influence susceptibility to vertebral deformity? European Vertebral Osteoporosis Study (EVOS) Group. Maturitas 32:179–187 10.1016/S0378-5122(99)00025-0 [DOI] [PubMed] [Google Scholar]
- 20.Tuomikoski P, Ylikorkala O, Mikkola TS (2015) Postmenopausal hot flushes and bone mineral density: a longitudinal study. Acta Obstet Gynecol Scand 94:198–203 10.1111/aogs.12546 [DOI] [PubMed] [Google Scholar]
- 21.Tural A, Yoldemir T, Erenus M (2009) Assessment of bone mineral density should be considered earlier in perimenopausal women with vasomotor symptoms. Int J Gynaecol Obstet 107:114–116 10.1016/j.ijgo.2009.07.015 [DOI] [PubMed] [Google Scholar]
- 22.Wong EMM, Tomlinson G, Pinto MM, Berger C, Cheung AM, Prior JC (2018) Women's Mid-Life Night Sweats and 2-Year Bone Mineral Density Changes: A Prospective, Observational Population-Based Investigation from the Canadian Multicentre Osteoporosis Study (CaMos). Int J Environ Res Public Health 15: [DOI] [PMC free article] [PubMed]
- 23.Grainge MJ, Coupland CA, Cliffe SJ, Chilvers CE, Hosking DJ (2001) Reproductive, menstrual and menopausal factors: which are associated with bone mineral density in early postmenopausal women? Osteoporos Int 12:777–787 10.1007/s001980170055 [DOI] [PubMed] [Google Scholar]
- 24.Jaber RM, Khalil IA, Almohtasib YS, Hamdan M, AlRyalat SA (2022) Association of menopausal symptom severity and osteoporotic fractures: a case-control study. J Women Aging 34:93–100 10.1080/08952841.2020.1803183 [DOI] [PubMed] [Google Scholar]
- 25.Naessen T, Persson I, Ljunghall S, Bergstrom R (1992) Women with climacteric symptoms: a target group for prevention of rapid bone loss and osteoporosis. Osteoporos Int 2:225–231 10.1007/BF01624146 [DOI] [PubMed] [Google Scholar]
- 26.Ahn S, Song R (2009) Bone mineral density and perceived menopausal symptoms: factors influencing low back pain in postmenopausal women. J Adv Nurs 65:1228–1236 10.1111/j.1365-2648.2009.04983.x [DOI] [PubMed] [Google Scholar]
- 27.Coronado PJ, Monroy M, Fasero M, Sanchez-Borrego R, Palacios S, Rejas J, Ruiz MA, AcgftsopvotC Short-Form (2021) Population-based norms for the Cervantes-SF short-form questionnaire assessing health-related quality of life in menopause. Maturitas 146:34–41 10.1016/j.maturitas.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 28.Crandall CJ, Larson J, Cauley JA, Schousboe JT, LaCroix AZ, Robbins JA, Watts NB, Ensrud KE (2019) Do Additional Clinical Risk Factors Improve the Performance of Fracture Risk Assessment Tool (FRAX) Among Postmenopausal Women? Findings From the Women’s Health Initiative Observational Study and Clinical Trials. JBMR Plus 3:e10239 10.1002/jbm4.10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hippisley-Cox J, Coupland C (2009) Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ 339:b4229 10.1136/bmj.b4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, Rosen HN, Weber DR, Zemel BS, Shepherd JA (2019) Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J Clin Densitom 22:453-471 [DOI] [PubMed]
- 31.Anagnostis P, Bosdou JK, Vaitsi K, Goulis DG, Lambrinoudaki I (2021) Estrogen and bones after menopause: a reappraisal of data and future perspectives. Hormones (Athens) 20:13–21 10.1007/s42000-020-00218-6 [DOI] [PubMed] [Google Scholar]
- 32.Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ, Lee JS, Karlamangla AS (2012) Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res 27:111–118 10.1002/jbmr.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anagnostis P, Siolos P, Gkekas NK et al (2019) Association between age at menopause and fracture risk: a systematic review and meta-analysis. Endocrine 63:213–224 10.1007/s12020-018-1746-6 [DOI] [PubMed] [Google Scholar]
- 34.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG (2005) Hot flushes during the menopause transition: a longitudinal study in Australian-born women. Menopause 12:460–467 10.1097/01.GME.0000155200.80687.BE [DOI] [PubMed] [Google Scholar]
- 35.Overlie I, Moen MH, Holte A, Finset A (2002) Androgens and estrogens in relation to hot flushes during the menopausal transition. Maturitas 41:69–77 10.1016/S0378-5122(01)00256-0 [DOI] [PubMed] [Google Scholar]
- 36.Randolph JF Jr, Sowers M, Bondarenko I, Gold EB, Greendale GA, Bromberger JT, Brockwell SE, Matthews KA (2005) The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab 90:6106–6112 10.1210/jc.2005-1374 [DOI] [PubMed] [Google Scholar]
- 37.Mashchak CA, Kletzky OA, Artal R, Mishell DR Jr (1984) The relation of physiological changes to subjective symptoms in postmenopausal women with and without hot flushes. Maturitas 6:301–308 10.1016/0378-5122(84)90001-X [DOI] [PubMed] [Google Scholar]
- 38.Kondo H, Nifuji A, Takeda S, Ezura Y, Rittling SR, Denhardt DT, Nakashima K, Karsenty G, Noda M (2005) Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J Biol Chem 280:30192–30200 10.1074/jbc.M504179200 [DOI] [PubMed] [Google Scholar]
- 39.Togari A (2002) Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech 58:77–84 10.1002/jemt.10121 [DOI] [PubMed] [Google Scholar]
- 40.Cignarelli M, Cicinelli E, Corso M, Cospite MR, Garruti G, Tafaro E, Giorgino R, Schonauer S (1989) Biophysical and endocrine-metabolic changes during menopausal hot flashes: increase in plasma free fatty acid and norepinephrine levels. Gynecol Obstet Invest 27:34–37 10.1159/000293612 [DOI] [PubMed] [Google Scholar]
- 41.Hildreth KL, Ozemek C, Kohrt WM, Blatchford PJ, Moreau KL (2018) Vascular dysfunction across the stages of the menopausal transition is associated with menopausal symptoms and quality of life. Menopause 25:1011–1019 10.1097/GME.0000000000001112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang WY, Hsin IL, Chen DR, Chang CC, Kor CT, Chen TY, Wu HM (2017) Circulating interleukin-8 and tumor necrosis factor-alpha are associated with hot flashes in healthy postmenopausal women. PLoS One 12:e0184011 10.1371/journal.pone.0184011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manolagas SC, Almeida M (2007) Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol 21:2605–2614 10.1210/me.2007-0259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file3 Funnel plots assessing publication bias for each outcome and p-value from Egger’s test: a) risk of fracture (p=0.425); b) low BMD (p=0.470); c) LS BMD (p=0.06); d) FN BMD (p=0.11); e) TH BMD, p=0.08 (TIFF 29512 KB)