Abstract
Summary
Review of medical records from 173 women with osteoporosis who received abaloparatide treatment revealed that 96.0% had at least one visit for osteoporosis management and 55.5% had medication support group access. The most common reasons for discontinuing treatment were financial (31.2%) and tolerability (22.8%). Most patients (64.8%) completed treatment as prescribed.
Purpose
Abaloparatide is approved for the treatment of women with postmenopausal osteoporosis at high risk for fracture. This study evaluated real-world treatment patterns for patients new to abaloparatide, regardless of osteoporosis treatment history.
Methods
Data for patients with ≥ 1 prescription for abaloparatide were collected retrospectively from six academic and clinical practice settings across the US.
Results
A total of 173 patients were enrolled (mean [SD] age, 69.8 [7.4] years). At the time of abaloparatide treatment initiation, 78.6% had received other osteoporosis medications. Mean (SD) time from discontinuation of osteoporosis medications prior to initiation of abaloparatide was 1.7 (3.2) years. Twenty-four months of follow-up data from the initiation date of abaloparatide was collected from 94.0% of patients and 6.0% of patients had 12–24 months of follow-up. During the follow-up period, 96.0% of patients had at least one visit for osteoporosis management and 55.5% had access to a medication support program. The median duration of therapy was 18.6 months and 105/162 (64.8%) completed abaloparatide treatment as prescribed. The most common reasons for treatment discontinuation were financial (31.2%) and tolerability (22.8%). Following completion of a course of treatment with abaloparatide, 82/162 (50.6%) patients transitioned to another osteoporosis medication. The median time between abaloparatide treatment course completion and the initiation of follow-on medication was 21 days.
Conclusion
Most patients completed treatment with abaloparatide as prescribed, and over half continued with an antiresorptive agent. This favorable conduct may be the result of regular follow-up visits and accessibility to both medication and patient support services.
Keywords: Abaloparatide, Anabolic therapy, Osteoporosis, Treatment patterns, Treatment persistence
Introduction
Osteoporosis (OP) is a systemic skeletal disease associated with low bone mineral density (BMD), microarchitectural deterioration, and an increased risk of fracture [1]. The estimated prevalence of OP in the US is 10.2 million with an additional 43.4 million patients with low bone mass [2]. Osteoporosis-related fractures result in significant reductions in health-related quality of life, which persist years after the occurrence of the fracture and are greater in patients who sustain more than one fracture over time [3].
In addition to the disease burden, economic consequences are also increased for both the patient and society. In 2016, approximately 1.8 million Medicare beneficiaries sustained an average of 2.1 OP-related fractures at a total estimated cost of $5.7 billion for subsequent fractures alone [4]. Management of sentinel fractures and the patients’ underlying OP is of paramount importance for reducing the economic burden of disease, given the high, and often imminent, risk for a subsequent fracture, as well as the high incremental cost associated with these events among patients incurring a subsequent fracture versus those without a subsequent fracture [5].
Clinical practice guidelines recommend anabolic therapies for the treatment of patients at very high risk for fractures [6]. Patients with a recent fracture in the past 12 months, with a history of multiple fractures, who have sustained a fracture while on approved osteoporosis therapy or while on medications causing bone loss, patients with a very low T-score, or at a high risk for falls or at a high risk for fracture by fracture risk assessment tool (FRAX) may be categorized as such [6, 7]. Despite these recommendations to test and/or treat patients for osteoporosis following a fracture event, the majority of fracture patients remain undiagnosed and untreated [8]. A large proportion of patients who begin therapy discontinue treatment [9], and most receive little to no benefit from that treatment. Any benefits realized may wane quickly, thus perpetuating the increased risk for fracture [10, 11]. Low reported satisfaction with treatments for osteoporosis is associated with increased rates of discontinuation and changes in therapy during the first year of treatment. Changes in treatment may also be due to the financial or clinical burden associated with the disease. Limited knowledge on the management of OP and inadequate follow-up may also contribute to discontinuation of treatment [12, 13].
Abaloparatide (TYMLOS©, Radius Health, Inc., Boston, MA) is a parathyroid hormone-related protein analogue (PTHrP [1–34]) indicated for the treatment of postmenopausal women with osteoporosis at high risk of fracture, and as treatment to increase bone density in men with osteoporosis at high risk of fracture [14]. In an 18-month, phase 3, double-blind, randomized controlled trial (Abaloparatide Comparator Trial [ACTIVE]) of postmenopausal women with OP, abaloparatide significantly reduced the relative risk of vertebral (86%, P < 0.001) and nonvertebral (43%, P = 0.049) fractures compared to placebo, and reduced the risk of major osteoporotic fractures (55.0%, P = 0.03) compared to teriparatide, with comparable safety across treatment groups [15].
In a real-world study, the effectiveness and cardiovascular safety of abaloparatide were compared with teriparatide in propensity score–matched patients (N = 11,616 per treatment cohort) for 19 months following the index prescription date. The nonvertebral fracture event rate was 2.9% with abaloparatide and 3.2% with teriparatide (P = NS). The hip fracture event rate was lower for abaloparatide (1.0%) than for teriparatide (1.3%; P = 0.04) [16]. The risks for major adverse cardiovascular events (MACE) with and without a diagnosis of heart failure were comparable for the two anabolic agents.
In a real-world study of patient experience (HEOR-001), most patients reported a high level of satisfaction with abaloparatide and that it was convenient and easy to use [17]. Data on abaloparatide real-world treatment patterns and patient journey have not been previously evaluated. Furthermore, reasons for treatment change cannot be ascertained from secondary data sources (e.g., claims data, electronic health records). The objective of this study (HEOR-002) was to evaluate the management of postmenopausal osteoporosis in women treated with abaloparatide, as well as treatment patterns in the 24-month period after treatment initiation. Persistence with abaloparatide was evaluated, as well as duration of treatment with prior OP therapies, and time between discontinuation of prior therapy and initiation of abaloparatide. This study was completed prior to the approval for male indication and therefore does not include assessment of abaloparatide use in men.
Methods
Study design
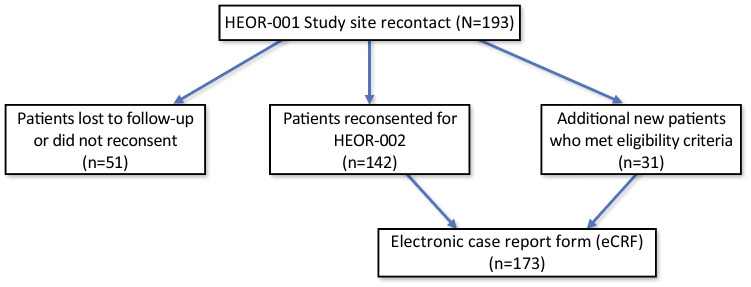
This is a multicenter, observational study with primary data collected from six geographically distinct bone health centers across the US. Eight participating sites from the aforementioned patient experience study (HEOR-001) [17] were recontacted to participate in the follow-up study. Of these, seven sites agreed to participate and six provided data. The sites included both community and academic-affiliated physician practices to improve the generalizability of findings. Of 193 original participants from HEOR-001, 142 were re-enrolled in the HEOR-002 study. The remaining 51 patients were lost to follow-up or did not provide informed consent for the new study. Thirty-one new patients were enrolled in the study to partially replace those patients, bringing the total sample to 173 participants (Fig. 1). The study was approved by an Institutional Review Board (IRB) at each site or at a central IRB if the institution did not have one.
Fig. 1.
Patient enrollment. Of the original 193 patients recontacted from HEOR-001, 51 patients were lost to follow up and 142 patients reconsented to participate in HEOR-002. An additional 31 new patients met eligibility criteria for a total of 173 patients participating in HEOR-002. Primary patient data from the 173 participants was collected and entered into an electronic case report form (eCRF)
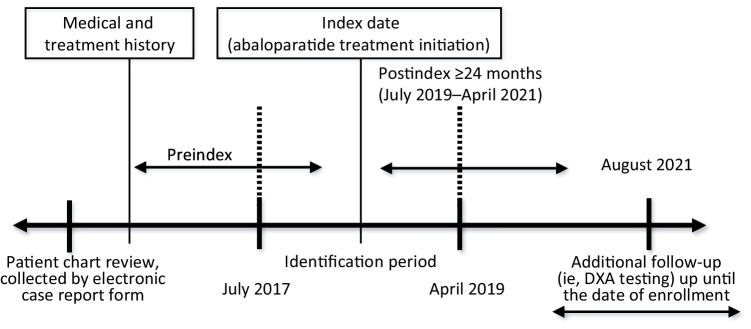
Patients with an abaloparatide prescription between July 2017 and April 2019 (identification period) were identified. Prescription data were gathered by completing a standardized electronic case report form (eCRF) by the clinician or researcher based on patient medical records. The follow-up time for osteoporosis treatment was 24 months from the date of the first abaloparatide prescription. DXA results were included up to the date of study enrollment. Medical and treatment history as well as follow-up treatment patterns were extracted retrospectively from patients’ medical records through August 2021 (Fig. 2).
Fig. 2.
Study design. Patient data collected from electronic case report form (eCRF) were reviewed for medical and treatment history during the preindex period. The index date refers to the date of abaloparatide treatment initiation, which occurred during the identification period between July 2017 and April 2019. The postindex period was a period of ≥ 24 months from the date of abaloparatide treatment initiation, between July 2019 and April 2021. Additional follow-up (i.e., DXA testing) occurred up until the date of study enrollment
Inclusion criteria
The study included postmenopausal women with a physician-confirmed diagnosis of osteoporosis considered at high risk for fracture. All patients had to have ≥ 1 prescriptions for abaloparatide for at least a month. Patients re-enrolled from the HEOR-001 study were required to have at least 24 months of follow-up information from the initiation of treatment with abaloparatide, while patients newly enrolled in the study were required to have at least 12 months of follow-up information. Patients were also required to be treated in an outpatient setting at the time of study enrollment. All patients provided voluntary informed consent for the use of their information.
Exclusion criteria
Patients with Paget disease of bone, preexisting hypercalcemia, primary hyperparathyroidism, urolithiasis, hypercalciuria, skeletal malignancies, and those participating in any clinical trial at the inception of the study were excluded. All patients were new to abaloparatide, though some were not anabolic-naïve and had switched from teriparatide. Patients with prior treatment with any OP medications were included.
Source of data
Primary data were collected by the physician or a delegated healthcare professional from the participating sites and entered an eCRF using secure and password protected, electronic data capture (EDC) software. Data storage was compliant with the Health Insurance Portability and Accountability Act (HIPAA), and the EDC software was certified to be compliant with the FDA’s 21 CRF Part 11 regulation. No patient’s identity or medical records were disclosed for the purposes of this study. Only data de-identified in compliance with 45 CFR 164.514(a)-(c) and accessed using HIPAA-compliant procedures were used. Pharmacy claims data were not accessible or utilized for this study.
Variable definitions
Patients were categorized based on whether they (1) discontinued abaloparatide and initiated another OP treatment prior to 24 months of anabolic treatment or (2) discontinued therapy with one OP medication and did not start any other OP medications. Treatment gap was defined as a deliberate break in treatment as instructed by a physician. An exploratory analysis of change in BMD T-score was done in a subgroup of patients for whom baseline, and follow-up data were available. Baseline data were from DXA scans done any time from 6 months prior to abaloparatide initiation up to 1 month after initiation. When there were multiple DXA scans, the scan done closest to the initiation date was used. Follow-up data were from DXA scans done between 9 and 15 months of abaloparatide initiation. When multiple DXA scans were available, the scan closest to 12 months post initiation was used. Lastly, 2-year evaluation data were from DXA scans done between 21 and 27 months of abaloparatide initiation. When multiple DXA scans were available, the scan closest to 24 months post abaloparatide initiation was used.
Statistical analyses
The primary objective of this study was descriptive in nature with no formal sample size calculations. For continuous variables, number of patients, mean, standard deviation, median, interquartile range (Q1 and Q3), and minimum and maximum values are provided. For categorical variables, the number and percentage of patients in each category are provided. All analyses were conducted using Stata 16.0 or later.
Missing data and imputations
Any variables with over 50% missing data were analyzed to identify any systematic differences in comparison to patients without missing data. Partial dates where only month and year were known were assigned full date data using the midpoint (15th) of the month in order to create a full date.
Results
The total sample for the study was 173 patients (Fig. 1). Approximately 24 months of follow-up data were collected from 94.0% of the patients, and the remaining 6.0% had between 12 and 24 months of available follow-up data.
Demographic and clinical characteristics
Patient mean (SD) age was 69.8 (7.4) years (Table 1). Mean (SD) body mass index (BMI) was 24.8 kg/m2 (5.1) and 43.4% had a BMI in the overweight (25.0 to 29.9 kg/m2) or obese (30.0 kg/m2 or higher) range. The most common chronic preexisting conditions were cardiovascular disease (50.3%), gastrointestinal conditions (32.4%), and endocrine disorders (24.9%).
Table 1.
Patient characteristics
| Patient characteristics | N = 173 |
|---|---|
|
Age (years), mean ± SD minimum, maximum |
69.8 (7.4) 54, 93 |
|
BMI (kg/m2), mean ± SD minimum, maximum |
24.8 (5.1) 16.6, 44.6 |
| Concomitant conditions, n (%) | |
| Cardiovascular | 87 (50.3) |
| GI conditions | 56 (32.4) |
| Endocrine disorders | 43 (24.9) |
| Psychiatric disorders | 38 (22.0) |
| Cancer/hematological disorders | 15 (8.7) |
| Renal disease | 2 (1.2) |
| Others | 41 (23.7) |
| None | 35 (20.2) |
| N = 138 | |
|
Duration of OP (months), mean ± SD minimum, maximum |
96.2 (61.4) 19.5, 302.0 |
BMI body mass index, GI gastrointestinal, OP osteoporosis, SD standard deviation
Baseline history of osteoporosis and treatment
Mean (SD) time since diagnosis of OP at the time of study enrollment was 96.2 (61.4) months. Just over half of patients (52.0%) were diagnosed through routine screening, 28.9% were diagnosed at the time of fracture, and 20% were diagnosed due to risk factors or OP symptoms. Patients could have multiple circumstances leading to OP diagnosis. Bone mineral density assessments by DXA were used to confirm a diagnosis of OP in 82.1%, and conventional x-ray confirmed the diagnosis in 12.1% of patients. Most patients (162/173 [93.6%]) had ≥ 1 DXA scans after initiation of treatment with abaloparatide. The median baseline T-scores were − 2.50, − 2.00, and − 2.85 for femoral neck, total hip, and lumbar spine, respectively.
Most patients (136/173 [78.6%]) had prior treatment for osteoporosis before abaloparatide and (45/136 [33.1%]) had previously been treated with an anabolic drug. The mean (SD) time between discontinuation of a prior OP medication and initiation of abaloparatide was 1.7 (3.2) years. Twenty-four percent (42/173) of patients changed treatment from teriparatide to abaloparatide, and the reasons for switching included a mandatory formulary switch (33.3%), financial/copay concerns (19.0%), tolerability issues with teriparatide (19.0%), lack of efficacy (9.5%), and hypercalcemia (4.8%). For the remaining patients, the reason for switching was unknown or not available.
Follow-up monitoring
In the 2-year follow-up period after beginning abaloparatide, 96.0% of patients visited with a healthcare professional for their osteoporosis, with a median of 3 visits. Over half (55.5%) received drug support for their osteoporosis medication. Of these, 26.0% were participants in a support program provided by Radius Health, Inc. Of 54 patients with OP fractures since diagnosis, 27.8% participated in a fracture liaison service program (FLS; a program to support patients in the weeks after a fracture has been surgically treated).
Central DXA scans were used most frequently (93.6%) to monitor patients; 8.1% used conventional x-ray; 7.5% assessed vertebral fractures by spine radiography or vertebral fracture analysis (VFA). Bone turnover markers (BTMs) were assessed in (70/173 [40.5%] patients) at some point following their diagnosis, with a median of 3 assessments. Of those evaluated with BTMs, 65 patients received at least one postbaseline BTM assessment, with 46 (65.7%) for P1CP or procollagen type 1 N-terminal propeptide (formation markers) and 26 (37.1%) for markers of resorption urinary or serum collagen type 1 cross-linked C-telopeptide.
Treatment patterns following abaloparatide treatment initiation
Over half of patients (84/158 [53.2%]) were treated with abaloparatide for ≥ 18 months (Table 2). The median duration of anabolic therapy for patients who completed treatment was 18.6 months. Mean (SD) duration of abaloparatide therapy was 17.2 (8.1) months and was longer for patients who were previously treated for OP (18.0 [8.2] months) compared to those without a history of OP treatment (14.6 [7.3] months). Median duration of cumulative exposure to anabolics, including patients who stopped and restarted therapy, was 22.5 months. Study sites were asked to indicate whether patients adhered to treatment (took medication as prescribed). About 5.6% of patients had a gap in therapy with a mean (SD) of 62.4 (90.5) days. The most common reasons for discontinuation of treatment were financial in nature (31.2%) and tolerability issues (22.8%) (Table 3).
Table 2.
Treatment patterns following initiation of abaloparatide
| Abaloparatide treatment duration (N = 158) | ||
| At least 18 months (≥ 547 days), (n, %) | 84 | 53.2 |
| Less than 18 months (< 547 days), (n, %) | 74 | 46.8 |
| At least 24 months (≥ 730 days), (n, %) | 44 | 27.8 |
| Mean (days/months) | 524.1 | 17.2 |
| Median (days/months) | 567.0 | 18.6 |
| Standard deviation (days/months) | 246.7 | 8.1 |
| Teriparatide duration before abaloparatide initiation (N = 42) | ||
| Mean (days/months) | 368.0 | 12.1 |
| Median (days/months) | 328.5 | 10.8 |
| Standard deviation (days/months) | 346.8 | 11.4 |
| Discontinued abaloparatide and initiated another OP treatment prior to 24 months of anabolic treatment (out of the 74 patients who discontinued within 18 months) | ||
| Total patients who have discontinued within 18 months AND previously received teriparatide, (n, %) | 31 | 41.9 |
| Total anabolic exposure (N = 158) | ||
| Mean (days/months) | 608.3 | 20.0 |
| Standard deviation (days/months) | 301.4 | 9.9 |
| Median (days/months) | 684.7 | 22.5 |
| Interquartile range (days/months) | 406.7, 753.4 | 0.95, 88.6 |
OP osteoporosis
Table 3.
Reason for discontinuation of treatment with abaloparatide
| n (%) | |
|---|---|
| Total enrolled (N = 162) | |
| Patients completed the course of treatment | 105 (64.8) |
| Patients who discontinued treatment | 57 (35.2) |
| Reasons for treatment discontinuation (N = 57) | |
| Financial reasons/copay | 18 (31.2) |
| Poor tolerabilitya | 13 (22.8) |
| Other, reason not included | 12 (21.1) |
| Mandatory formulary switch to another treatment | 5 (8.8) |
| Do not know | 4 (7.0) |
| Patient experience of new conditions | 3 (5.3) |
| Patient requested treatment change | 2 (3.5) |
| Lack of treatment efficacy | 2 (3.5) |
aAmong patients who discontinued abaloparatide due to poor tolerability, one patient each reported: chronic fatigue; developed itching; dizziness and heart palpitations; fatigue and generalized aching; fatigue, weakness, and memory issues; heart pounding/palpitations; heart racing; hypercalcemia; palpitations and intermittent nausea; patient did not feel well on abaloparatide; reported decreased mentation; ringing in ears; and severe gastrointestinal issues, nausea, and vomiting
Treatment after abaloparatide
Fifty-one percent (82/162) continued treatment with another OP medication after their course of abaloparatide within the 24-month follow-up period. Denosumab was the most common initial follow-on therapy (48.8%), followed by zoledronic acid (20.8%). The median time between the completion of treatment with abaloparatide and the beginning of the next therapy was 21 days with a maximum reported time between therapies of 12.3 months. Seventy-two of 82 (87.8%) patients were still on their initial follow-on OP therapy at the end of the study.
Additional treatment following abaloparatide
Fewer than 5% of patients received more than one treatment after abaloparatide and within 24 months of abaloparatide initiation, including alendronate or zoledronic acid. The median time from stopping the first follow-on OP drug after abaloparatide to the second OP drug (index to index) was 3 days. Of the seven patients who switched to a second drug, four (57.1%) did so due to tolerability with the first treatment that followed abaloparatide. For 5/7 (71.4%) patients who started a second follow-on treatment, that therapy was still ongoing at the end of the study period and was to be continued indefinitely if tolerated in 57.1%.
DXA results
Although DXA scan results were available for most patients during the follow-up period, only 48/173 (27.7%) patients (Table 4) had BMD results within the necessary time frames for inclusion in the baseline, 12 months, and 24 months analyses, as described in the methods. Median BMD T-scores increased from baseline (femoral neck, − 2.5; total hip, − 2.0; lumbar spine, − 2.9) compared with 12 months (femoral neck, − 2.4; total hip, − 1.9; lumbar spine, − 1.7) and 24 months (femoral neck, − 2.3; total hip, − 1.8; lumbar spine, − 1.7).
Table 4.
Median BMD T-score changes following abaloparatide
| N | Baseline | N | 12 months | N | 24 months | |
|---|---|---|---|---|---|---|
| Femoral neck | 48 | − 2.5 | 45 | − 2.4 | 36 | − 2.3 |
| Total hip | 51 | − 2.0 | 42 | − 1.9 | 39 | − 1.8 |
| Lumbar spine | 50 | − 2.9 | 43 | − 1.7 | 39 | − 1.7 |
BMD bone mineral density
Discussion
This study was conducted to evaluate treatment patterns with abaloparatide in real-world clinical practice settings. The majority of patients completed 18 months or longer of abaloparatide treatment. Increases in median BMD T-scores were observed in a subset of patients for whom baseline and follow-up data were available.
Disease monitoring of OP occurred more often in this population of patients than in previously reported studies (HEDIS 2020). Persistence with abaloparatide was similar to persistence rates reported in The Medication Use Patterns, Treatment Satisfaction, and Inadequate Control of Osteoporosis Study (MUSIC-OS), an observational study to identify treatment patterns and adherence to oral pharmacological therapy for osteoporosis [9]. In that study, 86.9% of patients had been taking oral therapy for approximately 4 years and, of these, 49.2% had an adherence evaluation of osteoporosis (ADEOS) score of ≥ 20, indicating a high probability of medication adherence. However, persistence rates were affected by GI symptoms, with ADEOS scores ≥ 20 in 45.5% of patients with GI symptoms, compared with 57.6% of patients without GI symptoms. In a retrospective study of postmenopausal women after a fracture, persistence rates for oral bisphosphonates were lower, ranging from 31.7 to 43.4% at 12 months and 19.6 to 23.7% at 24 months [18]. A study by Cheng et al. [19] also found persistence rates among women treated with bisphosphonates to be low, with approximately 35% of patients remaining on therapy after 12 months. Contributing factors to the higher persistence rates with abaloparatide in this study were close monitoring at the participating bone health centers and a good tolerability profile. Of note, only half of patients transitioned to another treatment after abaloparatide. Although follow-up duration was similar for all patients in the study, some patients did not have a follow-up appointment during this period, which may have led to an underestimation of subsequent treatment.
Discontinuation of therapy is associated with increased risk of fracture and higher medical costs [9, 20, 21]. Patients’ reasons for discontinuing treatment include concerns about drug side effects, perceived lack of efficacy, difficulty taking the medication as directed, and medication expense. Low adherence in the Medicare population has been shown to be an independent predictor of future fracture risk [22]. Nearly 60% (34,483/57,913) of women on Medicare did not adhere to osteoporosis treatment in the first year [23]. Lack of adherence was associated with a 20% greater risk of any fracture (odds ratio, 1.2; 95% CI, 1.07–1.35) and higher medical costs (cost ratio, 1.13; 95% CI, 1.06–1.21).
There are two potential reasons why we observed a greater persistence rate for this study. Consistent with a previously published abaloparatide patient experience study by Gold et al. [17], most patients reported high satisfaction with the abaloparatide regimen as it seemed to minimally disrupt their activities of daily living (85%) and was convenient to fit into their daily schedule (84%). All reported taking abaloparatide as directed, and 93% reported never deliberately missing a dose. In the current study, all patients reported having access to their provider for help with their therapy or were participating in a patient support program. Adequate support from a healthcare team has been associated with greater adherence and persistence [17].
Selection bias should be considered as a limitation of this study, due to the nonrandom selection of sites participating in the study. The study sites were chosen based on their prior involvement with HEOR-001. In addition, patients needed to be willing to participate, which may skew enrollment towards those who have had an influential experience. We reported for HEOR-001 that patients had a generally positive experience with abaloparatide. Having access to healthcare support and treatment may be associated with higher socioeconomic status overestimating the favorable outcomes reported here.
Though some patients were lost to follow-up, baseline characteristics of patients who re-enrolled in the current study after the HEOR-001 study and the newly added patients were similar. Generalizability of this study is limited given the use of a convenience sample and the small sample size. However, to address this limitation, the sites were selected from diverse geographical locations in the US and included both academic and community practices. We also did not observe any differences in the patient characteristics between those who participated and completed the current study and those who did not reconsent to participate or who were lost to follow-up.
This is the first study of treatment patterns of patients initiating treatment with abaloparatide in real-world clinical practice settings. Primary data collection allowed for a broad capture rate of disease and treatment history including data on variables not readily available from claims and electronic health records (e.g., BMI and BMD). Furthermore, reasons for switches were available and could provide insights to improve adherence/persistence with therapy.
Many patients who prescribed abaloparatide in this real-world study completed their course of treatment as prescribed. The observed treatment pattern reported may reflect a high level of provider support, regular follow-up and access to patient support, and educational programs. The findings establish the association of regular monitoring/provider support and patient satisfaction with the treatment regimen with persistence, allowing for the potential of improved bone health outcomes.
Acknowledgements
Funding for this study was provided by Radius Health, Inc. (Radius). All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. Editorial support (Allyson Lehrman, DPM and Lacy Miron, PharmD, assembling tables and figures, collating author comments, copyediting, fact checking, and referencing) and graphic services were provided by AOIC, LLC and were funded by Radius. Artificial intelligence (AI) technologies such as Language Learning Models, chatbots, and image creators were not used in the production of this work.
Data availability
Data that underlie the results reported in a published article may be requested for further research 6 months after completion of FDA or EMA regulatory review of a marketing application (if applicable) or 18 months after trial completion (whichever is latest). Radius will review requests individually to determine whether (i) the requests are legitimate and relevant and meet sound scientific research principles, and (ii) are within the scope of the participants’ informed consent. Prior to making data available, requestors will be required to agree in writing to certain obligations, including without limitation, compliance with applicable privacy and other laws and regulations. Proposals should be directed to info@radiuspharm.com.
Declarations
Conflict of interest
DTG is a consultant for Radius Health, Inc. (Radius). TBeckett is a former speaker for Radius, Amgen, and Eli Lilly and an investigator for Radius, and her institution received funding to conduct the study reported here. CD has participated in advisory boards and is a speaker for Amgen, Eli Lilly, and Radius. ALJ is a speaker for Amgen. MM has no conflicts of interest to disclose. AM and TBailey are employees of Adelphi Real World, Bollington, UK, and are paid consultants of Radius. LP and YW are employees of Radius. JC and SAW are former employees of Radius. SAW is currently a consultant for Radius. JMK is a consultant for Radius and Alexion, a speaker for Alexion, Amgen, and Radius, and has participated in advisory boards for Alexion, Amgen, and Radius.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, Siris ES (2022) The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 33(10):2049–2102. 10.1007/s00198-021-05900-y 10.1007/s00198-021-05900-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bone Health and Osteoporosis Foundation. 54 Million Americans Affected by Osteoporosis and Low Bone Mass. June 2, 2014. Accessed November 21, 2022. https://www.bonehealthandosteoporosis.org/2014/06/02/54-million-americans-affected-by-osteoporosis-and-low-bone-mass/
- 3.Gold T, Williams SA, Weiss RJ, Wang Y, Watkins C, Carroll J, Middleton C, Silverman S (2019) Impact of fractures on quality of life in patients with osteoporosis: a US cross-sectional survey. J Drug Assess 8(1):175–183. 10.1080/21556660.2019.1677674 10.1080/21556660.2019.1677674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen D, Pelizzari P, Pyenson B. Medicare cost of osteoporotic fractures - 2021 updated report. The clinical and cost burden of fractures associated with osteoporosis. Milliman Research Report. March 2021. Accessed July 19, 2023. https://www.milliman.com/en/insight/-/media/milliman/pdfs/2021-articles/3-30-21-Medicare-Cost-Osteoporotic-Fractures.ashx
- 5.Williams SA, Daigle SG, Weiss R, Wang Y, Arora T, Curtis JR (2021) Economic burden of osteoporosis-related fractures in the US Medicare population. Ann Pharmacother 55(7):821–829. 10.1177/1060028020970518 10.1177/1060028020970518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa WNB (2020) American association of clinical endocrinologists/american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr Pract 26(Suppl 1):1–46. 10.4158/gl-2020-0524suppl 10.4158/gl-2020-0524suppl [DOI] [PubMed] [Google Scholar]
- 7.Arceo-Mendoza RM, Camacho PM (2021) Postmenopausal osteoporosis: latest guidelines. Endocrinol Metab Clin North Am 50(2):167–178 10.1016/j.ecl.2021.03.009 [DOI] [PubMed] [Google Scholar]
- 8.Diffenderfer BW, Wang Y, Pearman L, Pyrih N, Williams SA (2023) Real-world management of patients with osteoporosis at very high risk for fracture. J Am Acad Orthop Surg 31(6):e327–e335 10.5435/JAAOS-D-22-00476 [DOI] [PubMed] [Google Scholar]
- 9.Modi A, Sen S, Adachi JD, Adami S, Cortet B, Cooper AL, Geusens P, Mellström D, Weaver J, van den Burgh JP, Nguyen AM, Sajjan S, MUSIC-OS Study Group (2016) Gastrointestinal symptoms and association with medication use patterns, adherence, treatment satisfaction, quality of life, and resource use in osteoporosis: baseline results of the MUSIC-OS study. Osteoporos Int 27(3):1227–1238. 10.1007/s00198-015-3388-3 10.1007/s00198-015-3388-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett-Connor E, Wade SW, Do TP, Satram-Hoang S, Stewart R, Gao G, Macarios D (2012) Treatment satisfaction and persistence among postmenopausal women on osteoporosis medications: 12-month results from POSSIBLE US™. Osteoporos Int 23(2):733–741. 10.1007/s00198-011-1620-3 10.1007/s00198-011-1620-3 [DOI] [PubMed] [Google Scholar]
- 11.Siris ES, Selby PL, Saag KG, Borgström F, Herings RM, Silverman SL (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122(2 Suppl):S3-13. 10.1016/j.amjmed.2008.12.002 10.1016/j.amjmed.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 12.Beaudart C, Silverman S, Gold DT, Williams SA, Weiss R, Hiligsmann M (2022) A qualitative study to assess US patient preferences between new transdermal system and injectable anabolic therapies for osteoporosis treatment. Arch Osteoporos 17(1):57. 10.1007/s11657-022-01075-z 10.1007/s11657-022-01075-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewiecki EM, Leader D, Weiss R, Williams SA (2019) Challenges in osteoporosis awareness and management: results from a survey of US postmenopausal women. J Drug Assess 8(1):25–31. 10.1080/21556660.2019.1579728 10.1080/21556660.2019.1579728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tymlos. Prescribing information. Radius Health Inc; 2017. Revised June 2023. Accessed November 16, 2023. https://radiuspharm.com/wp-content/uploads/tymlos/tymlos-prescribing-information.pdf
- 15.Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CAF, Hu M-Y, Harris AG, Fitzpatrick LA, Cosman F, Christiansen C, ACTIVE Study Investigators (2016) Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316(7):722–733. 10.1001/jama.2016.11136 10.1001/jama.2016.11136 [DOI] [PubMed] [Google Scholar]
- 16.Cosman F, Cooper C, Wang Y, Mitlak B, Varughese S, Williams SA (2022) Comparative effectiveness and cardiovascular safety of abaloparatide and teriparatide in postmenopausal women new to anabolic therapy: a US administrative claims database study. Osteoporos Int 33(8):1703–1714. 10.1007/s00198-022-06413-y 10.1007/s00198-022-06413-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold DT, Weiss R, Beckett T, Deal C, Epstein RS, James AL, Kernaghan JM, Mohseni M, Spiegel M, Vokes T, Roberts J, Bailey T, Wang Y, Williams SA (2021) Abaloparatide real-world patient experience study. JBMR Plus 5(3):e10457. 10.1002/jbm4.10457 10.1002/jbm4.10457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durden E, Pinto L, Lopez-Gonzalez L, Juneau P, Barron R (2017) Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos 12(1):22. 10.1007/s11657-0170316-5 10.1007/s11657-0170316-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng L-I, Durden E, Limone B, Radbill L, Juneau PL, Spangler L, Mirza FM, Stolshek BS (2015) Persistance and compliance with osteroporosis therapies among women in a commercially insured population in the United States. J Manag Care Spec Pharm 21(9):824–833a. 10.18553/jmcp.2015.21.9.824 10.18553/jmcp.2015.21.9.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halpern R, Becker L, Iqbal SU, Kazis LE, Macarios D, Badamgarav E (2011) The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm 17(1):25–39. 10.18553/jmcp.2011.17.1.25 10.18553/jmcp.2011.17.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross S, Samuels E, Gairy K, Iqbal S, Badamgarav E, Siris E (2011) A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health 14(4):571–581. 10.1016/j.jval.2010.11.010 10.1016/j.jval.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 22.Keshishian A, Boytsov N, Burge R, Krohn K, Lombard L, Zhang X, Xie L, Baser O (2017) Examining the effect of medication adherence on risk of subsequent fracture among women with a fragility fracture in the U.S. Medicare population. J Manag Care Spec Pharm 23(11):1178–1190. 10.18553/jmcp.2017.17054 10.18553/jmcp.2017.17054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modi A, Siris ES, Tang J, Sen S (2015) Cost and consequences of noncompliance with osteoporosis treatment among women initiating therapy. Curr Med Res Opin 31(4):757–765. 10.1185/03007995.2015.1016605 10.1185/03007995.2015.1016605 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that underlie the results reported in a published article may be requested for further research 6 months after completion of FDA or EMA regulatory review of a marketing application (if applicable) or 18 months after trial completion (whichever is latest). Radius will review requests individually to determine whether (i) the requests are legitimate and relevant and meet sound scientific research principles, and (ii) are within the scope of the participants’ informed consent. Prior to making data available, requestors will be required to agree in writing to certain obligations, including without limitation, compliance with applicable privacy and other laws and regulations. Proposals should be directed to info@radiuspharm.com.