Abstract
The innate immune system exhibits features of memory, termed trained immunity, which promote faster and more robust responsiveness to heterologous challenges. Innate immune memory is sustained through epigenetic modifications, affecting gene accessibility, and promoting a tailored gene transcription for an enhanced immune response. Alterations in the epigenetic landscape are intertwined with metabolic rewiring. Here, we review the metabolic pathways that underscore the induction and maintenance of trained immunity, including glycolysis, oxidative phosphorylation, the tricarboxylic acid cycle, and amino acid and lipid metabolism. The intricate interplay of these pathways is pivotal for establishing innate immune memory in distinct cellular compartments. We explore in particular the case of resident lung alveolar macrophages. We propose that leveraging the memory of the innate immune system may present therapeutic potential. Specifically, targeting the metabolic programs of innate immune cells is an emerging strategy for clinical interventions, either to boost immune responses in immunosuppressed conditions or to mitigate maladaptive activation in hyperinflammatory diseases.
Keywords: Trained immunity, Metabolism, Epigenetics, Therapy
Trained Immunity as the Memory of the Innate Immune System
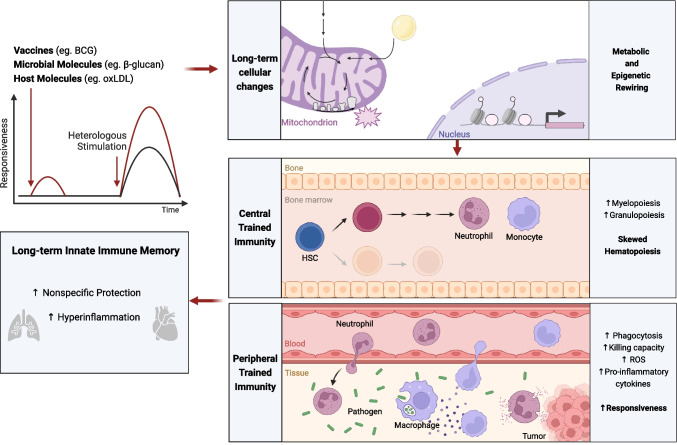
The immune system consists of two components: the innate system, which provides immediate but less specific defence; and the adaptive system, which offers highly specific responses but takes days to weeks to develop. Immunological memory is classically attributed to the adaptive immune system, which allows for faster and more effective responses after repeated exposures to the same antigen. Vaccines leverage this process by inducing specific adaptive immune responses, offering long-lasting protection against targeted pathogens [1]. However, living systems without an adaptive immune system still develop increased resistance to subsequent infections. For instance, plants can establish an immune response against a pathogen that provides protection against distinct subsequent infections [2]. This systemic acquired resistance (SAR) demonstrates that the innate immune system can exhibit memory characteristics. Human and murine models also suggest that the innate immune system possesses memory features, referred to as trained immunity or innate immune memory (Fig. 1) [3]. Specifically, challenged innate immune cells acquire memory features that allow for a faster and more robust response to a secondary event in an antigen-agnostic fashion. This protection conferred by trained immunity, although of shorter duration than the adaptive immune memory, can last from months to a few years.
Fig. 1.
Summary of the players involved in innate immune memory. Trained immunity inducers, such as live-attenuated vaccines or microbial and host molecules may trigger metabolic and epigenetic changes. These alterations support a hematopoietic bias towards myelopoiesis and granulopoiesis (i.e. central trained immunity), which leads to the production of trained innate immune cells (eg. neutrophils and monocytes/macrophages). Trained innate immune cells show enhanced effector functions (i.e. peripheral trained immunity), which confers protection against infections or enhances antitumoral activity, or may become maladaptive in the case of hyperinflammatory conditions. Figure created with Biorender.com
Different epidemiological studies have reported that live-attenuated vaccines have non-specific benefits. Namely, the widely used vaccine Bacille Calmette-Guérin (BCG), administered to target tuberculosis, has been shown to confer protection against pathogens other than Mycobacterium tuberculosis. Low birth-weight neonates vaccinated with BCG exhibited lower all-cause mortality, primarily through the prevention of sepsis and respiratory tract infection [4, 5]. Indeed, whole blood stimulation assays showed that BCG-vaccinated infants had increased production of cytokines upon stimulation with the fungus Candida albicans or the TLR1/2 agonist Pam3Cys [6]. In elderly individuals, BCG vaccination has also been shown to decrease respiratory infections, highlighting the potential of BCG-induced trained immunity for the broad protection of distinct vulnerable populations [7]. Live-attenuated viral vaccines, such as the oral polio vaccine (OPV) and the measles, mumps, and rubella (MMR) vaccine have also been shown to confer non-specific protection, since both OPV and MMR have reduced infant mortality, independently of the protection against the target infections [8, 9].
In addition to vaccines, other agents also induce innate immune memory programs. C. albicans, or the fungal cell wall component β-glucan, enhanced monocytic proinflammatory cytokines production after a secondary unrelated stimulation [10]. Endogenous oxidized low-density lipoprotein (oxLDL) induced a long-lasting proinflammatory phenotype in monocytes, which has been associated with atherosclerosis and foam cell formation [11]. Also, myeloid cells of mice fed a western-type diet exhibited a long-lasting trained immunity phenotype [12]. Other inducers of trained immunity have been identified, such as extracellular labile heme that increased mouse survival in a polymicrobial sepsis model [13].
Innate immune memory is supported by epigenetic modifications affecting gene accessibility, and thus conferring long-term potential for tailored gene transcription, which promote a quick and heightened immune response [14]. Epigenetic memory has been reported not only in short-lived peripheric innate immune cells, but also in their long-lived progenitors. Inducers of innate immune memory skew bone marrow hematopoietic stem cells (HSC) differentiation towards myelopoiesis, with production of innate immune cells with increased effector functions. Indeed, HSCs of mice exposed to lipopolysaccharides exhibited conserved epigenetic marks for at least 12 weeks, which increased the expression of immune-related genes after a subsequent infection with Pseudomonas aeruginosa [15]. Similarly, BCG or β-glucan administration conferred protection of mice against tuberculosis and chemotherapy-induced myeloablation, respectively, via changes at the levels of HSCs [16, 17]. The establishment of long-term central innate immune memory was also shown in a human BCG vaccination model, wherein HSCs were transcriptionally distinct 90 days after BCG vaccination when compared to prior to vaccination [18]. The epigenetic changes of progenitor cells may underline the long-lasting protection of inducers of trained immunity. However, changes of progenitor compartments may also lead to a long-term maladaptive innate immune memory, with exacerbated inflammatory responses, such as in patients with atherosclerosis [19] or even after severe COVID-19 infection [20]. Also, an experimental model of periodontitis-induced maladaptive myelopoiesis, which rendered the mice more susceptible to arthritis, pointed to inappropriate induction of trained immunity in the bone marrow progenitors as an amplifier of inflammatory comorbidities [21]. In addition to HSC memory, epigenetic changes in mice have also been linked to the transmission of trained immunity traits through generations [22]. Specifically, the progeny of male mice previously infected with C. albicans were protected against heterologous infections. This protection derived from differences in DNA methylation are present in the sperm DNA of the infected parental mice [22].
Alterations to the epigenetic landscape are interwoven with metabolic changes, which together influence the establishment of innate immune memory. The integration of both extracellular metabolites from the microenvironment and intracellular metabolites with distinct transcriptional programs contribute to the establishment of innate immune memory. Metabolites act as signalling molecules, cofactors and substrates that modulate protein activity and ultimately contribute to cell function. In this review, we discuss the metabolic pathways that support the initiation and maintenance of innate immune memory, which are tightly connected with the molecular and epigenetic control of trained immunity. Here, we also suggest that metabolism is an attractive target for future therapeutic strategies, either to potentiate immune responses in immunosuppressed conditions or to decrease maladaptive activation of the innate immune system in hyperinflammatory diseases.
Metabolic Modulation Supports Trained Immunity
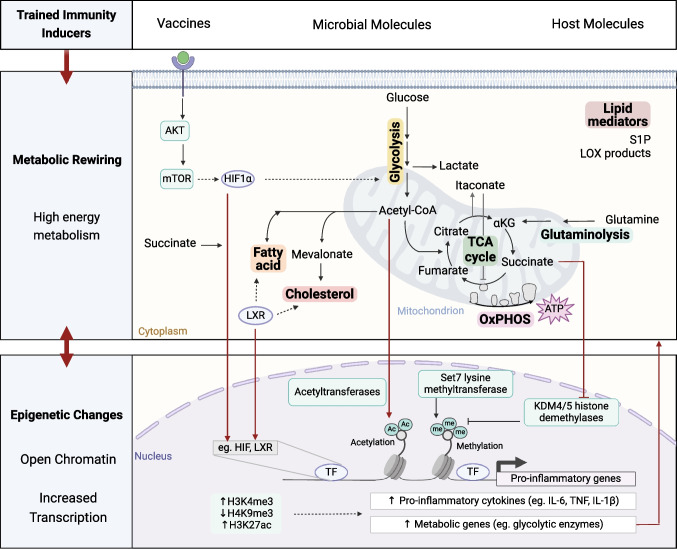
Immune cells are finely attuned to extracellular cues and are able to quickly respond to microenvironmental changes. The rapid rewiring of metabolic pathways produces alterations in the bioenergetic profile of the cell and promotes the synthesis of signaling metabolites that ultimately affect cellular functions. Innate immune memory has been shown to be supported by different metabolic pathways, such as glycolysis, oxidative phosphorylation (OXPHOS), the tricarboxylic acid (TCA) cycle, amino acid, and lipid metabolism (Fig. 2).
Fig. 2.
Metabolic pathways that support innate immune memory and their interaction with epigenetic regulators. The upregulation of multiple metabolic pathways supports the establishment of trained immunity in distinct cell types by providing energy, deferential building blocks and by modulating protein activity. Metabolites enriched upon trained immunity may activate transcription factors and modulate the activity of epigenetic enzymes. These promote an epigenetic signature that enables the increased transcription of pro-inflammatory and metabolic genes, which in turn amplify the existing metabolic shift and ultimately confer a sustained increase in effector functions. (S1P sphingosine-1-phosphate; LOX lipoxygenase; αKG α-ketoglutarate; TCA tricarboxylic acid; OxPHOS oxidative phosphorylation; AKT protein kinase B; mTOR mammalian target of rapamycin; HIF1α hypoxia inducible factor 1α; LXR liver X receptor; TF transcription factor; Ac acetyl; me methyl). Figure created with Biorender.com
Glycolysis and Oxidative Phosphorylation
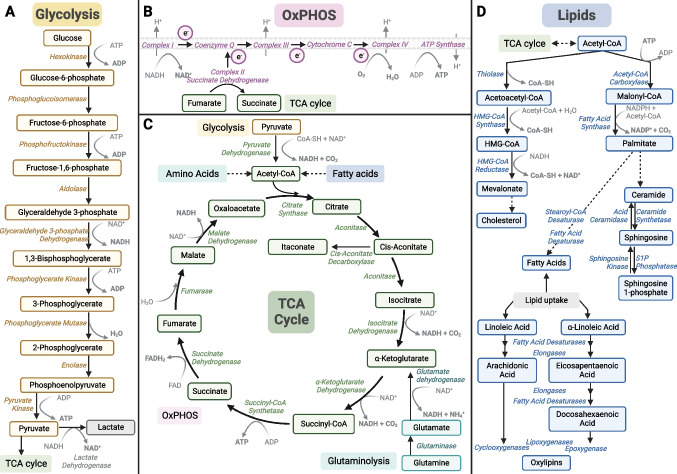
Glycolysis involves the rapid degradation of glucose with production of pyruvate, energy and reducing power in the form of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NADH) respectively. Pyruvate can then fuel the TCA cycle or be oxidized into lactate (Fig. 3A). The upregulation of glycolysis, with increased glucose consumption and release of lactate, has been described in distinct cell types and in response to different inducers of innate immune memory. For example, glycolysis was upregulated in neutrophils isolated from BCG vaccinated individuals, revealing increased amounts of lactate secretion. Enhanced lactate production was correlated with enhanced fungicidal activity, possibly due to the associated increase in the release of reactive oxygen species (ROS) [23]. ROS were also increased in neutrophils of mice exposed to β-glucan [24]. BCG, β-glucan and oxLDL have also been shown to induce glycolysis in monocytes [25–27]. Indeed, the concomitant pharmacological inhibition of glucose uptake and exposure to the BCG or oxLDL led to the long-term decrease of responsiveness of human trained macrophages, while the inhibition of glycolysis of control cells did not affect their long-term cytokine production capacity [26, 27]. In addition, glycolysis may also be relevant in the establishment of central innate immune memory. Namely, hematopoietic stem cells of mice treated with β-glucan presented an increased glycolytic rate, and administration of β-glucan together with a glucose uptake inhibitor decreased the myeloid bias of hematopoietic stem cells characteristic of trained mice [17]. This increase in glycolysis was associated with changes in the epigenome, namely the increased deposition of the transcriptional permissive mark H3K4me3 at regions associated with the promoters of glycolytic enzymes [25–27]. The upregulation of glycolytic genes might be regulated by the protein kinase B (AKT) – mammalian target of rapamycin (mTOR)—hypoxia inducible factor (HIF)-1α pathway, ultimately contributing for the increased responsiveness characteristic of a trained cell.
Fig. 3.
Schematic overview of the major metabolic pathways involved in innate immune memory, such as (a) glycolysis, (b) oxidative phosphorylation (OxPHOS), (c) tricarboxylic acid (TCA), glutaminolysis and (d) fatty acid, cholesterol, sphingolipid and oxylipin synthesis. Figure created with Biorender.com
Indeed, inhibition of mTOR with rapamycin or of HIF-1α with ascorbate decreased the pro-inflammatory cytokine production of β-glucan or BCG treated monocytes [25, 26]. Also, the myeloid specific deletion of HIF1A compromised the survival of β-glucan trained mice to a S. aureus infection [25]. In humans, oral metformin treatment decreased the ex vivo induction of BCG- and oxLDL-induced trained immunity in monocytes [26, 27]. Metformin is an antihyperglycemic drug known to inhibit mTOR through activation of AMP-activated protein kinase (AMPK). In addition, it also dampens oxidative phosphorylation (OXPHOS), by inhibition of complex I of the electron transport chain. It is important to highlight that mTOR and HIF-1α not only have a role in the activation of glycolysis but can also modulate other pathways. For example, chromatin immunoprecipitation followed by sequencing analysis of HIF-binding sites has identified approximately 500 sites across the genome [28]. At the metabolic level, HIF-1α has been shown to decrease the transcription of the TCA cycle enzyme succinate dehydrogenase (SDH) [29]. HIF-1α may also enhance the expression of glutamine transporters, thus increasing the intracellular concentration of glutamate [30]. Glutamate in turn may be converted to α-ketoglutarate, a metabolite of the TCA cycle. Of note, the consumption of glutamine to feed the TCA cycle, also known as glutaminolysis, has also been shown to play a role in innate immune memory, as discussed in the later sections of this review.
OXPHOS includes the oxidation of NADH, the creation of a proton gradient which is used by F0F1-ATP synthase to synthesize ATP, and the reduction of oxygen (Fig. 3B). Innate immune memory rely on a highly energetic metabolism, with the concomitantly increase of glycolysis and oxygen consumption, as observed for BCG, oxLDL and β-glucan [26] [27] [31]. Indeed, monocytes treated with the ATP synthase inhibitor oligomycin, followed by exposure to memory inducing stimuli such as β-glucan or oxLDL, presented a decreased long-term cytokine producing capacity when compared to trained macrophages that were not exposed to oligomycin [31, 32]. The potential role of OXPHOS in innate immune memory was also highlighted by suggestive associations between common single nucleotide polymorphisms (SNPs) in OXPHOS-related genes and the variability in the responsiveness of β-glucan- or oxLDL- trained monocytes [31, 32].
The increased mitochondrial metabolism of innate memory cells contributes to the increase in energy stores but also potentiates the production of mitochondrial ROS. Indeed, human monocytes exposed to oxLDL exhibited higher levels of mitochondrial ROS [33]. Similarly, neutrophils of zebrafish previously exposed to the bacteria Shigella respond to stimulation with higher levels of mitochondrial ROS, when compared to naïve neutrophils [34]. A sublethal dose of Salmonella enterica also generated neutrophils that respond to stimulation by producing increased amounts of mitochondrial ROS [35]. In this study, S. enterica infection rewired HSC towards the generation of neutrophils with enhanced bactericidal activity, which was phenocopied by overexpression of the transcription factor CCAAT/enhancer binding protein beta (C/EBPβ). C/EBPβ was also involved in LPS-induced memory of mice HSC, by targeting myeloid enhancers and potentially promoting myelopoiesis [15].
In addition to monocytes and neutrophils, lymphoid cell populations such as the natural killer (NK) cells have also shown memory characteristics. NK cells isolated from BCG-vaccinated individuals have also shown increased proinflammatory cytokine production following ex vivo heterologous stimulation [36]. Curiously, NK cells of human cytomegalovirus (HCMV) seropositive individuals exhibit heightened glycolysis, mitochondrial oxidative metabolism, and mitochondrial ROS, relative to NK cells of HCMV-seronegative individuals. This metabolic rewiring and increase in the capacity to produce IFN-γ was dependent on a chromatin modifying transcriptional regulator [37]. Thus, NK cell memory is potentially regulated by metabolic and epigenetic changes, similarly to monocytes [38].
Together, these findings suggest a relevant role for glycolysis, OXPHOS and also mitochondria metabolism and function in innate immune memory.
Tricarboxylic Acid Cycle
The increase of oxidative phosphorylation and TCA cycle activity is characteristic of different trained immunity programs. The TCA cycle is a fundamental mitochondrial process that, through a series of oxidative reactions, reduces NADH and FADH2 coenzymes, contributing directly to the electron transport chain and ATP production (Fig. 3C). The TCA cycle also integrates several anabolic and catabolic pathways and produces metabolic intermediates [39]. Trained immunity is supported by the accumulation of TCA cycle intermediate metabolites. Upon induction and maintenance of trained immunity, two main carbon sources are responsible for the supplementation of TCA cycle metabolites: (i) pyruvate-derived acetyl-CoA and (ii) glutamine-derived α-ketoglutarate.
The former derives from the upregulation of glycolysis, which produces increased levels of pyruvate. Once imported into the mitochondria, pyruvate is oxidized into acetyl-CoA. β-oxidation of fatty acids also feeds the TCA cycle by producing acetyl-CoA and NADH and FADH2. Acetyl-CoA not only fuels the TCA cycle but also acts as a source of acetyl groups for histone acetyltransferases. Indeed, the increase in the intracellular concentration of acetyl-CoA promotes histone 3 lysine 27 acetylation (H3K27ac) and leads to the upregulation of glycolysis-related genes, such as hexokinase 2, phosphofructokinase and lactate dehydrogenase [40]. Particularly, a short exposure to exogenous acetyl-CoA increased the capacity of human monocytes to produce IL-6 upon TLR2 stimulation [41]. Acetyl-CoA can be metabolised and condensed with oxaloacetate to form the TCA cycle intermediate citrate. Citrate levels in stimulated macrophages are central for the induction of proinflammatory factors such as ROS, nitric oxide (NO), and prostaglandins [42]. In the cytosol, citrate can also be cleaved to regenerate Acetyl-CoA and used as a precursor of lipid biosynthesis.
In addition to glycolysis, glutaminolysis fuels the TCA cycle and is necessary for the establishment of trained immunity [43]. Glutamine is converted to glutamate, which in turn is synthesised into alpha-ketoglutarate (α-KG) (Fig. 3C). α-KG is a cofactor necessary for the activity of α-KG dependent dioxygenases (α-KGDD). However, trained monocytes are also characterized by the accumulation of the TCA cycle metabolites succinate and fumarate. These metabolites compete with α-KG and inhibit the activity of multiple α-KGDD [44]. Among the α-KGDD family are the prolyl hydroxylase domain (PHD) enzymes, which hydroxylate the transcription factor HIF-1α, promoting its constitutive degradation. Stabilization of HIF-1α is crucial for the induction of trained immunity, promoting the induction of IL-1β transcription, glycolysis, and expression of histone demethylases [25, 45, 46].
The α-KGDD family also includes histone and DNA demethylases. Particularly, fumarate accumulation in human monocytes was shown to increase the production of pro-inflammatory cytokines after stimulation, while also increasing the deposition of H3K4me3 at regions associated with the promoters of the pro-inflammatory genes TNF and IL6. The enrichment of this transcriptional-permissive H3K4me3 mark was associated with a fumarate-induced decrease in KDM5 activity, a histone H3K4 demethylase [43]. Other α-KGDD have also been implicated in the establishment of innate immune memory. The pharmacological inhibition of the H3K9 histone demethylase KDM4 decreased glycolysis and the capacity of β-glucan trained human monocytes to secrete IL-6 and TNF upon TLR4 stimulation. Accordingly, inhibition of KDM4 increased the deposition of the repressor mark H3K9me3 at regions associated with promoters of IL6 and TNF of β-glucan trained cells [47]. KDM4 expression was also increased in stimulated myeloid progenitor cells of mice trained with a western-type diet [12]. This interplay between metabolic intermediators and the activity of epigenetic enzymes illustrates the cross-regulation of metabolism and epigenetics that underlines innate immune memory [14, 48].
The upregulation of the TCA cycle promotes an accumulation of succinate in trained monocytes [49]. Succinate can be oxidised to fumarate by succinate dehydrogenase (SDH), also known as complex II of the electron transport chain. SDH is a bridge enzyme between the TCA cycle and the OXPHOS, since the oxidation of succinate into fumarate promotes the transference of an electron along the electron transport chain. Genetic human studies have revealed the presence of individual SNPs in SDH genes, which were associated with changes in the capacity of pro-inflammatory cytokine production [49]. Furthermore, the transcription of SDHB and SDHD were increased in human macrophages trained with β-glucan [31]. The increase in SDH transcription was accompanied by the enrichment of TCA cycle metabolites, which were ablated by the pharmacological inhibition of the lysine methyltransferase Set7. Similarly, bone marrow cells of mice exposed to β-glucan exhibited a long-term increase in SDHB that was not observed in β-glucan-trained SET7 deficient animals. The transcription of SDHB appears to be regulated by the acquisition of H3K4me1 at SDHB enhancer regions which is deposited by the activity of Set7 induced by β-glucan [31]. These data highlight that the enzymes that participate in the TCA cycle are also regulated at the epigenetic level, particularly through the action of Set7.
SDH activity is not only regulated at the transcriptional but also at the protein level, namely by Itaconate, a competitive inhibitor of SDH [50]. Itaconate is produced by aconitate decarboxylase 1 (ACOD1) from cis-aconitate, an intermediate of the TCA cycle. It is synthesised in pro-inflammatory macrophages [51] and triggers anti-inflammatory and antioxidant responses [52–54]. Itaconate not only inhibits SDH but may also affects other metabolic pathways by reacting with thiol groups [55]. Itaconate and its derivatives have been reported to alkylate the ROS scavenger molecule glutathione [53], and different glycolytic enzymes [52, 55, 56]. In line with the anti-inflammatory activity of itaconate, β-glucan restored the cytokine production capacity of LPS-tolerized monocytes, possibly by inhibiting itaconate production and re-establishing SDH function [49]. Also, supplementation of monocytes with the derivative dimethyl itaconate led to the inhibition of β-glucan-induced trained immunity [49]. In contrast, monocytes exposed to dimethyl itaconate in the absence of other stimuli exhibited features of trained immunity, with metabolic, transcriptional, epigenetic and pro-inflammatory changes similar to those induced by the prototypical inducer of trained immunity β-glucan [57]. DMI administration also increased mice survival to S. aureus infection. Dimethyl itaconate-induced trained immunity possibly involved glutathione metabolism. Glutathione was shown to also be involved in vitro β-glucan-induced trained immunity [58] and its pharmacological inhibition reduces the cytokine production of both DMI- and β-glucan-trained macrophages [57, 58]. Itaconate may also modulate the epigenetic landscape of innate immune cells. Of note, itaconate was shown to inhibit TET DNA dioxygenases upon LPS stimulation, and may also suppress the activity of other α-KGDD [59]. However, its role in the epigenetic control of trained immunity has not been addressed. Overall, itaconate and its derivatives have a dual anti- and pro-inflammatory role in trained immunity that is context dependent.
Amino acids
Amino acids have a central role in cellular metabolism and innate immunity activation. Amino acids are not only the building blocks of proteins but also act as precursors of metabolites involved in the trained immunity phenotype. As discussed above, glutaminolysis is essential for trained immunity induction since it replenishes the TCA cycle via α-ketoglutarate. Indeed, pharmacological inhibition of glutaminase blunted the stimulated production of IL-6 and TNF by β-glucan-trained human monocytes. In line with this, glutaminolysis inhibition also decreased the deposition of H3K4me3 at regions associated with the promoters of the IL6 and TNF genes [43]. In addition to glutamine, human monocytes exposed to β-glucan also exhibited increased consumption of aspartate and methionine [43], however, the role of these amino acids in the establishment of innate immune memory remains to be explored further. Methionine, in particular, is the precursor of S-adenosylmethionine (SAM), a methyl donor critical for DNA and histone methylation. LPS-stimulated macrophages upregulate the methionine cycle to fuel the production of SAM which in turn contributes to histone methylation that promotes IL-1β production [60]. Different amino acids have been shown to influence the activation of innate immune cells, however their contribution to the establishment of memory is underexplored. More studies are warranted to obtain a clearer understanding of their specific role in both immunometabolism rewiring and epigenetic modifications driving innate immune memory.
Lipid metabolism
Lipid metabolism has also been implicated in different programs of innate immune memory. Cells acquire fatty acids through both de novo lipogenesis and by uptake from the environment (Fig. 3D). Dietary fatty acids can act as inflammatory stimuli and their involvement in trained immunity has been reported [61]. A diet rich in saturated fatty acids altered the relative populations of hematopoietic stem cells in mice and increased the responsiveness of bone marrow and circulating monocytes to ex vivo TLR4 stimulation. Particularly, the saturated fatty acid palmitate contributed to the increased response of macrophages, and palmitate exposure was sufficient to enhance the clearance of C. albicans from infected mice that lack mature T and B cells [61]. Similarly, a genetic mouse model of atherosclerosis fed a high-fat diet, which induces transient hypercholesterolemia, exhibited a shift of the hematopoietic compartment towards an enrichment of granulocyte-monocyte progenitors [12]. This myeloid bias was sustained, even after the mice returned to a standard chow diet and the cholesterol levels normalized. These results point to the capacity of dietary compounds to modulate innate immune responses. Of note, a high-salt diet was also recently shown to induce a persistent immune memory imprinted in the hematopoietic compartment, with a transcriptional increase of genes involved in glycolysis and fatty acid metabolism [62].
In addition to dietary enrichment of cholesterol, the intracellular upregulation of cholesterol pathways has also been implicated in trained immunity. Namely, various species of cholesterol esters were enriched in hematopoietic stem cells isolated from β-glucan-trained mice. In this model, the pharmacological inhibition of the cholesterol pathway decreased the hematopoietic myeloid bias induced by β-glucan [17]. Mevalonate, synthetized from acetyl-CoA, may be used for the production of cholesterol and it is thought to be the bioactive metabolite of this pathway in the induction of innate immune memory. Accordingly, the inhibition of mevalonate production decreased lactate secretion, H3K4me3 deposition at regions associated with the promoter of TNF and ultimately the production of TNF, which were enhanced in BCG- or oxLDL- trained monocytes [63]. In line with this, a constitutive trained immunity phenotype was observed in hyper immunoglobulin D syndrome patients, who present with mevalonate accumulation [63]. Thus, mevalonate has been shown to induce maladaptive trained immunity, that leads to the sterile inflammatory attacks observed in these patients, while also contributing to the protection conferred by different stimuli such as β-glucan, BCG and oxLDL [17, 63].
A master regulator of fatty acid metabolism, the nuclear liver X receptor (LXR), was shown to also control the induction of trained immunity. The pharmacological inhibition of this transcription factor decreased the secretion of IL-6 and TNF by stimulated macrophages previously exposed to oxLDL or BCG [41, 64]. LXR inhibition also dampened the metabolic and epigenetic rewiring induced by oxLDL-induced trained immunity, as observed with the decrease in lactate secretion and the reduced H3K4me3 deposition at regions associated with the promoters of IL6 and TNF [64]. Interestingly, the activation of LXR was sufficient to phenocopy the increased responsiveness, metabolic and epigenetic changes of a trained immune cell. Particularly, the increased capacity for IL-6 and TNF secretion induced by LXR activation was dependent on the mevalonate pathway [41]. Thus, highlighting the different contexts of innate immune memory activation in which mevalonate participates.
Trained immunity has also been associated with other lipid metabolic pathways. A lipidomic analysis performed of hematopoietic progenitors isolated from β-glucan-trained mice revealed an enrichment for shorter and more saturated fatty acids, and a decrease in lipids containing arachidonic acid [17]. In contrast, a recent study reported that long-chain polyunsaturated fatty acids support BCG-induced increased responsiveness in human monocytes [65]. In this study, arachidonic acid levels were not modulated by β-glucan or BCG, instead, a tailored production of lipid mediators was observed. Particularly, lipid mediators produced by lipoxygenases (LOX) were enriched in BCG exposed monocytes, and the inhibition of 5- or 12-LOX activity dampened the effect of BCG on cytokine production. Interestingly, 12-LOX derived lipid mediators were increased in monocytes isolated from individuals that had been vaccinated one month prior with BCG [65]. Lipid mediators are produced through the enzymatic oxidation of polyunsaturated fatty acids and a wealth of studies have lately underscored that they are involved in different phases of host defense, from inflammation and induction of innate immunity, to modulation and resolving processes [66]. From that perspective it is not surprising that they are involved in the induction and modulation of trained immunity, and they could potentially represent therapeutic targets in diseases in which trained immunity is involved. In addition to LOX-derived lipid mediators, another lipid mediator has also been implicated in the induction of trained immunity. Sphingosine-1-phosphate abundance was increased in whole particle β-glucan exposed murine macrophages, and inhibition of the production of sphingosine-1-phosphate attenuated TNF secretion [67]. The authors suggest that sphingosine-1-phosphate may promote mitochondrial fission, which in turn was essential for the increased responsiveness and oxygen consumption triggered by whole particle β-glucan [67]. In accordance, a recent study also reported that pharmacological modulation of sphingolipid metabolism affected C. albicans-induced trained immunity in human monocytes [68]. Particularly, inhibition of the enzyme acid ceramidase, which converts ceramide to sphingosine, dampened the boosted cytokine production, glycolytic rate and OxPHOS potentiated by C. albicans.
Local Innate Immune Memory: the Case of the Lung
Innate immune memory has mostly been explored in a systemic context, in which peripheral innate memory cells are continuously replenished by HSC that retain epigenetic memory [16–19, 21]. However, some studies suggest that innate immune memory is also induced and maintained in self-contained tissues [69, 70]. Particularly, respiratory infections have been a focus of study, possibly due to the preferential heterologous protection provided by BCG vaccination in humans [4, 7]. Particularly, alveolar macrophages (AMs), the resident macrophages of the airways, have been cells of interest since they act as the primary immune sentinels of the respiratory tract. However, it is important to note that resident AM originate from the yolk sac during embryonic haematopoiesis, and not from circulating monocytes [71]. Thus, there are subsets of lung tissue-resident macrophages that have different ontologies and possibly distinct immunological functions [72]. Consequently, it is important to employ strategies that allow for accurate cellular identification that consider the similarities between monocytes recruited to the lung and the resident AM population. Not only ontology but also environment may affect cell function. It is relevant to highlight that glucose concentrations in the airways are lower than in circulation [73], which might influence the metabolic pathways employed by the resident cells and not fully recapitulate the metabolic rewiring that takes place in other tissues or culture media conditions.
In mouse models, acute respiratory viral infections have been shown to induce memory features in alveolar macrophages (AM), protecting mice from subsequent bacterial infections [74] and promoting long-term antitumor immunity [75]. In particular, trained AM demonstrated a markedly increased glycolytic metabolism, which was ablated by T cells depletion or IFNγ deficiency. The authors reported that IFN-γ from effector CD8 + T cells was necessary for AM memory formation [74]. Similarly, IFN-γ has also been shown to be crucial for central trained immunity, since HSC of IFN-γ-receptor deficient mice failed to expand upon BCG vaccination [16]. AM with long-term memory induced by viral infection also exhibited increased oxygen consumption rates, which were fuelled by fatty acids and glucose oxidation [75]. Fatty acid oxidation has also been shown to participate in AM memory formation upon mouse exposure to ambient amounts of lipopolysaccharides [76]. Another murine study reported that LPS inhalation induced trained immunity in AM, which exhibited increased efferocytosis efficiency and transcriptional upregulation of lipid and arginine metabolism, ultimately enhancing inflammation resolution [77].
In addition to the local triggering of innate memory, the increase in lung immunity has also been reported upon systemic induction of trained immunity. BCG subcutaneous immunization of mice led to increased glycolysis and oxidative phosphorylation of AM [78]. However, it also altered intestinal microbiota, and promoted the levels of intestinal short-chain fatty acids (SCFA) carnitine and butyrate. The authors reported that this enrichment in the intestine was similarly found in the serum and lungs of BCG vaccinated animals. Via microbiota transplantation models or by introducing SCFA in the drinking water of naïve mice, the authors suggest that there is a causal relationship between BCG-altered microbiota and AM memory. Curiously, the gut microbiome has also been implicated in innate immune memory features in humans. In a human BCG vaccination cohort, the abundance of Roseburia in stool samples negatively correlated with ex vivo circulating innate immune responsiveness, possibly due to the modulation of plasma phenylalanine metabolism [79]. Particularly in human AM isolated from sputum, BCG vaccination decreased the expression of surface activation markers, but the functional consequences remain to be investigated [80].
Implications for Disease and Future Therapies
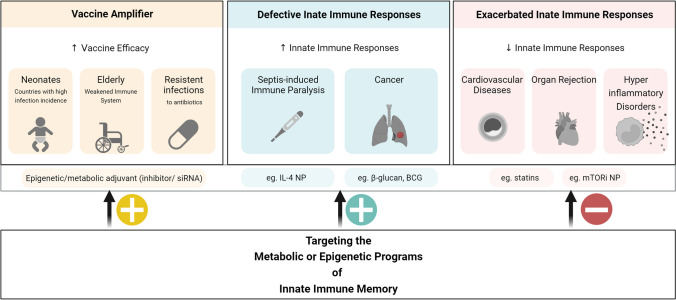
The growing field of trained immunity, highlighted by metabolic changes in myeloid and lymphoid cells, offers new treatment options for many diseases where imbalanced immune responses play a key role. The close relationship between cellular metabolism and trained immunity not only clarifies the mechanics of immune responses, but also suggests a new approach for therapeutic interventions. Drug-based modulation, similar to vaccination strategies used in the adaptive immune system, may have potential in using the memory-like properties of the innate immune system for disease prevention and treatment. This immunometabolic connection outlines a hopeful path for therapeutic targeting, where metabolic pathways and their interactions with immune responses can be adjusted to lessen or prevent disease conditions (Fig. 4).
Fig. 4.
Clinical scenarios that might benefit from the modulation of innate immune memory and possible therapeutic approaches. The therapeutic use of innate immune memory may encompass the improvement of vaccines and the regulation of compromised or hyperactive immune responses. The design of vaccine amplifiers that trigger innate immune memory might not only enhance the pathogen-specific responses induced by the classical vaccine formulations, but also provide cross-protection to unrelated infections. This increased non-specific protection would be of particular interest in countries of high infection burden and to counter the rise in antibiotic resistant pathogens. Individuals with a weakened immune system could also benefit from a boosted innate immunity, such as in the case of sepsis or cancer. However, the dampening of innate immune memory could also be a strategy in conditions of exacerbated immune responses, as for example in cardiovascular diseases and graft versus host disease. In order to target innate immune memory programs, the use of prototypical inducers such as BCG or β-glucan has been suggested. Also, the use of metabolic or epigenetic modulators has been hypostatised. The targeted delivery of mRNA, siRNA, inhibitors, or cytokines could be performed by nanoparticles (NP) carries administered locally or systemically. Figure created with Biorender.com
Heterologous Effects of BCG Vaccination
As outlined in previous sections, the BCG vaccine induces non-specific protection against heterologous infections through the induction of trained immunity. A study involving 325 individuals demonstrated that BCG vaccination influences circulating metabolite concentrations which, in turn, impact trained immunity responses such as increased IL-1β and TNF production upon stimulation with the gram-positive bacteria Staphylococcus aureus. The metabolic foundation of trained immunity encompasses more than just glycolysis and the tricarboxylic acid cycle, extending to lipid and amino acid metabolism, thus illustrating a complex metabolic network driving immune responses. In this regard, taurine metabolism was also identified as a new metabolic pathway associated with BCG-induced trained immunity [81]. Notably, alterations in cholesterol metabolism, a key player in atherosclerosis, have also been connected to the modulation of trained immunity, suggesting a potential therapeutic target. The application of statins, known for regulating cholesterol levels, might impact trained immunity and contribute to atherosclerosis management. However, an interventional study in patients with familial hypercholesterolemia showed that whereas monocytes from these patients present a trained immunity phenotype, treatment with statins does not revert this pro-inflammatory phenotype [82].
Vaccine Adjuvants as Innate Immune Amplifiers
The advent of pharmacological amplification posits a transformative approach to bolster the effectiveness of vaccines, focusing on the fine-tuning of metabolic and epigenetic mechanisms within immune cells. This paradigm leverages the modulation of metabolic pathways, to augment both specific and nonspecific immune responses post-vaccination. For instance, the modulation of glycolysis through compounds like 2-deoxyglucose, or TCA cycle and OXPHOS through substances like dichloroacetate [83], showcases a prospect of enhancing immune responses.
In the global fight against antimicrobial resistance (AMR), vaccines stand as a formidable first line of defence, with the potential to drastically reduce the utilization and thus resistance to antibiotics. This could be achieved through the addition of molecules that are able to modulate the most important metabolic pathways and potentiate trained immunity responses. This idea extends to providing a robust immune response against emerging infections, even when specific vaccines are yet under development, thus acting as a bridge to curb morbidity and mortality during the interim. The envisioned new generation of vaccines, accentuated with metabolic amplifiers, aims at tackling both communicable and non-communicable diseases. The "amplifier" component in vaccines, as proposed, would be able to enhance the memory and effector functions of immune cells, broadening the scope of vaccine efficacy beyond specific antigenic targets to a wider array of pathogens, including those resistant to current antimicrobial treatments. The implications of this novel vaccination strategy resonate profoundly in populations with compromised immune responses such as the elderly and immunocompromised individuals. The underlying principle of augmenting innate immune responses through metabolic modulation could provide an adjuvant strategy to the diminished vaccine efficacy in these groups. The utilization of live vaccines like BCG has shown promise in enhancing immune responses in the elderly, indicating the potential of trained immunity in this demographic [7]. As the world grapples with multiple infectious diseases, alongside an increasing prevalence of AMR, the exploration of metabolic modulators as vaccine amplifiers opens a promising avenue. The envisioned advancements in vaccine technology underscore a concerted effort towards unlocking the full potential of vaccines, aligning with a broader strategy to combat infectious diseases through metabolic tuning, thereby opening new horizons in the realm of immunotherapy and disease control.
Potential of Nanomedicines
One significant technological development is the control of trained immunity through nanomedicine, which appears promising particularly in diseases like cancer and inflammation-related syndromes. The control achieved through epigenetic and metabolic reprogramming of hematopoietic stem and progenitor cells underlines the potential of targeting metabolic pathways to adjust trained immunity for cancer treatment. The chance to deliver metabolic modifiers or immune-modifying agents accurately to specific cell types or tissues is promising for precisely adjusting trained immunity based on the situation. Nanoparticles that can deliver small molecule inhibitors, siRNA, or other agents could offer a way to adjust the metabolic and epigenetic foundations of trained immunity with high accuracy and low systemic toxicity.
For instance, the phenomenon of sepsis and the resulting immune paralysis significantly highlight the intricate role of metabolic regulation in trained immunity. In scenarios of sepsis, a state of immune paralysis often ensues, marked by a compensatory anti-inflammatory response syndrome which augments the risk of secondary infections and death [84]. The process of metabolic reprogramming is pivotal in directing immune responses during sepsis, and in turn, modulating the state of immune paralysis. A recent study used nanomedicine to target interleukin-4 (IL-4) to myeloid cells to alleviate sepsis-induced immunoparalysis, utilizing the principles of trained immunity. In cultured primary human monocytes, IL-4 inhibits acute inflammation, while simultaneously inducing a long-lasting innate immune memory. To harness this dual characteristic of IL-4 in vivo, a fusion protein of apolipoprotein A1 (apoA1) and IL-4 was developed, integrating the cytokine into a lipid nanoparticle. When injected intravenously, the apoA1-IL-4-embedding nanoparticle targets myeloid-cell-rich haematopoietic organs, particularly the spleen and bone marrow. The study further demonstrated that IL-4 nanotherapy resolved immunoparalysis in mice with lipopolysaccharide-induced hyperinflammation, as well as in ex vivo human sepsis models and in experimental endotoxemia. These findings advocate for the translational development of nanoparticle formulations of apoA1-IL-4 for the treatment of patients with sepsis who are at risk of immunoparalysis-induced complications [85].
The field of cancer provides a crucial setting to examine the complicated interaction between metabolic processes and trained immunity. The cancer-induced changes in the tumor microenvironment (TME) and systemic metabolism create a detailed scenario where trained immunity could act as both a foe and friend. A deep understanding of these dynamics is crucial for creating innovative therapeutic strategies. Metabolic reprogramming is a common feature in both cancer cells and immune cells. Within the TME, trained immune cells show altered metabolic profiles that can either encourage or halt tumor progression. Triggering trained immunity in pro-tumorigenic macrophages is also a potential strategy to manage tumor metastasis. In this regard, in vivo treatment with whole beta-glucan particle led to a trained immunity behaviour in lung interstitial macrophages, resulting in the blocking of tumor metastasis and extending survival in multiple metastatic mouse models. As discussed previously, this trained immunity was driven by the metabolite sphingosine-1-phosphate, highlighting the importance of metabolic intermediates in adjusting trained immunity and possibly using it for cancer treatment [67]. Furthermore, the immunological behaviour of trained immunity in cancer might change based on the level of metabolic reprogramming brought on by the extent of training, suggesting a complicated interaction between metabolic signals and trained immunity activation. Considering the anti-cancer findings, the metabolic reprogramming observed in alveolar macrophages (AMs) post-influenza infection, as depicted by elevated glycolysis, PI3K–Akt, HIF-1, and mTOR signalling, underscores the therapeutic promise of leveraging trained immunity against cancer. The enhanced oxygen consumption and glycolytic rate reflect a prolonged metabolic rewiring instrumental in fortifying antitumor responses [75]. These findings accentuate the potential of trained immunity, driven by metabolic adaptations, as a viable approach to bolster antitumor defences, thereby broadening the spectrum of cancer therapeutic strategies.
Steps from bench to bedside
Given these findings, a deeper investigation into how metabolism regulates trained immunity in different diseases is necessary to develop new treatment strategies. The complex metabolic pathways linked with trained immunity suggest many potential targets for treatment. For example, targeting metabolic enzymes like glycolytic enzymes or key parts of lipid metabolism could have a modifying effect on trained immunity, and in turn, change the progression of a disease. Regulating trained immunity through metabolic pathways could also work alongside existing treatments to boost effectiveness and improve results in a wide range of diseases, including infectious diseases, autoimmune disorders, and cancers. New techniques like metabolomics and single-cell sequencing provide exceptional insights into the metabolic basis of trained immunity. These techniques allow for a detailed examination of the metabolic changes that come with trained immunity and possible implications in disease. For instance, identifying metabolic markers that characterize trained immune cells can reveal new targets for treatments. A deeper understanding of how metabolic pathways change in response to various immune challenges will help improve treatment strategies to adjust trained immunity.
Moreover, developing these ideas into clinical practice requires a strong regulatory framework to ensure the safety and effectiveness of treatments targeting trained immunity. The variation in metabolic reprogramming across different people and disease conditions is challenging and calls for a personalized approach to treatment. Discovery and validation of biomarkers will be crucial for categorizing patients and monitoring treatment responses. It is also important to consider the possible unintended effects and the long-term implications of adjusting trained immunity. A detailed understanding of the balance between immunological and metabolic factors is vital to avoid unexpected outcomes and to fully use the potential of trained immunity approaches.
In conclusion, the growing understanding of the close relationship between metabolism and trained immunity highlights new treatment possibilities. The ability to regulate trained immunity through metabolic interventions could lead to a new wave of treatments for a wide range of diseases. Ongoing research and collaboration across different disciplines will be crucial to uncover the full range of therapeutic possibilities offered by adjusting the metabolic aspects of trained immunity, thus marking a new phase of understanding and managing diseases.
Author contributions
AVF, JD-A and LMMP wrote draft; all authors critically corrected the manuscript.
Funding
MGN was supported by an ERC Advanced Grant (#833247) and a Spinoza Grant of the Netherlands Organization for Scientific Research. J.D.-A. is supported by The Netherlands Organization for Scientific Research (VENI grant 09150161910024 and Off Road 04510012010022).
Data availability
Not applicable for a review.
Declarations
MGN is a scientific founder of Lemba Therapeutics, TTxD and Biotrip. LABJ is scientific founder of TTxD, Lemba Therapeutics and Salvina Therapeutics. The other authors have no relevant financial or non-financial interests to disclose.
Footnotes
This contribution is part of the special issue “Immuno - Metabolism of Myeloid Cells” organized by Prof. Florent Ginhoux and Dr. Nicolas Venteclef.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Delany I, Rappuoli R, De Gregorio E (2014) Vaccines for the 21st century. EMBO Mol Med 6:708–720. 10.1002/emmm.201403876 10.1002/emmm.201403876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu ZQ, Dong X (2013) Systemic acquired resistance: Turning local infection into global defense. Annu Rev Plant Biol 64:839–863. 10.1146/annurev-arplant-042811-105606 10.1146/annurev-arplant-042811-105606 [DOI] [PubMed] [Google Scholar]
- 3.Netea MG, Joosten LAB, Latz E, et al (2016) Trained immunity: A program of innate immune memory in health and disease. Science 352:aaf1098. 10.1126/science.aaf1098 [DOI] [PMC free article] [PubMed]
- 4.Aaby P, Roth A, Ravn H et al (2011) Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J Infect Dis 204:245–252. 10.1093/infdis/jir240 10.1093/infdis/jir240 [DOI] [PubMed] [Google Scholar]
- 5.Prentice S, Nassanga B, Webb EL et al (2021) BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect Dis 21:993–1003. 10.1016/S1473-3099(20)30653-8/ATTACHMENT/76920E16-B3DA-46FC-AE93-A0D8EAB9B7FE/MMC3.PDF 10.1016/S1473-3099(20)30653-8/ATTACHMENT/76920E16-B3DA-46FC-AE93-A0D8EAB9B7FE/MMC3.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SG, Kleinnijenhuis J, Netea MG, Dockrell HM (2017) Whole Blood Profiling of Bacillus Calmette–Guérin-Induced Trained Innate Immunity in Infants Identifies Epidermal Growth Factor, IL-6, Platelet-Derived Growth Factor-AB/BB, and Natural Killer Cell Activation. Front Immunol 8:1–11. 10.3389/fimmu.2017.00644 10.3389/fimmu.2017.00644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S et al (2020) Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell 183:315–323. 10.1016/j.cell.2020.08.051 10.1016/j.cell.2020.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aaby P, Benn CS (2019) Developing the concept of beneficial non-specific effect of live vaccines with epidemiological studies. Clin Microbiol Infect 25:1459–1467. 10.1016/j.cmi.2019.08.011 10.1016/j.cmi.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 9.Sørup S, Benn CS, Poulsen A et al (2014) Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA 311:826–835. 10.1001/jama.2014.470 10.1001/jama.2014.470 [DOI] [PubMed] [Google Scholar]
- 10.Quintin J, Saeed S, Martens JHA et al (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12:223–232. 10.1016/j.chom.2012.06.006 10.1016/j.chom.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bekkering S, Quintin J, Joosten LAB et al (2014) Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. Arter Thromb Vasc Biol 34:1731–1738. 10.1161/ATVBAHA.114.303887 10.1161/ATVBAHA.114.303887 [DOI] [PubMed] [Google Scholar]
- 12.Christ A, Günther P, Lauterbach MAR et al (2018) Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 172:162-175.e14. 10.1016/j.cell.2017.12.013 10.1016/j.cell.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jentho E, Ruiz-Moreno C, Novakovic B et al (2021) Trained innate immunity, long-lasting epigenetic modulation, and skewed myelopoiesis by heme. Proc Natl Acad Sci 118:e2102698118. 10.1073/PNAS.2102698118 10.1073/PNAS.2102698118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanucchi S, Domínguez-Andrés J, Joosten LAB et al (2021) The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity 54:32–43. 10.1016/j.immuni.2020.10.011 10.1016/j.immuni.2020.10.011 [DOI] [PubMed] [Google Scholar]
- 15.de Laval B, Maurizio J, Kandalla PK et al (2020) C/EBPb-Dependent Epigenetic Memory Induces Trained Immunity in Hematopoietic Stem Cells. Cell Stem Cell 26:1–18. 10.1016/j.stem.2020.01.017 10.1016/j.stem.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann E, Sanz J, Dunn JL et al (2018) BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 172:176-190.e19. 10.1016/j.cell.2017.12.031 10.1016/j.cell.2017.12.031 [DOI] [PubMed] [Google Scholar]
- 17.Mitroulis I, Ruppova K, Wang B et al (2018) Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 172:147-161.e12. 10.1016/j.cell.2017.11.034 10.1016/j.cell.2017.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirovic B, Charlotte L, De Bree J et al (2020) Human BCG vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 28:1–13. 10.1016/j.chom.2020.05.014 10.1016/j.chom.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noz MP, Bekkering S, Groh L et al (2020) Reprogramming of bone marrow myeloid progenitor cells in patients with severe coronary artery disease. Elife 9:1–24. 10.7554/eLife.60939 10.7554/eLife.60939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong JG, Ravishankar A, Sharma S et al (2023) Epigenetic memory of coronavirus infection in innate immune cells and their progenitors. Cell 186:3882-3902.e24. 10.1016/j.cell.2023.07.019 10.1016/j.cell.2023.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Wang H, Yu X et al (2022) Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 185:1709-1727.e18. 10.1016/j.cell.2022.03.043 10.1016/j.cell.2022.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katzmarski N, Domínguez-Andrés J, Cirovic B, et al (2021) Transmission of trained immunity and heterologous resistance to infections across generations. Nat Immunol 22:1382–1390. 10.1038/s41590-021-01052-7 [DOI] [PubMed]
- 23.Moorlag SJCFM, Rodriguez-Rosales YA, Gillard J, et al (2020) BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep 33:108387. 10.1016/j.celrep.2020.108387 [DOI] [PMC free article] [PubMed]
- 24.Kalafati L, Kourtzelis I, Schulte-Schrepping J et al (2020) Innate Immune Training of Granulopoiesis Promotes Anti-tumor Activity. Cell 183:771-785.e12. 10.1016/j.cell.2020.09.058 10.1016/j.cell.2020.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng SC, Quintin J, Cramer RA, et al (2014) MTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684. 10.1126/science.1250684 [DOI] [PMC free article] [PubMed]
- 26.Arts RJW, Carvalho A, La Rocca C et al (2016) Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep 17:2562–2571. 10.1016/j.celrep.2016.11.011 10.1016/j.celrep.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keating ST, Groh L, Thiem K et al (2020) Rewiring of glucose metabolism defines trained immunity induced by oxidized low-density lipoprotein. J Mol Med 98:819–831. 10.1007/s00109-020-01915-w 10.1007/s00109-020-01915-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schödel J, Oikonomopoulos S, Ragoussis J et al (2011) High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 117:207–217. 10.1182/blood-2010-10-314427 10.1182/blood-2010-10-314427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meléndez-Rodríguez F, Urrutia AA, Lorendeau D et al (2019) HIF1α Suppresses Tumor Cell Proliferation through Inhibition of Aspartate Biosynthesis. Cell Rep 26:2257-2265.e4. 10.1016/j.celrep.2019.01.106 10.1016/j.celrep.2019.01.106 [DOI] [PubMed] [Google Scholar]
- 30.Morotti M, Bridges E, Valli A et al (2019) Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc Natl Acad Sci 116:12452–12461. 10.1073/pnas.1818521116 10.1073/pnas.1818521116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keating S, Groh L, van der Heijden C et al (2020) The Set7 Lysine Methyltransferase Regulates Plasticity in Oxidative Phosphorylation Necessary for Trained Immunity Induced by Beta-Glucan. Cell Rep 31:107548. 10.2139/ssrn.3444608 10.2139/ssrn.3444608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groh LA, Ferreira A V, Helder L, et al (2021) oxLDL-Induced Trained Immunity Is Dependent on Mitochondrial Metabolic Reprogramming. Immunometabolism 3:e210025. 10.20900/immunometab20210025 [DOI] [PMC free article] [PubMed]
- 33.Sohrabi Y, Lagache SMM, Schnack L et al (2019) mTOR-Dependent Oxidative Stress Regulates oxLDL-Induced Trained Innate Immunity in Human Monocytes. Front Immunol 9:3155. 10.3389/fimmu.2018.03155 10.3389/fimmu.2018.03155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomes MC, Brokatzky D, Bielecka MK et al (2023) Shigella induces epigenetic reprogramming of zebrafish neutrophils. Sci Adv 9:ead9706. 10.1126/sciadv.adf9706 10.1126/sciadv.adf9706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darroch H, Keerthisinghe P, Sung YJ et al (2023) Infection-experienced HSPCs protect against infections by generating neutrophils with enhanced mitochondrial bactericidal activity. Sci Adv 9:eadf9904. 10.1126/sciadv.adf9904 10.1126/sciadv.adf9904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinnijenhuis J, Quintin J, Preijers F et al (2014) BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol 155:213–219. 10.1016/j.clim.2014.10.005 10.1016/j.clim.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cichocki F, Wu CY, Zhang B et al (2018) ARID5B regulates metabolic programming in human adaptive NK cells. J Exp Med 215:2379–2395. 10.1084/jem.20172168 10.1084/jem.20172168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasid O, Chevalier C, Camarasa TMN et al (2019) H3K4me1 Supports Memory-like NK Cells Induced by Systemic Inflammation. Cell Rep 29:3933-3945.e3. 10.1016/j.celrep.2019.11.043 10.1016/j.celrep.2019.11.043 [DOI] [PubMed] [Google Scholar]
- 39.Ryan DG, O’Neill LAJ (2020) Krebs Cycle Reborn in Macrophage Immunometabolism. Annu Rev Immunol 38:289–313. 10.1146/annurev-immunol-081619-104850 [DOI] [PubMed]
- 40.Wellen KE, Hatzivassiliou G, Sachdeva UM, et al (2009) ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324:1076–80. 10.1126/science.1164097 [DOI] [PMC free article] [PubMed]
- 41.Sohrabi Y, Sonntag GVH, Braun LC et al (2020) LXR Activation Induces a Proinflammatory Trained Innate Immunity-Phenotype in Human Monocytes. Front Immunol 11:353. 10.3389/fimmu.2020.00353 10.3389/fimmu.2020.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viola A, Munari F, Sánchez-Rodríguez R et al (2019) The metabolic signature of macrophage responses. Front Immunol 10:1462 10.3389/fimmu.2019.01462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arts RJW, Novakovic B, ter Horst R et al (2016) Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab 24:807–819. 10.1016/j.cmet.2016.10.008 10.1016/j.cmet.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao M, Yang H, Xu W et al (2012) Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev 26:1326–1338. 10.1101/gad.191056.112 10.1101/gad.191056.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tannahill GM, Curtis AM, Adamik J et al (2013) Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496:238–242. 10.1038/nature11986 10.1038/nature11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batie M, Frost J, Frost M, et al Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science 363:1222–1226. 10.1126/science.aau5870 [DOI] [PubMed]
- 47.Moorlag SJCFM, Matzaraki V, Puffelen JH et al (2021) An integrative genomics approach identifies KDM4 as a modulator of trained immunity. Eur J Immunol. 10.1002/EJI.202149577 10.1002/EJI.202149577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martínez-Reyes I, Chandel NS (2020) Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 11:1–11. 10.1038/s41467-019-13668-3 10.1038/s41467-019-13668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Domínguez-Andrés J, Novakovic B, Li Y et al (2019) The Itaconate Pathway Is a Central Regulatory Node Linking Innate Immune Tolerance and Trained Immunity. Cell Metab 29:211-220.e5. 10.1016/j.cmet.2018.09.003 10.1016/j.cmet.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 50.Cordes T, Wallace M, Michelucci A et al (2016) Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem 291:14274–14284. 10.1074/jbc.M115.685792 10.1074/jbc.M115.685792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michelucci A, Cordes T, Ghelfi J et al (2013) Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A 110:7820–7825. 10.1073/pnas.1218599110 10.1073/pnas.1218599110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mills EL, Ryan DG, Prag HA et al (2018) Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556:113–117. 10.1038/nature25986 10.1038/nature25986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bambouskova M, Gorvel L, Lampropoulou V et al (2018) Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature 556:501–504. 10.1038/s41586-018-0052-z 10.1038/s41586-018-0052-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hooftman A, Angiari S, Hester S et al (2020) The Immunomodulatory Metabolite Itaconate Modifies NLRP3 and Inhibits Inflammasome Activation. Cell Metab 32:1–11. 10.1016/j.cmet.2020.07.016 10.1016/j.cmet.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin W, Qin K, Zhang Y, et al (2019) S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat Chem Biol 15:983–991. 10.1038/s41589-019-0323-5 [DOI] [PubMed]
- 56.Liao ST, Han C, Xu DQ et al (2019) 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat Commun 10:5091. 10.1038/s41467-019-13078-5 10.1038/s41467-019-13078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferreira A V., Kostidis S, Groh LA, et al (2023) Dimethyl itaconate induces long-term innate immune responses and confers protection against infection. Cell Rep 42:112658. 10.1016/j.celrep.2023.112658 [DOI] [PubMed]
- 58.Ferreira AV, Koeken VACM, Matzaraki V et al (2021) Glutathione Metabolism Contributes to the Induction of Trained Immunity. Cells 10:971. 10.3390/cells10050971 10.3390/cells10050971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen L-L, Morcelle C, Cheng Z-L et al (2022) Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses. Nat Cell Biol 2022:1–11. 10.1038/s41556-022-00853-8 10.1038/s41556-022-00853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu W, Wang Z, Zhang K et al (2019) One-Carbon Metabolism Supports S-Adenosylmethionine and Histone Methylation to Drive Inflammatory Macrophages. Mol Cell 75:1147–1160. 10.1016/j.molcel.2019.06.039 10.1016/j.molcel.2019.06.039 [DOI] [PubMed] [Google Scholar]
- 61.Seufert AL, Hickman JW, Traxler SK, et al (2022) Enriched dietary saturated fatty acids induce trained immunity via ceramide production that enhances severity of endotoxemia and clearance of infection. Elife 11:e76744. 10.7554/eLife.76744 [DOI] [PMC free article] [PubMed]
- 62.Lin T, Jiang D, Chen W, et al (2023) Trained immunity induced by high-salt diet impedes stroke recovery. EMBO Rep 1–22. 10.15252/embr.202357164 [DOI] [PMC free article] [PubMed]
- 63.Bekkering S, Arts RJW, Novakovic B et al (2018) Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 172:135-146.e9. 10.1016/j.cell.2017.11.025 10.1016/j.cell.2017.11.025 [DOI] [PubMed] [Google Scholar]
- 64.Findeisen HM, Voges VC, Braun LC et al (2022) LXRalphaRegulates oxLDL-Induced Trained Immunity in Macrophages. Int J Mol Sci 23:6166. 10.3390/IJMS23116166 10.3390/IJMS23116166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreira A V, Alarcon-barrera JC, Domínguez-andrés J, et al (2023) Fatty acid desaturation and lipoxygenase pathways support trained immunity. Nat Commun 14:7385. 10.1038/s41467-023-43315-x [DOI] [PMC free article] [PubMed]
- 66.Buckley CD, Gilroy DW, Serhan CN (2014) Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity 40:315–327. 10.1016/J.IMMUNI.2014.02.009 10.1016/J.IMMUNI.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding C, Shrestha R, Zhu X et al (2023) Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis. Nat Immunol 24:239–254. 10.1038/s41590-022-01388-8 10.1038/s41590-022-01388-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rother N, Yanginlar C, Prévot G et al (2023) Acid ceramidase regulates innate immune memory. Cell Rep 42:113458. 10.1016/j.celrep.2023.113458 10.1016/j.celrep.2023.113458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naik S, Larsen SB, Gomez NC et al (2017) Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550:475–480. 10.1038/nature24271 10.1038/nature24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wendeln AC, Degenhardt K, Kaurani L et al (2018) Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556:332–338. 10.1038/s41586-018-0023-4 10.1038/s41586-018-0023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guilliams M, De Kleer I, Henri S et al (2013) Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210:1977–1992. 10.1084/jem.20131199 10.1084/jem.20131199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aegerter H, Lambrecht BN, Jakubzick CV (2022) Biology of lung macrophages in health and disease. Immunity 55:1564–1580. 10.1016/j.immuni.2022.08.010 10.1016/j.immuni.2022.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gill SK, Hui K, Farne H et al (2016) Increased airway glucose increases airway bacterial load in hyperglycaemia. Sci Rep 6:1–10. 10.1038/srep27636 10.1038/srep27636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao Y, Jeyanathan M, Haddadi S et al (2018) Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell 175:1634–1650. 10.1016/j.cell.2018.09.042 10.1016/j.cell.2018.09.042 [DOI] [PubMed] [Google Scholar]
- 75.Wang T, Zhang J, Wang Y et al (2023) Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs. Nat Immunol 24:423–438. 10.1038/s41590-023-01428-x 10.1038/s41590-023-01428-x [DOI] [PubMed] [Google Scholar]
- 76.Zahalka S, Starkl P, Watzenboeck ML et al (2022) Trained immunity of alveolar macrophages requires metabolic rewiring and type 1 interferon signaling. Mucosal Immunol 15:896–907. 10.1038/s41385-022-00528-5 10.1038/s41385-022-00528-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakraborty S, Singh A, Wang L, et al (2023) Trained immunity of alveolar macrophages enhances injury resolution via KLF4-MERTK-mediated efferocytosis. J Exp Med 220:e20221388. 10.1084/jem.20221388 [DOI] [PMC free article] [PubMed]
- 78.Jeyanathan M, Vaseghi-Shanjani M, Afkhami S, et al (2022) Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut–lung axis. Nat Immunol 23:1687–1702. 10.1038/s41590-022-01354-4 [DOI] [PMC free article] [PubMed]
- 79.Stražar M, Mourits VP, Koeken VACM et al (2021) The influence of the gut microbiome on BCG-induced trained immunity. Genome Biol 22:1–22. 10.1186/s13059-021-02482-0 10.1186/s13059-021-02482-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koeken VACM, van der Pasch ES, Leijte GP et al (2020) The effect of BCG vaccination on alveolar macrophages obtained from induced sputum from healthy volunteers. Cytokine 133:155135. 10.1016/j.cyto.2020.155135 10.1016/j.cyto.2020.155135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koeken VACM, Qi C, Mourits VP et al (2022) Plasma metabolome predicts trained immunity responses after antituberculosis BCG vaccination. PLoS Biol 20:e3001765. 10.1371/journal.pbio.3001765 10.1371/journal.pbio.3001765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bekkering S, Stiekema LCA, Bernelot Moens S et al (2019) Treatment with Statins Does Not Revert Trained Immunity in Patients with Familial Hypercholesterolemia. Cell Metab 30:1–2. 10.1016/j.cmet.2019.05.014 10.1016/j.cmet.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 83.Oh TS, Zabalawi M, Jain S, et al (2022) Dichloroacetate improves systemic energy balance and feeding behavior during sepsis. JCI Insight 7:e153944. 10.1172/jci.insight.153944 [DOI] [PMC free article] [PubMed]
- 84.Nakamori Y, Park EJ, Shimaoka M (2021) Immune Deregulation in Sepsis and Septic Shock: Reversing Immune Paralysis by Targeting PD-1/PD-L1 Pathway. Front Immunol 11:1–14. 10.3389/fimmu.2020.624279 10.3389/fimmu.2020.624279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schrijver DP, Röring RJ, Deckers J et al (2023) Resolving sepsis-induced immunoparalysis via trained immunity by targeting interleukin-4 to myeloid cells. Nat Biomed Eng. 10.1038/s41551-023-01050-0 10.1038/s41551-023-01050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable for a review.