Highlights
-
•
As expected, a higher volume of physical activity was associated with reduced odds of both hypertension and obesity.
-
•
When considering the volume of physical activity, the frequency or intensity of physical activity did not significantly impact the odds of hypertension.
-
•
An increased proportion of vigorous physical activity relative to the total volume was associated with a small additional reduction in the odds of obesity.
-
•
Overall, physical activity volume seems to be more important than specific patterns of accumulation for preventing hypertension and obesity in women.
Keywords: Physical activity, Frequency, Intensity, Incidences, Epidemiology, Women
Abstract
Background
Optimal patterns of accrual of recommended levels of physical activity (PA) for prevention of hypertension and obesity are not known. The overall aim of this study was to investigate whether different patterns of accumulation of PA are differentially associated with hypertension and obesity in Australian women over 21 years. Specifically, we investigated whether, for the same weekly volume of PA, the number of sessions (frequency) and vigorousness of PA (intensity) were associated with a reduction in the occurrence of hypertension and obesity in women.
Methods
Data from the 1973–1978 and 1946–1951 cohorts of the Australian Longitudinal Study on Women's Health were analyzed (n = 20,588; 12%–16% with a Bachelor's or higher degree). Self-reported PA, hypertension, height, and weight were collected using mail surveys every 3 years from 1998/2000 to 2019/2021. Generalized Estimating Equation models with a 3-year lag model were used to investigate the association of PA volume (metabolic equivalent min/week) (none; 33–499; 500–999; ≥1000, weekly frequency (none; 1–2 times; 3–4 times; 5–7 times; ≥8 times), and the proportion of vigorous PA to total volume of PA (none; 0%; 1%–33%; 34%–66%; 67%–100%) with odds of hypertension and obesity from 2000 to 2021.
Results
The cumulative incidence of hypertension was 6% in the 1973–1978 and 23% in the 1946–1951 cohort; 27% of women in the 1973–1978; and 15% in the 1946–1951 cohort developed obesity over the period. Overall, a higher volume of PA was associated with reduced odds of hypertension and obesity. When the volume of PA was considered, the odds of hypertension did not vary according to the frequency or intensity of PA. However, increased proportion of vigorous PA to the total volume of PA was associated with a small additional reduction in the risk of obesity.
Conclusion
PA volume appears to be more important than the pattern of accumulation for the prevention of hypertension and obesity. Incorporating more sessions, particularly of vigorous-intensity PA, may provide extra benefits for the prevention of obesity.
Graphical Abstract
1. Introduction
Non-communicable chronic diseases are a major concern worldwide. Most non-communicable chronic diseases and their symptoms become more common with aging and, once present, continue to persist or deteriorate.1 For example, it is expected that by 2066, between 21% and 23% of the total population in Australia will be over 65 years, and most will have at least 2 long-term chronic conditions.2 This scenario will exponentially burden the healthcare system. Hypertension and obesity are major risk factors for the development of cardiovascular and metabolic conditions. In Australia, it has been estimated that 1 in 3 adults have hypertension, and a similar proportion has obesity.3 Thus, identifying patterns of key risk factors for hypertension and obesity in young and mid-age adults is vital for developing preventive strategies, which could have long-lasting benefits as the population ages.
Physical activity (PA) is a well-known modifiable risk factor for the prevention and management of hypertension and obesity.4, 5, 6 National and international guidelines focus on the weekly volume of PA and encourage adults to accumulate 150–300 min per week of moderate PA (MPA), 75–150 min per week of vigorous PA (VPA), or a combination of both intensities.4,7, 8, 9 These guidelines were developed under the assumption that weekly frequency and regularity of PA are not essential, and that activities of moderate and vigorous intensity are similarly associated with most health outcomes at the same volume of PA.4,7, 8, 9
Studies have suggested that mid-age and older adults who achieve the PA guidelines in 1–2 sessions per week (the weekend warrior pattern) have a similar risk of all-cause mortality10,11 and a similar prevalence of psychological distress to those who do more frequent activities.12 In mid-age Australian adults, associations of participation in a wide variety of sports and recreational activities once a week with reduced odds of hypertension, diabetes, and obesity over 6 years are similar to associations observed for participation 2 or more times per week.13 Some researchers have suggested that VPA might yield additional health benefits to those that are already well known to be associated with total volume of PA. For example, using self-reported data, Hamer and colleagues12 showed that Scottish and British women, but not men, who undertook VPA as part of their weekly PA had lower odds of psychological distress than women who did not include vigorous activities in their routine. Using data from more than 400,000 Americans, Wang and colleagues14 found that the proportion of VPA to total PA was associated with an extra reduction in the risk of all-cause and cardiovascular disease mortality. Pavey et al.,15 showed that, among women, the risk of hypertension was slightly lower for moderate and VPA than for moderate only PA across the entire range of PA levels, but this difference was only significant at the highest PA level. Furthermore, research using data from device-measured PA has shown inconsistent findings regarding the optimal patterns of accumulation of PA and occurrence of diseases.16,17 Overall, findings from previous studies highlight inconsistencies in our knowledge about the optimal patterns of activity accumulation and emphasize the need for further research to investigative the complexity of the relationships between a variety of patterns of PA and health outcomes.
The effects of patterns of accumulation of PA on the aetiology of cardiovascular and metabolic chronic conditions remain unclear. To date, most studies have explored the associations between patterns of PA accumulation and all-cause and cause-specific mortality. As the risks of onset of chronic diseases increase markedly from young to older adulthood,1 the role of frequency and intensity of PA in reducing the risk of major non-communicable chronic diseases should be investigated further. A systematic review that included 17 meta-analyses of data from adults with normal blood pressure, prehypertension, and hypertension reported insufficient evidence on whether frequency or intensity of PA influences the associations between PA and hypertension.18,19 Moreover, no studies have examined the association between long-term PA patterns and hypertension and obesity in women. Thus, the overarching aim was to investigate whether different patterns of accumulation of PA are differentially associated with hypertension and obesity in Australian women over 21 years of age. Specifically, we investigated whether for the same weekly volume of PA the number of sessions (frequency) and vigorousness of PA (intensity) were associated with a reduction in the occurrence of hypertension and obesity in women.
2. Methods
2.1. Sample
We used data from the 1946–1951 and 1973–1978 cohorts of the Australian Longitudinal Study on Women's Health (ALSWH). This is a longitudinal study of women's health that started in 1996 (Survey 1) and has been collecting health information every 3 years since 1998 and 2000 (Survey 2) for the 1946–1951 and 1973–1978 cohorts, respectively. Participants were randomly selected from the Medicare (Australian national health insurance) database, which includes all Australian citizens and permanent residents. At baseline, participants were largely representative of Australian women the same age in terms of area of residence, state and territory distribution, and marital status. To provide robustness and improve the power of the analyses, data from both cohorts were pooled. Further details of recruitment and design are available in previous publications.20 All participants provided written informed consent. The study was approved by the Human Research Ethics Committees of the University of Queensland and the University of Newcastle (H-076-0795 (2004/HE000224)).
2.2. PA
In each survey of both cohorts, PA was assessed using a modified version of the Active Australia Survey, which has acceptable levels of reliability and validity.21 This questionnaire measures the frequency and total duration of walking (for exercise/recreation or to get to and from places), MPA and VPA in the last week. The weekly volume of PA in metabolic equivalent (MET)-min for each participant was calculated as the sum of minutes spent walking and in MPA (weighted by an MET value of 3.33) and the duration of VPA (weighted by an MET value of 6.66). Participants were classified into groups according to their weekly volume of PA (none (<33 MET-min/week); low (33–499 MET-min/week); moderate (500–999 MET-min/week); or high (≥1000 MET-min/week)), number of sessions per week (none; 1–2; 3–4; 5–7; or ≥8), and proportion of VPA to total volume of PA (none; 1%–33%; 34%–66%; or 67%–100%). To explore the potential associations of volume, frequency, and intensity of PA with hypertension and obesity, joint variables of volume and frequency and volume and intensity were also created.
2.3. Outcome variables
Participants reported height, weight, and whether they had any specified long-term conditions, at all surveys. Hypertension was defined as a positive answer to the following question: “Have you ever been told by a doctor or nurse that you have hypertension?” Body mass index (BMI) was calculated as self-reported weight (kg)/height (m)2, and obesity was defined as BMI ≥ 30.0 kg/m2.22 To estimate the incidence of hypertension and obesity, those women who reported hypertension or had obesity in Surveys 1 and 2 were excluded. Thus, all the analyses were based on new cases of hypertension and obesity from 1998 (Survey 2, age 47–52) to 2019 (Survey 9, age 68–73) for the 1946–1951 cohort, and from 2000 (Survey 2, age 22–27) to 2021 (Survey 9, age 43–49) for the 1973–1978 cohort.
2.4. Covariates
Questions about socio-demographic characteristics were asked at each survey. For this study, variables were only included in the analytical models if the same questions were asked in both cohorts. Based on literature, the following variables were selected as covariates for multivariate models: age, education, area of residence, country of birth, hours of paid work, marital status and the number of children, smoking (current and former status), and alcohol (frequency and the number of standard drinks consumed on each occasion).
2.5. Statistical analyses
Analyses were performed in 2023 using Stata software 18.0 (StataCorp LLC., College Station, TX, USA). Descriptive analyses were conducted to summarize the sample characteristics of each cohort at baseline and to describe the distribution of participants according to PA variables (volume, frequency, and intensity) in each survey. First, individual associations of volume, frequency, and intensity of PA with hypertension and obesity were investigated. Second, the associations of frequency and intensity with hypertension and obesity, stratified by volume of PA, were examined. Third, joint variables (volume–frequency; volume–intensity) were derived and used as predictors of cumulative incidence of hypertension and obesity. Crude and multivariate Generalized Estimating Equation models with a 3-year lag model, in which PA variables at Surveys 2–8 were matched with hypertension and obesity measured at Surveys 3–9, were used to estimate odds ratios and respective 95% confidence intervals (95%CIs) of the associations. For the adjusted analyses, the models included the health condition in the preceding observation and the variables described in the covariates section. These covariates were included in the multivariable models as they are known to be potential confounders for the association between PA and long-term health conditions. To test the robustness of the findings and account for residual confounding caused by unmeasured variables, E-values23 for the associations of PA variables with hypertension and obesity were used to estimate the strength of the association (on the odds ratio scale) of an unmeasured confounder, with both exposure and outcome needed to negate the observed associations. In our analyses, an E-value of 2.0, for example, implies that an unmeasured confounder (e.g., diet) would need to increase both the likelihood of being in the exposure group of interest and the risk for the outcome of interest by 2-fold above the measured covariates to negate the associations observed in the study.
3. Results
From the 27,961 women who returned a completed survey at baseline (Survey 1, 1996), 25,068 responded to at least one of the subsequent surveys; 21,264 women responded to at least 4 surveys, and 10,036 women completed all surveys. The analytical sample included 20,588 women who did not have hypertension or obesity at baseline (Survey 1 or Survey 2). Sociodemographic characteristics of women whose data are included in the analyses are presented in Table 1. At Survey 2 (1998/2000), 9% and 16% of women in the 1973–1978 and 1945–1951 cohorts, respectively, did not report PA. Between 1998/2000 and 2019/2021, this proportion varied between 8%–13% in the 1973–1978 cohort and 13%–15% in the 1946–1951 cohort; 50%–60% of participants met the PA guidelines over the period. From Survey 2 to Survey 9, 6% of women in the 1973–1978 and 23% in the 1946–1951 cohort developed hypertension, while 27% and 15% of women in the 1973–1978 and 1946–1951 cohorts (respectively) developed obesity in the same period (Fig. 1).
Table 1.
Sociographic and behavioral characteristics of the analytical sample at Survey 1 (1996).
| 1973–1978 cohorta (n = 11,971) |
1946–1951 cohortb (n = 8617) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Highest qualification | ||||
| No formal qualification | 295 | 2.5 | 1373 | 16.1 |
| High school | 8120 | 68.2 | 4103 | 48.0 |
| Trade/certificate/diploma | 2094 | 17.6 | 1737 | 20.3 |
| Tertiary (Bachelor's or higher degree) | 1398 | 11.7 | 1328 | 15.6 |
| Marital status | ||||
| Married or de facto | 2497 | 21.0 | 7118 | 83.0 |
| Single, separated, or widowed | 9416 | 79.0 | 1459 | 17.0 |
| Smoking status | ||||
| Never | 6151 | 53.8 | 4373 | 52.4 |
| Former | 1670 | 14.6 | 2382 | 28.6 |
| Current | 3620 | 31.6 | 1588 | 19.0 |
| Alcohol consumption | ||||
| Low-risk drinker | 6182 | 52.2 | 4429 | 51.9 |
| Non-drinker | 1061 | 9.0 | 1204 | 14.1 |
| Rarely drinks | 3955 | 33.4 | 2467 | 28.9 |
| Risky/high-risk drinker | 637 | 5.4 | 433 | 5.1 |
| Physical activity (MET-min/week)c | ||||
| None | 708 | 9.3 | 1106 | 16.0 |
| <500 | 2374 | 31.3 | 1952 | 28.3 |
| 500–999 | 1833 | 24.2 | 1670 | 24.2 |
| ≥1000 | 2670 | 35.2 | 2176 | 31.5 |
| Frequency of physical activity (sessions/week)c | ||||
| None | 708 | 9.4 | 1106 | 16.3 |
| 1–2 | 1269 | 16.8 | 937 | 13.8 |
| 3–4 | 1614 | 21.4 | 1507 | 22.2 |
| 5–7 | 1917 | 25.4 | 1796 | 26.4 |
| ≥8 | 2046 | 27.1 | 1449 | 21.3 |
| Proportion of VPA to total physical activity volume (%)c | ||||
| None | 708 | 9.3 | 1106 | 16.0 |
| 0 | 3337 | 44.0 | 4549 | 65.9 |
| 1–33 | 703 | 9.3 | 381 | 5.5 |
| 34–66 | 1664 | 21.9 | 542 | 7.9 |
| 67–100 | 1173 | 15.5 | 326 | 4.7 |
Note: Percentages may not add up to 100% due to rounding.
Abbreviations: MET = metabolic equivalent; VPA = vigorous physical activity.
Average age at baseline: 20.5 ± 1.5 years (mean ± SD).
Average age at baseline: 47.5 ± 1.5 years.
Data from Survey 2.
Fig. 1.
Cumulative prevalence of (A) hypertension and (B) obesity in Australian women. At Survey 3, the average age of participants in the 1973–1978 and 1946–1951 cohorts was 27.6 ± 1.5 years and 52.5 ± 1.5 years (mean ± SD), respectively. At Survey 9, the average age of participants in the 1973–1978 and 1946–1951 cohorts was 45.5 ± 1.5 years and 70.8 ± 1.5 years, respectively. Surveys were conducted every 3 years.
Distributions of PA volume (MET-min/week) from Survey 2 to Survey 9, according to frequency and the proportion of total activity that was VPA, are presented in Fig. 2. Overall, women who reported higher frequencies of PA had the highest PA volumes; the volume did not substantially vary according to the proportion of VPA in the total volume (Fig. 2).
Fig. 2.
Distribution of physical activity volume (MET-min/week) from Survey 2 to Survey 9 according to (A) frequency and (B) proportion of total that was vigorous physical activity. MET = metabolic equivalent; PA = physical activity.
Associations between total PA, frequency, and intensity with odds of hypertension and obesity after 3 years are presented in Table 2. Overall, higher levels of PA, regardless of the measure (volume, frequency, or intensity) were associated with lower risks of hypertension and obesity. Compared with participants who reported no PA, those who reported ≥1000 MET-min/week had 16% (95%CI: 0.76–0.93) lower risk of hypertension and 27% (95%CI: 0.66–0.80) lower risk of obesity. Inverse dose–response associations of similar magnitude between frequency of PA and the proportion of VPA to total volume of PA were also observed for both health conditions (Table 2). The magnitude of unmeasured confounding needed to negate the associations described in Table 2 ranged between 1.46 (for the association between 33–499 MET-min/week and obesity) and 2.55 (for the association between 1%–33% of vigorous from the total volume of PA and obesity).
Table 2.
Associations of PA volume, frequency, and intensity with odds of hypertension and obesity from 2000 to 2021.
| PA | PA cases | Total | Model 1a | Model 2b | E-valuec |
|---|---|---|---|---|---|
| n | n | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Hypertension | |||||
| MET-min/week | |||||
| None | 1053 | 9082 | 1.00 | 1.00 | |
| 33–499 | 1758 | 20,932 | 0.93 (0.84–1.02) | 0.95 (0.86–1.05) | NA |
| 500–999 | 1627 | 17,601 | 0.91 (0.82–1.00) | 0.93 (0.84–1.03) | NA |
| ≥1000 | 2456 | 26,402 | 0.81 (0.74–0.89) | 0.84 (0.76–0.93) | 1.67 (1.36) |
| Frequency | |||||
| None | 1053 | 9082 | 1.00 | 1.00 | |
| 1–2 | 762 | 9710 | 0.88 (0.78–0.98) | 0.89 (0.80–1.01) | NA |
| 3–4 | 1328 | 14,518 | 0.94 (0.85–1.05) | 0.97 (0.88–1.08) | NA |
| 5–7 | 1988 | 20,558 | 0.87 (0.79–0.96) | 0.89 (0.81–0.99) | 1.50 (1.11) |
| ≥8 | 1685 | 19,361 | 0.82 (0.74–0.90) | 0.85 (0.77–0.94) | 1.63 (1.32) |
| The proportion of VPA to total volume PA (%)d | |||||
| None | 1053 | 7964 | 1.00 | 1.00 | |
| 0 | 4135 | 40,246 | 0.92 (0.85–1.01) | 0.95 (0.86–1.03) | NA |
| 1–33 | 535 | 6253 | 0.80 (0.70–0.91) | 0.83 (0.73–0.95) | 1.70 (1.29) |
| 34–66 | 820 | 11,446 | 0.77 (0.68–0.86) | 0.79 (0.70–0.89) | 1.85 (1.50) |
| 67–100 | 351 | 6990 | 0.74 (0.64-0.86) | 0.79 (0.68–0.91) | 1.85 (1.43) |
| Obesity | |||||
| MET-min/week | |||||
| None | 1640 | 8567 | 1.00 | 1.00 | |
| 33–499 | 2981 | 20,227 | 0.87 (0.79–0.95) | 0.90 (0.82–0.99) | 1.46 (1.11) |
| 500–999 | 2099 | 17,202 | 0.79 (0.72–0.87) | 0.84 (0.76–0.92) | 1.67 (1.39) |
| ≥1000 | 2497 | 25,903 | 0.69 (0.63–0.76) | 0.73 (0.66–0.80) | 2.08 (1.81) |
| Frequency | |||||
| None | 1640 | 8567 | 1.00 | 1.00 | |
| 1–2 | 1386 | 9308 | 0.90 (0.81–1.00) | 0.93 (0.83–1.04) | NA |
| 3–4 | 1784 | 14,171 | 0.78 (0.71–0.86) | 0.82 (0.74–0.91) | 1.74 (1.43) |
| 5–7 | 2357 | 20,095 | 0.77 (0.70–0.85) | 0.80 (0.73–0.88) | 1.81 (1.53) |
| ≥8 | 1902 | 19,009 | 0.70 (0.64–0.77) | 0.74 (0.67–0.82) | 2.04 (1.74) |
| Proportion of VPA to total volume PA (%)d | |||||
| None | 1640 | 8567 | 1.00 | 1.00 | |
| 0 | 5252 | 38,735 | 0.85 (0.78–0.93) | 0.88 (0.81–0.96) | 1.53 (1.25) |
| 1–33 | 532 | 6158 | 0.61 (0.53–0.69) | 0.63 (0.55–0.73) | 2.55 (2.08) |
| 34–66 | 1109 | 11,396 | 0.66 (0.59–0.74) | 0.70 (0.62–0.78) | 2.21 (1.88) |
| 67–100 | 684 | 7043 | 0.69 (0.61–0.78) | 0.73 (0.64–0.83) | 2.08 (1.70) |
Abbreviations: 95%CI = 95% confidence interval; MET = metabolic equivalent; NA = not available; OR = odds ratio; PA = physical activity; VPA = vigorous physical activity.
Adjusted for age and health condition in the preceding observation.
Adjusted for age, health condition in the preceding observation, education, marital status, smoking, and alcohol consumption.
Conditional on the measured covariates included in Model 2, the E-value represents the minimum strength of association that a potential unmeasured confounder would need to have with both the PA and health condition variables to explain the observed associations. Values in brackets represent the lower limit of the 95%CI.
n in the analytical models ranged between 70,226 and 62,220 observations from 15,245 to 15,596 participants.
Associations of the frequency and intensity of PA with the odds of hypertension and obesity at different volumes of PA are described in Table 3. For the same volume of PA, a higher number of sessions and a greater proportion of VPA were not associated with the odds of hypertension. However, lower odds of hypertension were associated with a higher proportion of VPA in relation to the total volume among highly active women (≥1000 MET-min/week). No clear associations between the frequency of PA and the odds of obesity were observed. Higher proportions of VPA were associated with lower odds of obesity at all levels of PA, although a clear dose–response relationship was not observed (Table 3).
Table 3.
Relative risks for the association of frequency of PA, and the proportion of VPA to total PA volume, with hypertension and obesity, stratified by total PA.
| Total PA (MET-min/week) |
||||||
|---|---|---|---|---|---|---|
| <500 |
500–999 |
≥1000 |
||||
| Cases/person-time | OR (95%CI) | Cases/person-time | OR (95%CI) | Cases/person-time | OR (95%CI) | |
| Hypertension | ||||||
| Frequency | ||||||
| 1–2 | 587/7823 | 1.00 | 120/1265 | 1.00 | 36/403 | 1.00 |
| 3–4 | 569/6587 | 1.13 (0.98–1.30) | 501/5272 | 1.00 (0.77–1.29) | 219/2305 | 1.30 (0.83–2.05) |
| 5–7 | 384/4109 | 1.07 (0.91–1.26) | 687/7227 | 0.87 (0.67–1.11) | 870/8721 | 1.26 (0.82–1.94) |
| ≥8 | 117/1553 | 1.02 (0.80–1.30) | 277/3278 | 0.94 (0.71–1.23) | 1256/14,071 | 1.19 (0.78–1.83) |
| Proportion of VPA to total PA volume (%) | ||||||
| 0 | 1576/18,363 | 1.00 | 1247/11,503 | 1.00 | 1198/9422 | 1.00 |
| 1–33 | 22/257 | 1.39 (0.85–2.28) | 85/1238 | 0.91 (0.70–1.19) | 417/4621 | 0.88 (0.76–1.01) |
| 34–66 | 71/967 | 1.08 (0.81–1.45) | 180/3011 | 0.84 (0.69–1.02) | 548/7172 | 0.82 (0.72–0.94) |
| 67–100 | 35/829 | 0.74 (0.51–1.09) | 74/1460 | 0.90 (0.68–1.19) | 236/4537 | 0.83 (0.70–0.99) |
| Obesity | ||||||
| Frequency | ||||||
| 1–2 | 1159/7521 | 1.00 | 151/1213 | 1.00 | 50/385 | 1.00 |
| 3–4 | 847/6401 | 0.81 (0.71–0.92) | 678/5166 | 1.06 (0.83–1.36) | 223/2270 | 0.93 (0.60–1.44) |
| 5–7 | 594/3996 | 0.98 (0.85–1.14) | 836/7061 | 0.92 (0.72–1.18) | 876/8584 | 0.98 (0.65–1.48) |
| ≥8 | 244/1524 | 0.92 (0.75–1.14) | 367/3232 | 0.97 (0.75–1.27) | 1245/13,807 | 0.93 (0.62–1.40) |
| Proportion of VPA to total PA volume (%) | ||||||
| 0 | 2688/17,734 | 1.00 | 1450/11,123 | 1.00 | 1006/9014 | 1.00 |
| 1–33 | 26/255 | 0.60 (0.35–1.02) | 112/1214 | 0.78 (0.60–1.01) | 378/4562 | 0.78 (0.66–0.91) |
| 34–66 | 120/956 | 0.92 (0.71–1.19) | 327/3016 | 0.79 (0.67–0.94) | 637/7140 | 0.85 (0.74–0.97) |
| 67–100 | 87/814 | 0.66 (0.49–0.89) | 170/1484 | 0.95 (0.76–1.19) | 413/4582 | 0.90 (0.77–1.06) |
Note: Adjusted for age, health condition in the preceding observation, education, marital status, smoking, and alcohol consumption.
Abbreviations: 95%CI = 95% confidence interval; MET = metabolic equivalent; OR = odds ratio; PA = physical activity; VPA = vigorous physical activity.
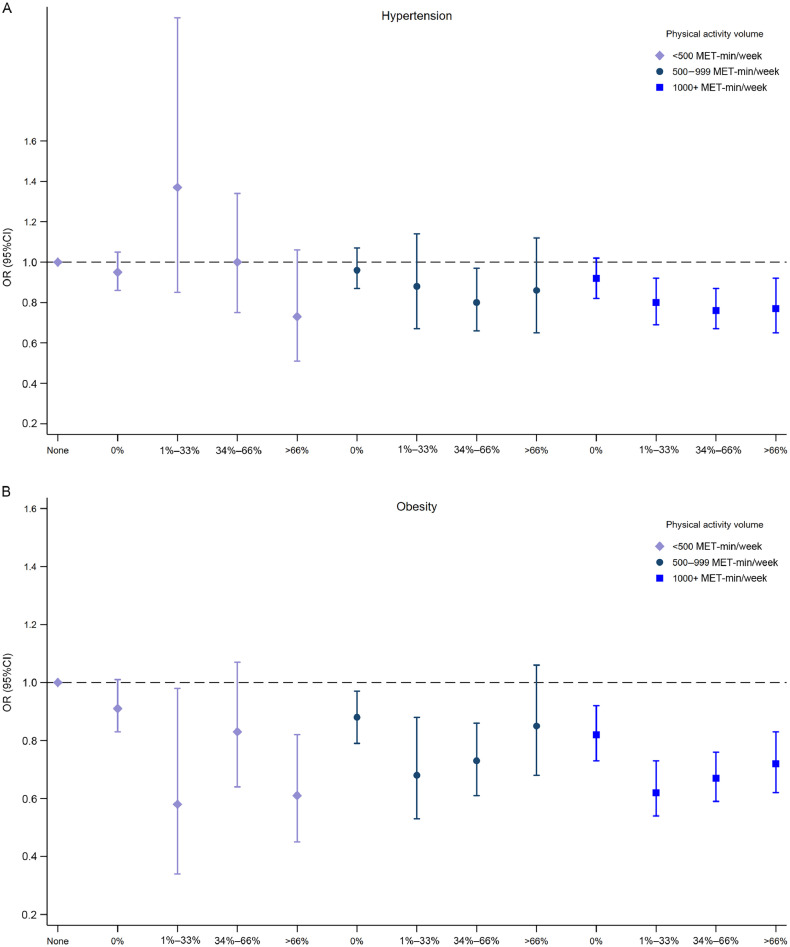
The joint associations of frequency and volume and of intensity and volume with hypertension and obesity are presented in Fig. 3, Fig. 4, respectively. Overall, the results were consistent with those observed in Table 3; there were no clear associations to suggest that frequency influenced the associations of PA with hypertension and obesity. However, there was a tendency for higher proportions of VPA to be associated with lower odds of obesity. Compared with inactive participants, women who accumulated ≥1000 MET-min/week and did not report VPAs had 15% lower odds (95%CI: 0.75–0.96) of obesity, whereas those with 1%–33% of vigorous activities had 37% lower odds of obesity (95%CI: 0.53–0.75).
Fig. 3.
Adjusted ORs for the joint associations between total PA (MET-min/week) and frequency of PA (x axis) with (A) hypertension and (B) obesity. Adjusted for age, health condition in the preceding observation, education, marital status, smoking, and alcohol consumption. 95%CI = 95% confidence interval; MET = metabolic equivalent; OR = odds ratio; PA = physical activity.
Fig. 4.
Adjusted ORs for the joint associations between total PA (MET-min/week) and proportion of vigorous to total volume of PA (x axis) with (A) hypertension and (B) obesity. Adjusted for age, health condition in the preceding observation, education, marital status, smoking, and alcohol consumption. 95%CI = 95% confidence interval; MET = metabolic equivalent; OR = odds ratio; PA = physical activity.
4. Discussion
In this study, we investigated whether different patterns of PA are differentially associated with hypertension and obesity in Australian women. Our findings suggest that even a few sessions are associated with reduced odds of hypertension and obesity. Furthermore, the inclusion of VPA, particularly for women with high volumes of PA, may provide small additional benefits for the prevention of obesity. To our knowledge, this is one of the first epidemiological studies investigating the extent to which frequency and intensity of PA contribute to the well-known benefits of volume of PA on women's health. Our findings indicate that promoting PA, even in a single session, might lead to improvements in health at the population level, and that volume of PA appears to be more important than the pattern of accumulation.
The potential benefits of frequency of PA have been assessed in previous studies. Using data from a nationwide cohort in the Republic of Korea, Jeong and colleagues24 showed that engaging in PA 1–2 times per week might be beneficial for preventing stroke in mid-age adults. In mid-age Australian adults, performing any of a variety of PAs (e.g., running, cycling, tennis, team sports, exercise classes, and resistance training) with at least weekly frequency was associated with lower odds of hypertension and obesity over 6 years.13 In accordance with previous studies, our findings suggest that recommending even 1 weekly session of PA may be a useful public health strategy for the prevention of hypertension and obesity.
Our results show that the inclusion of some vigorous activity may lead to greater reductions in the odds of hypertension and obesity over 21 years, particularly among those who are highly active. This finding is in line with previous analysis from the 1946–1951 cohort, which indicated that accumulation of 500–1000 MET-min/week of VPA was associated with a 27% risk reduction in hypertension, compared to an 18% risk reduction when the same level of PA was accumulated from moderate intensity activities.15 Additionally, data from the Women's Health Initiative Observational Study suggest that women who met PA recommendations through either walking, an MPA, or VPAs all had similar reductions in risk of cardiovascular disease compared to those with low levels of activity.25
Improvements in cardiorespiratory fitness (peak rate of oxygen consumption), cardiac stroke volume, blood pressure, body composition, and lipid profiles might explain the additional health benefits associated with VPA.26, 27, 28 A meta-analysis of 27 randomized controlled trials that compared the effects of higher and lower intensity aerobic PA on chronic inflammation in adults showed that PA intensity did not influence chronic inflammatory responses, although exploratory analyses suggested that higher intensity PA may be more efficacious than lower intensity for middle-aged adults.29 However, in another meta-analysis of randomized controlled trials, Keating and colleagues30 found that high-intensity interval training (which is a VPA) is likely to provide similar benefits to MPA in terms of body fat reduction.
It has been suggested that the lack of adjustment for total volume of PA in most studies, along with inconsistent methods for defining components of PA, may prevent firm conclusions from being drawn regarding which patterns of duration, intensity, and weekly frequency are most important for health benefits among adults.31 Although our findings suggest that PA accumulated in different patterns of frequency and intensity are associated with reduced risk of hypertension and obesity and that the addition of VPA may provide extra health benefits, these findings should be interpreted with caution. As frequency and intensity of PA are highly correlated with PA volume, methodological approaches used to detangle these components from one another often fail to adequately investigate each component in isolation from the others.
Some limitations of this study should be acknowledged. Self-report measures used to collect information about PA, hypertension, and BMI might have introduced bias in the results. While issues regarding underestimation of the exposure and outcomes could affect the estimates of prevalence and incidence, which are not within the scope of this study, this error is likely to be non-differential due to the nature of self-reporting and may lead to more conservative findings of the associations between exposure and outcomes. Although the analyses were adjusted for a variety of sociodemographic, behavioral, and health-related variables that were asked in both cohorts, residual confounding due to diet and other unmeasured variables cannot be disregarded. E-values suggested that the magnitude of the association of a potential unmeasured confounder with both PA and outcome variables needed to negate the observed associations was relatively high (odds ratio > 1.5), suggesting that the associations we found were relatively robust to unmeasured confounding. Despite the analytical approach used in our study, which provides insights into the longitudinal relationships between exposure and outcome variables, bias resulting from time-dependent relationships between exposure, confounders, and outcomes cannot be ruled out based on our findings. However, since our study included repeated measures of the outcome, we adjusted the models for the outcome variable in the previous survey to minimize bias from time-dependent relationships and unmeasured factors. Our findings may be attributable to a difference in report precision between MPA and VPA. Women may report VPA more precisely than MPA, which would lead to stronger associations between exposure and outcome variables. Our findings should be interpreted with caution as multiple comparisons may have increased the potential for type I errors. Last, missing data due to loss to follow-up and death may have introduced bias and diluted the magnitude of the associations observed in the study.
The strengths of this study include 21 years of repeated data collection from large population-based cohorts of women with high ongoing participation rates. More than 80% of the women in the original cohorts were included in these analyses, and >60% responded to at least 7 out of 8 possible surveys. This enabled the use of statistical approaches that account for multiple observations from each participant and for the time-varying nature of the exposure, outcome, and confounder variables. This approach accounts for within-participant variability, and hence minimizes variability due to random error in the measures. We pooled data from 2 large cohorts, which ensured the necessary statistical power to investigate the potential health benefits associated with patterns of accumulation of PA that are less common.
5. Conclusion
These findings show positive associations between at least weekly participation in PA with 2 important long-term health conditions in women, namely, hypertension and obesity. From a public health perspective, the results reinforce the importance of overall volume of PA, regardless of frequency or intensity. Practitioners and public health messages should encourage women to achieve the PA guidelines using a combination of activities of varying frequencies and intensities that fit their life constraints and preferences.
Acknowledgments
The research on which this paper is based was conducted as part of the Australian Longitudinal Study on Women's Health by the University of Queensland and the University of Newcastle. We are grateful to the Australian Government Department of Health and Aged Care for funding and to the women who provided the survey data. GIM is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (APP2008702). BPN is supported by the National Council for Scientific and Technological Developments- CNPq (process number 308772/2022-9).
Authors’ contributions
GIM conceived the study and performed the analyses, participated in the study design, and reviewed the literature; WJB conceived the study; DD, SEK, BPN, and RB participated in the study design and reviewed the literature. All authors contributed to multiple revisions of the article. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Data availability statement
Data are available upon reasonable request. Access to Australian Longitudinal Study on Women's Health (ALSWH) dataset requires approval from the ALSWH Data Access Committee. More information can be found at the ALSWH website: https://alswh.org.au/for-data-users/.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Dash SR, Hoare E, Varsamis P, Jennings GLR, Kingwell BA. Sex-specific lifestyle and biomedical risk factors for chronic disease among early-middle, middle and older aged Australian adults. Int J Environ Res Public Health. 2019;16:224. doi: 10.3390/ijerph16020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare. Older Australia at a glance. Available at:https://www.aihw.gov.au/reports/older-people/older-australia-at-a-glance/. [accessed 23.04.2023].
- 3.Australian Institute of Health and Welfare . AIHW; Canberra: 2018. Australia's health 2018. Australia's health series No. 16. AUS 221. [Google Scholar]
- 4.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson NA, Sultana RN, Brown WJ, Bauman AE, Gill T. Physical activity in the management of obesity in adults: A position statement from Exercise and Sport Science Australia. J Sci Med Sport. 2021;24:1245–1254. doi: 10.1016/j.jsams.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Sharman JE, Smart NA, Coombes JS, Stowasser M. Exercise and Sport Science Australia position stand update on exercise and hypertension. J Hum Hypertens. 2019;33:837–843. doi: 10.1038/s41371-019-0266-z. [DOI] [PubMed] [Google Scholar]
- 7.Brown WJ, Bauman AE, Bull F, Burton NW. Development of evidence-based physical activity recommendations for adults (18‒64 years). Report prepared for the Australian Government Department of Health, August 2012. Available at:http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-phys-act-guidelines/$File/DEB-PAR-Adults-18-64years.pdf. [accessed 23.04.2023]
- 8.Umpierre D, Coelho-Ravagnani C, Cecilia Tenorio M, et al. Physical activity guidelines for the Brazilian population: Recommendations report. J Phys Act Health. 2022;19:374–381. doi: 10.1123/jpah.2021-0757. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services . 2nd ed. U.S. Department of Health and Human Services; Washington, DC: 2018. Physical Activity Guidelines for Americans. [Google Scholar]
- 10.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS., Jr The weekend warrior and risk of mortality. Am J Epidemiol. 2004;160:636–641. doi: 10.1093/aje/kwh274. [DOI] [PubMed] [Google Scholar]
- 11.O'Donovan G, Lee IM, Hamer M, Stamatakis E. Association of “weekend warrior” and other leisure time physical activity patterns with risks for all-cause, cardiovascular disease, and cancer mortality. JAMA Intern Med. 2017;177:335–342. doi: 10.1001/jamainternmed.2016.8014. [DOI] [PubMed] [Google Scholar]
- 12.Hamer M, Biddle SJH, Stamatakis E. Weekend warrior physical activity pattern and common mental disorder: A population wide study of 108,011 British adults. Int J Behav Nutr Phys Act. 2017;14:96. doi: 10.1186/s12966-017-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mielke GI, Bailey TG, Burton NW, Brown WJ. Participation in sports/recreational activities and incidence of hypertension, diabetes, and obesity in adults. Scand J Med Sci Sports. 2020;30:2390–2398. doi: 10.1111/sms.13795. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Nie J, Ferrari G, et al. Association of physical activity intensity with mortality: A national cohort study of 403,681 US Adults. JAMA Intern Med. 2021;181:203–211. doi: 10.1001/jamainternmed.2020.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavey TG, Peeters G, Bauman AE, Rey-Lopez JP, Rezende LFM. Does vigorous physical activity provide additional benefits beyond those of moderate? Med Sci Sports Exerc. 2013;45:1948–1955. doi: 10.1249/MSS.0b013e3182940b91. [DOI] [PubMed] [Google Scholar]
- 16.Ho FK, Zhou Z, Petermann-Rocha F, et al. Association between device-measured physical activity and incident heart failure: A prospective cohort study of 94,739 UK Biobank participants. Circulation. 2022;146:883–891. doi: 10.1161/CIRCULATIONAHA.122.059663. [DOI] [PubMed] [Google Scholar]
- 17.Strain T, Wijndaele K, Dempsey PC, et al. Wearable-device-measured physical activity and future health risk. Nat Med. 2020;26:1385–1391. doi: 10.1038/s41591-020-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pescatello LS, Buchner DM, Jakicic JM, et al. Physical activity to prevent and treat hypertension: A systematic review. Med Sci Sports Exerc. 2019;51:1314–1323. doi: 10.1249/MSS.0000000000001943. [DOI] [PubMed] [Google Scholar]
- 19.Keating SE, Coombes JS, Stowasser M, Bailey TG. The role of exercise in patients with obesity and hypertension. Curr Hypertens Rep. 2020;22:77. doi: 10.1007/s11906-020-01087-5. [DOI] [PubMed] [Google Scholar]
- 20.Dobson AJ, Hockey R, Brown WJ, et al. Cohort profile update: Australian Longitudinal Study on Women's Health. Int J Epidemiol. 2015;44 doi: 10.1093/ije/dyv110. [DOI] [PubMed] [Google Scholar]
- 21.Brown WJ, Burton NW, Marshall AL, Miller YD. Reliability and validity of a modified self-administered version of the active Australia Physical Activity Survey in a sample of mid-age women. Aust N Z J Public Health. 2008;32:535–541. doi: 10.1111/j.1753-6405.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 22.Burton NW, Brown W, Dobson A. Accuracy of body mass index estimated from self-reported height and weight in mid-aged Australian women. Aust N Z J Public Health. 2010;34:620–623. doi: 10.1111/j.1753-6405.2010.00618.x. [DOI] [PubMed] [Google Scholar]
- 23.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 24.Jeong HG, Kim DY, Kang DW, et al. Physical activity frequency and the risk of stroke: A nationwide cohort study in Korea. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 26.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 27.Swain DP, Franklin BA. VO2 reserve and the minimal intensity for improving cardiorespiratory fitness. Med Sci Sports Exerc. 2002;34:152–157. doi: 10.1097/00005768-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Powell KE, Paluch AE, Blair SN. Physical activity for health: What kind? How much? How intense? On top of what? Annu Rev Public Health. 2011;32:349–365. doi: 10.1146/annurev-publhealth-031210-101151. [DOI] [PubMed] [Google Scholar]
- 29.Rose GL, Skinner TL, Mielke GI, Schaumberg MA. The effect of exercise intensity on chronic inflammation: A systematic review and meta-analysis. J Sci Med Sport. 2021;24:345–351. doi: 10.1016/j.jsams.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Keating SE, Johnson NA, Mielke GI, Coombes JS. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18:943–964. doi: 10.1111/obr.12536. [DOI] [PubMed] [Google Scholar]
- 31.Brady R, Brown WJ, Hillsdon M, Mielke GI. Patterns of accelerometer-measured physical activity and health outcomes in adults: A systematic review. Med Sci Sports Exerc. 2022;54:1155–1166. doi: 10.1249/MSS.0000000000002900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Access to Australian Longitudinal Study on Women's Health (ALSWH) dataset requires approval from the ALSWH Data Access Committee. More information can be found at the ALSWH website: https://alswh.org.au/for-data-users/.