Abstract
Background
The introduction of the 1 mm cut-off for resection margin according to the Leeds Pathology Protocol has transformed the concept of surgical radicality. Its impact on nodal-positive resected pancreatic ductal adenocarcinoma patients is unclear. The aim of this study was to analyse the effect of margin clearance on survival among resected, nodal-positive pancreatic ductal adenocarcinoma patients whose specimens were analysed according to the Leeds Pathology Protocol.
Methods
Data were collected retrospectively from multicentre clinical databases. Resected patients with nodal involvement were included. Overall survival and disease-free survival were analysed according to minimum reported margin clearances of 0, 0.5, 1, and 2 mm. The results are reported separately for patients who had not undergone venous resection and for patients for whom data were available regarding the superior mesenteric vein-facing margin or the vein specimen. The eighth edition of TNM classification by the AJCC was used.
Results
The study comprised 290 stage IIB patients and 215 stage III patients without venous resection. The superior mesenteric vein margin analysis comprised 127 stage IIB patients and 198 stage III patients. The different resection margin distances were not associated with overall survival and disease-free survival among patients without venous resection (P > 0.050). Receiving adjuvant therapy was associated with longer overall survival among stage IIB patients (P = 0.034) and stage III patients (P = 0.003) and with longer disease-free survival among stage III patients (P < 0.001).
Conclusions
In this study, a margin clearance greater than 1 mm showed no clear effect on overall survival in pancreatic ductal adenocarcinoma patients with nodal involvement, whereas adjuvant therapy was confirmed to be essential to ensure longer overall survival.
This is a retrospective multicentre study on the effect of margin clearance on survival among pancreatic ductal adenocarcinoma patients with nodal involvement. This study shows that a margin clearance greater than 1 mm does not result in a better prognosis. Oncological therapy remains an essential way to improve the prognosis among these patients.
Introduction
The prognosis among patients undergoing surgical resection of pancreatic ductal adenocarcinoma (PDAC) has recently improved with the introduction of chemotherapy combinations like folinic acid-fluorouracil-irinotecan-oxaliplatin (FOLFIRINOX), neoadjuvant therapy, and better surgical options for patients previously deemed unresectable, such as those with venous and arterial resections1. The radicality of the surgery is determined according to the margin clearance in the resection specimen. When this margin clearance is greater than 1 mm, the tumour is considered radically resected (R0 resection), whereas, when it is not, the resection is considered to be an R1 resection2. In 2006, a new axial slicing technique protocol called the Leeds Pathology Protocol (LEEPP) was introduced3,4. In this protocol, the anterior, posterior, and superior mesenteric vein (SMV-facing margin, previously groove margin) margins are coloured, followed by slicing the specimen perpendicularly to the duodenal axis. This new method led to the detection of significantly more R1 resections, in which the posterior resection margin was most often affected, followed by the SMV-facing margin1. How feasible categorizing R0 and R1 resections is and what their impact is on overall survival (OS) remain open questions. As most studies have only focused on the current R1 definition, data on other margin clearances is lacking. The results regarding the current R0/R1 definition and its impact on survival5,6 are inconsistent, although they mostly point towards shorter OS in the case of an R1 resection7. The aim of this multicentre retrospective study was to investigate the role of different resection margin distances on the survival of patients with PDAC and nodal involvement. With increasing numbers of patients undergoing surgery for tumours involving the SMV/portal vein (PV), a subgroup analysis included those patients with detailed information on the SMV margin status.
Methods
This multicentre retrospective study was approved by the Ethics Committee of each participating centre. Only centres evaluating surgical specimens according to a standardized axial method (Leeds3 or Royal Colleges of Pathologists8 protocol) were eligible for inclusion in the study. The participating centres were: Birmingham University Hospital National Health Service Foundation Trust, UK; Klinikum rechts der Isar, Technical University of Munich, Germany; Karolinska Institute, Stockholm, Sweden; Tampere University Hospital, Tampere, Finland; IRCCS Ospedale San Raffaele, Milan, Italy; Amsterdam UMC, Amsterdam, The Netherlands; Vilnius University Hospital, Vilnius, Lithuania; and Acibadem Mehmet Ali Aydinlar University Hospital, Istanbul, Turkey.
Patients who had undergone a pancreatic resection for PDAC with nodal involvement (stage IIB according to the seventh edition of TNM classification by the AJCC) between 2012 and 2017 were included. Patients with no data available on resection margins, with R2 resections, with an unknown number of positive lymph nodes, with an arterial resection, who received neoadjuvant therapy, or with a distant metastasis (M1) were excluded from the study.
Preoperative, histopathological, and oncological follow-up data were retrieved from patient files. All data available on margin clearance at specific sites (SMV-facing margin, superior mesenteric artery-facing margin, and anterior, posterior, and pancreatic transection margins) were gathered, as well as data on the pathology of the portal vein (PV) or SMV specimen if these were resected. Follow-up data regarding adjuvant therapy, disease-free survival (DFS), and OS were recorded retrospectively.
The tumours were recategorized according to the eighth edition of TNM classification by the AJCC to stage IIB (less than or equal to three positive regional lymph nodes) and stage III (greater than three positive regional lymph nodes) PDAC9. Data were subsequently analysed separately for stage IIB and stage III PDAC.
The margin clearance of the patients who had not undergone a venous resection was analysed according to the minimum reported margin (MRM), at four different cut-offs: 0, greater than 0.5, greater than 1, and greater than 2 mm. In addition, whether a wider SMV-facing margin achieved by a PV/SMV resection leads to longer survival was analysed in a separate analysis. This analysis included both patients without a venous resection, but with data on SMV-facing margin distances, and patients with a venous resection and data available on venous specimens. This analysis is referred to as SMV margin analysis and the results are reported according to the following categories: cancer involvement in the venous specimen; no cancer involvement in the venous specimen; SMV-facing margin clearance less than or equal to 0.5 mm; and SMV-facing margin clearance greater than 0.5 mm.
Primary outcomes were OS and DFS in relation to the resection margin clearance. Patients who died within 90 days after surgery were excluded from the survival analysis.
Statistical analysis was performed using SPSS® (IBM, Armonk, NY, USA; Statistics for Windows, Version 26.0, 2019). The results are reported as n (%) and median (interquartile range (i.q.r.)). Univariable analysis was performed using Pearson’s chi-squared test and Cox regression analysis. HRs for margins and the use of adjuvant therapy are presented centre adjusted. Multivariable analysis was performed using Cox regression analysis. A variable was included in the multivariable analysis when the P value was <0.100 in the univariable analysis. Kaplan–Meier survival curves are presented and differences are presented with log rank P values.
Results
Patient characteristics
A total of 653 patients with nodal involvement were identified. Of these, 290 stage IIB patients and 215 stage III patients underwent a pancreatic resection without a venous resection. Out of these patients, 86 stage IIB patients and 91 stage III patients had detailed information on the SMV-facing margin available. A total of 41 stage IIB patients and 107 stage III patients underwent a venous resection. This resulted in a total of 127 stage IIB patients and 198 stage III patients for the SMV margin analysis. The most common resection was a pancreatoduodenectomy.
Without venous resection
The median preoperative carbohydrate antigen 19-9 (CA19-9) concentration was 140 and 143 kU/l for stage IIB patients and stage III patients respectively. The proportion of patients receiving adjuvant therapy was 67% (193 of 290) for stage IIB patients and 59.1% (127 of 215) for stage III patients. See Table 1.
Table 1.
Characteristics of patients with stage IIB and III pancreatic ductal adenocarcinoma and histopathological findings for pancreatic specimens
| Without venous resection | SMV margin analysis | |||
|---|---|---|---|---|
| Stage IIB | Stage III | Stage IIB | Stage III | |
| Total | 290 | 215 | 127 | 198 |
| Sex | ||||
| Male | 160 (55.2) | 122 (56.7) | 65 (51.2) | 94 (47.5) |
| Female | 1230 (44.8) | 93 (43.3) | 62 (48.8) | 104 (52.5) |
| Age (years), median (i.q.r.) | 68 (61–74) | 68 (62–74) | 67 (63–74) | 69 (62–74) |
| Preoperative BMI (kg/m2), median (i.q.r.) | 24 (22–27) | 24 (22–28) | 24 (22–28) | 24 (22–28) |
| Unknown | 65 (22.4) | 51 (17) | 16 (12.6) | 25 (12.3) |
| ASA grade | ||||
| I–II | 219 (75.5) | 157 (73.0) | 97 (76.4) | 148 (74.7) |
| III–IV | 70 (24.1) | 56 (26.0) | 29 (22.8) | 48 (24.2) |
| Unknown | 1 (0.3) | 2 (0.9) | 1 (0.8) | 2 (1.0) |
| Preoperative CA19-9 (kU/l), median (i.q.r.) | 140 (32–530) | 143 (38–624) | 112 (27–498) | 153 (34–660) |
| Unknown | 82 (28.3) | 59 (20) | 14 (11) | 28 (13.9) |
| Type of surgery | ||||
| Pancreatoduodenectomy | 235 (81.0) | 202 (94) | 120 (94.5) | 178 (89.9) |
| Distal pancreatectomy | 38 (13.1) | 6 (2.8) | 3 (2.4) | 7 (3.5) |
| Total pancreatectomy | 17 (5.9) | 7 (3.3) | 4 (3.1) | 13 (6.6) |
| Venous resection | NA | NA | 40 (31.5) | 107 (54.0) |
| Adjuvant therapy | 193 (66.6) | 127 (59.1) | 93 (73.2) | 128 (64.6) |
| Unknown | 26 (9.0) | 25 (11.6) | 16 (12.5) | 17 (8.4) |
| Tumour size (mm), median (i.q.r.) | 30 (25–40) | 30 (25–40) | 30 (21–35) | 33 (25–40) |
| Unknown | 10 (3.4) | 5 (1.7) | 1 (0.78) | 3 (1.5) |
| T stage | ||||
| T1–2 | 199 (68.6) | 146 (67.9) | 101 (79.5) | 129 (63.9) |
| T3 | 91 (31.4) | 69 (32.1) | 22 (17.3) | 67 (33.8) |
| Unknown | 13 (4.5) | 5 (2.3) | 4 (3.1) | 5 (2.5) |
| Differentiation stage | ||||
| 1–2 | 171 (59.2) | 128 (59.5) | 74 (58.1) | 120 (60.6) |
| 3–4 | 109 (37.7) | 76 (35.3) | 46 (36.5) | 73 (36.9) |
| Unknown | 9 (3.1) | 11 (5.1) | 6 (4.8) | 5 (2.5) |
| Number of harvested lymph nodes, median (i.q.r.) | 22 (16–32) | 22 (15–31) | 24 (16–32) | 25 (19–37) |
| Perineural invasion | 229 (79.0) | 188 (87.4) | 114 (89.8) | 180 (90.8) |
| Unknown | 12 (4.2) | 7 (3.2) | 3 (2.4) | 4 (2.0) |
| Angioinvasion | 144 (49.7) | 162 (75.3) | 73 (57.5) | 134 (67.7) |
| Unknown | 29 (10.0) | 22 (10.2) | 14 (11.0) | 17 (8.6) |
| Minimum reported margin | ||||
| Margin 0 mm | 55 (19.0) | 77 (35.8) | NA | NA |
| Unknown | 23 (7.9) | 16 (7.4) | – | – |
| Margin >0.5 mm | 186 (64.1) | 97 (45.1) | NA | NA |
| Unknown | 20 (6.9) | 16 (7.4) | – | – |
| Margin >1 mm | 97 (33.4) | 77 (35.8) | NA | NA |
| Unknown | – | – | – | – |
| Margin >2 mm | 38 (13.1) | 18 (8.4) | NA | NA |
| Unknown | 40 (13.8) | 51 (23.7) | – | – |
| SMV margin analysis | ||||
| SMV margin >0.5 mm | NA | NA | 46 (36.2) | 41 (20.7) |
| SMV margin ≤0.5 mm | NA | NA | 40 (31.5) | 50 (25.3) |
| No cancer in the vein specimen | NA | NA | 16 (12.6) | 37 (18.7) |
| Cancer in the vein specimen | NA | NA | 25 (19.7) | 70 (35.4) |
Values are n (%) unless otherwise indicated. i.q.r., interquartile range; NA, not available; CA19-9, carbohydrate antigen 19-9; SMV, superior mesenteric vein.
SMV margin analysis
The median preoperative CA19-9 concentration was 112 and 153 kU/l for stage IIB patients and stage III patients respectively. The proportion of patients receiving adjuvant therapy was 73.2% (93 of 127) for stage IIB patients and 64.6% (128 of 198) for stage III patients. See Table 1.
Postoperative histopathological findings
Without venous resection
The median tumour size was 30 for both stage IIB patients and stage III patients. According to the eighth edition of TNM classification by the AJCC, 68.6% (199 of 290) of stage IIB patients and 67.9% (146 of 215) of stage III patients had a T1–2 tumour. Perineural invasion was present among 79.0% (229 of 290) and 87.4% (188 of 215) of stage IIB patients and stage III patients respectively. The differentiation stage was low for 59.2% (171 of 290) of stage IIB patients and 59.5% (128 of 215) of stage III patients. Among stage IIB patients and stage III patients, 67% (193 of 290) and 64.2% (138 of 215) respectively had an MRM of less than or equal to 1 mm. See Table 1.
SMV margin analysis
The median tumour size was 30 and 33 mm for stage IIB patients and stage III patients respectively. A T1–2 tumour was found in 79.5% (101 of 127) of stage IIB patients and in 63.9% (129 of 198) of stage III patients. Perineural invasion was detected in 89.8% (114 of 127) and 91.1% (180 of 198) of stage IIB patients and stage III patients respectively. The differentiation stage was low for 58.1% (74 of 127) of stage IIB patients and 60.69% (120 of 198) of stage III patients. Venous resection was performed on 31.5% (40 of 127) of stage IIB patients and on 54.0% (107 of 198) of stage III patients. Of these patients, 40% (16 of 40) of stage IIB patients and 35% (37 of 107) of stage III patients had no malignancy in the final vein specimen. See Table 1.
Survival analysis
Postoperative mortality
Thirty-day mortality was 0.9% among stage IIB patients and 2.1% among stage III patients. Ninety-day mortality was 3.0% among stage IIB patients and 5.2% among stage III patients.
Without venous resection
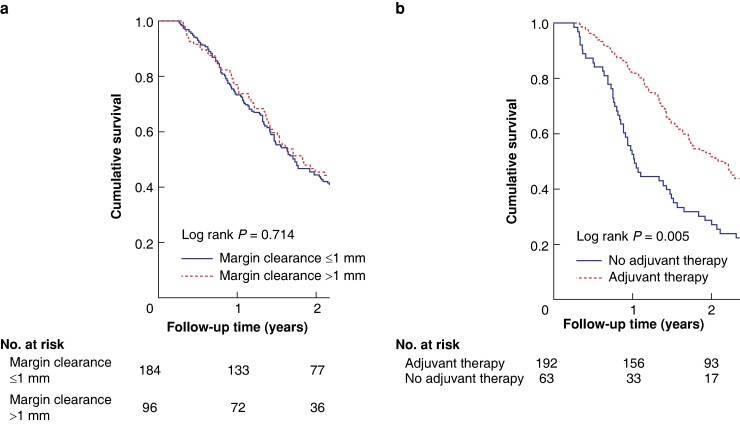
The median OS was 19.2 months among stage IIB patients. In the multivariable analysis, T stage T3 (HR 2.27, 95% c.i. 1.48 to 3.48; P < 0.001) and differentiation stage 3–4 (HR 1.47, 95% c.i. 1.06 to 2.02; P = 0.020) were associated with shorter OS. Among stage IIB patients, the multivariable analysis showed that receiving adjuvant therapy (HR 0.69, 95% c.i. 0.48 to 0.97; P = 0.034) and greater than 0 mm margin clearance (HR 0.59, 95% c.i. 0.38 to 0.91; P = 0.018) were associated with longer OS. A greater than 0 mm margin clearance did not show a significant survival benefit in Kaplan–Meier analysis (log rank P = 0.692). See Fig. 1 and Table 2.
Fig. 1.
Kaplan–Meier survival curves of stage IIB patients
a In relation to 1 mm cut-off. b In relation to adjuvant therapy.
Table 2.
Overall survival analysis: without venous resection
| Stage IIB | Stage III | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR (95% c.i.) | P | HR (95% c.i.) | P | HR (95% c.i.) | P | HR (95% c.i.) | P | |
| Age (>65 versus ≤65 years) | 1.08 (0.82,1.43) | 0.594 | – | – | 1.08 (0.84,1.49) | 0.636 | – | – |
| Sex (female versus male) | 0.95 (0.73,1.23) | 0.687 | – | – | 0.71 (0.52,0.96) | 0.028 | 0.66 (0.47,0.92) | 0.015 |
| BMI >25 kg/m2 (yes versus no) | 0.86 (0.65,1.16) | 0.324 | – | – | 0.89 (0.65,1.22) | 0.462 | – | – |
| ASA grade (III–IV versus I–II) | 0.98 (0.72,1.33) | 0.885 | – | – | 1.48 (1.05,2.08) | 0.024 | 1.49 (1.02,2.18) | 0.040 |
| ASA grade (unknown versus I–II) | 1.14 (0.16,8.14) | 0.898 | – | – | 1.00 (0.14,7.19) | 0.999 | 1.00 (0.13,7.81) | 0.996 |
| CA19-9 (per 100 kU/l increase) | 1.01 (1.00,1.11) | 0.153 | – | – | 1.01 (1.00,1.02) | 0.043 | 1.01 (1.00,1.02) | 0.005 |
| Tumour size (per cm increase) | 1.17 (1.06,1.29) | 0.001 | 1.03 (0.88,1.21) | 0.731 | 1.06 (0.95,1.19) | 0.309 | – | – |
| T stage (T3 versus T1–2) | 2.24 (1.64,2.98) | <0.001 | 2.27 (1.48,3.48) | <0.001 | 0.93 (0.67,1.27) | 0.660 | 0.86 (0.58,1.26) | 0.431 |
| T stage (unknown versus T1–2) | 1.69 (0.83,3.45) | 0.152 | – | – | 0.19 (0.03,1.35) | 0.096 | 0.15 (0.02,1.11) | 0.063 |
| Perineural invasion (yes versus no) | 0.90 (0.64,1.27) | 0.546 | – | – | 1.11 (0.62,2.01) | 0.723 | – | – |
| Perineural invasion (unknown versus no) | 0.69 (0.33,1.42) | 0.314 | – | – | 1.17 (0.41,3.32) | 0.775 | – | – |
| Angioinvasion (yes versus no) | 0.89 (0.68,1.17) | 0.410 | – | – | 1.25 (0.79,1.96) | 0.344 | 1.62 (0.94,2.79) | 0.085 |
| Angioinvasion (unknown versus no) | 1.17 (0.75,1.85) | 0.492 | – | – | 2.08 (1.12,3.86) | 0.020 | 3.36 (1.51,7.47) | 0.003 |
| Differentiation stage (3–4 versus 1–2) | 1.52 (1.16,2.00) | 0.003 | 1.47 (1.06,2.02) | 0.020 | 1.39 (1.00,1.93) | 0.050 | 2.14 (1.36,3.36) | 0.001 |
| Differentiation stage (unknown versus 1–2) | 2.08 (1.05,4.10) | 0.035 | 1.62 (0.72,3.65) | 0.244 | 1.24 (0.57,2.66) | 0.589 | 0.82 (0.31,2.21) | 0.697 |
| Adjuvant therapy* (yes versus no) | 0.66 (0.47,0.92) | 0.016 | 0.69 (0.48,0.97) | 0.034 | 0.61 (0.42,0.87) | 0.006 | 0.56 (0.38,0.82) | 0.003 |
| Adjuvant therapy* (unknown versus no) | 0.92 (0.47,1.78) | 0.796 | 0.83 (0.40,1.71) | 0.607 | 0.70 (0.39,1.27) | 0.238 | 0.43 (0.21,0.88) | 0.022 |
| MRM >0 mm* (yes versus no) | 0.60 (0.40,0.91) | 0.016 | 0.59 (0.38,0.91) | 0.018 | 1.04 (0.68,1.58) | 0.857 | – | – |
| MRM >0 mm* (unknown versus no) | 0.68 (0.37,1.27) | 0.227 | 0.64 (0.33,1.21) | 0.167 | 1.54 (0.83,2.86) | 0.172 | – | – |
| MRM >0.5 mm* (yes versus no) | 0.76 (0.53,1.10) | 0.147 | – | – | 1.13 (0.76,1.69) | 0.538 | – | – |
| MRM >0.5 mm* (unknown versus no) | 0.70 (0.37,1.32) | 0.272 | – | – | 1.61 (0.89,2.93) | 0.117 | – | – |
| MRM >1 mm* (yes versus no) | 0.94 (0.69,1.28) | 0.947 | – | – | 1.16 (0.79,1.69) | 0.433 | – | – |
| MRM >2 mm* (yes versus no) | 1.05 (0.70,1.58) | 0.812 | – | – | 1.57 (0.84,2.92) | 0.158 | – | – |
| MRM >2 mm* (unknown versus no) | 0.80 (0.48,1.32) | 0.381 | – | – | 1.06 (0.69,1.64) | 0.782 | – | – |
*Centre adjusted. CA19-9, carbohydrate antigen 19-9; MRM, minimum reported margin.
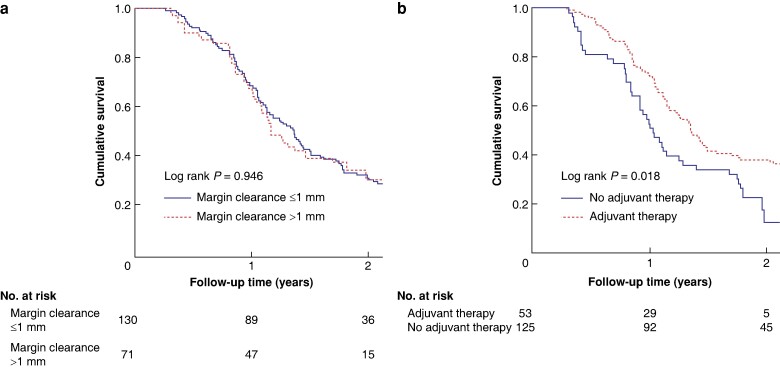
Among stage III patients, the multivariable analysis showed that a higher preoperative CA19-9 concentration (HR 1.01, 95% c.i. 1.00 to 1.02; P = 0.005), differentiation stage 3–4 (HR 2.14, 95% c.i. 1.36 to 3.36; P = 0.001), and ASA grade III–IV (HR 1.49, 95% c.i. 1.02 to 2.18; P = 0.040) were associated with shorter OS, whereas receiving adjuvant therapy (HR 0.56, 95% c.i. 0.38 to 0.82; P = 0.003) and female sex (HR 0.66, 95% c.i. 0.47 to 0.92; P = 0.015) were associated with longer OS. The median survival was 14.4 months among stage III patients. See Fig. 2 and Table 2.
Fig. 2.
Kaplan–Meier survival curves of stage III patients
a In relation to 1 mm cut-off. b In relation to adjuvant therapy.
SMV margin analysis
Among stage IIB patients, the multivariable analysis showed that T stage T3 (HR 2.29, 95% c.i. 1.05 to 5.02; P = 0.038) and differentiation stage 3–4 (HR 2.29, 95% c.i. 1.33 to 3.95; P = 0.003) were associated with shorter OS, whereas receiving adjuvant therapy (HR 0.31, 95% c.i. 0.15 to 0.62; P < 0.001) and histopathological cancer involvement in the vein specimen (HR 0.47, 95% c.i. 0.24 to 0.93; P = 0.030) were associated with longer OS. See Table 3.
Table 3.
Overall survival analysis: SMV margin analysis
| Stage IIB | Stage III | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR (95% c.i.) | P | HR (95% c.i.) | P | HR (95% c.i.) | P | HR (95% c.i.) | P | |
| Age (>65 versus ≤65 years) | 0.93 (0.60,1.43) | 0.728 | – | – | 1.05 (0.76,1.46) | 0.766 | – | – |
| Sex (female versus male) | 0.90 (0.61,1.34) | 0.605 | – | – | 1.13 (0.84,1.53) | 0.425 | – | – |
| BMI >25 kg/m2 (yes versus no) | 1.34 (0.87,2.07) | 0.188 | – | – | 0.88 (0.64,1.22) | 0.450 | – | – |
| ASA grade (III–IV versus I–II) | 1.27 (0.80,2.00) | 0.311 | – | – | 1.16 (0.818,1.63) | 0.412 | – | – |
| ASA grade (unknown versus I–II) | 1.34 (0.19,9.70) | 0.774 | – | – | 0.99 (0.14,7.09) | 0.990 | – | – |
| CA19-9 (per 100 kU/l increase) | 1.01 (1.00,1.02) | 0.049 | 1.01 (0.99,1.03) | 0.374 | 1.01 (1.00,1.02) | 0.018 | 1.01 (1.00,1.02) | 0.013 |
| Tumour size (per cm increase) | 1.21 (1.04,1.40) | 0.015 | 1.01 (0.85,1.43) | 0.48 | 1.04 (0.95,1.15) | 0.393 | – | – |
| T stage (T3 versus T1–2) | 1.74 (1.07,2.83) | 0.025 | 2.29 (1.05,5.02) | 0.038 | 1.05 (0.764,1.45) | 0.761 | 1.14 (0.75,1.73) | 0.547 |
| T stage (unknown versus T1–2) | 1.06 (0.14,7.78) | 0.955 | 0.11 (0.01,1.74) | 0.118 | 0.16 (0.02,1.14) | 0.138 | 0.22 (0.03,1.63) | 0.138 |
| Perineural invasion (yes versus no) | 0.95 (0.47,1.90) | 0.879 | 0.70 (0.32,1.53) | 0.368 | 1.05 (0.59,1.86) | 0.874 | 0.97 (0.46,2.06) | 0.944 |
| Perineural (unknown versus no) | 6.71 (1.74,25.9) | 0.006 | 15.5 (1.48,161) | 0.022 | 11.9 (2.51,56.0) | 0.002 | 2.80 (0.38,20.8) | 0.315 |
| Angioinvasion (yes versus no) | 1.20 (0.78,1.86) | 0.410 | 1.05 (0.60,1.85) | 0.861 | 1.04 (0.73,1.48) | 0.822 | 0.71 (0.42,1.19) | 0.195 |
| Angioinvasion (unknown versus no) | 2.94 (1.54,5.57) | 0.001 | 1.51 (0.58,3.91) | 0.400 | 2.63 (1.43,4.84) | 0.002 | 1.49 (0.68,3.25) | 0.316 |
| Differentiation stage (3–4 versus 1–2) | 1.94 (1.25,3.00) | 0.003 | 2.29 (1.33,3.95) | 0.003 | 1.17 (0.86,1.61) | 0.321 | 1.23 (0.89,1.89) | 0.350 |
| Differentiation stage (unknown versus 1–2) | 4.48 (1.87,10.7) | <0.011 | 1.20 (0.33,4.34) | 0.786 | 4.49 (1.41,14.4) | 0.011 | 3.45 (0.77,15.8) | 0.107 |
| Adjuvant therapy* (yes versus no) | 0.37 (0.20,0.69) | 0.002 | 0.31 (0.15,0.62) | <0.001 | 0.48 (0.33,0.69) | <0.001 | 0.47 (0.30,0.72) | <0.001 |
| Adjuvant therapy* (unknown versus no) | 0.36 (0.14,0.90) | 0.029 | 0.19 (0.06,0.57) | 0.003 | 0.82 (0.42,1.59) | 0.556 | 0.71 (0.33,1.55) | 0.389 |
| SMV margin status* (>0.5 versus ≤0.5 mm) | 0.79 (0.46,1.34) | 0.381 | 0.84 (0.45,1.56) | 0.572 | 0.99 (0.59,1.65) | 0.954 | – | – |
| No cancer in the vein specimen | 1.17 (0.57,2.42) | 0.675 | 1.86 (0.81,4.28) | 0.144 | 0.90 (0.54,1.49) | 0.671 | – | – |
| Cancer in the vein specimen | 0.52 (0.28,0.96) | 0.037 | 0.47 (0.24,0.93) | 0.030 | 1.22 (0.75,1.99) | 0.422 | – | – |
*Centre adjusted. CA19-9, carbohydrate antigen 19-9.
Among stage III patients, the multivariable analysis showed that receiving adjuvant therapy (HR 0.47, 95% c.i. 0.30 to 0.72; P < 0.001) was associated with longer OS, whereas a higher preoperative CA19-9 concentration (HR 1.01, 95% c.i. 1.00 to 1.02; P = 0.013) was associated with shorter OS. See Table 3.
Disease-free survival
Without venous resection
Among stage IIB patients, the multivariable analysis showed that increased tumour size (HR 1.33, 95% c.i. 1.15 to 1.55; P < 0.001) and a higher preoperative CA19-9 concentration (HR 1.13, 95% c.i. 1.00 to 1.03; P = 0.035) were associated with shorter DFS. Among stage III patients, the multivariable analysis showed that receiving adjuvant therapy (HR 0.35, 95% c.i. 0.23 to 0.55; P < 0.001) and a BMI greater than 25 kg/m2 (HR 0.61, 95% c.i. 0.42 to 0.90; P = 0.012) were associated with longer DFS, whereas a higher preoperative CA19-9 concentration (HR 1.02, 95% c.i. 1.01 to 1.03; P < 0.001) was associated with shorter DFS. See Table 4.
Table 4.
Disease-free survival analysis: without venous resection
| Stage IIB | Stage III | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR (95% c.i.) | P | HR (95% c.i.) | P | HR (95% c.i.) | P | HR (95% c.i.) | P | |
| Age (>65 versus ≤65 years) | 0.98 (0.72,1.33) | 0.875 | – | – | 1.27 (0.87,1.84) | 0.211 | – | – |
| Sex (female versus male) | 0.96 (0.71,1.29) | 0.785 | – | – | 0.81 (0.57,1.15) | 0.235 | – | – |
| BMI >25 kg/m2 (yes versus no) | 0.89 (0.59,1.13) | 0.225 | – | – | 0.69 (0.49,0.99) | 0.044 | 0.61 (0.42,0.90) | 0.012 |
| ASA grade (III–IV versus I–II) | 0.99 (0.70,1.39) | 0.943 | – | – | 1.39 (0.95,2.04) | 0.095 | 1.52 (1.00,2.31) | 0.050 |
| ASA grade (unknown versus III–IV) | 3.57 (0.50,25.8) | 0.207 | – | – | 5.69 (0.78,41.7) | 0.087 | 2.89 (0.38,21.9) | 0.306 |
| CA19-9 (per 100 kU/l increase) | 1.01 (1.00,1.02) | 0.037 | 1.01 (1.00,1.03) | 0.035 | 1.01 (1.00,1.02) | 0.003 | 1.02 (1.01,1.03) | <0.001 |
| Tumour size (per cm increase) | 1.18 (1.05,1.32) | 0.004 | 1.33 (1.15,1.55) | <0.001 | 1.05 (0.92,1.20) | 0.513 | – | – |
| T stage (T3 versus T1–2) | 2.20 (1.58,3.06) | <0.001 | – | – | 1.04 (0.72,1.50) | 0.839 | – | – |
| T stage (T3 versus unknown) | 0.60 (0.19,1.89) | 0.382 | – | – | 0.72 (0.23,2.29) | 0.579 | – | – |
| Perineural invasion (yes versus no) | 1.60 (1.04,2.46) | 0.034 | 1.06 (0.59,1.88) | 0.856 | 0.73 (0.42,1.28) | 0.276 | – | – |
| Perineural invasion (unknown versus no) | 1.22 (0.55,2.71) | 0.634 | 0.94 (0.34,2.63) | 0.903 | 1.43 (0.51,4.00) | 0.492 | – | – |
| Angioinvasion (yes versus no) | 1.22 (0.89,1.67) | 0.214 | – | – | 1.15 (0.69,1.93) | 0.589 | – | – |
| Angioinvasion (unknown versus no) | 1.46 (0.88,2.42) | 0.145 | – | – | 1.76 (0.86,3.60) | 0.124 | – | – |
| Differentiation stage (3–4 versus 1–2) | 1.14 (0.83,1.56) | 0.409 | – | – | 1.11 (0.77,1.60) | 0.575 | – | – |
| Differentiation stage (unknown versus 1–2) | 1.69 (0.78,3.63) | 0.184 | – | – | 1.51 (0.65,3.48) | 0.337 | – | – |
| Adjuvant therapy* (yes versus no) | 0.88 (0.59,1.30) | 0.511 | 0.68 (0.43,1.08) | 0.105 | 0.38 (0.25,0.57) | <0.001 | 0.35 (0.23,0.55) | <0.001 |
| Adjuvant therapy* (unknown versus no) | 0.43 (0.18,1.03) | 0.058 | 0.38 (0.16,0.93) | 0.034 | 0.29 (0.14,0.59) | <0.001 | 0.31 (0.154,0.64) | 0.001 |
| MRM cut-off >0 mm* (yes versus no) | 1.07 (0.67,1.72) | 0.767 | – | – | 1.05 (0.67,1.67) | 0.824 | – | – |
| MRM cut-off >0 mm* (unknown versus no) | 1.21 (0.60,2.41) | 0.596 | – | – | 1.68 (0.82,3.4) | 0.153 | – | – |
| MRM cut-off >0.5 mm* (yes versus no) | 0.94 (0.64,1.40) | 0.777 | – | – | 0.97 (0.63,1.49) | 0.883 | – | – |
| MRM cut-off >0.5 mm* (unknown versus no) | 0.95 (0.48,1.88) | 0.881 | – | – | 1.60 (0.80,3.17) | 0.183 | – | – |
| MRM cut-off >1 mm* (yes versus no) | 0.97 (0.69,1.35) | 0.837 | – | – | 1.09 (0.72,1.65) | 0.617 | – | – |
| MRM cut-off >2 mm* (yes versus no) | 1.03 (0.65,1.63) | 0.903 | – | – | 1.59 (0.81,3.12) | 0.176 | – | – |
| MRM cut-off >2 mm* (unknown versus no) | 0.97 (0.56,1.67) | 0.911 | – | – | 1.08 (0.66,1.73) | 0.774 | – | – |
*Centre adjusted. CA19-9, carbohydrate antigen 19-9; MRM, minimum reported margin.
Local recurrence was not associated with margin widths among stage IIB patients or stage III patients (stage IIB: P = 0.800 for margin clearance greater than 0 mm, P = 0.731 for margin clearance greater than 0.5 mm, P = 0.398 for margin clearance greater than 1 mm, and P = 0.200 for margin clearance greater than 2 mm; stage III: P = 0.467 for margin clearance greater than 0 mm, P = 0.536 for margin clearance greater than 0.5 mm, P = 0.913 for margin clerance greater than 1 mm, and P = 0.426 for margin clearance greater than 2 mm).
SMV margin analysis
Among stage IIB patients, the multivariable analysis showed that T stage T3 (HR 8.01, 95% c.i. 3.352 to 19.2; P < 0.001) was associated with shorter DFS, whereas an SMV margin greater than 0.5 mm (HR 0.35, 95% c.i. 0.17 to 0.69; P = 0.002) and female sex (HR 0.59, 95% c.i. 0.36 to 0.96; P = 0.032) were associated with longer DFS. Among stage III patients, the multivariable analysis showed that receiving adjuvant therapy (HR 0.60, 95% c.i. 0.36 to 0.98; P = 0.041) was associated with longer DFS, whereas angioinvasion (HR 0.46, 95% c.i. 0.25 to 0.85; P = 0.013), a higher preoperative CA19-9 concentration (HR 1.01, 95% c.i. 1.00 to 1.02; P = 0.008), and differentiation stage 3–4 (HR 1.83, 95% c.i. 1.09 to 3.08; P = 0.023) were associated with shorter DFS. See Table 5.
Table 5.
Disease-free survival analysis: SMV margin analysis
| Stage IIB | Stage III | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR (95% c.i.) | P | HR (95% c.i.) | P | HR (95% c.i.) | P | HR (95% c.i.) | P | |
| Age (>65 versus ≤65 years) | 0.92 (0.58,1.47) | 0.739 | – | – | 0.78 (0.54,1.12) | 0.179 | – | – |
| Sex (female versus male) | 0.65 (0.42,1.00) | 0.049 | 0.59 (0.36,0.96) | 0.032 | 1.28 (0.90,1.81) | 0.170 | – | – |
| BMI >25 kg/m2 (yes versus no) | 0.98 (0.60,1.62) | 0.947 | – | – | 1.36 (0.94,1.96) | 0.104 | – | – |
| ASA grade (III–IV versus I–II) | 0.95 (0.57,1.58) | 0.852 | – | – | 0.90 (0.59,1.36) | 0.631 | 1.26 (0.78,2.20) | 0.341 |
| ASA grade (unknown versus I–II) | 3.12 (0.43,23.0) | 0.263 | – | – | 7.65 (1.03,56.8) | 0.047 | 5.28 (0.64,43.9) | 0.124 |
| CA19-9 (per 100 kU/l increase) | 1.01 (1.00,1.02) | 0.127 | – | – | 1.01 (1.00,1.02) | 0.060 | 1.01 (1.00,1.02) | 0.008 |
| Tumour size (per cm increase) | 1.21 (1.05,1.39) | 0.007 | 0.82 (0.62,1.09) | 0.171 | 1.00 (0.90,1.11) | 0.978 | – | – |
| T stage (T3 versus T1–2) | 3.24 (1.90,5.55) | <0.001 | 8.01 (3.35,19.2) | <0.001 | 1.10 (0.76,1.58) | 0.62 | – | – |
| T stage (unknown versus T1–2) | 0.65 (0.09,4.73) | 0.670 | 0.21 (0.02,2.45) | 0.215 | 0.35 (0.09,1.43) | 0.144 | – | – |
| Perineural invasion (yes versus no) | 1.12 (0.49,2.59) | 0.786 | – | – | 1.48 (0.72,3.05) | 0.291 | 1.44 (0.53,3.96) | 0.477 |
| Perineural (unknown versus no) | 3.00 (0.60,15.1) | 0.182 | – | – | 15.6 (3.09,78.5) | <0.001 | 6.32 (0.69,57.8) | 0.10 |
| Angioinvasion (yes versus no) | 1.08 (0.68,1.74) | 0.739 | 0.90 (0.48,1.67) | 0.736 | 1.19 (0.79,1.77) | 0.410 | 0.46 (0.25,0.85) | 0.013 |
| Angioinvasion (unknown versus no) | 2.26 (1.11,4.60) | 0.025 | 1.76 (0.70,4.47) | 0.23 | 1.82 (0.83,4.22) | 0.075 | 0.45 (1.01,24.0) | 0.113 |
| Differentiation stage (3–4 versus 1–2) | 1.32 (0.84,2.09) | 0.235 | 1.53 (0.84,2.79) | 0.165 | 0.90 (0.62,1.29) | 0.556 | 1.83 (1.09,3.08) | 0.023 |
| Differentiation stage (unknown versus 1–2) | 2.90 (1.14,7.39) | 0.026 | 3.88 (1.23,12.3) | 0.021 | 6.73 (2.05,22.1) | 0.002 | 4.93 (1.01,24.0) | 0.048 |
| Adjuvant therapy* (yes versus no) | 0.79 (0.39,1.62) | 0.524 | 0.62 (0.28,1.38) | 0.242 | 0.57 (0.36,0.89) | 0.013 | 0.60 (0.36,0.98) | 0.041 |
| Adjuvant therapy* (unknown versus no) | 0.36 (0.12,1.08) | 0.067 | 0.38 (0.11,1.32) | 0.126 | 0.30 (0.12,0.75) | 0.009 | 0.17 (0.06,0.50) | 0.001 |
| SMV margin status* (>0.5 versus ≤0.5 mm) | 0.51 (0.28,0.92) | 0.024 | 0.35 (0.17,0.69) | 0.002 | 0.66 (0.36,1.20) | 0.174 | – | – |
| No cancer in the vein specimen | 0.91 (0.44,1.88) | 0.911 | 0.72 (0.31,1.67) | 0.446 | 1.26 (0.70,2.28) | 0.437 | – | – |
| Cancer in the vein specimen | 0.63 (0.33,1.21) | 0.166 | 0.65 (0.33,1.30) | 0.222 | 1.52 (0.89,2.60) | 0.126 | – | – |
*Centre adjusted. CA19-9, carbohydrate antigen 19-9; SMV, superior mesenteric vein.
Discussion
In this study, a margin clearance greater than 1 mm showed no clear effect on OS in PDAC patients with nodal involvement, whereas adjuvant therapy was confirmed to be essential to ensure longer OS.
Previous articles have reported survival advantages for a margin clearance greater than 1 mm10–12, but most of them also included patients with no nodal involvement (stages I and IIA). Also, various meta-analyses have reported survival benefits of a greater than 1 mm margin clearance, but they were often biased by different slicing techniques, the definition of margins, and the incomplete data on oncological treatment7,13,14.
It has been reported that, after meticulous pathological analysis of a specimen, a greater than 1 mm margin clearance is detected among a minority of patients15. The results of the present study demonstrate the same effect. Moreover, the location and the size of a tumour affect the potential margin widths. Increasing margin clearance towards the anterior surface is impossible, but in the transection line of the pancreas the margin width can be expanded until a total pancreatectomy is performed. In this study, the T stage T3 was a prognostic factor for shorter OS, demonstrating the importance of tumour size.
Another challenge regarding resection classification is how to define the margin clearance when a venous resection is performed. The invasion of the SMV-facing margin, especially beyond the adventitia of the PV, has been reported to be associated with a poorer prognosis16, although the margin itself is wider after the venous resection. A study by Kleive et al.17 demonstrated that the margin clearance at the SMV-facing margin was frequently less than or equal to 1 mm, especially for large tumours with a broad invasive front, and concluded that it is not feasible to achieve a margin clearance greater than 1 mm. The SMV margin analysis showed that a positive margin on the vein specimen was associated with longer OS among stage IIB patients. This result may be explained by a potentially more aggressive oncological approach among these patients.
This study demonstrated that evaluation of venous infiltration intraoperatively can be difficult and inaccurate, showing that nearly 40% of venous specimens had no malignancy during the final pathological examination. These data are concordant with the results of a large study by Delpero et al.18, which reported that 46% of patients with a venous resection had no vein infiltration.
In this study, adjuvant therapy was associated with longer OS in the multivariable analysis of both stage IIB and stage III patients. The DFS analysis showed a beneficial effect of adjuvant therapy on DFS for stage III patients, but not for stage IIB patients. This may be explained by varying follow-up schemes in the participating centres when the date of the detection of local recurrence or distant metastasis is associated with the follow-up intervals.
The main strength of this study is the use of the standardized histological, axial slicing protocol (LEEPP). Moreover, patients were recategorized according to the eighth edition of TNM classification by the AJCC to stage IIB and stage III PDAC subgroups. The new categories take into consideration the effect of the nodal involvement on survival. Thus, the application of the eighth edition of TNM classification decreased the potential bias caused by the nodal status in the results. In addition, data on a 1 mm margin clearance were available in all reports, which enabled the analysis of the current R0 definition among patients who underwent a pancreatic resection without a venous resection. The exclusion of patients who underwent neoadjuvant therapy allowed direct evaluation of the effect of surgical radicality on survival. The analysis was centre adjusted to decrease the impact of centre-related differences.
Some limitations need to be acknowledged. The retrospective and multicentre nature of the study challenged data recording completeness. This might have led to an underpowered analysis, especially with regard to the SMV margin analysis. During recent years, preoperative neoadjuvant therapy has become a common strategy when there is a suspicion of any vessel involvement in the preoperative imaging. The effect of margin clearance on survival after neoadjuvant therapy could not be determined in this study due to the limitations in the dataset. Despite the standardized histopathological protocol, the majority of the pathology reports contained incomplete data on the margin clearance for different margin sites. This is most likely due to a clinical policy not to report the margin widths when the margin clearance was less than 1 mm for one margin, as that held no clinical relevance for the current R0 or R1 definition.
In conclusion, adjuvant therapy plays an essential role in the prognosis among patients with PDAC with nodal involvement. The margin clearance greater than 1 mm showed no significant association with OS in this study. As earlier studies have reported, true margin clearance analysis requires meticulous sampling of the specimens and, without this, the microscopic spread of PDAC may not be noticed. Whether the same quality of margin clearance evaluation can be achieved in clinical work as in a research setting is uncertain. On the other hand, challenges exist regarding how to interpret margin clearance after a vein resection and/or when a greater than 1 mm margin is impossible to achieve due to tumour location. This study emphasizes the role of postoperative oncological therapy and diminishes the role of 1 mm margin clearance as a prognostic factor. In the future, neoadjuvant therapy may decrease infiltrative growth and systemic spread and improve OS, especially in borderline resectable PDAC patients.
Acknowledgements
The authors thank Professor Caroline Verbeke for valuable comments during the study and Virginia Mattila for proofreading the manuscript.
Contributor Information
Reea P Ahola, Department of Gastroenterology and Gastrointestinal Surgery, Tampere University Hospital and Tampere University, Tampere, Finland.
Eline S Zwart, Department of Surgery, Amsterdam UMC, Amsterdam, Cancer Center Amsterdam, Amsterdam, The Netherlands.
Benediktas Kurlinkus, Clinic of Gastroenterology, Nephrourology and Surgery, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
Asif Halimi, Division of Surgery, CLINITECH, Karolinska Institute, Stockholm, Sweden; Department of Surgical and Perioperative Sciences, Surgery, Umeå University, Umeå, Sweden.
Bengi S Yilmaz, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany.
Giulio Belfiori, Division of Pancreatic Surgery, Vita Salute San Raffaele University, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Keith Roberts, Department of Surgery, Birmingham University Hospital National Health Service Foundation Trust, Birmingham, UK.
Rupaly Pande, Department of Surgery, Birmingham University Hospital National Health Service Foundation Trust, Birmingham, UK.
Hasan A Al-Saffar, Division of Surgery, CLINITECH, Karolinska Institute, Stockholm, Sweden.
Patrick Maisonneuve, Division of Epidemiology and Biostatistics, IEO European Institute of Oncology IRCCS, Milan, Italy.
Güralp O Ceyhan, HPB Unit, Department of General Surgery, School of Medicine, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey.
Johanna Laukkarinen, Department of Gastroenterology and Gastrointestinal Surgery, Tampere University Hospital and Tampere University, Tampere, Finland; Department of Surgery, Tampere University, Tampere, Finland.
Funding
This study was conducted as a project of the Ninth Pancreas 2000 Education and Research Programme, funded by Acibadem Mehmet Ali Aydinlar University, the Karolinska Institute, and the European Pancreatic Club (EPC). The authors appreciate the support and funding from: the Cancer Center Amsterdam Foundation (grant number 2017-4-09), The Netherlands (CCA) to E.S.Z.; the Lithuanian Society of Gastroenterology, Lithuania to B.K.; State Research Funding (VTR), Finland and the Sigrid Jusélius Foundation, Finland to R.P.A. and J.L.; the Italian Ministry of Health via Ricerca Corrente and 5 per 1000 funds to P.M. The funders had no involvement in the study design, data collection, data analysis, manuscript preparation, or publication decisions.
Author contributions
Reea P. Ahola (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing—original draft, Writing—review & editing), Eline S. Zwart (Data curation, Funding acquisition, Investigation, Methodology, Resources, Writing—original draft, Writing—review & editing), Benediktas Kurlinkus (Data curation, Funding acquisition, Investigation, Methodology, Resources, Writing—original draft, Writing—review & editing), Asif Halimi (Data curation, Funding acquisition, Investigation, Methodology, Writing—original draft, Writing—review & editing), Bengi S. Yilmaz (Data curation, Investigation, Writing—original draft, Writing—review & editing), Giulio Belfiori (Data curation, Writing—original draft, Writing—review & editing), Keith Roberts (Data curation, Investigation, Writing—original draft, Writing—review & editing), Rupaly Pande (Data curation, Writing—original draft, Writing—review & editing), Hasan A. Al-Saffar (Data curation, Writing—review & editing), Patrick Maisonneuve (Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing), Güralp O. Ceyhan (Investigation, Methodology, Resources, Supervision, Writing—original draft, Writing—review & editing), and Johanna Laukkarinen (Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing—original draft, Writing—review & editing)
Disclosure
The authors declare no conflicts of interest.
Data availability
Data were handled according to personal data legislation and individual data are not available.
References
- 1. Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant treatment in pancreatic cancer. Front Oncol 2020;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hermanek PSL. TNM Classification of Malignant Tumors (4th edn). Berlin: Springer-Verlag, 1987 [Google Scholar]
- 3. Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232–1237 [DOI] [PubMed] [Google Scholar]
- 4. Verbeke CS, Gladhaug IP. Dissection of pancreatic resection specimens. Surg Pathol Clin 2016;9:523–538 [DOI] [PubMed] [Google Scholar]
- 5. Janot MS, Kersting S, Belyaev O, Matuschek A, Chromik AM, Suelberg D et al. Can the new RCP R0/R1 classification predict the clinical outcome in ductal adenocarcinoma of the pancreatic head? Langenbeck’s Arch Surg 2012;397:917–925 [DOI] [PubMed] [Google Scholar]
- 6. Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB (Oxford) 2009;11:18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurlinkus B, Ahola R, Zwart E, Halimi A, Yilmaz BS, Ceyhan GO et al. In the era of the Leeds protocol: a systematic review and a meta-analysis on the effect of resection margins on survival among pancreatic ductal adenocarcinoma patients. Scand J Surg 2020;109:11–17 [DOI] [PubMed] [Google Scholar]
- 8. The Royal College of Pathologists . Cancer datasets and tissue pathways. 2019. https://www.rcpath.org/profession/guidelines/cancer-datasets-and-tissue-pathways.html
- 9. Allen PJ, Kuk D, Castillo CF-D, Basturk O, Wolfgang CL, Cameron JL et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg 2017;265:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghaneh P, Kleeff J, Halloran CM, Raraty M, Jackson R, Melling J et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg 2019;269:520–529 [DOI] [PubMed] [Google Scholar]
- 11. Osipov A, Nissen N, Rutgers J, Dhall D, Naziri J, Chopra S et al. Redefining the positive margin in pancreatic cancer: impact on patterns of failure, long-term survival and adjuvant therapy. Ann Surg Oncol 2017;24:3674–3682 [DOI] [PubMed] [Google Scholar]
- 12. Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF-D, Deshpande V et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 2013;257:731–736 [DOI] [PubMed] [Google Scholar]
- 13. Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JHG, Bakkevold KE et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75–83; discussion 83 [DOI] [PubMed] [Google Scholar]
- 14. Chandrasegaram MD, Goldstein D, Simes J, Gebski V, Kench JG, Gill AJ et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg 2015;102:1459–1472 [DOI] [PubMed] [Google Scholar]
- 15. Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008;15:1651–1660 [DOI] [PubMed] [Google Scholar]
- 16. Fukuda S, Oussoultzoglou E, Bachellier P, Rosso E, Nakano H, Audet M et al. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch Surg 2007;142:172–179; discussion 180 [DOI] [PubMed] [Google Scholar]
- 17. Kleive D, Labori KJ, Line P-D, Gladhaug IP, Verbeke CS. Pancreatoduodenectomy with venous resection for ductal adenocarcinoma rarely achieves complete (R0) resection. HPB (Oxford) 2020;22:50–57 [DOI] [PubMed] [Google Scholar]
- 18. Delpero JR, Boher JM, Sauvanet A, Le Treut YP, Sa-Cunha A, Mabrut JY et al. Pancreatic adenocarcinoma with venous involvement: is up-front synchronous portal-superior mesenteric vein resection still justified? A survey of the Association Française de Chirurgie. Ann Surg Oncol 2015;22:1874–1883 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were handled according to personal data legislation and individual data are not available.
References
- 1. Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant treatment in pancreatic cancer. Front Oncol 2020;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hermanek PSL. TNM Classification of Malignant Tumors (4th edn). Berlin: Springer-Verlag, 1987 [Google Scholar]
- 3. Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232–1237 [DOI] [PubMed] [Google Scholar]
- 4. Verbeke CS, Gladhaug IP. Dissection of pancreatic resection specimens. Surg Pathol Clin 2016;9:523–538 [DOI] [PubMed] [Google Scholar]
- 5. Janot MS, Kersting S, Belyaev O, Matuschek A, Chromik AM, Suelberg D et al. Can the new RCP R0/R1 classification predict the clinical outcome in ductal adenocarcinoma of the pancreatic head? Langenbeck’s Arch Surg 2012;397:917–925 [DOI] [PubMed] [Google Scholar]
- 6. Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB (Oxford) 2009;11:18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurlinkus B, Ahola R, Zwart E, Halimi A, Yilmaz BS, Ceyhan GO et al. In the era of the Leeds protocol: a systematic review and a meta-analysis on the effect of resection margins on survival among pancreatic ductal adenocarcinoma patients. Scand J Surg 2020;109:11–17 [DOI] [PubMed] [Google Scholar]
- 8. The Royal College of Pathologists . Cancer datasets and tissue pathways. 2019. https://www.rcpath.org/profession/guidelines/cancer-datasets-and-tissue-pathways.html
- 9. Allen PJ, Kuk D, Castillo CF-D, Basturk O, Wolfgang CL, Cameron JL et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg 2017;265:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghaneh P, Kleeff J, Halloran CM, Raraty M, Jackson R, Melling J et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg 2019;269:520–529 [DOI] [PubMed] [Google Scholar]
- 11. Osipov A, Nissen N, Rutgers J, Dhall D, Naziri J, Chopra S et al. Redefining the positive margin in pancreatic cancer: impact on patterns of failure, long-term survival and adjuvant therapy. Ann Surg Oncol 2017;24:3674–3682 [DOI] [PubMed] [Google Scholar]
- 12. Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF-D, Deshpande V et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 2013;257:731–736 [DOI] [PubMed] [Google Scholar]
- 13. Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JHG, Bakkevold KE et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75–83; discussion 83 [DOI] [PubMed] [Google Scholar]
- 14. Chandrasegaram MD, Goldstein D, Simes J, Gebski V, Kench JG, Gill AJ et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg 2015;102:1459–1472 [DOI] [PubMed] [Google Scholar]
- 15. Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008;15:1651–1660 [DOI] [PubMed] [Google Scholar]
- 16. Fukuda S, Oussoultzoglou E, Bachellier P, Rosso E, Nakano H, Audet M et al. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch Surg 2007;142:172–179; discussion 180 [DOI] [PubMed] [Google Scholar]
- 17. Kleive D, Labori KJ, Line P-D, Gladhaug IP, Verbeke CS. Pancreatoduodenectomy with venous resection for ductal adenocarcinoma rarely achieves complete (R0) resection. HPB (Oxford) 2020;22:50–57 [DOI] [PubMed] [Google Scholar]
- 18. Delpero JR, Boher JM, Sauvanet A, Le Treut YP, Sa-Cunha A, Mabrut JY et al. Pancreatic adenocarcinoma with venous involvement: is up-front synchronous portal-superior mesenteric vein resection still justified? A survey of the Association Française de Chirurgie. Ann Surg Oncol 2015;22:1874–1883 [DOI] [PubMed] [Google Scholar]