Abstract
Objective:
The objective of this study was to investigate the correlation between metformin exposure and the incidence of lactic acidosis in critically ill patients.
Methods:
The patients with type 2 diabetes mellitus (T2DM) were included from Medical Information Mart for Intensive Care IV database (MIMIC-IV). The primary outcome was the incidence of lactic acidosis. The secondary outcomes were lactate level and in-hospital mortality. Propensity score matching (PSM) method was adopted to reduce bias of the confounders. The multivariate logistic regression was used to explore the correlation between metformin exposure and the incidence of lactic acidosis. Subgroup analysis and sensitivity analysis were used to test the stability of the conclusion.
Results:
We included 4939 patients. There were 2070 patients in the metformin group, and 2869 patients in the nonmetformin group. The frequency of lactic acidosis was 5.7% (118/2070) in the metformin group and it was 4.3% (122/2869) in the nonmetformin group. There was a statistically significant difference between the two groups (P < 0.05). The lactate level in the metformin group was higher than in the nonmetformin group (2.78 ± 2.23 vs. 2.45 ± 2.24, P < 0.001). After PSM, the frequency of lactic acidosis (6.3% vs. 3.7%, P < 0.001) and lactate level (2.85 ± 2.38 vs. 2.40 ± 2.14, P < 0.001) were significantly higher in the metformin group compared with the nonmetformin group. In multivariate logistic models, the frequency of lactic acidosis was obviously increased in metformin group, and the adjusted odds ratio (OR) of metformin exposure was 1.852 (95% confidence interval (CI) = 1.298–2.643, P < 0.001). The results were consistent with subgroup analysis except for respiratory failure subgroup. Metformin exposure increased lactate level but did not affect the frequency of lactic acidosis in patients of respiratory failure with hypercapnia. However, the in-hospital mortality between metformin and nonmetformin group had no obvious difference (P = 0.215). In sensitivity analysis, metformin exposure showed similar effect as the original cohort.
Conclusions:
In critically ill patients with T2DM, metformin exposure elevated the incidence of lactic acidosis except for patients of respiratory failure with hypercapnia, but did not affect the in-hospital mortality.
Keywords: Metformin, lactic acidosis, type 2 diabetes mellitus, critically ill patients
Introduction
Metformin is the first-line drug for type 2 diabetes mellitus (T2DM). In addition to its glucose-lowering effect, metformin also has benefits in many other aspects such as cardiovascular protection, 1 anti-inflammatory, 2 antitumor, 3 and anti-aging. 4 Some studies showed that metformin reduced the risk of cardiovascular morbidity and all-cause mortality, as it improved insulin resistance and ameliorated metabolic disturbance in patients with arteriosclerotic cardiovascular disease (ASCVD).1,5
Metformin is safe for most patients with low risk of hypoglycemia. The most common adverse effect is gastrointestinal intolerance at the initial use stage, which can be relieved gradually over time. However, it has a rare but life-threatening adverse effect, which is called metformin-associated lactic acidosis (MALA). The incidence of MALA was only 2 to 9 cases per 100,000 patients/year, but the mortality was as high as 25.4% to 50%.6,7
Metformin is not the only causation inducing lactic acidosis (LA). LA is defined as hyperlactatemia (plasma lactate level is more than 5 mmol/L) and acidosis (the pH value of blood gas is <7.35). According to the etiology of LA, it can be divided into type A, B, or D. Type A is caused by tissue hypoxia (reduced supply or increased demand of oxygen), such as shock, respiratory failure, heart failure, anemia, malignant neoplasm, inflammation, convulsion, and muscle spasm. Type B is caused by lactate removal impairment (inflammation, liver disease, or renal insufficiency), drug (metformin, ethanol, and so on), genetic diseases (deficiency of mitochondrial or glycolysis-related genes). Type D occurs in patients with short bowel syndrome, that lactobacillus converts glucose into D-lactate. 8 All these diseases are the risk factors for LA.
Nevertheless, the association between metformin exposure and LA remains controversial. Some studies showed that metformin did not increase the risk of developing LA.9–12 Patients suffering from MALA had other hyperlactatemia-related risk factors such as acute hypoxia and acute kidney insufficiency.6,13–15 LA happened more frequently in critically ill patients with multiple risk factors of LA. 16 To investigate whether there was a correlation between LA and metformin exposure in critically ill patients, we conducted a retrospective observational study using a large, publicly available database.
Methods
Data sources
This study used the Medical Information Mart for Intensive Care (MIMIC)-IV database (Version 2.2), which was established by the Massachusetts Institute of Technology. This database contained the medical records of 53,000 patients in intensive care unit (ICU) between 2008 and 2019 at Beth Israel Deaconess Medical Center. The study was exempt from approval by our Institutional Review Board (IRB) because it used a public, de-identified database with prior IRB approval. Our access to the database had been approved by the institutional review committee of the Massachusetts Institute of Technology (Cambridge, Massachusetts, USA). Tong completed the Collaborative Institutional Training Initiative course named “Data or Specimens Only Research” and obtained the certification (certification number: 44627934). The research team was finally qualified to use the database and extract data.
Study population and data extraction
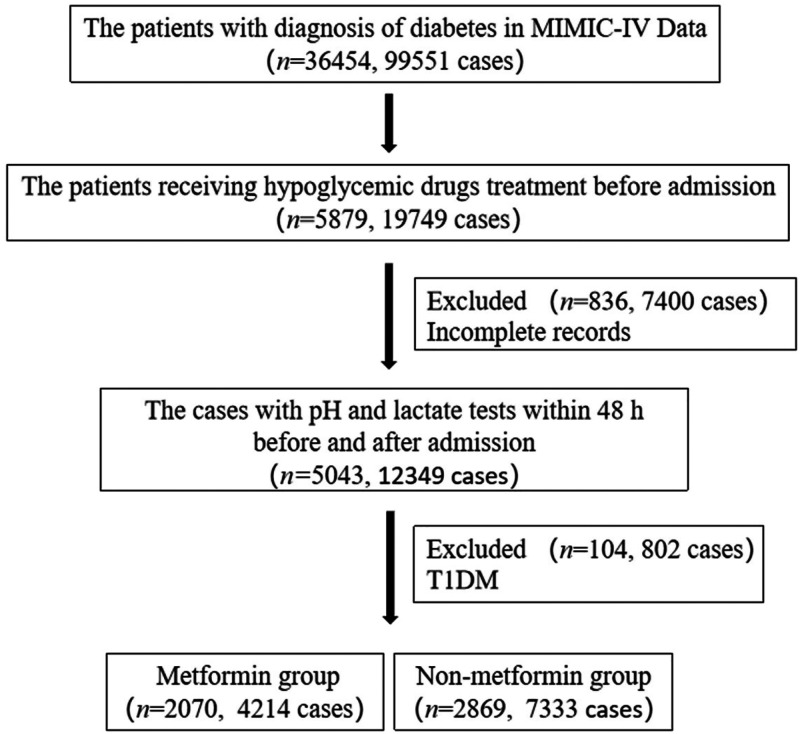
We extracted diabetes patients who were taking hypoglycemic drugs in the pre-admission records. All patients with T2DM aged 18 years or older were initially included in the study. The diagnosis of T2DM was based on the International Classification of Disease (ICD), 9th or 10th Revision. 17 We excluded patients who did not undergo lactate or pH detection within 48 h after admission. We also excluded the patients with type 1 diabetes mellitus. Included patients were subdivided into two groups according to pre-admission metformin exposure. Pre-admission metformin exposure was defined as a record of using metformin in “medrecon” in MIMIC-IV. The study flowchart was shown in Figure 1.
Figure 1.
Flowchart of data extraction.
PostgreSQL (version 15.1.1) and ICD-9, ICD-10 codes were used to extract data. This study included the following variables: demographic features (age and gender),body mass index (BMI), heart failure, respiratory failure, anemia, renal insufficiency, liver disease, shock, malignancy, inflammation, convulsion, alcohol use, smoking, coronary heart disease, cerebrovascular disease, hypertension, and hyperlipidemia.
Outcomes
The primary outcome was the incidence of LA. The secondary outcomes were lactate level and in-hospital mortality.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and were compared using the t test. The categorical variables were expressed as a frequency (percentage) and were compared using the Chi-square or Fishers exact test. Multivariate modeling of the relationship between metformin exposure and LA in patients was determined using a logistic regression model. The effect of metformin exposure was expressed by odds ratio (OR) with 95% confidence interval (CI). Multiple linear regression was performed to determine the connection between metformin exposure and lactate level.
Propensity score-matching (PSM) 18 method was applied to adjust the imbalanced confounders. A multivariate logistic regression model was performed to evaluate the scores of PSM for metformin exposure of all the patients. PSM method was performed with replacement at a 1 : 1 matching ratio via nearest neighbor, and a caliper width of 0.01 was used in present analysis. All possible confounders were included in the PSM method.
Sensitivity analysis and subgroup analysis were constructed to demonstrate the robustness of our results. The variables with missing values <10% were replaced with the mean of that variable. All statistical analyses were performed using SPSS 26.0. A two-tailed test was performed, and P < 0.05 was considered statistically significant.
Results
Characteristics of included participants
In the MIMIC-IV database, after screening for inclusion and exclusion criteria, a total of 4939 patients were included in the study cohort. The baseline information was shown in Table 1. There were 2070 patients in the metformin group, and there were 2869 patients in the nonmetformin group. The frequency of LA was 5.7% (118/2070) in the metformin group and it was 4.3% (122/2869) in the nonmetformin group. There was a statistically significant difference between the two groups (P < 0.05). The lactate level in the metformin group was higher than in the nonmetformin group (2.78 ± 2.23 vs. 2.45 ± 2.24, P < 0.001).
Table 1.
Baseline characteristics and demographics of all patients.
| Characteristic | Metformin group | Nonmetformin group | P value |
|---|---|---|---|
| All patients | 2070 | 2869 | |
| Gender, n (%) | 0.404 | ||
| Male | 1147 (55.4) | 1624 (56.6) | |
| Female | 923 (44.6) | 1245 (43.4) | |
| Age (years, mean ± SD) | 69.27 ± 12.77 | 71.49 ± 12.25 | 0.001 |
| BMI,mean ± SD | 31.15 ± 7.32 | 30.97 ± 7.49 | 0.398 |
| Heart failure, n (%) | 666 (32.2) | 1413 (49.3) | 0.001 |
| Respiratory failure, n (%) | 876 (42.3) | 1294 (45.1) | 0.052 |
| Anemia, n (%) | 794 (38.4) | 1473 (51.3) | 0.001 |
| Renal insufficiency, n (%) | 540 (26.1) | 1716 (59.8) | 0.001 |
| Liver disease, n (%) | 344 (16.6) | 576 (20.1) | 0.002 |
| Shock, n (%) | 584 (28.2) | 865 (30.1) | 0.140 |
| Malignancy, n (%) | 627 (30.3) | 878 (30.6) | 0.814 |
| Inflammation, n (%) | 1229 (59.4) | 1857 (64.7) | 0.001 |
| Convulsion, n (%) | 124 (6.0) | 176 (6.1) | 0.834 |
| Alcohol use, n (%) | 62 (3.0) | 74 (2.6) | 0.378 |
| Smoking, n (%) | 938 (45.3) | 1274 (44.4) | 0.526 |
| Coronary heart disease, n (%) | 842 (40.7) | 1411 (49.2) | 0.001 |
| Cerebrovascular disease, n (%) | 496 (24.0) | 848 (29.6) | 0.001 |
| Hypertension, n (%) | 1118 (54.0) | 725 (25.3) | 0.001 |
| Hyperlipidemia, n (%) | 1695 (81.9) | 1932 (67.3) | 0.001 |
| LA, n (%) | 118 (5.7) | 122 (4.3) | 0.020 |
| Lactate〔median (IQR)〕 | 2.2 (1.6–3.1) | 1.80 (1.3–2.6) | 0.001 |
| In-hospital mortality, n (%) | 232 (11.2) | 290 (10.1) | 0.215 |
| 28-Day mortality, n (%) | 218 (10.5) | 264 (9.2) | 0.12 |
BMI: body mass index; IQR: interquartile range; LA: lactic acidosis.
The patients in the nonmetformin group were more likely to report some comorbidities such as heart failure, anemia, renal insufficiency, liver disease, and inflammation, which also were the risk factors for LA. Nevertheless, The patients in the nonmetformin group had a lower lactate level and frequency of LA. The percentages of age, heart failure, anemia, renal insufficiency, liver disease, inflammation, coronary heart disease, cerebrovascular disease, hypertension, and hyperlipidemia were significantly higher in the metformin group than in the nonmetformin group.
Outcome after propensity score-matching
After PSM, of 1529 pairs matched (Table 2). The PSM method effectively reduced the bias of possible confounding factors. No significant differences were found in all matched factors except cerebrovascular disease (P < 0.05). In the matched cohort, the frequency of LA (6.3% vs. 3.7%, P < 0.001) and lactate level (2.85 ± 2.38 vs. 2.40 ± 2.14, P < 0.001) were obviously higher in the metformin group compared with the nonmetformin group, which was the same as with the original data.
Table 2.
The baseline information and outcomes after propensity score matching.
| Characteristic | Metformin group | Nonmetformin group | P value |
|---|---|---|---|
| All patients | 1529 | 1529 | |
| Gender, n (%) | 0.611 | ||
| Male | 844 (55.2) | 830 (54.3) | |
| Female | 685 (44.8) | 699 (45.7) | |
| Age (years, mean ± SD) | 70.11 ± 12.69 | 70.33 ± 12.51 | 0.641 |
| BMI, mean ± SD | 31.06 ± 7.39 | 30.95 ± 7.54 | 0.683 |
| Heart failure, n (%) | 580 (37.9) | 589 (38.5) | 0.738 |
| Respiratory failure, n (%) | 642 (42.0) | 668 (43.7) | 0.342 |
| Anemia, n (%) | 645 (42.2) | 626 (40.9) | 0.486 |
| Renal insufficiency, n (%) | 524 (34.3) | 500 (32.7) | 0.358 |
| Liver disease, n (%) | 285 (18.6) | 296 (19.4) | 0.612 |
| Shock, n (%) | 431 (28.2) | 440 (28.8) | 0.718 |
| Malignancy, n (%) | 491 (32.1) | 510 (33.4) | 0.464 |
| Inflammation, n (%) | 955 (62.5) | 957 (62.6) | 0.940 |
| Convulsion, n (%) | 91 (6.0) | 90 (5.9) | 0.939 |
| Alcohol use, n (%) | 44 (2.9) | 43 (2.8) | 0.913 |
| Smoking, n (%) | 683 (44.7) | 680 (44.5) | 0.913 |
| Coronary heart disease, n (%) | 658 (43.0) | 649 (42.4) | 0.742 |
| Cerebrovascular disease, n (%) | 378 (24.7) | 390 (25.5) | 0.018 |
| Hypertension, n (%) | 675 (44.1) | 660 (43.2) | 0.584 |
| Hyperlipidemia, n (%) | 1188 (77.7) | 1143 (74.8) | 0.056 |
| LA, n (%) | 96 (6.3) | 56 (3.7) | 0.001 |
| Lactate〔median (IQR)〕 | 2.2 (1.6–3.2) | 1.8 (1.4–2.6) | 0.001 |
| In-hospital mortality, n (%) | 176 (11.5) | 165 (10.8) | 0.527 |
| 28-Day mortality, n (%) | 164 (10.7) | 156 (10.2) | 0.636 |
BMI: body mass index; IQR: interquartile range; LA: lactic acidosis.
Association between metformin exposure and LA in patients
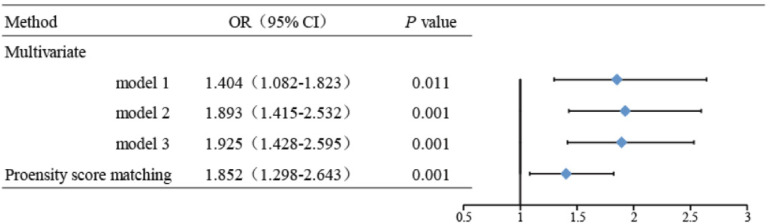
As shown in Figure 2, the OR of metformin exposure was 1.404 (95% CI = 1.082–1.823, P < 0.05) in multivariate logistic model. After adjusting for possible confounders, the adjusted OR was 1.925 (95% CI = 1.428–2.595, P < 0.001, Model 3). In the matched cohort, all weighted variables had been balanced between the two groups. After correction for confounders, the metformin exposure associated with a 1.852-fold (95% CI = 1.298–2.643, P < 0.001) increase in the risk of LA.
Figure 2.
Association between metformin exposure and LA. Model 1: adjusted for age, gender and BMI. Model 2: adjusted for age, gender, BMI, heart failure, respiratory failure, anemia, renal insufficiency, liver disease, shock, malignancy, inflammation, convulsion, and alcohol use. Model 3: adjusted for age, gender, BMI, heart failure, respiratory failure, anemia, renal insufficiency, liver disease, shock, malignancy, inflammation, convulsion, alcohol use, smoking, coronaryheartdisease, cerebrovasculardisease, hypertension, and hyperlipidemia. BMI: body mass index; LA: lactic acidosis.
Then the level of lactate was compared between metformin group and nonmetformin group. The results showed that metformin exposure was related to the increase of lactate level (β = 0.139, 95% CI = 0.441–0.579, P < 0.001).
Subgroup analysis
Table 3 indicated as to whether the correlation between metformin exposure and LA was stable across subgroups. Subgroup analysis was performed according to risk factors for LA. Except for respiratory failure subgroups, the other subgroups had a higher frequency of LA and higher lactate level in metformin group (Tables 3 and 4).
Table 3.
Results of subgroup analyses of metformin exposure and LA according to clinical characteristics.
| Subgroups | Metformin group | Nonmetformin group | Adjusted OR (95% CI) | P for interaction |
|---|---|---|---|---|
| Age | 0.298 | |||
| >65 | 78 (6.0) | 95 (4.8) | 1.679 (1.181–2.386) | |
| ≤65 | 40 (5.1) | 27 (3.1) | 2.869 (1.577–5.218) | |
| BMI | 0.397 | |||
| ≤24 | 14 (4.9) | 19 (4.1) | 1.574 (0.674–3.673) | |
| 24 < BMI≤28 | 21 (5.2) | 16 (2.9) | 3.916 (1.746–8.781) | |
| >28 | 83 (6.0) | 87 (4.7) | 1.651 (1.151–2.368) | |
| Heart failure | 0.102 | |||
| Yes | 50 (7.5) | 61 (4.3) | 2.265 (1.453–3.532) | |
| No | 68 (4.8) | 61 (4.2) | 1.643 (1.092–2.472) | |
| Anemia | 0.652 | |||
| Yes | 62 (7.8) | 68 (4.6) | 2.286 (1.510–3.461) | |
| No | 56 (4.4) | 54 (3.9) | 1.595 (1.031–2.468) | |
| Renal insufficiency | 0.105 | |||
| Yes | 61 (11.3) | 97 (5.7) | 1.828 (1.255–2.663) | |
| No | 57 (3.7) | 25 (2.2) | 1.825 (1.109–3.004) | |
| Liver disease | 0.654 | |||
| Yes | 26 (7.6) | 48 (8.3) | 1.052 (0.588–1.881) | |
| No | 92 (5.3) | 74 (3.2) | 2.415 (1687–3.458) | |
| Shock | 0.009 | |||
| Yes | 82 (14.0) | 101 (11.7) | 1.630 (1.147–2.316) | |
| No | 36 (2.4) | 21 (1.0) | 2.943 (1.617–5.356) | |
| Malignancy | 0.045 | |||
| Yes | 29 (4.6) | 29 (3.3) | 1.927 (1.054–3.523) | |
| No | 89 (6.2) | 93 (4.7) | 1.950 (1.378–2.759) | |
| Inflammation | 0.998 | |||
| Yes | 84 (6.8) | 98 (5.3) | 1.761 (1.246–2.490) | |
| No | 34 (4.0) | 24 (2.4) | 2.561 (1.384–4.741) | |
| Convulsion | 0.392 | |||
| Yes | 10 (8.1) | 6 (3.4) | 5.241 (1.277–21.518) | |
| No | 108 (5.5) | 116 (4.3) | 1.808 (1.327–2.463) | |
| Alcohol use | 0.152 | |||
| Yes | 8 (12.9) | 3 (4.1) | 41.755 (2.421–720.15) | |
| No | 110 (5.5) | 119 (4.3) | 1.871 (1.379–2.540) | |
| Respiratory failure | 0.148 | |||
| 0 | 34 (1383) | 38 (1916) | 1.555 (0.885–2.734) | |
| 1 | 56 (443) | 51 (617) | 2.091 (1.339–3.267) | |
| 2 | 28 (244) | 33 (336) | 1.246 (0.659–2.354) |
0: no respiratory failure.
1: respiratory failure without hypercapnia.
2: respiratory failure with hypercapnia.
BMI: body mass index; CI: confidence interval; LA: lactic acidosis; OR: odds ratio.
Table 4.
Results of subgroup analyses of metformin exposure and lactate level according to clinical characteristics.
| Subgroups | Metformin group | Nonmetformin group | P | P for interaction |
|---|---|---|---|---|
| Age | 0.155 | |||
| >65 | 2.2 (1.6–3.2) | 1.8 (1.4–2.6) | 0.001 | |
| ≤65 | 2.0 (1.5–3.0) | 1.8 (1.3–2.7) | 0.004 | |
| BMI | 0.979 | |||
| ≤24 | 2.2 (1.5–3.3) | 1.7 (1.3–2.6) | 0.005 | |
| 24 < BMI ≤ 28 | 2.2 (1.6–3.3) | 1.8 (1.4–2.6) | 0.023 | |
| >28 | 2.2 (1.6–3.1) | 1.8 (1.3–2.6) | 0.001 | |
| Heart failure | 0.333 | |||
| Yes | 2.2 (1.6–3.2) | 1.8 (1.4–2.6) | 0.001 | |
| No | 2.2 (1.6–3.1) | 1.8 (1.3–2.7) | 0.001 | |
| Anemia | 0.013 | |||
| Yes | 2.3 (1.6–3.5) | 1.8 (1.3–2.6) | 0.001 | |
| No | 2.1 (1.6–3.0) | 1.8 (1.4–2.6) | 0.009 | |
| Renal insufficiency | 0.001 | |||
| Yes | 2.4 (1.7–3.8) | 1.8 (1.3–2.7) | 0.001 | |
| No | 2.1 (1.5–3.0) | 1.8 (1.4–2.5) | 0.001 | |
| Liver disease | 0.449 | |||
| Yes | 2.5 (1.7–3.9) | 2.2 (1.5–3.3) | 0.206 | |
| No | 2.1 (1.6–3.0) | 1.8 (1.3–2.5) | 0.001 | |
| Shock | 0.218 | |||
| Yes | 2.7 (1.8–4.2) | 2.3 (1.6–3.8) | 0.162 | |
| No | 2.0 (1.5–2.8) | 1.7 (1.3–2.3) | 0.001 | |
| Malignancy | 0.904 | |||
| Yes | 2.1 (1.5–3.1) | 1.8 (1.3–2.6) | 0.001 | |
| No | 2.2 (1.6–3.2) | 1.8 (1.3–2.6) | 0.001 | |
| Inflammation | 0.208 | |||
| Yes | 2.2 (1.6–3.3) | 1.9 (1.4–2.8) | 0.001 | |
| No | 2.1 (1.5–2.9) | 1.7 (1.3–2.3) | 0.001 | |
| Convulsion | 0.218 | |||
| Yes | 2.3 (1.7–3.8) | 1.8 (1.4–2.5) | 0.003 | |
| No | 2.2 (1.6–3.1) | 1.8 (1.3–2.6) | 0.001 | |
| Alcohol use | 0.088 | |||
| Yes | 2.6 (1.6–5.2) | 2.2 (1.6–3.0) | 0.015 | |
| No | 2.2 (1.6–3.1) | 1.8 (1.3–2.6) | 0.001 | |
| Smoking | 0.043 | |||
| Yes | 2.1 (1.5–3.0) | 1.8 (1.3–2.6) | 0.032 | |
| No | 2.3 (1.6–3.3) | 1.8 (1.4–2.6) | 0.001 | |
| Coronaryheartdisease | 0.512 | |||
| Yes | 2.2 (1.6–3.2) | 1.8 (1.4–2.6) | 0.004 | |
| No | 2.2 (1.5–3.1) | 1.8 (1.3–2.6) | 0.001 | |
| AKI | 0.157 | |||
| Yes | 2.2 (1.6–3.2) | 1.9 (1.4–2.7) | 0.809 | |
| No | 2.2 (1.6–3.1) | 1.8 (1.3–2.6) | 0.001 | |
| Cerebrovascular disease | 0.247 | |||
| Yes | 2.91 ± 2.45 | 2.68 ± 2.69 | 0.121 | |
| No | 2.75 ± 2.15 | 2.35 ± 2.02 | 0.001 | |
| Hypertension | 0.042 | |||
| Yes | 2.2 (1.6–3.1) | 1.9 (1.3–2.7) | 0.029 | |
| No | 2.2 (1.6–3.2) | 1.8 (1.3–2.6) | 0.001 | |
| Hyperlipidemia | 0.681 | |||
| Yes | 2.2 (1.6–3.1) | 1.8 (1.3–2.6) | 0.001 | |
| No | 2.2 (1.6–3.3) | 1.9 (1.4–2.7) | 0.007 | |
| Respiratory failure | 0.016 | |||
| 0 | 2.0 (1.5–2.7) | 1.7 (1.3–2.3) | 0.001 | |
| 1 | 2.9 (2.0–4.3) | 2.2 (1.6–3.5) | 0.001 | |
| 2 | 2.3 (1.7–3.7) | 2.2 (1.5–3.5) | 0.438 |
0: no respiratory failure.
1: respiratory failure without hypercapnia.
2: respiratory failure with hypercapnia.
AKI: acute kidney injury; BMI: body mass index.
In respiratory failure subgroup, metformin exposure increased the frequency of LA and lactate level in patients of respiratory failure without hypercapnia. Nevertheless, metformin exposure increased lactate level but did not affect the frequency of LA in patients of respiratory failure with hypercapnia. The results of subgroup analysis showed that there was a significant interaction between shock (P = 0.009), malignancy (P = 0.045), and metformin exposure (Table 3). In Table 4, a significant interaction between anemia (P = 0.013), renal insufficiency (P = 0.001), and metformin exposure were observed.
Association between metformin exposure and the mortality in patients
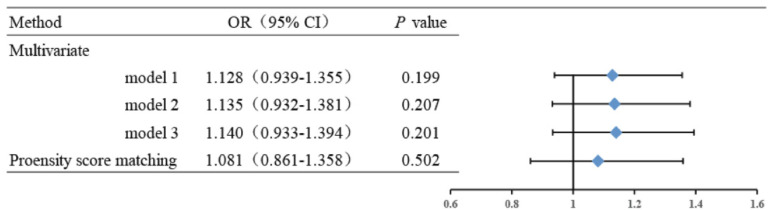
The in-hospital mortality was analyzed in this study. The in-hospital mortality between metformin and nonmetformin group had no obviously difference (P = 0.215), which was 11.2% (232/2070) in the metformin group and 10.1% (290/2869) in the nonmetformin group (Table 1). After PSM, The in-hospital mortality was 11.5% in the metformin group and 10.8% in the nonmetformin group, which also had no significant difference (P = 0.527) either (Table 2). In the multivariate logistic regression model and subgroup analysis, metformin exposure still showed no impact on the mortality after adjusting the confounders (Figure 3 and Table 5).
Figure 3.
Association between metformin exposure and in-hospital mortality. Model 1: adjusted for age, gender and BMI. Model 2: adjusted for age, gender, BMI, heart failure, respiratory failure, anemia, renal insufficiency, liver disease, shock, malignancy, inflammation, convulsion, and alcohol use. Model 3: adjusted for age, gender, BMI, heart failure, respiratory failure, anemia, renal insufficiency, liver disease, shock, malignancy, inflammation, convulsion, alcohol use, smoking, coronary heart disease, cerebrovascular disease, hypertension, and hyperlipidemia. BMI: body mass index; LA: lactic acidosis.
Table 5.
Results of subgroup analyses of metformin exposure and in-hospital mortality according to clinical characteristics.
| Subgroups | Metformin group | Nonmetformin group | Adjusted OR (95% CI) | P for interaction |
|---|---|---|---|---|
| Age | 0.087 | |||
| >65 | 140 (10.9) | 207 (10.4) | 1.100 (0.859–1.410) | |
| ≤65 | 92 (11.8) | 83 (9.4) | 1.232 (0.870–1.744) | |
| BMI | 0.799 | |||
| ≤24 | 32 (11.3) | 49 (10.7) | 0.919 (0.544–1.554) | |
| 24 < BMI ≤ 28 | 43 (10.6) | 63 (11.3) | 1.012 (0.632–1.620) | |
| >28 | 157 (11.4) | 178 (9.6) | 1.264 (0.985–1.622) | |
| Heart failure | 0.841 | |||
| Yes | 70 (10.5) | 136 (9.6) | 1.192 (0.858–1.655) | |
| No | 162 (11.5) | 154 (10.6) | 1.127 (0.873–1.454) | |
| Anemia | 0.539 | |||
| Yes | 101 (12.7) | 158 (10.7) | 1.268 (0.942–1.707) | |
| No | 131 (10.3) | 132 (9.5) | 1.044 (0.794–1.372) | |
| Renal insufficiency | 0.347 | |||
| Yes | 67 (12.4) | 173 (10.1) | 1.238 (0.901–1.700) | |
| No | 165 (10.8) | 117 (10.1) | 1.030 (0.795–1.335) | |
| Liver disease | 0.809 | |||
| Yes | 33 (9.6) | 51 (8.9) | 1.202 (0.727–1.987) | |
| No | 199 (11.5) | 239 (10.4) | 1.119 (0.897–1.395) | |
| Shock | 0.212 | |||
| Yes | 63 (10.8) | 99 (11.4) | 1.107 (0.767–1.596) | |
| No | 169 (11.4) | 191 (9.5) | 1.177 (0.924–1.500) | |
| Malignancy | 0.137 | |||
| Yes | 66 (10.5) | 100 (11.4) | 0.969 (0.678–1.385) | |
| No | 166 (11.5) | 190 (9.5) | 1.239 (0.969–1.584) | |
| Inflammation | 0.511 | |||
| Yes | 136 (11.1) | 192 (10.3) | 1.143 (0.887–1.473) | |
| No | 96 (11.4) | 98 (9.7) | 1.130 (0.809–1.577) | |
| Convulsion | 0.72 | |||
| Yes | 17 (13.7) | 20 (11.4) | 1.934 (0.811–4.610) | |
| No | 215 (11.0) | 270 (10.0) | 1.125 (0.913–1.385) | |
| Alcohol use | 0.79 | |||
| Yes | 8 (12.9) | 10 (13.5) | 1.270 (0.306–5.266) | |
| No | 224 (11.2) | 280 (10.0) | 1.133 (0.923–1.389) | |
| Lactate level | 0.223 | |||
| ≤2 | 98 (10.3) | 168 (9.8) | 0.995 (0.746–1.326) | |
| 2 < Lactate level≤5 | 117 (12.7) | 102 (10.9) | 1.313 (0.962–1.791) | |
| >5 | 17 (8.9) | 20 (9.7) | 0.956 (0.435–2.098) | |
| Respiratory failure | 0.237 | |||
| 0 | 145 (10.5) | 195 (10.2) | 0.946 (0.736–1.216) | |
| 1 | 58 (13.1) | 62 (10.0) | 1.412 (0.934–2.136) | |
| 2 | 29 (11.9) | 33 (9.9) | 1.475 (0.831–2.620) |
0: no respiratory failure.
1: respiratory failure without hypercapnia.
2: respiratory failure with hypercapnia.
BMI: body mass index; CI: confidence interval; OR: odds ratio.
Sensitivity analysis
In order to test the robustness of our findings, we analyzed the correlation between metformin exposure and the frequency of LA based on patient's last admission. As some patients had several admissions, sensitivity analysis included a total of 11547 cases. There were 4214 cases in the metformin group, and there were 7333 cases in the nonmetformin group. The baseline information was shown in Supplemental Table S1. Metformin exposure elevated the frequency of LA and lactate level in original cohort (P < 0.001 and P < 0.001, respectively). After PSM, of 3663 pairs matched (Supplemental Table S2). No significant differences were found in all matched factors (P > 0.05). In the matched cohort, the frequency of LA (3.8% vs. 1.9%, P < 0.001) and lactate level (2.66 ± 1.95 vs. 2.17 ± 1.55, P < 0.001) were significantly higher in the metformin group compared with the nonmetformin group, which was the same as with the original data. In the multivariate logistic regression model, metformin exposure still elevated the frequency of LA (OR = 2.107, 95% CI = 1.560–2.844, P < 0.001) after adjusting the confounders (Supplemental Figure S1). In subgroup analysis, metformin exposure did not affect the frequency of LA in patients of respiratory failure (OR = 1.252, 95% CI = 0.726–2.160, P = 0.418) with hypercapnia, even though it increased lactate level (3.10 ± 2.59 vs. 2.77 ± 2.27, P = 0.033) (Supplemental Table S3).
Discussion
In this retrospective control study, metformin exposure increased the incidence of LA and plasma lactate level in critically ill patients, but had no impact on in-hospital mortality. Our study included 4939 critically ill patients. In order to make our results more reliable, we applied PSM analysis and multivariate logistic regression to verify our conclusion.
The reason why metformin elevated plasma lactate level was related to the molecular mechanism of metformin, which had not been fully elucidated. The major mechanism of metformin was to inhibit mitochondrial respiratory chain complex Ⅰ. The inhibition of mitochondrial respiratory chain elevated the ratio of adenosine monophosphate (AMP)/adenosine triphosphate, afterwards it increased glucose uptake and improved mitochondria function by activating AMP-activated protein kinase. On the other hand, it also led to the accumulation of glycolytic metabolites by the inhibition of oxidative phosphorylation, resulting in an increase of lactate. 19 On the contrary, Wang et al. 20 showed that pharmacological metformin concentration improved mitochondrial respiration and increased oxygen consumption by increasing mitochondrial density, while supra-pharmacological metformin concentrations reduced mitochondrial respiration. It implied that pharmacological metformin concentration might not increase serum lactate level. However, these animal trials were conducted in the absence of risk factors for LA and might not be suitable for the clinical context of critically ill patients.
More and more clinicians felt puzzled that whether metformin induce LA or not. First of all, Kajbaf et al. 21 queried the quality of past reports for MALA, which only 10.4% of cases had rigorous criteria. Some reports for MALA was in fact LA induced by other risk as hypoxia and sepsis. Secondly, animal trials showed that metformin accumulation induced by renal failure did not increase in the lactate concentration. 22 Finally, Salpeter et al. 23 conducted a meta-analysis of 347 prospective comparative and observational cohort studies. The result showed that there was no significant difference between metformin group and control group in blood lactate concentration and the risk of developing LA, which failed to indicate the correlation between the metformin exposure and LA. Some studies also displayed that metformin did not affect the incidence of LA in the general population.9–12,24–26 Of note, these patients had relatively low-risk profile of LA. The effect of metformin on lactate metabolism might be diluted. LA happened more frequently in critically ill patients. Some studies showed that metformin exposure elevated plasma lactate level in critically ill patients or sepsis patients.27–29 The results were consistent with our findings. What was more, most patients (98.7%) suffering from LA in our study had at least one more risk factor, and had more risk factors than non-LA patients. This study implied that metformin did not affect the frequency of LA, if patients did not have any other risk factors for LA, as reported by the COSMIC Approach Study. 30 It was worth noting that we should interrupt the use of metformin when patients had other risk factors for LA.
Furthermore, we wondered whether the relationship between metformin exposure and LA was in agreement with the outcomes of different subgroups. We conducted subgroup analysis according to the risk factors of LA. Tissue hypoxia (including shock, respiratory failure, heart failure, and anemia), excessive energy expenditure (malignancy, inflammation, and convulsion) and impaired clearance of lactate (liver disease, alcohol abuse, and renal insufficiency) increased lactate level and the risk of causing LA. To our surprise, metformin exposure raised the risk of inducing LA except subgroup of respiratory failure with hypercapnia. In subgroup of respiratory failure with hypercapnia, metformin exposure elevated plasma lactate level, but did not affect the frequency of LA. The result was consistent with the sensitivity analysis. Metformin inhibited mitochondrial respiratory chain. As a result, metformin reduced O2 expenditure and CO2 production in patients of respiratory failure with hypercapnia, thus inhibiting respiratory acidosis. More evidences were needed to prove this conclusion. In subgroup of liver disease, metformin exposure had no effect on the frequency of LA. In subgroup of convulsion, metformin exposure, and convulsion had synergistic interaction on the frequency of LA and plasma lactate level. It implied that convulsion or muscle spasm might also be contraindication of metformin, which was never mentioned before.
In order to discuss whether metformin exposure affected the mortality of critically ill patients, we chose the last admission of every patient. We found that there was no difference between metformin users and metformin nonusers. UKPDS shown that metformin exposure reduced the all-in mortality of T2DM patients. 31 This was at least partially attributed to the reduction of ROS production. Metformin could reduce ROS production by inhibiting mitochondrial complex I. Oxidative stress was relative to many disease such as inflammation, cancer, cardiac vascular disease and neurodegenerative diseases. Improving oxidative stress response could improve the prognosis of these diseases. On the other hand, metformin improved metabolism such as hyperlipidemia, obesity and nonalcoholic fatty liver disease. Unfortunately, following studies and meta-analysis did not show the benefits of metformin on all-in mortality and cardiovascular death.32–34 Recent studies showed that metformin might reduce the mortality of critically ill or sepsis patients, which contradicted our research. In Yang's study, renal insufficiency patients were excluded. 35 As was known to all, renal failure might lead to the accumulation of metformin and a decrease in lactate clearance, which impaired the benefits of metformin. Posma et al. 28 held the view that similar lactate levels were associated with a lower mortality rate in metformin users compared with metformin nonusers. Nevertheless, we did not find such trend in subgroups of different lactate levels. Metformin use was frequently discontinued in diabetic patients who admitted to the ICU. This might attenuate the impact of metformin on all-in mortality. Nevertheless, Jo et al. 36 also showed that pre-admission metformin exposure was not associated with reduced 30-day mortality among acuterespiratory distress syndrome patients with DM, which was consistent with our research.
This study also had several limitations and biases. First of all, like other retrospective analyses, residual confounders such as duration of the disease, value of glycosylated hemoglobin (HbA1c), plasma metformin level, doses of metformin potentially exist and genetic heterogeneity. However, there was no exact relationship plasma metformin level and LA. Daniel Lalau showed that one case about high plasma metformin level and normal plasma lactate level, however another case displayed normal plasma metformin level and high plasma lactate level. 37 The value of HbA1c also had no clear correlation with LA, which was defective in 78% cases, although there were no significant differences between metformin users and nonusers. Genetic heterogeneity could not be avoided in clinical research. Patients with mitochondrial genetic disorders might be more susceptible to LA after taking metformin. However, the incidence of mitochondrial genetic disorders was very low, and it had little impact on such a large sample of research. 38 We adjusted for possible confounders and minimized the influence of risk factors for LA that might lead to outcome bias through the PSM and subgroup analysis. Secondly, some patients did not have pre-admission medications, and we had to exclude these patients. To reduce bias, we only collected patients with T2DM and provided pre-admission medications, which might also induce selection bias. Thirdly, the diagnostic information in this database covered all the diagnoses throughout the hospitalization process, but it could not distinguish the subsequence of disease occurrence, which would lead to information bias. For example, patients might suffer from acute heart failure after LA, so heart failure was not the cause of LA in these patients. Finally, the causes of death were not recorded in the database, we could not conduct a competing risk analysis.
Conclusion
In critically ill patients with T2DM, metformin exposure elevated the incidence of LA except for patients of respiratory failure with hypercapnia, but did not affect the in-hospital mortality. Further clinical trials were required to confirm and validate this association.
Supplemental Material
Supplemental material, sj-docx-1-sci-10.1177_00368504241262116 for Metformin exposure and the incidence of lactic acidosis in critically ill patients with T2DM: A retrospective cohort study by Jingkai Tong, Xin Li, Tong Liu and Ming Liu in Science Progress
Acknowledgements
We would like to express our gratitude to MIMIC database and GitHub staff for providing data and data analysis code to us. We also appreciate the efforts of all staff from the MIT Laboratory for Computational Physiology.
Footnotes
Authors’ contributions: ML has made substantial contributions to the conception revised the manuscript critically. JT has contributed to acquisition, analysis, and interpretation of the data. TL and XL have participated in drafting the manuscript, especially improving the fluency and accuracy of language. All authors read and approved the final version of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the National Key R&D Program 2019YFA0802502 and 2022YFE0131400, Special Research Fund for Central Universities Peking Union Medical College 3332021083, and also by the National Natural Science Foundation of China 82220108014 and 81830025.
ORCID iD: Ming Liu https://orcid.org/0009-0002-2906-5054
Supplemental material: Supplemental material for this article is available online.
References
- 1.Li JZ, Li YR. Cardiovascular protection by metformin: latest advances in basic and clinical research. Cardiology 2023; 148: 374–384. [DOI] [PubMed] [Google Scholar]
- 2.Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol 2021; 320: C873–C879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamarudin MNA, Sarker MMR, Zhou JR, et al. Metformin in colorectal cancer: molecular mechanism, preclinical and clinical aspects. J Exp Clin Cancer Res 2019; 38: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Gan D, Lin S, et al. Metformin in aging and aging-related diseases: clinical applications and relevant mechanisms. Theranostics 2022; 12: 2722–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016; 164: 740–751. [DOI] [PubMed] [Google Scholar]
- 6.Huang W, Castelino RL, Peterson GM. Adverse event notifications implicating metformin with lactic acidosis in Australia. J Diabetes Complicat 2015; 29: 1261–1265. [DOI] [PubMed] [Google Scholar]
- 7.Wang GS, Hoyte C. Review of biguanide (metformin) toxicity. J Intensive Care Med 2019; 34: 863–876. [DOI] [PubMed] [Google Scholar]
- 8.Zanza C, Facelli V, Romenskaya T, et al. Lactic acidosis related to pharmacotherapy and human diseases. Pharmaceuticals (Basel) 2022; 15: 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanatani T, Sai K, Tohkin M, et al. Impact of Japanese regulatory action on metformin-associated lactic acidosis in type II diabetes patients. Int J Clin Pharm 2015; 37: 537–545. [DOI] [PubMed] [Google Scholar]
- 10.Chang CH, Sakaguchi M, Dolin P. Epidemiology of lactic acidosis in type 2 diabetes patients with metformin in Japan. Pharmacoepidemiol Drug Saf 2016; 25: 1196–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deden LN, Aarts EO, Aelfers SCW, et al. Risk of metformin-associated lactic acidosis (MALA) in patients after gastric bypass surgery. Obes Surg 2018; 28: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 12.Aharaz A, Pottegård A, Henriksen DP, et al. Risk of lactic acidosis in type 2 diabetes patients using metformin: a case control study. PLoS ONE 2018; 13: e0196122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renda F, Mura P, Finco G, et al. Metformin-associated lactic acidosis requiring hospitalization. A national 10 year survey and a systematic literature review. Eur Rev Med Pharmacol Sci 2013; 17: 45–49. [PubMed] [Google Scholar]
- 14.Peña Porta JM, Villafuerte Ledesma HM, Vicente de Vera Floristán C, et al. Incidence, factors related to presentation, course and mortality of metformin-associated lactic acidosis in the healthcare area of a tertiary hospital. Nefrologia (Engl Ed) 2019; 39: 35–43. [DOI] [PubMed] [Google Scholar]
- 15.Kuan IHS, Savage RL, Duffull SB, et al. The association between metformin therapy and lactic acidosis. Drug Saf 2019; 42: 1449–1469. [DOI] [PubMed] [Google Scholar]
- 16.Zanza C, Facelli V, Romenskaya T, et al. Lactic acidosis related to pharmacotherapy and human diseases. Pharmaceuticals (Basel) 2022; 15: 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal A, Gluckman TJ, Tcheng JE. What's in a name? The new ICD-10 (10th revision of the international statistical classification of diseases and related health problems) codes and type 2 myocardial infarction. Circulation 2017; 136: 1180–1182. [DOI] [PubMed] [Google Scholar]
- 18.Kane LT, Fang T, Galetta MS, et al. Propensity score matching: a statistical method. Clin Spine Surg 2020; 33: 120–122. [DOI] [PubMed] [Google Scholar]
- 19.Feng J, Wang X, Ye X, et al. Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res 2022; 177: 106114. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, An H, Liu T, et al. Metformin improves mitochondrial respiratory activity through activation of AMPK. Cell Rep 2019; 29: 1511–1523.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajbaf F, Lalau JD. The criteria for metformin-associated lactic acidosis: the quality of reporting in a large pharmacovigilance database. Diabet Med 2013; 30: 345–348. [DOI] [PubMed] [Google Scholar]
- 22.Barthelmebs M, Wiernsperger N, Krieger JP, et al. Mild acute renal failure potentiates metformin accumulation in the diabetic rat kidney without further impairment of renal function. Diabetes Metab 2003; 29: 163–170. [DOI] [PubMed] [Google Scholar]
- 23.Salpeter SR, Greyber E, Pasternak GA, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2010; 2010: CD002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee EY, Hwang S, Lee YH, et al. Association between metformin use and risk of lactic acidosis or elevated lactate concentration in type 2 diabetes. Yonsei Med J 2017; 58: 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang W, Castelino RL, Peterson GM. Lactate levels with chronic metformin use: a narrative review. Clin Drug Investig 2017; 37: 991–1007. [DOI] [PubMed] [Google Scholar]
- 26.Koren S, Zilberman-Itskovich S, Koren R, et al. Metformin does not induce hyperlactatemia in patients admitted to internal medicine ward. Isr Med Assoc J 2017; 19: 300–303. [PubMed] [Google Scholar]
- 27.Green JP, Berger T, Garg N, et al. Impact of metformin use on the prognostic value of lactate in sepsis. Am J Emerg Med 2012; 30: 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posma RA, Frøslev T, Jespersen B, et al. Prognostic impact of elevated lactate levels on mortality in critically ill patients with and without preadmission metformin treatment: a Danish registry-based cohort study. Ann Intensive Care 2020; 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen FC, Kung CT, Cheng HH, et al. Metformin affects Serum lactate levels in predicting mortality of patients with sepsis and bacteremia. J Clin Med 2019; 8: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cryer DR, Nicholas SP, Henry DH, et al. Comparative outcomes study of metformin intervention versus conventional approach the COSMIC Approach Study. Diabetes Care 2005; 28: 539–543. [DOI] [PubMed] [Google Scholar]
- 31.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 32.Li T, Providencia R, Mu N, et al. Association of metformin monotherapy or combined therapy with cardiovascular risks in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2021; 20: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CG, Heckman-Stoddard B, Dabelea D, et al. Effect of metformin and lifestyle interventions on mortality in the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care 2021; 44: 2775–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Z, Zhang H, Wu C, et al. Effect of metformin on adverse outcomes in T2DM patients: systemic review and meta-analysis of observational studies. Front Cardiovasc Med 2022; 9: 944902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Q, Zheng J, Wen D, et al. Association between metformin use on admission and outcomes in intensive care unit patients with acute kidney injury and type 2 diabetes: a retrospective cohort study. J Crit Care 2021; 62: 206–211. [DOI] [PubMed] [Google Scholar]
- 36.Jo YS, Choi SM, Lee J, et al. Effect of preadmission metformin use on clinical outcome of acute respiratory distress syndrome among critically ill patients with diabetes. Tuberc Respir Dis (Seoul) 2017; 80: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalau JD, Kajbaf F, Protti A, et al. Metformin-associated lactic acidosis (MALA): moving towards a new paradigm. Diabetes Obes Metab 2017; 19: 1502–1512. [DOI] [PubMed] [Google Scholar]
- 38.Shin HJ, Na JH, Lee YM. A case of exacerbated encephalopathy with stroke-like episodes and lactic acidosis triggered by metformin in a patient with MELAS. Neurol Sci 2024; 45: 2337–2339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sci-10.1177_00368504241262116 for Metformin exposure and the incidence of lactic acidosis in critically ill patients with T2DM: A retrospective cohort study by Jingkai Tong, Xin Li, Tong Liu and Ming Liu in Science Progress