Abstract
Background:
Pelvic organ prolapse is a descent of the vaginal compartments and the surrounding organ due to loss of support of the vaginal tissue. It has a significant psychological, physical, and social impact that affects women’s quality of life. However, its true prevalence is unknown due to the variability in the methods used to diagnose the disorder.
Objectives:
This study aimed to determine the prevalence of pelvic organ prolapse and its associated risk factors among women in Sidama region, Ethiopia.
Study design:
A community-based cross-sectional survey was conducted in the Dale–Wonsho Health and Demographic Surveillance Site, Sidama region, from March to October 2023.
Methods:
A multi-stage stratified cluster sampling was used to select a sample of 816 women. Anatomical prolapse was diagnosed based on the standardized pelvic organ prolapse quantification method, and symptomatic prolapse was assessed by patient-reported symptoms. A complex survey-based modified Poisson regression was used to assess the risk factors associated with prolapse.
Results:
A total of 815 participated in the interview, and 779 (95.6%) underwent pelvic examination to assess for prolapse status. Anatomical prolapse (Stages II–IV) was observed in 241 (30.9%; 95% confidence interval = 24–38.7) of the participants. The prevalence of symptomatic pelvic organ prolapse was 78.5% (95% confidence interval = 69.1–85.7) among women with anatomical prolapse (189/241). This prevalence falls to 24.27% (95% confidence interval = 19.98–29.16) for the total sample population. Higher frequency of childbirth, prolonged heavy lifting activities, and prolonged labor increased the likelihood of developing anatomical prolapse. Childbirth at an early age and prolonged heavy lifting activities were significantly associated with symptomatic prolapse.
Conclusion:
Anatomical prolapse and symptomatic prolapse are high in the study area. Parity, prolonged heavy lifting, prolonged labor, and early age childbirth were associated with pelvic organ prolapse. Community-based education and interventions that focus on the modification of risk factors are needed.
Keywords: anatomical prolapse, Ethiopia, pelvic organ prolapse, POP-Q, prolapse symptoms
Plain language summary
A study on pelvic organ prolapse confirmed through physical examination and symptoms of prolapse assessed among women in Sidama region of Ethiopia
Why was the study done? Pelvic organ prolapse occurs when one or more vaginal compartments and their surrounding organs drop from their normal position. Imagine the pelvic floor as a supportive sling made of muscles, ligaments, and tissues. These structures usually hold organs, such as vagina, bladder, uterus, and rectum in place. However, when the pelvic floor weakens due to factors, such as pregnancy, childbirth, or menopause, these organs bulge. Symptoms may include feeling a tissue bulge near the vaginal opening, pelvic pressure, lower back pain, urinary changes, and even difficulty keeping in a tampon. However, evidence about how common the problem is in the Sidama region is limited. This may hinder the need for efforts to be taken in identifying and treating the disorder. What did the researchers do? The research team assessed the symptoms of prolapse by asking women through house-to-house visits invited them to the nearby health facility and conducted pelvic examination to confirm the presence of prolapse. Women’s characteristics that can be related to prolapse were also assessed through interview. What did the researchers find? A total of 815 women participated in interviews on prolapse symptoms, and 779 underwent pelvic examination. Among those examined, one in three women (241/779) has a physically confirmed prolapse. Among the confirmed prolapses, 189 women reported symptom of prolapse. Women who have birth many times, who work on prolonged heavy lifting activities, and who have a history of labor that lasted more than 24 h have a high chance to develop prolapse. Similarly, those who gave birth before the age of 18 years and those engaged in prolonged heavy lifting activities have higher chance of developing prolapse symptoms. What do the findings mean? The findings showed that prolapse is common in the Sidama region of Ethiopia and that it needs attention of stakeholders.
Introduction
Pelvic organ prolapse (POP) is one of the pelvic floor disorders in women characterized by the descent of one of the vaginal walls, cervix, uterus, bladder, or rectum into the vaginal lumen.1,2 It is a common condition for which women of all ages seek medical and surgical treatment. 3 The prevalence varies based on the diagnosis method used. A recent (2022) review conducted by the International Urogynecology Consultation Committee shows that the prevalence of POP varies widely (1%–65%) based on whether it is assessed by symptoms (1%–31%), pelvic examination (10%–50%), or both (20%–65%). 4 Even though there is no clear and universally agreed definition of POP, 5 it is recommended to describe clinically significant prolapse by using both anatomical staging and patient-reported symptoms. 6
The risk of POP is increased in low- and middle-income countries due to higher parity, early marriage, greater numbers of vaginal deliveries, and more frequent heavy manual work.7 –9 The risk factors that have been identified so far are both non-modifiable (family history, ethnicity, and age) and modifiable (obesity, underweight, chronic malnutrition, heavy lifting, chronic medical problems, parity, and age at first birth).1,2,10 –12
POP causes a sensation of vaginal bulge, voiding and defecatory dysfunction, and sexual dysfunction which adversely affects the quality of life.13,14 Studies from both high- and low-income countries revealed that POP patients were struggling to handle their daily activities due to the symptoms of POP.15,16 POP has also a significant psychological impact on women. 17
In Ethiopia, the prevalence of symptomatic POP varies from 1%–30% based on the population category, study setting, and diagnosis method used.18 –21 This approach may underestimate the prevalence due to the asymptomatic nature of POP. Objectively measured POP at the community level reaches up to 56.3% 20 based on the simplified pelvic organ prolapse (sPOP) assessment method. None of these studies have used the standard pelvic organ prolapse quantification (POP-Q) system. The POP-Q system is specific and objectively quantifies the prolapse by using a measuring instrument (centimeters), and nine points in the vagina and the tissues around it. The simplified version of the POP-Q (sPOP) system reduces the number of points to only four points (anterior, posterior, apex/cuff, and cervix) for measurement and does not necessarily use exact measurements.22,23 A review of POP anatomical assessments recommended the use of standardized POP-Q for effective communication between clinicians, reproducible evaluation, meaningful comparison of studies, and comparison of different populations. 24 Therefore, this study aimed to assess the prevalence of POP based on both standard POP-Q and subjective symptoms and assessed risk factors for POP among women in Dale and Wonsho districts, Sidama region, Ethiopia.
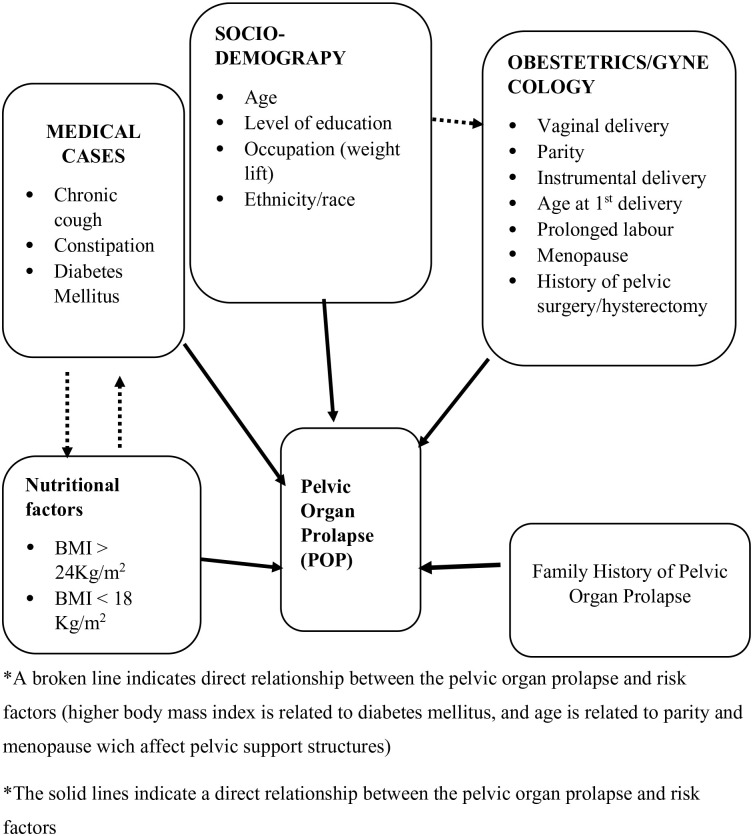
Conceptual framework
Based on the findings of different literature, the following conceptual framework was developed (Figure 1). The conceptual framework shows that different risk factors cause POP either through direct damage of pelvic muscles (levator ani muscle and its nerve supply) or by causing the collagen to weaken.25 –31
Figure 1.
Conceptual framework of risk factors affecting pelvic organ prolapse.
Methods and materials
Study setting
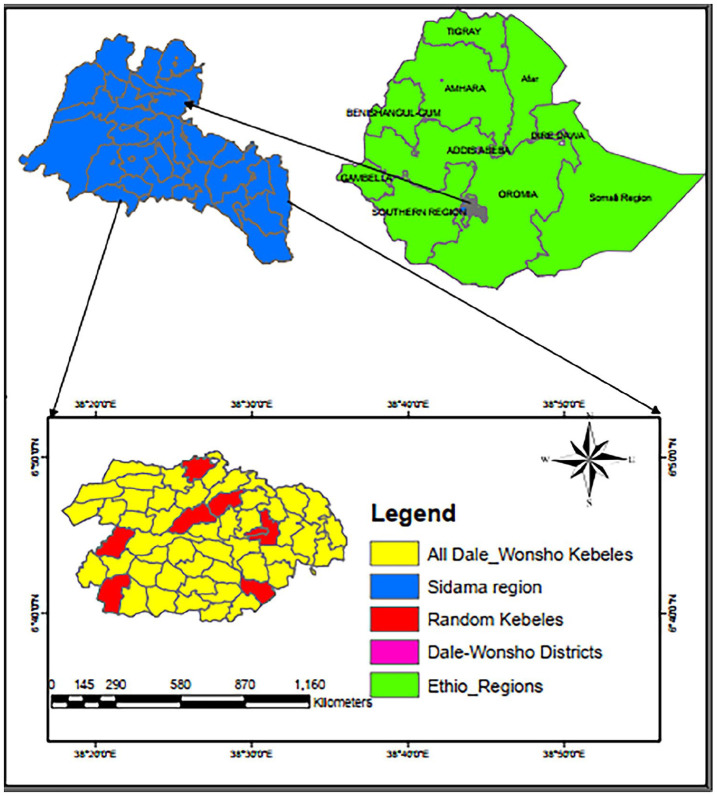
This study was conducted in the Dale and Wonsho districts of Sidama National Regional State, Ethiopia, over a period of 8 months from 13 March to 28 October 2023. Both districts are known for their highly dense population and coffee production 32 (Figure 2). Dale district has 10 health centers and 33 health posts. Hamlin Fistula Center, which provides care for fistula cases and POP, is located in Yirgalem town in Dale district. Wonsho district has five health centers and 17 health posts. According to the 2021 report of Sidama Regional Health Bureau, the total population of Dale district is 254,653 and that of Wonsho district is 129,730. 33 According to the Ethiopian Central Statistical Agency, the female population accounts for 49.7% of the Dale district population and 49.2% of the Wonsho population. 34
Figure 2.
A map of study area in Dale and Wonsho districts, Sidama region, Ethiopia.
In 2017, Hawassa University established its own Health and Demographic Surveillance System (HDSS) site in the Dale and Wonsho districts of the Sidama region. 32 The HDSS site includes 10 rural and two urban kebeles (small administrative units in Ethiopia). The surveillance site is referred to as the Dale–Wonsho Health and Demographic Surveillance Site (D–W HDSS). The main purpose of establishing the HDSS was to provide a background context for clinical trials and generate longitudinal epidemiological data.
Study design and population
A community-based cross-sectional study was conducted by using subjective and objective measure among women who have given birth (at least once) or are ⩾18 years, and whose household information is found in the database of the Health and Demographic Survey of Hawassa University. Women who were pregnant (confirmed or suspected), in the immediate postpartum period (6 weeks), or unable to participate due to sickness were excluded.
Sample size and sampling technique
The sample size was calculated using Open-Epi version 3.1 both for the prevalence of the POP and its associated risk factors. The sample size for the prevalence study was calculated with the assumptions of 56.3% prevalence of POP and a non-response rate of 7%, 20 95% confidence interval, and a design effect of 2. The calculated sample size was 813. The sample size for associated risk factors was also estimated for variables that were significantly associated with anatomical prolapse in a previous study conducted in northern Ethiopia. 20 The variable names, assumptions considered, and their respective results were summarized in a table (Supplemental File 1). A maximum sample size was obtained from the first objective which was 813. To select an equal number of participants, 101.6 women from each cluster, the size was approximated to 102 for each cluster, and the final sample size was adjusted to 816. This was considered maximum and adequate to address the remaining objectives.
Hawassa University HDSS site was initially stratified into two districts (Dale and Wonsho). Then, representative kebeles (small administrative units in Ethiopia) were considered as “a cluster” for this study and selected from both districts (six kebeles from Dale district and two kebeles from Wonsho district) randomly. The number of households in each kebele ranges from 640 to 2200. Based on complex sample survey assumption, an equal number of eligible women from each kebele/cluster were selected using computer-generated random numbers (based on the list of their house numbers). Therefore, this study included two strata: Dale and Wonsho districts; eight clusters (kebeles), and 816 eligible women. The interview was conducted with the female head of the selected households.
Study variables and measurements
The outcome variables of this study were anatomical POP and symptomatic POP hereafter called “anatomical prolapse” and “symptomatic prolapse,” respectively. The anatomical prolapse is a POP of Stage ⩾II on the POP-Q system. There are nine measurement points in the POP-Q system. All are measured at maximum valsalva, except total vaginal length (TVL) which is measured at rest. Measurements are expressed in centimeters to the nearest 0.5 cm. The nine measurement points in and around the vagina are as follows: Genital hiatus (GH) measured from the middle of the external urethral meatus to the posterior aspect of the hymenal remnant, perineal body (PB) measured from the posterior aspect of the hymenal remnant to the middle of the anal opening, point C is the lowest part of cervix (or vaginal cuff), and point D is the topmost part of the posterior vaginal wall (posterior vaginal pouch). Both points are measured relative to the plane of hymenal remnants. A hymenal remnant is chosen as it is a clearly defined and easily identifiable structure. TVL is measured from hymenal remnant to point D. Point Aa is an arbitrary fixed point on the anterior vaginal wall, which is 3 cm back from the middle of the external urethral meatus in normal cases. Point Ba is the lowest part of the upper anterior vagina. This point is not a fixed point like Aa. It can be anywhere along the vaginal wall above the first 3 cm (Aa). Points Ap and Bp are similar to points Aa and Ba but found on the posterior vaginal wall. These measures are translated into a staging system as follows: Stage 0: Points Aa, Ba, Ap, and Bp are all at −3 cm and C and D are at greater than/equal to TVL—2 cm; Stage I: the criteria for stage zero are not met, and the leading edge of the prolapse is greater than 1 cm above the hymen; Stage II: the leading edge of the prolapse is between 1 cm above or below the hymen; Stage III: the leading edge is greater than 1 cm beyond the hymen but less than TVL—2 cm from the hymen; and Stage IV: the leading edge of the prolapse is greater than TVL—2 cm beyond the hymenal remnant.22,35,36
Symptomatic prolapse is considered when anatomical prolapse (Stage ⩾II) and prolapse symptom are reported. Symptoms of POP were collected by using the Sidaamu Afoo version of the pelvic organ prolapse symptom score (POP-SS) questionnaire, 37 which has seven questions with a 5-point Likert-type scale that ranges from 0 to 4 (0 = never felt symptom, 1 = occasionally, 2 = sometimes, 3 = most of the time, and 4 = all of the time), a higher score indicating severe symptoms. 38 A response score different from zero indicates the presence of symptoms. Women were also asked which symptom they felt for a long time and how long she has the symptom.
The independent variables include sociodemographic characteristics (level of education, residence, occupation, ethnicity, age in years, and marital status), lifestyle variables (smoking habit and average hours of heavy lifting per day/carrying big baby, water, sand, and others), obstetrics and reproductive characteristics (mode of delivery, number of childbirth, place of delivery, history of a length of labor more than 24 h, age at first marriage, and age at first childbirth), medical and surgical history (chronic cough/more than 3 weeks, constipation/infrequent stooling and difficulty passing stool, diabetes mellitus, body mass index, history of pelvic surgery, trauma, and family history of POP).
Data collection tools and procedures
The data collection tool has six parts (sections). Part I is about the sociodemographic characteristics of the participants which has eight items; part II focuses on the POP-related lifestyle of the participants, which has six items; part III contains 14 items that ask about the obstetrics and gynecologic history of the participants; part IV is about the wealth index of the study participants’ household, which has 28 items; part V is about POP-SS; and part VI is about objectively measured POP and related measures, which have 14 measurements. The questionnaires used to collect data about sociodemographic characteristics, lifestyle, obstetrics, and gynecologic history were developed from a review of related literature.39 –43 It was initially developed in the English version and translated into the local language (Sidaamu Afoo) (Supplemental File 2). The POP-SS tool was used to assess prolapse symptoms by asking the participants how often they have had the following symptoms in the past 4 weeks: (1) A feeling of something coming down from the vagina? (2) a feeling of discomfort/pain in the vagina? (3) a heaviness/dragging feeling in the lower abdomen? (4) a dragging feeling in the lower back? (5) a difficulty in emptying the bladder? (6) a feeling of incomplete bladder emptying? and (7) a feeling of incomplete bowel emptying? The total score was calculated by summing up all responses to the seven-question test. The questionnaire was previously translated to a local language and validated in the same study area among women with POP who were admitted to Yirgalem Hamlin Fistula Center. 37 The tool was valid and took 15–20 min to complete.
The data were collected using a Kobo Toolbox. Subjective data were collected by eight nurses through home-to-home visits. All study participants were invited for a pelvic examination at a nearby health facility and given an appointment (date and time) irrespective of their symptoms. The collected data were uploaded to the European Union Kobo Toolbox Server daily and monitored by the principal investigator.
POP quantification was done by two staff members of Yirgalem Hamlin Fistula Center. They are para-medical professionals (called “Health Officers” in Ethiopia) who have special training in fistula and POP management. Women empty their bladders and lie in a lithotomy position. The prolapse degree was measured by a ruler (spatula marked with a centimeter) used to measure the prolapse of nine points in and around the vagina.
Data collectors and field supervisors were trained for 3 days on the purpose of the study, the use of the Kobo tool, and each item in the questionnaire. Similarly, pelvic floor examiners were trained by a urogynaecologist at the Hamlin Fistula Center to minimize discrepancies across examiners. As part of the pre-test, they performed 10 POP-Qs under the supervision of the urogynecologist on patients admitted to Hamlin Fistula Center for POP. At the data collection time, they were kept blinded about participant’s background information including symptom status.
This article was prepared according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline, and the checklist was submitted as an additional file (Supplemental File 3).
Statistical analysis
The data set was downloaded from the Kobo Toolbox server and exported to Microsoft Excel 2013. This was imported to Stata version 17 software (StataCorp LLC, College Station, Texas, USA) for data cleaning, recode, and analysis. All forms of data analysis were done taking the nature of complex sampling design into account. The design variables used to declare the complex sample were comprised of the stratum, cluster, and weighting variables. In this study, the stratum variable was the districts (Dale and Wonsho) and the cluster variable was kebele. The weighting variable was created from the product of cluster selection weight and household selection weights. Then to normalize the weights, the survey weight of each unit is divided by the unweighted average of the survey weights. Complex sample summary measures for categorical variables were presented in terms of frequency and percentages. For continuous variables, mean and standard deviation or median and interquartile range (IQR) were presented. The wealth index was determined using principal component analysis44,45 based on 28 items.
Complex sample modified-Poisson regression was used to investigate the association between the outcome variables (anatomical prolapse and symptomatic prolapse) and potential risk factors. In cross-sectional studies, the measure of choice is the prevalence ratio which can be either directly estimated by statistical models46,47 or transformed from odds ratios obtained from logistic regression. 48 However, when the prevalence of outcome of interest is common, the odds ratio overestimates the prevalence ratio.46,47 The prevalence ratio is easier to interpret and understand than the odds ratio by non-epidemiologists. In addition, the model-based estimation is more appropriate for controlling confounders.46,49 The Poisson regression equation is as follows: log (π/t) =β0 + β1X1 + . . . +βkXk 46 where π is the event, t is the time followed up, and xs are the covariates. When a constant time is assigned to everyone in the study (modified Poisson regression), the estimated episode of interest is equal to prevalence ratio. The relative risk estimate of a given covariate is eβ.46,50 In complex sample analysis, even though the application of robust standard error is not allowed with the svy prefix, the inherent robustness in the svy estimation ensures that Poisson regression accounts for the survey design characteristics. 51
Variables with p values less than or equal to 0.25 on bivariable analysis and other important variables (supported by literature) 52 were included in the multivariable model. The model was refined repeatedly by excluding variables which does not significantly impact the overall model fit or the parameters of the remaining individual variables. This process was continued until model significance and goodness of fit were achieved. Adjusted Wald test was used to assess the overall significance of a model by comparing the full model against the reduced (null) model. 53 The test result showed that the full model is significantly different from the reduced model (anatomical prolapse p = 0.005 and symptomatic prolapse p = 0.034), suggesting that the variables included in the full model are important in explaining the outcome variable. The association of outcome variables and risk factors was expressed in terms of adjusted prevalence ratio (APR) with a 95% confidence interval. A multivariable linear regression model was used to assess multicollinearity among independent variables. A variance inflation factor (VIF) of less than 5 was taken as suggestive of less multicollinearity. 54 The data set on which this study is based is provided as an additional file (Supplemental File 4).
Results
Sociodemographic characteristics
A total of 816 women were selected, and 815 (99.9%) participated in the interview and were invited for pelvic examination at a nearby health center. One woman did not wish to participate in the study. Among women who were invited, 779 (95.6%) underwent examination to assess for POP. The age of the participants ranged from 18 to 85 years with a mean age of 41.5 years and a standard deviation of 12.75 years. Seven hundred and fifteen (87.7%) of the participants were rural residents.
All participants experienced vaginal birth (100%). The median number of childbirths was 4 (IQR = 3–5) with a minimum of one and maximum of nine frequencies of childbirth. The minimum age at first childbirth was 14 years, and the maximum age was 35 years. Table 1 depicts the sociodemographic and reproductive health characteristics of study participants.
Table 1.
Sociodemographic and reproductive health characteristics of study participants involved in the prevalence of pelvic organ prolapse in Sidama region, Ethiopia, 2024 (weighted n = 815).
| Variables | Category | Frequency | % |
|---|---|---|---|
| Districts | Dale | 590 | 72.4 |
| Wonsho | 225 | 27.6 | |
| Residence | Urban | 101 | 12.4 |
| Rural | 714 | 87.6 | |
| Marital status | Married | 739 | 90.6 |
| Others | 77 | 9.4 | |
| Level of education | No formal education | 572 | 70.2 |
| Primary school | 161 | 19.7 | |
| Secondary school and above | 82 | 10.1 | |
| Religion | Protestant | 715 | 87.7 |
| Orthodox | 79 | 9.7 | |
| Others | 21 | 2.6 | |
| Occupation | Employed | 35 | 4.3 |
| Non-employed | 780 | 95.7 | |
| Ethnicity | Sidama | 809 | 99.3 |
| Others¥ | 6 | 0.7 | |
| Wealth index | Poorest | 166 | 20.34 |
| Poorer | 170 | 20.8 | |
| Middle | 163 | 20.1 | |
| Rich | 156 | 19.2 | |
| Richest | 160 | 19.6 | |
| Chronic cough (>3 weeks) | No | 567 | 69.6 |
| Yes | 248 | 30.4 | |
| Constipation | No | 607 | 74.5 |
| Yes | 208 | 25.5 | |
| Family history of POP | No | 582 | 71.4 |
| Yes | 233 | 28.6 | |
| History of pelvic surgery | No | 665 | 81.5 |
| Yes | 150 | 18.5 | |
| Body mass index (kg/m2) | <18.5 | 248 | 30.5 |
| −24.9 | 481 | 59 | |
| ⩾25 | 86 | 10.5 | |
| Abortion experience | No | 745 | 92 |
| Yes | 70 | 8 | |
| History of homebirth | No | 197 | 24.2 |
| Yes | 618 | 75.8 | |
| A place for the last childbirth | Home | 528 | 64.8 |
| Health facility | 287 | 35.2 | |
| Prolonged labor | No | 472 | 58 |
| Yes | 343 | 42 | |
| Menopause | No | 545 | 66.8 |
| Yes | 270 | 33.2 |
Note. Others: never married, widowed, and divorced; Others: Muslim and Catholic; Others¥: Amhara and Gurage Oromo. POP: pelvic organ prolapse.
Anatomical pelvic organ prolapse (POP-Q)
Based on the result of the POP-Q, among the 779 women who received pelvic examination, 324 (41.5%; 95% CI = 31.9–51.9) had some degree of prolapse (Stages I–IV). Among these, Stage I prolapse was found in 83 (10.7%), Stage II in 169 (21.7%), Stage III in 46 (5.5%) and Stage IV in 26 (3.3%). Anatomical prolapse (Stages II–IV) were observed in 241 (30.9%; 95% CI = 24–38.7) of the women (Table 2).
Table 2.
Pelvic organ prolapse characteristics among women in Sidama region, Ethiopia, 2024.
| Variables | Categories | Anatomical prolapse (Stage ⩾II, n = 241) | Symptomatic prolapse (n = 189) |
|---|---|---|---|
| Number (%) | Number (%) | ||
| Stage of prolapse | Stage II | 169 (70.2) | 119 (70.2) |
| Stage III | 46 (19.1) | 44 (95) | |
| Stage IV | 26 (10.7) | 26 (100) | |
| Prolapsed site | Anterior prolapse | 211 (87.7) | 167 (88.5) |
| Posterior prolapse | 148 (61.3) | 126 (66.4) | |
| Apical prolapse | 56 (23.4) | 54 (28.7) | |
| Multiple sites a | 127 (52.5) | 110 (58.2) |
Multiple sites prolapse: at least two of the following: anterior, posterior, and apical prolapses.
Symptomatic POP
The prevalence of symptomatic POP (in women with anatomical prolapse of Stages II and above) was 78.5% (189/241) (95% CI = 69.1–85.7). This prevalence fell to 24.27% (95% CI = 19.98–29.16) for the total population who underwent pelvic examination (Table 2). Among the symptomatic prolapse cases (n = 189), the most commonly reported symptoms were a feeling of discomfort/pain in the vagina (98.4%) and a feeling of something passing through the vagina (96.8%) (Figure 3). The most chronic symptom was a feeling of something passing through the vagina (46.7%). The remaining symptoms were as follows: a feeling of discomfort/pain in the vagina (14.6%), dragging pain in the lower back 12%, a feeling of unemptied bowel (8.4%), a need to strain/push to urinate (7.9%), a feeling of unemptied bladder (5.9%), and a dragging/heaviness feeling in the lower abdomen (4.6%). The duration of symptoms ranges from 5 months to 20 years with a median of 48 months (IQR = 42.1–53.9 months). Pelvic organ prolapse symptom (POP-S) was reported by 213 (27.4%; 95% CI = 22–33.5) of participants who underwent pelvic examination (779).
Figure 3.
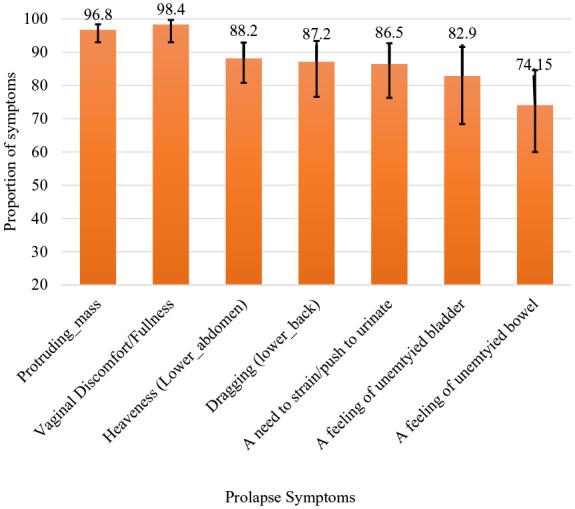
Proportion of prolapse symptoms reported by women in Sidama region, Ethiopia, 2024.
Factors associated with anatomical prolapse (Stage ⩾II)
In a modified Poisson regression, average heavy lifting hours per day, number of childbirths, and experience of prolonged labor (>24 h) were significantly associated with anatomical prolapse. An one-unit increase in the average daily heavy lifting hours led to a 26% increase in the prevalence of anatomical prolapse (APR = 1.26; 95% CI = 1.1–11.4). The risk of developing anatomical prolapse increased by 17% for each unit increase in the number of childbirth (APR = 1.7; 95% CI = 1.1–1.24). Women who experienced prolonged labor (labor lasting more than 24 h) had a 32% higher likelihood of developing anatomical prolapse (APR = 1.32; 95% CI = 1.1–1.56) (Table 3).
Table 3.
Risk factors for anatomical pelvic organ prolapse in Sidama region, Ethiopia, 2024 (weighted n = 779).
| Variables | Anatomical prolapse (n = 779) | CPR (95% CI) | APR (95% CI) | |
|---|---|---|---|---|
| Yes (%) | No (%) | |||
| Average heavy lifting hours/day | 1.34 (1.9–1.5)* | 1.26 (1.14–1.4)** | ||
| Childbirth frequency | 1.31 (1.2–1.4)* | 1.17 (1.1–1.24)** | ||
| Chronic cough (>3 weeks) | ||||
| No | 138 (25) | 405 (75) | Ref | Ref |
| Yes | 103 (44) | 134 (56) | 1.72 (1–2.8)* | 1.28 (0.85–1.9) |
| Prolonged labor (>24 h) | ||||
| No | 110 (24) | 342 (76) | Ref | Ref |
| Yes | 131 (40) | 196 (60) | 1.65 (1.2–2.3)* | 1.32 (1.1–1.56)** |
| Constipation (>3 months) | ||||
| No | 163 (28) | 410 (72) | Ref | Ref |
| Yes | 78 (38) | 129 (62) | 1.3 (1.1–1.7)* | 1.04 (0.86–1.27) |
| Age at first delivery (years) | ||||
| <18 | 115 (49.7) | 117 (50.3) | 2.17 (1.38–3.4)* | 1.57 (0.97–2.56) |
| ⩾18 | 125 (23) | 421 (77) | Ref | Ref |
Significant at p < 0.25; **Highly significant at p < 0.01; Ref: reference category; CPR: crude prevalence ratio; APR: adjusted prevalence ratio; CI: confidence interval.
Factors associated with symptomatic prolapse
An one-unit increase in the average daily heavy lifting hours increased the likelihood of developing symptomatic prolapse by 16% (APR = 1.16; 95% CI = 1.1–1.28). Women who gave birth before the age of 18 years had a 29% higher likelihood of developing symptomatic prolapse (APR = 1.29; 95% CI = 1.1–1.52) (Table 4).
Table 4.
Risk factors associated with symptomatic pelvic organ prolapse in Sidama region, Ethiopia, 2024 (weighted n = 241).
| Variables | Symptomatic prolapse (n = 241) | CPR (95% CI) | APR (95% CI) | |
|---|---|---|---|---|
| Yes (%) | No (%) | |||
| Average daily heavy lifting | 1.18 (1.03–1.33)* | 1.16 (1.1–1.28)** | ||
| Childbirth frequency | 1.01 (1–1.14)* | 1.01 (0.97–1.1) | ||
| Chronic cough (>3 weeks) | ||||
| No | 100 (73) | 38 (27) | Ref | Ref |
| Yes | 89 (86.3) | 14 (13.7) | 1.19 (0.98–1.43)* | 1.13 (0.99–1.3) |
| Prolonged labor (>24 h) | ||||
| No | 85 (78) | 25 (22) | Ref | Ref |
| Yes | 104 (79) | 27 (21) | 1.02 (0.84–1.2)* | 0.94 (0.8–1.1) |
| Family history of POP | ||||
| No | 126 (75) | 42 (25) | Ref | Ref |
| Yes | 64 (86.5) | 10 (13.5) | 1.15 (1.02–1.3)* | 1.1 (0.86–1.41) |
| Age at first delivery (years) | ||||
| <18 | 105 (91) | 11 (9) | 1.35 (1.13–1.6)* | 1.29 (1.1–1.52)** |
| ⩾18 | 84 (67) | 41 (33) | Ref | Ref |
Significant at p < 0.25; **Highly significant at p < 0.01; Ref: reference category; CPR: crude prevalence ratio; APR: adjusted prevalence ratio; CI: confidence interval.
Discussion
This survey showed that almost one-third (30.9%) of the women in the study area had anatomical POP as confirmed clinically. The majority of the prolapse cases were Stage II (70.2%), while Stage III was observed in 19.1% and Stage IV in 10.7%. Among those with anatomical prolapse, 78.4% had symptomatic prolapse which accounts for 24.3% of the total sample. The likelihood of developing anatomical prolapse increased by 17% for one-unit increase in the frequency of childbirth, 26% with one-unit increase in average heavy lifting hours per day, and 32% with experiencing prolonged labor (⩾24 h). Similarly, the likelihood of developing symptomatic prolapse was increased by 16% with prolonged heavy lifting hours and 29% with early childbirth (less than 18 years).
The prevalence of anatomical prolapse (30.9%) in this study is similar to a previous study conducted in Referral Hospitals of Amhara region, Ethiopia (31.8%), 68 tertiary hospitals in Uganda (27.5%), 55 and a community-based study in Turkey (27.1%). 27 However, this prevalence is higher than the prevalence reported from Pakistan (10.3%), 56 and the pooled prevalences reported from Ethiopia (22.7%–23.5%).57,58 The discrepancy could be the result of the difference in prevalence calculation and prolapse measurement and population studied. The prevalence from Pakistan was calculated for the source population (5064) but only 551 participants were clinically examined (among whom 521 had a prolapse). Since POP is usually asymptomatic,59 –61 those who were not examined may have some degree of prolapse. The two systematic reviews and meta-analyses from Ethiopia included POP prevalences that were estimated by symptomatic approach which can underestimate the prevalence. In addition, the prevalence estimates were based on primary studies involving different populations such as facility-based and community-based studies. The reviews also encompassed studies that specifically focused on reproductive-age females. Furthermore, where the primary studies did not report the prevalence (such as case–control studies), manual calculation was used to estimate the prevalence. 57
On the contrary, the prevalence of anatomical prolapse in this study is much lower than the prevalence reported in earlier studies from northern Ethiopia (55.1%–56.3%)20,62 and Tanzania (64.6%). 63 This discrepancy could be due to the difference in measurement techniques, study subjects, and prevalence estimation methods. Previous reports from Ethiopia used the sPOP quantification method but this study used the standardized POP-Q method. This difference in measurement method and interobserver differences may have caused the discrepancy. In Tanzania, the study subjects were of higher parity (median =5, and IQR = 0–14), but in this study, the median number of childbirths was 4 (IQR = 3–5). In addition, the current prevalence was estimated based on complex survey analysis which takes into account the effect of complex design but the previous studies did not. This may affect both the prevalence estimation and their respective standard errors.64,65 The discrepancy could be also secondary to the difference in genetic background even though the evidence is limited. A systematic review and meta-analysis showed that individual studies were of small sample size and often of poor quality. 66 It was stated that the genetic contributions to POP remain poorly understood, and much work was required to establish the role of genes in the pathogenesis of POP. 67
The most common stage of prolapse in this study was Stage II prolapse (70.2%). This is similar to previous reports from Tanzania (63.6%) 63 and Uganda (63.4%). 55 This indicates that POP is very common in the community.
We found a lower prevalence of symptomatic prolapse (24.3%) as compared to the anatomical prolapse (30.9%). It is expected to see lower correlation between anatomical prolapse and self-reported prolapse symptoms.27,55,63,68 The discrepancy is likely because most women will only experience symptoms when the leading edge of the prolapse approaches the hymen. As a result, prolapse above the hymen may be asymptomatic. 69 In addition, there may be under-reporting as the issue is sensitive and women may be reluctant to acknowledge this problem. Compared with previous studies, this study revealed a higher prevalence of reported symptomatic prolapse (78.4%) among women with anatomical prolapse. This might be due to the utilization of a locally validated tool that is more appropriate for participants to understand and respond. Indeed, a previous study from northern Ethiopia revealed that symptomatic prolapse was reported only in 46% of anatomical prolapse cases in a population where 56.4% were thought to have anatomical prolapsed. 20 This difference may also be related to the difference in measuring anatomical prolapse.
We observed that POP-Ss were more common in higher degrees of prolapse which is expected. In this study, prolapse symptoms were experienced by all women with Stage IV prolapse and 95% of women with Stage III prolapse. This is similar to previous reports where self-report for genital prolapse was 95% from Nepal, 70 94% of women undergoing surgery for POP in Uganda, 71 and 97.9% of women with Stage III or more in Ethiopia. 20 Prolapse symptoms were reported by 70% of Stage II patients in this study and 40.1% of previous studies from Ethiopia. This indicates that at least 30% of women with Stage II prolapse may remain asymptomatic.
In this study, the most commonly reported prolapse symptoms were a feeling of discomfort/pain in the vagina (98.4%) and a feeling of something passing through the vagina (96.8%). It was evidenced that women with POP beyond the hymen have increased symptoms that help to define symptomatic prolapsed. 59 A sense of bulging or protrusion in the vagina was considered as the most specific symptom.72,73 This finding highlights the importance of clinicians’ and researchers’ awareness of the significance of population specific symptoms for prolapse identification.
This study showed a significant association between anatomical prolapse and the frequency of childbirth. This is supported by findings from China, 74 Turkey, 27 Pakistan, 56 Tanzania, 63 Uganda, 55 and Ethiopia.20,57,75 POP is caused by injury to its supportive structures (levator ani muscle or its nerve supply and connective tissue). Vaginal delivery directly disrupts this structure due to overstretching, comprehension, and avulsion during childbirth. 76 Similarly, additional vaginal birth causes repeated trauma to these structures causing organ prolapsed. 77 In this study, all participants experienced vaginal birth and majority of them gave birth more than one time. This result has both clinical and public health significance. It informs health care providers to counsel women on the impact of multiple childbirth and the importance of preventive measures and to individualize management plans.
In this study, an one-unit increase in the average daily heavy lifting hours leads to a higher likelihood of both anatomical and symptomatic prolapses. Similar findings were reported previously from Ethiopia,57,68,75 Nepal, 78 and Tanzania. 63 It was evidenced that lifting larger weights and strenuous physical activities affect pelvic ligaments and supporting structures.63,69,79 Thus, this study shows the need for education on handling heavy weight/load, workplace adjustment, and early interventions.
Women who faced prolonged labor (more than 24 h) had a higher likelihood of developing anatomical prolapse. Similar findings were reported from India, 80 Uganda, 55 Tanzania, 63 and Ethiopia.57,75 In addition to the possibility of pelvic floor damage in the case of prolonged labor, pudendal nerve damage was seen in women who labor ⩾20 h. 81 In this study area, women are still prone to prolonged labor due to a higher prevalence of home delivery (64%), early age childbirth, and lack of transportation (87.6% living in rural areas).
This study showed that early age childbirth (at less than 18 years) was significantly associated with symptomatic prolapse. Similar results were reported from India 82 and Ethiopia where childbirth before the age of 20 years was associated with POP. 68 This could be related to the immaturity of the birth canal and supportive ligaments. Prolapse risk is also increased with multiple deliveries. This study indicates the importance of delaying early childbirth, family planning, birth spacing, and proactive management of pregnancy and childbirth.
This study has many strengths. The study employed a complex survey analysis, which accounted for the effect of a complex sampling design. In addition, the large sample size and high response rate enhance the likelihood that the results can be generalized to the target population in the Sidama region, Ethiopia. To our knowledge, this study is the first to utilize a standardized POP-Q method of anatomical assessment of prolapse for a community-based study in Ethiopia. Furthermore, the assessment of POP-Ss was conducted using a locally validated questionnaire.
This study also had some limitations. The study relied on an interviewer-administered questionnaire to assess risk factors for prolapse. However, the way this questionnaire was administered could have influenced participants’ responses. Participants may have misreported their prolapse symptoms, wealth index items, and age due to social desirability bias. Most patients may also not know their exact age. Consequently, age was not found to be associated with prolapse in this study. Similarly, they may not be sure about their family history of prolapse. Most participants had no formal education and were rural residents. Risk factors for prolapse were assessed through interviews. These factors may lead to overestimation or underestimation of the effects of risk factors. Therefore, when interpreting the study results, these issues should be taken into consideration.
Conclusion
The prevalence of both anatomical and symptomatic prolapses is high in the Sidama region of Ethiopia. Prolonged labor, increased parity, and prolonged duration of heavy lifting were risk factors significantly associated with anatomical prolapse. Early age childbirth and prolonged duration of heavy lifting were risk factors strongly associated with symptomatic POP. All risk factors identified in this study are preventable or at least modifiable. Thus, we recommend community-based interventions that focus on health education on risk factors, intrapartum care that prevents prolonged labor, and family planning services.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057241265078 for Prevalence and risk factors of pelvic organ prolapse among women in Sidama region, Ethiopia: A community-based survey by Melese Siyoum, Rahel Nardos, Wondwosen Teklesilasie and Ayalew Astatkie in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455057241265078 for Prevalence and risk factors of pelvic organ prolapse among women in Sidama region, Ethiopia: A community-based survey by Melese Siyoum, Rahel Nardos, Wondwosen Teklesilasie and Ayalew Astatkie in Women’s Health
Supplemental material, sj-docx-3-whe-10.1177_17455057241265078 for Prevalence and risk factors of pelvic organ prolapse among women in Sidama region, Ethiopia: A community-based survey by Melese Siyoum, Rahel Nardos, Wondwosen Teklesilasie and Ayalew Astatkie in Women’s Health
Supplemental material, sj-dta-4-whe-10.1177_17455057241265078 for Prevalence and risk factors of pelvic organ prolapse among women in Sidama region, Ethiopia: A community-based survey by Melese Siyoum, Rahel Nardos, Wondwosen Teklesilasie and Ayalew Astatkie in Women’s Health
Acknowledgments
The authors would like to thank our sponsors (Hawassa University, Minnesota University, Worldwide Fistula Fund, and Maternal Health Fund) for their financial support, study participants, and data collectors. They also thank Dr. Biniyam Sirak (urogynecologist) for his professional support and training data collectors on POP-Q, and Dr. Karen Gold and Joseph Kinahan (Maternal Health Fund), Soja Orlowski (Worldwide Fistula Fund), and Katie Brown (University of Minnesota) for their facilitation in transferring funds.
Footnotes
ORCID iDs: Melese Siyoum  https://orcid.org/0000-0001-5451-5665
https://orcid.org/0000-0001-5451-5665
Ayalew Astatkie  https://orcid.org/0000-0002-4840-9601
https://orcid.org/0000-0002-4840-9601
Supplemental material: Supplemental material for this article is available online.
Declarations
Ethics approval and consent to participate: Ethical approval was obtained from Hawassa University, College of Medicine and Health Sciences, Institutional Review Board (Ref. No. IRB/157/14). Support letter was also obtained from Sidama Region Public Health Institute and the respective health facilities of the study area. A written voluntarily informed consent was obtained from study participants. Considering the sensitive nature of the condition, data about self-reported prolapse symptoms were collected in a private place at the home of the participants. A pelvic examination was performed in the delivery room where physical privacy and information confidentiality were ensured. The pelvic examination has some physical discomfort and psychological distress in exposing the private part. Travel cost to a health facility for pelvic examination was compensated with 200 Ethiopian Birr which was approximately 4 US Dollars. Participants with advanced prolapse (Stage IV) were linked to Yirgalem Hamline Fistula Center for treatment, and symptomatic prolapse Stage I–III cases were enrolled in an interventional study, which is a consecutive part of this project.
Consent for publication: Not applicable.
Author contribution(s): Melese Siyoum: Conceptualization; Formal analysis; Funding acquisition; Methodology; Validation; Writing—original draft; Writing – review & editing.
Rahel Nardos: Fund acquisition; Methodology; Validation; Writing – review & editing.
Wondwosen Teklesilasie: Methodology; Validation; Writing – review & editing.
Ayalew Astatkie: Conceptualization; Formal analysis; Methodology; Validation; Writing – review & editing.
All authors read and approved the final article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The payment for data collectors, participant transportation, and supervisor per diems was sponsored by Hawassa University, University of Minnesota, Worldwide Fistula Fund, and Maternal Health Fund. The funders have no role in designing the study, data management, and publication.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The data set used for this study is available within the article and also as a supplementary file.
References
- 1. Haylen B, Maher C, Barber M. International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecol J 2016; 27: 655–684. [DOI] [PubMed] [Google Scholar]
- 2. Haylen BT, De Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010; 29: 4–20. [DOI] [PubMed] [Google Scholar]
- 3. Shah AD, Kohli N, Rajan SS, et al. The age distribution, rates, and types of surgery for pelvic organ prolapse in the USA. Int Urogynecol J Pelvic Floor Dysfunct 2008; 19(3): 421–428. [DOI] [PubMed] [Google Scholar]
- 4. Brown HW, Hegde A, Huebner M, et al. International urogynecology consultation chapter 1 committee 2: epidemiology of pelvic organ prolapse: prevalence, incidence, natural history, and service needs. Int Urogynecol J 2022; 33(2): 173–187. [DOI] [PubMed] [Google Scholar]
- 5. Ilunga-Mbaya E, Mukwege D, Tshilobo PL, et al. Pelvic organs prolapse in low-resources countries: epidemiology, risk factors, quality of life. Narrative Review. Open J Urol 2023; 13: 238–250. [Google Scholar]
- 6. Gutman RE, Ford DE, Quiroz LH, et al. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms? Am J Obstet Gynecol 2008; 199(6): 683e1–683.e6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker GJ, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J 2011; 22(2): 127–135. [DOI] [PubMed] [Google Scholar]
- 8. Lien YS, Chen GD, Ng SC. Prevalence of and risk factors for pelvic organ prolapse and lower urinary tract symptoms among women in rural Nepal. Int J Gynaecol Obstet 2012; 119(2): 185–188. [DOI] [PubMed] [Google Scholar]
- 9. Islam RM, Oldroyd J, Rana J, et al. Prevalence of symptomatic pelvic floor disorders in community-dwelling women in low- and middle-income countries: a systematic review and meta-analysis. Int Urogynecol J 2019; 30(12): 2001–2011. [DOI] [PubMed] [Google Scholar]
- 10. Vergeldt TF, Weemhoff M, IntHout J, et al. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int Urogynecol J 2015; 26: 1559–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mothes AR, Radosa MP, Altendorf-Hofmann A, et al. Risk index for pelvic organ prolapse based on established individual risk factors. Arch Gynecol Obstet 2016; 293(3): 617–624. [DOI] [PubMed] [Google Scholar]
- 12. Bradshaw KD, Corton MM, Halvorson LM, et al. Williams gynecology. New York: McGraw-Hill Education LLC, 2016. [Google Scholar]
- 13. Iglesia C, Smithling KR. Pelvic organ prolapse. Am Fam Physician 2017; 96: 179–185. [PubMed] [Google Scholar]
- 14. American College of Obstetricians and Gynecologists and American Urogynecologic Society. Pelvic organ prolapse. Female Pelvic Med Reconstr Surg 2019; 25: 397–408. [DOI] [PubMed] [Google Scholar]
- 15. Gjerde JL, Rortveit G, Muleta M, et al. Living with pelvic organ prolapse: voices of women from Amhara region, Ethiopia. Int Urogynecol J 2017; 28(3): 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mirskaya M, Lindgren E-C, Carlsson M. Online reported women’s experiences of symptomatic pelvic organ prolapse after vaginal birth. BMC Women’s Health 2019; 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeleke BM, Ayele TA, Woldetsadik MA, et al. Depression among women with obstetric fistula, and pelvic organ prolapse in northwest Ethiopia. BMC Psychiatry 2013; 13: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ballard K, Ayenachew F, Wright J, et al. Prevalence of obstetric fistula and symptomatic pelvic organ prolapse in rural Ethiopia. Int Urogynecol J 2016; 27(7): 1063–1067. [DOI] [PubMed] [Google Scholar]
- 19. Hambisa HD, Birku Z, Gedamu S. Magnitude of symptomatic pelvic floor dysfunction and associated factors amongst women in Western Ethiopia: a cross-sectional study. Inquiry 2023; 60: 469580231171309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belayneh T, Gebeyehu A, Adefris M, et al. Pelvic organ prolapse in Northwest Ethiopia: a population-based study. Int Urogynecol J 2020; 31(9): 1873–1881. [DOI] [PubMed] [Google Scholar]
- 21. Kebede BN, Hayelom DH, Birgoda GT, et al. Prevalence of pelvic floor disorder and associated factors among women in Arba Minch Health and Demographic surveillance site, Gamo Zone, Southern Ethiopia, 2021. Front Urol 2023; 3: 1196925. [Google Scholar]
- 22. Manonai J, Mouritsen L, Palma P, et al. The inter-system association between the simplified pelvic organ prolapse quantification system (S-POP) and the standard pelvic organ prolapse quantification system (POPQ) in describing pelvic organ prolapse. Int Urogynecol J 2011; 22(3): 347–352. [DOI] [PubMed] [Google Scholar]
- 23. Swift S, Morris S, McKinnie V, et al. Validation of a simplified technique for using the POPQ pelvic organ prolapse classification system. Int Urogynecol J Pelvic Floor Dysfunct 2006; 17(6): 615–620. [DOI] [PubMed] [Google Scholar]
- 24. Sioutis D, Reid F. Pelvic organ prolapse: anatomical and functional assessment. Obstet Gynaecol Reprod Med 2017; 27: 57–64. [Google Scholar]
- 25. Abdool Z, Dietz HP, Lindeque BG. Ethnic differences in the levator hiatus and pelvic organ descent: a prospective observational study. Ultrasound Obstet Gynecol 2017; 50(2): 242–246. [DOI] [PubMed] [Google Scholar]
- 26. Allen-Brady K, Norton PA, Hill AJ, et al. Risk of pelvic organ prolapse treatment based on extended family history. Am J Obstet Gynecol 2020; 223(1): 105.e1–105.e8. [DOI] [PubMed] [Google Scholar]
- 27. Aytan H, Ertunç D, Tok EC, et al. Prevalence of pelvic organ prolapse and related factors in a general female population. Turk J Obstet Gynecol 2014; 11(3): 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beketie ED, Tafese WT, Assefa ZM, et al. Symptomatic pelvic floor disorders and its associated factors in South-Central Ethiopia. PLoS ONE 2021; 16(7): e0254050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leng B, Zhou Y, Du S, et al. Association between delivery mode and pelvic organ prolapse: a meta-analysis of observational studies. Eur J Obstet Gynecol Reprod Biol 2019; 235: 19–25. [DOI] [PubMed] [Google Scholar]
- 30. Maxwell M, Berry K, Wane S, et al. Pelvic floor muscle training for women with pelvic organ prolapse: the PROPEL realist evaluation. Health Serv Deliv Res 2020; 8: 1–132. [PubMed] [Google Scholar]
- 31. Nygaard I, Bradley C, Brandt D, et al. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol 2004; 104(3): 489–497. [DOI] [PubMed] [Google Scholar]
- 32. Debiso AT, Tefera K, Asseffa NA, et al. Cohort profile: the Dale–Wonsho health and demographic surveillance system, Southern Ethiopia. Int J Epidemiol 2024; 53: dyae018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sidama Region Health Bureau. Health facility data with Catchment Population (Updated Meskerem 2014 E.C). Hawassa, Ethiopia: Sidama Region Health Bureau, 2021. [Google Scholar]
- 34. Central Statistics Agency. Population projection of Ethiopia for all regions at wereda level from 2014–2017. Addis Ababa: Central Statistical Agency, 2013. [Google Scholar]
- 35. Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996; 175(1): 10–17. [DOI] [PubMed] [Google Scholar]
- 36. Fiona R. Assessment of pelvic organ prolapse: a practical guide to the pelvic organ prolapse quantification. Obstet Gynecol Reprod Health 2014; 24: 170–176. [Google Scholar]
- 37. Siyoum M, Teklesilasie W, Nardos R, et al. Reliability and validity of the Sidaamu Afoo version of the pelvic organ prolapse symptom score questionnaire. BMC Womens Health 2023; 23: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hagen S, Glazener C, Sinclair L, et al. Psychometric properties of the pelvic organ prolapse symptom score. BJOG 2009; 116(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 39. Awwad J, Sayegh R, Yeretzian J, et al. Prevalence, risk factors, and predictors of pelvic organ prolapse: a community-based study. Menopause 2012; 19(11): 1235–1241. [DOI] [PubMed] [Google Scholar]
- 40. Henok A. Prevalence and factors associated with pelvic organ prolapse among pedestrian back-loading women in Bench Maji Zone. Ethiop J Health Sci 2017; 27(3): 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lawrence JM, Lukacz ES, Nager CW, et al. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet Gynecol 2008; 111(3): 678–685. [DOI] [PubMed] [Google Scholar]
- 42. Horst W, do Valle JB, Silva JC, et al. Pelvic organ prolapse: prevalence and risk factors in a Brazilian population. Int Urogynecol J 2017; 28(8): 1165–1170. [DOI] [PubMed] [Google Scholar]
- 43. Cheung RYK, Chan SSC, Shek KL, et al. Pelvic organ prolapse in Caucasian and East Asian women: a comparative study. Ultrasound Obstet Gynecol 2019; 53(4): 541–545. [DOI] [PubMed] [Google Scholar]
- 44. Poirier MJ, Grépin KA, Grignon M. Approaches and alternatives to the wealth index to measure socioeconomic status using survey data: a critical interpretive synthesis. Soc Indic Res 2020; 148: 1–46. [Google Scholar]
- 45. Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan 2006; 21(6): 459–468. [DOI] [PubMed] [Google Scholar]
- 46. Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003; 3: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706. [DOI] [PubMed] [Google Scholar]
- 48. Zocchetti C, Consonni D, Bertazzi PA. Estimation of prevalence rate ratios from cross-sectional data. Int J Epidemiol 1995; 24: 1064–1065. [DOI] [PubMed] [Google Scholar]
- 49. Miettinen OS, Cook EF. Confounding: essence and detection. Am J Epidemiol 1981; 114(4): 593–603. [DOI] [PubMed] [Google Scholar]
- 50. Coutinho LM, Scazufca M, Menezes PR. Methods for estimating prevalence ratios in cross-sectional studies. Rev Saude Publica 2008; 42(6): 992–998. [PubMed] [Google Scholar]
- 51. StataCorp LP. Stata survey data reference manual. College Station, TX: StataCorp LP, 1985. [Google Scholar]
- 52. Hosmer DW, Jr, Lemeshow S, Sturdivant RX. Applied logistic regression. Hoboken, NJ: John Wiley & Sons, 2013. [Google Scholar]
- 53. Williams R. Analyzing Complex Survey Data: some key issues to be aware of University of Notre Dame, https://www3.nd.edu/~rwilliam/stats2/SvyCautions.pdf (2015, accessed 20 June 2022).
- 54. Gareth J, Daniela W, Trevor H, et al. An introduction to statistical learning: with applications in R. London: Springer, 2013. [Google Scholar]
- 55. Tugume R, Lugobe HM, Kato PK, et al. Pelvic organ prolapse and its associated factors among women attending the Gynecology Outpatient Clinic at a Tertiary Hospital in Southwestern Uganda. Int J Womens Health 2022; 14: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jokhio AH, Rizvi RM, MacArthur C. Prevalence of pelvic organ prolapse in women, associated factors and impact on quality of life in rural Pakistan: population-based study. BMC Womens Health 2020; 20: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Addisu D, Mekie M, Belachew YY, et al. The prevalence of pelvic organ prolapse and associated factors in Ethiopia: a systematic review and meta-analysis. Front Med 2023; 10: 1193069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gedefaw G, Demis A. Burden of pelvic organ prolapse in Ethiopia: a systematic review and meta-analysis. BMC Womens Health 2020; 20: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse? Am J Obstet Gynecol 2003; 189(2): 372–377. [DOI] [PubMed] [Google Scholar]
- 60. Chow D, Rodríguez LV. Epidemiology and prevalence of pelvic organ prolapse. Curr Opin Urol 2013; 23: 293–298. [DOI] [PubMed] [Google Scholar]
- 61. Collins S, Lewicky-Gaupp C. Pelvic organ prolapse. Gastroenterol Clin 2022; 51: 177–193. [DOI] [PubMed] [Google Scholar]
- 62. Megabiaw B, Adefris M, Rortveit G, et al. Pelvic floor disorders among women in Dabat district, northwest Ethiopia: a pilot study. Int Urogynecol J 2013; 24(7): 1135–1143. [DOI] [PubMed] [Google Scholar]
- 63. Masenga GG, Shayo BC, Rasch V. Prevalence and risk factors for pelvic organ prolapse in Kilimanjaro, Tanzania: a population based study in Tanzanian rural community. PLoS ONE 2018; 13(4): e0195910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lepkowski JM. Statistical methodologies for analyzing a complex sample survey. Hyattsville, MD: US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Health Statistics, 1988. [Google Scholar]
- 65. Heeringa SG, West BT, Berglund PA. Applied survey data analysis. New York: Chapman and Hall/CRC, 2010. [Google Scholar]
- 66. Ward RM, Velez Edwards DR, Edwards T, et al. Genetic epidemiology of pelvic organ prolapse: a systematic review. Am J Obstet Gynecol 2014; 211(4): 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Allen-Brady K, Chua JW, Cuffolo R, et al. Systematic review and meta-analysis of genetic association studies of pelvic organ prolapse. Int Urogynecol J 2022; 33: 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Muche HA, Kassie FY, Biweta MA, et al. Prevalence and associated factors of pelvic organ prolapse among women attending gynecologic clinic in referral hospitals of Amhara Regional State, Ethiopia. Int Urogynecol J 2021; 32(6): 1419–1426. [DOI] [PubMed] [Google Scholar]
- 69. Barber MD. Pelvic organ prolapse. BMJ 2016; 354: i3853. [DOI] [PubMed] [Google Scholar]
- 70. Bonetti TR, Erpelding A, Pathak LR. Listening to “felt needs”: investigating genital prolapse in western Nepal. Reprod Health Matters 2004; 12(23): 166–175. [DOI] [PubMed] [Google Scholar]
- 71. Byamugisha J, Justus B, Kakaire O, et al. Characteristics and outcomes of patients with pelvic organ prolapse: an analysis of data from Mulago National Referral Hospital from 2007–2016. Afr Health Sci 2023; 23(1): 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Manonai J, Wattanayingcharoenchai R. Relationship between pelvic floor symptoms and POP-Q measurements. Neurourol Urodyn 2016; 35(6): 724–727. [DOI] [PubMed] [Google Scholar]
- 73. Kuncharapu I, Majeroni BA, Johnson DW. Pelvic organ prolapse. Am Fam Physician 2010; 81: 1111–1117. [PubMed] [Google Scholar]
- 74. Li Z, Xu T, Li Z, et al. An epidemiologic study on symptomatic pelvic organ prolapse in obese Chinese women: a population-based study in China. Diabetes Metab Syndr Obes 2018; 11: 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Borsamo A, Oumer M, Worku A, et al. Associated factors of pelvic organ prolapse among patients at public Hospitals of Southern Ethiopia: a case-control study design. PLoS ONE 2023; 18(1): e0278461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dietz HP. Pelvic floor trauma in childbirth. Aust N Z J Obstet Gynaecol 2013; 53: 220–230. [DOI] [PubMed] [Google Scholar]
- 77. Yeniel AÖ, Ergenoglu AM, Askar N, et al. How do delivery mode and parity affect pelvic organ prolapse. Acta Obstet Gynecol Scand 2013; 92(7): 847–851. [DOI] [PubMed] [Google Scholar]
- 78. Bodner-Adler B, Shrivastava C, Bodner K. Risk factors for uterine prolapse in Nepal. Int Urogynecol J 2007; 18: 1343–1346. [DOI] [PubMed] [Google Scholar]
- 79. Akmel M, Segni H. Pelvic organ prolapse in Jimma University specialized hospital, Southwest Ethiopia. Ethiop J Health Sci 2012; 22(2): 85–92. [PMC free article] [PubMed] [Google Scholar]
- 80. Joseph N, Krishnan C, Reddy BA, et al. Clinical profile of uterine prolapse cases in South India. J Obstet Gynaecol India 2016; 66(Suppl. 1): 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labour: prospective study before and after childbirth. Br J Obstet Gynaecol 1994; 101(1): 22–28. [DOI] [PubMed] [Google Scholar]
- 82. Verma D. Factors associated with pelvic organ prolapse: a prospective study in a tertiary care hospital in Northern India. Int J Reproduc Contracept Obstet Gynecol 2016; 5: 1862–1865. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057241265078 for Prevalence and risk factors of pelvic organ prolapse among women in Sidama region, Ethiopia: A community-based survey by Melese Siyoum, Rahel Nardos, Wondwosen Teklesilasie and Ayalew Astatkie in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455057241265078 for Prevalence and risk factors of pelvic organ prolapse among women in Sidama region, Ethiopia: A community-based survey by Melese Siyoum, Rahel Nardos, Wondwosen Teklesilasie and Ayalew Astatkie in Women’s Health
Supplemental material, sj-docx-3-whe-10.1177_17455057241265078 for Prevalence and risk factors of pelvic organ prolapse among women in Sidama region, Ethiopia: A community-based survey by Melese Siyoum, Rahel Nardos, Wondwosen Teklesilasie and Ayalew Astatkie in Women’s Health
Supplemental material, sj-dta-4-whe-10.1177_17455057241265078 for Prevalence and risk factors of pelvic organ prolapse among women in Sidama region, Ethiopia: A community-based survey by Melese Siyoum, Rahel Nardos, Wondwosen Teklesilasie and Ayalew Astatkie in Women’s Health