Abstract
Inebilizumab is one of the monoclonal antibodies approved as maintenance therapy for aquaporin-4 immunoglobulin G-seropositive neuromyelitis optica spectrum disorder (NMOSD). It is a humanized monoclonal antibody targeting cluster of differentiation 19 (CD19). Common adverse reactions include urinary tract infections, nasopharyngitis, arthralgia, infusion reactions, headaches and a decrease in immunoglobulin levels. Here, we present a case of an NMOSD patient who experienced transient hyperCKaemia after the use of inebilizumab. The adverse reactions of this very rare monoclonal antibody drug improved after discontinuation.
Keywords: adverse effect, case report, hyperCKaemia, inebilizumab, neuromyelitis optica spectrum disorder
Introduction
Inebilizumab is a humanized monoclonal antibody targeting CD19 found on B cells and is highly effective in reducing the risk of attack of neuromyelitis optica spectrum disorder (NMOSD). 1 The study demonstrates that compared to placebo, inebilizumab significantly reduces 3-month Expanded Disability Status Scale (EDSS)-confirmed disability progression. Furthermore, it is not influenced by baseline disability, prestudy attack frequency and disease duration. The average EDSS score improved with long-term treatment. 2 It is a monoclonal antibody approved as a maintenance therapy for aquaporin-4 immunoglobulin G (AQP4-IgG)-seropositive NMOSD. According to the recommendations of the randomized control trial study protocol, the initial dose was 300 mg administered intravenously on the first day, followed by another 300 mg intravenous infusion on the 15th day and subsequent doses of 300 mg intravenous infusion every 6 months from the first dose.1,3 Currently, reports on inebilizumab indicate that the main adverse effects are urinary tract infections, nasopharyngitis, arthralgia, infusion reactions, headaches and decreased immunoglobulin levels. 1 No cases of muscle damage following inebilizumab administration have been reported. HyperCKaemia is also associated with vigorous physical activity, muscle injury, pregnancy, alcohol or drug use, neoplasms, infections, endocrinopathies, haemopathies and neuromuscular disorders. However, there is limited knowledge regarding drug-induced hyperCKaemia. For example, adalimumab has previously been reported to cause muscle damage. The most common hypothesis for the induction of myositis by tumour necrosis factor (TNF)-α inhibitors is a disturbance in the cytokine balance associated with TNF-α inhibitors, which leads to myositis or other autoimmune diseases. 4 In this article, we describe an unusual case of a patient with NMOSD who experienced significant and transient elevation of creatinine kinase (CK) levels due to treatment with inebilizumab, which is short lived and has not resulted in profound consequences.
Case report
Our patient was a 42-year-old Han Chinese man with a medical history of NMOSD who presented to our hospital with the chief complaint of generalized muscle pain. At the age of 30 years (May 2011), the patient experienced sudden bilateral vision loss, only perceiving hand movements, accompanied by unsteady walking, numbness in the left lower limb, abdominal tightness and lumbar back pain. Magnetic resonance imaging (MRI) of the spinal cord at the first attack showed a long-segment T2 hyperintensity (Figure 1). Methylprednisolone sodium succinate was administered at 1000 mg for 5 days, followed by oral prednisone acetate at 60 mg, with a weekly reduction of 5 mg until discontinuation. The patient recovered well after being discharged from the hospital. However, the patient experienced three relapses over the subsequent 4 years, which improved after receiving hormone shock therapy each time. In 2015, the patient underwent antibody testing, which indicated AQP4-IgG-seropositivity (1:10). Spinal cord MRI revealed thoracic 2–4 vertebrae lesions (Figure 2). Combined with clinical presentation, laboratory tests and imaging results, the patient was diagnosed with NMOSD. Beginning in June 2015, mycophenolate mofetil was initiated at 750 mg twice daily; however, repeated attacks persisted. By April 2022, eight relapses had occurred. In April 2022, the patient was switched to rituximab (500 mg), administered once weekly for 2 consecutive weeks, followed by an infusion every 6 months, each time at a dosage of 500 mg. In November 2022, the patient experienced another severe spinal pain. Therefore, in September 2023, treatment was switched to inebilizumab [Figure 3(a)]. Before the administration of inebilizumab, the patient’s serum CK level remained normal at 88 U/L (1.5 μkat/L) [Figure 3(b)]. However, he developed generalized mild muscle pain and slight muscle weakness 10 days after the initial administration of 300 mg inebilizumab, which did not affect walking. During this period, the patients did not engage in strenuous exercise or physical exhaustion. We recommend that the patient undergo relevant haematological examinations, including electrolyte and muscle enzyme testing. His serum CK was elevated at 4676 U/L (78.0 μkat/L), rising to 5502 U/L (91.9 μkat/L) the following day [normal, 50–310 U/L (0.8–5.2 μkat/L)] and her serum lactate dehydrogenase (LDH) was elevated at 368 U/L (6.1 μkat/L), rising to 415 U/L (6.9 μkat/L) the following day [normal, 120–250 U/L (2.0–4.2 μkat/L)]. The aspartate transaminase (AST) level was elevated to 161 U/L (6.1 μkat/L) [normal, 15–40 U/L (0.3–0.7 μkat/L)], and the alanine transaminase (ALT) level was elevated to 62 U/L (1.0 μkat/L) [normal, 9–50 U/L (0.2–0.8 μkat/L)]. Simultaneously conducted cell subtype analysis shows B cell count 0 cell/mL (normal, 175–337 cell/μL), Natural Killer (NK) cell count 330 cell/μL (normal, 154–768 cell/μL) and the T-cell subtypes did not reveal any abnormalities. The patient’s serum anti-AQP4-antibody titer decreased to 1:32, which was a 10-fold reduction compared to before using inebilizumab. Serum AQP4-IgG was detected by a commercial cell-based assay from Kindstar Global, which is the large-scale high-end medical specialty inspection service inspection group in China. The examination for autoimmune diseases and the profile of myositis antibodies (against the antigens Jo-1, PL-7, PL-12, EJ, 0J, Zo, KS, Ha, Mi-2a, Mi-2β, T1F1γ, NXP2, MDA5, SAE1, SAE2, HMGCR, cN1A, CENP-B, Scl-70, RNA-PIII, Th/To, N0R-90, Fibrillarin, Ku, PM-Sc1100, PM-Sc175, Ro52) yielded negative results. Electromyography showed no abnormalities. He received 2000 mL of intravenous fluid (0.9% sodium chloride solution) daily, and on the 5th day of treatment, a follow-up CK test showed a significant decrease to 262 IU/L (normal, 19–226 IU/L), the LDH, AST and ALT returned to normal levels. Generalized muscle pain and limb weakness improved. After 2 weeks, the patient was again administered 300 mg of inebilizumab in accordance with the recommended regimen. However, on the 20th day after the second administration of the inebilizumab, again the patient experienced generalized muscle pain and slight limb weakness. His serum CK level was elevated at 4359 U/L (normal, 24–190 U/L). We recommend that the patient receive an intravenous infusion of saline and fluid supplementation to expedite excretion. Simultaneously, we tracked changes in CK levels in the patient, which gradually returned to normal after 27 days [Figure 3(b)]. During this period, the patient reported mild symptoms of muscle pain and weakness throughout the body, without significant difficulty in walking and daily activities. After the decrease in CK levels, both muscle pain and weakness completely disappeared. Continued follow-up of the patient revealed no further use of inebilizumab. At 6 months after inebilizumab, the patient switched to satralizumab and did not develop muscle soreness or weakness.
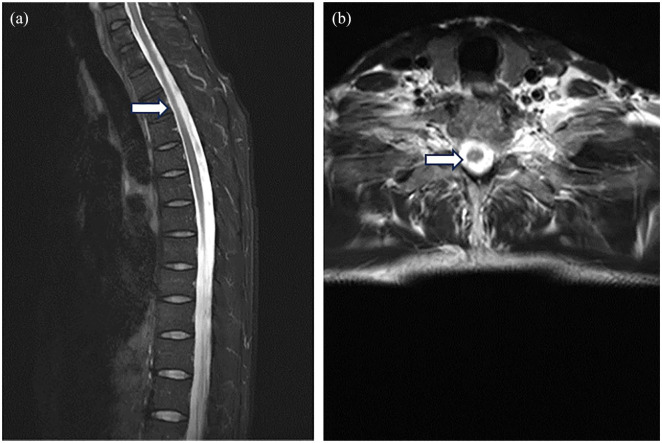
Figure 1.
Spinal cord MRI taken at the first attack. Sagittal (a) and axial (b) MR images of the thoracic spine show a long-segment T2 hyperintensity.
MRI, magnetic resonance imaging.
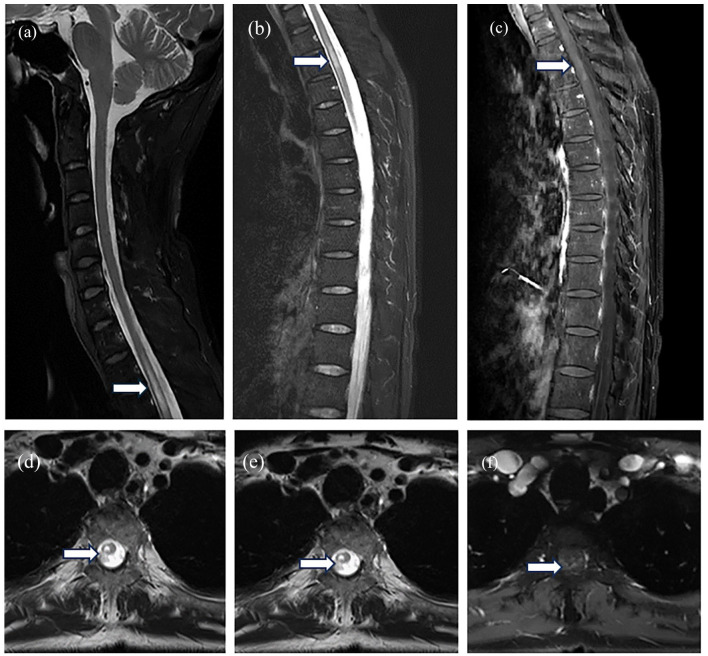
Figure 2.
Spinal cord MRI taken during the inflammatory myelitis episode. Sagittal (a–c) and axial (d–f) MR images of the thoracic spine show a long-segment T2 hyperintensity, with increased enhancement observed.
MRI, magnetic resonance imaging.
Figure 3.
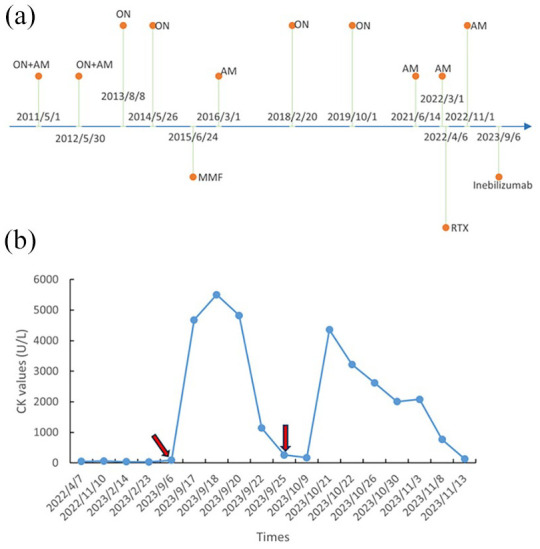
Timeline of the patient’s condition with relevant data of past episodes. (a) Relapse and treatment. (b) CK values. The red arrow indicates the time points of inebilizumab usage.
AM, acute myelitis; CK, creatinine kinase; MMF, mycophenolate mofetil; ON, optic neuritis; RTX, rituximab.
Discussion
Given the initiation of inebilizumab treatment, drug-induced hyperCKaemia was the most probable cause of the symptoms. Inebilizumab is a relatively new medication, and it is crucial to understand its potential side effects comprehensively. This incident is the first time we have observed muscle damage after using inebilizumab. In 2020, the Food and Drug Administration approved inebilizumab for the treatment of AQP4-seropositive NMOSD. The primary side effect of inebilizumab is an increased risk of infection. To our knowledge, this is the first reported case of hyperCKaemia following using inebilizumab and there are no clear mechanisms for this to occur with anti-CD19 treatment, whereas hyperCKaemia is well described to occur in NMOSD with AQP4 known to be expressed in muscle and muscle biopsy/pathology demonstrating antibody-mediated damage in NMOSD patients. 5 However, CK leakage due to antibody-mediated myolysis was excluded due to reduced AQP4-IgG titers. Furthermore, during this period, the patients did not engage in strenuous exercise, physical exhaustion or used other drugs that can cause hyperCKaemia. Both of the patient’s hyperCKaemia occurred after inebilizumab administration. Therefore, there is a connection between muscle damage and inebilizumab use. We conducted simultaneous testing of cellular subtypes and myositis antibodies, ruling out the reactive activation of NK cells or T cells due to the clearance of CD19 + B cells. There have been reports of monoclonal antibodies, possibly leading to the activation of autoimmune antibodies. 4 Our results also ruled out the possibility of inebilizumab-activating myositis antibodies. The elevation of the patient’s serum CK level was transient and did not cause persistent severe harm. However, the exact mechanisms underlying this phenomenon remain unclear.
In the long-term treatment of NMOSD, anti-CD20 antibodies such as rituximab, which deplete B cells, have become the preferred treatment method for NMOSD in recent years. 6 In addition, the monoclonal anti-CD19 antibody inebilizumab, which targets not only B cells but also plasmablasts that produce antibodies against CD19, has been shown to be beneficial in AQP4-IgG-positive NMOSD patients. Furthermore, tocilizumab and satralizumab, which inhibit the interleukin-6 (IL-6) pathway, have also been successful in treating NMOSD.7,8 C5 inhibitors such as eculizumab and ravulizumab, which inhibit terminal complement cascade, have been successfully used to prevent NMOSD attacks. 9 Since 2019, eculizumab, inebilizumab, satralizumab and ravulizumab have been approved for use in AQP4-IgG-positive NMOSD patients. 10 In the future, we need to further explore and study potential novel targets for the treatment of NMOSD, to provide greater assistance to clinical patients.
Conclusion
HyperCKaemia after inebilizumab administration is rarely observed. However, the specific mechanisms underlying this phenomenon remain unclear. Herein, we present a rare case of hyperCKaemia following using ibalizumab, which caused concern in the patient. However, after hydration therapy, the patient’s muscle enzymes quickly returned to normal. Whether similar occurrences in future patients will also be transient and mild remains to be further observed. It is important to emphasize that such adverse reactions are uncommon with B-cell-depleting monoclonal antibodies and can be alleviated with simple treatment after the discontinuation of inebilizumab.
Acknowledgments
We express our gratitude to all individuals who contributed to this study.
Footnotes
ORCID iD: Hongyu Zhou  https://orcid.org/0000-0002-7637-1305
https://orcid.org/0000-0002-7637-1305
Contributor Information
Xuefen Chen, Department of Neurology, West China Xiamen Hospital of Sichuan University, Xiamen, China; The Xiamen Key Laboratory of Psychoradiology and Neuromodulation, Xiamen, China.
Ziyan Shi, Department of Neurology, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
Rui Wang, Department of Neurology, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
Hongyu Zhou, Department of Neurology, West China Hospital, Sichuan University, No. 37 Guoxue Xiang, Chengdu, Sichuan 610041, China.
Declarations
Ethics approval and consent to participate: This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committees of West China Hospital, Sichuan University (approval number: 2018 [SHEN] 19). The patients provided the written informed consent to participate in this study.
Consent for publication: Written informed consent was obtained from the patients for the publication of medical data and related images.
Author contributions: Xuefen Chen: Data curation; Investigation; Visualization; Writing – original draft; Writing – review & editing.
Ziyan Shi: Data curation; Investigation; Methodology.
Rui Wang: Data curation; Investigation; Methodology; Resources.
Hongyu Zhou: Validation; Visualization; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: All the data produced or examined in the course of this study have been incorporated into this published article.
References
- 1. Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019; 394: 1352–1363. [DOI] [PubMed] [Google Scholar]
- 2. Marignier R, Bennett JL, Kim HJ, et al. Disability outcomes in the N-MOmentum trial of inebilizumab in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm 2021; 8: e978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frampton JE. Inebilizumab: first approval. Drugs 2020; 80: 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu SW, Velez NF, Lam C, et al. Dermatomyositis induced by anti-tumor necrosis factor in a patient with juvenile idiopathic arthritis. JAMA Dermatol 2013; 149: 1204–1208. [DOI] [PubMed] [Google Scholar]
- 5. He D, Li Y, Dai Q, et al. Myopathy associated with neuromyelitis optica spectrum disorders. Int J Neurosci 2016; 126: 863–866. [DOI] [PubMed] [Google Scholar]
- 6. Barreras P, Vasileiou ES, Filippatou AG, et al. Long-term effectiveness and safety of rituximab in neuromyelitis optica spectrum disorder and MOG antibody disease. Neurology 2022; 99: e2504–e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujihara K, Bennett JL, de Seze J, et al. Interleukin-6 in neuromyelitis optica spectrum disorder pathophysiology. Neurol Neuroimmunol Neuroinflamm 2020; 7: e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ringelstein M, Ayzenberg I, Lindenblatt G, et al. Interleukin-6 receptor blockade in treatment-refractory MOG-IgG-associated disease and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm 2022; 9: e1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asavapanumas N, Tradtrantip L, Verkman AS. Targeting the complement system in neuromyelitis optica spectrum disorder. Expert Opin Biol Ther 2021; 21: 1073–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waliszewska-Prosół M, Chojdak-Łukasiewicz J, Budrewicz S, et al. Neuromyelitis optica spectrum disorder treatment – current and future prospects. Int J Mol Sci 2021; 22: 2801. [DOI] [PMC free article] [PubMed] [Google Scholar]