Abstract
Diagnosing neoplastic fever requires excluding identifiable causes, making it a diagnostic challenge. Fever as a primary manifestation of pancreatic adenocarcinoma is uncommon with few cases reported in the literature. Here we present an unusual case of metastatic pancreatic adenocarcinoma primarily manifesting as pyrexia of unknown origin. A 63-year-old Sri Lankan male, a non-smoker who was diagnosed with diabetes, hypertension and dyslipidaemia presented with a history of fever, anorexia and weight loss for 2 months. Despite the completion of treatment for positive serology for paratyphi, his symptoms and inflammatory markers remained elevated while the rest of the infectious screening was negative. On further evaluation, the patient was found to have a hypodense distal pancreas with ring-enhancing multiple liver lesions on imaging. Histology confirmed pancreatic adenocarcinoma with liver metastasis. Atypical liver metastases may present with evidence of ring enhancement in computed tomography imaging; thus, the biopsy is mandatory for diagnosis and decision-making. Usually, tumours of the pancreatic tail are resectable but if they are associated with liver metastatic disease, surgical resection is not recommended because it is not potentially curative. Therefore, in the context of metastatic pancreatic adenocarcinoma, palliative chemotherapy and pharmacological management of fever are required.
Keywords: Fever of unknown origin, pancreatic adenocarcinoma, liver metastases
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy with an unfavourable prognosis. 1 It is the fourth leading cause of cancer-related mortality globally. 1 PDAC is more prevalent in the pancreatic head, occurring at twice the frequency compared to other regions of the pancreas. 1 Typical manifestations of PDAC involve anorexia, asthenia, weight loss, pain and obstructive jaundice. 2 Fever as a symptom or primary manifestation of PDAC is uncommon with few cases reported in the literature. 3
Diagnosing neoplastic fever typically requires excluding identifiable causes, making it a diagnostic challenge in cancer patients. 3 In this context, we present an unusual case of metastatic PDAC primarily manifesting as pyrexia of unknown origin (PUO).
Case presentation
A 63-year-old Sri Lankan male, a non-smoker who was diagnosed with diabetes, hypertension and dyslipidaemia presented with a history of fever, anorexia and loss of weight for 2 months. Earlier, he had been hospitalized and treated with antibiotics for paratyphoid fever due to positive serology for Salmonella paratyphi A (Agglutination test of 1:320). Despite completing the antibiotic course of meropenem and teicoplanin, his fever (100°F) persisted, leading to readmission.
He worked in a private firm as an accountant for several years with no recent travel outside the country. He had no notable family or sexual history. Over the 8 weeks, the fever persisted alongside dry cough, malaise, loss of appetite, loss of weight and more recent occurrences of feeling unfit. He denied experiencing gastrointestinal or urinary symptoms. His general and system examination was unremarkable. Notably, he had no rashes or joint swellings and exhibited no neurological signs.
Initially, he was evaluated at the medical unit for PUO. His basic haematology revealed a neutrophil leucocytosis (white blood cells: 24,470 × 109/L, neutrophils: 81.0%) and elevated inflammatory markers (C-reactive protein: 164.3 mg/L, erythrocyte sedimentation rate: 63 mm/first hour and procalcitonin: 8.650 ng/ml). His blood and urine cultures, as well as viral screening for Influenza A, B, COVID-19, and human immunodeficiency viruses (HIV) were negative (Table 1).
Table 1.
Summary of investigations to look for an infectious aetiology.
| Investigation | Results |
|---|---|
| Influenza virus antigens A and B | Negative |
| Urine culture | No growth |
| Blood culture | No growth |
| Malaria antigen rapid test Plasmodium falciparum and P. vivax antigens |
Negative |
| Widal test slide agglutination test Salmonella typhi O and S. typhi H: Salmonella paratyphi AH: |
Negative Negative Positive (dilution up to 1:320 ) |
| 2D and transthoracic echocardiogram | No vegetation |
| COVID-19 RT PCR; | Negative |
| HIV I and II antibodies rapid test | Negative |
| Blood picture | Mild normochromic normocytic anaemia was noted. Mild neutrophilia, eosinophilia and thrombocytosis appear reactive probably to infection/inflammation |
HIV: human immunodeficiency viruses.
Autoimmune and systemic vasculitis were ruled out through repeated autoimmune screening tests, including tests for antinuclear antibody (ANA), C-anti-neutrophil cytoplasmic antibody (ANCA) and P-ANCA, all of which returned negative except for ANA. Chest X-ray displayed no lesions. Additionally, the absence of lymph node enlargement and normal serum calcium levels ruled out a diagnosis of sarcoidosis, leading to the decision to halt further pulmonary investigations. The serological tests conducted for malaria showed negative. Additionally, 2D echocardiogram, trans-oesophageal echocardiogram and abdominal ultrasonography were carried out to eliminate the possibility of an underlying cause and were unremarkable.
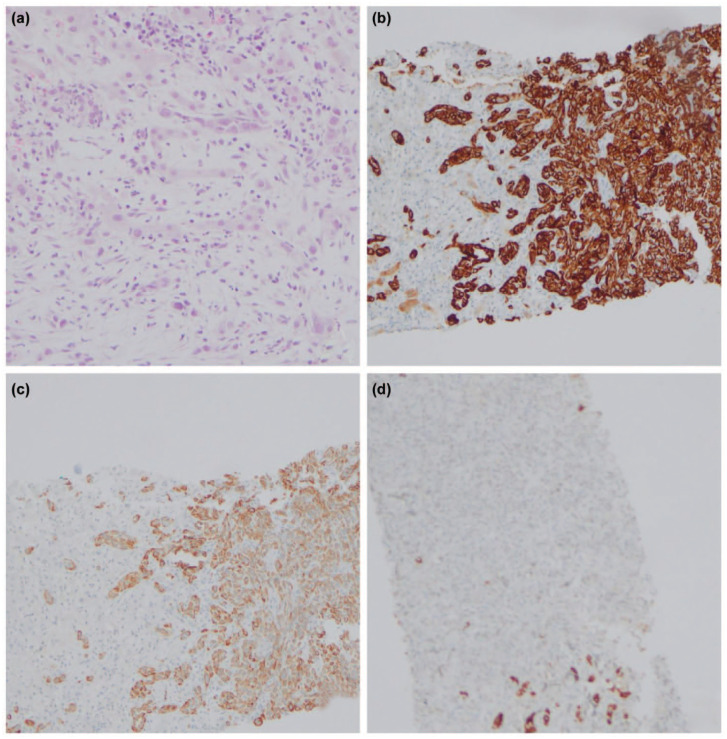
On further evaluation for fever and anorexia, computed tomography (CT) abdomen was performed, which revealed a hypodense segment in the pancreas involving the distal body and tail suspicious of malignancy (Figure 1) but with multiple ring-enhancing lesions in the liver in favour of infectious aetiology rather than malignancy (Figures 2 and 3). The alpha-fetoprotein level was 2.89 IU/ml and CA 19.9 level was 156.6 U/mL. Endoscopic ultrasound-guided biopsy from the abnormal hypodense segment of the pancreas was in favour of adenocarcinoma with a mucinous component. Hence, the CT-guided biopsy from the liver lesion was done and it confirmed metastatic deposits from known pancreatic adenocarcinoma (Figure 4).
Figure 1.
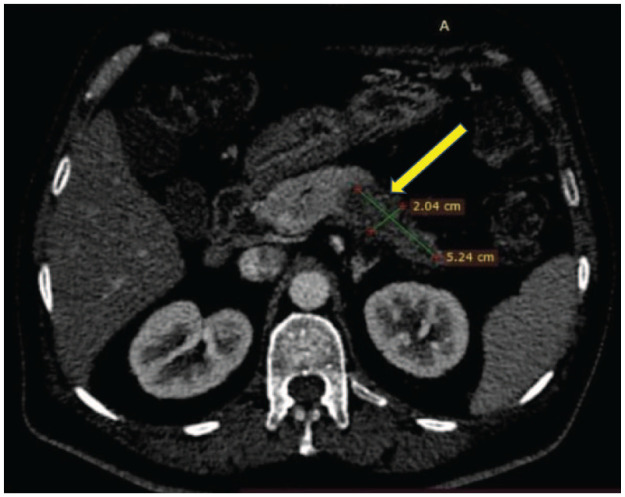
Computed tomography abdomen showing distal pancreatic lesion.
Figure 2.
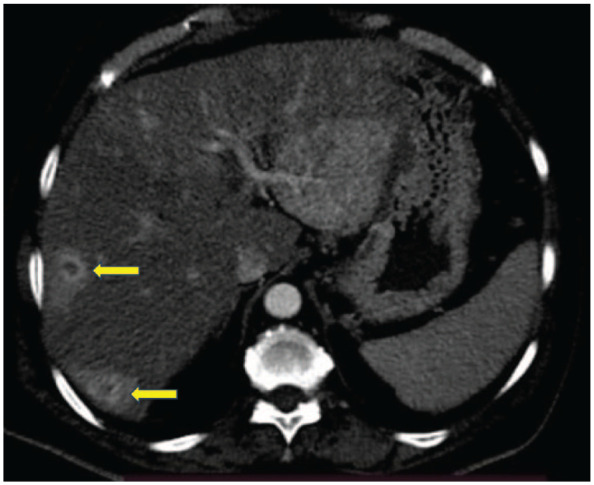
Computed tomography abdomen showing multiple ring-enhancing liver lesions (axial section).
Figure 3.
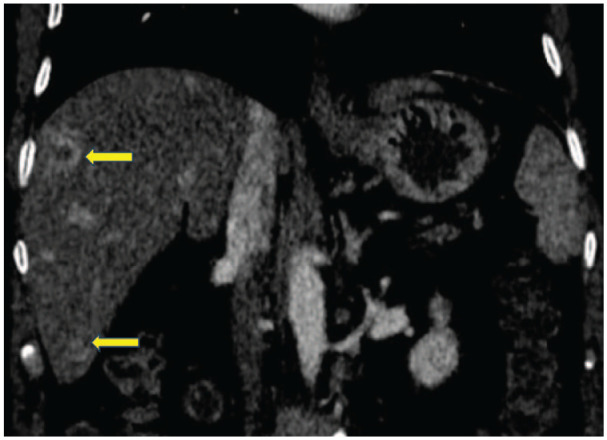
Computed tomography abdomen showing multiple ring-enhancing liver lesions (coronal section).
Figure 4.
Histology of the pancreas and the liver. (a) H&E staining magnification ×400. (b) CK 7 magnification ×200. (c) CK 19 magnification ×200. (d) HepPar 1 magnification ×400.
After confirming the diagnosis with the histology, the patient was directed to further palliative care. Meanwhile, symptomatic management was done to relieve his symptoms. Paracetamol was given as and when needed for fever, for which he responded well. In addition, multivitamin supplementation was done. His routine medications for other comorbidities were continued. Before he turned up for palliative chemotherapy, he succumbed to death.
Discussion
PUO is characterized by a body temperature of ⩾38.3°C on at least two occasions, lasting for ⩾3 weeks or involving multiple febrile episodes during this period. 4 A diagnosis remains elusive despite thorough history-taking and examination, along with comprehensive investigations, including routine blood tests, inflammatory markers, autoimmune screening, cultures, chest radiography and abdominal imaging. 4 Neoplastic fever, characterized by fever caused directly by cancer, is a well-documented phenomenon observed in patients with haematological malignancies, colon cancer, cholangiocarcinoma, hepatocellular carcinoma and renal cell carcinoma. 3 The diagnostic criteria for neoplastic fever are stringent and include temperature over 37.8°C at least once each day, duration of fever over 2 weeks, lack of evidence of infection on physical examination, laboratory examinations and imaging studies, absence of allergic mechanisms and lack of response of fever to an empiric, adequate antibiotic therapy for at least 7 days. 5 In our patient, all criteria were fulfilled except the presence of positive paratyphi serology. However, pyrexia persisted despite adequate course for paratyphi and empirical antibiotics.
This phenomenon is more commonly observed in the metastatic setting, particularly when metastases involve the liver. 6 The underlying mechanism of neoplastic fever is not clearly understood. One proposed explanation suggests that in response to a tumour, cancer cells or host cells may generate specific pyrogenic cytokines like interleukin (IL)-1, IL-6, IL-8, tumour necrosis factor (TNF)-α and interferon. 7 These cytokines have the potential to activate the anterior preoptic nuclei of the hypothalamus, leading to an elevation in the body temperature set point by inducing prostaglandin E2. 7 Additional mechanisms contributing to neoplastic fever involve tumour necrosis, the liberation of TNF and the release of other pyrogens from deceased tissue. 7
Distinguishing between infectious fever and neoplastic fever is crucial, as the timely administration of appropriate antibiotics can significantly impact the outcome of patients with specific infections. 8 Once the diagnosis of neoplastic fever is confirmed, disease-specific treatments such as surgery, radiotherapy or chemotherapy may prove beneficial in alleviating the fever depending on the cancer type. 9 Furthermore, there have been reports indicating the substantial effectiveness of non-steroidal anti-inflammatory drugs in the management of neoplastic fever. These drugs include naproxen, diclofenac, indomethacin, rofecoxib and ibuprofen.3,10
In our patient, the diagnosis of PDAC was not suspected initially due to the positivity of paratyphoid serology. Although his inflammatory markers were elevated, serology, culture and imaging for suspected viral and bacterial infections were negative. Serology for autoimmune conditions was negative except for ANA which can be positive in 5% of the general population at 1:80 dilution. In elderly patients experiencing a decline in general health, the possibility of digestive cancers should be considered. While chronic fever is not a primary factor in this diagnostic process, the combination of advanced age and deteriorating health warrants investigation into potential neoplastic causes of digestive origin, typically through imaging studies such as thoraco-abdominal pelvic CT scans. Chronic fever of unknown origin is rare as a symptom in the clinical presentation of digestive cancers. There are several learning points from the reported patient. The patient had unusual ring-enhancing liver lesions which favoured liver abscesses rather than metastases. Therefore, a biopsy of the lesion was crucial for the diagnosis and decision-making regarding the management of PDAC. In this patient, investigation into potential neoplastic causes of digestive origin should have been considered earlier once infective causes are excluded or empirically treated. Unfortunately, owing to the histologically proven liver metastases, a curative resection was not feasible and the patient was referred to the oncologist for palliative chemotherapy. Our patient’s fever responded well to oral paracetamol; however, the fever recurred following the withdrawal of the drug.
Conclusion
We describe a rare case of metastatic pancreatic adenocarcinoma presenting as a fever of unknown origin. Liver metastases may present atypically as in this patient with evidence of ring enhancement in the CT imaging; thus, the biopsy is mandatory for the diagnosis and decision-making. If the tumour is deemed resectable, surgical intervention emerges as a secure and potentially curative treatment for both the fever and the underlying carcinoma. However, in the context of metastatic PDAC, palliative chemotherapy and pharmacological management of fever are required.
Acknowledgments
All the staff members who were involved in the management of this patient.
Footnotes
Author contributions: D.S. designed the case. C.N. and U.J. wrote the case. H.W. contributed to histopathological diagnosis. D.S. and N.F. were involved in the patient management and critical analysis of the paper.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: We have obtained written informed consent from the legally authorized representative of the deceased patient for the publication of this case report.
Research registration: Not applicable.
ORCID iDs: Umesh Jayarajah  https://orcid.org/0000-0002-0398-5197
https://orcid.org/0000-0002-0398-5197
Duminda Subasinghe  https://orcid.org/0000-0002-7342-0220
https://orcid.org/0000-0002-7342-0220
References
- 1. DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. Gastroenterology 1999; 117(6): 1464–1484. [DOI] [PubMed] [Google Scholar]
- 2. Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005; 7(5): 189–197. [DOI] [PubMed] [Google Scholar]
- 3. Shi N, Xing C, Chang X, et al. Pancreatic carcinoma masked as fever of unknown origin: a case report and comprehensive review of literature. Medicine (Baltimore) 2016; 95(35): e4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mulders-Manders C, Simon A, Bleeker-Rovers C. Fever of unknown origin. Clin Med (London, England) 2015; 15(3): 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foggo V, Cavenagh J. Malignant causes of fever of unknown origin. Clin Med (Lond) 2015; 15(3): 292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cvitkovic E, Bachouchi M, Boussen H, et al. Leukemoid reaction, bone marrow invasion, fever of unknown origin, and metastatic pattern in the natural history of advanced undifferentiated carcinoma of nasopharyngeal type: a review of 255 consecutive cases. J Clin Oncol 1993; 11(12): 2434–2442. [DOI] [PubMed] [Google Scholar]
- 7. Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer 2001; 92(6 Suppl): 1684–1688. [DOI] [PubMed] [Google Scholar]
- 8. Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002; 34(6): 730–751. [DOI] [PubMed] [Google Scholar]
- 9. Zee YK, Soo RA. Non-small cell lung cancer presenting with neoplastic fever at diagnosis and relapse. Int J Infect Dis 2010; 14(6): e518–e521. [DOI] [PubMed] [Google Scholar]
- 10. Kathula SK, Shah K, Polenakovik H, et al. Cyclo-oxygenase II inhibitors in the treatment of neoplastic fever. Support Care Cancer 2003; 11(4): 258–259. [DOI] [PubMed] [Google Scholar]