Abstract
Objective
Dysphagia is common in individuals who have undergone posterior fossa tumor (PFT) resection and negatively impacts on the individual’s quality of life, nutritional status, and overall health. We aimed to quantitatively synthesize data from studies of the prevalence of dysphagia following PFT resection.
Methods
PubMed, Web of Science, the Cochrane Library, Embase, China National Knowledge Infrastructure (CNKI), Wanfang database, and VIP database were searched for case-control and cross-sectional studies that evaluated the prevalence of dysphagia after PFT surgery. Meta-analyses were performed to determine the prevalence of dysphagia. Subgroup and meta-regression analyses were performed to determine the sources of heterogeneity among the studies.
Results
A total of 22 studies were included, involving 20,921 cases. A meta-analysis of the random-effects model showed that the pooled global prevalence of dysphagia following PFT resection was 21.7% (95% confidence interval: 16.9–26.6). The subgroup and meta-regression analyses demonstrated that participant age (P < 0.001), assessment methods (P = 0.004), and geographical region of the study participants (P = 0.001) were sources of heterogeneity among the studies.
Conclusions
Dysphagia has a high prevalence following PFT resection. Individuals with PFTs who are at a high risk for dysphagia should be identified early through screening. Multidisciplinary diagnosis and treatment of dysphagia are required to improve the outcomes in the early stages after PFT resection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-12656-1.
Keywords: Dysphagia, Posterior fossa tumor, Meta-analysis, Systematic review
Introduction
The posterior fossa is situated below the cerebellar tentorium and above the foramen magnum, which encompasses the fourth ventricle and cerebellum [1]. Although posterior fossa tumors (PFTs) are less commonly observed in adults [2], they are the most common solid tumors in the pediatric population, occurring at an incidence rate of 2–3.5 per 100,000 individuals [3, 4]. Microsurgery remains the preferred treatment for PFTs, particularly in cases involving malignancy, facial nerve dysfunction, hearing deterioration, and obstructive hydrocephalus [5].
The posterior fossa contain complex and vital structures [6], such as the cerebellum and brainstem, which play critical roles in the precise and efficient execution of speech and swallowing movements [7]. Surgical interventions for PFTs can lead to various serious adverse events, including posterior fossa syndrome/cerebellar mutism syndrome, muscle weakness, sensory changes, imbalance, cognitive dysfunction, and dysphagia [8]. Dysphagia, defined as impairment of the swallowing process, can occur if there is a malfunction with any part of the swallowing mechanism [9]. The two most common causes of dysphagia are (1) neurologic or anatomic injury of the cerebral cortex, brainstem, or cranial nerves (IX–XII) related to swallowing and (2) direct injury to the muscles of swallowing [9]. Anatomically adjacent to the site of PFTs, crucial structures such as brainstem, cerebellar tissue, or cranial nerves are prone to injury as a result of surgical removal, resulting in secondary focal neurological deficits [10]. In addition, dysphagia is also seen in cerebellar mutism or posterior fossa syndrome, which is a syndrome of clinical features after posterior fossa surgery characterized by mutism, emotional lability, hypotonia, and ataxia [11, 12]. In a study of 27 children with a PFT by Mei and colleagues, the incidence of postsurgical dysphagia was lower compared with a previous report that included only children with mutism after resection of PFTs [7, 13]. A possible reason for the discrepancy between these two studies relates to the presence of cerebellar mutism syndrome. Therefore, dysphagia is one of the commonly reported risks of surgical PFTs resection.
In patients with PFTs, dysphagia requires special attention as it can result in aspiration-induced lung diseases or malnutrition [14–16], leading to prolonged hospital stays and even mortality [9, 17]. The estimated annual cost of dysphagia in the US is $4–7 billion [18]. Furthermore, dysphagia has a profound negative impact on the emotional and mental well-being of patients, significantly affecting their quality of life [19].
Given the significant improvements in survival rates of PFTs resection in recent years, therapeutic strategies for the management of postoperative dysphagia and enhancement of the functional well-being have received increasing attention. Consequently, numerous studies have investigated the prevalence of dysphagia following PFT removal. However, no detailed and systematic meta-analysis has been conducted on the prevalence of dysphagia following PFT removal. While several studies have focused on patients with PFTs and reported the frequencies of dysphagia ranging from 33% to approximately 70% [1, 7, 11, 20], Ward et al. conducted a retrospective cross-sectional study involving 17,281 patients who underwent surgery for vestibular schwannoma, reporting a prevalence of dysphagia of 2.6% [21]. Studies of dysphagia following PFT removal had similar limitations, including small sample sizes and collection of data from a single healthcare setting. Consequently, there is limited information available on the global prevalence of dysphagia following PFT removal. Therefore, the aim of this study was to evaluate the prevalence of dysphagia following PFT removal, which could provide researchers and clinicians with the most recent information for the early detection, effective prevention, and adequate monitoring of postoperative dysphagia.
Methods
Protocol and registration
The review was prospectively registered in the PROSPERO database (registration no.: CRD42023441428).
Literature search
A comprehensive literature search was conducted using various databases, including PubMed, Web of Science, The Cochrane Library, Embase, China National Knowledge Infrastructure, Wanfang database, and VIP database. Articles published from database inception until March 31, 2023 were searched. The search strategy involved a combination of MeSH (Medical Subject Headings) and free-text terms. The key concepts used in the search were “dysphagia,” “posterior fossa tumors,” and “prevalence.” For each database, a tailored search query was developed to account for its unique characteristics. Supplementary material S1 provide a summary of the search strategy. In addition, manual searches of the reference lists of the retrieved articles were performed to identify any additional published studies relevant to the topic.
Inclusion and exclusion criteria
The review included observational studies, including case-control, cross-sectional, and longitudinal cohort studies. Studies that evaluated postoperative dysphagia in children and/or adult patients diagnosed with a PFT were included. We excluded reviews, meta-analyses, comments, meeting summaries, letters, duplicate literature, articles in languages other than English and Chinese, animal or cell studies, studies with incomplete information or missing data that could not be obtained through other sources, and studies focusing on patients who underwent posterior fossa surgery other than PFT resection.
Study selection and data extraction
Two investigators independently extracted the relevant information from articles that fulfilled the eligibility criteria and entered it into Excel worksheets. Disagreements between the investigators were resolved through consensus or discussion with a third investigator. The investigators extracted the basic information related to the study (name of the first author, publication year, region of the study population, and age range) and the main study characteristics (study design, total sample size, number of patients with dysphagia, assessment method of dysphagia, tumor site, and tumor types).
Quality assessment
Two investigators independently assessed the quality of the included studies using the Joanna Briggs Institute critical appraisal checklist (Supplementary Material S2). Disagreements in the quality assessments between the investigators were resolved through consensus. The scale includes nine items, with each item scored 0 (not qualified), 1 (mentioned but not described in detail), or 2 (detailed, comprehensive, and correct description). The sum of the domain scores (the highest possible score was 18), along with the percentage of the highest score, was computed for each study. A score > 70% of the maximum possible score denoted a high quality of the study.
Statistical analysis
Stata software (version 15.0; Stata Corp., College Station, TX, USA) was used to perform the meta-analyses, while the Review Manager software (RevMan 5.3; Cochrane Collaboration, Oxford, United Kingdom) was used to estimate the risk of bias. The primary outcome was the prevalence of dysphagia, recorded using basic descriptors (effect size [95% confidence interval]). Heterogeneity was evaluated based on the I2 values; I2 values > 75% indicated high heterogeneity among studies. The heterogeneity P-values were used to evaluate the potential heterogeneity among studies, with the level of significance set at 0.10. In cases of I2 ≤ 50%, which indicated low heterogeneity among studies, a fixed-effects model was used for analysis; otherwise, a random-effects model was used. If heterogeneity existed, subgroup analyses of the included studies were conducted according to the study design, publication year, age, assessment method of dysphagia, tumor sites, tumor types, and geographical region of the study population (such as North America, Europe, and East Asia). Moreover, we conducted univariate meta-regression to further investigate the cause of the heterogeneity.
Results
Search results
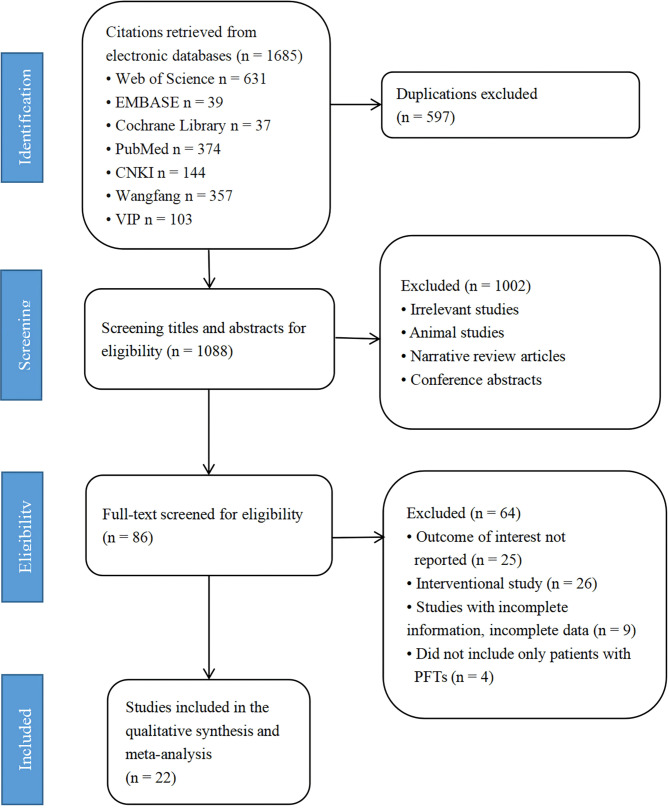
The initial search identified 1,646 articles, of which 22 fulfilled the eligibility criteria and were included in the quantitative analysis. A flow chart of article selection, along with the reasons of exclusion of the articles, is presented in Fig. 1.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses recommendations flowchart of search and selection of studies
Study characteristics
A total of 17 cross-sectional studies and 5 case-control studies were included in the analysis. All studies enrolled participants from clinical settings, except for the two epidemiological studies that analyzed population-level data [5, 21]. The sample size in these studies ranged from 11 to 17,281 participants. Of the 22 articles, 10 were published in Chinese and 12 in English. Seven studies [4, 7, 8, 10, 11, 22, 23] used instrumental and objective assessments, including video fluoroscopic swallowing study (VFSS) or fiberoptic endoscopic evaluation of swallowing (FEES). Among the remaining 15 studies, 8 [5, 21, 24–29] did not specify the assessment method, whereas the remaining 7 [1, 30–35] used screening tools, such as the water swallowing test (WST), repetitive saliva swallowing test (RSST), standardized swallowing assessment (SSA), clinical assessment of paediatric neurogenic dysphagia (CAPND), the dysphagia subscale of the Chinese scale of clinical neurologic deficit of stroke patients, and the dysphagia subscale of cerebellar mutism syndrome scale to detect dysphagia. Among the aforementioned screening tools, WST and SSA were the most commonly used. Table 1 summarizes the characteristics of the 22 studies.
Table 1.
Characteristics of the included studies
| First author [ref.] |
Year | Study design | Country | Sample Size, No. (M/F) | Age, ya | Number of patients with dysphagia | Tumor site (n) |
Tumor type (n) |
Assessment method for dysphagia | Methodological quality |
|---|---|---|---|---|---|---|---|---|---|---|
|
Wright [22] |
2023 | Cross-sectional | UK | 70 | 4.3 (0.8–19.8)b | 11 | 4th ventricle (3/23)d; Cerebellar hemisphere (0/19)d; Brain stem (2/15)d; CPA (6/13)d |
Medulloblastoma (3/14)e; Ependymoma (4/12)e; Embryonal tumour with multilayer rosettes (0/2)e; Glioma (3/33)e; Atypical teratoid rhabdoid tumour, (0/1)e; Other (0/8)e |
VFSS | High |
|
Li [30] |
2022 | Case-control | China | 228 (Anterior cranial fossa 104; Middle cranial fossa 66; Posterior fossa 58) | - | Anterior cranial fossa 3; Middle cranial fossa 7; Posterior fossa 14 |
Posterior fossa (58) |
Meningioma (84); Metastatic tumor (55); Glioma (49); Other (40) | WST | High |
|
Nasrollahi [24] |
2022 | Cross-sectional | USA | 798 (409/389) | - | 130 | Unclear |
Acoustic neuroma (798) |
- | High |
|
Li [21] |
2021 | Case-control | China | 98 (Anterior cranial fossa 32; Middle cranial fossa 38; Posterior fossa 28) |
No dysphagia: 55.14 ± 11.47 Dysphagia: 52.63 ± 17.90 |
Anterior cranial fossa 0; Middle cranial fossa 5; Posterior fossa 3 |
Posterior fossa (28) |
Meningioma (42); Metastatic tumor (28); Glioma (14); Other (14) | WST | High |
|
Zhang [32] |
2021 | Case-control | China | 147 (Posterior fossa 39; Non posterior fossa 108) |
No dysphagia: 52 (45–63)c Dysphagia: 59 (50–65)c |
Posterior fossa 18; Non posterior fossa 13 |
Posterior fossa (39) |
Unclear | SSA | High |
|
Alkins [5] |
2021 | Cross-sectional | Canada | 1456 (657/799) | 50.3 ± 15.3 | 285 | Unclear |
Vestibular schwannoma (1456) |
- | High |
|
Ricci [33] |
2021 | Cross-sectional | Italy | 30 (19/11) | 8 (3–10)c | 9 | Left cerebellar hemisphere (9); Right cerebellar hemisphere (6); Midline (vermis/4th ventricle, 15) |
Medulloblastoma (6); Pilocytic astrocytoma (15); Ependymoma (3); Difused glioma (3); Cavernoma (1); Papilloma (1); Ganglioglioma (1) |
Cerebellar mutism syndrome scale | Moderate |
|
Yang [34] |
2020 | Case-control | China | 91 (61/30) | - | 32 | Cerebellar midline (61); Cerebellar hemisphere (30) | Medulloblastoma (45); Pilocytic astrocytoma (22); Ependymoma (10); Glioma (6); Other (8) | Dysphagia subscale of Chinese scale of the clinical neurologic deficit of stroke patients | High |
|
Goethe [4] |
2020 | Case-control | USA | 197 (112/85) |
No dysphagia: 7.2 ± 4.6 Dysphagia: 4.5 ± 3.4 |
43 | Cerebellum (8/82)d; 4th ventricle (25/96)d; Brainstem (6/13)d; CPA (4/9)d | Juvenile pilocytic astrocytoma (7/69)e; Medulloblastoma (13/66)e; Ependymoma (12/33)e; Atypical teratoid/rhabdoid tumor (4/10)e; Other (5/19)e | VFSS | High |
|
Lapa [10] |
2020 | Cross-sectional | Germany | 26 (13/13) | 49 ± 14 | 15 | Intraaxial paramedian (2); Petroclival (3); Tentorium (4); Cerebellum (1); Petrous bone and Foramen magnum (2); CN VIII (2); Median intraaxial cystic (2); CN IX u. X (1); Cerebellumintraaxial (2); CPA (5); Clivus (1); Intrameatal (1) | Meningioma (9); Vestibular schwannoma (6); Metastases (5); other (6) | WST and FEES | High |
|
Wang [35] |
2018 | Cross-sectional | China | 41 (15/26) | 49.6 ± 12.9 | 25 |
Cerebellum and 4th ventricle (20); CPA (20); Petroclival (1) |
Unclear | RSST and WST and SSA | High |
|
Chen [25] |
2017 | Cross-sectional | China | 106 (59/47) | 33.6 (9–57)b | 1 | 4th ventricle (81); Brainstem (25) | Medulloblastoma (53); Pilocytic astrocytoma (3); Ependymoma (23); Cerebral cavernous angiomas (18); Choroid plexus papilloma (2); other (7) | - | Moderate |
|
Lee [8] |
2016 | Cross-sectional | Korea | 183 (108/75) | - | 39 | Unclear | Medulloblastoma (71); Pilocytic astrocytoma (49); Ependymoma (26); Atypical teratoid rhabdoid tumor (12); Vestibular schwannoma (6); Choroid plexus papilloma (5); Other (14) | VFSS | High |
|
Zhang [26] |
2012 | Cross-sectional | China | 31 (17/14) | 26.5 (14–62)b | 1 | 4th ventricle (31) | Medulloblastoma (6); Pilocytic astrocytoma (4); Ependymoma (12); Angioblastoma (5); Choroid plexus papilloma (2); Other (2) | - | Moderate |
|
Wu [27] |
2012 | Cross-sectional | China | 115 (79/36) | 13.5 (1–34)b | 11 | 4th ventricle (94); Cerebellum hemisphere (19); CPA (2) | Medulloblastoma (115) | - | Moderate |
|
Ward [21] |
2012 | Cross-sectional | USA | 17,281 | 50.4 (18–97)b | 443 | Unclear | Vestibular schwannoma (17,281) | - | High |
|
Mei [7] |
2011 | Cross-sectional | Australia | 27 (18/9) | 6.7 (2.0–13.8)b | 9 |
4th ventricle (26); Brainstem (22); Cerebellar midline (12); Cerebellum left hemisphere (8); Cerebellum right hemisphere (3) |
Medulloblastoma (11); Pilocytic astrocytoma (9); Ependymoma (4); Ganglioglioma (1); Germinoma (1); Atypical teratoid/rhabdoid tumour (1) | VFSS | High |
|
Starmer [23] |
2011 | Cross-sectional | USA | 181 (76/105) | 49 (20–81)b | 57 | CPA (181) | Acoustic neuromas (168); Other (Meningioma, Hamartoma, Rhabdomyoma, and Epidermoid, 13) | VFSS | High |
|
Chen [29] |
2010 | Cross-sectional | China | 44 (24/20) | 28.3 (2–56)b | 3 | 4th ventricle (44) | Medulloblastoma (9); Pilocytic astrocytoma (2); Ependymoma (25); Angioblastoma (3); Choroid plexus papilloma (2); Other (3) | - | Moderate |
|
Deng [28] |
2009 | Cross-sectional | China | 84 (48/36) | 5.4 (3–7)b | 6 | 4th ventricle (84) | Glioma (84) | - | High |
|
Morgan [1] |
2008 | Cross-sectional | UK | 11 (9/2) | 8.0 ± 2.9 | 8 |
4th ventricle, Brainstem (1); 4th ventricle, Cerebellum (6); Cerebellum (4) |
Pilocytic astrocytoma (4); Medulloblastoma (6); Choroids plexus papillpma (1) | Clinical assessment of paediatric neurogenic dysphagia | High |
|
Newman [11] |
2006 | Cross-sectional | USA | 24 (15/9) | 5.9 ± 5.3 | 7 | CPA (7); 4th ventricle (4); Brain stem (5); Midbrain (1); Cerebellum (1); Unclear (6) | Ependymoma (12); Malignant fibrous histiocytoma (1); Glioma (2); Astrocytoma (3); Medulloblastoma (2); Unclear (4) | VFSS | High |
M = male; F = female; UK = United Kingdom; USA = United States; CPA = cerebellopontine angle; CN = cranial nerve; VFSS = video fluoroscopic swallowing study; WST = water swallowing test; SSA = standard swallowing assessment; FEES = fiberoptic endoscopic evaluation of swallowing; RSST = Repeat saliva swallowing test.
aValues are expressed as mean ± SD, unless indicated otherwise.
bMedian (range).
cMedian (interquartile range).
d(Number of patients with dysphagia after surgery at a specific tumor site/Number of patients at a specific tumor site).
e(Number of patients with dysphagia after surgery for a specific tumor type/Number of patients with a specific tumor type).
Risk of bias
The mean total quality score was 13.41 ± 1.53, with 17 (77.3%) studies exhibiting a high quality and the remaining 5 (22.7%) demonstrating a moderate risk of bias. Furthermore, 17 (77.3%) of the 22 studies recruited participants from a single healthcare center, resulting in a high risk of bias related to the sample timeframe. Most studies had unclear risk of bias on the sample size or sample coverage. Figure 2 provides a summary of the risk of bias assessment for the included studies.
Fig. 2.
Risk of bias. A: Bar chart demonstrating the authors’ judgements on each methodological quality item presented as percentages for all included studies. B: Summary of authors’ judgements on each risk of bias item for all included studies (n = 22)
Prevalence of dysphagia following PFT resection
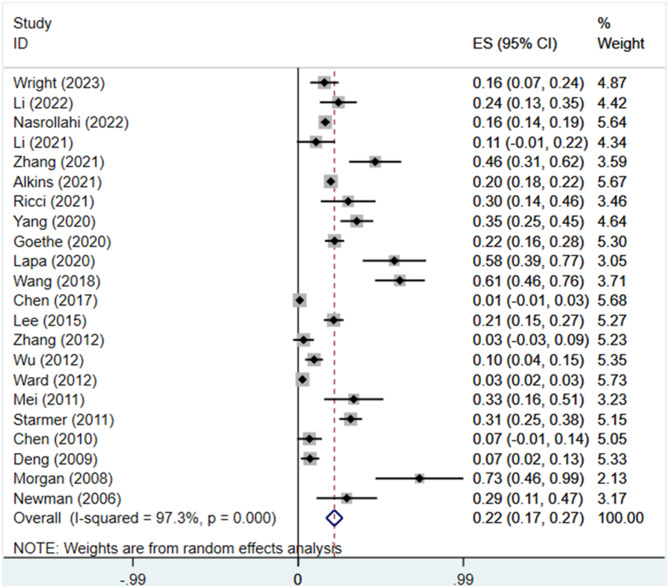
In total, the 22 studies included 20,921 participants who had undergone PFT resection. The prevalence rate of postoperative dysphagia showed a wide range (0.9–72.7%) among the included studies. The random-effects meta-analysis resulted in a summary estimate of 21.7% (95% CI: 16.9–26.6) (Fig. 3). There was significant heterogeneity among the included studies (I2 = 97.3%; P < 0.001).
Fig. 3.
The forest plot of the overall estimation of the prevalence of dysphagial following posterior fossa tumor resection based on the random efects model. ES, effect size; CI, confidence interval
Subgroup and meta-regression analyses
The subgroup analyses were conducted according to the study design, publication year, age, assessment method of dysphagia, and the geographical region of the study population. Table 2 presents the summary of subgroup analyses and heterogeneity assessments. The subgroup analyses demonstrated high heterogeneity among the included studies. Accordingly, meta-regression analyses were performed to explore the sources of the heterogeneity. As shown in Table 2, the meta-regression analyses showed that the age of the participants (P < 0.001), assessment methods for dysphagia (P = 0.004), and geographical region of the study population (P = 0.001) were significantly associated with heterogeneity among the included studies.
Table 2.
Subgroup and meta-regression analyses of the prevalence of dysphagia following PFT resection
| Subgroup | Heterogeneity test | Effects model | Pooled prevalence rate % (95% CI) |
Coefficient (95% CI) | SE | P > | t | | |
|---|---|---|---|---|---|---|---|
| I2 | P | ||||||
| Study design | 0.177 (-0.086–0.440) | 0.126 | 0.175 | ||||
| Cross-sectional (n = 17) | 97.5% | < 0.001 | Random effects | 20.0 (14.7–25.2) | |||
| Case–control (n = 5) | 78.1% | 0.001 | Random effects | 26.7 (17.1–36.3) | |||
| Year of publication | 0.114 (-0.170–0.398) | 0.136 | 0.412 | ||||
| 2006–2013 (n = 9) | 93.7% | < 0.001 | Random effects | 16.8 (9.0–24.5) | |||
| 2014–2023 (n = 13) | 96.4% | < 0.001 | Random effects | 25.7 (18.6–32.7) | |||
| Sample size | 0.106 (-0.103–0.314) | 0.100 | 0.302 | ||||
| 10–99 (n = 14) | 90.9% | < 0.001 | Random effects | 28.8 (19.3–38.4) | 0.183 (-0.051–0.416) | 0.111 | 0.118 |
| 100–999 (n = 5) | 97.8% | < 0.001 | Random effects | 18.1 (7.2–29.0) | 0.077 (-0.188–0.341) | 0.126 | 0.551 |
| > 1000 (n = 3) | 99.3% | < 0.001 | Random effects | 10.6 (-2.4–23.5) | Reference | ||
| Age, y | 0.272 (0.151–0.392) | 0.058 | < 0.001 | ||||
| < 18 (n = 9) | 84.5% | < 0.001 | Random effects | 25.9 (17.8–33.9) | Reference | ||
| ≥ 18 (n = 9) | 98.6% | < 0.001 | Random effects | 27.6 (18.4–36.8) | 0.009 (-0.159–0.178) | 0.080 | 0.908 |
| Uncategorized (n = 4) | 71.7% | 0.014 | Random effects | 4.7 (0.1–9.2) | -0.221 (-0.428 – -0.013) | 0.099 | 0.038 |
| Assessment method | 0.329 (0.117–0.541) | 0.101 | 0.004 | ||||
| Simple symptom screening (n = 2) | 0.0% | 0.596 | Random effects | 33.8 (25.4–42.2) | Reference | ||
| Bedside assessments (n = 5) | 90.5% | < 0.001 | Random effects | 41.4 (20.3–62.4) | 0.060 (-0.194–0.314) | 0.138 | 0.628 |
| Instrumental and objective assessments (n = 7) | 74.0% | 0.001 | Random effects | 27.2 (20.2–34.1) | -0.046 (-0.285–0.193) | 0.114 | 0.689 |
| Undeclared (n = 8) | 98.2% | < 0.001 | Random effects | 8.4 (2.6–14.1) | -0.246 (-0.477– -0.015) | 0.110 | 0.038 |
| Region | 0.408 (0.202–0.614) | 0.098 | 0.001 | ||||
| North America (n = 6) | 99.0% | < 0.001 | Random effects | 19.4 (9.4–29.4) | -0.212 (-0.447–0.023) | 0.112 | 0.074 |
| East Asia (n = 11) | 94.4% | < 0.001 | Random effects | 19.0 (11.0–26.9) | -0.211 (-0.467–0.045) | 0.122 | 0.100 |
| Europe (n = 4) | 89.5% | < 0.001 | Random effects | 42.2 (17.3–67.0) | Reference | ||
| Australia (n = 1) | - | - | - | 33.3 (15.6–51.1) | -0.075 (-0.535–0.386) | 0.219 | 0.738 |
CI = confidence interval; SE = standard error.
Given that two studies showed the incidence of postoperative dysphagia for each tumor site or tumor type, while other studies either only focused on a tumor site (e.g., CPA, fourth ventricle) or a tumor type (e.g., medulloblastoma, acoustic neuroma), or did not clarify the specific site and type of PFTs, it was difficult to conduct subgroup analysis based on tumor site or tumor type among all included studies. Therefore, we excluded studies that did not clarify tumor sites or types, reorganized the data, and then conducted subgroup analysis according to tumor sites and tumor types (Table 3). Figure 4 provides the forest plots of the pooled prevalences of dysphagial following PFT resection based on different tumor sites and types.
Table 3.
Subgroup analyses of the prevalence of dysphagia following PFT resection according to tumor site and tumor type
| Subgroup | Heterogeneity test | Effects model | Pooled prevalence rate % (95% CI) |
|
|---|---|---|---|---|
| I2 | P | |||
| Tumor site | ||||
| CPA (n = 6 ) | 98.9% | < 0.001 | Random effects | 21.8 (11.0–32.7) |
| 4th ventricle (n = 5 ) | 78.8% | 0.001 | Random effects | 10.7 (3.2–18.1) |
| Cerebellum (n = 3) | 96.1% | < 0.001 | Random effects | 25.0 (–5.3–55.2) |
| Brainstem (n = 2) | 75.1% | 0.045 | Random effects | 28.0 (–4.0–60.0) |
| Tumor type | ||||
| Medulloblastoma (n = 3) | 49.6% | 0.138 | Random effects | 14.6 (6.4–22.8) |
| Ependymoma (n = 2) | 0.0% | 0.850 | Random effects | 35.5 (21.6–49.5) |
| Vestibular schwannoma/Acoustic neuroma (n = 3) | 99.5% | < 0.001 | Random effects | 12.8 (0.1–25.4) |
| Glioma (n = 3) | 0.0% | 0.799 | Random effects | 8.4 (4.4–12.4) |
CPA = cerebellopontine angle; CI = confidence interval; SE = standard error.
Fig. 4.
The forest plot of the overall estimation of the prevalence of dysphagia following PFT resection based on different tumor sites (A) and types (B). CPA = cerebellopontine angle; ES, effect size; CI, confidence interval
Discussion
This review quantitatively synthesized the current evidence on the prevalence of dysphagia following PFT resection. The analysis included 22 studies with 20,921 participants, and found a pooled global prevalence of 21.7%, which is slightly higher than the estimated prevalence rate of approximately 20% in the general population reported in a national health interview survey [36]. Rajat et al. [37]. reported the global prevalence of oropharyngeal dysphagia in different populations to be 43.8%. Our findings are not consistent with those of previous studies, which can be attributed to the inclusion of a large number of stroke and dementia patients in the systematic review and meta-analysis conducted by Rajat et al., while the present study focused on patients who had undergone PFT resection.
Due to the deep anatomical position and small space of the posterior cranial fossa, the posterior cranial nerves (IX–XII) located adjacent to the PFT are vulnerable to injury and traction during the prolonged duration of tumor resection, resulting in hypoesthesia, loss of function of the glossopharyngeal nerve, and atrophy of the lingual muscles [35]. Moreover, postoperative brain edema may compress the aforementioned nerves and lead to dysphagia [38]. In addition, the use of tracheal intubation during the perioperative period can also affect the occurrence of dysphagia. Studies have shown that post-extubation dysphagia is common in patients admitted to the intensive care unit [17]. Our study found that patients who have undergone PFT removal are at a high risk of postoperative dysphagia. Consequently, clinicians should pay attention to dysphagia in these patients, as aspiration pneumonia caused by dysphagia is one of the leading causes of death [9]. The other complications of dysphagia include bronchospasm, chronic malnutrition and weight loss, muscle wasting, and dehydration [9]. Dysphagia is a primary reason for delayed hospital discharge and increased need for healthcare services [18, 19, 39–41]. Therefore, it is essential to identify dysphagia early in patients who have undergone PFT removal.
Dysphagia, a symptom of swallowing impairment, can occur in cases of swallowing dysfunction [9]. The lack of uniform definitions of dysphagia and significant methodological variability among the included studies made it difficult to perform synthesis of the prevalence data. In the present review, eight different definitions of dysphagia were used by the included studies. Based on the assessment methods for dysphagia, we divided the studies into four subgroups: simple symptom screening, bedside assessments (WST, RSST, SSA, and CAPND), instrumental and objective assessments (VFSS and FEES), and undeclared methods. The prevalence of dysphagia was 33.8% (95% CI: 25.4–42.2), 41.4% (95% CI: 20.3–62.4), 27.2% (95% CI: 20.2–34.1), and 8.4% (95% CI: 2.6–14.1) in these subgroups, respectively. In the simple symptom screening studies, patients were evaluated for dysphagia based on the presence or absence of symptoms related to difficulty in eating or drinking water, which is a convenient and suitable method for the rapid identification of the risk of dysphagia in clinical practice. The bedside clinical swallowing examination is conducted by trained healthcare professionals, typically speech-language pathologists [9]. This test assesses the swallowing with foods and liquids of different consistencies, and provides additional information about the patient’s cognition, phonation, cranial nerve function involved in swallowing, speech intelligibility, and cough strength to evaluate the patient’s ability to handle secretions [9, 42]. However, bedside assessment is inappropriate to monitor for silent aspiration or motility issues; for these reasons, instrumental and objective assessments may be performed. VFSS and FEES enable the visualization of the swallowing mechanism and provide detailed information on the swallowing function [9]. They involve the use of specialized equipment, which require the presence of an imaging specialist and a speech-language pathologist. However, these assessment methods are invasive and may damage the body. Therefore, VFSS and FEES should not be used as initial diagnostic tools if a structural cause of dysphagia is suspected and there is no concern for aspiration. In the present study, the meta-regression analyses showed that the difference in the assessment methods for dysphagia was one possible cause of heterogeneity among the studies (P = 0.004). The results indicate the importance of selecting an appropriate assessment method for dysphagia following PFT resection, suggesting that clinicians should conduct instrumental and objective assessments of swallowing in a timely manner, where appropriate, in addition to the bedside assessment [9].
The prevalence of dysphagia varies with age. Rajati et al. reported that the prevalence of oropharyngeal dysphagia increased with increasing age [37]. Wilkins et al. found that patients with dysphagia in primary care were more likely to be older than those without dysphagia (mean ages of 48.1 and 45.7 years in patients with and without dysphagia, respectively; P = 0.001) [43]. Other studies have identified a prevalence of dysphagia of 40–50% among older individuals residing in long-term care facilities [44]. A previous survey [36] showed that a significant proportion of respondents identified “advancing age” as an etiology for their swallowing problems. This may be because of the deteriorating swallowing reflex and body function of older individuals, which leads to the loss of muscle strength and nerve functions that control eating, thereby predisposing to dysphagia [30]. Although the aforementioned studies have focused on the evaluation and management of dysphagia in adults, the prevalence of dysphagia in the pediatric population also requires attention, as dysphagia is a common pediatric disease, although it is not as common in children as in the older population [9]. This review found that the estimated pooled prevalence of dysphagia was 25.9% (95% CI: 17.8–33.9) in pediatric patients and 27.6% (95% CI: 18.4–36.8) in adults. These results suggest that age was a source of heterogeneity among the included studies (P < 0.001). The current data from adult patients were not restricted to older patients, which may explain the lack of significant difference in the prevalence of dysphagia between children and adults. Dysphagia in the pediatric population also requires early detection and treatment to prevent malnutrition and delayed developmental milestones, which may negatively impact the patient health.
Considering the differences between adults and children with the respect to the tumor types and surgical strategies of PFTs, we conducted subgroup analyses of tumor site and tumor type separately. Among all the structures involved in the included studies, the highest prevalence of dysphagia after PFTs resection was in the brainstem (28.0%, 95% CI: − 4.0–60.0), followed by the cerebellum (25.0%, 95% CI: − 5.3–55.2), CPA (21.8%, 95% CI: 11.0–32.7), and the fourth ventricle (10.7%, 95% CI: 3.2–18.1). Tumors in the brainstem may compromise the lower cranial nerves, affecting pharyngeal function and swallowing ability [7]. Goethe and colleague reported that patients who developed dysphagia post-operatively were more likely to have brainstem involvement [4]. PFTs resection in brainstem is more challenging, leading to a risk for injury to key structures and has been associated with postoperative dysphagia. In addition to site, tumor type is also one of the factors contributing to postoperative dysphagia. The results of subgroup analysis showed that the pooled prevalence of postoperative dysphagia in ependymoma, medulloblastoma, vestibular schwannoma/acoustic neuroma, and glioma subgroup was 35.5% (95% CI: 21.6–49.5), 14.6% (95% CI: 6.4–22.8), 12.8% (95% CI: 0.1–25.4), and 8.4% (95% CI: 4.4–12.4), respectively. Previous report has concluded that ependymoma were associated with increased risk of postoperative dysphagia [8]. A possible reason is that ependymomas and medulloblastomas are closely related to cranial nerves and the dorsal brainstem [22]. The lower cranial nerves involved in swallowing have a greater risk of injury in surgical removal of ependymomas and medulloblastomas compared to other PFTs.
It should be noted that our study only summarized the prevalence rate of postoperative dysphagia in different tumor sites and types, and provided insufficient evidence regarding the risk factors for dysphagia in patients undergoing surgery for PFTs. Moreover, there are many other factors that were not included in this study. For example, radiation intervention for head and neck cancer has been recognized as a potentially dose-limiting toxicity to swallowing function [45]. Neuropathy and fibrosis of the oral and pharyngeal musculature may persist for a long time after the completion of treatment, ultimately impairing the motor function of key swallowing structures and leading to long-term dysphagia [45]. Previous study has pointed that half of head and neck cancer patients undergoing radiationtherapy may have significant dysphagia [46]. Given the importance associated with management of swallowing dysfunction, further research is needed to explore the risk factors for dysphagia after PFTs resection.
The meta-regression analysis showed that the geographical region of the study population (P = 0.001) was a significant source of heterogeneity among the included studies. According to the subgroup analyses based on different regions, the highest prevalence of dysphagia was found in Europe (42.2%, 95% CI: 17.3–67.0), followed by Australia (33.3%, 95% CI: 15.6–51.1), North America (19.4%, 95% CI: 9.4–29.4), and East Asia (19.0%, 95% CI: 11.0–26.9). Rajati et al. also confirmed substantial variations in the pooled prevalence of oropharyngeal dysphagia between different regions and countries [37]. One explanation for this variation is the population structure in different countries of the world [37]. In addition, the differences in the use of advanced techniques may be one of the reasons for the variations in the results across different regions. In this study, VFSS and FEES were used more frequently in North American and European studies. However, nearly half of the Asian studies did not specify the assessment methods for dysphagia. It is necessary to carefully evaluate the prevalence of dysphagia in different regions to emphasize that clinicians should pay attention to this symptom and its consequences.
Despite the rigorous methodology of this review, our study had certain limitations. First, most of the included studies had a relatively short follow-up and a retrospective or cross-sectional design, leading to the possibility of high levels of selection bias. Second, there was significant heterogeneity among the included studies. Furthermore, some of the included studies had small sample sizes and variations in the assessment protocols. In addition, combining pediatric and adult patients may complicate the analysis due to significant differences the nature of PFTs. A focus on pediatric or adult patients alone might provide clearer insights. Despite these limitations, this review represents the first known meta-analysis of the prevalence of dysphagia following PFT resection. Our results have significant implications for clinical practice as our findings emphasize that clinicians should pay attention to the occurrence of dysphagia in patients who have undergone PFT and provide foundations for targeted treatment strategies.
Conclusions
This systematic review and meta-analysis revealed a high prevalence of dysphagia following PFT resection. Individuals who have undergone PFT resection are at a high risk of dysphagia and should be identified early through screening methods. Multidisciplinary diagnosis and treatment of dysphagia is required to improve the outcomes in the early stages after PFT resection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Z. Zhou and Q. Zhao contributed to the study conception and design. Material preparation, data collection and analysis were performed by Y. Wang, X. Zhang, J. Huang, and Y. Duan. The first draft of the manuscript was written by Y. Duan. Z. Zhou and Q. Zhao commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Young Talents Training Foundation in Nursing of Sun Yat-sen University, China (grant no.: N2022Y05).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The study was approved by the Bioethics Committee of the Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China (No. B2024-133-01). All procedures performed in studies involving human paticipants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Human ethics and consent to Participate declarations
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuyu Duan, Yueli Wang and Xiaowei Zhang contributed equally to this study.
Contributor Information
Zhihuan Zhou, Email: zhouzhh@sysucc.org.cn.
Qinqin Zhao, Email: zhaoqq@sysucc.org.cn.
References
- 1.Morgan AT, Sell D, Ryan M, Raynsford E, Hayward R. Pre and post-surgical dysphagia outcome associated with posterior fossa tumour in children. J NEURO-ONCOL. 2008;87(3):347–54. 10.1007/s11060-008-9524-6 [DOI] [PubMed] [Google Scholar]
- 2.Mora P, Pons A, Cos M, Camins A, Muntané A, Aguilera C, Arús C, Majós C. Magnetic resonance spectroscopy in posterior fossa tumours: the tumour spectroscopic signature may improve discrimination in adults among haemangioblastoma, ependymal tumours, medulloblastoma, and metastasis. EUR RADIOL. 2019;29(6):2792–801. 10.1007/s00330-018-5879-z [DOI] [PubMed] [Google Scholar]
- 3.Lin CT, Riva-Cambrin JK. Management of posterior fossa tumors and hydrocephalus in children: a review. CHILD NERV SYST. 2015;31(10):1781–9. 10.1007/s00381-015-2781-8 [DOI] [PubMed] [Google Scholar]
- 4.Goethe EA, Gadgil N, Stormes K, Wassef A, LoPresti M, Lam S. Predicting dysphagia in children undergoing surgery for posterior fossa tumors. CHILD NERV SYST. 2020;36(5):925–31. 10.1007/s00381-019-04468-7 [DOI] [PubMed] [Google Scholar]
- 5.Alkins RD, Newsted D, Nguyen P, Campbell RJ, Beyea JA. Predictors of postoperative complications in vestibular Schwannoma Surgery-A Population-based study. OTOL NEUROTOL. 2021;42(7):1067–73. 10.1097/MAO.0000000000003107 [DOI] [PubMed] [Google Scholar]
- 6.Formentin C, Matias LG, de Souza RDSL, de Almeida R, Joaquim AF, Ghizoni E. Anatomy of the posterior Fossa: a comprehensive description for pediatric brain tumors. CHILD NERV SYST. 2024;40(3):613–24. 10.1007/s00381-023-06220-8 [DOI] [PubMed] [Google Scholar]
- 7.Mei C, Morgan AT. Incidence of mutism, dysarthria and dysphagia associated with childhood posterior fossa tumour. CHILD NERV SYST. 2011;27(7):1129–36. 10.1007/s00381-011-1433-x [DOI] [PubMed] [Google Scholar]
- 8.Lee WH, Oh BM, Seo HG, Kim SK, Phi JH, Chong S, Han TR. One-year outcome of postoperative swallowing impairment in pediatric patients with posterior fossa brain tumor. J NEURO-ONCOL. 2016;127(1):73–81. 10.1007/s11060-015-2010-z [DOI] [PubMed] [Google Scholar]
- 9.McCarty EB, Chao TN. Dysphagia and Swallowing disorders. MED CLIN N AM. 2021;105(5):939–54. 10.1016/j.mcna.2021.05.013 [DOI] [PubMed] [Google Scholar]
- 10.Lapa S, Quick-Weller J, Nasari C, Dziewas R, Gessler F, Wagner M, Warnecke T, Hattingen E, Seifert V, Konczalla J. Pre- and post-surgical dysphagia in adults with tumors of the posterior Fossa: a prospective blinded study. CANCERS. 2020;12(9):2561. 10.3390/cancers12092561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman LA, Boop FA, Sanford RA, Thompson JW, Temple CK, Duntsch CD. Postoperative swallowing function after posterior fossa tumor resection in pediatric patients. CHILD NERV SYST. 2006;22(10):1296–300. 10.1007/s00381-006-0065-z [DOI] [PubMed] [Google Scholar]
- 12.Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, Dias MS, Allen JC. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the children’s Oncology Group. J NEUROSURG. 2006;105(6 Suppl):444–51. [DOI] [PubMed] [Google Scholar]
- 13.Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. NEUROSURGERY 1995, 37(5):885–893. [DOI] [PubMed]
- 14.Popman A, Richter M, Allen J, Wham C. High nutrition risk is associated with higher risk of dysphagia in advanced age adults newly admitted to hospital. NUTR DIET. 2018;75(1):52–8. 10.1111/1747-0080.12385 [DOI] [PubMed] [Google Scholar]
- 15.Pavithran J, Puthiyottil IV, Narayan M, Vidhyadharan S, Menon JR, Iyer S. Observations from a pediatric dysphagia clinic: characteristics of children at risk of aspiration pneumonia. LARYNGOSCOPE. 2019;129(11):2614–8. 10.1002/lary.27654 [DOI] [PubMed] [Google Scholar]
- 16.Marin S, Serra-Prat M, Ortega O, Clavé P. Cost of oropharyngeal dysphagia after stroke: protocol for a systematic review. BMJ OPEN. 2018;8(12):e22775. 10.1136/bmjopen-2018-022775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schefold JC, Berger D, Zürcher P, Lensch M, Perren A, Jakob SM, Parviainen I, Takala J. Dysphagia in mechanically ventilated ICU patients (DYnAMICS): a prospective observational trial. CRIT CARE MED. 2017;45(12):2061–9. 10.1097/CCM.0000000000002765 [DOI] [PubMed] [Google Scholar]
- 18.Patel DA, Krishnaswami S, Steger E, Conover E, Vaezi MF, Ciucci MR, Francis DO. Economic and survival burden of dysphagia among inpatients in the United States. DIS ESOPHAGUS. 2018;31(1):1–7. 10.1093/dote/dox131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancaster J. Dysphagia: its nature, assessment and management. Br J Community Nurs 2015, Suppl Nutrition:S28-S32. [DOI] [PubMed]
- 20.Huber JF, Bradley K, Spiegler B, Dennis M. Long-term neuromotor speech deficits in survivors of childhood posterior fossa tumors: effects of tumor type, radiation, age at diagnosis, and survival years. J CHILD NEUROL. 2007;22(7):848–54. 10.1177/0883073807303995 [DOI] [PubMed] [Google Scholar]
- 21.Ward BK, Francis HW, Best SR, Starmer HM, Akst LM, Gourin CG. National prevalence and impact of perioperative vagus nerve injury in vestibular schwannoma. LARYNGOSCOPE. 2012;122(12):2824–31. 10.1002/lary.23605 [DOI] [PubMed] [Google Scholar]
- 22.Wright SH, Blumenow W, Kumar R, Mallucci C, Felton A, McMahon S, Hennigan D, Avula S, Pizer B. Prevalence of dysphagia following posterior fossa tumour resection in children: the Alder Hey experience. CHILD NERV SYST. 2023;39(3):609–16. 10.1007/s00381-022-05774-3 [DOI] [PubMed] [Google Scholar]
- 23.Starmer HM, Best SR, Agrawal Y, Chien WW, Hillel AT, Francis HW, Tamargo RJ, Akst LM. Prevalence, characteristics, and management of swallowing disorders following cerebellopontine angle surgery. OTOLARYNG HEAD NECK. 2012;146(3):419–25. 10.1177/0194599811429259 [DOI] [PubMed] [Google Scholar]
- 24.Nasrollahi TS, Shahrestani S, Borrelli M, Raskin J, Hopp ML, Wu AW, Miller ME, Wong YT. Analysis of readmissions data among frail and non-frail patients presenting for acoustic neuroma. J CLIN NEUROSCI. 2022;99:82–8. 10.1016/j.jocn.2022.03.013 [DOI] [PubMed] [Google Scholar]
- 25.CHEN L, XU R, WEI Q, LI Y, LI W, GAO YUB, ZHAO J. Clinical study of microsurgery resection for the fourth ventricle and brain stem tumor through cerebellomedullary fissure approach[In Chinese]. J Clin Neurosurg. 2017;14(5):360–5. 10.3171/2016.6.JNS152946 [DOI] [Google Scholar]
- 26.Zhang W, Liu X, Lai X, Ye M, Huang J, Xie F, Liang M. Microsurgery of tumors in the fourth ventricle (31 cases report)[In Chinese]. Chin J Neuro-Oncology. 2012;10(4):246–9. [Google Scholar]
- 27.WU D, WANG C, XU S, PANG Q. Modified operation and comprehensive analysis of diagnosis and management of medulloblastoma[In Chinese]. J SHANDONG Univ (HEALTH SCIENCES). 2012;50(1):101–4. [Google Scholar]
- 28.Deng Z, Yang W, Yang F, Shi S, Tian X, Chen C, Zhang J. Perioperative Management of preschoolers with 4th ventricle glioma: a report of 84 cases[In Chinese]. Chin Gen Pract. 2009;12(11):998–9. [Google Scholar]
- 29.Chen Y. Clinical review of microsurgery treatment of theFourth Ventricular tumors: Report of 44 cases[In Chinese]. China Medical University; 2010.
- 30.LI H, LI M, XIAO S, HOU Y, WANG S, ZHAO Y. Construction of postoperative aspiration risk prediction model for Brain Tumor Patients[In Chinese]. Military Nurs. 2022;39(8):54–7. [Google Scholar]
- 31.LI M, SHI Q, LU J, TIAN L, LI H, YAN L. Status quo and influence factors of postoperative aspiration in patients with brain tumors[In Chinese]. Chin Nurs Res. 2021;35(14):2593–6. [Google Scholar]
- 32.Zhang L, Yu L, Zeng L, Li R, Chen D. Risk factors of acquired swallowing dysfunction in patients undergoing brain tumor resection[In Chinese]. Chin J Mod Nurs. 2021;27(20):2691–6. [Google Scholar]
- 33.Ricci FS, D’Alessandro R, Somà A, Salvalaggio A, Rossi F, Rampone S, Gamberini G, Davico C, Peretta P, Cacciacarne M, et al. Development and application of a diagnostic and severity scale to grade post-operative pediatric cerebellar mutism syndrome. EUR J PEDIATR. 2022;181(3):941–50. 10.1007/s00431-021-04290-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.YANG W, GE M, SUN H, JI Y, WANG R, YANG X, ZHANG T, ZHANG J. Clinical characteristics of cerebellar mutism syndrome in children[In Chinese]. Chin J Nerv Mental Dis. 2020;46(7):385–9. [Google Scholar]
- 35.Wang F, Yao Z, Chen L, Ji C. Early identification and rehabilitation care of post-extubation dysphagia in patients undergoing surgical treatment of posterior cranial fossa tumor[In Chinese]. J Nurs Sci. 2018;33(9):4. [Google Scholar]
- 36.Bhattacharyya N. The prevalence of dysphagia among adults in the United States. OTOLARYNG HEAD NECK. 2014;151(5):765–9. 10.1177/0194599814549156 [DOI] [PubMed] [Google Scholar]
- 37.Rajati F, Ahmadi N, Naghibzadeh ZA, Kazeminia M. The global prevalence of oropharyngeal dysphagia in different populations: a systematic review and meta-analysis. J TRANSL MED. 2022;20(1):175. 10.1186/s12967-022-03380-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.QI C, ZHANG Y, LI C, YANG Q, LI Y, GAO T, Xiaoliang DU. Risk factors of acquired dysphagia in patients after posterior cranial fossa surgery[In Chinese]. Chin Nurs Res. 2023;37(7):1158–63. [Google Scholar]
- 39.Maeshima S, Osawa A, Miyazaki Y, Seki Y, Miura C, Tazawa Y, Tanahashi N. Influence of dysphagia on short-term outcome in patients with acute stroke. AM J PHYS MED REHAB. 2011;90(4):316–20. 10.1097/PHM.0b013e31820b13b2 [DOI] [PubMed] [Google Scholar]
- 40.Jukic PN, Orlandoni P, Dell’Aquila G, Carrieri B, Eusebi P, Landi F, Volpato S, Zuliani G, Lattanzio F, Cherubini A. Dysphagia in nursing home residents: management and outcomes. J AM MED DIR ASSOC. 2019;20(2):147–51. 10.1016/j.jamda.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 41.Attrill S, White S, Murray J, Hammond S, Doeltgen S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: a systematic review. BMC HEALTH SERV RES. 2018;18(1):594. 10.1186/s12913-018-3376-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdel JA, Katzka DA, Castell DO. Approach to the patient with dysphagia. AM J MED. 2015;128(10):1117–38. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins T, Gillies RA, Thomas AM, Wagner PJ. The prevalence of dysphagia in primary care patients: a HamesNet Research Network study. J AM BOARD FAM MED. 2007;20(2):144–50. 10.3122/jabfm.2007.02.060045 [DOI] [PubMed] [Google Scholar]
- 44.Mahboubi H, Verma SP. Swallowing disorders in the ambulatory medical setting. OTOLARYNG HEAD NECK. 2014;150(4):563–7. 10.1177/0194599813518017 [DOI] [PubMed] [Google Scholar]
- 45.Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn GB, Moore MW, Holsinger FC. Late dysphagia after radiotherapy-based treatment of head and neck cancer. CANCER-AM CANCER SOC. 2012;118(23):5793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J CLIN ONCOL. 2006;24(17):2636–43. 10.1200/JCO.2006.06.0079 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.