Abstract
Background
Hyperglycemia is a rapidly increasing risk factor for cancer mortality worldwide. However, the dose‒response relationship between glucose levels and all-cause mortality in cancer survivors is still uncertain.
Methods
We enrolled 4,491 cancer survivors (weighted population 19,465,739) from the 1999–2019 National Health and Nutrition Examination Survey (NHANES). Cancer survivors were defined based on the question of whether they had ever been diagnosed with cancer by a doctor or a health professional. Hemoglobin A1c (HbA1c) was selected in this study as a stable marker of glucose level. Mortality was ascertained by linkage to National Death Index records until December 31, 2019. Cox proportional hazard, Kaplan‒Meier survival curves and Restricted cubic spline regression models were used to evaluate the associations between HbA1c and all-cause mortality risk in cancer survivors.
Results
In NHANES, after adjusting for confounders, HbA1c had an independent nonlinear association with increased all-cause mortality in cancer survivors (nonlinear P value < 0.05). The threshold value for HbA1c was 5.4%, and the HRs (95% CI) below and above the threshold value were 0.917 (0.856,0.983) and 1.026 (1.010,1.043), respectively. Similar associations were found between fasting glucose and all-cause mortality in cancer survivors, and the threshold value was 5.7 mmol/L.
Conclusions
HbA1c was nonlinearly associated with all-cause mortality in cancer survivors, and the critical value of HbA1c in decreased mortality was 5.4%, suggesting optimal glucose management in cancer survivors may be a key to preventing premature death in cancer survivors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-19545-z.
Keywords: Cancer survivors, HbA1c, Fasting glucose, All-cause mortality, NHANES
Background
In the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019, we found that hyperglycemia is a rapidly increasing risk factor for cancer mortality worldwide over the past 30 years, and it has become a serious public health problem that requires urgent attention. Studies have already reported that hyperglycemia or diabetes is associated with poor prognosis in various cancers, including breast [1], gastric [2], and colorectal [3, 4] etc. [5, 6]. Hyperglycemia may support cancer progression through multiple mechanisms, including the promotion of tumor cell proliferation, invasion, and migration, as well as the induction of apoptosis resistance and chemoresistance [7–9]. It has also been shown that hyperglycemia or diabetes increases cancer mortality [10–15]. A study also observed a U-shaped relationship between glucose levels (HbA1c) and cancer mortality in female[16]. However, there are also studies proposed that higher glucose levels are not associated with the survival of patients with cancer [17, 18]. Therefore, the relationship between glucose levels and all-cause mortality in cancer survivors has been inconclusive. Additionally, the dose‒response relationship is still uncertain. To further enrich this research field, we explored the association between glucose levels and all-cause mortality in cancer survivors based on the 1999–2019 National Health and Nutrition Examination Survey (NHANES).
Methods
Population of the study
In this analysis, the subjects were selected based on asking them if they had ever been diagnosed with cancer by a doctor or a health professional [19]. NHANES asked people aged 20 and older about their history of cancer [20]. In addition, cases with ineligibility for mortality or missing HbA1c values were excluded. Individuals with abnormal HbA1c (5 SD) were also excluded. The data cleaning algorithm was shown in Supplemental Figure S1, and the final sample size was 4,491.
Study variables
In NHANES, hemoglobin A1c (HbA1c) was selected to represent the glucose level in this study, and HbA1c represents the average blood glucose level in 2–3 months, thus providing a stable marker of glucose level [21–23]. HbA1c and fasting glucose were obtained from NHANES laboratory files. The confounders included in the present study were mainly based on the study by Cao C et al. [19] and included age, sex, race/ethnicity, BMI, education level, family income-poverty ratio, alcohol use, smoking status, ideal physical activity, Healthy Eating Index (HEI) score, self-reported hypertension, hypercholesterolemia, cardiovascular disease (CVD), years since first cancer diagnosis, use of prescription medications, and cancer sites. Data on gender, age, race/ethnicity, education level, family income, smoking status, physical activity, dietary status, disease and cancer status, prescription medications use were obtained from household interviews with NHANES using a standardized questionnaire. BMI and blood pressure were obtained through NHANES examination measurements. The primary outcome variable was all-cause mortality, and secondary outcome variables were CVD and cancer-related mortality. Mortality data from NHANES (1999–2018) were provided by the National Centre for Health Statistics using probabilistic record matching with death certificate data found in the National Death Index (NCHS Linked Mortality File) by December 31, 2019. Furthermore, CVD and cancer-related mortality were determined using the International Statistical Classification of Diseases, 10th Revision (ICD-10) codes.
Race/ethnicity was divided into the following five categories: Mexican American, Non-Hispanic Black, Non-Hispanic White, Other Hispanic or Other Race. Education level was divided into the following five categories: < 9th grade, 9-11th grade, high school graduate or equivalent, some college or AA degree, and college graduate or above. The family income-poverty ratio was divided into ≤ 1.0, 1.0–3.0 and > 3.0 [24]. Smoking status was divided into never smokers, former smokers, and current smokers. Ideal physical activity was defined as ≥ 150 min of moderate-intensity activities per week, ≥ 75 min of vigorous-intensity activities per week, or an equivalent combination of both [25]. Hypertension was defined as (1) informed by a physician, (2) taking medication for hypertension, or (3) average blood pressure reaching the diagnostic value of hypertension (SBP ≥ 140 or DBP ≥ 90). Hypercholesterolemia was defined as (1) hypertriglyceridemia: TG ≥ 1.7 mmol/L; (2) hypercholesterolemia: high total cholesterol (TC ≥ 5.18 mmol/L), high LDL (LDL ≥ 3.37 mmol/L), low HDL (male: HDL < 1.04 mmol/L, female: HDL < 1.30 mol/L); or (3) use of lipid-lowering drugs. Cardiovascular disease was defined as having been told by a physician that the patient had one or more of the following conditions: coronary heart disease, congestive heart failure, heart attack, stroke, and angina. The years since cancer diagnosis was defined as the participant's age minus the age at which the cancer was first reported. The Healthy Eating Index 2015 (HEI-2015) was used to assess the quality of diet [26], and the HEI values were taken as the mean of the first and second 24-h dietary recall interviews. The use of prescription medications was obtained from the Prescription Medications Questionnaire. Participants were asked if they had taken any prescription medications in the past 30 days and, if answered “yes”, were required to show the interviewers the containers or prescription printouts for verification. Antineoplastic use was defined as the use of at least one prescription medication classified as an antineoplastic. Cancer sites were determined based on the question “What kind of cancer was it?”. We classified cancers into eight groups based on these sites. The urinary and reproductive system cancers includes: Bladder, Kidney, Prostate, and Testis (testicular). The digestive system cancers includes: Colon, Esophagus (esophageal), Gallbladder, Pancreas (pancreatic), Liver, Stomach, and Rectum (rectal). The female reproductive system cancers includes: Breast, Cervix (cervical), Ovary (ovarian), and Uterus (uterine). The respiratory system cancers includes: Larynx/windpipe and Lung. The blood and lymphatic system cancers includes: Blood, Leukemia, and Lymphoma/Hodgkins disease. The skin cancer includes: Melanoma, Skin (non-melanoma), and Skin (don't know what kind). The other site cancers includes: Mouth/tongue/lip, Soft Tissue (muscle or fat), Thyroid, Bone, Brain, and Nervous System. Finally, the unknown or multiple types of cancers includes: Other, More than 2 kinds, and Don't know.
Multiple imputation was used for covariates with missing values [27]. The missing values of covariates are presented in Supplementary Table S1, and the most missing values were physical activity and HEI value, but physical activity and HEI value were very important. We used multiple interpolation methods to fill it, and the distribution of the filled results is similar to the actual distribution, as seen in Supplementary Figure S2. In the imputation process, we employed the Multiple Imputation by Chained Equations (MICE) package (version 3.14.0), adhering to the default settings [27]. Continuous variables were imputed using the “pmm” method, binary variables were imputed using the “logreg” method, and categorical variables were imputed using the “polyreg” method. We performed multiple imputation by creating 5 imputed datasets, followed by a pooling analysis to integrate the results across these datasets.
Statistical analyses
Due to the complex sampling design of NHANES, this study used and adjusted weights to account for multiple periods, allowing the outcomes to be extrapolated to the entire US population [19]. The study used multiple imputations to complement missing values. Individuals were divided into 4 groups based on the quartile of HbA1c: Quartile 1 (< 5.3%), Quartile 2 (5.3–5.6%), Quartile 3 (5.6–5.9%), and Quartile 4 (≥ 5.9%). Sample characteristics are presented as means (SEs) for normally distributed continuous variables, medians (interquartile ranges) for nonnormally distributed continuous variables, and numbers (percentages) for categorical variables. Kaplan‒Meier survival curve was plotted to describe all-cause mortality risk in the different subgroups. Cumulative incidence function curves were plotted to describe CVD and cancer-related mortality. Multiple models, adjusted for confounding factors using Cox proportional hazards models, were constructed to investigate the correlation between HbA1c levels and the risk of all-cause mortality. Cause-specific proportional hazards models were then used to analyze cause-specific mortality, including CVD and cancer-related mortality. In addition to the quartile-based analysis, we further categorized individuals into three subgroups based on HbA1c clinical thresholds: normal HbA1c level (< 5.7%), prediabetes HbA1c level (5.7–6.5%), and diabetes HbA1c level (≥ 6.5%). To examine the dose‒response relationship between HbA1c and mortality rate, we used a restricted cubic spline regression with three nodes (5th, 50th, and 75th) and adjusted the above variables in the model. If there was a non-linear relationship, two-piecewise linear regression models were used to explain how the associations differed based on the threshold point. The threshold value is determined by evaluating every possible value and selecting the threshold point with the highest likelihood. The differences in associations between two-piecewise linear regression models and one-line linear regression models were compared using the logarithmic likelihood ratio test.
The study was also stratified by age (≤ 70 years or > 70 years), sex (male or female), race/ethnicity (white or nonwhite), and BMI (< 30.0 or ≥ 30.0 kg/m2). In the sensitivity analysis, we excluded patients who died within 2 years of follow-up to lessen the probability of reverse causation [23]. Additionally, we examined the relationship between fasting glucose and all-cause mortality in cancer survivors. We also performed the same study in skin (non-melanoma), prostate and breast cancer survivors, which have a sample size above 600. All statistical analyses were conducted in R software (4.2.2) [28].
Results
Baseline characteristics by quartile of HbA1c
In this study, we enrolled 4,491 cancer survivors (weighted population 19,465,739) with a median (interquartile ranges) age of 65 (53, 75) years, and 42.254% men. The baseline characteristics grouped according to the quartile of HbA1c were shown in Table 1. Cancer survivors with higher HbA1c levels were older, more likely to be non-Hispanic black, have a higher BMI, be less likely to be alcohol users, have lower education levels and family income-poverty ratios, and be more likely to suffer from hypertension, hypercholesterolemia, CVD, respiratory system cancers and urinary and reproductive system cancers, more prescription medications use than those with lower HbA1c levels. More importantly, cancer survivors with higher HbA1c levels had higher all-cause and CVD-related mortality.
Table 1.
Baseline characteristics of participants among different HbA1c subgroups in NHANES 1999–2018
| Total | Subgroup 1 (≤ 5.3%) |
Subgroup 2 (5.3–5.6%) |
Subgroup 3 (5.6–5.9%) |
Subgroup 4 (> 5.9%) |
|
|---|---|---|---|---|---|
| Total, n | 4491 | 1156 | 1182 | 908 | 1245 |
| HbA1c (%) | 5.726(0.014) | 5.100(0.008) | 5.499(0.003) | 5.787(0.003) | 6.776(0.033) |
| Age (years) | 65(53,75) | 54(42,68) | 64(54,74) | 69(59,78) | 70(62,78) |
| Gender | |||||
| Female | 2372(57.746) | 670(63.742) | 624(56.511) | 485(59.012) | 593(50.420) |
| Male | 2119(42.254) | 486(36.258) | 558(43.489) | 423(40.988) | 652(49.580) |
| Race/ethnicity | |||||
| Mexican American | 313(2.298) | 75(1.882) | 71(2.181) | 56(2.086) | 111(3.164) |
| Non-Hispanic Black | 590(5.105) | 117(3.882) | 118(3.817) | 133(5.800) | 222(7.738) |
| Non-Hispanic White | 3172(86.929) | 879(89.724) | 892(88.307) | 632(86.036) | 769(82.299) |
| Other Hispanic | 221(2.330) | 49(1.795) | 56(2.745) | 42(1.869) | 74(2.891) |
| Other Race | 195(3.337) | 36(2.717) | 45(2.950) | 45(4.208) | 69(3.907) |
| BMI (kg/m2, n = 4373) | 28.757(0.119) | 26.932(0.213) | 28.007(0.207) | 29.378(0.247) | 31.579(0.297) |
| Alcohol user | |||||
| Yes | 2868(69.102) | 785(73.454) | 806(71.914) | 556(65.466) | 721(62.923) |
| No | 1323(25.419) | 272(19.767) | 316(23.929) | 299(29.021) | 436(31.666) |
| Unknown | 300(5.479) | 99(6.779) | 60(4.157) | 53(5.513) | 88(5.411) |
| Smoking status | |||||
| Never smoker | 1998(45.264) | 548(49.470) | 509(42.799) | 406(45.442) | 535(42.719) |
| Ever smoker | 1795(38.203) | 396(32.622) | 472(39.676) | 370(36.857) | 557(44.758) |
| Current smoker | 694(16.501) | 211(17.876) | 199(17.462) | 131(17.676) | 153(12.523) |
| Unknown | 4(0.032) | 1(0.032) | 2(0.063) | 1(0.025) | 0(0.000) |
| Education levels | |||||
| < 9th grade | 457(5.162) | 93(3.738) | 86(3.820) | 99(5.134) | 179(8.726) |
| 9-11th grade | 557(9.272) | 135(7.891) | 141(9.480) | 95(8.482) | 186(11.468) |
| High school graduate or equivalent | 1046(22.509) | 270(20.717) | 276(23.254) | 226(24.879) | 274(21.941) |
| Some college or AA degree | 1309(31.104) | 334(30.989) | 360(29.982) | 260(30.608) | 355(33.072) |
| College graduate or above | 1116(31.870) | 323(36.633) | 318(33.396) | 225(30.641) | 250(24.767) |
| Unknown | 6(0.083) | 1(0.032) | 1(0.068) | 3(0.256) | 1(0.026) |
| Family income-poverty ratio | |||||
| ≤ 1.0 | 586(8.982) | 161(9.469) | 138(8.544) | 107(7.848) | 180(9.840) |
| 1.0–3.0 | 1784(31.805) | 408(27.088) | 445(28.160) | 371(35.337) | 560(39.592) |
| > 3.0 | 1739(51.262) | 500(57.014) | 497(55.492) | 343(47.530) | 399(41.557) |
| Unknown | 382(7.951) | 87(6.429) | 102(7.804) | 87(9.285) | 106(9.011) |
| Ideal physical activity | |||||
| Yes | 1915(47.450) | 510(49.762) | 529(48.732) | 386(46.511) | 490(43.607) |
| No | 961(22.070) | 293(26.495) | 255(22.397) | 187(19.101) | 226(18.359) |
| Unknown | 1615(30.48) | 353(23.743) | 398(28.871) | 335(34.388) | 529(38.034) |
| HEI value (n = 3954) | 55.382(0.273) | 55.592(0.485) | 55.374(0.533) | 56.159(0.615) | 54.472(0.449) |
| Cancer duration | |||||
| ≤ 5 years | 1766(38.599) | 469(42.551) | 461(37.212) | 345(34.805) | 491(38.337) |
| 5–10 years | 963(21.788) | 249(21.484) | 251(23.103) | 203(22.990) | 260(19.536) |
| > 10 years | 1734(39.131) | 435(35.678) | 460(39.163) | 352(41.474) | 487(41.646) |
| Unknown | 28(0.482) | 3(0.287) | 10(0.522) | 8(0.731) | 7(0.481) |
| Hypertension | |||||
| Yes | 2956(59.482) | 598(43.795) | 739(56.977) | 620(64.938) | 999(78.549) |
| No | 1535(40.518) | 558(56.205) | 443(43.023) | 288(35.062) | 246(21.451) |
| Hypercholesterolemia | |||||
| Yes | 3597(80.424) | 807(70.073) | 939(80.818) | 754(85.254) | 1097(89.415) |
| No | 894(19.576) | 349(29.927) | 243(19.182) | 154(14.746) | 148(10.585) |
| CVD | |||||
| Yes | 1118(20.015) | 197(11.290) | 258(18.282) | 235(22.265) | 428(31.699) |
| No | 3373(79.985) | 959(88.710) | 924(81.718) | 673(77.735) | 817(68.301) |
| Use of prescription medications | |||||
| Antineoplastics | 363(8.130) | 98(9.008) | 106(8.663) | 67(7.242) | 92(7.056) |
| Other | 3484(75.624) | 798(67.393) | 868(71.417) | 733(81.154) | 1085(87.037) |
| No | 641(16.220) | 260(23.598) | 208(19.920) | 107(11.542) | 66(5.845) |
| Unknown | 3(0.026) | 0(0.001) | 0(0.000) | 1(0.062) | 2(0.062) |
| Cancer sites | |||||
| Urinary and Reproductive System Cancers | 791(12.120) | 154(8.372) | 180(11.286) | 186(14.143) | 271(16.371) |
| Digestive System Cancers | 315(5.018) | 70(4.457) | 69(3.629) | 58(6.110) | 118(6.583) |
| Female Reproductive System Cancers | 1112(25.145) | 307(26.524) | 294(25.161) | 223(24.563) | 288(23.812) |
| Respiratory System Cancers | 97(1.815) | 15(1.035) | 24(1.721) | 17(1.949) | 41(2.841) |
| Blood and Lymphatic System Cancers | 113(2.692) | 44(4.182) | 35(3.033) | 14(1.248) | 20(1.521) |
| Skin Cancers | 1216(34.363) | 357(38.542) | 347(35.195) | 232(32.457) | 280(29.454) |
| Other Site Cancers | 150(3.442) | 42(3.767) | 42(3.564) | 38(3.879) | 28(2.503) |
| Unknown or Multiple Types of Cancers | 697(15.404) | 167(13.122) | 191(16.411) | 140(15.651) | 199(16.914) |
| Outcome | |||||
| All-cause mortality | |||||
| Yes | 1590(26.197) | 373(20.946) | 405(25.121) | 330(29.255) | 482(31.853) |
| No | 2901(73.803) | 783(79.054) | 777(74.879) | 578(70.745) | 763(68.147) |
| CVD-related mortality | |||||
| Yes | 346(5.591) | 67(3.556) | 80(4.284) | 72(7.049) | 127(8.673) |
| No | 4145(94.409) | 1089(96.444) | 1102(95.716) | 836(92.951) | 1118(91.327) |
| Cancer-related mortality | |||||
| Yes | 472(7.892) | 124(7.671) | 124(7.831) | 94(9.055) | 130(7.290) |
| No | 4019(92.108) | 1032(92.329) | 1058(92.169) | 814(90.945) | 1115(92.710) |
Continuous variables for normally distributed are presented as the mean (SE), nonnormally distributed continuous variables are presented as the medians (interquartile ranges), and categorical variables are presented as numbers (percentages). All estimates accounted for the complex survey design
Association between HbA1c and the risk of mortality
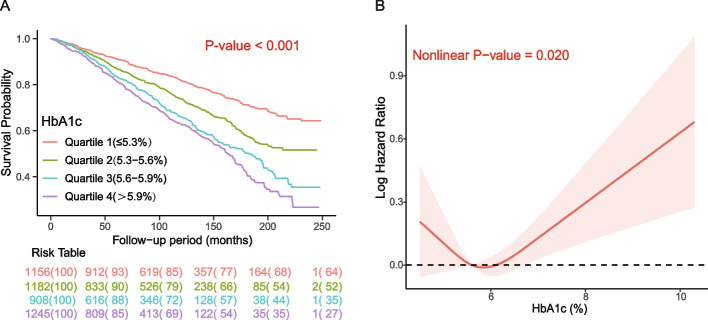
The Kaplan‒Meier survival curve stratified by the quartile of HbA1c was shown in Fig. 1-A, and the cumulative incidence of all-cause death decreased with increasing HbA1c (log-rank test, P < 0.001). Table 2 showed the results of the pooled multivariate regression analysis, after adjustment for multiple covariates, showing that the HR (95% CI) of HbA1c (per 0.2% increase) was 1.018 (1.002,1.034), and the P value was 0.029. The adjusted HRs (95% CIs) for the HbA1c subgroups (≤ 5.3, 5.3–5.6, 5.6–5.9, and > 5.9%) were 1.114 (0.940,1.319), 1.00 (reference value), 1.086 (0.910,1.295), and 1.172 (0.990,1.386), respectively (P trend = 0.273). Although no statistical significance was found, a U-shaped relationship with HR values was exhibited. Figure 1-B showed the dose‒response relationship between HbA1c and all-cause mortality, and a U-shaped relationship was observed after multivariate adjustment (non-linear P value = 0.022). When HbA1c was greater than the threshold of 5.4%, the HRs below and above the threshold point were 0.917 (95% CI, 0.856–0.983) and 1.026 (95% CI, 1.010–1.043), respectively (Table 3). The cumulative incidence function curves for CVD and cancer-related mortality were shown in Supplementary Figures S3-A and S4-A, respectively. The cumulative incidence of CVD-related mortality increased with increasing HbA1c levels (P < 0.001). No non-linear relationships were found for either CVD or cancer-related mortality, as indicated in Supplementary Figures S3-B and S4-B (all non-linear P values > 0.05). As presented in Table 2, the HRs associated with each 0.2% HbA1c increase were 1.040 (95% CI, 1.008–1.074) for CVD-related mortality and 0.979 (95% CI, 0.944–1.015) for cancer-related mortality. Furthermore, the adjusted HRs (95% CIs) for the HbA1c subgroups (≤ 5.3%, 5.3–5.6%, 5.6–5.9%, and > 5.9%) for CVD-related mortality were 1.226 (0.829,1.811), 1.000 (reference value), 1.430 (0.981,2.085), and 1.632 (1.158,2.300), respectively. For cancer-related mortality, the adjusted HRs (95% CIs) were 1.260 (0.919,1.729), 1.000 (reference value), 1.096 (0.757,1.586), and 0.854 (0.606,1.204), respectively. HRs for non-CVD and non-Cancer mortality among different subgroups were shown in Supplementary Table S2, a higher HbA1c level was not associated with non-CVD mortality, while HbA1c was associated with increased risks of non-Cancer mortality. Notably, among cancer survivors, higher HbA1c levels were associated with increased risks of CVD-related and non-Cancer mortality, however, they were not associated with cancer-related or non-CVD mortality.
Fig. 1.
Kaplan‒Meier curve for all-cause mortality categorized by different subgroups of HbA1c (A) and association between HbA1c and all-cause mortality (B). Note: A restricted cubic spline model was calculated after adjusting for age, sex, race/ethnicity, BMI, education level, family income-poverty ratio, alcohol use, smoking status, ideal physical activity, HEI score, self-reported hypertension, hypercholesterolemia, CVD, years since first cancer diagnosis, use of prescription medications, and cancer sites
Table 2.
Hazard ratios (95% CIs) for mortality among different HbA1c subgroups in NHANES 1999–2018
| HbA1c (per 0.2% increment) | Quartile 1 (≤ 5.3%) |
Quartile 2 (5.3–5.6%) |
Quartile 2 (5.6–5.9%) |
Quartile 4 (> 5.9%) |
P trend | |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| Model 1a | 1.025 (1.009,1.041) 0.002 | 1.040 (0.886,1.221) 0.632 | 1.000 | 1.051 (0.880,1.254) 0.584 | 1.240 (1.045,1.472) 0.014 | 0.022 |
| Model 2b | 1.018 (1.001,1.035) 0.036 | 1.095 (0.931,1.287) 0.272 | 1.000 | 1.072 (0.901,1.274) 0.434 | 1.188 (1.001,1.411) 0.048 | 0.169 |
| Model 3c | 1.018 (1.002,1.034) 0.029 | 1.114 (0.940,1.319) 0.213 | 1.000 | 1.086 (0.910,1.295) 0.360 | 1.172 (0.990,1.386) 0.065 | 0.273 |
| CVD-related mortality | ||||||
| Model 1a | 1.061 (1.035,1.088) < 0.001 | 1.114 (0.774,1.603) 0.562 | 1.000 | 1.431 (0.969,2.114) 0.072 | 1.966 (1.432,2.701) < 0.001 | < 0.001 |
| Model 2b | 1.049 (1.016,1.083) 0.004 | 1.167 (0.804,1.696) 0.416 | 1.000 | 1.429 (0.977,2.089) 0.066 | 1.782 (1.257,2.524) 0.001 | 0.006 |
| Model 3c | 1.04 (1.008,1.074) 0.014 | 1.226 (0.829,1.811) 0.307 | 1.000 | 1.430 (0.981,2.085) 0.063 | 1.632 (1.158,2.300) 0.005 | 0.032 |
| Cancer-related mortality | ||||||
| Model 1a | 0.986 (0.951,1.022) 0.435 | 1.146 (0.836,1.570) 0.398 | 1.000 | 1.072 (0.737,1.559) 0.715 | 0.901 (0.637,1.273) 0.554 | 0.218 |
| Model 2b | 0.974 (0.939,1.010) 0.154 | 1.245 (0.906,1.711) 0.177 | 1.000 | 1.076 (0.751,1.543) 0.689 | 0.832 (0.593,1.169) 0.290 | 0.029 |
| Model 3c | 0.979 (0.944,1.015) 0.252 | 1.260 (0.919,1.729) 0.152 | 1.000 | 1.096 (0.757,1.586) 0.627 | 0.854 (0.606,1.204) 0.368 | 0.049 |
aModel 1: adjusted for age
bModel 2: further adjusted (from Model 1) for sex, race/ethnicity, BMI, education level, family income-poverty ratio, alcohol use, smoking status, ideal physical activity, and HEI score
cModel 3: further adjusted (from Model 2) for self-reported hypertension, hypercholesterolemia, CVD, years since first cancer diagnosis, use of prescription medications, and cancer sites
Table 3.
Threhold effect analysis of HbA1c on all-cause mortality
| Threshold value | < threshold value (per 0.2% increment) | ≥ threshold value (per 0.2% increment) | P for log likelihood ratio test† | |
|---|---|---|---|---|
| All-cause mortality | 5.4 | 0.917 (0.856,0.983), 0.014 | 1.026 (1.010,1.043), 0.002 | 0.006 ~ 0.015 |
The two piecewise linear regression models were adjusted for age, sex, race/ethnicity, BMI, education level, family income-poverty ratio, alcohol use, smoking status, ideal physical activity, HEI score, self-reported hypertension, hypercholesterolemia, CVD, years since first cancer diagnosis, use of prescription medications, and cancer sites
† The P value for 5 imputed datasets range from 0.006 to 0.015, and the P for log likelihood ratio test < 0.05
Furthermore, when stratifying HbA1c by clinical cut-offs, the covariate-adjusted HRs for the subgroups (< 5.7, 5.7–6.5, and ≥ 6.5%) were as follows. For all-cause mortality: 1.00 (reference value), 1.028 (0.900,1.174) and 1.262 (1.045,1.523); for CVD-related mortality: 1.00 (reference value), 1.353 (1.020,1.795), and 1.600 (1.087,2.354); and for cancer-related mortality: 1.00 (reference value), 0.910 (0.695,1.191), and 0.782 (0.532,1.149). Cancer survivors with an HbA1c level of ≥ 6.5% had a higher risk of all-cause and CVD-related mortality (P < 0.05) compared to those with a normal HbA1c (< 5.7%). A significant trend (P < 0.05) indicates that the risk of all-cause and CVD-related mortality increases with higher HbA1c levels, as shown in Supplementary Table S3. For non-CVD and non-cancer mortality, we found that higher HbA1c levels were not associated with non-CVD mortality, but were associated with increased non-cancer mortality, as shown in Table S4.
A U-shaped relationship was also observed in fasting glucose samples (n = 2189) with a threshold of 5.7 mmol/L for all-cause mortality (Supplement Tables S5-7). Similar results were observed in breast cancer survivors (n = 659), prostate cancer survivors (n = 673), and skin (non-melanoma) cancer survivors (n = 698), and the threshold values were 5.5%, 5.3% and 6.0% respectively, as shown in Supplemental Tables S8-16. These were also similar when excluding participants who died within two years of follow-up, as shown in Supplemental Tables S17-19. Consistent results were obtained when analyses were stratified by sex, race/ethnicity and BMI (all P interaction > 0.010). At the same time, we found that age may influence the association between HbA1c and all-cause mortality risk (P interaction < 0.001). Among cancer survivors older than 70 years, increased HbA1c increased the risk of all-cause mortality, and the threshold value was 5.5%, while no such relationship was observed among those younger than 70 years, and the threshold value was 6.3% (Supplementary Table S20-25).
Discussion
In the past 30 years, hyperglycemia has rapidly become an increasing risk factor for mortality among cancer survivors worldwide. With the development of the economy and society, mortality attributed to hyperglycemia is increasing. Based on the heavy disease burden worldwide, how to obtain optimized glucose management in cancer survivors has become an urgent research direction.
Our results indicate that there is a U-shaped relationship between glucose levels and the risk of all-cause mortality among cancer survivors. Previous studies have shown that high HbA1c levels were associated with poor prognosis in patients with lung cancer [29, 30] and positively associated with glioblastoma cell proliferation [31]. However, there is still a lack of studies on the dose‒response relationship between glucose levels and all-cause mortality in cancer survivors. Our study investigated this relationship and found a U-shaped relationship between glucose levels and all-cause mortality, and the critical values of HbA1c or fasting glucose in the mortality decrease were 5.4% and 5.7 mmol/L, respectively. In breast, prostate and skin (non-melanoma) cancer survivors, the critical value of HbA1c were 5.5%, 5.3% and 6.0%. When HbA1c exceeds 5.4%, each 0.2% increase in HbA1c is associated with a 2.6% increase in the risk of all-cause mortality. The HR of 1.026 per 0.2% HbA1c increase may not be strong, but it is still clinically significant, as consecutive increments lead to a proportional rise in all-cause mortality (e.g., a 1.0% HbA1c increase results in a 13% increase in all-cause mortality). Additionally, even small increases in mortality can have a substantial impact at the population level.
In the dose–response analysis, an increased cancer-related mortality was observed at lower glucose levels. This observation may be attributed to the prevalence of cachexia and malnutrition among cancer survivors with low glucose. Both cachexia and malnutrition are considered indicative of more aggressive cancer phenotypes and are significant risk factors for higher cancer mortality [32, 33]. This study also observed a linear relationship between hyperglycemia and CVD-related mortality, whereas no significant association was found between hyperglycemia and cancer-related or non-CVD mortality. Therefore, it is hypothesized that the increased risk of all-cause mortality in cancer survivors with hyperglycemia may be attributed primarily to an elevated risk of CVD-related mortality. This suggests that hyperglycemia may exacerbate the risk of CVD-related mortality, subsequently increasing the all-cause mortality risk in cancer survivors. These findings are generally consistent with those reported by Mi-Hyang Jung et al., who observed a nonlinear relationship between glucose levels and CVD-related mortality among over 170,000 cancer survivors in Korea [34]. Specifically, they noted that beyond 90 mg/dL, an increase in fasting glucose levels was associated with an increased risk of CVD-related mortality. Multiple studies supported a positive correlation between hyperglycemia and the risk of CVD-related mortality [35–37]. The increase in glucose levels may increase the risk of CVD through various mechanisms. For instance, hyperglycemia impairs the function of endothelial cells, leading to the formation of atherosclerotic plaques and accelerating plaque rupture, thereby promoting the formation of vascular thrombi [38]. Additionally, hyperglycemia is associated with arterial stiffness, endothelial dysfunction, and increased intima-media thickness [39, 40]. These risk factors will significantly increase the CVD-related mortality among cancer survivors.
Given the prolonged survival observed in the majority of cancer survivors in this study, with 60.9% surviving beyond five years and 39.1% beyond ten years, CVD-related mortality can be more readily monitored and evaluated. However, we cannot discount the influence of hyperglycemia on the cancer-related mortality. Hyperglycemia may also affect cancer progression and prognosis through various pathways. For example, some studies have claimed that hyperglycemia accelerates ovarian tumor growth in a glucose concentration-dependent manner and significantly shortens overall patient survival. The mechanism may be due to the "Warburg" effect of glucose metabolism in tumor cells, where normal circulating glucose concentrations cannot meet the energy requirements of the tumor and constitute a limiting factor for cancer cell metabolism. Patients with hyperglycemia may have the potential to meet these energy requirements and promote cancer progression [41, 42]. It has also been reported that high blood glucose levels significantly increase breast cancer cell proliferation compared to low glucose levels. The mechanism may be that the epidermal growth factor receptor (EGFR) is activated by the guanosine triphosphatases (GTPases) Rac1 and Cdc42, which accelerate the cell cycle process and thus promote breast cancer cell proliferation [43]. On the other hand, hyperglycemia may promote tumor growth by increasing tumor microvessel density. Although hypoxia-inducible factor 1 (HIF-1) inhibitors can inhibit tumor growth, hyperglycemia blunts the effect of HIF-1 inhibitors. Meanwhile, glucagon-induced hyperglycemia affects the tumor microenvironment through the hypoxia-inducible factor 1 (HIF-1)-vascular endothelial growth factor (VEGF) pathway and promotes tumor growth and resistance to HIF-1 inhibitory therapy [44]. Hyperglycemia may also enhance the invasive ability of cancer cells; for example, it has been reported that diabetes can affect the malignant features of tumors in invasive ductal breast carcinoma (IDBC). The mechanism may be that high glucose concentrations in the tumor microenvironment enhance IDBC invasion by upregulating the expression of the glucose transporter 1 (Glut1)/matrix metalloproteinase 2 (MMP2)/matrix metalloproteinase 9 (MMP9) axis [45].
The present study has various strengths, such as a large sample size, prospective study design, and weighted analysis (allowing the results to be generalized to the entire United States). However, there were still several limitations of the study. First, cancer-related data in NHANES were obtained from participant self-reports, which may be subject to self-reporting bias. Second, NHANES database did not collect data on cancer stages or treatments. However, the absence of this data is an inevitable inherent limitation of retrospective observational studies. Lastly, we explored the association between glucose levels and mortality in cancer survivors in the United States population, which cannot be extended to global populations. Additionally, the sample size of other cancer subtypes in NHANES was too small for further analysis.
Conclusions
In summary, glucose management in cancer survivors is extremely important and may prevent premature death. The management of glucose levels in cancer survivors should be emphasized in clinical practice. Cancer survivors with hyperglycemia can be identified through glucose monitoring and screening and appropriately treated as early as possible to improve survival.
Supplementary Information
Acknowledgements
We thank all participants who volunteered as part of the NHANES. We also thank Yuzhuo Wang for her help with research methods.
Clinical trial registry
Not applicable, as this was based on an analysis of existing observational data from the NHANES.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- GBD
Global Burden of Diseases, Injuries, and Risk Factors Study
- HbA1c
Haemoglobin A1c
- CVD
Cardiovascular disease
- HEI-2015
Healthy Eating Index 2015
- EGFR
Epidermal growth factor receptor
- GTPases
Guanosine triphosphatases
- HIF-1
Hypoxia-inducible factor 1
- VEGF
Vascular endothelial growth factor
- IDBC
Invasive ductal breast carcinoma
- Glut1
Glucose transporter 1
- MMP2
Matrix metalloproteinase 2
- MMP9
Matrix metalloproteinase 9
Authors’ contributions
All authors have read and agreed to the published version of the manuscript. NL and YL contributed to the study design, manuscript conceptualization, supervision and preparation. JX and ZL conducted the formal analysis and drafted the paper. WLM, LH, SL and MX contributed to the interpretation of the data, and LR and RX critically reviewed the results and provided valuable input on revision. All authors read and approved the final manuscript.
Funding
This research was funded by the Research Personnel Cultivation Programme of Zhongda Hospital Southeast University (Grant no. CZXM-GSP-RC109), the National Key Research and Development Program of China (Grant no. 2023YFC3605800), the National High Level Hospital Clinical Research Funding and Elite Medical Professionals Project of China-Japan Friendship Hospital (Grant no. ZRJY2021-QM02) and Young Elite Scientist Sponsorship Program By BAST (Grant no. BYESS2023173).
Availability of data and materials
The NHANES database is available at https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the National Center for Health Statistics of the Center for Disease Control and Prevention Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Xie and Zeye Liu contributed equally to this work.
Contributor Information
Yun Liu, Email: liuyun@njmu.edu.cn.
Naifeng Liu, Email: liunf@seu.edu.cn.
References
- 1.Kang C, LeRoith D, Gallagher EJ. Diabetes, obesity, and breast cancer. Endocrinology. 2018;159:3801–12. 10.1210/en.2018-00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng J, Gao Y, Xie S-H, Santoni G, Lagergren J. Haemoglobin A1c and serum glucose levels and risk of gastric cancer: a systematic review and meta-analysis. Br J Cancer. 2022;126:1100–7. 10.1038/s41416-021-01693-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, He H, Zhang Q, Zhang Y. Fasting blood glucose was linearly associated with colorectal cancer risk in the population without self-reported diabetes mellitus history. Medicine. 2021;100:e26974. 10.1097/MD.0000000000026974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi J, Xiong L, Li J, et al. A linear dose-response relationship between fasting plasma glucose and colorectal cancer risk: systematic review and meta-analysis. Sci Rep. 2015;5:17591. 10.1038/srep17591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Beer JC, Liebenberg L. Does cancer risk increase with HbA1c, independent of diabetes? Br J Cancer. 2014;110:2361–8. 10.1038/bjc.2014.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob P, Chowdhury TA. Management of diabetes in patients with cancer. QJM. 2015;108:443–8. 10.1093/qjmed/hcu218 [DOI] [PubMed] [Google Scholar]
- 7.Dąbrowski M, Szymańska-Garbacz E, Miszczyszyn Z, Dereziński T, Czupryniak L. Risk factors for cancer development in type 2 diabetes: a retrospective case-control study. BMC Cancer. 2016;16:785. 10.1186/s12885-016-2836-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng CH. Type 2 diabetes mellitus and kidney cancer risk: a retrospective cohort analysis of the National Health Insurance. PLoS One. 2015;10:e0142480. 10.1371/journal.pone.0142480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Zhang X, Sang H, et al. Effects of hyperglycemia on the progression of tumor diseases. J Exp Clin Cancer Res. 2019;38:327. 10.1186/s13046-019-1309-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakehi E, Kotani K, Nakamura T, Takeshima T, Kajii E. Non-diabetic glucose levels and cancer mortality: a literature review. CDR. 2018;14:434–45. 10.2174/1573399813666170711142035 [DOI] [PubMed] [Google Scholar]
- 11.Ling S, Sweeting M, Zaccardi F, Adlam D, Kadam UT. Glycosylated haemoglobin and prognosis in 10,536 people with cancer and pre-existing diabetes: a meta-analysis with dose-response analysis. BMC Cancer. 2022;22:1048. 10.1186/s12885-022-10144-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferroni P, Formica V, Della-Morte D, et al. Prognostic value of glycated hemoglobin in colorectal cancer. WJG. 2016;22:9984. 10.3748/wjg.v22.i45.9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao CY, Luo SD, Chen WC, et al. Front Oncol. 2022;12:952616. 10.3389/fonc.2022.952616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang YL, Sheu WHH, Lin SY, Liou WS. Good glycaemic control is associated with a better prognosis in breast cancer patients with type 2 diabetes mellitus. Clin Exp Med. 2018;18:383–90. 10.1007/s10238-018-0497-2 [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Wu MF, Chang YL, Sheu WHH, Liou WS. Glycemic control was associated with nonprostate cancer and overall mortalities in diabetic patients with prostate cancer. J Chin Med Assoc. 2022;85:331–40. 10.1097/JCMA.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 16.Joshu CE, Prizment AE, Dluzniewski PJ, et al. Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990–2006. Int J Cancer. 2012;131:1667–77. 10.1002/ijc.27394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boursi B, Giantonio BJ, Lewis JD, Haynes K, Mamtani R, Yang Y-X. Serum glucose and hemoglobin A1C levels at cancer diagnosis and disease outcome. Eur J Cancer. 2016;59:90–8. 10.1016/j.ejca.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Xu W, Hu X, Yang X, Zhang M. The prognostic role of glycemia in patients with pancreatic carcinoma: a systematic review and meta-analysis. Front Oncol. 2022;12:780909. 10.3389/fonc.2022.780909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao C, Friedenreich CM, Yang L. Association of daily sitting time and leisure-time physical activity with survival among US cancer survivors. JAMA Oncol. 2022;8:395. 10.1001/jamaoncol.2021.6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. National health and nutrition examination survey: anthropometry procedures manual. Atlanta: Centers for Disease Control and Prevention. 2009. [Google Scholar]
- 21.Bennett CM, Guo M, Dharmage SC. HbA 1c as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med. 2007;24:333–43. 10.1111/j.1464-5491.2007.02106.x [DOI] [PubMed] [Google Scholar]
- 22.Sriwimol W, Choosongsang P, Choosongsang P, Petkliang W, Treerut P. Associations between HbA1c-derived estimated average glucose and fasting plasma glucose in patients with normal and abnormal hemoglobin patterns. Scand J Clin Lab Invest. 2022;82:192–8. 10.1080/00365513.2022.2040051 [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Peralta F, Choudhary P, Cosson E, Irace C, Rami-Merhar B, Seibold A. Understanding the clinical implications of differences between glucose management indicator and glycated haemoglobin. Diabetes Obes Metab. 2022;24:599–608. 10.1111/dom.14638 [DOI] [PubMed] [Google Scholar]
- 24.Wan Z, Guo J, Pan A, Chen C, Liu L, Liu G. Association of serum 25-hydroxyvitamin D concentrations with all-cause and cause-specific mortality among individuals with diabetes. Diabetes Care. 2021;44:350–7. 10.2337/dc20-1485 [DOI] [PubMed] [Google Scholar]
- 25.Sinha SK, Nicholas SB, Sung JH, et al. hs-CRP is associated with incident diabetic nephropathy: findings from the Jackson heart study. Diabetes Care. 2019;42:2083–9. 10.2337/dc18-2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118:1591–602. 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buuren SV, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Soft. 2011;45:45. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 28.Team R, Core R. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 29.Ogawa H, Fujibayashi Y, Nishikubo M, et al. Prognostic significance of preoperative haemoglobin A1c level in patients with lung adenocarcinoma. Interact Cardiovasc Thorac Surg. 2021;33:534–40. 10.1093/icvts/ivab140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motoishi M, Sawai S, Hori T, Yamashita N. The preoperative HbA1c level is an independent prognostic factor for the postoperative survival after resection of non-small cell lung cancer in elderly patients. Surg Today. 2018;48:517–24. 10.1007/s00595-017-1612-9 [DOI] [PubMed] [Google Scholar]
- 31.Orešković D, Raguž M, Predrijevac N, et al. Hemoglobin A1c in patients with glioblastoma-a preliminary study. World Neurosurg. 2020;141:e553–8. 10.1016/j.wneu.2020.05.231 [DOI] [PubMed] [Google Scholar]
- 32.Mariean CR, Tiucă OM, Mariean A, Cotoi OS. Cancer cachexia: new insights and future directions. Cancers. 2023;15:5590. 10.3390/cancers15235590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: clinical nutrition in cancer. Clin Nutr. 2021;40:2898–913. 10.1016/j.clnu.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 34.Jung MH, Yi SW, An SJ, Yi JJ, Ihm SH, Lee SY, et al. Associations between fasting glucose and cardiovascular disease mortality in cancer survivors: a population-based cohort study. Cardiometab Syndr J. 2024;4:9. 10.51789/cmsj.2024.4.e1 [DOI] [Google Scholar]
- 35.Barr ELM, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia. 2009;52:415–24. 10.1007/s00125-008-1246-y [DOI] [PubMed] [Google Scholar]
- 36.Sakurai M, Saitoh S, Miura K, Nakagawa H, Ohnishi H, Akasaka H, et al. HbA1c and the risks for all-cause and cardiovascular mortality in the general Japanese population. Diabetes Care. 2013;36:3759–65. 10.2337/dc12-2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sattar N, Rawshani A, Franzén S, Rawshani A, Svensson AM, Rosengren A, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks: findings from the Swedish National Diabetes Registry. Circulation. 2019;139:2228–37. 10.1161/CIRCULATIONAHA.118.037885 [DOI] [PubMed] [Google Scholar]
- 38.Park C, Guallar E, Linton JA, Lee D-C, Jang Y, Son DK, et al. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Diabetes Care. 2013;36:1988–93. 10.2337/dc12-1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Popele NM, Elizabeth Hak A, Mattace-Raso FUS, Bots ML, Van Der Kuip DAM, Reneman RS, et al. Impaired fasting glucose is associated with increased arterial stiffness in elderly people without diabetes mellitus: the Rotterdam study. J Am Geriatr Soc. 2006;54:397–404. 10.1111/j.1532-5415.2005.00614.x [DOI] [PubMed] [Google Scholar]
- 40.Thomas GN, Chook P, Qiao M, Huang XS, Leong HC, Celermajer DS, et al. Deleterious impact of “high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: the CATHAY study. ATVB. 2004;24:739–43. 10.1161/01.ATV.0000118015.26978.07 [DOI] [PubMed] [Google Scholar]
- 41.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kellenberger LD, Petrik J. Hyperglycemia promotes insulin-independent ovarian tumor growth. Gynecol Oncol. 2018;149:361–70. 10.1016/j.ygyno.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 43.Hou Y, Zhou M, Xie J, Chao P, Feng Q, Wu J. High glucose levels promote the proliferation of breast cancer cells through GTPases. BCTT. 2017;9:429–36. 10.2147/BCTT.S135665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Zhu YD, Gui Q, Wang XD, Zhu YX. Glucagon-induced angiogenesis and tumor growth through the HIF-1-VEGF-dependent pathway in hyperglycemic nude mice. Genet Mol Res. 2014;13:7173–83. 10.4238/2014.September.5.3 [DOI] [PubMed] [Google Scholar]
- 45.Sun XF, Shao YB, Liu MG, et al. High-concentration glucose enhances invasion in invasive ductal breast carcinoma by promoting Glut1/MMP2/MMP9 axis expression. Oncol Lett. 2017;13:2989–95. 10.3892/ol.2017.5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NHANES database is available at https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.