Abstract
Background
Studies have indicated an association between cesarean section (CS), especially elective CS, and an increased risk of celiac disease (CD), but the conclusions of other studies are contradictory. The primary aim of this study (CD-deliver-IT) was to evaluate the rate of CS in a large population of CD patients throughout Italy.
Methods
This national multicenter retrospective study was conducted between December 2020 and November 2021. The coordinating center was the Pediatric Gastroenterology and Liver Unit of Policlinico Umberto I, Sapienza, University of Rome, Lazio, Italy. Eleven other referral centers for CD have participated to the study. Each center has collected data on mode of delivery and perinatal period of all CD patients referring to the center in the last 40 years.
Results
Out of 3,259 CD patients recruited in different Italian regions, data on the mode of delivery were obtained from 3,234. One thousand nine hundred forty-one (1,941) patients (60%) were born vaginally and 1,293 (40%) by CS (8.3% emergency CS, 30.1% planned CS, 1.5% undefined CS). A statistically significant difference was found comparing median age at time of CD diagnosis of patients who were born by emergency CS (4 years, CI 95% 3.40–4.59), planned CS (7 years, CI 95% 6.02–7.97) and vaginal delivery (6 years, CI 95% 5.62–6.37) (log rank p < 0.0001).
Conclusions
This is the first Italian multicenter study aiming at evaluating the rate of CS in a large population of CD patients through Italy. The CS rate found in our CD patients is higher than rates reported in the general population over the last 40 years and emergency CS seems to be associated with an earlier onset of CD compared to vaginal delivery or elective CS in our large nationwide retrospective cohort. This suggests a potential role of the mode of delivery on the risk of developing CD and on its age of onset, but it is more likely that it works in concert with other perinatal factors. Further prospective studies on other perinatal factors potentially influencing gut microbiota are awaited in order to address heavy conflicting evidence reaming in this research field.
Keywords: Celiac disease, Mode of delivery, Cesarean section, Gut microbiota
Background
Celiac disease (CD) is an immune-mediated systemic disorder elicited by gluten and related prolamines in genetically susceptible individuals and characterized by the presence of a variable combination of gluten-dependent clinical manifestations, CD-specific antibodies, human leucocyte antigens (HLA)-DQ2 or HLA-DQ8 haplotypes, and enteropathy [1].
CD provides a unique model for autoimmune research, because the following key elements are known: the specific genes involved in its pathogenesis and the environmental trigger [2]. Although gluten consumption and certain HLA antigen genotypes are key factors for CD development, not all individuals with a predisposing genetic background develop loss of tolerance towards gluten [3, 4]: specifically, about 30% of the general population carry these genes [5], but only about 1% of the population develop CD [6]. This suggests that the risk is likely to be modified by other potential pathophysiological factors [7, 8], which are still to be clarified [9].
Several studies showed intestinal dysbiosis (altered gut microbiota composition or function) in patients with CD, either untreated or treated with a gluten-free diet [10–17]. Gut microbiota affects gut permeability and gut inflammatory activity (both directly and via the release of metabolites), which are suspected to play a role in increasing the risk of autoimmune disorders [18].
Mode of delivery is crucial for the acquisition of the microbiota after birth [19]. It has been hypothesized that infants delivered by cesarean section (CS) acquire different bacterial communities compared to infants born vaginally [19], with potential influence on the short and long-term immune responses to environmental factors, thereby predisposing to autoimmunity [18]. Although different studies have demonstrated the difference in early microbiome development between delivery modes, the underlying pathogenetic mechanisms have not yet been identified.
Studies have indicated an association between CS, especially elective CS [20], and an increased risk of CD [21–24], but the conclusions of other studies on this topic are contradictory [25–29].
Given this background, the primary aim of this study (CD-deliver-IT) was to evaluate the rate of CS in a large population of CD patients throughout Italy.
Methods
This national multicenter retrospective study was conducted between December 2020 and November 2021. The coordinating center was the Pediatric Gastroenterology and Liver Unit of Policlinico Umberto I, Sapienza, University of Rome, Lazio, Italy. Eleven other referral centers for CD have participated to the study (3 other centers located in Lazio, 2 respectively in Campania and in Calabria, 1 in each of these regions: Veneto, Liguria, Piedmont, Sicily).
Each center has collected data on mode of delivery and perinatal period of all CD patients referring to the center in the last 40 years.
These data were collected during planned follow up for CD or, alternatively, medical records were consulted to retrieve previously provided data. Collection of data through consultation of the Certificate of Delivery Assistance (CeDAP), which is the national source for vital birth information, was not possible. Required data were gender, date of birth, year of CD diagnosis, mode of delivery (elective CS or emergency CS or vaginal delivery), gestational age, birth weight, nationality and birthplace. Gestational age was defined as follows: preterm for babies delivered before 37 weeks of gestation, term birth for 37 0/7 weeks through 41 6/7 weeks, post-term if pregnancy has reached or extended beyond 42 0/7 weeks of gestation.
Unavailability of data relating the mode of delivery or unconfirmed CD diagnosis were considered exclusion criteria.
Continuous data were summarized by means (and standard deviation) and median (interquartile range). Categorical data were expressed as counts and percentages. The 95% confidence intervals of the rates was calculated (Wilson method). To compare continuous variables we performed t-test, while to compare categorical variables we used chi square test. We performed a Kaplan Maier method to evaluate the onset time of CD. The level of statistical significance was set at 0.05. All statistical analyzes were performed with STATA v.16.
To determine the sample size, it was assumed that the rate of CS is not very different from the rate at a national level; a sample of 1,372 subjects produced a 95% confidence interval, with a width equal to 5% when the hypothesized rate was equal to 32%.
Approximately 3000 patients have been screened in the study in order to identify the 1,372 subjects with the inclusion criteria, as per the planned size, for which all data have been collected according to CRF. Missing data imputation methods have not been used.
Results
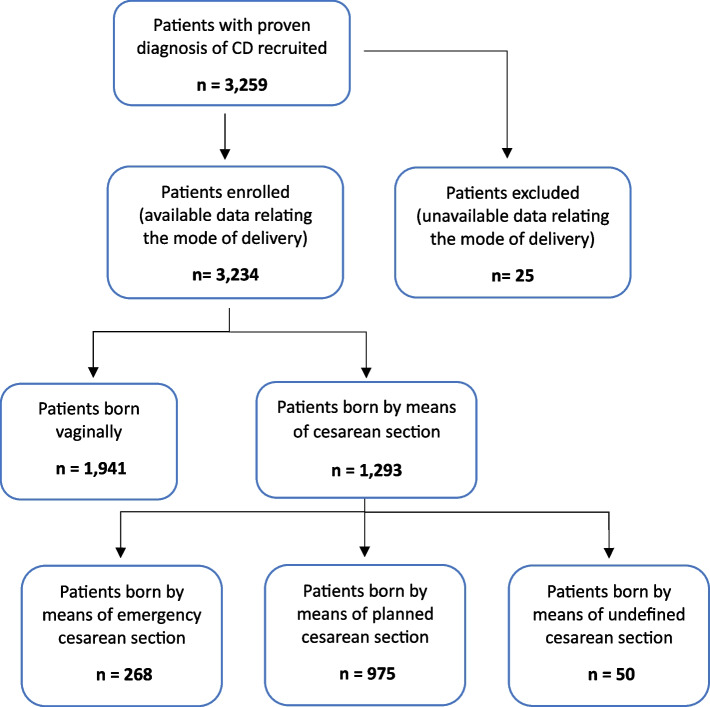
Out of 3,259 patients with proven diagnosis of CD recruited in twelve Gastroenterology Services of different Italian regions, data on the mode of delivery were obtained from 3,234 patients (Table 1). Twenty-five subjects were excluded due to unavailable data relating the mode of delivery (Fig. 1).
Table 1.
Italian regions of enrollment of patients included in the study
| Region of enrollment | Number of patients enrolled |
|---|---|
| Campania | 854 (26.4%) |
| Lazio | 826 (25.5%) |
| Liguria | 500 (15.5%) |
| Calabria | 414 (12.8%) |
| Piedmont | 322 (10%) |
| Sicily | 262 (8.1%) |
| Veneto | 56 (1.7%) |
Fig. 1.
Study population. CD = celiac disease
Out of 3,234 patients (M 1,164, 2 missing) age and age at time of CD diagnosis were known for 3,213 (99.4%) and 3,192 (98.7%) patients, respectively. Median age of patients included in the study was 14.0 years [IQR 9.0–18.0] and median age at time of CD diagnosis was 6.0 years [IQR 3.0–12.0].
As regards the mode of delivery, 1,941 (60%) were born vaginally and 1,293 (40%) by CS (8.3% emergency CS, 30.1% planned CS, 1.5% undefined CS) (Figs. 1 and 2).
Fig. 2.
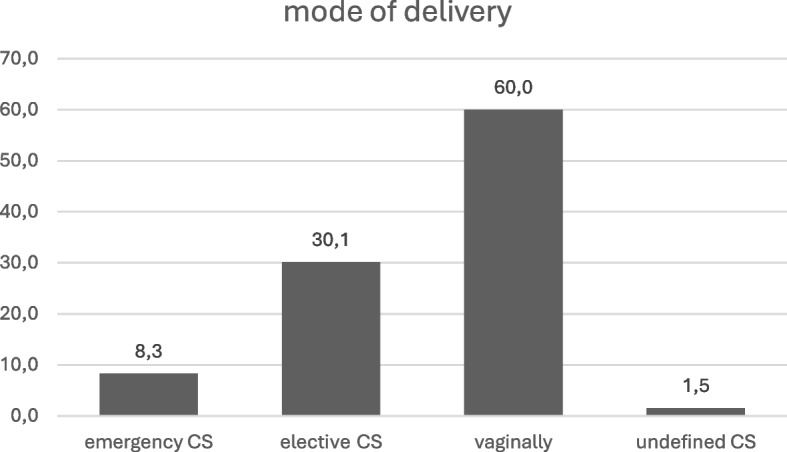
Mode of delivery of patients enrolled. CS = cesarean section
CS rate was also investigated by analyzing birth cohorts, and specifically: 1979–1989 (CS rate: 31%, CI95% 19.5–45), 1990–1999 (CS rate: 42.1%, CI95% 36.5–47.8), 2000–2009 (CS rate: 40.2%, CI95% 37.8–42.6) and 2010–2021 (CS rate: 39.5%, CI95% 36.7–42.3).
1745 newborn (54%) were born at term, 724 newborn (22.4%) were preterm, 263 newborn (8.1%) post-term; gestational age was unknown in 502 newborn (15.5%).
Nationality has been registered as Italian and foreign in 97.8% and 1.9% of patients respectively, whereas it was unknown in 0.3% of patients. Place of birth was recorded for 3,194 patients (98.8%) and it was a foreign state for 26 patients (0.8%); this data has not been retrieved for 40 (1.2%). Birthplace and region of enrollment were the same for 92% of patients.
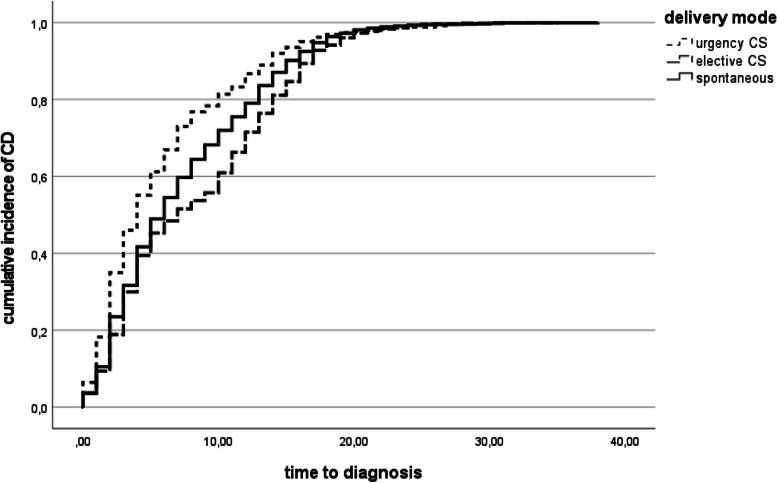
With regards to median age at time of CD diagnosis, it was 6 years for patients delivered naturally (CI 95% 5.62–6.37) and 6 years for those born by CS (CI 95% 5.42–6.57). When considering the type of CS, a statistically significant difference was found comparing median age at time of CD diagnosis of patients who were born by emergency CS (4 years, CI 95% 3.40–4.59), planned CS (7 years, CI 95% 6.02–7.97) and vaginal delivery (6 years, CI 95% 5.62–6.37) (log rank p < 0.001) (Fig. 3).
Fig. 3.
Median age at time of CD diagnosis when considering the type of CS. CS = cesarean section
Among patients who were born preterm, a statistically significant difference was found comparing median age at time of CD diagnosis of patients who were born by emergency CS (4 years, CI 95% 3.22–4.78) and those born by planned CS (6 years, CI 95% 4.76–7.23) (log rank p = 0.019).
When considering patients whose gestational age was at term, statistically significant differences with regard to median age at time of diagnosis were observed between patients born by emergency CS (3 years, CI 95% 2.05–3.94) and those born by planned CS (4 years, CI 95% 3.54–4.45) (log rank p = 0.001) and also when comparing patients born by emergency CS with those born by vaginal delivery (5 years, CI 95% 4.70–5.29) (log rank p = 0.001).
In post-term patients, median age at time of diagnosis was 4 years (CI 95% 2.41–5.58) in patients born by emergency CS and 12 years (CI 95% 9.11–14.88) in patients born by planned CS (log rank p < 0.001).
Considering data according to gender, statistically significant differences were found comparing median age at time of CD diagnosis both in males and females. Specifically, it was 4 years (CI 95% 2.82- 5.17) in males born by emergency CS and 7 years (CI 95% 5.33–8.66) in males born by planned CS (log rank p = 0.012). As regards females, a statistically significant difference was observed when comparing patients born by emergency CS (4 years, CI 95% 3.26–4.73) with those born by planned CS (7 years, CI 95% 5.56–8.43) (log rank p < 0.001) and with those born vaginally (6 years, CI 95% 5.51–6.48) (log rank p < 0.001).
Discussion
Our study depicts the mode of delivery of a large dataset of CD patients followed-up at referral centers. The CS rate in our CD patients was found to be 40% (95% CI 38.2–41.6), a higher rate compared to the CS rates recorded in the last 40 years in Italy in the general population. In fact, although the frequency of CS has increased significantly in Italy in recent decades (11.2% in 1980, 27.9% in 1996, 33.2% in 2000, 37.5% in 2010, 31.2% in 2021) (CeDAP registry), with a slightly decreasing trend in last years, the CS rate observed among our CD patients is higher than the CS rate in the respective years even when examining specific birth cohorts. Whether the higher rate of CS observed in our CD population could suggest a potential pathogenetic role of CS in CD it is not possible to evaluate given our study design.
We found that emergency CS seems to be associated with an earlier onset of CD compared to vaginal delivery or elective CS in 3,234 patients with diagnosed CD. Specifically, an earlier diagnosis of CD was found in children born by emergency CS compared to planned CS in the analysis of all patients enrolled and also in all subgroup analysis: this result was found in preterm, at-term and post-term patients and in both males and females. An earlier CD diagnosis in children born by emergency CS compared to children born vaginally was found analyzing all patients enrolled and in two subgroup analysis (at-term born babies and females).
Although our results may seem controversial, considering that infants delivered by emergency CS are exposed to the maternal vaginal microbiota during birth as well as those born naturally, the dogma that difference in early microbiome development between delivery modes is due to lack of vaginal exposure was recently challenged in a study by Mitchell et al. [30]. The similarity between infants delivered by CS before or after labor in that study is consistent with findings in the Baby Biome Study [31]: these authors have reported disrupted maternal transmission of Bacteroides strains and high level colonization by healthcare-associated opportunistic pathogens in babies born by CS and in those delivered vaginally with maternal antibiotic prophylaxis or not breastfed during the neonatal period. Indeed, it is recognized that the gut microbiota composition can be perturbed by other early life events, including feeding and antibiotic exposure [24, 31].
It is therefore understandable why the literature framework on this topic is wide and, often, contradictory. Some studies reported an increased risk of CD in individuals born by CS [20, 21, 32–34], whereas other studies did not [6, 22, 25, 26, 28, 29, 35–37].
Specifically, studies reporting an increased risk of CD in individuals born by CS have shown different evidence as regards the type of CS (planned or emergency CS) [20, 21, 32–34].
In the retrospective, multicenter, case–control study by Decker et al. [21] a significantly enhanced likelihood of being born by CS was found in children with CD compared with control subjects. A register-based national cohort study by Andersen et al. [32] found an increased risk of diabetes, arthritis, CD, and inflammatory bowel disease after CS compared with vaginal delivery: in a subgroup analysis, both acute and elective CS was associated with an increased risk of developing a chronic inflammatory disease. A recent study by Maleki et al. [34] concluded that babies born vaginally have a lower risk of developing CD than children born by CS.
On the other hand, Marild et al. [20] found a positive association between planned CS and later CD, but no increased risk of CD following emergency or any CS. Also analysis of data from a large United States-based mother- child cohort [33] revealed that being born by CS without labor may be associated with an increased risk for CD, while infants born by CS with labor did not.
The conclusions of other studies on this topic are different. According to the results of an Italian population-based birth cohort study published in 2014 perinatal factors, including CS, have little influence on the risk of childhood CD; otherwise, use of antibiotics and gastrointestinal infections in the first year of life may facilitate the early onset of CD by altering intestinal microflora and the gut mucosal barrier [25, 28]. One year later the Norwegian Mother and Child cohort study concluded that CD was not associated with mode of delivery [22]. In 2018 Sander et al. published the results of a large registry-based study, showing that mode of delivery was not associated with an increased risk of diagnosed CD [6] and the Teddy study of the same year went in a similar direction, finding that CS was not associated with increased risk for CD in the offspring [29]. The mode of delivery did not influence the risk of developing CD neither in a study by Lionetti et al. involving a cohort of children genetically predisposed to CD and prospectively followed from birth [35].
No associations between CS and CD were found in the study by Sevelsted et al. [26] analyzing children born by CS for risk of chronic immune diseases. On the other hand, a study published in 2021 on a cohort study of over 900,000 children showed that CS was not associated with the risk of hospitalization for a range of autoimmune disorders before 14 years of age, suggesting that this mode of delivery may not be related to the development of autoimmunity [36].
To summarize comprehensive data available, a recent systematic review and meta-analysis found that, compared with spontaneous birth, CS was not associated with an increased risk of CD and in subgroup analyses the association remained non-significant for both infants born after planned CS and emergency CS [37]. Authors underlines that all of studies in this meta-analysis did not report antibiotic exposure and thus they are unable to eliminate influences antibiotic on risk of CD. Moreover, they suggest that investigation of the association between CS and CD in children should take also breastfeeding into consideration. In addition to this, authors recognize that this meta-analysis is limited by the inclusion of exclusively observational studies, which are susceptible to confounding and that inadequate control for potential confounders may bias the results.
Among these studies with conflicting results, a potential explanation of an earlier diagnosis of CD in children born by emergency CS in our study could be found considering also other perinatal factors besides CS. Breastfeeding seems to moderate the effects of CS [38–40] and intrapartum antibiotics [41] on the early microbiota, producing a microbiota profile more similar to that of infants born vaginally or those not receiving antibiotics [42, 43]. Duration of breastfeeding is usually shorter in patients born by means of emergency CS compared to patients born by scheduled CS or spontaneous birth [44] and different studies have shown that prolonged breastfeeding is associated with a postponed diagnosis of CD [45–48]. Anyway, this perinatal factor was not investigated in our study.
Moreover, it might be reasonable to assume that emergency CS could imply an increased risk of infection, related to the indication for the procedure, for instance, the occurrence of an infection in the fetus or labor that has been prolonged, as well as the procedure itself [49]. The increased risk of infection entails a more frequent use of antibiotics in these babies and/or laboring mothers, and it is reported that exposure to early infections or antibiotics is associated to subsequent CD [50, 51]. Nevertheless, this hypothesis is not possible to be confirmed by our study design.
To our knowledge, this is the first Italian multicenter study aiming at evaluating the rate of CS in a large population of CD patients through Italy. The large population enrolled and the speed data collection represent strengths of this study. Moreover, only 0.8% of patients enrolled were born abroad, while the majority of CD patients included in this study were born in Italy (regions of Northern, Central and Southern of Italy), so data of this study could be applied to the whole Italian territory.
Nevertheless, we are aware of some limitations. The lack of information about other potential confounding factors, including perinatal and maternal factors, such as perinatal use of antibiotics, perinatal infections, duration of breastfeeding, family history for CD and social background is a limitation of our study. Moreover, data relating to the type of CS (planned or emergency CS) and to the gestational age were not available in 1.5% and 15.5% of patients respectively, although these percentages are low.
Conclusions
In conclusion, the CS rate found in our CD patients is higher than rates reported in the general population over the last 40 years and emergency CS seems to be associated with an earlier onset of CD compared to vaginal delivery or elective CS in our large nationwide retrospective cohort. This suggests that mode of delivery could have a potential role on the risk of developing CD and on its age of onset, but it is more likely that it works in concert with other perinatal factors. Further prospective studies on other perinatal factors potentially influencing gut microbiota are awaited in order to clarify this intriguing topic and to address heavy conflicting evidence reaming in this research field.
Acknowledgements
All the authors thank the Italian Society for Pediatric Gastroenterology, Hepatology and Nutrition (SIGENP) for the allowing space and discussion on this project in all the meetings.
We would like to thank the “Food-induced disease” study group of SIGENP: Luigi Principessa, Elisa D'Angelo, Basilio Malamisura, Angela Calvi, Noemi Zampatti, Ilaria Montafia, Antonella Diamanti, Pasquale Pisano.
Abbreviations
- CS
Cesarean section
- CD
Celiac disease
- HLA
Human leucocyte antigens
- CeDAP
Certificate of Delivery Assistance
Authors’ contributions
DI conceived and designed the study, enrolled participants, collected the data, and drafted the manuscript. FV conceived and designed the study, contributed data interpretation and drafted the manuscript. AV took care of statistical analyses and interpretation of data and drafted the manuscript. GDA enrolled participants, coordinated and supervised data collection. TP enrolled participants, collected the data and contributed data interpretation. MC enrolled participants, coordinated and supervised data collection. FM enrolled participants, collected the data and contributed data interpretation. AM enrolled participants, coordinated and supervised data collection. IR enrolled participants, collected the data and contributed data interpretation. LP enrolled participants, coordinated and supervised data collection. LG enrolled participants, collected the data and contributed data interpretation. FG enrolled participants, enrolled participants, coordinated and supervised data collection. MC enrolled participants, collected the data and contributed data interpretation. FF enrolled participants, enrolled participants, coordinated and supervised data collection. CMT enrolled participants, collected the data and contributed data interpretation. CP enrolled participants, collected the data and contributed data interpretation. MI enrolled participants, collected the data and contributed data interpretation. CB enrolled participants, collected the data and contributed data interpretation. RL contributed data analysis and interpretation and critically revised the manuscript. MM conceived and designed the study, supervised the whole project, contributed data interpretation, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
No financial assistance was received in support of the study. All authors benefited from an unconditional grant by Katia Scaiola, which covered the article publishing fee.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was launched after approval by the Ethical Committee of “Sapienza” University of Rome, Italy (December 2020, reference n. 6185, Prot. 0936/2020).
Participants were informed about the aim of the study, and a written consent was obtained for each patient, if the enrollment took place within a context where the patient or a parent of the patient was present. Written consent in young adults was provided by patients themselves; in case of patients under 18, a parental written consent and an age-adapted written consent were obtained; different information leaflets about the study were available in plain language and suitable for different age groups.
Where contacting data subjects to provide them with information on the processing of their data has proved impossible on organizational grounds, the processing of personal data was done without their informed consent on the basis of General Authorization to Process Personal Data for Scientific Research Purposes—1 March 2012.
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Monica Montuori, Email: m.montuori@policlinicoumberto1.it.
the “Food-induced disease” study group of SIGENP:
Luigi Principessa, Elisa D’Angelo, Basilio Malamisura, Angela Calvi, Noemi Zampatti, Ilaria Montafia, Antonella Diamanti, and Pasquale Pisano
References
- 1.Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54(1):136–60. 10.1097/MPG.0b013e31821a23d0 [DOI] [PubMed] [Google Scholar]
- 2.Leonard MM, Fasano A. Gluten and celiac disease risk: is it just a matter of quantity? JAMA. 2019;322(6):510–1. 10.1001/jama.2019.9678 [DOI] [PubMed] [Google Scholar]
- 3.Tjon JML, van Bergen J, Koning F. Celiac disease: how complicated can it get? Immunogenetics. 2010;62(10):641–51. 10.1007/s00251-010-0465-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronsson CA, Lee HS, Hård Af Segerstad EM, Uusitalo U, Yang J, Koletzko S, et al. Association of gluten intake during the first 5 years of life with incidence of celiac disease autoimmunity and celiac disease among children at increased risk. JAMA. 2019;322(6):514–23. 10.1001/jama.2019.10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madsen M, Svejgaard A, Graugaard B, Jakobsen BK, Morling N, Platz P, et al. HLA-B, -DR haplotype frequencies and gametic association in 1,204 unrelated Danes. Tissue Antigens. 1981;18(4):276–9. 10.1111/j.1399-0039.1981.tb01392.x [DOI] [PubMed] [Google Scholar]
- 6.Sander SD, Hansen AV, Størdal K, Nybo Andersen AM, Murray JA, Husby S. Mode of delivery is not associated with celiac disease. Clin Epidemiol. 2018;10:323–32. 10.2147/CLEP.S152168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356(6333):44–50. 10.1126/science.aah5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemppainen KM, Lynch KF, Liu E, Lönnrot M, Simell V, Briese T, et al. Factors that increase risk of celiac disease autoimmunity after a gastrointestinal infection in early life. Clin Gastroenterol Hepatol. 2017;15(5):694-702.e5. 10.1016/j.cgh.2016.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludvigsson JF, Green PH. The missing environmental factor in celiac disease. N Engl J Med. 2014;371(14):1341–3. 10.1056/NEJMe1408011 [DOI] [PubMed] [Google Scholar]
- 10.Cenit MC, Olivares M, Codoñer-Franch P, Sanz Y. Intestinal microbiota and celiac disease: cause, consequence or co-evolution? Nutrients. 2015;7(8):6900–23. 10.3390/nu7085314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdu EF, Galipeau HJ, Jabri B. el players in coeliac disease pathogenesis: role of the gut microbiota. Nat Rev Gastroenterol Hepatol. 2015;12(9):497–506. 10.1038/nrgastro.2015.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J, Kalliomäki M, Heilig HG, Palva A, Lähteenoja H, de Vos WM, et al. Duodenal microbiota composition and mucosal homeostasis in pediatric celiac disease. BMC Gastroenterol. 2013;13:113. 10.1186/1471-230X-13-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez E, Donat E, Ribes-Koninckx C, Fernández-Murga ML, Sanz Y. Duodenal-mucosal bacteria associated with celiac disease in children. Appl Environ Microbiol. 2013;79(18):5472–9. 10.1128/AEM.00869-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sellitto M, Bai G, Serena G, Fricke WF, Sturgeon C, Gajer P, et al. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS One. 2012;7(3):e33387. 10.1371/journal.pone.0033387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Cagno R, De Angelis M, De Pasquale I, Ndagijimana M, Vernocchi P, Ricciuti P, et al. Duodenal and faecal microbiota of celiac children: molecular, phenotype and metabolome characterization. BMC Microbiol. 2011;11:219. 10.1186/1471-2180-11-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 2008;8:232. 10.1186/1471-2180-8-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wacklin P, Laurikka P, Lindfors K, Collin P, Salmi T, Lähdeaho ML, et al. Altered duodenal microbiota composition in celiac disease patients suffering from persistent symptoms on a long-term gluten-free diet. Am J Gastroenterol. 2014;109(12):1933–41. 10.1038/ajg.2014.355 [DOI] [PubMed] [Google Scholar]
- 18.McLean MH, Dieguez D Jr, Miller LM, Young HA. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut. 2015;64(2):332–41. 10.1136/gutjnl-2014-308514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5. 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mårild K, Stephansson O, Montgomery S, Murray JA, Ludvigsson JF. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012;142(1):39–45. 10.1053/j.gastro.2011.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decker E, Engelmann G, Findeisen A, Gerner P, Laass M, Ney D, et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics. 2010;125(6):e1433–40. 10.1542/peds.2009-2260 [DOI] [PubMed] [Google Scholar]
- 22.Emilsson L, Magnus MC, Størdal K. Perinatal risk factors for development of celiac disease in children, based on the prospective Norwegian mother and child cohort study. Clin Gastroenterol Hepatol. 2015;13(5):921–7. 10.1016/j.cgh.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namatovu F, Olsson C, Lindkvist M, Myléus A, Högberg U, Ivarsson A, et al. Maternal and perinatal conditions and the risk of developing celiac disease during childhood. BMC Pediatr. 2016;16:77. 10.1186/s12887-016-0613-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard MM, Karathia H, Pujolassos M, Troisi J, Valitutti F, Subramanian P, et al. Multi-omics analysis reveals the influence of genetic and environmental risk factors on developing gut microbiota in infants at risk of celiac disease. Microbiome. 2020;8(1):130. 10.1186/s40168-020-00906-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts SE, Williams JG, Meddings D, Davidson R, Goldacre MJ. Perinatal risk factors and coeliac disease in children and young adults: a record linkage study. Aliment Pharmacol Ther. 2009;29(2):222–31. 10.1111/j.1365-2036.2008.03871.x [DOI] [PubMed] [Google Scholar]
- 26.Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135(1):e92–8. 10.1542/peds.2014-0596 [DOI] [PubMed] [Google Scholar]
- 27.Adlercreutz EH, Wingren CJ, Vincente RP, Merlo J, Agardh D. Perinatal risk factors increase the risk of being affected by both type 1 diabetes and coeliac disease. Acta Paediatr. 2015;104(2):178–84. 10.1111/apa.12836 [DOI] [PubMed] [Google Scholar]
- 28.Canova C, Zabeo V, Pitter G, Romor P, Baldovin T, Zanotti R, et al. Association of maternal education, early infections, and antibiotic use with celiac disease: a population-based birth cohort study in northeastern Italy. Am J Epidemiol. 2014;180(1):76–85. 10.1093/aje/kwu101 [DOI] [PubMed] [Google Scholar]
- 29.Koletzko S, Lee HS, Beyerlein A, Aronsson CA, Hummel M, Liu E, et al. Cesarean section on the risk of celiac disease in the offspring: the Teddy study. J Pediatr Gastroenterol Nutr. 2018;66(3):417–24. 10.1097/MPG.0000000000001682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell CM, Mazzoni C, Hogstrom L, Bryant A, Bergerat A, Cher A, et al. Delivery mode affects stability of early infant gut microbiota. Cell Rep Med. 2020;1(9):100156. 10.1016/j.xcrm.2020.100156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574(7776):117–21. 10.1038/s41586-019-1560-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen V, Möller S, Jensen PB, Møller FT, Green A. Caesarean delivery and risk of chronic inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, coeliac disease, and diabetes mellitus): a population based registry study of 2,699,479 births in Denmark during 1973–2016. Clin Epidemiol. 2020;12:287–93. 10.2147/CLEP.S229056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanpowpong P, Li S, Espinola JA, Santos LC, James KE, Powe CE, et al. Pregnancy- and birth-related risk factors for the development of childhood celiac disease. Acta Paediatr. 2023;112(5):1029–34. 10.1111/apa.16686 [DOI] [PubMed] [Google Scholar]
- 34.Maleki M, MontazeriFar F, Payandeh A, Azadbakht Z. Prevalence of celiac disease and its related factors in children aged 2–6 years old: a case-control study. Nutr Health. 2023:2601060231167456. 10.1177/02601060231167456. Online ahead of print. [DOI] [PubMed]
- 35.Lionetti E, Castellaneta S, Francavilla R, Pulvirenti A, Catassi C, SIGENP Working Group of Weaning and CD Risk. Mode of delivery and risk of celiac disease: risk of celiac disease and age at gluten introduction cohort study. J Pediatr. 2017;184:81-86.e2. 10.1016/j.jpeds.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 36.Soullane S, Henderson M, Kang H, Luu TM, Lee GE, Auger N. Cesarean delivery and risk of hospitalization for autoimmune disorders before 14 years of age. Eur J Pediatr. 2021;180(11):3359–66. 10.1007/s00431-021-04132-w [DOI] [PubMed] [Google Scholar]
- 37.Yang XY, Liu YH, Jiang HY, Ying XH. Cesarean section is not associated with increased risk of celiac disease in the offspring: a meta-analysis. J Matern Fetal Neonatal Med. 2022;35(25):9570–7. 10.1080/14767058.2022.2048813 [DOI] [PubMed] [Google Scholar]
- 38.Guo C, Zhou Q, Li M, Zhou L, Xu L, Zhang Y, et al. Breastfeeding restored the gut microbiota in caesarean section infants and lowered the infection risk in early life. BMC Pediatr. 2020;20(1):532. 10.1186/s12887-020-02433-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Qin S, Song Y, Feng Y, Lv N, Xue Y, et al. The perturbation of infant gut microbiota caused by cesarean delivery is partially restored by exclusive breastfeeding. Front Microbiol. 2019;10:598. 10.3389/fmicb.2019.00598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coker MO, Laue HE, Hoen AG, Hilliard M, Dade E, Li Z, et al. Infant feeding alters the longitudinal impact of birth mode on the development of the gut microbiota in the first year of life. Front Microbiol. 2021;12:642197. 10.3389/fmicb.2021.642197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983–93. 10.1111/1471-0528.13601 [DOI] [PubMed] [Google Scholar]
- 42.Davis EC, Wang M, Donovan SM. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes. 2017;8(2):143–71. 10.1080/19490976.2016.1278104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis EC, Dinsmoor AM, Wang M, Donovan SM. Microbiome composition in pediatric populations from birth to adolescence: impact of diet and prebiotic and probiotic interventions. Dig Dis Sci. 2020;65(3):706–22. 10.1007/s10620-020-06092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stokholm J, Thorsen J, Chawes BL, Schjørring S, Krogfelt KA, Bønnelykke K, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. 2016;138(3):881-889.e2. 10.1016/j.jaci.2016.01.028 [DOI] [PubMed] [Google Scholar]
- 45.Stevens FM, Egan-Mitchell B, Cryan E, McCarthy CF, McNicholl B. Reasing incidence of coeliac disease. Arch Dis Child. 1987;62(5):465–8. 10.1136/adc.62.5.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mäki M, Kallonen K, Lähdeaho ML, Visakorpi JK. Changing pattern of childhood coeliac disease in Finland. Acta Paediatr Scand. 1988;77(3):408–12. 10.1111/j.1651-2227.1988.tb10668.x [DOI] [PubMed] [Google Scholar]
- 47.Kelly DA, Phillips AD, Elliott EJ, Dias JA, Walker-Smith JA. Rise and fall of coeliac disease 1960–85. Arch Dis Child. 1989;64(8):1157–60. 10.1136/adc.64.8.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popp A, Mäki M. Changing pattern of childhood celiac disease epidemiology: contributing factors. Front Pediatr. 2019;7:357. 10.3389/fped.2019.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dencker A, Lyckestam Thelin I, Smith V, Lundgren I, Nilsson C, Li H, et al. Neonatal outcomes associated with mode of subsequent birth after a previous caesarean section in a first pregnancy: a Swedish population-based register study between 1999 and 2015. BMJ Paediatr Open. 2022;6(1):e001519. 10.1136/bmjpo-2022-001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dydensborg Sander S, Nybo Andersen AM, Murray JA, Karlstad Ø, Husby S, Størdal K. Association between antibiotics in the first year of life and celiac disease. Gastroenterology. 2019;156(8):2217–29. 10.1053/j.gastro.2019.02.039 [DOI] [PubMed] [Google Scholar]
- 51.Da Fonte TM, Valitutti F, Kenyon V, Locascio JJ, Montuori M, Francavilla R, et al. Zonulin as a biomarker for the development of celiac disease. Pediatrics. 2024;153(1):e2023063050. 10.1542/peds.2023-063050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.