Abstract
Background
Epigenetic dysregulation has been implicated in the development and progression of a variety of human diseases, but epigenetic changes are reversible, and epigenetic enzymes and regulatory proteins can be targeted using small molecules. Histone deacetylase inhibitors (HDACis), as a class of epigenetic drugs, are widely used to treat various cancers and other diseases involving abnormal gene expression.
Results
Specially, HDACis have emerged as a promising strategy to enhance the therapeutic effect of non-neoplastic conditions, including neurological disorders, cardiovascular diseases, renal diseases, autoimmune diseases, inflammatory diseases, infectious diseases and rare diseases, along with their related mechanisms. However, their clinical efficacy has been limited by drug resistance and toxicity.
Conclusions
To date, most clinical trials of HDAC inhibitors have been related to the treatment of cancer rather than the treatment of non-cancer diseases, for which experimental studies are gradually underway. Discussions regarding non-neoplastic diseases often concentrate on specific disease types. Therefore, this review highlights the development of HDACis and their potential therapeutic applications in non-neoplastic diseases, either as monotherapy or in combination with other drugs or therapies.
Keywords: Histone deacetylase, Histone deacetylase inhibitors, Non-neoplastic diseases
1. Introduction
Epigenetics encompasses a wide spectrum of genetic changesc in gene expression caused by environmental influences rather than alterations in DNA sequenc [1]. The main regulatory mechanisms of epigenetics include DNA methylation, histone modification, chromatin remodeling, and other processes [2]. Various enzymes involved in epigenetics play important roles in life processes, includinghistone deacetylase (HDAC). HDAC, an enzyme that acetylates histones and represses gene expression, is usually tightly controlled regarding expression and activity. Recent evidence indicates that aberrant activation or overproduction of HDACs is frequently found in many pathological processes involving systemic non-neoplastic diseases, including neurological diseases, cardiovascular disease (CVD), kidney disease, autoimmune diseases, inflammatory diseases, infectious diseases and rare diseases In specific, existing reports indicate that histone hypoacetylation and transcriptional changes are associated with various neurodegenerative diseases [3]. Then, HDAC2 phosphorylation plays an important role in pathological processes such as cardiac hypertrophy and heart failure [4]. Furthermore, class III HDACs modulate pathological changes in advanced diabetic nephropathy (DN) with oxidative stress, fibrosis, and EMT in renal tubules [[5], [6], [7]], class I HDACs are critical in reprogramming intestinal macrophages to adapt to chronic microbial exposure [8], and Class IIa HDACs can reversibly shuttle between the nucleus and cytoplasm after phosphorylation of specific serine residues [9]. Histone deacetylase inhibitors (HDACis) are compounds extensively researched over the years. Although hematological malignancies form the majority of clinical indications for HDACis [10], they are considered to have therapeutic potential for a wide range of other diseases, including (but not limited to) cancer, cardiovascular diseases, neurological diseases, renal diseases, infectious diseases, inflammatory diseases, autoimmune diseases, and depression [[11], [12], [13]], as shown in Fig. 1. Several phase I and phase II clinical trials involving HDACis are currently underway, and novel HDACis have improved safety, efficacy, brain penetration capability, and selectivity, dramatically improving treatment outcomes in various non-neoplastic indications [14].
Fig. 1.
HDACis in non-neoplastic diseases. HDACis: histone deacetylase inhibitors.
HDACs potentially play a role in nearly every aspect of health and disease. Here, the review particularly emphasizes details on HDACis and their potential therapeutic application and related mechanisms in various non-neoplastic diseases to understand further the relevant changes in HDAC activity in specific diseases and the corresponding effective therapeutic measures.
2. HDAC and HDACis
2.1. Overview of HDAC
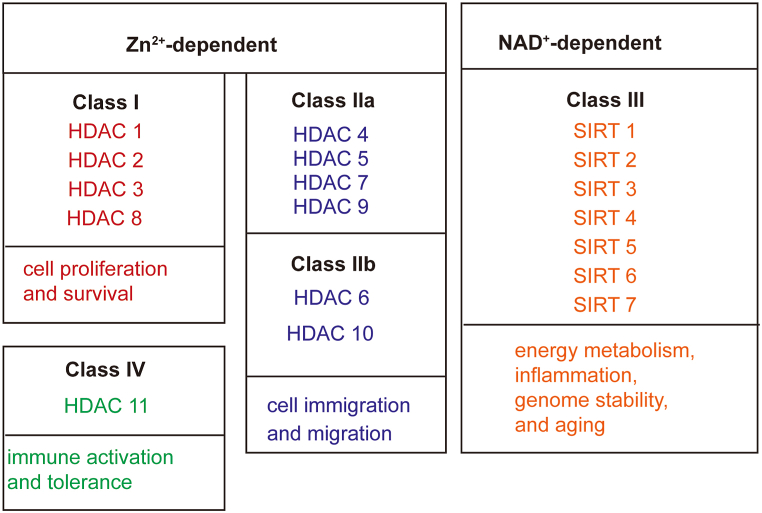
Histones are basic nuclear proteins that are the carriers of DNA in eukaryotes. Regulating gene expression through a series of post-translational modifications, including acetylation, phosphorylation, and methylation [15]. Histone acetylation is mainly regulated by histone acetyltransferases (HATs) and HDACs. HATs promote the acetylation of histones, loosen the chromatin structure, and promote the binding of corresponding transcription factors, thereby promoting gene expression. In contrast, HDACs deacetylate histones leadi to chromosome condensation and inhibition of gene transcription and translation [16]. HDAC, a common histone modification and a type of epigenetic regulation, affects chromosome packaging and DNA transcription. HDAC is a specific enzyme that induces the deacetylation of histone and non-histone proteins, playing an essential role in regulating physiological and pathological gene expression. The HDAC enzyme family in humans comprises 18 members and four classes (Fig. 2). Class I HDACs (HDAC-1, -2, -3, and -8) are essential for cell proliferation and survival; HDAC-1, -2, and -8 are often exclusively found in the nucleus, while HDAC-3 shuttles between the cytoplasm and nucleus [17]. Class II HDACs (HDAC4, 5, 7, and 9 [IIa]; HDAC6 and 10 [IIb]) are distributed throughout the nucleus and cytoplasm and have roles in cell migration and the inflammatory response [18]. Class III HDACs (sirtuins [Sirts]: Sirt1-7) are widely expressed and implicated in ageing, inflammation, energy metabolism, and genome stability [19]. Class IV HDACs (HDAC11), whose function is currently unclear, contribute to the balance between immune activation, tolerance, and evolutionary conservation [20]. Class I, II, and IV HDACs, known as “classical HDACs,” are Zinc-dependent enzymes, whereas class III sirtuins are NAD+-dependent enzymatic HDACs [21]. Aberrant expression and activation of HDACs have been reported to be related to tumors, and other non-neoplastic diseases, which is the focus of this review.
Fig. 2.
Classification of HDAC enzymes and their main roles. HDAC: Histone deacetylase.
2.2. Overview of HDACis
Most functions of HDACs have been revealed through the application of HDACis. HDACis, a class of natural and synthetic compounds, promote histone acetylation and chromatin remodeling, thereby facilitating the correct positioning of nucleosomes and restoring gene expression [22]. Besides histones, HDACi molecules have been found to modulate the acetylation levels of non-histone proteins, which has potential value in treating multiple disease loci [23]. HDACis have three structural domains: a zinc-binding domain, a surface recognition motif, and a linker region with a linear or circular structure [24], as illustrated in Fig. 3. HDACis can be classified into four categories based on their chemical structures as shown in Table 1: 1) short-chain fatty acids, such as sodium butyrate (NAB) and valproic acid (VPA); 2) cyclic peptides, such as Romidepsin and Trapoxin A; 3) hydroxamic acids, such as Trichostatin A (TSA) and Vorinostat (SAHA); and 4) benzamides drugs, such as entinostat and mocetinostat [25]. Hydroxamic acid, as a broad-spectrum class of HDACis, can inhibit both class I and II HDACs with nM potency. They are the most studied and have the highest molecular weight among the four HDACis, with short half-life but long-lasting effects [26]. Cyclic peptides are the most structurally complex group of HDACis, including depsipeptides, apixidine, and a group of cyclic hydroxamic acid-containing peptide molecules, which are generally considered class I HDACis. It is worth noting that the structures of such inhibitors are quite different, so their selectivities are also different [27]. Benzamide is an orally bioavailable drug that potently and selectively inhibits class I and IV HDAC enzymes [28]. Short-chain fatty acids seem to be the relatively moderate HDACi with some limited class I HDAC selectivity. Interestingly, recent evidence has revealed that Chinese herbal medicines and active ingredients, such as curcumin [29], ginsenoside [30], crotonin [31], cinnamic acid [32], baicalein [33], and emodin [31], also possess HDAC inhibition effects. Moreover, ketone bodies have been shown to function as endogenous HDACis [34,35]. Currently, five HDACis have been approved by the U.S. Food and Drug Administration for the treatment of blood cancers and certain solid tumors [36]. These five HDACs are effective but lack selectivity. They interact with other important metalloenzymes, resulting in cell cycle arrest, apoptosis, metastasis, and differentiation, leading to significant side effects such as nausea, vomiting, and fatigue, all of which are clinically manageable [37,38]. Although tumors have been the main target of HDACis, they have been proven effective in treating certain non-neoplastic diseases, as indicated in Table 2. The search for potential and selective inhibitors for the different isoforms of HDACs is currentlya major challenge, so current research focuses on the development of selective inhibitors of 18 HDAC subtypes. Chen et al. demonstrate that the addition of chidamide, a class I HDAC inhibitor, to a combination of the VEGFR inhibitor regorafenib and an anti-PD-1 antibody induces durable immunosuppression in the mouse colon cancer tumor microenvironment. Tumor-specific response [39]. Secondly, Susetyo et al. discussed the effect of the HDAC3 inhibitor RGFP966 on cerebellar morphological defects in perinatal hypothyroid mice. This inhibitor has been shown to induce histone hyperacetylation and promote transcription of thyroid hormone-responsive gene loci, potentially becoming a novel treatment for cerebellar abnormalities caused by hypothyroidism [40]. Another study by Wang et al. focused on the topic of selectivity and found that BG45, as a selective HDAC3 inhibitor, has potential and extended therapeutic potential for Alzheimer's disease (AD) by reducing inflammation and controlling the CREB/BDNF/NF-kB pathway [41]. Another reported zinc-binding moiety, the 1,2,4 trifluoromethyloxadiazole (TFMO) moiety, was used in the study of Bollmann et al. to generate class II selective compounds, and they developed a TMFO-based Class IIa inhibitor (YAK540), and analyzed the combined effect of YAK 540 and the proteasome inhibitor bortezomib in inducing leukemia cell apoptosis [42]. A different mode of action was tested in the study by Darwish et al. who worked to develop proteolytically targeting chimeras (PROTACs) of HDAC8 and study their activity against neuroblastoma. Studies have shown that the developed PROTAC strongly degrades HDAC8 protein in neuroblastoma cells. Since inhibition of HDAC8 in neuroblastoma cells is known to induce signs of neuronal differentiation, such as neurite outgrowth, the most promising PROTAC was investigated in combination with retinoic acid (ATRA), a known inducer of neuronal differentiation. Results showed that the combination significantly enhanced the differentiation phenotype compared to either substance alone [43].
Fig. 3.
Three structural domains of HDACis.
Table 1.
Classification and chemical structure of HDACis.
| Drug types | Drug names | Chemical structure |
|---|---|---|
| Short-chain fatty acids | Sodium butyrate (NAB) |  |
| Valproic acid (VPA) |  |
|
| Cyclic peptides | Romidepsin |  |
| Trapoxin A |  |
|
| Hydroxamic acid | Vorinostat (SAHA) |  |
| Trichostatin A (TSA) |  |
|
| Benzamides | Entinostat |  |
| Mocetinostat |  |
Table 2.
Research status of HDACis.
| Name of HDACis | Target | Status | Disease |
|---|---|---|---|
| Romidepsin | class I HDAC | animal model, cell experiment, phase 2A trial | AD [44]; atherosclerosis [45]; arthritis [46]; COVID-19 [47]; AIDS [48] |
| Apicidin | class I HDAC | animal model; cell experiment | AD [49]; PD [50]; cardiac diseases [51]; |
| MS-275 | class I HDAC | animal model | AD [5]; autism [52]; RA [38]; colitis [53]; diabetes [54] |
| RGFP966 | HDAC3 | animal model | HD [55]; AD [56,57]; atherosclerosis [58]; acute kidney injury [59]; inflammatory bowel disease [60]; allergic rhinitis [61]; hypothyroidism [62]; |
| WK2-16 | HDAC8 | animal model | neurodegenerative diseases [63]; sepsis [64]; heart failure [65] |
| TMP269 | Class IIa HDAC | cell experiment | stoke [66]; depression [67]; acute kidney injury [68]; autoimmune thyroid diseases [69]; rabies [70]; |
| LMK235 | HDAC4/5 | animal model | PD [71]; heart disease [72]; sepsis [73]; trigeminal neuropathic pain [74] |
| T2943 | HDAC5 | animal model | Depression [75] |
| Tubacin | HDAC6 | cell experiment, | PD [76]; hypertension [77]; Japanese encephalitis virus [78] |
| CKD-504 | HDAC6 | animal model | AD [13,79]; HD [79] |
| Resveratrol | SIRT1 | phase I clinical trials; animal model; | PD (NCT03095092, NCT03093389, NCT03095105, NCT03091543, NCT03094156 and NCT03097211); AD [80]; systemic lupus; diabetic kidney disease [81]; erythematosus [82]; arthritis [83]; |
| Honokiol | SIRT3 | animal model | AD [17]; PD [84]; heart disease [85] |
| FT895 | HDAC11 | animal model | depression [86]; systemic lupus erythematosus [87] |
3. Applications of HDACis in non-neoplastic diseases
3.1. HDACis and neurological diseases
The role of HDACs in regulating brain function, neurodevelopment, and degeneration has been examined using experimental models. For example, HDAC1 activity is crucial for repairing double-strand DNA breaks in neurons and has neuroprotective effects [88], and HDAC2 overexpression negatively regulates synaptic plasticity, synapse number, and dendritic spine density, leading to learning and memory dysfunction [89]. Class IIa HDACs (HDAC4, HDAC5, and HDAC9) play important roles in brain development and are involved in neurological functions such as long-term memory, depression, and neurological diseases [90]. HDAC11 in the hippocampus suggests that it may play an important role in learning and memory formation [91] Under pathological conditions, HDACs can directly or indirectly regulate the activity of key microtubule-associated protein and therefore affect pathogenesis [[92], [93], [94]]. HDAC6 may have important deacetylation functions in neurobiology and neuropathology as it plays a key role in regulating cellular responses to protein aggregation [95]. Recent discoveries have hinted that HDACis may have therapeutic value in treating various neurological diseases, particularly in the context of psychiatry and neurodegeneration [96,97]. VPA is still widely used as an anticonvulsant and mood stabilizer drug treating epilepsy and bipolar disorder as well as major depression, schizophrenia and migraines. Moreover, SAHA is reportedly currently in trials for epilepsy (NCT03056495) and AD (NCT03894826). So far, merely pan-HDAC inhibitors have been tested in preclinical studies. Over the years, the role of acetylation and individual HDACs in a range of neuropsychiatric disorders was elucidated, and a rational, targeted approach has been taken to study HDACis in these disorders.
I and II HDACis combat neurological diseases by reducing microglial activation in vivo and in vitro.
3.1.1. AD
AD is a chronic neurodegenerative disease which is characterized by cognitive impairment and memory deficits. However, current therapeutic remedies for AD provide only symptom relief and some degree of disease modification. HDACis exhibit neuroprotective properties in AD animal models, which is a promising strategy for brain diseases [98]. HDAC1, an 8-oxoguanine DNA glycosylase 1 (OGG1) deacetylase, alleviates 8-oxoguanine-induced oxidative DNA damage in the AD 5XFAD mouse model [99]. HDAC2 dysregulation leads to cholinergic nbM neuron dysfunction, NFT pathology, and cognitive decline during AD clinical progression [100]. A new type of selective HDAC2 inhibitor was found to reduce Aβ levels and further improve cognitive impairment by promoting the formation and growth of dendritic spine density [101]. In addition, entinostat, as a class I-specific HDAC inhibitor, has It exerts neuroprotective and anti-neuroinflammatory activities in the AD transgenic APP/PS1 mouse model [102]. HDAC3 may negatively regulate spatial memory and contribute to AD-related plasticity impairment, and inhibition of HDAC3 enhanced learning and memory in AD mice by decreasing tau phosphorylation and the buildup of Aβs and increasing the acetylation of histone H3 and H4 [103]. HDAC6 is the most important AD target among the HDAC homologous family and exerts neuroprotective effects by affecting protein aggregation and autophagy. The ubiquitin-binding domain at the C-terminus of HDAC6 plays an important role and affects actin and tubulin organization, Tau phosphorylation, and ApoE localization in neuronal cells [[104], [105], [106]]. Furthermore, HDAC6, which has a unique structure with two catalytic domains, targets many non-histone proteins, including α-tubulin, heat shock protein 90 (HSP-90), peroxiredoxin and tau protein [107]. For example, HDAC6 overexpression in AD disrupts mitochondrial microtubule-based transportation in a glycogen synthase kinase 3β-dependent manner [108]. Recently, two selective HDAC6is, Tubastatin A and ACY-1215, not only rescued cognitive and behavioral deficits without apparent side effects but also reduced amyloid-beta (Aβ) loading and tau hyperphosphorylation [109]. RGFP963 induced a transcriptional mechanism that enhanced synaptic efficacy and ultimately rescued learning and memory abilities in AD mouse models [16]. CKD-504 has recently been tested in AD studies with efficient blood-brain barrier permeability in AD patient-induced pluripotent stem cell (iPSC)-derived brain-like organoids and in AD-Like Pathology APP&Tau mice by decreasing pathologic tau proteins and rescuing synaptic pathology and cognitive deficits [110]. SIRTs regulate multiple processes related to the pathogenesis of AD, such as tau aggregation, mitochondrial dysfunction, oxidative stress, and neuroinflammation [111,112]. The emerging mechanisms in the neuroinflammation process mainly focus on microglial NF-κB signaling and inflammasome pathways [44].
Activation of SIRT1, SIRT2, SIRT3, and SIRT6 has been shown to have beneficial effects in preventing AD. For example, honokiol enhances antioxidant activity and mitochondrial function and reduces Aβ production by upregulating SIRT3 expression in Chinese hamster ovary cells (PS70 cells) carrying amyloid precursor protein and presenilin PS1 mutations [45]. The recently discovered SIRT6 small molecule activator MDL-811 deacetylates the enhancer of histone methyltransferase zeste homolog 2 (EZH2) and improves the EZH2/forkhead box C1 signaling pathway in microglia neuroinflammation in ischemic brain injury [46]. Combination of HDACis with other neuroprotective agents helps to achieve a better safety profile in chronic treatment. Combining vorinostat with tadalafil, a phosphodiesterase 5 inhibitor, attenuated cognitive deficits, LTP, and amyloid and tau protein pathology in AD mice, which was much better than each drug alone in terms of efficacy and duration of drug action [47]. Combining low-dose SAHA and curcumin improves therapeutic selectivity and provides comprehensive protection against Aβ-induced neuronal deficits [48]. It is worth noting that different stages of AD may produce specific markers, so stage-specific HDACis intervention therapy would be the best option. Although treatment with HDACis has been previously demonstrated to be beneficial in mitigating pathological characteristics associated with AD in mouse models or in vitro tests, HDACi-based therapies for AD patients have been unsuccessful. However, clinical trials are underway to overcome previously tested drugs' side effects and toxicity, showing promising results for future AD treatment [49].
3.1.2. Parkinson's disease (PD)
PD is a progressive disorder that affects the nervous system and the parts of the body controlled by the nerves. Although specific PD symptoms can be relieved with existing treatments, disease progression cannot be stopped [50]. PD studies have shown that dysregulation of histone acetylation levels is related to the pathogenesis of PD [51]. The balance of HDAC4 is critical for normal brain physiology, and HDAC4 aggregation can be observed in mutant dopaminergic neurons [52]. SIRT1 has been shown to act as a neuroprotective agent in H-SY5Y cells, possibly by downregulating the expression of NF-κβ and cleaved PARP1 and reducing phospho-α-Syn aggregation. Resveratrol targeting SIRT1 has been used to treat PD and has completed phase I clinical trials (NCT03095092, NCT03093389, ect). SIRT2 has been shown to enhance antioxidant defense mechanisms through deacetylation of FOXO3 [53]. Pinho et al. showed that targeting HDAC1 and HDAC6 isoforms was pharmacologically feasible in the zebrafish PD model and suggested that their inhibition might restore cellular metabolism in PD models. However, given the lack of improvement with exercise, HDAC1 or HDAC6 inhibitor therapy is unlikely to alleviate PD fully. Another study showed that using HDACis (i.e., VPA and NAB) reduced histone deacetylation, leading to chromatin relaxation and activation of multiple genes [54]. For instance, HDACi therapy induces the production of brain-derived neurotrophic factor, Bcl-133, Bcl-XL, glial-derived neurotrophic factor syn, p70, and heat shock protein [55]. Interestingly, HDAC inhibition is linked to reduced astrocyte- and T-cell-mediated inflammation [56]. Further studies found that HDAC inhibition improved -synuclein toxicity in cell culture and transgenic flies by reducing the targeting of -synuclein to the nucleus [57]. Moreover, in human-derived SK-N-SH and rat-derived MES 23.5 cells, SAHA partially protected against MPP(+)-mediated apoptosis and simultaneously increased histone acetylation increased, suggesting that SAHA preserves dopaminergic neurons [58]. Recent research has demonstrated that class IIa HDAC MC1568 administered peripherally exerted neuroprotective and behavioral improvement effects in a rat model of 6-OHDA lesions in the striatum of PD [59].
3.1.3. ALS
ALS is a group of progressive diseases that affect the nerve cells in the brain and spinal cord that control muscle movement. Elevated HDAC2 levels and reduced HDAC11 mRNA are known to be associated with apoptotic neuronal death in the brain tissue of ALS patients [60]. In the SOD1 mouse model, HDAC6 levels decreased with the onset of ALS symptoms before decreasing further with disease progression [61]. However, existing therapies cannot slow down disease progression [62]. There is strong evidence that HDACs can be pharmacologically inhibited with drugs like NAB and SAHA to enhance cell viability in ischemic stroke and ALS mouse models by promoting histone acetylation, gene transcription, and protein synthesis [63]. In SOD1(G93A) mice, combination pharmacological therapy with the class I HDACi lithium and VPA improves motor function, delays the onset of illness, and prolongs lifespan [64]. Furthermore, it has been demonstrated that resveratrol prevents motor neuron death and improves lifespan in a SOD1 (G1A) ALS mouse model by pharmacologically stimulating the class III HDAC sirtuin 1 and AMP-activated kinase [65]. However, silencing of HDAC4 in skeletal muscles resulted in the early development of ALS in mice, suggesting the possibility of a risk associated with using HDACis to treat ALS [113]. Several drug combinations are available for treating ALS, including the combination therapy of SAHA (pan-HDACi), RGFP109 (HDAC1/3 inhibitor), and arimoclomol, which rescues ALS-associated FUS mutations through DNA repair mechanisms [66]. According to a recent study, administering VPA and RESV to SOD1 (G93A) mice improved their motor function significantly, delayed the onset of illness, and increased survival. Interestingly, males exhibited earlier onset and slower disease progression than females in the vehicle-administration group [67]. In addition, NAB and sodium valproate are undergoing safety studies (NCT00107770) and double-blind randomized placebo-controlled trials (NCT00136110) in amyotrophic lateral sclerosis (ALS), respectively.
3.1.4. Huntington's disease (HD)
HD is an inherited disorder that causes nerve cells in parts of the brain to gradually break down and die. In HD pathology, impaired transcriptional activation and repression controlled by chromosomal acetylation are observed. Studies have shown that HDAC1 has a dual role in the regulation of neuronal viability. In cooperation with HDRP, HDAC1 promotes neuronal survival, but when interacting with HDAC3, HDAC1 promotes neuronal death [68]. The therapeutic efficacy of HDACis has been evaluated in various HD animal and cell models and shows promising therapeutic potential [69,70]. Hecklau et al. found that specific HDAC1 and HDAC3 inhibition had beneficial effects on transcriptional misregulation, coordination defects, and motor skill development [114]. Jia's experiments also support this conclusion [71]. Additional studies have verified the neuroprotective functions of the SIRT 2 inhibitor AK-7 in a HD mouse model. In 140CAG mice, the impact of AK-7 on aggregation was more potent, resulting in a 50 % reduction in inclusion bodies and in line with the pharmacological and genetic characteristics of SIRT2 inhibition seen in HD neurons in vitro [72]. Phenylbutyrate has been shown to have neuroprotective properties by Gardian et al. Specifically, 75-day administration of phenylbutyrate (HD-N171-82Q) to transgenic HD mice improved survival and reduced striatal degeneration and ventricular enlargement but did not affect motor coordination. Moreover, increased acetylation of histones H3 and H4 and the concomitant decrease in methylation of histone H3 in the striatum were observed following phenylbutyrate treatment [73]. In tgHD and BACHD cultures in vitro and in tgHD pups in vivo, low-dose LBH589 partially reverses behavioral abnormalities and restores abnormal neural differentiation [74]. Recently, Stott et al. identified a class IIa HDAC4 inhibitor, 5-(trifluoromethyl)-1,2,4-oxadiazole (TFMO, 12), which showed efficacy in preclinical models of HD [75]. In vitro and in vivo experiments show that the HDAC6 inhibitor CKD-504 reduces behavioral deficits, increases axonal transport and neuron number, restores synaptic function in corticostriatal circuits, and reduces mHTT accumulation, inflammation, and tau protein in YAC128 TG. Hyperphosphorylation improves HD pathology [76].
3.1.5. Depression
Depression (major depressive disorder, MDD) is a common and serious medical illness that negatively affects how you feel, the way you think, and how you act, involving genetic and environmental factors and affecting more than 16.31 billion people worldwide [77]. However, approximately 23 % of MDD patients are insensitive to common antidepressant drugs [78]. Class I HDACs and Class II HDACs are the most commonly used HDACs for modulating MDD [79,80]. Earlier studies have suggested a function for HDAC6 in mood regulation, with HDAC6-deficient mice treated with HDAC6 inhibitors exhibiting less anxiety and antidepressant behavior [81]. Moreover, class IIa HDACs (HDAC4, 5, and 9) are essential to the neurological function of depression [82]. Interestingly, HDAC expression has also been found in peripheral leukocytes of MDD patients [83]. Currently, the potential of various HDACis to show antidepressant effects and target HDACs and the genes encoding them has been studied and employed in animal models of MDD. Weaver et al. found that the HDACi TSA could reverse early maternal care-induced hippocampal transcriptome changes in rats [84]. Another HDAi, SAHA, exhibited an antidepressant-like response by modulating cytoplasmic HDAC6 levels [85]. VPA, an HDACi widely used in treating bipolar disorder, has also been shown to possess antidepressant properties. Studies have found that VPA affects BDNF, CORT, melanocortin-4 receptor, and glycogen synthase kinase 3β [86]. Entinostat, a particular class I HDAi, was detected to affect the expression of proteins, including BDNF, CORT, RAC1, CREB, and gap junction protein alpha 5, in mice suffering from chronic social defeat stress [87]. Furthermore, NAB was found to exert antidepressant effects by modifying the expression of transthyretin, serotonin 2A receptor, and BDNF [115]. Interestingly, combining the antidepressant fluoxetine and HDACis significantly reduced MDD-related behaviors, which implies that HDACis may be used along with common antidepressants soon [116]. Some herbal medicines have been clinically used to treat depression in China for a long time. Indeed, it has been shown that Xiaoyaosan, a Chinese herbal formula, can reduce the scores of the Hamilton scale and self-assessment depression scale in patients with depression [117]. Xiaoyaosan has also been shown to improve depression-like behaviors in CUMS-treated rats, chronically immobilized stressed rats, and CUMS-treated mice, possibly related to histone modifications [118].
3.2. HDACis and CVD
CVD is the primary cause of death in the world [119]. Hypertension and atherosclerosis are the most common cardiovascular diseases [120]. In the past decade, several studies have demonstrated the beneficial effects of HDACis on CVD, including anti-hypertensive effects, anti-inflammatory, anti-fibrotic, and anti-hypertrophic [121,122]. Regarding HDACis, clinical trials on CVDs have not yet been conducted, but many preclinical investigations have demonstrated that HDACis have a positive effect on CVDs. Recently, many traditional Chinese remedies with HDACis-like effects have gradually replaced traditional HDACis to treat CVDs [123].
3.2.1. Hypertension
Hypertension is when the pressure in your blood vessels is too high (140/90 mmHg or higher). Hypertension is a significant risk factor for developing CVDs, as well as for causing pathological disorders such as stroke, heart failure, and renal failure [124]. Although several anti-hypertensive drugs are available, optimal blood pressure control has not yet been achieved. Studies have found that HDACs are abnormally expressed and dysregulated in pathological situations such as cardiac hypertrophy [125], heart failure [126], arterial hypertension [127], and pulmonary hypertension [128]. It was found that VPA can reduce mean arterial pressure in spontaneously hypertensive rats [129] and systolic blood pressure in high-fat diet-fed mice [130] and deoxycorticosterone acetate (DOCA)-salt hypertensive rats [131]. Further studies have shown that VPA treatment decreased the elevated cardiac HDAC6 and HDAC8 activity in a hypertensive rat model [127], supported by drug research [132]. In rats treated with DOCA salt, Lee et al. found that Ivaltinostat, a pan-HDACi, attenuated systolic blood pressure by downregulating the angiotensinogen-renin component (approximately 170-135 mmHg, 207.8 ± 9.7–171.7 ± 9.8 mmHg, respectively) [133]. Ivaltinostat also reduced systolic blood pressure and diastolic blood pressure in high-fat diet-fed mice, and further studies found that ivaltinostat reduced the upregulation of HDAC1-3 and HDAC6 in kidney tissues [134]. Recent reports have shown that inhibiting the expression or activity of HDAC5 reduces vasoconstriction, vascular hypertrophy, and oxidative stress in hypertension models [135]. Notably, HDAC inhibition may have harmful consequences in some cases, such as leading to exaggerated vascular calcification [136].
3.2.2. Atherosclerosis
Atherosclerosis is a common condition that develops when a sticky substance called plaque builds up inside your arteries. Atherosclerotic disease is a major cause of adult morbidity and mortality. It has been identified that HDACs are involved in multiple processes of atherosclerosis formation, including elevated blood sugar and blood lipids, monocyte accumulation and migration, foam cell formation, smooth muscle cell (SMC) phenotype switching, fibrous cap formation, plaque disruption, thrombosis [137]. Regarding the function of individual HDACs, HDAC7 is involved in endothelial cell (EC) proliferation and migration and SMC proliferation, whereas HDAC3 regulates EC apoptosis [138]. In the stem cell theory of atherosclerosis, HDAC3 and HDAC7 have been shown to differentiate stem cells into ECs and SMCs, respectively [139]. Additionally, it has been found that two critical hemodynamic forces regulating EC dysfunction and function induce the activation of HDAC through different mechanisms [140]. For further validation, Romidepsin, an HDAC non-selective inhibitor, promotes the acetylation of non-histone substrates implicated in VCAM-1 transcription in EC, mediating the AS process [141]. Additionally, suppressing class I and class II HDAC activity by apicidine has been shown to inhibit proliferation and cell cycle arrest in the G1 phase of neonatal pulmonary artery SMCs. Butyrate inhibition of HDACs abolishes Akt activation and subsequent downstream targets of Akt, which promotes proliferation arrest [142]. Sodium valproate, a class I HDACi, promotes macrophage phenotypic switching, thereby delaying AS progression [143]. HDACis also inhibit the inflammatory response of atherosclerosis. Administration of TMP195, a specific inhibitor of class IIa HDAC, inhibits vital inflammatory pathways and attenuates atherogenesis in advanced AS, providing a new treatment method to reduce the consequences of vascular inflammation [144]. Moreover, Chen et al. found that a specific inhibitor of the HDAC3-specific inhibitor RGFP966 inhibits endothelial-to-mesenchymal transition by regulating the inflammatory response in AS [145].
3.2.3. Heart disease
Heart disease is a group of conditions that includes coronary heart disease, arrhythmias, heart failure, and valve disease. HDAC is vital in normal heart development, and the idea that abnormal HDAC causes heart disease has been strengthened by studies in HDAC knockout mice, such as Hdac2-, Hdac5-, and Hdac9-null, all of which have heart defects [146]. Moreover, conditional deletion of HDAC3 suggests that it is vital in cardiac function. Sirt1-deficient mice have cardiac, retinal, and skeletal defects [147]. Studies have shown that class I HDACs might be crucial for processing ions and preserving the integrity of the conduction system by regulating the transcription of target channel proteins or by directly binding to other proteins to maintain the conduction system of myocardial synchronous contraction [148]. Additionally, inhibiting type I HDACs can protect left ventricular systolic function after ischemia-reperfusion injury in isolated hearts. This cardioprotective effect is related to the nuclear localization of FOXO3a and the increased expression of its transcripts SOD2 and catalase [149]. Similar to the medical value illustrated by HDACis in animal models with left ventricular dysfunction [150], the therapeutic benefit of several commercially available broad-spectrum HDACis (SAHA) and class I HDAC targeting has been shown to successfully alleviate multiple pulmonary hypertension and right ventricular hypertrophy in a rodent model of pulmonary hypertension [151]. HDACis positively affect pulmonary vascular remodeling and stiffening and prevent permanent myocardial infarction [152]. VPA dramatically decreased infarct size in an experimental model of myocardial infarction via the Foxm1 pathway [153]. VPA also affects the acetylation of the mineralocorticoid receptor [131], and is linked to a decrease in cardiac fibrosis [154]. Also, in experimental models, VPA has been proven to prevent the development of atrial fibrillation [155]. The above-mentioned studies support the hypothesis that valproate is cardioprotective. Thus, HDAC inhibition appears to be a promising target for treating CVD.
3.3. HDACis and kidney disease
There is increasing evidence that HDAC is involved in developing and progressing various kidney diseases [156]. Class I HDAC isoforms were expressed in renal fibroblasts, the cortex of renal tubular cells, and the developing kidney, according to research on the expression profile and distribution of HDACs in the kidney [157]. Also, HDAC5, HDAC6 and HDAC11 have been found in the renal tubules [158,159]. SIRT1 is expressed in fibroblasts [160] and renal tubules [161]. Many preclinical studies have shown that HDACis exerts renoprotective effects by inhibiting inflammation, alloimmune response, cyst formation, fibrosis, angiogenesis, cell proliferation, and apoptosis [162].
3.3.1. DN
DN is a clinical syndrome characterized by persistent albuminuria and a progressive decline in renal function, and the term infers the presence of a typical pattern of glomerular disease. Diabetes-induced kidney disease is still the primary cause of chronic renal failure globally [163]. DN is characterized by accumulating extracellular matrix (ECM) proteins in the tubulointerstitium and glomerular mesangium, accompanied by tubular and glomerular basement membrane thickening, eventually progressing to tubulointerstitial and glomerulosclerosis fibrosis [164]. Over the past decade, several preclinical investigations have shown various HDACis' efficacy in DN research models [165,166]. Increased HDAC expression or activity has been commonly reported in mouse models of DN and patients with DN [167]. According to experimental results, administration with the class I selective HDACis VPA and SK-7041 lowers the expression of ECM components in renal epithelial cells (NRK52-E) in vitro [168]. Moreover, Gilbert et al. found that treatment with SAHA attenuated renal hypertrophy in rats by downregulating epidermal growth factor receptor (EGFR) expression [169]. Advani et al. demonstrated that SAHA effectively reduces mesangial matrix accumulation and albuminuria in STZ-diabetic wildtype mice [170]. Furthermore, Khan et al. showed that VPA reduces renal tubular damage, renal fibrosis, and proteinuria in STZ-diabetic rats [171]. The experimental results of Sun et al. also support this conclusion [172]. A recent study showed that administering VPA and SAHA attenuated proteinuria and reduced the development of glomerulosclerosis in several rodent models of glomerular injury [167]. Dong et al. demonstrated that NAB had similar effects and lessened programmed cell death, fibrosis, and oxidative damage in the DN mouse model [173]. In addition to DN, HDACs also regulate early DN by activating the EGFR signaling pathway [174]. For example, HDAC4 is deemed to be a critical epigenetic mediator during DN [175]; so, specific inhibition of HDAC4 can be used as a treatment for DN and related renal diseases [176], and VPA is occasionally used clinically for the treatment of painful neuropathy in patients with diabetes [177]. The effects of HDACs in diabetes are not only on the kidneys. Other metabolic benefits in treating diabetes also be provided by HDACis. For example, class I HDAC inhibition has been shown to improve insulin sensitivity in db/db mice by enhancing the oxidative metabolism of skeletal muscle and adipose tissue [178], while HDAC3 inhibition reduces pancreatic β-cell programmed cell death and increases β-cell growth, thereby preventing the onset of diabetes in non-obese diabetic mice [179].
Although HDACis effectively attenuate DN, combining HDACis and other inhibitors may have additive effects. No such studies have been conducted in DN. Still, it has been reported that the combination of ACE inhibitors and HDACis in HIV-associated nephropathy provides better kidney protection in mouse models. These two inhibitors can affect essential renal inflammation and fibrosis pathways, like TGF-β, NF-κB, mitogen-activated protein kinase, interleukin-1, and cell apoptosis signaling [180]. Therefore, examining the therapeutic effect of HDACis combined with other drugs in the context of DN is necessary.
3.3.2. Lupus nephritis (LN)
Lupus nephritis is kidney inflammation due to lupus, an autoimmune disease. Anti-dsDNA antibodies and immune complexes comprising antibodies are deposited in the small kidneys, a feature of LN. Glial annexin II and IFN-expression levels were found to be associated with the severity of LN [181]. Recent research has shown that the acetylation of histones and non-histones, among other epigenetic variables, is essential for the emergence and progression of LN [182]. It has been demonstrated that HDACis boost forkhead box P3 (Foxp3) Treg cells by maintaining Foxp3 lysine acetylation, which improves DNA binding [183]. Previous studies have shown that TSA fails to prevent the deposition of immune complexes in glomeruli in the MRL-lpr/lpr mouse model of lupus [184]. Recent research, however, has shown that HDAC inhibition affects LN. Specifically, ITF2357 decreased the expression of IL-6 and IL-10 mRNA in the glomeruli in a mouse model of LN. Furthermore, ITF2357 prevented glomerular deposition of C3 and IgG and renal glomerular basement membrane thickening [185]. CKD-506 is a selective HDAC6 inhibitor that inhibits the production of cytokines such as IL-10, IL-15, IL-17, and TNF-α and reduces CD4+-CD8-T cells in CD138 plasma cells, as well as CD25 cells and the Th1:Th2 ratio in the spleen to reduce the pathogenesis of SLE [186]. Other experiments also support this conclusion [187,188]. In a recent study, the administration of ACY-738 stopped LN progression by inhibiting IFN-α production by plasmacytoid dendritic cells [162]. These results suggest that HDAC6 inhibition could be a helpful approach to delaying LN progression.
3.3.3. Kidney injury
The progression and development of kidney injury eventually lead to renal interstitial fibrosis [189]. It has been reported that class I HDACs seem to play a major part in modulating renal fibrosis, and Class III HDACs are involved in regulating renal fibrosis [190]. Specific HDAC subtypes and their cellular origin (epithelial, endothelial, mesothelial) are deranged in most acute kidney injurymodels [191]. In all preclinical studies presented thus far, anti-fibrosis and anti-inflammation are the mechanisms underlying the renoprotective effect of HDACis in renal injury. Specifically, pan- or class I-selective HDACs have been shown to reduce the production of TGF-β, a main regulator of myofibroblast activation, lowering myogenesis fibroblast markers (e.g., vimentin alpha-smooth muscle actin) and reducing ECM (e.g., collagen, fibronectin) [192]. Another study showed that the administration of TSA also considerably inhibited the expression of α-SMA and fibronectin, two hallmarks of activated fibroblasts [193]. Marumo et al. demonstrated that HDAC1 and HDAC2 are essential for TNF-stimulated CSF-1 production [194]. Furthermore, daily intraperitoneal administration of TSA in unilateral ureteral occlusion mice caused increased infiltrating T regulatory cells (Tregs; CD4FOXP3) and decreased inflammatory T helper 17 (Th17; CD4IL-17) cells [195]. Moreover, renal fibroblast proliferation and STAT3 phosphorylation, a signal linked to renal fibroblast proliferation and the molecular onset of renal fibrosis, are both inhibited when HDAC1 or HDAC2 are silenced with targeted siRNA [196]. Mechanistic research has demonstrated that administration of entinostatinhibits the proliferation and activation of renal fibroblasts and can repress renal fibrosis through a mechanism involving the inactivation of TGF-β1/Smad3 and EGFR signaling pathways [158]. Moreover, SIRT1/2-selective inhibition has been shown to attenuate the progression of renal fibrosis by reducing the activation of renal fibroblasts [160]. In contrast, blockade of SIRT1/2 inhibited the activation of platelet-derived growth factor and EGFR, two growth factor receptors associated with renal fibrosis [197]. A recent study showed that HDAC6 activity suppression could prevent rhabdomyolysis-induced acute kidney injury; its renoprotective mechanism is related to inhibiting renal tubular cell apoptosis, inhibiting the inflammatory response, and reducing oxidative stress [198]. It cannot be ignored that HDACis can improve the regenerative capacity of injured kidneys by modulating the cell cycle [199].
3.4. HDACis and autoimmune diseases
HDACs have generated attention in immunology due to their ability to be involved in innate immune cell memory functions, as well as tolerance to endotoxin and T cell differentiation and activation [200]. Earlier studies have found that the effect of HDACis on the immune response has variable outcomes. For instance, TSA treatment in an SLE mouse model caused an increase in several inflammatory cytokines (including IFN-γ, IL-6, and IL-12) and decreased mRNA expression. TSA, phenylbutyrate [201], or FK228 [202] therapy lowered the expression of the pro-inflammatory cytokine TNF-α in two further early investigations utilizing rheumatoid arthritis animal models. Furthermore, immune cells isolated from patients receiving HDACis were also found to be less receptive to inflammatory stimuli [203]. Additionally, HDAC3 and HDAC6 inhibition exerted anti-inflammatory activity by reducing the production of pro-inflammatory cytokines and blocking the production of IL-6 and TNF-α, which led to improvements in clinical signs in SLE and colitis models [204]. Moreover, some recent studies on HDAC8 inhibitors have shown solid anti-inflammatory potential in autoimmune diseases preclinical models. For instance, a mouse model of ACY-738, CKD-506, and SLE was found to control T-cell and B-cell differentiation, restore abnormal B-cell development, and increase the proportion of splenic Tregs. Furthermore, HDAC6 inhibition considerably increased TGF-β in serum and decreased inflammatory cytokines like IL-17 and TNF-α in this model [186]. It was studied that ACY-738 delayed the onset of autoimmune encephalomyelitis and attenuated disease severity [205]. CKD-506 inhibited monocyte/macrocytic phagocytic inflammation, improved Treg function, and enhanced arthritis severity in a mouse model of rheumatoid arthritis [206]. In a mouse model of multiple sclerosis, TSA reduced the production of pro-inflammatory cytokines and improved multiple sclerosis-mediated pathological disruptions of myelin [207]. Notably, the results from a phase 2 study involving giveinostat, a benzamide targeting HDACs 1–10, showed histologic changes and some functional modifications in pediatric individuals with Duchenne muscular dystrophy [208]. Currently, two clinical trials in Duchenne muscular dystrophy (phase III: NCT0285179 and phase II/III: NCT03373968) and a phase 2 trial in Becker muscular dystrophy (NCT03238235) are being conducted. Givinostat has also been proven safe and effective in patients with juvenile idiopathic arthritis, according to the results of a phase 2 trial [209]. A recent study found the HDACi zabadinostat to be a systemic modulator of adaptive immunity with clinical benefit.
3.5. HDACis and inflammatory diseases
HDACis have recently received considerable attention in treating chronic inflammatory diseases, including in preclinical models of several inflammatory diseases, such as inflammatory bowel disease [210]. It has been reported that HDAC1, HDAC2, and HDAC3 affect the promoter activity of inflammatory genes. HDACs are critical regulators of various cellular functions, including inflammatory responses, and their dysregulation is closely related to multiple inflammatory diseases [211]. Studies have confirmed that lower dosages of HDACis are anti-inflammatory and reduce the production of pro-inflammatory cytokines. However, not all HDACis exhibit this property. Cantley et al. demonstrated that the anti-inflammatory effect of entinostat on human osteoclasts was only significant at higher doses [212]. Tatinrine [213] and entinostat [214] were the first potent, selective inhibitors of class I HDACs, and they have demonstrated treatment effects on inflammation in various models, including pancreatitis [215], chronic obstructive pulmonary disease [216], and inflammation associated with angiotensin II-induced hypertension [217]. These beneficial preclinical results were along with a significant reduction in multiple pro-inflammatory cytokines and leukocyte infiltration [218]. In mice given BML-19, the inflow of CD281 B lymphocytes into the inflamed colon was diminished in a DSS colitis model [219]. Furthermore, CKD-506 has been shown to inhibit macrophage conduction and NF-κB signaling in intestinal epithelial cells, leading to the amelioration of murine colitis [220]. It has been observed that HDACis, like TSA and SAHA, delay and lower NF-κB nuclear translocation and gene expression upon TNFα stimulation [221]. The capacity of HDACis to inhibit inflammation was also well-proven by Lee et al. [222]. This novel mentioned above HDACis exhibited nanomolar HDAC inhibitory potential and a strong inhibitory effect on the expression of inflammatory mediators in animal models.
3.6. HDACis and infectious diseases
Chidamide is a self-developed HDACi in China. According to a phase 1b/2a clinical trial, Chidamide can safely and effectively destroy HIV-1 latency in vivo and is a positive latency reversal drug [223]. The research by Kuai et al. also supports this view [224]. The following focuses on parasitic diseases and COVID-19.
3.6.1. Parasitic diseases
Parasitic diseases lead to severe global morbidity and mortality, particularly in underdeveloped areas. HDACis, used initially against cancer, are also being researched to target many parasitic diseases. Studies have found that HDACs have a critical function in human parasite disease. Specifically, TbHDAC1 and TbHDAC3 are essential for the survival of Trypanosoma brucei) [225], and PfHDAC1 is nuclear localized and expressed/transcribed in multiple life cycle stages of Plasmodium falciparum [226]. Toxoplasma gondii HDACs have also contributed to stage-specific gene regulation in various forms of this parasite (tachyzoite to tardyzoite forms) [227]. Various structural classes of HDACis have recently demonstrated positive results in treating parasitic diseases [228]. For example, the cyclic tetrapeptide HDACi apacillin shows strong in vitro action against T. gondii, P. falciparum, and other protozoan parasites [229]. However, short-chain fatty acids have weak inhibition against mammalian HDACs [230]. TSA is a potent inhibitor of P. falciparum growth in vitro [231], and TSA can partially inhibit the total sirtuin activity of protein lysates from five different life cycle phases [232]. Compared to TSA, SAHA is less active in vitro against laboratory lines of P. falciparum (IC50∼100–300 nM) but with improved parasite-specific selectivity [233]. Interestingly, low concentrations of SAHA, TSA, and criptaid (1–50 nM) significantly stimulated the proliferation and survival of T. gondii but inhibited the infectivity of tach P. falciparum yzoites [234]. Overall, the class III HDACi Sir2 inhibitor was moderately active against P. falciparum growth [235]. Recently, Vaca explored the combined potential of HDACis, TH65, TH92, and entinostat with ABZ and the combination potential of TH65 and TH92 with entinostat and found that all medication combinations exhibited effective anthelmintics in a time-dependent way effect [13].
3.6.2. Noval coronavirus disease (COVID-19)
COVID-19 is an infectious disease caused by the SARS-CoV-2 virus. Since its outbreak, COVID-19 caused by infection with SARS-CoV-2, has caused many deaths and has affected the lives of the global population in various ways. Given the involvement of immune system overactivation in the pathogenesis of COVID-19, immunosuppressive therapy is crucial for the therapeutic management of the condition, particularly in critically ill patients [236]. In mouse models, HDAC inhibition has been shown to reduce the morbidity related to severe COVID-19 by influencing both innate and adaptive immune systems [237]. As mentioned in other reports, the expression of the ACE2 gene is downregulated by histone modifications, including the EZH2-mediated deposition of H3K27me3. HDAC1/2 are genes that enhance the host immune response emerging targets of [238]. Therefore, HDACi, such as VPA against HDAC1/2, can be used clinically to defend against various diseases caused by coronaviruses, including COVID-19 [239]. HDACis and antiviral drugs have also shown efficacy against COVID-19 [240]. VPA may act on improving lung injury in acute respiratory distress syndrome caused by COVID-19. Indeed, VPA has been shown to reduce VEGF and angiogenic factors [241], inhibit IL -12 and TNF-α, reduce macrophage infiltration, and change the polarization of macrophages from T1 to the anti-inflammatory T2 phenotype [242]. In patients with COVID-19, endothelial dysfunction and consequent thrombosis have been identified as key mechanisms for targeting organ damage [243]. Additionally, studies have found that VPA has anti-platelet effects [244] and reduces the function of subendothelial cell dysfunction [245]. The effect of VPA on COVID-19 is now being assessed in clinical studies (e.g., NCT04513314) [246]. HDACis are considered promising treatments for COVID-19, given their anti-thrombotic and anti-inflammatory properties [247]. The incidence of thrombosis in an increasing number of hospitalized patients with COVID-19 infection is approximately 30 % [248]. Moreover, biomarkers of thrombosis, including D-dimer, fibrinogen, and vascular Willibrand factor, are increased in patients hospitalized with COVID-19 [249], and elevated levels of these coagulation factors are linked to an increased risk of critical illness and death [250]. The HDAC1is SAHA and Romidepsin lead to an increase in viral RNA, indicating that these proteins function in an antiviral manner [251]. Studies have found that HDAC2 inhibits the growth of antiviral response genes [252]. HDAC2 activates many interferon-specific genes via BRD4 to provide efficient antiviral responses [253]. It is anticipated that Nsp2 cleaves the nuclear localization signal of HDAC2, preventing the protein from entering the nucleus and suppressing various antiviral responses [254]. Furthermore, HDAC2 has been highlighted as an age-associated risk factor for SARS-CoV-2 infection, one of the potential determinants of sensitivity, as it is downregulated in the lungs of older people [255]. HDACs in macrophages show pro-inflammatory responses by increasing the levels of IL-1α, IL-1β, TNF-α, and IFN-γ. Specifically, HDAC2 changes monocytes and macrophages by impairing the activity of the NF-κB function of phagocytes and is central to SARS-strategy CoV-2's for evading the immune system. Moreover, nuclear localization of HDAC5 activates MCP-1 and TNF-α, triggering an inflammatory reaction [256].
3.7. HDACis and rare diseases
Rare diseases, often termed as orphan diseases, are health conditions that affect a small percentage of the population [257]. Global Genes has estimated that currently, approximately 10,000 rare diseases exist globally, with 80 % of these having identified genetic origin. HDACis have been tested for the treatment of different rare diseases, such as Rett syndrome (RTT), inherited retinal disorders (IRD), and so on [[258], [259], [260]]. Studies have shown that targeting MeCP2, the cause of RTT, HDACis plays a certain role, such as universal studies of the HDAC inhibitor trichostatin have shown that HDACs hyperacetylate histones in non-neuronal cells and reduce MeCP2 levels [259]. In addition, Mocetinostat and Entinostat, inhibitors of class I HDACs (HDAC1, HDAC2, HDAC3), also have similar effects [261,262]. A series of evidence suggests that MeCP2 abnormalities are associated with BDNF transport defects and microtubule dynamics, and inhibition of HDAC6 increases rapid axonal transport of BDNF and mitochondria in both anterograde and retrograde directions, enhancing synaptic activity [263]. In IRD, blocking histone deacetylation by specifically inhibiting HDAC1 can also block rhodopsin expression and rod photoreceptor cell development [264,265], and inhibiting HDAC3 has a certain protective effect on ganglion cell death [260]. HDAC6 inhibition has a significant protective role from oxidative stress-induced injuries and this is particularly important for photoreceptors and retinal cells which are exposed to high concentrations of reactive oxygen species (ROS) [266].
4. Conclusions
We know of at least 18 human HDAC enzymes, which help eradicate epigenetic modifications, help establish epigenetic dechromatin states, and modulate heritable alterations in gene expression. Over the past few decades, HDACis have received significant attention and have been clinically verified and evaluated as an antitumor drug class. Recently, the newly discovered HDACis have triggered an interest in identifying new indications, such as neurological, cardiovascular, inflammatory, autoimmune, and infectious diseases. Furthermore, the roles of HDACis in non-tumor diseases are different and are related to the type of disease, pathogenesis, and formation process. Therefore, more extensive and large-scale clinical investigations are required to determine the full potential of this class of drugs, and often their combination, for treating non-neoplastic conditions. Furthermore, it should be noted that several existing HDACis are working in combination, not only as a single drug. Here, we conducted a comprehensive literature review of the role of HDACis in non-neoplastic diseases. Soon, other non-neoplastic indications may enter clinical trials from the preclinical stage. We anticipate that the evaluation of HDACis in combination therapy will experience continuous growth to evaluate the possibility of maximizing their clinical efficacy and safety. Hopefully, the findings of previous, ongoing, and upcoming studies will soon be translated into novel medical protocols for treating non-neoplastic diseases.
Ethics declarations
Review and/or approval by an ethics committee was not needed for this study because there is not directedly involved in human and/or animal participants.
Consent for publication
Not applicable.
Data availability statement
Data associated with my study doesn't been deposited into a publicly available repository. No data was used for the research described in the article.
Funding
This research was funded by Shandong Provincial Natural Science Foundation of China (ZR2019MH096) and Health Promotion Project of China (BUHA-CPP-001).
CRediT authorship contribution statement
Chunxiao Zhou: Writing – original draft, Software, Methodology. Dengke Zhao: Investigation, Data curation. Chunyan Wu: Validation, Formal analysis. Zhimin Wu: Visualization, Software. Wen Zhang: Validation, Project administration. Shilv Chen: Supervision, Software. Xindong Zhao: Resources, Methodology. Shaoling Wu: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The chemical structures of Table 1 were created by Chenspider (http://www.chemspider.com).
Contributor Information
Chunxiao Zhou, Email: 2020020898@qdu.edu.cn.
Dengke Zhao, Email: qdzhaodengke@163.com.
Chunyan Wu, Email: wuchunyan0321@163.com.
Zhimin Wu, Email: wzm0905@163.com.
Wen Zhang, Email: m13709192196@163.com.
Shilv Chen, Email: sneeze99522@126.com.
Xindong Zhao, Email: zhaoxindong@yahoo.com.cn.
Shaoling Wu, Email: wushaoling@qdu.edu.cn.
Abbreviations
- HDACis
Histone deacetylase inhibitors
- HDAC
Histone deacetylase
- SIRT
sirtuins
- NAB
sodium butyrate
- VPA
valproic acid
- TSA
Trichostatin A
- SAHA
Vorinostat
- AD
Alzheimer's disease
- ALS
Amyotrophic lateral sclerosis
- Aβs
Amyloid-beta-peptides
- CREB
Element-binding protein
- PD
Parkinson's disease
- HD
Huntington's disease
- CVD
Cardiovascular disease
- SMC
smooth muscle cell
- EC
endothelial cell
- DN
Diabetic nephropathy
- ECM
Extracellular matrix
- LN
Lupus nephritis
- Foxp3
Forkhead box P3
- EGFR
Epidermal growth factor receptor
- MDD
Major depressive disorder
- COVID-19
Noval coronavirus disease
References
- 1.Hwang J.Y., Aromolaran K.A., Zukin R.S. Author Correction: the emerging field of epigenetics in neurodegeneration and neuroprotection. Nat. Rev. Neurosci. 2018;19(12):771. doi: 10.1038/s41583-018-0065-5. [DOI] [PubMed] [Google Scholar]
- 2.Stoccoro A., Coppedè F. Role of epigenetics in Alzheimer's disease pathogenesis. Neurodegener. Dis. Manag. 2018;8(3):181–193. doi: 10.2217/nmt-2018-0004. [DOI] [PubMed] [Google Scholar]
- 3.Shukla S., Tekwani B.L. Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front. Pharmacol. 2020;11:537. doi: 10.3389/fphar.2020.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon S., et al. PP2A negatively regulates the hypertrophic response by dephosphorylating HDAC2 S394 in the heart. Exp. Mol. Med. 2018;50(7):1–14. doi: 10.1038/s12276-018-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun H.-J., et al. Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. 2021;38 doi: 10.1016/j.redox.2020.101813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z., et al. Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway. 2019;508(2):398–404. doi: 10.1016/j.bbrc.2018.11.140. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z., et al. miR-133b and miR-199b knockdown attenuate TGF-β1-induced epithelial to mesenchymal transition and renal fibrosis by targeting SIRT1 in diabetic nephropathy. Eur. J. Pharmacol. 2018;837:96–104. doi: 10.1016/j.ejphar.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Sun R., Hedl M., Abraham C. Twist1 and Twist2 induce human macrophage memory upon chronic innate receptor treatment by HDAC-mediated deacetylation of cytokine promoters. J. Immunol. 2019;202(11):3297–3308. doi: 10.4049/jimmunol.1800757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aude-Garcia C., et al. Dual roles for MEF2A and MEF2D during human macrophage terminal differentiation and c-Jun expression. Biochem. J. 2010;430(2):237–244. doi: 10.1042/BJ20100131. [DOI] [PubMed] [Google Scholar]
- 10.Utsunomiya A., et al. Oral histone deacetylase inhibitor tucidinostat (HBI-8000) in patients with relapsed or refractory adult T-cell leukemia/lymphoma: phase IIb results. Cancer Sci. 2022;113(8):2778–2787. doi: 10.1111/cas.15431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinho B.R., et al. Pharmacological modulation of HDAC1 and HDAC6 in vivo in a zebrafish model: therapeutic implications for Parkinson's disease. Pharmacol. Res. 2016;103:328–339. doi: 10.1016/j.phrs.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Elfiky A.M.I., et al. Carboxylesterase-1 assisted targeting of HDAC inhibitors to mononuclear myeloid cells in inflammatory bowel disease. J Crohns Colitis. 2022;16(4):668–681. doi: 10.1093/ecco-jcc/jjab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaca H.R., et al. The potential for histone deacetylase (HDAC) inhibitors as cestocidal drugs. PLoS Neglected Trop. Dis. 2021;15(3) doi: 10.1371/journal.pntd.0009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malvaez M., et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc. Natl. Acad. Sci. U.S.A. 2013;110(7):2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angiolilli C., et al. Histone deacetylase 3 regulates the inflammatory gene expression programme of rheumatoid arthritis fibroblast-like synoviocytes. 2017;76(1):277–285. doi: 10.1136/annrheumdis-2015-209064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milazzo G., et al. Histone deacetylases (HDACs): evolution, specificity, role in transcriptional complexes, and pharmacological actionability. 2020;11(5):556. doi: 10.3390/genes11050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delcuve G.P., Khan D.H., Davie J.R. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin. Epigenet. 2012;4(1):5. doi: 10.1186/1868-7083-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaluza D., et al. Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin. EMBO J. 2011;30(20):4142–4156. doi: 10.1038/emboj.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders L.R., Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26(37):5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 20.Villagra A., Sotomayor E.M., Seto E. Histone deacetylases and the immunological network: implications in cancer and inflammation. Oncogene. 2010;29(2):157–173. doi: 10.1038/onc.2009.334. [DOI] [PubMed] [Google Scholar]
- 21.de Ruijter A.J., et al. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 2003;370(Pt 3):737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang-liang H., et al. Chemical constituents with HDAC inhibitory effects from Epimedium sagittatum. 2021;33(10):1681. [Google Scholar]
- 23.Singh B.N., et al. Nonhistone protein acetylation as cancer therapy targets. Expert Rev. Anticancer Ther. 2010;10(6):935–954. doi: 10.1586/era.10.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain D.K., et al. 2014. Hydroxamic Acid Based Histone Deacetylase Inhibitors: Present and Future Prospectives as Anticancer Agent. [Google Scholar]
- 25.Ververis K., et al. Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents. Biologics. 2013;7:47–60. doi: 10.2147/BTT.S29965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsing C.H., et al. Histone deacetylase inhibitor trichostatin A ameliorated endotoxin-induced neuroinflammation and cognitive dysfunction. Mediat. Inflamm. 2015;2015 doi: 10.1155/2015/163140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furumai R., et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62(17):4916–4921. [PubMed] [Google Scholar]
- 28.Trapani D., et al. Entinostat for the treatment of breast cancer. Expet Opin. Invest. Drugs. 2017;26(8):965–971. doi: 10.1080/13543784.2017.1353077. [DOI] [PubMed] [Google Scholar]
- 29.Soflaei S.S., et al. Curcumin: a natural pan-HDAC inhibitor in cancer. 2018;24(2):123–129. doi: 10.2174/1381612823666171114165051. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J.-S.J.C.T., Drugs H. 2018. Regulatory Mechanism of Ginsenoside Rd on Histone H3 Acetylation; pp. 2931–2936. [Google Scholar]
- 31.Wei Y., Guo Y., Lv S.J.J.o.E. Research on the progress of Traditional Chinese medicine components and preparations on histone deacetylase inhibitors-Like effects in the course of disease treatment. J. Ethnopharmacol. 2022;296 doi: 10.1016/j.jep.2022.115521. [DOI] [PubMed] [Google Scholar]
- 32.Ruwizhi N., B.A.J.I.j.o.m.s. Aderibigbe Cinnamic acid derivatives and their biological efficacy. 2020;21(16):5712. doi: 10.3390/ijms21165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X., et al. Natural HDAC‐1/8 inhibitor baicalein exerts therapeutic effect in CBF‐AML. 2020;10(4):e154. doi: 10.1002/ctm2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimazu T., et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muoio D.M., et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metabol. 2012;15(5):764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai M., et al. Discovery of a novel HDACi structure that inhibits the proliferation of ovarian cancer cells in vivo and in vitro. 2021;17(13):3493. doi: 10.7150/ijbs.62339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duenas-Gonzalez A., et al. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008;34(3):206–222. doi: 10.1016/j.ctrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Minucci S., Pelicci P.G. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 39.Chen J.S., et al. Chidamide plus tyrosine kinase inhibitor remodel the tumor immune microenvironment and reduce tumor progression when combined with immune checkpoint inhibitor in naïve and anti-PD-1 resistant CT26-bearing mice. Int. J. Mol. Sci. 2022;23(18) doi: 10.3390/ijms231810677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Susetyo A., et al. Histone deacetylase 3 inhibitor alleviates cerebellar defects in perinatal hypothyroid mice by stimulating histone acetylation and transcription at thyroid hormone-responsive gene loci. Int. J. Mol. Sci. 2022;23(14) doi: 10.3390/ijms23147869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C., et al. Selective targeting of class I HDAC reduces microglial inflammation in the entorhinal cortex of young APP/PS1 mice. Int. J. Mol. Sci. 2023;24(5) doi: 10.3390/ijms24054805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bollmann L.M., et al. The novel class IIa selective histone deacetylase inhibitor YAK540 is synergistic with bortezomib in leukemia cell lines. Int. J. Mol. Sci. 2022;23(21) doi: 10.3390/ijms232113398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darwish S., et al. Design, synthesis and biological characterization of histone deacetylase 8 (HDAC8) proteolysis targeting chimeras (PROTACs) with anti-neuroblastoma activity. Int. J. Mol. Sci. 2022;23(14) doi: 10.3390/ijms23147535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonda D.J., et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 2011;10(3):275–279. doi: 10.1016/S1474-4422(11)70013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramesh S., et al. SIRT3 activator Honokiol attenuates β-Amyloid by modulating amyloidogenic pathway. 2018;13(1) doi: 10.1371/journal.pone.0190350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He T., et al. A novel SIRT6 activator ameliorates neuroinflammation and ischemic brain injury via EZH2/FOXC1 axis. 2021;11(3):708–726. doi: 10.1016/j.apsb.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuadrado-Tejedor M., et al. Concomitant histone deacetylase and phosphodiesterase 5 inhibition synergistically prevents the disruption in synaptic plasticity and it reverses cognitive impairment in a mouse model of Alzheimer's disease. Clin. Epigenet. 2015;7:108. doi: 10.1186/s13148-015-0142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng J., et al. The anti-tumor histone deacetylase inhibitor SAHA and the natural flavonoid curcumin exhibit synergistic neuroprotection against amyloid-beta toxicity. 2014;9(1) doi: 10.1371/journal.pone.0085570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bondarev A.D., et al. Recent developments of HDAC inhibitors: emerging indications and novel molecules. Br. J. Clin. Pharmacol. 2021;87(12):4577–4597. doi: 10.1111/bcp.14889. [DOI] [PubMed] [Google Scholar]
- 50.Harrison I.F., Dexter D.T.J.P. therapeutics. Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson's disease? 2013;140(1):34–52. doi: 10.1016/j.pharmthera.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Park G., et al. Regulation of histone acetylation by autophagy in Parkinson disease. J. Biol. Chem. 2016;291(7):3531–3540. doi: 10.1074/jbc.M115.675488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Q., et al. Nuclear accumulation of histone deacetylase 4 (HDAC4) exerts neurotoxicity in models of Parkinson's disease. Mol. Neurobiol. 2017;54(9):6970–6983. doi: 10.1007/s12035-016-0199-2. [DOI] [PubMed] [Google Scholar]
- 53.Hou Y., et al. vol. 150. 2021. (Neuroprotective Effects of Short-Chain Fatty Acids in MPTP Induced Mice Model of Parkinson's Disease). [DOI] [PubMed] [Google Scholar]
- 54.Shukla S., Tekwani B.L. Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front. Pharmacol. 2020;11:537. doi: 10.3389/fphar.2020.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suh H.S., et al. Histone deacetylase inhibitors suppress the expression of inflammatory and innate immune response genes in human microglia and astrocytes. J. Neuroimmune Pharmacol. 2010;5(4):521–532. doi: 10.1007/s11481-010-9192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chuang D.M., et al. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32(11):591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kontopoulos E., Parvin J.D., Feany M.B. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 2006;15(20):3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- 58.Kidd S.K., Schneider J.S. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010;1354:172–178. doi: 10.1016/j.brainres.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazzocchi M., et al. Peripheral administration of the Class-IIa HDAC inhibitor MC1568 partially protects against nigrostriatal neurodegeneration in the striatal 6-OHDA rat model of Parkinson's disease. Brain Behav. Immun. 2022;102:151–160. doi: 10.1016/j.bbi.2022.02.025. [DOI] [PubMed] [Google Scholar]
- 60.Janssen C., et al. Differential histone deacetylase mRNA expression patterns in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2010;69(6):573–581. doi: 10.1097/NEN.0b013e3181ddd404. [DOI] [PubMed] [Google Scholar]
- 61.Du Z.W., et al. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun. 2015;6:6626. doi: 10.1038/ncomms7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bennett S.A., et al. Epigenetics in amyotrophic lateral sclerosis: a role for histone post-translational modifications in neurodegenerative disease. Transl. Res. 2019;204:19–30. doi: 10.1016/j.trsl.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lazo-Gómez R., et al. Histone deacetylases and their role in motor neuron degeneration. Front. Cell. Neurosci. 2013;7:243. doi: 10.3389/fncel.2013.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng H.L., et al. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience. 2008;155(3):567–572. doi: 10.1016/j.neuroscience.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mancuso R., et al. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics. 2014;11(2):419–432. doi: 10.1007/s13311-013-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuta R., et al. Depending on the stress, histone deacetylase inhibitors act as heat shock protein co-inducers in motor neurons and potentiate arimoclomol, exerting neuroprotection through multiple mechanisms in ALS models. Cell Stress Chaperones. 2020;25(1):173–191. doi: 10.1007/s12192-019-01064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bankole O., et al. Beneficial and sexually dimorphic response to combined HDAC inhibitor valproate and AMPK/SIRT1 pathway activator resveratrol in the treatment of ALS mice. Int. J. Mol. Sci. 2022;23(3) doi: 10.3390/ijms23031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bardai F.H., et al. Histone deacetylase-1 (HDAC1) is a molecular switch between neuronal survival and death. J. Biol. Chem. 2012;287(42):35444–35453. doi: 10.1074/jbc.M112.394544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pallos J., et al. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington's disease. Hum. Mol. Genet. 2008;17(23):3767–3775. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma S., Taliyan R. Transcriptional dysregulation in Huntington's disease: the role of histone deacetylases. Pharmacol. Res. 2015;100:157–169. doi: 10.1016/j.phrs.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Jia H., et al. HDAC inhibition imparts beneficial transgenerational effects in Huntington's disease mice via altered DNA and histone methylation. Proc. Natl. Acad. Sci. U.S.A. 2015;112(1):E56–E64. doi: 10.1073/pnas.1415195112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor D.M., et al. A brain-permeable small molecule reduces neuronal cholesterol by inhibiting activity of sirtuin 2 deacetylase. ACS Chem. Biol. 2011;6(6):540–546. doi: 10.1021/cb100376q. [DOI] [PubMed] [Google Scholar]
- 73.Gardian G., et al. Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington's disease. J. Biol. Chem. 2005;280(1):556–563. doi: 10.1074/jbc.M410210200. [DOI] [PubMed] [Google Scholar]
- 74.Siebzehnrübl F.A., et al. Early postnatal behavioral, cellular, and molecular changes in models of Huntington disease are reversible by HDAC inhibition. Proc. Natl. Acad. Sci. U.S.A. 2018;115(37):E8765–e8774. doi: 10.1073/pnas.1807962115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stott A.J., et al. Evaluation of 5-(Trifluoromethyl)-1,2,4-oxadiazole-Based class IIa HDAC inhibitors for huntington's disease. ACS Med. Chem. Lett. 2021;12(3):380–388. doi: 10.1021/acsmedchemlett.0c00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li E., et al. A novel HDAC6 inhibitor, CKD-504, is effective in treating preclinical models of huntington's disease. 2023;56(3):178. doi: 10.5483/BMBRep.2022-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bekhuis E., et al. The network structure of major depressive disorder, generalized anxiety disorder and somatic symptomatology. Psychol. Med. 2016;46(14):2989–2998. doi: 10.1017/S0033291716001550. [DOI] [PubMed] [Google Scholar]
- 78.Wang Q., Dwivedi Y. Advances in novel molecular targets for antidepressants. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;104 doi: 10.1016/j.pnpbp.2020.110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dunaway L.S., Pollock J.S. HDAC1: an environmental sensor regulating endothelial function. Cardiovasc. Res. 2022;118(8):1885–1903. doi: 10.1093/cvr/cvab198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uchida S., et al. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69(2):359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 81.Fukada M., et al. Loss of deacetylation activity of Hdac6 affects emotional behavior in mice. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Majdzadeh N., Morrison B.E., D'Mello S.R. Class IIA HDACs in the regulation of neurodegeneration. Front. Biosci. 2008;13:1072–1082. doi: 10.2741/2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calabrese V., et al. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxidants Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weaver I.C., Meaney M.J., Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc. Natl. Acad. Sci. U.S.A. 2006;103(9):3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calabrese F., et al. Modulation of neuronal plasticity following chronic concomitant administration of the novel antipsychotic lurasidone with the mood stabilizer valproic acid. Psychopharmacology (Berl) 2013;226(1):101–112. doi: 10.1007/s00213-012-2900-0. [DOI] [PubMed] [Google Scholar]
- 86.Wu H.F., et al. Alleviation of N-Methyl-D-Aspartate receptor-dependent long-term depression via regulation of the glycogen synthase kinase-3β pathway in the amygdala of a valproic acid-induced animal model of autism. Mol. Neurobiol. 2017;54(7):5264–5276. doi: 10.1007/s12035-016-0074-1. [DOI] [PubMed] [Google Scholar]
- 87.Golden S.A., et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat. Med. 2013;19(3):337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim D., et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60(5):803–817. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guan J.-S., et al. HDAC2 negatively regulates memory formation and synaptic plasticity. 2009;459(7243):55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Majdzadeh N., et al. Class IIA HDACs in the regulation of neurodegeneration. Front. Biosci.: J. Vis. Literacy. 2008;13:1072. doi: 10.2741/2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang X.J., Grégoire S. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol. Cell Biol. 2005;25(8):2873–2884. doi: 10.1128/MCB.25.8.2873-2884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harrison I.F., Powell N.M., Dexter D.T. The histone deacetylase inhibitor nicotinamide exacerbates neurodegeneration in the lactacystin rat model of Parkinson's disease. J. Neurochem. 2019;148(1):136–156. doi: 10.1111/jnc.14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szegő É.M., Outeiro T.F., Kazantsev A.G. Introductory Review on Sirtuins in Biology, Aging, and Disease. Elsevier; 2018. Sirtuins in brain and neurodegenerative disease; pp. 175–195. [Google Scholar]
- 94.Thomas E.A., D’Mell S.R. Complex neuroprotective and neurotoxic effects of histone deacetylases. J Neurochem, 2018;145(2):96–110. doi: 10.1111/jnc.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simões-Pires C., et al. HDAC6 as a target for neurodegenerative diseases: what makes it different from the other HDACs? 2013;8:1–16. doi: 10.1186/1750-1326-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Athira K.V., et al. An overview of the heterogeneity of major depressive disorder: current knowledge and future prospective. Curr. Neuropharmacol. 2020;18(3):168–187. doi: 10.2174/1570159X17666191001142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ziemka-Nalecz M., et al. Histone deacetylase inhibitors: a therapeutic key in neurological disorders? 2018;77(10):855–870. doi: 10.1093/jnen/nly073. [DOI] [PubMed] [Google Scholar]
- 98.Gräff J., Tsai L.H. The potential of HDAC inhibitors as cognitive enhancers. Annu. Rev. Pharmacol. Toxicol. 2013;53:311–330. doi: 10.1146/annurev-pharmtox-011112-140216. [DOI] [PubMed] [Google Scholar]
- 99.Pao P.-C., et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer's disease. 2020;11(1):2484. doi: 10.1038/s41467-020-16361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahady L., et al. HDAC2 dysregulation in the nucleus basalis of Meynert during the progression of Alzheimer's disease. Neuropathol. Appl. Neurobiol. 2019;45(4):380–397. doi: 10.1111/nan.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song J.M., et al. A mercaptoacetamide-based class II histone deacetylase inhibitor increases dendritic spine density via RasGRF1/ERK pathway. 2016;51(2):591–604. doi: 10.3233/JAD-150717. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Z.Y., Schluesener H.J. Oral administration of histone deacetylase inhibitor MS-275 ameliorates neuroinflammation and cerebral amyloidosis and improves behavior in a mouse model. J. Neuropathol. Exp. Neurol. 2013;72(3):178–185. doi: 10.1097/NEN.0b013e318283114a. [DOI] [PubMed] [Google Scholar]
- 103.Janczura K.J., et al. Inhibition of HDAC3 reverses Alzheimer's disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc. Natl. Acad. Sci. U.S.A. 2018;115(47):E11148–e11157. doi: 10.1073/pnas.1805436115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Balmik A.A., et al. The extracellular HDAC6 ZnF UBP domain modulates the actin network and post-translational modifications of Tau. Cell Commun. Signal. 2021;19(1):49. doi: 10.1186/s12964-021-00736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Balmik A.A., et al. HDAC6 ZnF UBP as the modifier of tau structure and function. Biochemistry. 2020;59(48):4546–4562. doi: 10.1021/acs.biochem.0c00585. [DOI] [PubMed] [Google Scholar]
- 106.Ding Y., et al. Inhibition of HDAC6 expression decreases brain injury induced by APOE4 and Aβ co-aggregation in rats. Mol. Med. Rep. 2019;20(4):3363–3370. doi: 10.3892/mmr.2019.10571. [DOI] [PubMed] [Google Scholar]
- 107.Hubbert C., et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 108.Zhang L., Sheng S., Qin C. The role of HDAC6 in Alzheimer's disease. J Alzheimers Dis. 2013;33(2):283–295. doi: 10.3233/JAD-2012-120727. [DOI] [PubMed] [Google Scholar]
- 109.Zhang L., et al. Tubastatin A/ACY-1215 improves cognition in Alzheimer's disease transgenic mice. J. Alzheim. Dis. 2014;41(4):1193–1205. doi: 10.3233/JAD-140066. [DOI] [PubMed] [Google Scholar]
- 110.Choi H., et al. Acetylation changes tau interactome to degrade tau in Alzheimer's disease animal and organoid models. Aging Cell. 2020;19(1) doi: 10.1111/acel.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jęśko H., et al. S irtuins and their roles in Brain Aging and Neurodegenerative disorders. Neurochem. Res. 2017;42:876–890. doi: 10.1007/s11064-016-2110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohamad Nasir N.F., Zainuddin A., Shamsuddin S. Emerging Roles of Sirtuin 6 in Alzheimer’s Disease. J Mol Neurosci. 2018;64(2):157–161. doi: 10.1007/s12031-017-1005-y. [DOI] [PubMed] [Google Scholar]
- 113.Pigna E., et al. Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis. EBioMedicine. 2019;40:717–732. doi: 10.1016/j.ebiom.2019.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hecklau K., et al. The effects of selective inhibition of histone deacetylase 1 and 3 in huntington's disease mice. Front. Mol. Neurosci. 2021;14 doi: 10.3389/fnmol.2021.616886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han A., et al. Possible additional antidepressant-like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology. 2014;81:292–302. doi: 10.1016/j.neuropharm.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 116.Feng D.D., et al. Nine traditional Chinese herbal formulas for the treatment of depression: an ethnopharmacology, phytochemistry, and pharmacology review. Neuropsychiatric Dis. Treat. 2016;12:2387–2402. doi: 10.2147/NDT.S114560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y., et al. Xiaoyaosan exerts antidepressant-like effects by regulating the functions of astrocytes and EAATs in the prefrontal cortex of mice. BMC Compl. Alternative Med. 2019;19(1):215. doi: 10.1186/s12906-019-2613-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Wang M., et al. Modified Xiaoyao San ameliorates depressive-like behaviors by triggering autophagosome formation to alleviate neuronal apoptosis. Biomed. Pharmacother. 2019;111:1057–1065. doi: 10.1016/j.biopha.2018.12.141. [DOI] [PubMed] [Google Scholar]
- 119.Hunter D.J., Reddy K.S. Noncommunicable diseases. N. Engl. J. Med. 2013;369(14):1336–1343. doi: 10.1056/NEJMra1109345. [DOI] [PubMed] [Google Scholar]
- 120.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 121.Iyer A., et al. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA‐salt hypertensive rats. 2010;159(7):1408–1417. doi: 10.1111/j.1476-5381.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cardinale J.P., et al. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. 2010;56(3):437–444. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Raj P., et al. A comprehensive analysis of the efficacy of resveratrol in atherosclerotic cardiovascular disease, myocardial infarction and heart failure. Molecules. 2021;26(21) doi: 10.3390/molecules26216600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wajngarten M., Silva G.S. Hypertension and stroke: update on treatment. Eur Cardiol, 2019;14(2):111–115. doi: 10.15420/ecr.2019.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kee H.J., et al. Activation of histone deacetylase 2 by inducible heat shock protein 70 in cardiac hypertrophy. Circ. Res. 2008;103(11):1259–1269. doi: 10.1161/01.RES.0000338570.27156.84. [DOI] [PubMed] [Google Scholar]
- 126.Zhao T., et al. Hdac8 inhibitor alleviates transverse aortic constriction-induced heart failure in mice by downregulating Ace1. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/6227330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kee H.J., et al. HDAC inhibition suppresses cardiac hypertrophy and fibrosis in DOCA-salt hypertensive rats via regulation of HDAC6/HDAC8 enzyme activity. Kidney Blood Press. Res. 2013;37(4–5):229–239. doi: 10.1159/000350148. [DOI] [PubMed] [Google Scholar]
- 128.Zhao L., et al. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation. 2012;126(4):455–467. doi: 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cardinale J.P., et al. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56(3):437–444. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choi J., et al. Role of the histone deacetylase inhibitor valproic acid in high-fat diet-induced hypertension via inhibition of HDAC1/angiotensin II axis. Int. J. Obes. 2017;41(11):1702–1709. doi: 10.1038/ijo.2017.166. [DOI] [PubMed] [Google Scholar]
- 131.Lee H.A., et al. Histone deacetylase inhibition attenuates transcriptional activity of mineralocorticoid receptor through its acetylation and prevents development of hypertension. Circ. Res. 2013;112(7):1004–1012. doi: 10.1161/CIRCRESAHA.113.301071. [DOI] [PubMed] [Google Scholar]
- 132.Chi Z., et al. Histone deacetylase 6 inhibitor tubastatin A attenuates angiotensin II-induced hypertension by preventing cystathionine γ-lyase protein degradation. Pharmacol. Res. 2019;146 doi: 10.1016/j.phrs.2019.104281. [DOI] [PubMed] [Google Scholar]
- 133.Yoon G.E., et al. Histone deacetylase inhibitor CG200745 ameliorates high-fat diet-induced hypertension via inhibition of angiotensin II production. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393(3):491–500. doi: 10.1007/s00210-019-01749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Landi F., et al. Body mass index is strongly associated with hypertension: results from the longevity check-up 7+ study. Nutrients. 2018;10(12) doi: 10.3390/nu10121976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bai L., et al. HDAC5 inhibition reduces angiotensin II-induced vascular contraction, hypertrophy, and oxidative stress in a mouse model. Biomed. Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111162. [DOI] [PubMed] [Google Scholar]
- 136.Azechi T., et al. Trichostatin A. An HDAC class I/II inhibitor, promotes Pi-induced vascular calcification via up-regulation of the expression of alkaline phosphatase. J. Atherosclerosis Thromb. 2013;20(6):538–547. doi: 10.5551/jat.15826. [DOI] [PubMed] [Google Scholar]
- 137.Zheng X.X., et al. Histone deacetylases and atherosclerosis. Atherosclerosis. 2015;240(2):355–366. doi: 10.1016/j.atherosclerosis.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 138.Bazou D., et al. Flow-induced HDAC1 phosphorylation and nuclear export in angiogenic sprouting. Sci. Rep. 2016;6 doi: 10.1038/srep34046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou B., et al. Splicing of histone deacetylase 7 modulates smooth muscle cell proliferation and neointima formation through nuclear β-catenin translocation. Arterioscler. Thromb. Vasc. Biol. 2011;31(11):2676–2684. doi: 10.1161/ATVBAHA.111.230888. [DOI] [PubMed] [Google Scholar]
- 140.Lee D.Y., et al. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc. Natl. Acad. Sci. U.S.A. 2012;109(6):1967–1972. doi: 10.1073/pnas.1121214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tambaro F.P., et al. Histone deacetylase inhibitors: clinical implications for hematological malignancies. Clin. Epigenet. 2010;1(1–2):25–44. doi: 10.1007/s13148-010-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao T.C., Wang Z., Zhao T.Y. The important role of histone deacetylases in modulating vascular physiology and arteriosclerosis. Atherosclerosis. 2020;303:36–42. doi: 10.1016/j.atherosclerosis.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen S., et al. Valproic acid: a new candidate of therapeutic application for the acute central nervous system injuries. Neurochem. Res. 2014;39(9):1621–1633. doi: 10.1007/s11064-014-1241-2. [DOI] [PubMed] [Google Scholar]
- 144.Asare Y., et al. Histone deacetylase 9 activates IKK to regulate atherosclerotic plaque vulnerability. Circ. Res. 2020;127(6):811–823. doi: 10.1161/CIRCRESAHA.120.316743. [DOI] [PubMed] [Google Scholar]
- 145.Chen L., et al. HDAC3 inhibitor suppresses endothelial-to-mesenchymal transition via modulating inflammatory response in atherosclerosis. Biochem. Pharmacol. 2021;192 doi: 10.1016/j.bcp.2021.114716. [DOI] [PubMed] [Google Scholar]
- 146.Haberland M., Montgomery R.L., Olson E.N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McBurney M.W., et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell Biol. 2003;23(1):38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Risebro C.A., et al. Epistatic rescue of Nkx2.5 adult cardiac conduction disease phenotypes by prospero-related homeobox protein 1 and HDAC3. Circ. Res. 2012;111(2):e19–e31. doi: 10.1161/CIRCRESAHA.111.260695. [DOI] [PubMed] [Google Scholar]
- 149.Cavasin M.A., Stenmark K.R., McKinsey T.A. Emerging roles for histone deacetylases in pulmonary hypertension and right ventricular remodeling (2013 Grover Conference series) Pulm. Circ. 2015;5(1):63–72. doi: 10.1086/679700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chelladurai P., Seeger W., Pullamsetti S.S. Epigenetic mechanisms in pulmonary arterial hypertension: the need for global perspectives. Eur. Respir. Rev. 2016;25(140):135–140. doi: 10.1183/16000617.0036-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chelladurai P., et al. Isoform-specific characterization of class I histone deacetylases and their therapeutic modulation in pulmonary hypertension. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-69737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cavasin M.A., et al. Selective class I histone deacetylase inhibition suppresses hypoxia-induced cardiopulmonary remodeling through an antiproliferative mechanism. Circ. Res. 2012;110(5):739–748. doi: 10.1161/CIRCRESAHA.111.258426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tian S., et al. HDAC inhibitor valproic acid protects heart function through Foxm1 pathway after acute myocardial infarction. EBioMedicine. 2019;39:83–94. doi: 10.1016/j.ebiom.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kang S.H., et al. Histone deacetylase inhibition attenuates cardiac hypertrophy and fibrosis through acetylation of mineralocorticoid receptor in spontaneously hypertensive rats. Mol. Pharmacol. 2015;87(5):782–791. doi: 10.1124/mol.114.096974. [DOI] [PubMed] [Google Scholar]
- 155.Scholz B., et al. HDAC (histone deacetylase) inhibitor valproic acid attenuates atrial remodeling and delays the onset of atrial fibrillation in mice. Circ Arrhythm Electrophysiol. 2019;12(3) doi: 10.1161/CIRCEP.118.007071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Choi S.Y., et al. Class I HDACs specifically regulate E-cadherin expression in human renal epithelial cells. J. Cell Mol. Med. 2016;20(12):2289–2298. doi: 10.1111/jcmm.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tang J., et al. Class I histone deacetylase activity is required for proliferation of renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2013;305(3):F244–F254. doi: 10.1152/ajprenal.00126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu W., et al. HDAC6 regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation in renal epithelial cells. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim J.I., et al. Gender-specific role of HDAC11 in kidney ischemia-and reperfusion-induced PAI-1 expression and injury. Am. J. Physiol. Ren. Physiol. 2013;305(1):F61–F70. doi: 10.1152/ajprenal.00015.2013. [DOI] [PubMed] [Google Scholar]
- 160.Ponnusamy M., et al. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J. Pharmacol. Exp. Therapeut. 2014;350(2):243–256. doi: 10.1124/jpet.113.212076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou X., et al. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J. Clin. Invest. 2013;123(7):3084–3098. doi: 10.1172/JCI64401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ren J., et al. Selective HDAC6 inhibition decreases early stage of lupus nephritis by down-regulating both innate and adaptive immune responses. Clin. Exp. Immunol. 2018;191(1):19–31. doi: 10.1111/cei.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reidy K., et al. Molecular mechanisms of diabetic kidney disease. J. Clin. Invest. 2014;124(6):2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mauer S.M., et al. Structural-functional relationships in diabetic nephropathy. J. Clin. Invest. 1984;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gilbert R.E., et al. Histone deacetylase inhibition attenuates diabetes-associated kidney growth: potential role for epigenetic modification of the epidermal growth factor receptor. Kidney Int. 2011;79(12):1312–1321. doi: 10.1038/ki.2011.39. [DOI] [PubMed] [Google Scholar]
- 166.Advani A., et al. Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am. J. Pathol. 2011;178(5):2205–2214. doi: 10.1016/j.ajpath.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Inoue K., et al. Podocyte histone deacetylase activity regulates murine and human glomerular diseases. J. Clin. Invest. 2019;129(3):1295–1313. doi: 10.1172/JCI124030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Noh H., et al. Histone deacetylase-2 is a key regulator of diabetes-and transforming growth factor-β1-induced renal injury. 2009;297(3):F729–F739. doi: 10.1152/ajprenal.00086.2009. [DOI] [PubMed] [Google Scholar]
- 169.Gilbert R.E., et al. Histone deacetylase inhibition attenuates diabetes-associated kidney growth: potential role for epigenetic modification of the epidermal growth factor receptor. 2011;79(12):1312–1321. doi: 10.1038/ki.2011.39. [DOI] [PubMed] [Google Scholar]
- 170.Advani A., et al. Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. 2011;178(5):2205–2214. doi: 10.1016/j.ajpath.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Khan S., et al. Valproate attenuates the proteinuria, podocyte and renal injury by facilitating autophagy and inactivation of NF-κB/iNOS signaling in diabetic rat. Biochimie. 2015;110:1–16. doi: 10.1016/j.biochi.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 172.Sun X.Y., et al. Valproate attenuates diabetic nephropathy through inhibition of endoplasmic reticulum stress-induced apoptosis. Mol. Med. Rep. 2016;13(1):661–668. doi: 10.3892/mmr.2015.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dong W., et al. Sodium butyrate activates NRF2 to ameliorate diabetic nephropathy possibly via inhibition of HDAC. J. Endocrinol. 2017;232(1):71–83. doi: 10.1530/JOE-16-0322. [DOI] [PubMed] [Google Scholar]
- 174.Chen J., Chen J.K., Harris R.C. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J. Am. Soc. Nephrol. 2015;26(5):1115–1125. doi: 10.1681/ASN.2014020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin C.-L., et al. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J. Am. Soc. Nephrol. 2014;25(8):1698–1709. doi: 10.1681/ASN.2013050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang X., et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014;86(4):712–725. doi: 10.1038/ki.2014.111. [DOI] [PubMed] [Google Scholar]
- 177.Kochar D.K., et al. Sodium valproate for painful diabetic neuropathy: a randomized double-blind placebo-controlled study. QJM. 2004;97(1):33–38. doi: 10.1093/qjmed/hch007. [DOI] [PubMed] [Google Scholar]
- 178.Galmozzi A., et al. Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes. 2013;62(3):732–742. doi: 10.2337/db12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dirice E., et al. Isoform-selective inhibitor of histone deacetylase 3 (HDAC3) limits pancreatic islet infiltration and protects female nonobese diabetic mice from diabetes. J. Biol. Chem. 2017;292(43):17598–17608. doi: 10.1074/jbc.M117.804328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhong Y., et al. Renoprotective effect of combined inhibition of angiotensin-converting enzyme and histone deacetylase. 2013;24(5):801–811. doi: 10.1681/ASN.2012060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abdel Galil S.M., et al. Interferon alpha gene expression and serum level association with low vitamin D levels in Egyptian female patients with systemic lupus erythematosus. Lupus. 2018;27(2):199–209. doi: 10.1177/0961203317716321. [DOI] [PubMed] [Google Scholar]
- 182.Hedrich C.M. Epigenetics in SLE. Curr. Rheumatol. Rep. 2017;19(9):58. doi: 10.1007/s11926-017-0685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Beier U.H., et al. Histone/protein deacetylases control Foxp3 expression and the heat shock response of T-regulatory cells. Curr. Opin. Immunol. 2011;23(5):670–678. doi: 10.1016/j.coi.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mishra N., et al. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J. Clin. Invest. 2003;111(4):539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Regna N.L., et al. Class I and II histone deacetylase inhibition by ITF2357 reduces SLE pathogenesis in vivo. Clin. Immunol. 2014;151(1):29–42. doi: 10.1016/j.clim.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Choi E.W., et al. CKD-506, a novel HDAC6-selective inhibitor, improves renal outcomes and survival in a mouse model of systemic lupus erythematosus. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-35602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Suso J.P., et al. Profile of BAFF and its receptors' expression in lupus nephritis is associated with pathological classes. Lupus. 2018;27(5):708–715. doi: 10.1177/0961203317739132. [DOI] [PubMed] [Google Scholar]
- 188.Ruggenenti P., Fervenza F.C., Remuzzi G. Treatment of membranous nephropathy: time for a paradigm shift. Nat. Rev. Nephrol. 2017;13(9):563–579. doi: 10.1038/nrneph.2017.92. [DOI] [PubMed] [Google Scholar]
- 189.Neilson E.G. Mechanisms of disease: fibroblasts—a new look at an old problem. Nat. Clin. Pract. Nephrol. 2006;2(2):101–108. doi: 10.1038/ncpneph0093. [DOI] [PubMed] [Google Scholar]
- 190.Manson S.R., et al. HDAC dependent transcriptional repression of Bmp-7 potentiates TGF-β mediated renal fibrosis in obstructive uropathy. J. Urol. 2014;191(1):242–252. doi: 10.1016/j.juro.2013.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hyndman K.A., et al. Dynamic changes in histone deacetylases following kidney ischemia-reperfusion injury are critical for promoting proximal tubule proliferation. Am. J. Physiol. Ren. Physiol. 2019;316(5):F875–f888. doi: 10.1152/ajprenal.00499.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Humphreys B.D. Mechanisms of renal fibrosis. Annu. Rev. Physiol. 2018;80:309–326. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 193.Pang M., et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Ren. Physiol. 2009;297(4):F996–F1005. doi: 10.1152/ajprenal.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Marumo T., et al. Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury. Am. J. Physiol. Ren. Physiol. 2010;298(1):F133–F141. doi: 10.1152/ajprenal.00400.2009. [DOI] [PubMed] [Google Scholar]
- 195.Wu W.P., et al. The attenuation of renal fibrosis by histone deacetylase inhibitors is associated with the plasticity of FOXP3(+)IL-17(+) T cells. BMC Nephrol. 2017;18(1):225. doi: 10.1186/s12882-017-0630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pang M., et al. Histone deacetylase 1/2 mediates proliferation of renal interstitial fibroblasts and expression of cell cycle proteins. J. Cell. Biochem. 2011;112(8):2138–2148. doi: 10.1002/jcb.23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu N., et al. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J. Am. Soc. Nephrol. 2012;23(5):854–867. doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shi Y., et al. Inhibition of HDAC6 protects against rhabdomyolysis-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2017;312(3):F502–f515. doi: 10.1152/ajprenal.00546.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Novitskaya T., et al. A PTBA small molecule enhances recovery and reduces postinjury fibrosis after aristolochic acid-induced kidney injury. Am. J. Physiol. Ren. Physiol. 2014;306(5):F496–F504. doi: 10.1152/ajprenal.00534.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ghiboub M., et al. HDAC3 mediates the inflammatory response and LPS tolerance in human monocytes and macrophages. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.550769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chung Y.L., et al. A therapeutic strategy uses histone deacetylase inhibitors to modulate the expression of genes involved in the pathogenesis of rheumatoid arthritis. Mol. Ther. 2003;8(5):707–717. doi: 10.1016/s1525-0016(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 202.Nishida K., et al. Histone deacetylase inhibitor suppression of autoantibody-mediated arthritis in mice via regulation of p16INK4a and p21(WAF1/Cip1) expression. Arthritis Rheum. 2004;50(10):3365–3376. doi: 10.1002/art.20709. [DOI] [PubMed] [Google Scholar]
- 203.Brinkmann C.R., et al. Treatment of HIV-infected individuals with the histone deacetylase inhibitor panobinostat results in increased numbers of regulatory T cells and limits ex vivo lipopolysaccharide-induced inflammatory responses. mSphere. 2018;3(1):e00616–e00617. doi: 10.1128/mSphere.00616-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Furlan A., et al. Pharmacokinetics, safety and inducible cytokine responses during a phase 1 trial of the oral histone deacetylase inhibitor ITF2357 (givinostat) Mol. Med. 2011;17(5–6):353–362. doi: 10.2119/molmed.2011.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.LoPresti P. The selective HDAC6 inhibitor ACY-738 impacts memory and disease regulation in an animal model of multiple sclerosis. Front. Neurol. 2019;10:519. doi: 10.3389/fneur.2019.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shuttleworth S.J., Bailey S.G., Townsend P.A. Histone Deacetylase inhibitors: new promise in the treatment of immune and inflammatory diseases. Curr. Drug Targets. 2010;11(11):1430–1438. doi: 10.2174/1389450111009011430. [DOI] [PubMed] [Google Scholar]
- 207.Faraco G., Cavone L., Chiarugi A. The therapeutic potential of HDAC inhibitors in the treatment of multiple sclerosis. Mol. Med. 2011;17(5–6):442–447. doi: 10.2119/molmed.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bettica P., et al. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 2016;26(10):643–649. doi: 10.1016/j.nmd.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 209.Vojinovic J., et al. Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2011;63(5):1452–1458. doi: 10.1002/art.30238. [DOI] [PubMed] [Google Scholar]
- 210.Friedrich M., et al. HDAC inhibitors promote intestinal epithelial regeneration via autocrine TGFβ1 signalling in inflammation. Mucosal Immunol. 2019;12(3):656–667. doi: 10.1038/s41385-019-0135-7. [DOI] [PubMed] [Google Scholar]
- 211.Busslinger M., Tarakhovsky A. Epigenetic control of immunity. Cold Spring Harbor Perspect. Biol. 2014;6(6) doi: 10.1101/cshperspect.a019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shakespear M.R., et al. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32(7):335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 213.Moradei O.M., et al. Novel aminophenyl benzamide-type histone deacetylase inhibitors with enhanced potency and selectivity. J. Med. Chem. 2007;50(23):5543–5546. doi: 10.1021/jm701079h. [DOI] [PubMed] [Google Scholar]
- 214.Saito A., et al. A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc. Natl. Acad. Sci. U.S.A. 1999;96(8):4592–4597. doi: 10.1073/pnas.96.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nadeem A., et al. Imiquimod-induced psoriasis-like skin inflammation is suppressed by BET bromodomain inhibitor in mice through RORC/IL-17A pathway modulation. Pharmacol. Res. 2015;99:248–257. doi: 10.1016/j.phrs.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 216.Leus N.G., et al. HDAC1-3 inhibitor MS-275 enhances IL10 expression in RAW264.7 macrophages and reduces cigarette smoke-induced airway inflammation in mice. Sci. Rep. 2017;7 doi: 10.1038/srep45047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ryu Y., et al. Class I histone deacetylase inhibitor MS-275 attenuates vasoconstriction and inflammation in angiotensin II-induced hypertension. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0213186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lin H.S., et al. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br. J. Pharmacol. 2007;150(7):862–872. doi: 10.1038/sj.bjp.0707165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Do A., et al. An HDAC6 inhibitor confers protection and selectively inhibits B-cell infiltration in DSS-induced colitis in mice. J. Pharmacol. Exp. Therapeut. 2017;360(1):140–151. doi: 10.1124/jpet.116.236711. [DOI] [PubMed] [Google Scholar]
- 220.Lee J.W., et al. Novel histone deacetylase 6 inhibitor CKD-506 inhibits NF-κB signaling in intestinal epithelial cells and macrophages and ameliorates acute and chronic murine colitis. Inflamm. Bowel Dis. 2020;26(6):852–862. doi: 10.1093/ibd/izz317. [DOI] [PubMed] [Google Scholar]
- 221.Imre G., et al. Histone deacetylase inhibitors suppress the inducibility of nuclear factor-kappaB by tumor necrosis factor-alpha receptor-1 down-regulation. Cancer Res. 2006;66(10):5409–5418. doi: 10.1158/0008-5472.CAN-05-4225. [DOI] [PubMed] [Google Scholar]
- 222.Lee H.Y., et al. 1-arylsulfonyl-5-(N-hydroxyacrylamide)indolines histone deacetylase inhibitors are potent cytokine release suppressors. Chembiochem. 2013;14(10):1248–1254. doi: 10.1002/cbic.201300201. [DOI] [PubMed] [Google Scholar]
- 223.Li J.H., et al. The histone deacetylase inhibitor chidamide induces intermittent viraemia in HIV-infected patients on suppressive antiretroviral therapy. HIV Med. 2020;21(11):747–757. doi: 10.1111/hiv.13027. [DOI] [PubMed] [Google Scholar]
- 224.Kuai Q., et al. Histone deacetylase inhibitor chidamide promotes reactivation of latent human immunodeficiency virus by introducing histone acetylation. J. Med. Virol. 2018;90(9):1478–1485. doi: 10.1002/jmv.25207. [DOI] [PubMed] [Google Scholar]
- 225.Ingram A.K., Horn D. Histone deacetylases in Trypanosoma brucei: two are essential and another is required for normal cell cycle progression. Mol. Microbiol. 2002;45(1):89–97. doi: 10.1046/j.1365-2958.2002.03018.x. [DOI] [PubMed] [Google Scholar]
- 226.Aurrecoechea C., et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37(Database issue):D539–D543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Saksouk N., et al. Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol. Cell Biol. 2005;25(23):10301–10314. doi: 10.1128/MCB.25.23.10301-10314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Scholte L.L.S., et al. Evolutionary relationships among protein lysine deacetylases of parasites causing neglected diseases. Infect. Genet. Evol. 2017;53:175–188. doi: 10.1016/j.meegid.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 229.Bougdour A., et al. Drug inhibition of HDAC3 and epigenetic control of differentiation in Apicomplexa parasites. J. Exp. Med. 2009;206(4):953–966. doi: 10.1084/jem.20082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Strobl J.S., et al. Scriptaid and suberoylanilide hydroxamic acid are histone deacetylase inhibitors with potent anti-Toxoplasma gondii activity in vitro. J. Parasitol. 2007;93(3):694–700. doi: 10.1645/GE-1043R.1. [DOI] [PubMed] [Google Scholar]
- 231.Vaca H.R., et al. Histone deacetylase enzymes as potential drug targets of Neglected Tropical Diseases caused by cestodes. Int J Parasitol Drugs Drug Resist. 2019;9:120–132. doi: 10.1016/j.ijpddr.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dubois F., et al. Histone deacetylase inhibitors induce apoptosis, histone hyperacetylation and up-regulation of gene transcription in Schistosoma mansoni. Mol. Biochem. Parasitol. 2009;168(1):7–15. doi: 10.1016/j.molbiopara.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 233.Dow G.S., et al. Antimalarial activity of phenylthiazolyl-bearing hydroxamate-based histone deacetylase inhibitors. Antimicrob. Agents Chemother. 2008;52(10):3467–3477. doi: 10.1128/AAC.00439-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Prusty D., et al. Nicotinamide inhibits Plasmodium falciparum Sir2 activity in vitro and parasite growth. FEMS Microbiol. Lett. 2008;282(2):266–272. doi: 10.1111/j.1574-6968.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 235.Chakrabarty S.P., et al. Development and characterization of lysine based tripeptide analogues as inhibitors of Sir2 activity. Bioorg. Med. Chem. 2009;17(23):8060–8072. doi: 10.1016/j.bmc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 236.Cruz-Topete D., Cidlowski J.A. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22(1–2):20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ripamonti C., et al. HDAC inhibition as potential therapeutic strategy to restore the deregulated immune response in severe COVID-19. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.841716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chai P., et al. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: a pan-cancer analysis. J. Hematol. Oncol. 2020;13(1):43. doi: 10.1186/s13045-020-00883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hung I.F., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nehme Z., Pasquereau S., Herbein G. Control of viral infections by epigenetic-targeted therapy. Clin. Epigenet. 2019;11(1):55. doi: 10.1186/s13148-019-0654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhao Y., et al. Valproic acid inhibits the angiogenic potential of cervical cancer cells via HIF-1α/VEGF signals. Clin. Transl. Oncol. 2016;18(11):1123–1130. doi: 10.1007/s12094-016-1494-0. [DOI] [PubMed] [Google Scholar]
- 242.Wu C., et al. Histone deacetylase inhibition by sodium valproate regulates polarization of macrophage subsets. DNA Cell Biol. 2012;31(4):592–599. doi: 10.1089/dna.2011.1401. [DOI] [PubMed] [Google Scholar]
- 243.Bikdeli B., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Larsson P., et al. Valproic acid selectively increases vascular endothelial tissue-type plasminogen activator production and reduces thrombus formation in the mouse. J. Thromb. Haemostasis. 2016;14(12):2496–2508. doi: 10.1111/jth.13527. [DOI] [PubMed] [Google Scholar]
- 245.Causey M.W., et al. Valproic acid reversed pathologic endothelial cell gene expression profile associated with ischemia-reperfusion injury in a swine hemorrhagic shock model. J. Vasc. Surg. 2012;55(4):1096–1103.e51. doi: 10.1016/j.jvs.2011.08.060. [DOI] [PubMed] [Google Scholar]
- 246.clinicaltrials.gov. [cited 2020 14 August]; Available from: https://clinicaltrials.gov/ct2/show/NCT04513314.
- 247.Pitt B., et al. Potential repurposing of the HDAC inhibitor valproic acid for patients with COVID-19. Eur. J. Pharmacol. 2021;898 doi: 10.1016/j.ejphar.2021.173988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Klok F.A., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Panigada M., et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemostasis. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang F., et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5(10) doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ravindran V., et al. Discovery of host-directed modulators of virus infection by probing the SARS-CoV-2-host protein-protein interaction network. Briefings Bioinf. 2022;23(6) doi: 10.1093/bib/bbac456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Crimi E., et al. Epigenetic susceptibility to severe respiratory viral infections and its therapeutic implications: a narrative review. Br. J. Anaesth. 2020;125(6):1002–1017. doi: 10.1016/j.bja.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Xu P., et al. NOS1 inhibits the interferon response of cancer cells by S-nitrosylation of HDAC2. J. Exp. Clin. Cancer Res. 2019;38(1):483. doi: 10.1186/s13046-019-1448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Marié I.J., Chang H.M., Levy D.E. HDAC stimulates gene expression through BRD4 availability in response to IFN and in interferonopathies. J. Exp. Med. 2018;215(12):3194–3212. doi: 10.1084/jem.20180520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Eastman A.J., et al. Epigenetic stabilization of DC and DC precursor classical activation by TNFα contributes to protective T cell polarization. Sci. Adv. 2019;5(12):eaaw9051. doi: 10.1126/sciadv.aaw9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Weiss U., et al. Inhibition of HDAC enzymes contributes to differential expression of pro-inflammatory proteins in the TLR-4 signaling cascade. Int. J. Mol. Sci. 2020;21(23) doi: 10.3390/ijms21238943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Richter T., et al. Rare disease terminology and definitions-A systematic global review: report of the ISPOR rare disease special interest group. Value Health. 2015;18(6):906–914. doi: 10.1016/j.jval.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 258.Butler K.V., et al. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J. Am. Chem. Soc. 2010;132(31):10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Good K.V., et al. Trichostatin A decreases the levels of MeCP2 expression and phosphorylation and increases its chromatin binding affinity. Epigenetics. 2017;12(11):934–944. doi: 10.1080/15592294.2017.1380760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schmitt H.M., et al. Targeting HDAC3 in the DBA/2J spontaneous mouse model of glaucoma. Exp. Eye Res. 2020;200 doi: 10.1016/j.exer.2020.108244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Unterman I., et al. Expanding the MECP2 network using comparative genomics reveals potential therapeutic targets for Rett syndrome. Elife. 2021;10 doi: 10.7554/eLife.67085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Park S.Y., Kim J.S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp. Mol. Med. 2020;52(2):204–212. doi: 10.1038/s12276-020-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gao Y.S., Hubbert C.C., Yao T.P. The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation. J. Biol. Chem. 2010;285(15):11219–11226. doi: 10.1074/jbc.M109.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ferreira R.C., et al. Histone deacetylase 1 is essential for rod photoreceptor differentiation by regulating acetylation at histone H3 lysine 9 and histone H4 lysine 12 in the mouse retina. J. Biol. Chem. 2017;292(6):2422–2440. doi: 10.1074/jbc.M116.756643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Popova E.Y., et al. Inhibition of epigenetic modifiers LSD1 and HDAC1 blocks rod photoreceptor death in mouse models of retinitis pigmentosa. J. Neurosci. 2021;41(31):6775–6792. doi: 10.1523/JNEUROSCI.3102-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Roche J., Bertrand P. Inside HDACs with more selective HDAC inhibitors. Eur. J. Med. Chem. 2016;121:451–483. doi: 10.1016/j.ejmech.2016.05.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with my study doesn't been deposited into a publicly available repository. No data was used for the research described in the article.