Abstract
An estimated 45% of adult Americans currently have high blood pressure (HBP). Effective blood pressure (BP) control is essential for preventing major adverse events from cardiovascular and other vascular-related diseases, such as chronic kidney disease, stroke and dementia. A large and growing number of medical professional societies, health care organizations, and governmental agencies have now endorsed a clinical practice guideline-based target for adequate control of HBP to a systolic BP of less than 130 mm Hg. However, adequate BP control to this goal has been recently estimated to be as low as 30%. The first and most important steps to guide effective BP control include accurate, standardized BP measurement and formal assessment of overall atherosclerotic cardiovascular disease risk. In addition to appropriate pharmacologic treatment, optimal BP management must also include multifaceted guideline-directed lifestyle modifications. High-quality evidence now supports effective uniform HBP control that is consistently achievable for most of people from diverse backgrounds. This can be accomplished through identification and prioritization of social determinants of health enabled by shared decision making that is delivered via team-based care. Such integrated approaches can have a substantial impact for simultaneously reducing several major modifiable atherosclerotic cardiovascular disease risk factors. Hence, moving the “Big Needle” of improved overall cardiovascular, kidney, and brain health of the US population must no longer be solely relegated to primary care and will require a major and coordinated reprioritization of capital and evidence-based human resource allocations by all health care stakeholder organizations.
Since 2019, the American Heart Association (AHA) and American Stroke Association have jointly published their annual summary of Heart Disease and Stroke Statistics, defining high blood pressure (HBP) on the basis of the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults (The 2017 HBP Guideline) staging classification (Table 1)1: “The following definition of HBP has been proposed for surveillance: systolic blood pressure (SBP) ≥130 mm Hg, diastolic blood pressure (DBP) ≥80 mm Hg, or self-reported antihypertensive medicine use, or having been told previously, at least twice, by a physician or other health professional that one has HBP.”2 These 11 specialty societies include the American College of Cardiology (ACC); AHA; American Association of Physician Assistants (AAPA); Association of Black Cardiologists (ABC); American College of Preventive Medicine (ACPM); American Geriatrics Society (AGS); American Pharmacists Association (APhA); American Association for Preventive Cardiologists (ASPC); American Society of Hypertension (ASH); National Medical Association (NMA); and Preventive Cardiology Nurses Association (PCNA).
Table 1.
Blood Pressure Classification From the 2017 ACC/AHA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adultsa,b,1
| Normal | Less than 120/80 mm Hg |
|---|---|
| Elevated | SBP between 120 and 129 mm Hg and DBP less than 80 mm Hg |
| Stage 1 | SBP between 130 and 139 mm Hg or DBP between 80 and 89 mm Hg |
| Stage 2 | SBP at least 140 mm Hg or DBP at least 90 mm Hg |
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Hypertensive crisis: SBP greater than 180 mm Hg and/or DBP greater than 120 mm Hg, with patients needing prompt changes in medication if there are no other indications of problems or immediate hospitalization if there are signs of organ damage.
Growing Consensus for a Lower HBP Control Target
In addition to these original endorsers of the 2017 HBP Guideline, many other US professional organizations, such as the American Diabetes Association, large health systems, payers, as well as certain federal and state government agencies, have also adopted a standard target of SBP less than 130 mm Hg for adequate control of HBP (Supplemental Appendix 1, available online at http://www.mcpiqojournal.org).
The current chronic kidney disease guidelines from the Kidney Disease: Improving Global Outcomes Blood Pressure Work Group, which now recommends controlling HBP to a target goal of SBP less than 120 mm Hg for most adult patients with chronic kidney disease not receiving dialysis when tolerated.3 Furthermore, a recently published Post Hoc Analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) has also shown that older patients, those most functionally impacted by incident cardiovascular disease (CVD), even adults up to the age of 85 years with frailty and HBP, achieve equal benefit from intensive blood pressure (BP) control to the more intensive goal of SBP less than 120 mm Hg when compared with patients younger than 65 years and that these benefits are seen without an increased risk of serious adverse events.4,5Additional evidence derived from SPRINT demonstrates a lower risk of left ventricular conduction disease,6 improved secondary prevention in patients with stroke or cerebral transient ischemic attacks,7 and greater absolute cognitive benefit among those at higher risk of dementia for people with HBP treated to the lower target.8 The recent Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients and the China Rural Hypertension Control Project, not included in recent metanalyses and reviews, further support the finding of the SPRINT trial for the impact of intensive BP control markedly reducing incident CVD events.9,10
Conflicting Guidelines Cause Uncertainty
Despite this growing consensus, some key regulatory agencies and professional medical societies have not yet accepted this target for adequate HBP control. Conflicting definitions for hypertension control remain in place for several regulatory organizations and a very few remaining professional societies, including the Centers for Medicare and Medicaid (CMS),11 the National Committee on Quality Assurance (NCQA),12 US Health and Human Services “Million Hearts” initiative,13 and 2 major US physician organizations, the American College of Physicians and American Academy of Family Physicians, which now promote conflicting definitions for adequate HBP control of SBP less than 140 mm Hg14 and SBP less than 140 or less than 135 mm Hg, respectively.15 The recently published 2023 European Society of Hypertension guidelines for the management of arterial hypertension also has different definitions for adequate control, but now align more closely with the 2017 ACC AHA et al HBP Guideline.16 Although ACC and AHA jointly published a new peer-reviewed performance measure of adequate HBP control to SBP less than 130 mm Hg for both stage 2 and stage 1 HBP in 2019,17 adoption and use of these criteria have not been done by CMS and NCQA. The subsequent failure to align treatment goals across multiple major US medical specialty societies has resulted in widespread confusion and widespread clinical inertia among health care providers and, hence, continued poor hypertension control on a national level and, in particular, for persons most vulnerable to the complications of hypertension.
The Global Impact of Uncontrolled HBP
High blood pressure remains the most prevalent modifiable risk factor for the development of morbidity and mortality in the world. According to the most recently available data from AHA, stage 2 HBP (defined as 140/90 mm Hg) was 1 of the 5 leading risk factors for the burden of disease (years of life lost and disability-adjusted life years) in all regions of the world except Oceania and eastern, central, and western sub-Saharan Africa.2 In the United States, more than 1 in 4 (28%) of individuals with stage 2 HBP are not controlled to less than 140/90 mm Hg. This percentage has remained unchanged or worse for the past decade despite continuous public reporting of the current quality measure by CMS, many other health insurers, and other governmental agencies such as the Office of the National Coordinator for Health Information Technology (ONC) in a large variety of value-based payment programs.11,12 In fact, the recent COVID pandemic was associated with a dramatic decline in this quality measure in 202018 in spite of growing evidence supporting substantial health benefits from adequately treating SBP to less than 130 mm Hg.5
Using the current evidence and this lower consensus-based HBP control target, it is now estimated that 45% of the US population, or more than 115 million adults aged 18 years and older and not including an additional unknown number of children aged 8–17 years have or are at risk for developing stage 1 or stage 2 HBP.2,19 Hence, if the current quality measure deployed by NCQA, CMS, and other payers redefined “adequate control” to this lower target and included adults and children with stage 1 HBP, many experts expect control rates to be reported as low as 30%.
2017 HBP Guideline Recommendation 1: Ensuring Accurate BP Measurement
Consistent, accurate measurement of BP for any given patient is also of critical importance for both people with and without HBP. This statement has been strongly reflected in the 2017 HBP Guideline, reinforced by the SPRINT trial and by national and international efforts to standardize and encourage consistent BP measurements obtained within health care settings as well as through properly validated self-monitored BP devices commonly used by patients in the home across many countries worldwide.1,20, 21, 22, 23 It should also be noted that a growing number of commercially available BP measurement devices have been validated for clinical accuracy as determined through the US Blood Pressure Validated Device Listing and internationally.24,25 Accurate BP measurement is obtained when a number of important preparatory steps are taken in advance of each reading in accordance with checklists and infographics such as those used in the AHA/AMA “target BP” initiative.26,27 The importance of accurate BP measurement in both health care and home settings cannot be overemphasized, given the well-documented and quantified inaccuracies of as much as 20 mm Hg that may occur with improper technique, as outlined in Table 2.20,22 Recently published and highly publicized data also demonstrate that choosing an incorrect cuff size for any given individual (miscuffing) results in strikingly inaccurate BP measurements.28
Table 2.
| Source | Range of mean error in SBP (mm Hg) | Range of mean error in DBP (mm Hg) |
|---|---|---|
| Patient related | ||
| Acute meal ingestion | −6 | −5 to −2 |
| Acute caffeine use | +3 to +14 | +2 to +13 |
| Acute nicotine use | +3 to +25 | +3 to +18 |
| Bladder distension | +4 to +33 | +3 to +19 |
| White-coat effect | Up to +26 | Up to +21 |
| Procedure related | ||
| Insufficient rest | +4 to + 12 | +2 to +4 |
| Legs crossed at knees | +3 to + 15 | +1 to +11 |
| Arm lower than heart level | +4 to +23 | +3 to +12 |
| Talking during measurement | +4 to +19 | +5 to +14 |
| Fast deflation rate | −9 to −3 | +2 to +6 |
| Equipment related | ||
| Automated device variabilityb | −4 to +17 | −8 to +10 |
| Too small a cuff | +2 to +11 | +2 to +7 |
| Too large a cuff | −4 to −1 | −5 to −1 |
| Observer related | ||
| Terminal digit preference for zero (rounding off during auscultatory measurements) | Up to 79% overrepresentation of terminal zero | Up to 79% overrepresentation of terminal zero |
| Reliance on a single measurement | +3 to +10 | −2 to +1 |
| Hearing deficit | −2 to −0.1 | +1 to +4 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
To optimize automated device accuracy, use a validated device.
In 2020, the US Preventive Services Task Force published an Updated Evidence Report that noted that screening for HBP using office-based BP measurement had major accuracy limitations, including misdiagnosis, although direct harms of measurement were minimal.29 Hence, for some patients, 24-hour ambulatory BP monitoring (ABPM) remains an important method for the diagnosis of HBP. The 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA HBP Guideline assigned a class IIa recommendation (the benefit exceeds the risk, making the treatment reasonable) for the use of ABPM to detect masked hypertension among adults with an office BP close to an office SBP 120 to less than 130 mm Hg and not taking antihypertensive medication. The technology and techniques to obtain ABPM measurements have markedly improved over time.1 Optical photoplethysmographic technology has also been found to provide effective ABPM that may improve both access to and acceptance of more regular and high-quality monitoring than is offered by outpatient assessments measured during clinical encounters.30 However, ABPM is not widely available in primary care settings and can be expensive and time consuming.31 For now, obtaining accurate office and home BP measurements using externally validated oscillometric devices remain the most commonly recommended method in daily clinical practice settings.
2017 HBP Guideline Recommendation 2: Evaluating Cardiovascular Risk
On the basis of most recently available mortality data from 2020, heart disease and stroke currently claim more lives each year than cancer and chronic lower respiratory disease combined, accounting for a crude worldwide prevalence of atherosclerotic cardiovascular disease (ASCVD) of 608 million cases, an increase of 29% when compared with similar data from the previous decade ending in 2010.2 A number of well-documented individual ASCVD risk factors, including age, BP, hyperlipidemia, diabetes, and smoking tobacco are often diagnosed and treated in a piecemeal manner. Other factors, including noncardiac inflammatory conditions, environmental and social determinants/drivers of health (SDoH), as well as a number of genetic factors, add further considerable complexities to implementing successful clinical- and patient-level evidence-based management strategies shown to effectively reduce overall ASCVD risk.32, 33, 34, 35
2017 HBP Guideline Recommendation 3: the Importance of Classifying HBP
To correctly classify each patient with HBP in accordance with 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline recommendations and those from other important groups (eg, American Diabetes Association, Kidney Disease: Improving Global Outcomes, and AAP, as noted in Supplemental Appendix 1), it is, hence, first necessary to estimate ASCVD risk and obtain consistently accurate BP measurements. The ACC provides an easy to access online calculator that forecasts the potential impact of various interventions that can reduce ASCVD risk for those without previous cardiovascular disease.36 However, there is little evidence of its regular use in daily practice, although this tool has been already readily incorporated into many electronic health records systems. More recently, AHA has developed the online Predicting Risk of CVD EVENTs calculator, which demonstrates good discrimination and calibration in the overall population and among demographic and cardiovascular-kidney-metabolic subgroups (eg, obesity, diabetes, and chronic kidney disease).37,38 Table 3 outlines the 2017 HBP Guideline classification taxonomy, which provides further guidance regarding both pharmacologic and nonpharmacologic treatment decisions necessary to achieve optimal BP control. The use of BP-lowering medication and guideline-directed lifestyle modifications (GDLMs) consisting of weight loss, dietary sodium reduction and potassium supplementation, healthy dietary pattern, physical activity, and limited alcohol consumption are recommended for patients with stage 2 HBP regardless of ASCVD risk and for those with stage 1 HBP with high ASCVD risk.39 For patients with stage 1 HBP and low ASCVD risk as well as those with elevated BP (SBP between 120 and 129 mm Hg), GDLMs may also be highly effective in reducing HBP without medication. Regardless of the class of HBP, both clinicians and patients should prioritize their efforts to continue to promote GDLMs, which may lower BP by as much as 11 mm Hg and, hence, further lower overall ASCVD risk (also associated with other comorbidities) through successful adoption of these nonpharmacologic interventions (Table 4).1
Table 3.
| Stage 2 high BP | Stage 1 high BP | Elevated BP | |
|---|---|---|---|
| ASCVD risk ≥10% | COR/LOE 1A | COR LOE 1A | Not recommended |
| ASCVD risk <10% | COR LOE 1 C-LD | Not initially recommended | Not recommended |
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; COR, class of recommendation; LOE, level of evidence.
Nonpharmacologic lifestyle modifications recommended for all (COR LOE 1A). For older adults (65 years or older) with hypertension and a high burden of comorbidity and limited life expectancy, clinical judgment, patient preference, and a team-based approach to assess risk/benefit is reasonable for decisions regarding intensity of BP-lowering and choice of antihypertensive drugs (COR LOEIIa-CEO).
Table 4.
ACC/AHA Guideline-Directed Nonpharmacologic Interventions for Treatment and Prevention of Hypertensiona,1
| ACC/AHA COR/LOE | Nonpharmacologic intervention | Dose | Approximate impact on SBP |
|
|---|---|---|---|---|
| Hypertension (mm Hg) | Normotension (mm Hg) | |||
| 1-A: Physical activity | Aerobic |
|
−5 to 8 | −2 to 4 |
| Dynamic resistance |
|
−4 | −2 | |
| Isometric resistance |
|
−5 | −4 | |
| 1-A: Moderation in alcohol intake | Alcohol consumption | In individuals who drink alcohol, reduce alcoholb to:
|
−4 | −3 |
| 1-A: Weight loss | Weight/body fat | Best goal is ideal body weight, but aim for at least a 1-kg reduction in body weight for most adults who are overweight. Expect about 1 mm Hg for every 1-kg reduction in body weight | −5 | −2 to 3 |
| 1-A: Healthy diet | DASH dietary pattern | Consume a diet rich in fruits, vegetables, whole grains, and low-fat dairy products, with reduced content of saturated and total fat | −11 | −3 |
| 1-A: Reduced intake of dietary sodium | Dietary sodium | Optimal goal is <1500 mg/d, but aim for at least a 1000-mg/d reduction in most adults | −5 to 6 | −2 to 3 |
| 1-A: Enhanced intake of dietary potassium | Dietary potassium | Aim for 3500-5000 mg/d, preferably by consumption of a diet rich in potassium | −4 to 5 | −2 |
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; COR, class of recommendation; LOE, level of evidence; oz, ounces; SBP, systolic blood pressure.
In the United States, one “standard” drink contains roughly 14 g of pure alcohol, which is typically found in 12 oz of regular beer (usually about 5% alcohol), 5 oz of wine (usually about 12% alcohol), and 1.5 oz of distilled spirits (usually about 40% alcohol).
The Importance of Integrating HBP Control with Overall ASCVD Risk Reduction
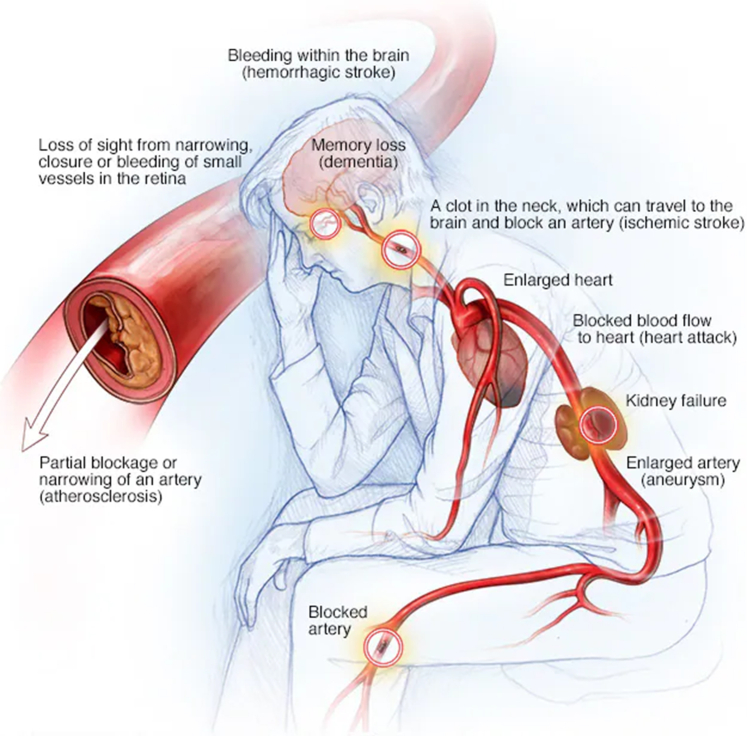
Current evidence indicates that comprehensive, overall ASCVD risk assessment and reduction should focus on the vascular system as the target organ, and not just isolate focus on the end organs for each individual ASCVD risk factor. Figure40 provides an excellent visualization of the importance of this unifying concept of the vascular system as the target organ for ASCVD risk reduction. In response to these challenges, the ACC has recently published an Expert Consensus Decision Pathway for Integrating Atherosclerotic Cardiovascular Disease and Multimorbidity Treatment: A Framework for Pragmatic, Patient-Centered Care:
Figure.
Focus on the vascular system as the overall target organ for blood pressure control and atherosclerotic cardiovascular disease risk reduction, not just the end organs. From Mayo Foundation for Medical Education and Research,40 with permission.
“Fractionated care plans, polypharmacy, side effects, drug–drug interactions, and financial toxicity can all begin to accumulate as patients acquire multimorbidity and advancing age. Clinicians caring for these patients need better guidance on how to mitigate ASCVD progression and major adverse cardiovascular events within the context of other chronic conditions, multiple guideline-directed medical therapies, changing prognoses, and patient preferences for care…. Accordingly, care for patients must shift from recommending all evidence-based options to an approach that prioritizes therapies with the greatest expected benefit/harm profile, while aligning with patients’ goals and preferences.”41
The development and implementation of such a comprehensive integrated approach has also been emphasized in the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease42 as well as 8 specific overarching health system interventions known as “The Blueprint for Change.”43 Thus, there is evolving and highly supportive evidence that such integrated approaches can not only have a highly substantial impact in lowering BP to normal levels but also control other ASCVD risk factors, not just with medications but also with the successful adoption of patient-centered GDLMs.44, 45, 46 Newly published data pooled and harmonized individual-level data from 112 cohort studies conducted in 34 countries and 8 geographic regions participating in the Global Cardiovascular Risk Consortium have confirmed the 5 modifiable risk factors associated with cardiovascular disease and death from any cause, with HBP considered to be the leading risk factor for cardiovascular disease responsible for up to 13.5% of all deaths annually worldwide.47 In addition, growing evidence supports the notion that comprehensive joint risk factor control is associated with lower risks of CVD and that a high degree of risk factor control (including HBP, body mass index, low density lipoprotein cholesterol, hemoglobin A1c, albuminuria, smoking, and physical activity) may considerably attenuate the excess risk of CVD among patients with HBP.48 Hence, adequate control of HBP must be effectively integrated with simultaneous efforts to also reduce other modifiable ASCVD risk factors, with the goal of promoting the overall health of the macrovascular and microvascular system as the target organ, rather than just solely focusing on individual risk factors and specific end organs (Figure).40
The Golden Triangle
In 2020 the National Heart, Lung, and Blood Institute (NHLBI) and the Division for Heart Disease and Stroke Prevention of the CDC convened a virtual workshop with multidisciplinary national experts who noted the vital need for an evidence-based guideline approach for the triad of (1) shared decision making through (2) evidence-based team care to (3) address social determinants of health as a necessary requirement in achieving effective HBP control.49 The detailed specifications for these 3 evidence-based strategies will now be referred to as the Golden Triangle, which have been codified in the 2019 AHA/ACC Clinical Performance and Quality Measures for Adults With High Blood Pressure and are included in Supplemental Appendix 2 (available online at http://www.mcpiqojournal.org).17 The multiple complexities of assuring accurate BP measurement in all settings; estimating ASCVD risk; identifying the correct BP stage; prescribing the right medication; providing personalized, tailored, and patient-centered education and motivation for the adoption of successful GDLMs, and integrating all of these actions in full synergy with other ASCVD and multimorbidity treatments for millions of people requires that the Golden Triangle be an essential component of a more effective multidisciplinary care delivery system that not only incorporates and supports but also enhances what has traditionally been accomplished in primary care settings.
Eliminating Health Care Disparities is Essential
The US Healthy People 2030 framework defines SDoH (also referred to as drivers) using the multifactorial domains of education, economic stability, social context, neighborhood environment, and health care access.50 The AHA has formally established its 2030 impact goal of equitably improving state-specific variations of cardiovascular-related healthy life expectancy, including eliminating race and ethnicity as well as urban vs rural residence–related differences.51 No surprise, uncontrolled HBP leading to the development of ASCVD remains a very large contributor to these disparities and inequities in life expectancy for Black Americans.52 Furthermore, the economic burden of health inequities and disparities at the US national and state levels for major racial and ethnic minority population groups (American Indian and Alaska Native, Latino/Hispanic, Black, Native Hawaiian, and Other Pacific Islander) is relevant.53 Recent data have found that specific SDoHs, including low education, low income, living in a health professional shortage area, disadvantaged neighborhood, and high-poverty zip code, have contributed to the excess likelihood of uncontrolled BP among Black compared with that in White adults.54,55 For patients traditionally underserved by the health system, strategies to improve BP control have been shown to be effective by integrating health care resources within these populations.56,57 Hence, the elimination of these differences, as continually emphasized by the AHA, is imperative to achieving effective control of HBP for most to a SBP level of less than 130 mm Hg at the population level worldwide.58
The Current Care Delivery System Must Change
Even when ASCVD risk can be appropriately evaluated and addressed in daily medical practice, “clinical inertia” within our health care system has long existed for both clinicians and patients to follow the most current clinical recommendations for implementing effective GDLMs and pharmacologic treatments. The daily burdens of time constraints and associated additional unreimbursed practice costs required to simply obtain accurate BP measurements and assure adherence to prescribed treatments in the absence of symptoms, both discourage and confuse patients and impede their clinicians from taking the best course of actions necessary to improve outcomes. Therefore, it is no longer tenable or acceptable for health system executives and governance to relegate accountability for the care of patients with HBP solely to primary care. Instead, they must provide the appropriate capital investments, guideline driven human resources, and clinical practice support for new care delivery infrastructures designed to ensure the effective control of this highly prevalent risk factor and achievement of BP-lowering GDLMs and medication adherence via the Golden Triangle platform of team-based care, shared decision making, and assessment of SDoH for every patient that they touch.
To achieve a more cohesive system of guideline-based care delivery improvements for millions of adults and children with inadequately controlled stage 1 and stage 2 HBP, leaders from federal and state governments, major integrated health systems, and payers must collaborate to provide unified, up-to-date criteria for adequate BP control; proper financial, managerial, clinical, and operational expertise; as well as visible, committed organizational leadership support and full accountability at the local, regional, and national levels. Stronger health system alliances and congruent and coherent alignment with insurers, employers, public health, community health safety net organizations, professional societies, quality measurement developers, large and small biopharmaceutical and medical device firms, digital health information technology companies, and governmental health agencies are vitally needed.
The Blueprint for Change: Moving a Big Needle
Achieving adequate control of HBP along with other ASCVD risk factors will also require these groups to jointly support a fully redesigned, integrated system of care. This “Blueprint for Change”59 must simultaneously ensure reliable, accurate BP measurement, ongoing reduction of ASCVD risk through promotion of GDLMs delivered by the Golden Triangle of identification of the most relevant social drivers/determinants of individual health, effective use of shared decision making from evidence-based, expert-driven care teams (summarized in Supplemental Appendix 3, available online at http://www.mcpiqojournal.org).43 No doubt, the current health care delivery infrastructure and economic resources that support care of major acute and chronic CVD are vastly different than what is needed to achieve health promotion, maintenance, and prevention necessary to reduce major adverse cardiac events, but it is, nonetheless, imperative to devote more effort to successful HBP control and other ASCVD risk reduction efforts.60 As an important starting point, in 2021 the NHLBI and the Division for Heart Disease and Stroke Prevention of the CDC convened a multidisciplinary group of national experts to inform the surgeon general’s call to action to control hypertension.49 Finally, the most currently available data from National Health and Nutrition Examination Survey indicate that the decline in CVD-related mortality among US adults with HBP appears to have stalled.61 Henceforth, moving the “Big Needle” of improved overall cardiovascular, renal, and brain health of the US population will require a major and coordinated reprioritization of both capital and human resource allocations by all of these stakeholders.
Potential Competing Interests
Dr Casey reports participation as follows: National Committee for Quality Assurance (NCQA) Cardiovascular Measure Advisory Panel (no financial remuneration); National Hypertension Control Initiative (NHCI) Advisory Board (no financial remuneration); and Advisory Board for Collaboration Oriented Approach for Controlling High Blood Pressure (COACH) Oregon Health and Science University Grant Number U18 HS026849. Dr Blood reports institutional grants from Eli Lilly, Boehringer Ingelheim, Milestone Therapeutics, and Novo Nordisk; consulting fees from Color Health, Arsenal Capital Partners, Milestone Therapeutics, Novo Nordisk, Signum Technologies, Knownwell Health, Porter Health, and Scriptchain; honoraria from Medscape ; travel support from American College of Cardiology and American Heart Association; U.S. Patent application No 16/636,524 entitled “Smartphone Application for Medical Image Data Sharing and Team Activation”; participation in data safety monitoring board of HI-Pro; equity options in Knownwell and Porter Health. Dr Persell receives research funding paid to Northwestern University from Omron Healthcare, National Institutes of Health, Agency for Healthcare Reseach and Quality, Health Resources and Services Administration, and Northwestern Memorial Healthcare; consulting fees from RAND; and honoraria from Pri-Med, Omron Healthcare, and National Committee for Quality Assurance. Dr Pohlman reports no competing interests. Dr Williamson reports grants from National Institutes of Health, Alzheimer’s Association, and Biogen; honorarium from University of New South Wales and Arbor Acres Retirement Community; and participation in the boards of Impact of Intensive Treatment of Systolic Blood Pressure on Brain Perfusion, Amyloid and Tau in Older Adults, National Institute on Aging; Pharmaceutical Assistance to the Aged and Disabled, National Institute on Aging; and Blood Pressure Control Target in Diabetes, National Research and Development Institute of China.
Footnotes
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA /ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. Published correction appears in J Am Coll Cardiol. 2018;71(19):2275-2279. [DOI] [PubMed] [Google Scholar]
- 2.Martin S.S., Aday A.W., Almarzooq Z.I., et al. 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation. 2024;149(8):e347–e913. doi: 10.1161/CIR.0000000000001209. Published correction appears in Circulation. 2024;149(19):e1164. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z., Du X., Hua C., et al. The effect of frailty on the efficacy and safety of intensive blood pressure control: a post hoc analysis of the SPRINT trial. Circulation. 2023;148(7):565–574. doi: 10.1161/CIRCULATIONAHA.123.064003. [DOI] [PubMed] [Google Scholar]
- 5.Derington C.G., Bress A.P., Berchie R.O., et al. Estimated population health benefits of intensive systolic blood pressure treatment among SPRINT-eligible US adults. Am J Hypertens. 2023;36(9):498–508. doi: 10.1093/ajh/hpad047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frimodt-Møller E.K., Vittinghoff E., Kaur G., Biering-Sørensen T., Soliman E.Z., Marcus G.M. Association between intensive vs standard blood pressure control and incident left ventricular conduction disease: a post hoc analysis of the SPRINT randomized clinical trial. JAMA Cardiol. 2023;8(6):612–616. doi: 10.1001/jamacardio.2023.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu C.Y., Saver J.L., Ovbiagele B., Wu Y., Cheng C.Y., Lee M. Association between magnitude of differential blood pressure reduction and secondary stroke prevention: a meta-analysis and meta-regression. JAMA Neurol. 2023;80(5):506–515. doi: 10.1001/jamaneurol.2023.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghazi L., Shen J., Ying J., et al. Identifying patients for intensive blood pressure treatment based on cognitive benefit: a secondary analysis of the SPRINT randomized clinical trial. JAMA Netw Open. 2023;6(5) doi: 10.1001/jamanetworkopen.2023.14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W., Zhang S., Deng Y., et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385(14):1268–1279. doi: 10.1056/NEJMoa2111437. [DOI] [PubMed] [Google Scholar]
- 10.He J., Ouyang N., Guo X., et al. Effectiveness of a non-physician community health-care provider-led intensive blood pressure intervention versus usual care on cardiovascular disease (CRHCP): an open-label, blinded-endpoint, cluster-randomised trial. Lancet. 2023;401(10380):928–938. doi: 10.1016/S0140-6736(22)02603-4. [DOI] [PubMed] [Google Scholar]
- 11.Controlling high blood pressure eCQI Resource Center (healthit.gov) https://ecqi.healthit.gov/ecqm/ec/2023/cms165v11
- 12.Controlling high blood pressure—NCQA. https://www.ncqa.org/hedis/measures/controlling-high-blood-pressure/
- 13.Hypertension control challenge Million Hearts® (hhs.gov) https://millionhearts.hhs.gov/partners-progress/champions/challenge.html
- 14.Qaseem A., Wilt T.J., Rich R., et al. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430–437. doi: 10.7326/M16-1785. Published correction appears in Ann Intern Med. 2018;168(7):530-532. [DOI] [PubMed] [Google Scholar]
- 15.News Staff, News A.A.F.P. AAFP issues new clinical practice guideline on hypertension. Ann Fam Med. 2023;21(2):190–191. doi: 10.1370/afm.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whelton P.K., Flack J.M., Jennings G., Schutte A., Wang J., Touyz R.M. Editors’ commentary on the 2023 ESH management of arterial hypertension guidelines. Hypertension. 2023;80(9):1795–1799. doi: 10.1161/HYPERTENSIONAHA.123.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey D.E., Jr., Thomas R.J., Bhalla V., et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circ Cardiovasc Qual Outcomes. 2019;12(11) doi: 10.1161/HCQ.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamberlain A.M., Cooper-DeHoff R.M., Fontil V., et al. Disruption in blood pressure control with the COVID-19 pandemic: the PCORnet blood pressure control laboratory. Mayo Clin Proc. 2023;98(5):662–675. doi: 10.1016/j.mayocp.2022.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley M., Hernandez A.K., Kuznia A.L. High blood pressure in children and adolescents. Am Fam Physician. 2018;98(8):486–494. [PubMed] [Google Scholar]
- 20.Padwal R., Campbell N.R.C., Schutte A.E., et al. Optimizing observer performance of clinic blood pressure measurement: a position statement from the Lancet Commission on Hypertension Group. J Hypertens. 2019;37(9):1737–1745. doi: 10.1097/HJH.0000000000002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimbo D., Artinian N.T., Basile J.N., et al. Self-measured blood pressure monitoring at home: a joint policy statement from the American Heart Association and American Medical Association. Circulation. 2020;142(4):e42–e63. doi: 10.1161/CIR.0000000000000803. Published correction appears in Circulation. 2020;142(4):e64. [DOI] [PubMed] [Google Scholar]
- 22.Ordunez P., Lombardi C., Picone D.S., et al. HEARTS in the Americas: a global example of using clinically validated automated blood pressure devices in cardiovascular disease prevention and management in primary health care settings. J Hum Hypertens. 2023;37(2):126–129. doi: 10.1038/s41371-022-00659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancia G., Kreutz R., Brunström M., et al. 2023 ESH guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the the International Society of Hypertension (ISH) and the European Renal Association (ERA) J Hypertens. 2023;41(12):1874–2071. doi: 10.1097/HJH.0000000000003480. Published correction appears in J Hypertens. 2024;42(1):194. [DOI] [PubMed] [Google Scholar]
- 24.US blood pressure validated device listing. https://www.validatebp.org/
- 25.Picone D.S., Bui T., Chapman N., et al. Prevalence of validated blood pressure measuring devices being sold by Amazon: 12-month prospective analysis across 10 countries. J Hypertens. 2023;41(suppl 1):e137–e138. doi: 10.1097/01.hjh.0000914116.21861.af. [DOI] [Google Scholar]
- 26.Muntner P., Shimbo D., Carey R.M., et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73(5):e35–e66. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SMBP infographic—Target:BP. American Medical Association. targetbp.org
- 28.Ishigami J., Charleston J., Miller E.R., Matsushita K., Appel L.J., Brady T.M. Effects of cuff size on the accuracy of blood pressure readings: the Cuff(SZ) randomized crossover trial. JAMA Intern Med. 2023;183(10):1061–1068. doi: 10.1001/jamainternmed.2023.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guirguis-Blake J.M., Evans C.V., Webber E.M., Coppola E.L., Perdue L.A., Weyrich M.S. Screening for hypertension in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(16):1657–1669. doi: 10.1001/jama.2020.21669. [DOI] [PubMed] [Google Scholar]
- 30.Vybornova A., Polychronopoulou E., Wurzner-Ghajarzadeh A., Fallet S., Sola J., Wuerzner G. Blood pressure from the optical Aktiia Bracelet: a 1-month validation study using an extended ISO81060-2 protocol adapted for a cuffless wrist device. Blood Press Monit. 2021;26(4):305–311. doi: 10.1097/MBP.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stergiou G.S., Palatini P., Parati G., et al. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39(7):1293–1302. doi: 10.1097/HJH.0000000000002843. [DOI] [PubMed] [Google Scholar]
- 32.Wong N.D., Budoff M.J., Ferdinand K., et al. Atherosclerotic cardiovascular disease risk assessment: an American Society for Preventive Cardiology clinical practice statement. Am J Prev Cardiol. 2022;10 doi: 10.1016/j.ajpc.2022.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casey D.E. In: Transforming Healthcare: an Insider’s Look on Why and How. 1st ed. Menacker M., editor. Wiley; 2022. Forward. [Google Scholar]
- 34.Casey D.E., Kopolow A., Solid C. Major economic losses associated with inadequate control of high blood pressure: time for a major change. Popul Health Manag. 2022;25(3):291–293. doi: 10.1089/pop.2022.0002. [DOI] [PubMed] [Google Scholar]
- 35.Alpert J.S. New coronary heart disease risk factors. Am J Med. 2023;136(4):331–332. doi: 10.1016/j.amjmed.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 36.ASCVD risk estimator + (acc.org) https://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/
- 37.PREVENT online calculator—professional heart daily American Heart Association. https://professional.heart.org/en/guidelines-and-statements/prevent-calculator
- 38.Khan S.S., Matsushita K., Sang Y., et al. Development and validation of the American Heart Association’s PREVENT equations. Circulation. 2024;149(6):430–449. doi: 10.1161/CIRCULATIONAHA.123.067626. Published correction appears in Circulation. 2024;149(11):e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carey R.M., Moran A.E., Whelton P.K. Treatment of hypertension: a review. JAMA. 2022;328(18):1849–1861. doi: 10.1001/jama.2022.19590. [DOI] [PubMed] [Google Scholar]
- 40.Mayo Clinic High blood pressure dangers: hypertension’s effects on your body. https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/in-depth/high-blood-pressure/art-20045868
- 41.Writing Committee, Birtcher K.K., Allen L.A., et al. 2022 ACC Expert consensus decision pathway for integrating atherosclerotic cardiovascular disease and multimorbidity treatment: a framework for pragmatic, patient-centered care: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(3):292–317. doi: 10.1016/j.jacc.2022.08.754. [DOI] [PubMed] [Google Scholar]
- 42.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. Published correction appears in Circulation. 2020;141(16):e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casey D.E., Jr., Daniel D.M., Bhatt J., et al. Controlling high blood pressure: an evidence-based blueprint for change. Am J Med Qual. 2022;37(1):22–31. doi: 10.1097/01.JMQ.0000749856.90491.43. [DOI] [PubMed] [Google Scholar]
- 44.Blood A.J., Cannon C.P., Gordon W.J., et al. Results of a remotely delivered hypertension and lipid program in more than 10 000 patients across a diverse health care network. JAMA Cardiol. 2023;8(1):12–21. doi: 10.1001/jamacardio.2022.4018. Published correction appears in JAMA Cardiol. 2023;8(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brettler J.W., Arcila G.P.G., Aumala T., et al. Drivers and scorecards to improve hypertension control in primary care practice: recommendations from the HEARTS in the Americas Innovation Group. Lancet Reg Health Am. 2022;9 doi: 10.1016/j.lana.2022.100223. None. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu E.Y.T., Wan E.Y.F., Mak I.L., et al. Assessment of hypertension complications and health service use 5 years after implementation of a multicomponent intervention. JAMA Netw Open. 2023;6(5) doi: 10.1001/jamanetworkopen.2023.15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Global Cardiovascular Risk Consortium. Magnussen C., Ojeda F.M., et al. Global effect of modifiable risk factors on cardiovascular disease and mortality. N Engl J Med. 2023;389(14):1273–1285. doi: 10.1056/NEJMoa2206916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kou M., Wang X., Ma H., Li X., Heianza Y., Qi L. Degree of risk factor control and incident cardiovascular diseases in patients with hypertension. Mayo Clin Proc. 2024;99(3):387–399. doi: 10.1016/j.mayocp.2023.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Commodore-Mensah Y., Loustalot F., Himmelfarb C.D., et al. Proceedings from a National Heart, Lung, and Blood Institute and the Centers for Disease Control and prevention workshop to control hypertension. Am J Hypertens. 2022;35(3):232–243. doi: 10.1093/ajh/hpab182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Social determinants of health—healthy people 2030. health.gov
- 51.Angell S.Y., McConnell M.V., Anderson C.A.M., et al. The American Heart Association 2030 impact goal: a presidential advisory from the American Heart Association. Circulation. 2020;141(9):e120–e138. doi: 10.1161/CIR.0000000000000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caraballo C., Massey D.S., Ndumele C.D., et al. Excess mortality and years of potential life lost among the black population in the US, 1999-2020. JAMA. 2023;329(19):1662–1670. doi: 10.1001/jama.2023.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaVeist T.A., Pérez-Stable E.J., Richard P., et al. The economic burden of racial, ethnic, and educational health inequities in the US. JAMA. 2023;329(19):1682–1692. doi: 10.1001/jama.2023.5965. [DOI] [PubMed] [Google Scholar]
- 54.More than half of U.S. adults don’t know heart disease is leading cause of death, despite 100-year reign. https://newsroom.heart.org/news/more-than-half-of-u-s-adults-dont-know-heart-disease-is-leading-cause-of-death-despite-100-year-reign?utm_campaign=sciencenews23-24&utm_source=science-news&utm_medium=phd-link&utm_content=phd-01-24-24
- 55.Magnani J.W. Hypertension-a social disease in need of social solutions. Hypertension. 2023;80(7):1414–1416. doi: 10.1161/HYPERTENSIONAHA.123.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh H., Fulton J., Mirzazada S., Saragosa M., Uleryk E.M., Nelson M.L.A. Community-based culturally tailored education programs for black communities with cardiovascular disease, diabetes, hypertension, and stroke: systematic review findings. J Racial Ethn Health Disparities. 2023;10(6):2986–3006. doi: 10.1007/s40615-022-01474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Victor R.G., Lynch K., Li N., et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378(14):1291–1301. doi: 10.1056/NEJMoa1717250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colvin C.L., Kalejaiye A., Ogedegbe G., Commodore-Mensah Y. Advancing equity in blood pressure control: a response to the surgeon general’s call-to-action. Am J Hypertens. 2022;35(3):217–224. doi: 10.1093/ajh/hpab187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casey D. The ACMQ 2019 quality institute: blueprint for change. Am J Med Qual. 2019;34(4):418–419. doi: 10.1177/1062860619865077. [DOI] [PubMed] [Google Scholar]
- 60.Casey D.E., Oglesby B., Pohlman D. The rising prevalence of multiple chronic conditions among US adults with hypertension. Am J Hypertens. 2024;37(7):449–451. doi: 10.1093/ajh/hpae045. [DOI] [PubMed] [Google Scholar]
- 61.Choi E., Shimbo D., Chen L., et al. Trends in all-cause, cardiovascular, and noncardiovascular mortality among US adults with hypertension. Hypertension. 2024;81(5):1055–1064. doi: 10.1161/HYPERTENSIONAHA.123.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.