Abstract
Tau protein aggregation is a defining characteristic of Alzheimer's disease (AD), leading to the formation of neurofibrillary tangles that disrupt neural communication and ultimately result in cognitive decline. Nanotechnology presents novel strategies for both diagnosing and treating Alzheimer's disease. Nanotechnology. It has become a revolutionary tool in the fight against Alzheimer's disease, particularly in addressing the pathological accumulation of tau protein. This review explores the relationship between tau-related neurophysiology and the utilization of nanotechnology for AD treatment, focusing on the application of nanomaterials to regulate tau phosphorylation, hinder tau aggregation and propagation, stabilize microtubules, eliminate pathological tau and emphasize the potential of nanotechnology in developing personalized therapies and monitoring treatment responses in AD patients. This review combines tau-related neurophysiology with nanotechnology to provide new insights for further understanding and treating Alzheimer's disease.
Keywords: Nanotechnology, Tau protein, Alzheimer's disease, Tau phosphorylation
Graphical abstract
1. Introduction
Alzheimer's disease (AD), a progressive neurodegenerative disease, is caused by the accumulation of aberrant protein aggregates in the brain. Notably, the tau protein occupies a prominent position among these aggregates, assuming the role of a pivotal participant within the intricate tapestry of the disease's pathophysiology [1]. Besides AD, tau aggregates have been detected in more than 20 distinct neurodegenerative conditions collectively called “tauopathies” [2]. With AD as the outlier, the majority of these disorders manifest without amyloid accumulation, and a significant proportion are linked to tau mutations [3]. These observations strongly imply that the impairment of tau function and/or the aggregation of tangles contribute to the pathogenesis of these diseases as this inquiry delves into the multifaceted functions attributed to tau in the context of Alzheimer's, a more lucid representation materializes, elucidating its integral contributions to the continuum of disease advancement. Therefore, Tau is receiving increasing attention as a potential alternative therapeutic target [4].The FDA has not approved any drugs targeting Tau, and some drugs are undergoing clinical trials [5]. Their objectives include inhibiting the hyperphosphorylation of Tau [6], promoting the degradation of Tau, preventing the aggregation of Tau, and improving cerebral blood circulation. Researchers are also exploring and attempting immunotherapy to address the issues related to Tau protein [7]. Pharmaceutical interventions for Alzheimer's and other neurodegenerative disorders face several challenges. These include the necessity for high drug doses, which can lead to safety concerns, the drugs' difficulty in crossing the blood-brain barrier to reach the intended target, and the frequent interruption of treatment due to these limitations [8]. The application of nanotechnology offers significant potential to mitigate these issues. Nanoparticles play a crucial role in reducing drug toxicity through their capacity to enable the controlled release of therapeutic agents at specific target sites [9]. Moreover, their capacity to traverse the blood-brain barrier allows for the diagnostic and therapeutic application of nanoparticles in neurological disorders. The precise and efficient delivery of medications to specific targets at the appropriate time, achieved through nanotechnology-based drug delivery systems, continues to represent a significant scientific and therapeutic benchmark.
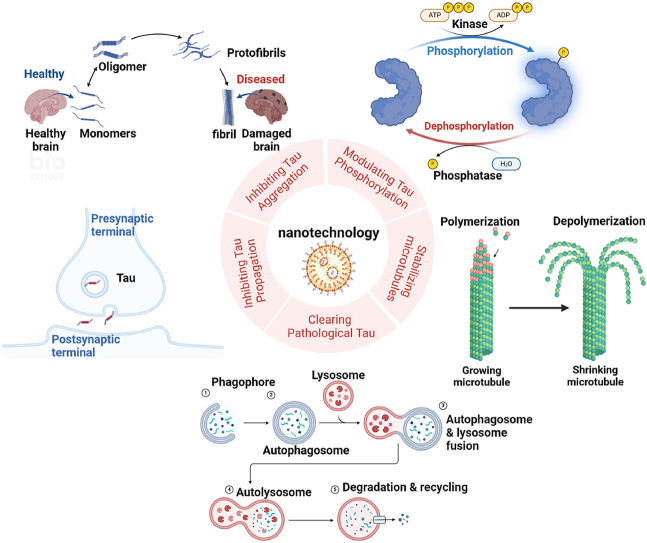
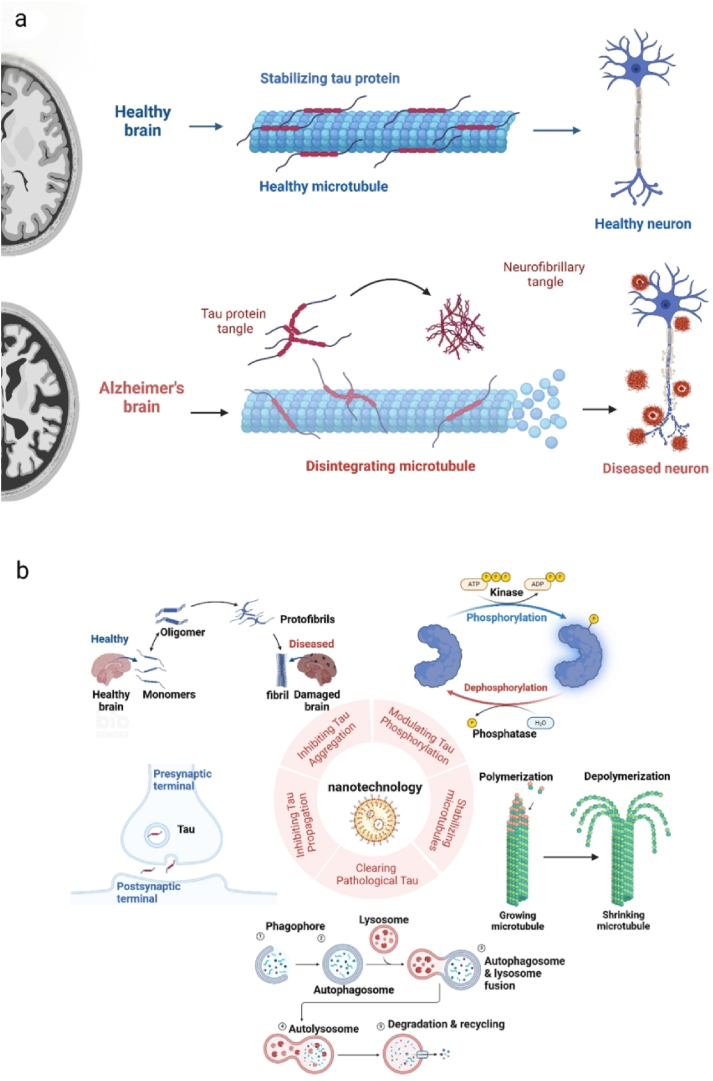
Nanotechnology is a rapidly growing field that involves manipulating and engineering nanoscale materials and devices, ranging between 1 and 100 nm. This technology can potentially revolutionize various sectors, such as energy, electronics, materials science, and medicine. One of the most promising applications of nanotechnology is in medicine. Nano-sized particles and devices can be engineered to specifically target diseased cells, delivering drugs or other therapeutic agents directly to the disease site [10]. These nanoparticles (NPs) can also be used for imaging, diagnosis, and monitoring of diseases, enabling early treatment [11]. Thus, it provides a new arsenal of tools to combat tau protein aggregation [12]. By using nanoparticle-based drug delivery systems, medications can be precisely targeted to affected areas, reducing side effects and maximizing therapeutic effectiveness. This targeted approach holds immense promise in halting disease progression and preserving cognitive function [13]. This review explores the potential of utilizing various NPs in detecting and treating AD through different methods. By introducing the application of NPs, we hope to shed light on the disease process and open up new research avenues for the future as shown in Fig. 1.
Fig. 1.
Tau pathology and nanotechnology applications (a) Pathology of Alzheimer's disease in a healthy brain, tau proteins stabilize the microtubules, which are responsible for keeping the structure and function of nerve cells. In AD, tau proteins become abnormal and begin to accumulate in the brain. This accumulation results in the formation of NFTs. (b) The application of nanotechnology in treating tau pathology in Alzheimer's disease includes regulating tau phosphorylation, inhibiting tau aggregation and diffusion, stabilizing microtubules, and promoting tau clearance. Created with BioRender.com.
2. The tau protein
2.1. Structure and function
The tau protein, first discovered in the 1970s, is a highly abundant neuronal protein that predominantly localizes to axons [14]. It has a crucial role in maintaining the structural integrity of neurons and regulating microtubule dynamics, which are essential for intracellular transport, neuronal development, and synaptic function.
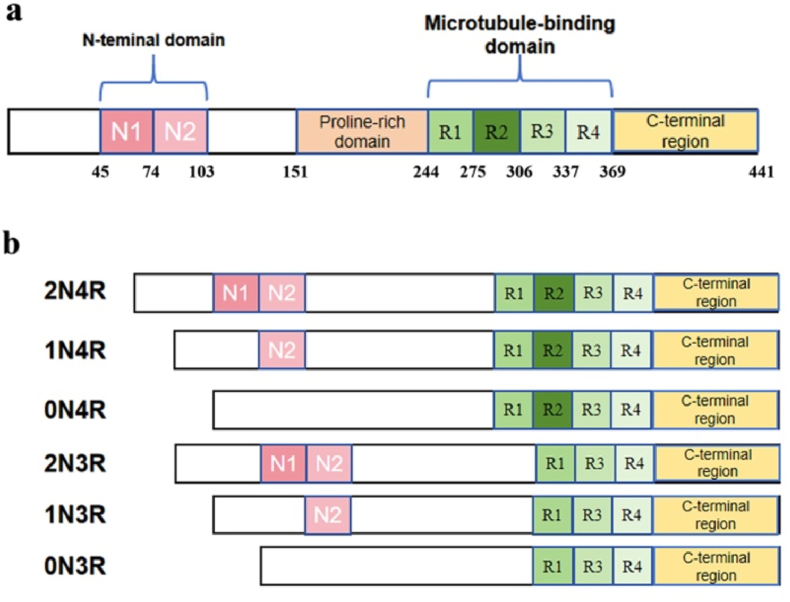
The MAPT encodes the tau protein, and through alternative splicing, a process that involves 8 out of the total 16 exons, a spectrum of 6 isoforms emerges in the central nervous system (CNS), complemented by an additional 6 isoforms in the peripheral nervous system (PNS) [15]. These isoforms exhibit varying molecular weights, ranging from 58 kDa to 66 kDa, with a singular isoform of 110 kDa also present [16,17]. Comprising four distinct primary domains as shown in Fig. 2, the tau protein's destiny is sculpted by alternative splicing, particularly within the N-terminal projection region and the microtubule-binding domain (MBD). This process yields two prominent isoforms: the 3-repeat (3 R) and the 4-repeat (4 R) tau. In the symphony of the adult human brain, the two isoforms harmonize in a balanced 1:1 ratio. Notably, the 3 R tau assumes prominence during development. In contrast, the 4 R tau takes the lead during adulthood [18]. Distinguished by their capabilities, the 4 R tau shines with heightened proficiency in shepherding microtubule assembly, a fact that surpasses the abilities of 3 R tau [19,20]. Perturbations in the equilibrium between the 3 R and 4 R tau isoforms have been linked to AD and other tauopathies, subjects extensively explored in comprehensive reviews [21].
Fig. 2.
The structure and isoforms of tau. (a) Domains of 2N4R tau. The structure of Tau protein consists of an N-terminal domain, alkaline proline-rich region, microtubule binding region, and C-terminal domain. (b) The isoforms of tau. The Tau protein can be cleaved to form six mRNA combinations and ultimately translated into six protein isomers.
Tau stabilizes microtubules by binding and promoting microtubule assembly [22]. This interaction ensures the proper organization of microtubules and facilitates axonal transport. In addition to its role in microtubule stabilization, tau participates in synaptic plasticity and signal transduction [23,24].
2.2. Post-translational modifications
Tau undergoes post-translational modifications, including phosphorylation [25], acetylation, glycation, and truncation. Phosphorylation is the most extensively studied modification and is critical in tau's function and dysfunction [26]. Tau phosphorylation holds significant implications in AD and has undergone extensive investigation [27,28]. This is primarily because neurofibrillary tangles (NFTs) extracted from AD-afflicted brains are rich in heavily phosphorylated tau variants. The biochemical analysis of tau phosphorylation, particularly at sites linked to the disease, indicates that phosphorylation decreases tau's ability to combine microtubules [29,30]. Furthermore, it prompts tau to self-assemble and become tangles and filaments, causing the charge and structure of the MBD to change [31]. Mass spectrometry assessment of tau has identified many pathological phosphorylation sites have been identified [32]. Up to now, roughly 45 of the 85 potential phosphorylation sites within tau have been identified. Many of those exhibit varying phosphorylation in brains affected by frontotemporal dementia (FTD), or AD but not in healthy controls [33,34].
2.3. Pathological aggregation and propagation
Tauopathies are characterized by the accumulation of abnormal tau aggregates in the brain. AD is the most prevalent tauopathy; tau aggregates and amyloid-beta plaques are hallmarks of AD pathology. FTD is another major tauopathy where tau aggregation leads to neuronal dysfunction and cell loss in the frontal and temporal lobes [35,36]. It is still unclear whether the detrimental components involve insoluble aggregates, pre-fibril soluble tau oligomers, fragments derived from pre-existing aggregates, or the transformation of soluble tau into aggregates. The prolonged period of cognitive deterioration in Alzheimer's disease emphasizes a slow, cumulative toxic mechanism. Similarly, the formation of initial aggregates exhibits gradual progression, with subsequent fragmentation significantly expediting the creation of additional aggregates [37].
Tau can spread throughout the brain in a prion-like manner, including cellular absorption, template seeding, secretion, and cell movement through synaptic and non-synaptic routes [38,39], propagating pathological changes from cell to cell [40].The tau's propagation can be segmented into 3 stages: (1) An abnormal tau variant is released into the extracellular space by the donor cell; (2) The aberrant tau out of a cell is taken up by the next cells; (3) The pathological tau assimilated by recipient cells to become new intracellular aggregates [41]. Many of researches both in vitro and in vivo have elucidated neurons how release and internalize tau, providing insights into the dissemination of tau-related disorder in Alzheimer's disease.
The tau protein's vital key in keeping neuronal function and structure is well-established. However, its involvement in neurodegenerative diseases highlights the need for a deeper understanding of its complex biology and the development of effective therapeutic interventions. In conclusion, the tau protein's multifaceted functions and implications in neurodegenerative disorders make it a subject of intense research. Advances in our understanding of tau biology could potentially lead to breakthroughs in treating tauopathies and making individuals affected by these debilitating conditions have a better quality of life.
3. Tau immunization
Tau protein misfolding and aggregation are hallmark features of AD progression. Tau immunization aims to stimulate the immune system to recognize and target these pathological tau aggregates. By generating antibodies against tau, this approach seeks to prevent or clear tau aggregates from the brain, potentially halting or slowing down disease progression [42,43]. Preclinical investigations utilizing diverse animal models have substantiated the feasibility and efficacy of tau immunization [44]. These studies have collectively demonstrated the amelioration of tau pathology, concomitant with improvements in cognitive function and the preservation of neuronal integrity post-immunization. Encouragingly, select experimental vaccines have transitioned into nascent-phase clinical trials, engendering insight into their safety profile and potential therapeutic impact on human subjects [45].
3.1. Active immunization
Active immunization involves providing the body with target proteins and adjuvants to stimulate the body's immune response. Active immunization prompts the body to generate antibodies spontaneously, usually leading to a long-lasting effect. Here, two reported vaccines are introduced. The first one, AADvac1, has its antigenic epitope selected from monoclonal antibody DC8E8 in vitro experiments [46]. It primarily targets the mutual lease of Tau proteins in the human body. Immunizing Tau transgenic rat AD models with this vaccine reduces levels of both early and late pathological forms of Tau [47]. Furthermore, significant improvements are observed in behavioral tests. Following successful outcomes in animal models, Austrian researchers conducted a 12-week Phase I clinical trial, which evaluated the vaccine's safety in humans for the first time. Clinical trial results showed that AADvac1 exhibits good safety and immunogenicity. However, this vaccine's Phase II clinical trial results are still pending. The other vaccine, ACI-35, is lipid-based and contains 16 synthetic copies of Tau fragments phosphorylated at protein pathology residues S396 and S404 [48]. Preclinical trials conducted on Tau-P301L mice receiving long-term vaccine administration did not exhibit adverse symptoms such as inflammation, and their lifespan was increased post-vaccination [49]. Currently, safety trials for this vaccine in humans are ongoing.
3.2. Passive immunization
Passive immunity is safer than active immunity because it doesn't require the patient to generate antibodies themselves, shortening the immune response time and reducing the risk of adverse immune reactions. Passive immunity also provides higher specificity for target epitopes. Adjusting treatment plans based on disease stage becomes feasible due to the changing distribution of antigenic determinants with disease progression. In Tau immunotherapy, anti-Tau antibodies penetrate the brain, bind with intraneuronal lysosomal markers, and hinder pathological Tau propagation. However, intracellularly targeting Tau might be more effective against pathological proteins, as most are inside cells.
BMS-986168 is a humanized IgG4 monoclonal antibody that targets N-terminal fragments of Tau (eTau) that come from pluripotent stem cells of familial Alzheimer's disease patients [50]. When added to neuronal cultures, these fragments lead to hyperactivity and increased amyloid beta (Aβ) production. BMS-986168 significantly reduces Tau and Aβ levels in animal tissue interstitial fluid and exhibits safety and tolerability in human trials. Ongoing clinical trials involve other relevant patients [51]. ABBV-8E12, a humanized lgG4 monoclonal antibody, targets amino acids 25–30 of extracellular Tau, inhibiting exogenous Tau aggregation into NFTs. In animal tests, brain-injected ABBV-8E12 reduces Tau aggregation and hyperphosphorylation, enhancing cognitive levels in mice. Results of ongoing human trials are pending [52,53]. Similar antibodies include RG7345, a humanized monoclonal antibody targeting Tau phosphorylation site S422 prominent in neuronal dendrites [54]. Tau phosphorylation at this site is pathological. RO7105705, an lgG4 anti-Tau antibody, primarily targets extracellular Tau, potentially curbing microglial activation and alleviating AD-related inflammation [55]. BIIB076 binds monomeric and preformed Tau fibrils and human brain Tau. UCB0107, a monoclonal antibody under testing in PSP patients, targets amino acids 235–246 of tau [56].
Tau immunity, as a promising scientific approach, has emerged in ongoing explorations, and nanotechnology, as an emerging technology, has provided new insights and methods for regulating tau immunity. Utilizing nanotechnology for vaccine delivery, nanocarriers can enhance the stability and specificity of vaccines, effectively delivering tau protein vaccines to the immune system, activating the body's immune response, and promoting the recognition and clearance of tau protein. Nanotechnology can also modulate the immune system response, enhancing immune cell recognition and clearance capabilities for tau protein. By designing appropriate nanoparticles or nanomaterials, immune cell activity and function can be regulated, improving the efficacy of immune therapy. Nanotechnology shows high feasibility in regulating Tau immunity, offering innovative solutions to address the therapeutic challenges of Tau protein-related diseases, and unlocking the mysteries of AD. Despite facing various challenges, progress in tauopathies, designing immune frameworks, and transitioning to clinical evaluation indicates this is a continuously evolving frontier field. Future research should further explore the application of nanotechnology in personalized therapy, improving brain penetration, clinical trial validation, and multidisciplinary collaboration, advancing the widespread application of nanotechnology in regulating Tau immunity.
4. The application of nanotechnology
4.1. Nanotechnology in modulating tau phosphorylation
Although the tau protein is subject to an array of post-translational modifications, including glycosylation and non-enzymatic glycation, phosphorylation emerges as the predominant modification. Tau is characterized as a protein that is extensively phosphorylated, and the extent of its phosphorylation is essential for regulating its biological functions. Research indicates that roughly 30 distinct sites within the tau protein undergo phosphorylation under normal circumstances. A substantial proportion of these phosphorylation sites are situated on serine and threonine residues close to proline, with 17 such sites incorporated into the longest tau isoform detected in the human brain [57]. Tau phosphorylation is crucial for regulating its normal functions, such as maintaining microtubule stability, as well as its participation in pathological processes that lead to the formation of neuronal filaments associated with neurodegenerative diseases. Several therapeutic strategies have been proposed to target the hyperphosphorylation of tau protein in AD, including tau kinase inhibitors, tau phosphatase activators, and gene therapy utilizing small interfering RNA (siRNA) technology. These strategies aim to modulate the phosphorylation state of tau, thereby preventing the formation of neurofibrillary tangles and improving cognitive function.
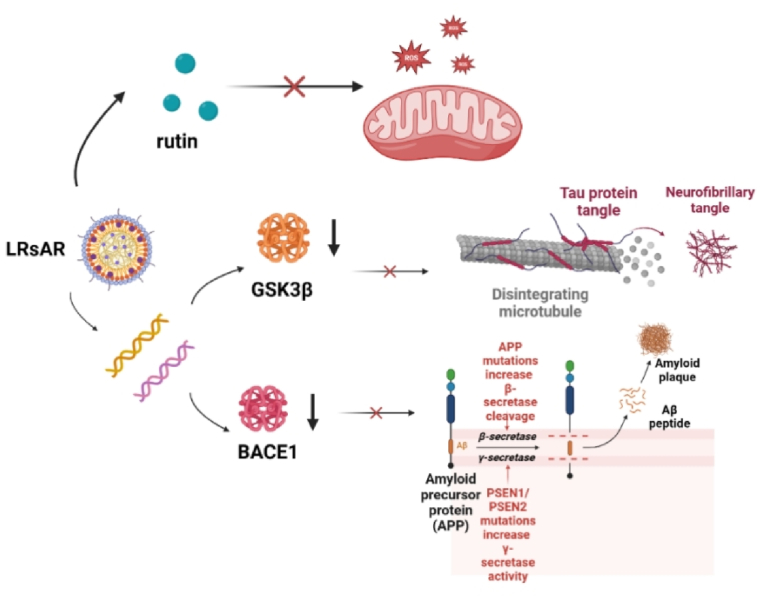
Gene therapy using siRNA can specifically silence target genes, thereby inhibiting the production of phosphorylated Tau (p-Tau). However, due to enzymatic degradation and immune clearance, nucleic acid drugs are often characterized by poor bioavailability. Nanocarriers have emerged as a solution to overcome this challenge by creating a protective environment for nucleic acid drugs, safeguarding them from deactivation during circulation in the body. Therefore, nucleic acid drug delivery systems based on nanocarriers have made significant advancements in recent years [58]. For example, gene drugs LRsGAR were developed by encapsulating siGSK-3β and bovine serum albumin within two-dimensional magnesium/aluminum nanoparticles modified with targeting peptides. The nanomedicine utilizes the widely used nanotargeted delivery platform, layered double hydroxide nanoparticles (LDH NP), which can efficiently load various proteins and peptides [59]. The LDH NP surface is modified with low-density lipoprotein receptor 1 (LRP1) ligand angiotensin II (Ang2) and nicotinic acetylcholine receptor (nAChR) ligand rabies virus glycoprotein 29 (RVG29) [60].The Ang2 on the surface of the nanoparticles can interact with LRP1 to enable the nanomedicine to cross the blood-brain barrier, and LRP1 further targets neurons through nAChR mediated by RVG29. Administration of LRsGAR via intravenous injection notably decreased GSK3β mRNA levels in the hippocampus and cortex of P301S AD mice, resulting in a reduction in p-Tau accumulation in the brain, as illustrated in Fig. 3 [61].
Fig. 3.
Therapeutic mechanism of LRsAR nanomedicine. In neuronal cells, the endosome escape of siBACE1 or siGSK3β in LRsAR nanomedicine can downregulate the expression of BACE1 or GSK3β, reduce the production of Aβ or the hyperphosphorylation of Tau, while releasing rutin to rescue mitochondrial function and decrease ROS. Created with BioRender.com.
During the phosphorylation process of Tau, various kinases, such as glycogen synthase kinase 3β (GSK3β), cyclin-dependent kinase 5 (cdk5), and c-Jun N-terminal kinase (JNK), all play roles, with each kinase associated with specific phosphorylation sites [62]. Among these kinases, GSK3β is the key player in phosphorylation and serves as a primary target for inhibiting Tau phosphorylation. Several inhibitors targeting GSK3β have been developed and utilized in brain-targeted delivery systems. For example, Ji et al. designed chondroitin sulfate selenium (CS@Se) to regulate GSK3β activation for Tau dephosphorylation by regulating the activation of GSK3β. CS@Se can weaken the hyperphosphorylation of tau by adjusting GSK3β′s expression, inhibiting the Ser396 and Ser404 sites on the tau protein, and reducing the p-tau levels in AD mice by 50 %. Furthermore, CS@Se activates the extracellular signal-regulated kinase 1/2 (ERK 1/2) and p38 mitogen-activated protein kinase (p38 MAPK) pathways, suppresses nuclear factor-κB (NF-κB) translocation, and regulates the expression of pro-inflammatory cytokines [11].
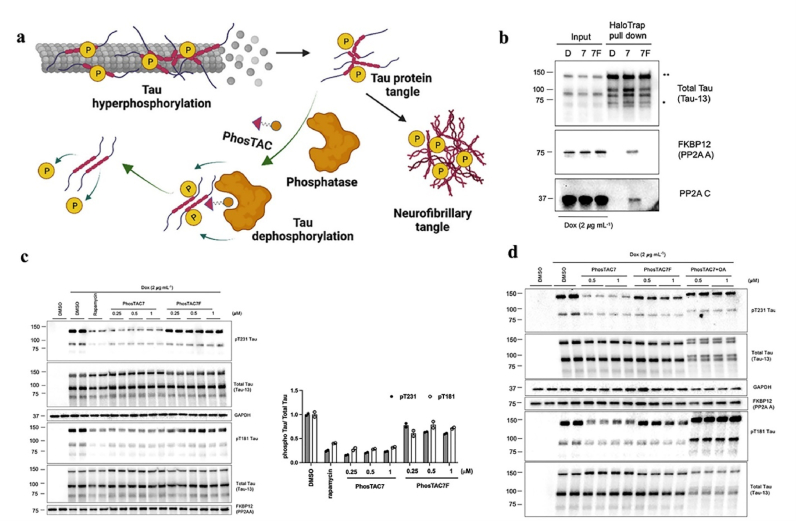
Compared to kinases, the number of phosphatases involved in tau dephosphorylation is relatively limited. Protein phosphatase 2 A (PP2A) is the primary tau phosphatase class responsible for 70 % of tau phosphatase activity, making it a key target for dysregulation intervention [63]. Sodium selenate (VEL015) has effectively reduced tau phosphorylation levels in both laboratory and in vivo settings. As a targeted activator for PP2A, it can reinforce the PP2A-tau protein complexes [64]. In another study, the author has developed a method to remove excessive phosphorylation of endogenous tau protein. This method is called PhosTAC (Phosphorylation-Targeting Chimera) technology. Through this method, a small molecule PhosTAC was designed to facilitate the development of a stable ternary complex between endogenous tau and the dephosphorylating enzyme PP2A, leading to rapid, efficient, and sustained dephosphorylation of tau, as depicted in Fig. 4. The authors utilized small-molecule PhosTACs to recruit tau to PP2A, a natural tau phosphatase, resulting in the formation of stable ternary complexes and subsequent effective dephosphorylation of tau. This process enhanced tau protein degradation, as confirmed by mass spectrometry data indicating the downregulation of multiple phosphorylation sites on tau [65].
Fig. 4.
PhosTAC-Induced Tau Dephosphorylation in a PP2A-Dependent Manner. (a) Therapeutic mechanism of PhosTAC. (b) PhosTAC-induced stable ternary complex with tau and PP2A. (c) PhosTAC7 but not the inactive PhosTAC7F induced tau dephosphorylation. (d) PhosTAC7-induced tau dephosphorylation via PP2A [65].
The excessive phosphorylation of tau protein is a key factor in the development of Alzheimer's disease, making the targeting of this process a promising therapeutic approach for combating the disease. With advancements in our comprehension of the mechanisms behind tau phosphorylation, we anticipate the emergence of increasingly precise and potent therapies for AD in the coming years.
4.2. Nanotechnology in inhibiting tau aggregation
The formation of intracellular Tau aggregates is facilitated by the microtubule-binding domain, which interacts with sites Ser214 and Glu372 [66]. The MBD tightly binds to microtubules and facilitates the assembly of microtubule dimers. Within the third repeat sequence lies a hexapeptide motif VQIVYK essential for generating protofilaments [67]. This motif, along with the VQIINK hexapeptide motif, promotes the formation of β-sheet structures necessary for Tau aggregation [68]. Disruption of the local structure surrounding VQIVYK due to mutations in the FTD domain of Tau protein can lead to spontaneous aggregation [69]. In response to these characteristics, researchers have developed a novel intracellular pathological tau protein-targeting nanoparticle chaperone (Tau-nChap) that can be used as a potential therapy for AD. This nanoparticle chaperone is engineered with the VQIINK hexapeptide from tau protein on the surface of self-assembled micelles containing hydrophobic domains and confined spaces akin to chaperones. Tau-nChap leverages its lysosome-responsive surface to escape lysosomes and enter the cytoplasm, addressing the challenge of intracellular tau protein localization. Once inside the cell, Tau-nChap selectively captures pathological tau proteins without disrupting the normal functions of tau protein. This selective binding is achieved through the synergistic action of the tau protein recognition peptide VQIINK and the confined hydrophobic domains on the surface, effectively preventing tau protein aggregation [70]. Ultimately, this pathological tau protein-targeting nanoparticle chaperone demonstrates the potential to reduce neuronal toxicity associated with tau proteins, thereby ameliorating cognitive impairment in AD model mice. This customized tau protein nanoparticle chaperone presents a promising strategy for the targeted treatment of AD and provides insights into addressing other neurodegenerative diseases caused by specific pathogenic proteins, such as Parkinson's disease and Huntington's disease.
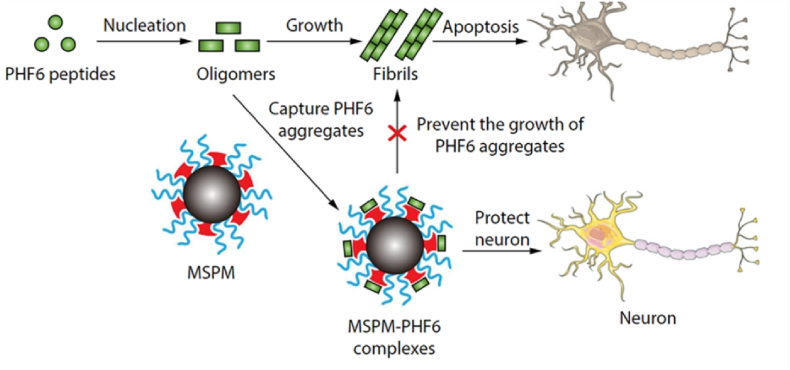
Additionally, various nanomaterials have shown the ability to modulate tau aggregation. Nanomaterials with hydrophobic surfaces have been found to interact with PHF6, leading to significantly enhanced regulatory effects due to the large surface area of interaction. Scientists have developed a biomimetic nanoparticle chaperone utilizing a composite shell-crosslinked polymer micelle, presenting a novel therapy targeting tau proteins in Alzheimer's disease, as illustrated in Fig. 5. The surface of this nanoparticle chaperone contains phase-separated domains resembling natural molecular chaperones, which not only effectively bind to PHF6 to impede its aggregation and prevent the internalization of PHF6 species into neurons but also facilitate the proteasomal degradation of PHF6 aggregates. This process ultimately leads to a significant reduction in neuronal toxicity caused by PHF6 [71]. This biomimetic nanoparticle chaperone provides a new approach for treating Alzheimer's disease by targeting tau protein aggregation.
Fig. 5.
By capturing the PHF6 species, the MSPM inhibits PHF6 aggregation and mitigates PHF6-mediated cytotoxicity, thus protecting neurons against apoptosis [71].
Methylene blue (MB) is a small molecule inhibitor of tau aggregation, and researchers have designed and synthesized an H2O2-responsive multifunctional nanocomposite UCNPs@mSiO2-MB@AuNPs (abbreviated as USMA). This material releases gold nanoparticles (AuNPs) and the small molecule inhibitor MB upon H2O2 stimulation, inhibiting the aggregation of Aβ and Tau, thus exploring an efficient treatment for AD [72]. It is important to note that the material is traceable, allowing for the monitoring of methylene blue release through its up-conversion fluorescence, which offers insights into the methylene blue content in the lesion area. Significantly, USMA demonstrates the ability to effectively mitigate the cytotoxicity resulting from Aβ and Tau aggregation.
In summary, nanotechnology for inhibiting tau aggregation holds great promise in AD treatment, offering a wide range of applications and research directions. These technologies are expected to improve the delivery and efficacy of existing drugs and enable the development of new treatment approaches.
4.3. Nanotechnology in inhibiting tau propagation and stabilizing microtubules
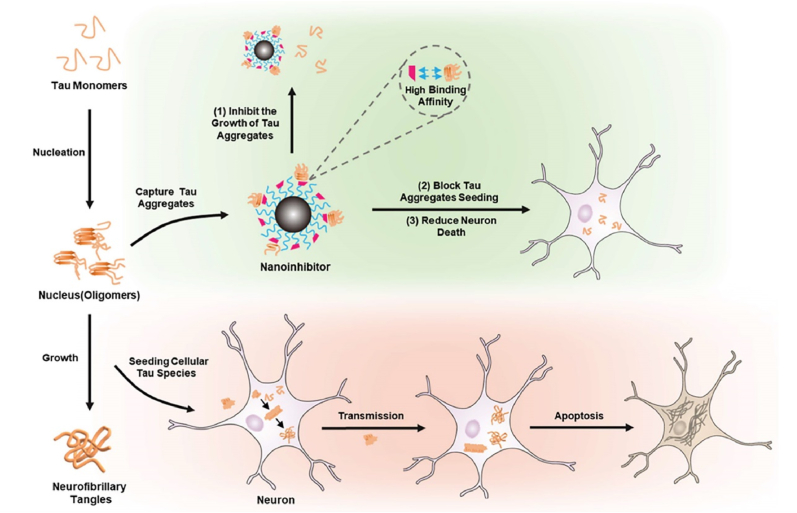
Research in tau transgenic mice has clearly illustrated the transmission of tau pathology from one brain region to another [38]. Injecting tau oligomers derived from AD patients into the hippocampus of wild-type mice has been shown to induce memory deficits and lead to the spread of phosphorylated Tau to various brain regions, such as the cortex, corpus callosum, and hypothalamus [73]. The precise mechanisms underlying the inter-neuronal movement of tau protein remain incompletely understood. Recent investigations have started to delve into potential pathways, including exosome release, synaptic transmission, and prion-like propagation of tau protein, highlighting the long-distance dissemination of toxic tau aggregates and the initiation of similar aggregates in remote brain areas. In response to these spreading mechanisms, scientists have sought to combat Alzheimer's disease by impeding the spread of tau protein. A novel tau-targeted therapy for AD has been developed in the form of a multifunctional nanoinhibitor [74].This NanoTLK is crafted as a small-sized particle with a hydrophobic core comprising poly(ε-caprolactone) (PCL) and a hydrophilic shell composed of poly(ethylene glycol) (PEG). The surface of the nanocontainer is uniformly coated with tau-binding peptides, specifically (D)-TLKIVW (TLK). The TLK peptides on the nanocontainer's surface can bind to tau aggregates, which contain the specific motif VQIVYK as shown in Fig. 6. This specific binding allows the nanocontainer to efficiently intercept the propagation of tau aggregates and mitigate the neuronal toxicity caused by tau aggregation. Additionally, the nanocontainer's interaction with tau can promote the enzymatic breakdown of tau aggregates, thereby supporting the balance of tau metabolism within cells. Through the innovative application of nanotechnology, the nanomaterials overcome the shortcoming of conventional blockers that cannot cross the BBB. Furthermore, they are meticulously surface-engineered with a variety of ligands capable of selectively binding to the targeted sites. This strategic enhancement significantly boosts the efficiency of drug delivery to the brain, effectively halting the progression of tau aggregates. The hydrophobic nature of PCL allows the nanocontainer to self-assemble into a stable structure in aqueous environments, while the PEG layer aids in maintaining micelle integrity and preventing non-specific interactions with cellular components. However, when utilizing PCL, which is a hydrophobic material, certain factors related to performance and safety must also be considered. Hydrophobicity affects the solubility of nanomaterials in water, leading to precipitation, which can reduce delivery efficiency and therapeutic efficacy. It also influences the penetration of the BBB and uptake by brain cells, potentially causing toxic effects on brain tissue. Overall, this innovative nanoscale approach holds significant potential as a therapeutic avenue for Alzheimer's disease.
Fig. 6.
Illustration of the Regulation Mechanism of the Nano inhibitor on Tau Protein. Nano inhibitors significantly reduce tau-mediated cytotoxicity by effectively inhibiting tau protein aggregation, recognizing tau aggregates, and blocking their inoculation in nerve cells through multivalent binding to aggregators. Moreover, the tau complex formed after binding is more easily degraded [74].
Microtubules are vital cytoskeleton components and perform numerous important biological functions within cells. In neurons, their role is particularly crucial, as they are involved in the growth of axons, the branching of dendrites, and the release of neurotransmitters [24]. Abnormal aggregation of microtubule proteins and microtubule fragmentation contribute to the pathological processes seen in AD. Therefore, targeting microtubule stability for treatment holds significant value in AD therapy. The laboratory setting has demonstrated that paclitaxel is capable of inducing microtubule assembly. This compound achieves its effect by binding to the taxane binding site within the microtubule lumen, which is close to the tau protein, displacing the tau protein from the microtubules. Paclitaxel represents the first tested microtubule stabilizer in animal models of neurodegenerative tauopathies. It is believed that the interaction between paclitaxel and the taxane site on β-tubulin promotes microtubule stability by inducing conformational changes in the β-tubulin M-loop, leading to stronger lateral interactions between neighboring protofilaments [75]. Many studies have already explored using paclitaxel combined with other materials to assemble into nanodrugs, thereby enhancing drug delivery efficiency and reducing toxicity [76]. Consequently, using nanocarriers to deliver paclitaxel will have great potential for future clinical applications.
4.4. Nanotechnology in clearing pathological tau
Pathological tau is a key neurotoxic factor in AD, which leads to the progressive cognitive decline and neurodegeneration observed in patients. Tau protein is typically found in the brain where it plays a crucial role in stabilizing microtubules, essential for preserving the structure and function of neurons. However, in AD, tau protein becomes hyperphosphorylated and forms insoluble aggregates, known as neurofibrillary tangles, which disrupt microtubule function and lead to neuronal dysfunction and death. Therefore, clearing pathological tau is a critical objective in treating AD. By removing these aggregates, it may be possible to restore the stability and function of microtubules, which could potentially reverse the cognitive and neurodegenerative symptoms of the disease. Strategies are currently being explored to achieve this goal, including using tau-specific immunotherapy, inhibiting tau phosphorylation, and enhancing tau clearance by autophagy or other cellular degradation pathways.
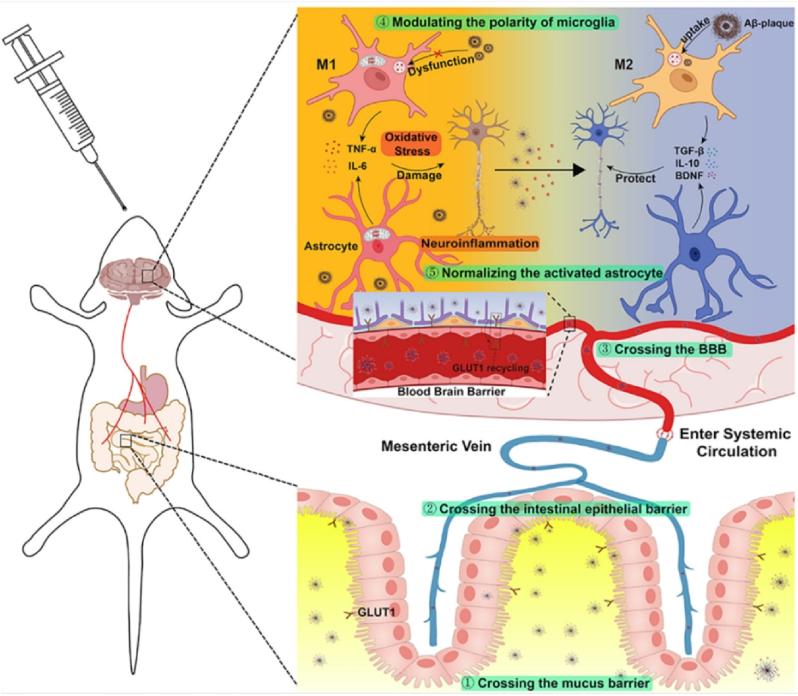
Researchers have developed a complex oral nanoparticle for the multi-target treatment of AD based on the characteristics of natural physiological barriers. Initially, the study selected PLGA-PEG as the nanocarrier scaffold due to its good stability and safety. PLGA-PEG is a hydrophilic polymer due to the presence of poly (ethylene glycol) (PEG) segments. This hydrophilic nature allows PLGA-PEG to dissolve in aqueous environments, which is essential for drug delivery systems that need to traverse through mucus, a gel-like substance that covers the mucosal surfaces in the body. The ability of PLGA-PEG to interact with water helps it to dissolve and mix with the mucus, facilitating its penetration. The negative charge of PLGA-PEG can lead to electrostatic repulsion with the mucus, which actually facilitates penetration. This is because the repulsive force between like charges can create gaps or pores in the mucus barrier, making it easier for PLGA-PEG to pass through the mucin barrier. The study leveraged the shared characteristics of intestinal epithelial cells and brain endothelial cells to target glucose transporter 1 (GLUT1) receptors, which are highly expressed in both cell types. By grafting α-mannopyranoside to the PEG terminal, the nanoparticles were able to co-target the intestinal epithelial barrier (IEB) and BBB. To enhance the nanoparticles' ability to traverse the BBB, a blood sugar control strategy was utilized to relocate GLUT1 from the luminal to the apical plasma membrane, facilitating the penetration of mannose-modified nanoparticles through the BBB and their accumulation in the brain. Subsequently, the encapsulated fingolimod (FTY) was released within neurons to modulate the aberrant activation of microglia and astrocytes: (1) Microglia transitioned from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype, restoring their ability to phagocytize toxic substances like Aβ and Tau while decreasing the release of inflammatory mediators. (2) Normalization of overactive astrocytes resulted in reduced secretion of pro-inflammatory factors and increased release of anti-inflammatory and neuroprotective factors, as illustrated in Fig. 7. By concurrently diminishing elevated inflammation levels and alleviating oxidative stress, the study effectively reshaped the abnormal lesion microenvironment [77].
Fig. 7.
Illustrates a schematic diagram depicting how nanoparticles achieve brain targeting through oral administration. The released FTY within the mannose-modified nanoparticles regulates the polarization of microglia from the pro-inflammatory M1 state to the anti-inflammatory M2 state and normalizes the activation of astrocytes. This process enhances the clearance of toxic protein Aβ, reduces oxidative stress, and mitigates neuroinflammation [77].
Autophagy, a crucial cellular degradation system responsible for clearing damaged intracellular components, plays a vital role in maintaining cellular homeostasis. Dysregulated autophagy has been implicated in the accumulation of hyperphosphorylated and aggregated tau proteins observed in the progression of AD [78].Modulating the autophagy process can aid in the clearance of pathological tau aggregates. In a recent study, researchers developed a tau protein-targeting nanoparticle assembly (TNH) by combining cellulose nanocrystals (CNPs), an anti-tau antibody (AT8), and magnetic mesoporous silica nanoparticles (M-MSN). CNPs induce autophagy, while the surface modification of M-MSN enhances autophagic flux by promoting good dispersibility [79]. AT8 specifically binds to the phosphorylation sites (Ser202/Thr205) in pathological tau [80], and as a targeting ligand coupled to the nanoparticle assembly, it confers tau affinity and promotes retention in tau disease-related cells [81]. Additionally, iron oxide nanoparticles encapsulated within the core of mesoporous silica nanoparticles enable monitoring of drug delivery and treatment efficacy through their magnetic resonance imaging (MRI) properties [82]. TNH selectively accumulates in the hippocampi affected by tau protein pathology, where it activates autophagy by inhibiting the mammalian target of rapamycin (mTOR) and promoting the activation of transcription factor EB (TFEB) [83]. This targeted approach enhances autophagic flux, leading to the detoxification of pathological tau, preservation of neuronal viability, and attenuation of cognitive decline in Alzheimer's disease.
Overall, nanotechnology plays a crucial role in developing these treatment strategies. Drug delivery systems based on nanoparticles can enhance the delivery of tau-targeting drugs to the brain, improve their efficacy, and reduce side effects. Clearing pathological tau represents a promising new approach to treating Alzheimer's disease, with the potential to decelerate or halt disease progression and enhance the quality of life for individuals affected by the condition.
As explored, nanomaterials are increasingly recognized as promising strategies to tackle current obstacles in targeted drug delivery and surmounting barriers for drug passage across the BBB in the treatment of neurodegenerative disorders. Nanotechnology offers several advantages in improving the pathological mechanisms of AD. The BBB, which acts as a protective and dynamic interface, can hinder drug transport into the CNS [84]. However, NPs, with their high surface-to-volume ratio and long circulation times, can overcome these barriers and deliver drugs specifically to the target site, reducing side effects and increasing treatment effectiveness. They can achieve this through cellular absorption, modification with targeting groups, and transcellular lipophilic pathways [85]. Furthermore, nanotechnology has advantages in drug delivery and improved imaging and disease diagnosis. NPs can be integrated with specific biomarkers to detect certain histological phenotypes via ultrasound and measure biomarkers. Nanotechnology also serves as a highly sensitive disease detector for early diagnostic purposes, given its stability, reliability, and performance in medical imaging-based detection [86].
However, due to their special physical and chemical properties, nanoparticles can more easily cross the BBB than larger molecular drugs, which raises concerns about their potential toxicity. While NPs offer various advantages, their constituents, such as nucleic acids, antibody fragments, peptides, and proteins, can trigger immunotoxic responses [87]. Clinical experiments can identify acute nanoparticle toxicity, yet it is essential to consider the potential chronic toxicity from prolonged exposure and accumulation. Currently, there is a lack of experimental studies on living organisms to assess chronic toxicity and adverse effects of nanoparticles [88]. Additionally, the consumption of high quantities of nanocarriers containing surfactants and cosurfactants due to low encapsulation efficacy may lead to severe adverse effects. Some nanoparticles may not be efficiently eliminated by clearance systems, resulting in brain accumulation and cytotoxicity. Prolonged nanoparticle accumulation in the brain can cause injuries [89]. The production of nanoparticles is a complex and costly process requiring specific ingredients, instruments, and optimal conditions, especially for multifunctional nanoparticles with preventive and therapeutic roles. Therefore, further experimental studies are crucial to reduce the clinical application costs of nanotechnology in medical care. Clear guidelines and regulations are essential to ensure the safe and ethical development and utilization of these technologies.
5. Conclusion and future perspectives
Nanotechnology has the potential to revolutionize AD research and treatment by providing new solutions for diagnosis, treatment, and prevention of this debilitating disease. Nanosensors can detect AD biomarkers with high sensitivity and specificity in biological samples, enabling early detection and intervention. NPs can be used for targeted drug delivery to the brain, overcoming the challenges posed by the BBB. They can exploit endogenous transport mechanisms or actively target specific cell types in the brain, resulting in more precise drug delivery, reduced systemic toxicity, and enhanced therapeutic efficacy. Additionally, nanotechnology can prevent AD by delivering neuroprotective agents to the brain, scavenging ROS, and modulating pathological processes associated with AD.
However, the transition of nanotechnology into clinical applications for the diagnosis and treatment of AD hinges on the paramount importance of biocompatibility. Primarily, the efficacy of nanomaterials is paramount, as their size and shape significantly influence their distribution and bioavailability within the body. Nanomaterials with excessive size may struggle to penetrate the blood-brain barrier, whereas those with specific shapes may exhibit enhanced targeting capabilities for particular cell types. Stability is another critical requirement, as nanomaterials must maintain their integrity to prevent the premature release or degradation of active ingredients during storage and use. Additionally, the controlled release of therapeutic agents is essential for achieving sustained therapeutic outcomes.
Safety is a critical factor and a significant challenge currently faced by the application of nanotechnology in AD. The application of nanotechnology requires that the nanomaterials and their degradation products be non-toxic and well-tolerated by human tissues, without triggering inflammatory responses or immune system dysregulation. The potential for DNA damage or mutations must be minimized, particularly the toxicity to neurons and other brain tissues, as AD primarily affects these areas. A thorough understanding of the metabolic pathways of nanomaterials is essential to ensure that their degradation rates are consistent with those of biological tissues, to avoid any long-term toxicity.
The synthesis of nanomaterials is a complex and costly process, requiring specific ingredients, equipment, and optimal conditions, especially for multifunctional nanomaterials intended for preventive and therapeutic purposes. Therefore, further experimental research is necessary to reduce the clinical application costs of nanotechnology in patient healthcare. Clear guidelines and regulations are also needed to ensure the safe and responsible development and use of these technologies.
Moreover, ethical implications must be carefully considered when deploying nanotechnology in human subjects. By taking these factors into comprehensive account, we can ensure that the application of nanomaterials in AD treatment is not only safe but also highly effective.
In the summery, the future of nanotechnology in AD research appears promising, with several potential directions for further exploration and development. One of the future perspectives of nanotechnology in AD research is the identification of novel drug targets for more effective treatment. Current therapeutic approaches primarily target Aβ plaques and tau protein tangles, which are hallmark pathological features of AD. However, recent research suggests that several other potential targets could be explored. Another exciting future perspective is the development of nanomedicines with multiple therapeutic functions to treat different aspects of AD simultaneously. AD is a complex disease with multiple pathological processes, including neuroinflammation, oxidative stress, protein aggregation, and synaptic dysfunction. Nanotechnology offers the possibility of designing multifunctional NPs that can target multiple pathological processes simultaneously. These NPs can carry different therapeutic agents, such as antioxidants, anti-inflammatory drugs, and agents that modulate protein aggregation, to provide a comprehensive and synergistic approach to AD treatment. By combining different therapeutic functions into a single nanomedicine, researchers can enhance treatment efficacy and reduce the need for multiple drug administrations. Integrating nanotechnology with other emerging technologies is another promising future perspective in AD research. Nanotechnology can complement and synergize with other fields, such as artificial intelligence (AI), genomics, and stem cell research, to advance our understanding of AD and develop personalized treatment strategies. For example, nanosensors combined with AI algorithms can enable real-time monitoring of AD biomarkers, providing valuable insights into disease progression and response to treatment. Furthermore, integrating nanotechnology with genomics can facilitate the development of personalized nanomedicines tailored to an individual's genetic profile. Additionally, nanotechnology can enhance the delivery and differentiation of stem cells for regenerative therapies in AD. By integrating nanotechnology with other emerging technologies, researchers can unlock new possibilities for precision medicine and personalized treatments in AD. These future perspectives can revolutionize our understanding of AD and pave the way for more effective diagnostic tools, targeted drug delivery systems, and neuroprotective strategies. With continued research and collaboration, the full potential of nanotechnology in the fight against AD can be realized, bringing us closer to improved patient outcomes and, ultimately, a cure for this devastating neurodegenerative disorder.
CRediT authorship contribution statement
Rongrong Ma: Writing – original draft, Software. Qianwen Mu: Writing – original draft. Yue Xi: Writing – review & editing. Gang Liu: Writing – review & editing. Chao Liu: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Rongrong Ma and Qianwen Mu contributed equally to this work. This work was sponsored by the National Natural Science Foundation of China (81925019, U22A20333, 82272144), the Guangdong Basic and Applied Basic Research Foundation (2020A1515110456, 2022J011403, 2020Y4003), and the Shenzhen Science and Technology Innovation Commission (JCYJ20220530143411025, JCYJ20230807091404010, B2302014).
Contributor Information
Rongrong Ma, Email: 21620200156469@stu.xmu.edu.cn.
Gang Liu, Email: gangliu.cmitm@xmu.edu.cn.
Chao Liu, Email: liuchao66888@xmu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Lee V.M., Balin B.J., Otvos L., Jr., Trojanowski J.Q. Science. 1991;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs G.G. Handb. Clin. Neurol. 2017;145:355–368. doi: 10.1016/b978-0-12-802395-2.00025-0. [DOI] [PubMed] [Google Scholar]
- 3.Wood J.G., Mirra S.S., Pollock N.J., Binder L.I. Proc. Natl. Acad. Sci. U. S. A. 1986;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Yu Y. J. Neuroinflammation. 2023;20(1):165. doi: 10.1186/s12974-023-02853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings J., Zhou Y., Lee G., Zhong K., Fonseca J., Cheng F. Alzheimers Dement (N Y) 2023;9(2) doi: 10.1002/trc2.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Höglinger G.U., Huppertz H.J., Wagenpfeil S., Andrés M.V., Belloch V., León T., Del Ser T. Mov. Disord. 2014;29(4):479–487. doi: 10.1002/mds.25815. [DOI] [PubMed] [Google Scholar]
- 7.Albert M., Mairet-Coello G., Danis C., Lieger S., Caillierez R., Carrier S., Skrobala E., Landrieu I., Michel A., Schmitt M., Citron M., Downey P., Courade J.P., Buée L., Colin M. Brain. 2019;142(6):1736–1750. doi: 10.1093/brain/awz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Liu C., Zheng Z., Chen S., Pang X., Xiang X., Tang J., Ren E., Chen Y., You M., Wang X., Chen X., Luo W., Liu G., Xia N. Adv. Mater. 2019;31(17) doi: 10.1002/adma.201808294. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca-Santos B., Gremião M.P., Chorilli M. Int. J. Nanomed. 2015;10:4981–5003. doi: 10.2147/ijn.S87148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An X., Xiang W., Liu X., Li S., Xu Z., He P., Ge R.L., Tang F., Cheng Z., Liu C., Liu G. Angew Chem. Int. Ed. Engl. 2024;63(13) doi: 10.1002/anie.202319489. [DOI] [PubMed] [Google Scholar]
- 11.Ji D., Wu X., Li D., Liu P., Zhang S., Gao D., Gao F., Zhang M., Xiao Y. Int. J. Biol. Macromol. 2020;154:233–245. doi: 10.1016/j.ijbiomac.2020.03.079. [DOI] [PubMed] [Google Scholar]
- 12.Song N., Sun S., Chen K., Wang Y., Wang H., Meng J., Guo M., Zhang X.D., Zhang R. J. Contr. Release. 2023;360:392–417. doi: 10.1016/j.jconrel.2023.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Hersh A.M., Alomari S., Tyler B.M. Int. J. Mol. Sci. 2022;23(8) doi: 10.3390/ijms23084153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weingarten M.D., Lockwood A.H., Hwo S.Y., Kirschner M.W. Proc. Natl. Acad. Sci. U. S. A. 1975;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goedert M., Spillantini M.G., Jakes R., Rutherford D., Crowther R.A. Neuron. 1989;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 16.Andreadis A. Biochim. Biophys. Acta. 2005;1739(2–3):91–103. doi: 10.1016/j.bbadis.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Goate A., Chartier-Harlin M.C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L., et al. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 18.Hong M., Zhukareva V., Vogelsberg-Ragaglia V., Wszolek Z., Reed L., Miller B.I., Geschwind D.H., Bird T.D., McKeel D., Goate A., Morris J.C., Wilhelmsen K.C., Schellenberg G.D., Trojanowski J.Q., Lee V.M. Science. 1998;282(5395):1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 19.Neve R.L., Harris P., Kosik K.S., Kurnit D.M., Donlon T.A. Brain Res. 1986;387(3):271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- 20.El Mammeri N., Dregni A.J., Duan P., Wang H.K., Hong M. Sci. Adv. 2022;8(29) doi: 10.1126/sciadv.abo4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Souza I., Schellenberg G.D. Biochim. Biophys. Acta. 2005;1739(2–3):104–115. doi: 10.1016/j.bbadis.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Cleveland D.W., Hwo S.Y., Kirschner M.W. J. Mol. Biol. 1977;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- 23.Shaw-Smith C., Pittman A.M., Willatt L., Martin H., Rickman L., Gribble S., Curley R., Cumming S., Dunn C., Kalaitzopoulos D., Porter K., Prigmore E., Krepischi-Santos A.C., Varela M.C., Koiffmann C.P., Lees A.J., Rosenberg C., Firth H.V., de Silva R., Carter N.P. Nat. Genet. 2006;38(9):1032–1037. doi: 10.1038/ng1858. [DOI] [PubMed] [Google Scholar]
- 24.Harada A., Oguchi K., Okabe S., Kuno J., Terada S., Ohshima T., Sato-Yoshitake R., Takei Y., Noda T., Hirokawa N. Nature. 1994;369(6480):488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- 25.Bramblett G.T., Goedert M., Jakes R., Merrick S.E., Trojanowski J.Q., Lee V.M. Neuron. 1993;10(6):1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- 26.Noble W., Hanger D.P., Miller C.C., Lovestone S. Front. Neurol. 2013;4:83. doi: 10.3389/fneur.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billingsley M.L., Kincaid R.L. Biochem. J. 1997;323(Pt 3):577–591. doi: 10.1042/bj3230577. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanger D.P., Anderton B.H., Noble W. Trends Mol. Med. 2009;15(3):112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Lindwall G., Cole R.D. J. Biol. Chem. 1984;259(8):5301–5305. [PubMed] [Google Scholar]
- 30.Mandelkow E., von Bergen M., Biernat J., Mandelkow E.M. Brain Pathol. 2007;17(1):83–90. doi: 10.1111/j.1750-3639.2007.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond E., Pires G., MacMurray C., Askenazi M., Nayak S., Bourdon M., Safar J., Ueberheide B., Wisniewski T. Brain. 2020;143(9):2803–2817. doi: 10.1093/brain/awaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbier P., Zejneli O., Martinho M., Lasorsa A., Belle V., Smet-Nocca C., Tsvetkov P.O., Devred F., Landrieu I. Front. Aging Neurosci. 2019;11:204. doi: 10.3389/fnagi.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris M., Maeda S., Vossel K., Mucke L. Neuron. 2011;70(3):410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina M., Avila J. Expert Rev. Neurother. 2015;15(1):115–122. doi: 10.1586/14737175.2015.1000864. [DOI] [PubMed] [Google Scholar]
- 35.Hof P.R., Bouras C., Buée L., Delacourte A., Perl D.P., Morrison J.H. Acta Neuropathol. 1992;85(1):23–30. doi: 10.1007/bf00304630. [DOI] [PubMed] [Google Scholar]
- 36.Geddes J.F., Hughes A.J., Lees A.J., Daniel S.E. Brain. 1993;116(Pt 1):281–302. doi: 10.1093/brain/116.1.281. [DOI] [PubMed] [Google Scholar]
- 37.Kundel F., Hong L., Falcon B., McEwan W.A., Michaels T.C.T., Meisl G., Esteras N., Abramov A.Y., Knowles T.J.P., Goedert M., Klenerman D. ACS Chem. Neurosci. 2018;9(6):1276–1282. doi: 10.1021/acschemneuro.8b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen K.L., Yu R.J., Zhong C.B., Wang Z., Xie B.K., Ma H., Ao M., Zheng P., Ewing A.G., Long Y.T. Angew Chem. Int. Ed. Engl. 2024 doi: 10.1002/anie.202406677. [DOI] [PubMed] [Google Scholar]
- 39.Kfoury N., Holmes B.B., Jiang H., Holtzman D.M., Diamond M.I. J. Biol. Chem. 2012;287(23):19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J.W., Hussaini S.A., Bastille I.M., Rodriguez G.A., Mrejeru A., Rilett K., Sanders D.W., Cook C., Fu H., Boonen R.A., Herman M., Nahmani E., Emrani S., Figueroa Y.H., Diamond M.I., Clelland C.L., Wray S., Duff K.E. Nat. Neurosci. 2016;19(8):1085–1092. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clavaguera F., Bolmont T., Crowther R.A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A.K., Beibel M., Staufenbiel M., Jucker M., Goedert M., Tolnay M. Nat. Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aina A., Hsueh S.C.C., Gibbs E., Peng X., Cashman N.R., Plotkin S.S. ACS Chem. Neurosci. 2023;14(15):2603–2617. doi: 10.1021/acschemneuro.3c00007. [DOI] [PubMed] [Google Scholar]
- 43.Guo T., Noble W., Hanger D.P. Acta Neuropathol. 2017;133(5):665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D., Zhang W., Ming C., Gao X., Yuan H., Lin X., Mao X., Wang C., Guo X., Du Y., Shao L., Yang R., Lin Z., Wu X., Huang T.Y., Wang Z., Zhang Y.W., Xu H., Zhao Y. Neuron. 2024;112(10):1676–1693.e12. doi: 10.1016/j.neuron.2024.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Mably A.J., Kanmert D., Mc Donald J.M., Liu W., Caldarone B.J., Lemere C.A., O'Nuallain B., Kosik K.S., Walsh D.M. Neurobiol. Aging. 2015;36(3):1316–1332. doi: 10.1016/j.neurobiolaging.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Kontsekova E., Zilka N., Kovacech B., Novak P., Novak M. Alzheimer's Res. Ther. 2014;6(4):44. doi: 10.1186/alzrt278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novak P., Schmidt R., Kontsekova E., Zilka N., Kovacech B., Skrabana R., Vince-Kazmerova Z., Katina S., Fialova L., Prcina M., Parrak V., Dal-Bianco P., Brunner M., Staffen W., Rainer M., Ondrus M., Ropele S., Smisek M., Sivak R., Winblad B., Novak M. Lancet Neurol. 2017;16(2):123–134. doi: 10.1016/s1474-4422(16)30331-3. [DOI] [PubMed] [Google Scholar]
- 48.Theunis C., Crespo-Biel N., Gafner V., Pihlgren M., López-Deber M.P., Reis P., Hickman D.T., Adolfsson O., Chuard N., Ndao D.M., Borghgraef P., Devijver H., Van Leuven F., Pfeifer A., Muhs A. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panza F., Solfrizzi V., Seripa D., Imbimbo B.P., Lozupone M., Santamato A., Tortelli R., Galizia I., Prete C., Daniele A., Pilotto A., Greco A., Logroscino G. Immunotherapy. 2016;8(9):1119–1134. doi: 10.2217/imt-2016-0019. [DOI] [PubMed] [Google Scholar]
- 50.Bright J., Hussain S., Dang V., Wright S., Cooper B., Byun T., Ramos C., Singh A., Parry G., Stagliano N., Griswold-Prenner I. Neurobiol. Aging. 2015;36(2):693–709. doi: 10.1016/j.neurobiolaging.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Qureshi I.A., Tirucherai G., Ahlijanian M.K., Kolaitis G., Bechtold C., Grundman M. Alzheimers Dement (N Y) 2018;4:746–755. doi: 10.1016/j.trci.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanamandra K., Jiang H., Mahan T.E., Maloney S.E., Wozniak D.F., Diamond M.I., Holtzman D.M. Ann Clin Transl Neurol. 2015;2(3):278–288. doi: 10.1002/acn3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanamandra K., Kfoury N., Jiang H., Mahan T.E., Ma S., Maloney S.E., Wozniak D.F., Diamond M.I., Holtzman D.M. Neuron. 2013;80(2):402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buée L., Bussière T., Buée-Scherrer V., Delacourte A., Hof P.R. Brain Res Brain Res Rev. 2000;33(1):95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 55.Lee S.H., Le Pichon C.E., Adolfsson O., Gafner V., Pihlgren M., Lin H., Solanoy H., Brendza R., Ngu H., Foreman O., Chan R., Ernst J.A., DiCara D., Hotzel I., Srinivasan K., Hansen D.V., Atwal J., Lu Y., Bumbaca D., Pfeifer A., Watts R.J., Muhs A., Scearce-Levie K., Ayalon G. Cell Rep. 2016;16(6):1690–1700. doi: 10.1016/j.celrep.2016.06.099. [DOI] [PubMed] [Google Scholar]
- 56.Novak P., Kontsekova E., Zilka N., Novak M. Front. Neurosci. 2018;12:798. doi: 10.3389/fnins.2018.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drechsel D.N., Hyman A.A., Cobb M.H., Kirschner M.W. Mol. Biol. Cell. 1992;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aimo A., Castiglione V., Rapezzi C., Franzini M., Panichella G., Vergaro G., Gillmore J., Fontana M., Passino C., Emdin M. Nat. Rev. Cardiol. 2022;19(10):655–667. doi: 10.1038/s41569-022-00683-z. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L.-x., Xie X.-x., Liu D.-q., Xu Z.P., Liu R.-t. Biomaterials. 2018;174:54–66. doi: 10.1016/j.biomaterials.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 60.Cheng W., Su Y.-L., Hsu H.-H., Lin Y.-H., Chu L.-A., Huang W.-C., Lu Y.-J., Chiang C.-S., Hu S.-H. ACS Nano. 2022;16(3):4014–4027. doi: 10.1021/acsnano.1c09601. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L., Hou S., Movahedi F., Li Z., Li L., Hu J., Jia Y., Huang Y., Zhu J., Sun X., Zeng L., Liu R., Xu Z.P. Nano Today. 2023;49 doi: 10.1016/j.nantod.2023.101788. [DOI] [Google Scholar]
- 62.Himmelstein D.S., Ward S.M., Lancia J.K., Patterson K.R., Binder L.I. Pharmacol. Ther. 2012;136(1):8–22. doi: 10.1016/j.pharmthera.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin L., Latypova X., Wilson C.M., Magnaudeix A., Perrin M.L., Terro F. Ageing Res. Rev. 2013;12(1):39–49. doi: 10.1016/j.arr.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Malpas C.B., Vivash L., Genc S., Saling M.M., Desmond P., Steward C., Hicks R.J., Callahan J., Brodtmann A., Collins S., Macfarlane S., Corcoran N.M., Hovens C.M., Velakoulis D., O'Brien T.J. J Alzheimers Dis. 2016;54(1):223–232. doi: 10.3233/jad-160544. [DOI] [PubMed] [Google Scholar]
- 65.Hu Z., Chen P.H., Li W., Douglas T., Hines J., Liu Y., Crews C.M. J. Am. Chem. Soc. 2023 doi: 10.1021/jacs.2c11706. [DOI] [PubMed] [Google Scholar]
- 66.Fauquant C., Redeker V., Landrieu I., Wieruszeski J.M., Verdegem D., Laprévote O., Lippens G., Gigant B., Knossow M. J. Biol. Chem. 2011;286(38):33358–33368. doi: 10.1074/jbc.M111.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daebel V., Chinnathambi S., Biernat J., Schwalbe M., Habenstein B., Loquet A., Akoury E., Tepper K., Müller H., Baldus M., Griesinger C., Zweckstetter M., Mandelkow E., Vijayan V., Lange A. J. Am. Chem. Soc. 2012;134(34):13982–13989. doi: 10.1021/ja305470p. [DOI] [PubMed] [Google Scholar]
- 68.von Bergen M., Barghorn S., Li L., Marx A., Biernat J., Mandelkow E.M., Mandelkow E. J. Biol. Chem. 2001;276(51):48165–48174. doi: 10.1074/jbc.M105196200. [DOI] [PubMed] [Google Scholar]
- 69.Chen D., Joachimiak L.A. Methods Mol. Biol. 2023;2551:189–201. doi: 10.1007/978-1-0716-2597-2_13. [DOI] [PubMed] [Google Scholar]
- 70.Xu L., Ding Y., Ma F., Chen Y., Chen G., Zhu L., Long J., Ma R., Liu Y., Liu J., Huang F., Shi L. Nano Today. 2022;43 doi: 10.1016/j.nantod.2022.101388. [DOI] [Google Scholar]
- 71.Zhu L., Zhang M.-Q., Jing H.-R., Zhang X.-P., Xu L.-L., Ma R.-J., Huang F., Shi L.-Q. Chin. J. Polym. Sci. 2022;40(9):1062–1070. doi: 10.1007/s10118-022-2799-9. [DOI] [Google Scholar]
- 72.Qiao L., Shen Y., Zhang S., Wang M., Lv G., Dou Q., Li C. BMEMat. 2023;1(1) doi: 10.1002/bmm2.12011. [DOI] [Google Scholar]
- 73.Lasagna-Reeves C.A., Castillo-Carranza D.L., Sengupta U., Guerrero-Munoz M.J., Kiritoshi T., Neugebauer V., Jackson G.R., Kayed R. Sci. Rep. 2012;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu L., Xu L., Wu X., Deng F., Ma R., Liu Y., Huang F., Shi L. ACS Appl. Mater. Interfaces. 2021;13(20):23328–23338. doi: 10.1021/acsami.1c00257. [DOI] [PubMed] [Google Scholar]
- 75.Ballatore C., Brunden K.R., Huryn D.M., Trojanowski J.Q., Lee V.M., Smith A.B. 3rd, J Med Chem. 2012;55(21):8979–8996. doi: 10.1021/jm301079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li L., Liu T., Zuo S., Li Y., Zhao E., Lu Q., Wang D., Sun Y., He Z., Sun B., Sun J. Adv. Mater. 2024;36(4) doi: 10.1002/adma.202310633. [DOI] [PubMed] [Google Scholar]
- 77.Lei T., Yang Z., Jiang C., Wang X., Yang W., Yang X., Xie R., Tong F., Xia X., Huang Q., Du Y., Huang Y., Gao H. ACS Nano. 2024;18(4):3234–3250. doi: 10.1021/acsnano.3c09715. [DOI] [PubMed] [Google Scholar]
- 78.Scrivo A., Bourdenx M., Pampliega O., Cuervo A.M. Lancet Neurol. 2018;17(9):802–815. doi: 10.1016/s1474-4422(18)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song W., Soo Lee S., Savini M., Popp L., Colvin V.L., Segatori L. ACS Nano. 2014;8(10):10328–10342. doi: 10.1021/nn505073u. [DOI] [PubMed] [Google Scholar]
- 80.Wang P., Joberty G., Buist A., Vanoosthuyse A., Stancu I.C., Vasconcelos B., Pierrot N., Faelth-Savitski M., Kienlen-Campard P., Octave J.N., Bantscheff M., Drewes G., Moechars D., Dewachter I. Acta Neuropathol. 2017;133(5):731–749. doi: 10.1007/s00401-016-1663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Congdon E.E., Gu J., Sait H.B., Sigurdsson E.M. J. Biol. Chem. 2013;288(49):35452–35465. doi: 10.1074/jbc.M113.491001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ling D., Lee N., Hyeon T. Acc. Chem. Res. 2015;48(5):1276–1285. doi: 10.1021/acs.accounts.5b00038. [DOI] [PubMed] [Google Scholar]
- 83.Sun H., Zhong Y., Zhu X., Liao H., Lee J., Chen Y., Ma L., Ren J., Zhao M., Tu M., Li F., Zhang H., Tian M., Ling D. ACS Nano. 2021;15(3):5263–5275. doi: 10.1021/acsnano.0c10690. [DOI] [PubMed] [Google Scholar]
- 84.Karthivashan G., Ganesan P., Park S.Y., Kim J.S., Choi D.K. Drug Deliv. 2018;25(1):307–320. doi: 10.1080/10717544.2018.1428243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson B., Samanta M.K., Santhi K., Kumar K.P., Paramakrishnan N., Suresh B. Brain Res. 2008;1200:159–168. doi: 10.1016/j.brainres.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 86.Laroui H., Rakhya P., Xiao B., Viennois E., Merlin D. Dig. Liver Dis. 2013;45(12):995–1002. doi: 10.1016/j.dld.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang S., Su R., Nie S., Sun M., Zhang J., Wu D., Moustaid-Moussa N. J. Nutr. Biochem. 2014;25(4):363–376. doi: 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Desai N. AAPS J. 2012;14(2):282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu Y.L., Gao J.Q. Int. J. Pharm. 2010;394(1–2):115–121. doi: 10.1016/j.ijpharm.2010.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.