Abstract
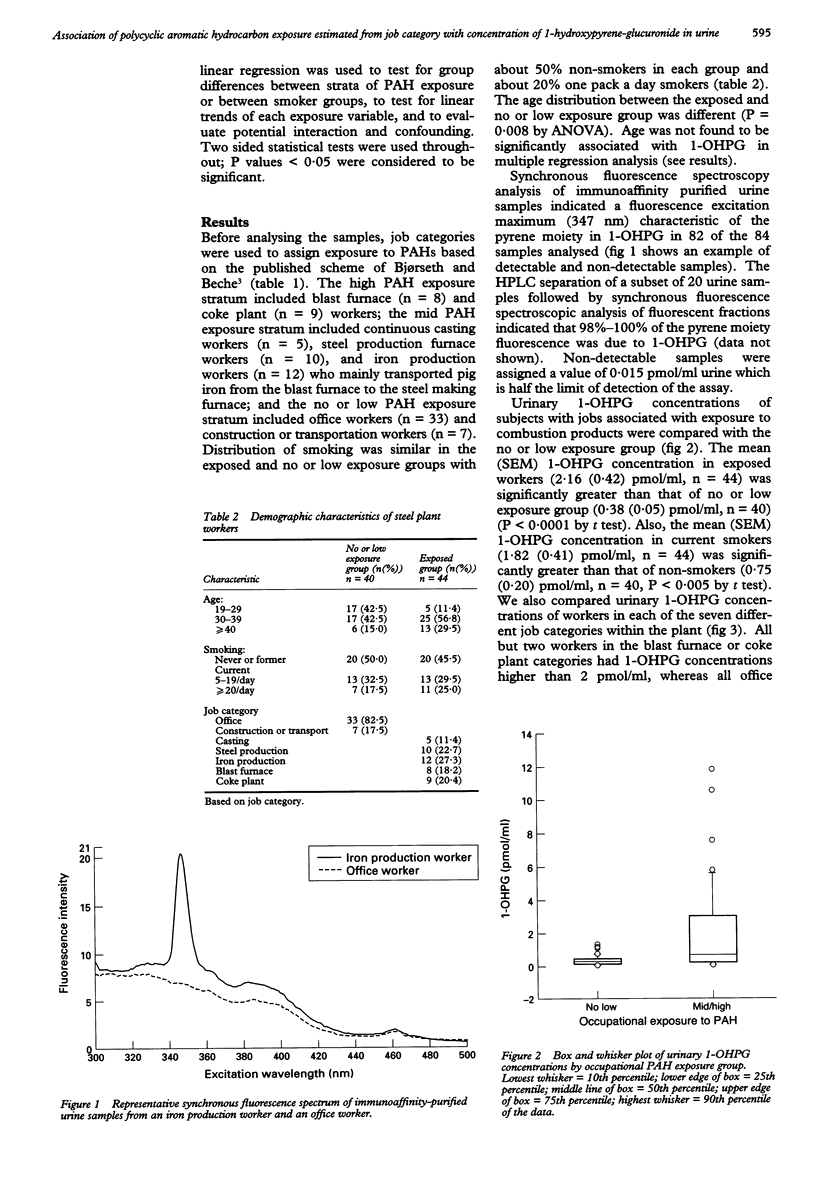
OBJECTIVES--Increased risk of lung cancer has been associated with employment in the steel industry. This association is thought to be due in part to increased concentrations of polycyclic aromatic hydrocarbons (PAHs) in air found in this work environment. Measurement of PAH metabolites in human urine provides a means of assessing individual internal dose of PAHs. This study examined the relative contribution of occupation and smoking to urinary concentration of 1-hydroxypyrene glucuronide (1-OHPG) among a group of workers at a steel plant. METHODS--Concentrations of 1-OHPG in urine from 44 workers with jobs associated with increased air concentrations of PAHs and 40 workers with jobs with low or no exposure to PAHs were measured. 20 workers in each group were not current smokers. Urinary 1-OHPG was measured by synchronous fluorescence spectroscopy after immunoaffinity chromatography specific for PAH metabolites. RESULTS--Mean (SEM) urinary 1-OHPG concentration was 2.16 (0.42) pmol/ml urine among the 44 occupationally exposed workers compared with 0.38 (0.05) among the 40 workers with no or low exposure (P < 0.0001). Mean urinary 1-OHPG concentration was 1.82 (0.41) pmol/ml urine among the 44 current smokers compared with 0.75 (0.20) among the 40 non-smokers (P < 0.005). Mean 1-OHPG concentrations in non-smokers were 0.26 (n = 20), 0.70 (n = 15), and 2.84 pmol/ml urine (n = 5) for strata of exposure to PAHs (no or low, mid, and high) based on job category; the corresponding values in smokers were 0.55 (n = 20), 0.94 (n = 12), and 4.91 pmol/ml (n = 12), respectively. Multiple linear regression showed significant differences between subjects in different PAH exposure with increased concentrations of 1-OHPG in urine. Amounts of foods containing PAHs ingested by this group of workers were relatively low and did not contribute significantly to urinary 1-OHPG concentrations. CONCLUSIONS--These results indicate that 1-OHPG is a common urinary metabolite in people with recent occupational exposure to PAHs and is associated with both job category and estimated stratum of PAH exposure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher G., Bjørseth A. Determination of exposure to polycyclic aromatic hydrocarbons by analysis of human urine. Cancer Lett. 1983 Jan;17(3):301–311. doi: 10.1016/0304-3835(83)90168-4. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Gennart J. P., Mercado-Calderon F., Delavignette J. P., Cupers L., Lauwerys R. Evaluation of exposure to polycyclic aromatic hydrocarbons in a coke production and a graphite electrode manufacturing plant: assessment of urinary excretion of 1-hydroxypyrene as a biological indicator of exposure. Br J Ind Med. 1992 Nov;49(11):761–768. doi: 10.1136/oem.49.11.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clonfero E., Zordan M., Venier P., Paleologo M., Levis A. G., Cottica D., Pozzoli L., Jongeneelen F. J., Bos R. P., Anzion R. B. Biological monitoring of human exposure to coal tar. Urinary excretion of total polycyclic aromatic hydrocarbons, 1-hydroxypyrene and mutagens in psoriatic patients. Int Arch Occup Environ Health. 1989;61(6):363–368. doi: 10.1007/BF00381025. [DOI] [PubMed] [Google Scholar]
- Fannick N., Gonshor L. T., Shockley J., Jr Exposure to coal tar pitch volatiles at coke ovens. Am Ind Hyg Assoc J. 1972 Jul;33(7):461–468. doi: 10.1080/0002889728506687. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Henderson P. T. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr. 1987 Jan 23;413:227–232. doi: 10.1016/0378-4347(87)80230-x. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Leijdekkers C. M., Bos R. P., Henderson P. T. 1-hydroxypyrene in human urine after exposure to coal tar and a coal tar derived product. Int Arch Occup Environ Health. 1985;57(1):47–55. doi: 10.1007/BF00383545. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Scheepers P. T., Bos R. P., Henderson P. T., Nijenhuis E. H., Veenstra S. J., Brouns R. M., Winkes A. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann Occup Hyg. 1988;32(1):35–43. doi: 10.1093/annhyg/32.1.35. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J. Biological exposure limit for occupational exposure to coal tar pitch volatiles at cokeovens. Int Arch Occup Environ Health. 1992;63(8):511–516. doi: 10.1007/BF00386338. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Bos R. P., Anzion R. B., Theuws J. L., Henderson P. T. Biological monitoring of polycyclic aromatic hydrocarbons. Metabolites in urine. Scand J Work Environ Health. 1986 Apr;12(2):137–143. doi: 10.5271/sjweh.2166. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Bos R. P. Excretion of pyrene and hydroxypyrene in urine. Cancer Lett. 1990 May 30;51(2):175–179. doi: 10.1016/0304-3835(90)90054-2. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Leijdekkers C. M., Bos R. P., Theuws J. L., Henderson P. T. Excretion of 3-hydroxy-benzo(a)pyrene and mutagenicity in rat urine after exposure to benzo(a)pyrene. J Appl Toxicol. 1985 Oct;5(5):277–282. doi: 10.1002/jat.2550050503. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Scheepers P. T., Groenendijk A., Van Aerts L. A., Anzion R. B., Bos R. P., Veenstra S. J. Airborne concentrations, skin contamination, and urinary metabolite excretion of polycyclic aromatic hydrocarbons among paving workers exposed to coal tar derived road tars. Am Ind Hyg Assoc J. 1988 Dec;49(12):600–607. doi: 10.1080/15298668891380312. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., van Leeuwen F. E., Oosterink S., Anzion R. B., van der Loop F., Bos R. P., van Veen H. G. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med. 1990 Jul;47(7):454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. H., Rothman N., Poirier M. C., Greenberg A., Hsu C. H., Schwartz B. S., Baser M. E., Groopman J. D., Weston A., Strickland P. T. Interindividual differences in the concentration of 1-hydroxypyrene-glucuronide in urine and polycyclic aromatic hydrocarbon-DNA adducts in peripheral white blood cells after charbroiled beef consumption. Carcinogenesis. 1995 May;16(5):1079–1085. doi: 10.1093/carcin/16.5.1079. [DOI] [PubMed] [Google Scholar]
- Keimig S. D., Kirby K. W., Morgan D. P., Keiser J. E., Hubert T. D. Identification of 1-hydroxypyrene as a major metabolite of pyrene in pig urine. Xenobiotica. 1983 Jul;13(7):415–420. doi: 10.3109/00498258309052279. [DOI] [PubMed] [Google Scholar]
- Omland O., Sherson D., Hansen A. M., Sigsgaard T., Autrup H., Overgaard E. Exposure of iron foundry workers to polycyclic aromatic hydrocarbons: benzo(a)pyrene-albumin adducts and 1-hydroxypyrene as biomarkers for exposure. Occup Environ Med. 1994 Aug;51(8):513–518. doi: 10.1136/oem.51.8.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella R. M., Hemminki K., Tang D. L., Paik M., Ottman R., Young T. L., Savela K., Vodickova L., Dickey C., Whyatt R. Polycyclic aromatic hydrocarbon-DNA adducts in white blood cells and urinary 1-hydroxypyrene in foundry workers. Cancer Epidemiol Biomarkers Prev. 1993 Jan-Feb;2(1):59–62. [PubMed] [Google Scholar]
- Santella R. M., Lin C. D., Cleveland W. L., Weinstein I. B. Monoclonal antibodies to DNA modified by a benzo[a]pyrene diol epoxide. Carcinogenesis. 1984 Mar;5(3):373–377. doi: 10.1093/carcin/5.3.373. [DOI] [PubMed] [Google Scholar]
- Sherson D., Sigsgaard T., Overgaard E., Loft S., Poulsen H. E., Jongeneelen F. J. Interaction of smoking, uptake of polycyclic aromatic hydrocarbons, and cytochrome P450IA2 activity among foundry workers. Br J Ind Med. 1992 Mar;49(3):197–202. doi: 10.1136/oem.49.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland P. T., Kang D., Bowman E. D., Fitzwilliam A., Downing T. E., Rothman N., Groopman J. D., Weston A. Identification of 1-hydroxypyrene glucuronide as a major pyrene metabolite in human urine by synchronous fluorescence spectroscopy and gas chromatography-mass spectrometry. Carcinogenesis. 1994 Mar;15(3):483–487. doi: 10.1093/carcin/15.3.483. [DOI] [PubMed] [Google Scholar]
- Van Rooij J. G., Van Lieshout E. M., Bodelier-Bade M. M., Jongeneelen F. J. Effect of the reduction of skin contamination on the internal dose of creosote workers exposed to polycyclic aromatic hydrocarbons. Scand J Work Environ Health. 1993 Jun;19(3):200–207. doi: 10.5271/sjweh.1322. [DOI] [PubMed] [Google Scholar]
- Venier P., Clonfero E., Cottica D., Gava C., Zordan M., Pozzoli L., Levis A. G. Mutagenic activity and polycyclic aromatic hydrocarbon levels in urine of workers exposed to coal tar pitch volatiles in an anode plant. Carcinogenesis. 1985 May;6(5):749–752. doi: 10.1093/carcin/6.5.749. [DOI] [PubMed] [Google Scholar]
- van Schooten F. J., Jongeneelen F. J., Hillebrand M. J., van Leeuwen F. E., de Looff A. J., Dijkmans A. P., van Rooij J. G., den Engelse L., Kriek E. Polycyclic aromatic hydrocarbon-DNA adducts in white blood cell DNA and 1-hydroxypyrene in the urine from aluminum workers: relation with job category and synergistic effect of smoking. Cancer Epidemiol Biomarkers Prev. 1995 Jan-Feb;4(1):69–77. [PubMed] [Google Scholar]



