Abstract
The murine coronavirus spike (S) protein contains a leucine zipper domain which is highly conserved among coronaviruses. To assess the role of this leucine zipper domain in S-induced cell-to-cell fusion, the six heptadic leucine and isoleucine residues were replaced with alanine by site-directed mutagenesis. The mutant S proteins were analyzed for cell-to-cell membrane fusion activity as well as for progress through the glycoprotein maturation process, including intracellular glycosylation, oligomerization, and cell surface expression. Single-alanine-substitution mutations had minimal, if any, effects on S-induced cell-to-cell fusion. Significant reduction in fusion activity was observed, however, when two of the four middle heptadic leucine or isoleucine residues were replaced with alanine. Double alanine substitutions that involved either of the two end heptadic leucine residues did not significantly affect fusion. All double-substitution mutant S proteins displayed levels of endoglycosidase H resistance and cell surface expression similar to those of the wild-type S. However, fusion-defective double-alanine-substitution mutants exhibited defects in S oligomerization. These results indicate that the leucine zipper domain plays a role in S-induced cell-to-cell fusion and that the ability of S to induce fusion may be dependent on the oligomeric structure of S.
Numerous studies have established that entry of enveloped virus into host cells requires membrane fusion between virus and host cell. For most animal viruses this fusion function is mediated by a single envelope glycoprotein on virions. The spike (S) protein is such a protein for murine coronaviruses (43). The S protein can be visualized by electron microscopy as peplomer projections from virions; each peplomer is thought to be composed of three oligomerized S protein molecules (16). Before being assembled into virion membranes, the S protein undergoes a complex posttranslational intracellular maturation process (8). The processing of S protein starts with cotranslational glycosylation of a newly synthesized S polypeptide in the endoplasmic reticulum (ER) into a 150-kDa form (8), which is later slowly oligomerized in the ER (47) and transported to the Golgi apparatus, where S is further processed into a 180-kDa endoglycosidase H (endo H)-resistant form (32). The S protein is subsequently cleaved by host cell proteases (21, 44) into two similarly sized subunits: S1 and S2. The C-terminal S2 subunit, which associates noncovalently with the N-terminal S1, anchors the S protein to the membrane through a transmembrane domain (14), while the S1 subunit contains the receptor binding activity of the S protein (9, 45).
During infection, not all processed S protein molecules are assembled into virions but instead some of them are transported by the host cell secretory system to the host cell surface. A similar processing route occurs for the recombinant S protein molecules expressed in the absence of other coronavirus proteins, using a vaccinia virus-based expression system (3, 15, 28). These non-virion-associated recombinant S protein molecules are capable of inducing cell-to-cell fusion, demonstrating that the S protein alone contains all the sequences that are necessary for its fusogenicity. Further analysis of the S protein sequence reveals that it has the structural features common to many other fusogenic viral glycoproteins, such as influenza virus HA, paramyxovirus F, and the human immunodeficiency virus (HIV) env protein (26). These features include the fact that S is a type I membrane protein and contains a fusion peptide-like region (28) as well as two heptad repeat regions in the membrane-anchored S2 subunit (10).
The shorter heptad repeat region of S is adjacent to the transmembrane domain and consists of a leucine zipper motif, which is highly conserved among coronaviruses (4). The leucine zipper motif, characterized as a sequence of leucine residues repeated every seven amino acids, was first described in DNA binding proteins such as c-Myc, c-Jun and GCN4 (27); it was shown to play an essential role in protein dimerization required for DNA binding (25, 39). Subsequently, leucine zipper-like motifs were also identified as domains in many viral glycoproteins (6, 17) and shown to be highly conserved among members of the same virus family. Since many fusogenic viral glycoproteins exist in oligomeric forms (18), it is conceivable that their leucine zipper domains provide an associative force for adjacent subunits to maintain a proper oligomeric structure. Studies of leucine zipper domains of several retroviral (12, 19, 38) and paramyxoviral (5, 40) fusion proteins reveal that they are all essential for viral infectivity and fusion. The sequence determinants of HIV env oligomerization have been mapped to its leucine zipper domain (2, 36). Moreover, this domain, when fused with the maltose-binding protein of Escherichia coli, is capable of conferring a tetrameric structure on this normally monomeric protein (42). To analyze the role of the leucine zipper motif in the structure and function of the S protein, we performed site-directed mutagenesis by substituting alanine for the heptadic leucine residues, either singly or in combinations of two residues. The effects on cell-to-cell membrane fusion, processing, and cell surface expression of such mutant S proteins were examined. Our results show that substitution of alanine for leucine residues, whether individually or in combination, had minimal, if any, effects on the processing and cell surface expression of the S protein. Double alanine substitutions that involved two of the four middle leucine residues abrogated the S-induced cell-to-cell fusion, while the ones that involved either of the two end leucine residues had minimal, if any, effects on cell-to-cell fusion. The loss of fusogenic activity in S appears to be correlated with defects in its oligomerization.
MATERIALS AND METHODS
Cell lines.
DBT cells (21) and BHK-21 cells (20) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (GIBCO/BRL) unless otherwise indicated.
Alanine substitution mutagenesis.
Mutagenesis was performed on plasmid pINT2, which contains the full-length mouse hepatitis virus (MHV) A59 spike gene (28). The mutant S plasmid clones were generated by oligonucleotide-directed PCR mutagenesis (1). The desired heptadic leucine- or isoleucine-to-alanine codon changes were incorporated into a PCR-amplified fragment by using the 5′ flanking primer wzl-15 (28) and the 3′ flanking primer wzl-43 (5′-GGGGGATCCAGGCCATTTCACATACATTTC-3′) and a series of mutagenic primer pairs (Table 1). The PCR fragments were digested with restriction enzymes MluI and NdeI and cloned into the corresponding sites of pINT2 to replace the corresponding wild-type fragments. For generating single leucine-to-alanine mutants, pINT2 was used as the template for PCR. Double-alanine-substitution mutants were generated by the same process as the single mutants except that the PCR templates were plasmids containing corresponding single leucine-to-alanine codon substitutions instead of pINT2. The presence of targeted mutations in all plasmid constructs was verified by DNA sequencing.
TABLE 1.
Oligonucleotides for alanine substitution PCR mutagenesis
| Namea | Positionb | Sequencec | Mutationd |
|---|---|---|---|
| wz167A | 3628–3669 | CAGACGTCTATTGCGCCTGATgcATCTCTCGATTTCGAGAAG | L1217A |
| wz167B | 3651–3628 | TgcCTAGGACGCAATAGACGTCTG | |
| wz168A | 3652–3690 | TCTCTCGATTTCGAGAAGgcAAATGTTACTTTGCTGGAC | L1224A |
| wz168B | 3672–3652 | TgcCTTCTCGAAATCGAGAGA | |
| wz169A | 3673–3711 | AATGTTACTTTGCTGGACgcGACGTATGAGATGAACAGG | L1231A |
| wz169B | 3693–3673 | CgcGTCCAGCAAAGTAACATT | |
| wz170A | 3694–3732 | ACGTATGAGATGAACAGGgcTCAGGATGCAATTAAGAAG | I1238A |
| wz170B | 3714–3694 | AgcCCTGTTCATCTCATACGT | |
| wz171A | 3715–3753 | CAGGATGCAATTAAGAAGgcAAATGAGAGCTACATCAAC | L1245A |
| wz171B | 3735–3715 | TgcCTTCTTAATTGCATCCTG | |
| wz172A | 3736–3774 | AATGAGAGCTACATCAACgcCAAGGAAGTTGGCACATAT | L1252A |
| wz172B | 3756–3736 | GgcGTTGATGTAGCTCTCATT |
Two partially complementary primers (A and B) were used in each PCR mutagenesis for the introduction of alanine codon substitutions for heptadic leucine and isoleucine codons.
The region of the MHV-A59 S sequence in each oligonucleotide is marked by the positions of the 5′ and 3′ nucleotides separated by a dash. The position of the 5′ nucleotide is shown first for each oligonucleotide. All positions are numbered in reference to the first nucleotide of the S coding sequence.
Oligonucleotide sequences are shown starting with the 5′ nucleotide. Capital letters indicate that the sequence is exactly the same as the wild-type S DNA sequence. Lowercase letters indicate the mismatched nucleotides designed for specific mutations.
The designated amino acid substitutions resulting from using each mutagenic primer pair.
Cell-to-cell fusion assay.
A cell-to-cell fusion assay with β-galactosidase (β-Gal) as the reporter enzyme to indicate the level of fusion was performed as previously described (28). Briefly, the donor group of DBT cells were infected with vaccinia virus vTF7-3 (22) and then transfected with plasmids containing either wild-type or mutant S genes by using Lipofectamine (GIBCO/BRL). The recipient group of DBT cells were transfected with plasmid pG1NTβGal (33). After 4 h of transfection, the donor cells were washed once with DMEM and then resupplied with DMEM. The recipient cells were trypsinized, washed once with DMEM, resuspended in DMEM with 2% fetal bovine serum, plated on top of the donor cell monolayer, and incubated overnight. A 3:1 excess of recipient cells to donor cells was used to ensure that donor cells fused with the surrounding recipient cells. The cell monolayers were lysed in 1% NP-40. Equal volumes of cell lysates and the chlorophenol red–β-d-galactopyranoside substrate solution (28) were mixed, and β-Gal activity was determined from the substrate hydrolysis rate, measured with purified E. coli β-Gal (Boehringer Mannheim) as a standard. At least three independent experiments were performed for each mutant S gene, and triplicates were assayed for each experiment. The standard deviations among different experiments were less than 25%.
Infection and transfection.
DBT cells were seeded in T25 flasks (Falcon) at 106 per flask 40 h before being infected with vTF7-3 at 5 PFU/cell. After 1 h of infection at 37°C, the cells were washed once with DMEM and subjected to SuperFect (Qiagen)-mediated transfection. For each sample, 10 μl of SuperFect and 8 μg of plasmid DNA were mixed in 200 μl of Opti-MEM (GIBCO/BRL) and incubated at room temperature for 30 min. The mixtures were diluted to 2 ml with Opti-MEM prior to addition to the cell monolayers. After a 4-h incubation at 37°C, the cells were refed with 2 ml of fresh Opti-MEM and incubated at 37°C for 2 h before further analysis.
Metabolic labeling, immunoprecipitation, and endo H analysis.
DBT cells were infected with vTF7-3 and transfected with plasmids containing either wild-type or mutant S genes as described above. The cells were washed once with methionine- and cysteine-free DMEM and incubated for 1 h at 37°C with 2 ml of methionine- and cysteine-free DMEM containing 20 μl of 35S Express protein labeling mix (110 μCi/ml; Dupont NEN). The cells were then refed with 2 ml of Opti-MEM containing nonradioactive methionine and cysteine (2 mM each) and incubated for 2 h before lysis with 0.7 ml of lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% NP-40, 0.5% sodium deoxycholate, 10 mM phenylmethylsulfonyl fluoride). Cell debris and nuclei were cleared from the lysates by centrifugation for 10 min at 13,000 × g at 4°C. The supernatants were collected into fresh Eppendorf tubes and stored at −80°C. Immunoprecipitation of the radiolabeled S proteins and subsequent endo H analysis were performed as previously described (28).
Surface expression of S proteins.
BHK-21 cells were infected with vTF7-3 and transfected with plasmids containing either wild-type or mutant S genes as described above. Cell surface expression of S proteins was examined by flow cytometry analysis as previously described (28).
Sucrose gradient analysis.
Sedimentation in sucrose gradients was used to examine the oligomeric status of the S proteins. DBT cells were infected with vTF7-3 and transfected with plasmids containing either wild-type or mutant S genes as described above. The cells were washed once and incubated with DMEM free of methionine and cysteine for 20 min. The cells were then refed with 2 ml of methionine- and cysteine-free DMEM containing 20 μl of 35S Express protein labeling mix and incubated for 20 min. The cells were then incubated with 2 ml of DMEM containing nonradioactive methionine and cysteine (2 mM each) and incubated for 2 h before lysis with Triton X-100 lysis buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA [pH 8.0], 150 mM NaCl, 1% Triton X-100). Cell lysates containing equal amounts of radioactive label (107 trichloroacetic acid-precipitable cpm) were layered on top of linear 5 to 20% sucrose gradients prepared in sucrose gradient buffer (50 mM Tris [pH 7.5], 5 mM EDTA [pH 8.0], 150 mM NaCl, 0.1% Triton X-100). The gradients were centrifuged for 17 h at 35,000 rpm at 4°C with an SW41 rotor (Beckman). A total of 18 fractions were collected for each gradient. The radiolabeled S proteins in each fraction were immunoprecipitated with 2 μl of anti-S AO4 serum as previously described (28).
RESULTS
Mutagenesis of heptadic leucine and isoleucine residues by alanine substitution.
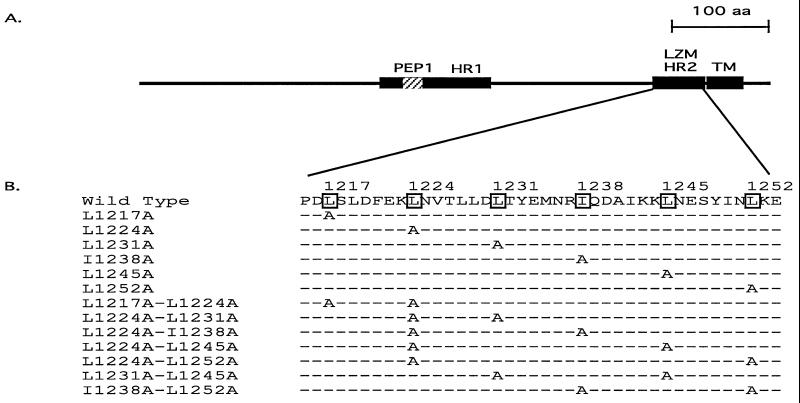
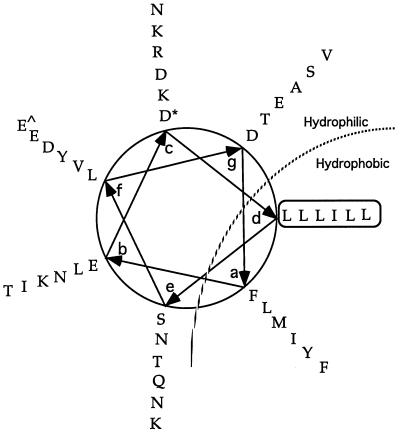
The leucine zipper domain of the MHV-A59 S protein is located adjacent to the transmembrane domain (Fig. 1A) and contains six heptadic leucine and isoleucine residues, with isoleucine at the fourth heptadic position and leucine at the other five heptadic positions (Fig. 1B) (For simplicity, they will be referred to hereafter as heptadic leucine residues.) This region is highly conserved, as previously reported (4). Modeling this region into an alpha helix reveals a typical amphipathic structure (Fig. 2). The a and d positions in the amphipathic helix are occupied respectively by a chain of bulky hydrophobic amino acid residues (FLMIYF) and the heptadic leucine chain (LLLILL), forming the putative hydrophobic face of the helix. The hydrophilic face of the helix contains two amino acid chains consisting of only charged and polar amino acid residues at positions c (NKRDKD) and e (SNTQNK). To analyze the role of the leucine zipper domain in S-induced cell-to-cell fusion, alanine substitution mutagenesis was performed on the heptadic leucine residues (LLLILL). The reasons we chose alanine over other amino acids as a substituting residue for leucine are (i) alanine has a strong propensity to form an alpha helix and its substitution has been documented to have minimal disruptive effects on the alpha-helical structure characteristic of a leucine zipper domain in proteins or model synthetic peptides (23, 31, 34, 52) and (ii) alanine lacks the bulky hydrophobic side chain of a leucine residue, which is believed to be the major hydrophobic force for a leucine zipper domain to facilitate protein interaction (35). Thus, replacement of heptadic leucine residues with alanine would likely influence only the biological properties derived from the bulky hydrophobic side chain of a leucine residue while having minimal effects on the overall alpha-helical structure. In several studies, alanine substitution mutagenesis has also been used successfully to analyze the role of leucine zipper domains in membrane fusion and virus entry mediated by the corresponding viral glycoproteins (19, 37, 38, 40, 51).
FIG. 1.
The leucine zipper domain of the MHV-A59 S protein. (A) Schematic diagram of MHV-A59 S2 subunit showing the relative location of the leucine zipper domain in relation to other distinctive domains in S2. The thin black line represents the entire S2 region. Distinctive domains are shown as rectangles with their names above. LZM, leucine zipper domain (4); HR1 and HR2, heptad repeat regions 1 and 2 (14); TM, transmembrane domain (30); PEP1, fusion peptide-like region (28). The region representing PEP1 is hatched since it is part of HR1. LZM and HR2 represent the same region of MHV-A59 S. (B) Amino acid sequence of the leucine zipper domain of wild-type and mutant S proteins. The heptadic leucine and isoleucine residues are boxed, and their corresponding positions are marked above in reference to the starting methionine residue of the S protein. The leucine zipper domain sequences of single- or double-alanine-substitution mutants are listed below the wild-type sequence. Leucine- or isoleucine-to-alanine substitutions in those mutants are indicated by the letter A, while the dashed lines represent sequences identical to that of the wild-type S. aa, amino acids.
FIG. 2.
Helical-wheel representation of the leucine zipper domain of the MHV-A59 S protein. The leucine zipper domain of the MHV-A59 S protein is modeled as an alpha-helical structure. The starting and ending amino acid residues in the helical wheel are marked with “∗” and “^,” respectively. The heptadic leucine and isoleucine residues are boxed. The amphipathic nature of the helix is indicated by a dotted line with putative hydrophilic and hydrophobic sides marked.
All six heptadic leucine residues in MHV-A59 S were initially replaced with alanine individually (L1217A, L1224A, L1231A, I1238A, L1245A, and L1252A [Table 1]). Since a heptad repeat of three consecutive leucine residues was found to be sufficient to assemble Lac repressor dimers into tetramers via formation of a shortened coiled coil (11), it seemed likely that single leucine-to-alanine substitutions might be insufficient to prevent the functioning of the leucine zipper domain. Thus, we carried out substitution of two heptadic leucine residues concomitantly with alanine (L1217A-L1224A, L1224A-L1231A, L1224A-I1238A, L1224A-L1245A, L1224A-L1252A, L1231A-L1245A, and I1238A-L1252A [Table 1]). The resulting double-substitution mutants generated a variety of heptadic leucine patterns that contain two to four consecutive heptadic leucine residues. The positions of all the mutations generated are indicated in Fig. 1.
Substitution of a single heptadic leucine residue exhibited minimal effects on S-induced cell-to-cell fusion.
We initially measured the effects of single alanine substitutions on S-induced cell-to-cell fusion. The level of fusion was measured both quantitatively and qualitatively with a previously described fusion assay, using E. coli β-Gal as a reporter enzyme to reflect the extent of fusion (28). The relative level of β-Gal activity among mutant S proteins, when expressed as a percentage of the β-Gal activity obtained from the wild-type S protein, was approximately proportional to the level of cell-to-cell fusion as reflected by the size of the syncytium under a microscope. The fusion-negative phenotype corresponded to a percentage of less than 10%, while a fusion phenotype similar to that of the wild-type S had percentages over 70%. Intermediate fusion phenotypes were reflected by percentages between 10 and 70%. Quantitation of the β-Gal activities for the six single-alanine-substitution mutants showed that they all retained more than 80% of the β-Gal activity measured for the wild-type S protein, indicating that single substitutions had minimal effects on S fusogenicity (Table 2). Further examination, under the microscope, of the syncytium formation of these mutants revealed that there was little difference in terms of the syncytium size produced by mutant and wild-type S proteins (data not shown). The results indicate that the S protein can tolerate single alanine substitution for any heptadic leucine residue without significantly affecting its fusogenicity.
TABLE 2.
Fusion activity and cell surface expression of leucine zipper mutant S glycoproteins
| Name | Substitutiona | Fusion activityb | Oligomerizationc | Endo H resistanced | Surface expressione |
|---|---|---|---|---|---|
| Wild type | LLLILL | 100 ± 19 | + | + | 100 ± 16 |
| L1217A | ALLILL | 91 ± 15 | ND | + | ND |
| L1224A | LALILL | 81 ± 12 | ND | + | ND |
| L1231A | LLAILL | 89 ± 17 | ND | + | ND |
| I1238A | LLLALL | 84 ± 9 | ND | + | ND |
| L1245A | LLLIAL | 87 ± 16 | ND | + | ND |
| L1252A | LLLILA | 96 ± 11 | ND | + | ND |
| L1217A-L1224A | AALILL | 64 ± 11 | + | + | 94 ± 12 |
| L1224A-L1231A | LAAILL | 8 ± 3 | − | + | 78 ± 15 |
| L1224A-I1238A | LALALL | 19 ± 5 | − | + | 75 ± 10 |
| L1224A-L1245A | LALIAL | 7 ± 3 | − | + | 83 ± 14 |
| L1224A-I1252A | LALILA | 81 ± 18 | + | + | 80 ± 13 |
| I1231A-L1245A | LLAIAL | 9 ± 2 | − | + | 73 ± 11 |
| I1238A-L1252A | LLLALA | 103 ± 21 | + | + | 69 ± 14 |
Heptadic leucine and isoleucine residues in the leucine zipper domain of MHV-A59 S protein are shown. Alanine substitutions are indicated for each leucine zipper mutant.
Reported as the percentage of the β-Gal to be activity produced in samples expressing wild-type S protein, which is considered to be 100% (see Materials and Methods). All data are averages from at least three experiments, each of which was performed in triplicate analysis. The standard deviations are also shown.
Oligomerization status as determined by sucrose gradient assay. +, oligomeric forms of S were detected; −, oligomeric forms of S were missing for the respective leucine zipper mutants. Representative oligomerization profiles are shown in Fig. 4. ND, not determined.
Glycosylation status as determined by endo H resistance assay as shown in Fig. 3. +, S protein was endo H resistant.
Reported as the percentage of the mean fluorescence intensity values compared with those of samples expressing the wild-type S protein after subtracting background values obtained for mock-transfected samples. Experiments were repeated twice, with a standard deviation of less than 20%. ND, not determined.
Substitution of alanine for two heptadic leucine residues concomitantly exhibited variable effects on S-induced cell-to-cell fusion.
Examination of seven different double-alanine-substitution mutants by the β-Gal fusion assay resulted in three types of fusion phenotypes (Table 2). Three of the above-mentioned mutants (L1217A-L1224A, L1224A-L252A, and I1238A-L1252A) had fusion phenotypes similar to that of the wild-type S protein, while L1217A-L1224A resulted in slightly less fusion. Three other mutants (L1224A-L1231A, L1224A-L1245A, and I1238A-L1245A) completely lost the ability to induce syncytium formation, as indicated by the minimal levels of β-Gal activity. Mutant L1224A-I1238A exhibited a greatly reduced level of fusion activity; the syncytia it induced were very small. Closer scrutiny of the leucine-to-alanine substitution patterns in these mutants revealed that all three fusion-positive double-substitution mutants involved the substitution of heptadic leucine residues at either end of the leucine zipper domain (L1217 and L1252). However, drastic reduction of S fusion activity occurred when two of the other four middle heptadic leucine residues were simultaneously replaced with alanine. Thus, the results suggest that the four middle heptadic leucine residues may be the backbone in maintaining the proper leucine zipper structure necessary for S fusogenicity.
Alanine substitutions did not affect the ability of S to acquire endo H resistance.
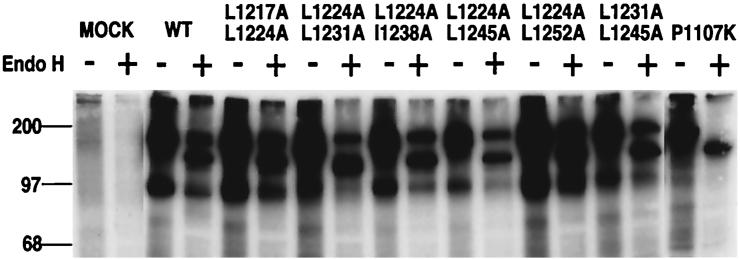
To analyze whether the mutant S proteins undergo intracellular maturation similar to that of the wild-type S protein, they were expressed in cells by using a vaccinia virus-based expression system and the resistance to endo H digestion was measured. Endo H resistance is acquired through modification of the oligosaccharide portion of the glycoproteins in the medial Golgi apparatus (24). It is believed to be an important parameter in measuring whether the transport of glycoproteins from the ER to the Golgi is accomplished. Proteins that otherwise are misfolded, misassembled, or processed aberrantly usually fail to escape from the ER due to a cellular quality control system (18), making these proteins unable to gain endo H resistance in the Golgi. Thus, the acquisition of endo H resistance is an indication that the glycoproteins are folded and processed correctly.
Cells expressing wild-type and mutant S proteins were labeled with [35S]methionine and [35S]cysteine and chased with nonradioactive methionine and cysteine before being subjected to lysis. S proteins were immunoprecipitated from the lysates with anti-S AO4 serum, incubated with or without endo H, and examined by SDS-polyacrylamide gel electrophoresis (PAGE). A portion of wild-type S protein became endo H resistant after a 2-h chase (Fig. 3), consistent with previous observations (3, 28). The incomplete acquisition of endo H resistance is probably due to the low rate of processing of coronavirus S, when expressed with a vaccinia virus-based system (46). The double-alanine-substitution mutants, whether fusion positive or negative, all displayed endo H profiles similar to that of the wild type (Fig. 3), suggesting that these mutant proteins were processed in a manner similar to that of the wild-type S. Thus, it is unlikely that the loss of fusogenicity of four double-alanine-substitution mutants (L1224A-L1231A, L1224A-L1245A, I1238A-L1245A, and L1224A-I1238A) was a direct result of a gross conformational difference between the mutant S proteins and the wild-type S. As expected, the endo H profiles of all single-alanine-substitution mutants were similar to that of the wild-type S protein (data not shown). P1107K, an endo H-sensitive mutant S protein previously described (28), was included as a negative control (Fig. 3).
FIG. 3.
Endo H resistance of double-alanine-substitution mutants. Cells expressing the wild-type or mutant S proteins were labeled with [35S]methionine and [35S]cysteine and then chased with excessive nonradioactive methionine and cysteine. The cells were subsequently lysed, and the lysates were subjected to immunoprecipitation with goat anti-S serum AO4. The immunoprecipitated S proteins were split into two aliquots, incubated overnight either with (+) or without (−) endo Hf, subjected to SDS-PAGE, and detected by autoradiography. The name of each mutant analyzed for endo H resistance is indicated at the top of its lane. An endo H-sensitive mutant S protein, P1107K (28), was included as a negative control. The positions of molecular weight markers are indicated on the left.
Alanine substitutions had little effect on the cell surface expression of S.
To examine whether the loss of fusogenicity of mutant S proteins was due to reduced levels of cell surface expression of those proteins, cell sorting flow cytometry (fluorescence-activated cell sorter) analysis was applied to cells expressing wild-type or mutant S proteins. The levels of mutant S proteins that were transported on the cell surface were indicated by the mean surface fluorescence intensity value for each mutant as a percentage of that of the wild-type S after subtracting the background (28). As shown in Table 2, fusion-positive and fusion-negative double-alanine-substitution mutants exhibited similar levels of surface expression, although their levels were slightly lower than that of the wild-type S. These results demonstrate that the significant reduction in the ability to induce fusion by certain double-alanine-substitution mutants is not due to an insufficient amount of S proteins available on the cell surface.
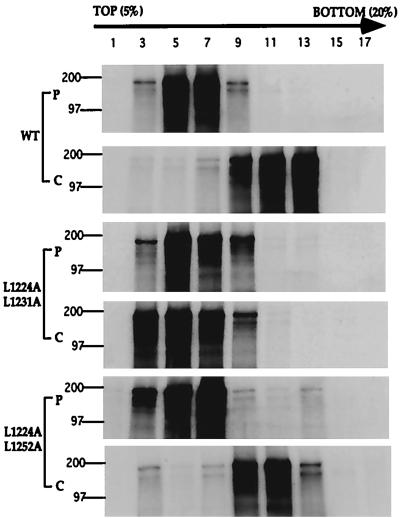
Mutant S proteins that were fusion negative exhibited defects in oligomerization.
To determine whether mutations in the leucine zipper domain affect the oligomerization of S, the mutant S proteins were assayed by sucrose gradient centrifugation. Cells were infected with vTF7-3, transfected with plasmids encoding either the wild-type or one of the mutant S proteins, subjected to pulse-chase labeling with [35S]methionine and [35S]cysteine, and subsequently lysed. The lysates were layered on 5 to 20% sucrose gradients, and 18 fractions were collected after centrifugation. The radiolabeled S proteins in the fractions were immunoprecipitated with anti-S AO4 serum, subjected to SDS-PAGE, and detected by autoradiography. As shown in Fig. 4A, after 20 min of pulse labeling, the wild-type S proteins were detected primarily in fractions 5 and 7. After a 2-h chase with nonradioactive methionine and cysteine, the wild-type S proteins were detected primarily in fractions 11 and 13, indicating S proteins were converting from a monomeric form into an oligomeric form (Fig. 4B). The fusion-positive mutant L1224A-L1252A showed a gradient shift similar to that of the wild-type S after a 2-h chase (Fig. 4E and F), indicating that it is oligomerized similarly to the wild-type S. However, the fusion-negative mutant L1224A-L1231A did not display a similar shift in the gradient after a 2-h chase, suggesting that the mutant S proteins had a defect in oligomerization (Fig. 4C and D). Sucrose gradient analyses were also carried out on all other fusion-negative mutant S proteins; the proteins were detected by Western blotting, and the results were consistent with those from the pulse-chase labeling experiments (data not shown). These findings demonstrate that the loss of S fusogenicity is correlated with the absence of oligomeric forms of S, as measured by this technique (Table 2).
FIG. 4.
Sucrose gradient analysis of the wild-type and mutant S proteins. Cells were infected with vTF7-3 and transfected with plasmids containing either wild-type or mutant S genes. Cell lysates were prepared either after the cells were pulse labeled with [35S]methionine and [35S]cysteine or after the cells were pulse labeled and chased with nonradioactive methionine and cysteine. The cell lysates containing equal amounts of trichbroacetic acid-precipitable cpm were subsequently layered on top of the 5 to 20% sucrose gradients and subjected to centrifugation. The sucrose gradients were fractionated, and the proteins in the gradient fractions were immunoprecipitated with anti-S AO4 antibody, subjected to SDS-PAGE, and detected by autoradiography (see Materials and Methods). The sucrose gradient profiles of cell lysates containing the wild-type S protein (WT), the fusion-negative mutant S protein (L1224A-L1231A), and the fusion-positive mutant S protein (L1224A-L1252A) are shown as representative samples. P and C, profiles from the pulse labeling and from the pulse labeling followed by a chase with nonradioactive methionine and cysteine, respectively. The lanes contain samples from the odd-numbered sucrose gradient fractions. Fraction 1 represents the top of the gradient, while fraction 17 represents the bottom.
DISCUSSION
Despite numerous studies of the relationship of coronavirus S protein structure and S-induced membrane fusion, little was known about the possible role of the highly conserved leucine zipper domain in cell-to-cell fusion (4). To characterize this distinctive structural feature, we performed alanine substitution mutagenesis on heptadic leucine residues. Several possible factors that may indirectly affect the fusogenicity of S, including cell surface expression, acquisition of endo H resistance, and oligomerization, were also analyzed. The mutagenesis results indicate that the leucine zipper domain is critical for S to accomplish its cell-to-cell fusion function and that the fusion function may be dependent on the oligomeric structure of S that is maintained by the leucine zipper domain.
Although alanine substitutions within the leucine zipper domain had only minimal, if any, effects on the acquisition of endo H resistance and the cell surface expression of S, significant effects on its fusogenicity and oligomerization were observed with some of the analyzed mutants. While single alanine substitutions had little effect on the ability of S to induce cell-to-cell fusion, substitution of at least two heptadic leucine residues (L1224A-L1231A, L1224A-I1238A, L1224A-L1245A, and L1231A-L1245A) was able to dramatically reduce S-induced cell-to-cell fusion. However, not all leucine residues were of equal importance in affecting S fusogenicity, since some double-alanine-substitution mutants (L1217A-L1224A, L1224A-L1252A, and I1238A-L1252A) displayed wild-type levels of cell-to-cell fusion activity. Further comparison of substitution patterns between fusion-positive and fusion-negative mutants indicates that the two leucine residues (L1227 and L1252) at the ends of the leucine zipper domain were much more tolerant of alanine substitution than the ones in the middle. It appears that these two leucine residues are dispensable and the four middle heptadic leucine residues are sufficient to maintain the fusion activity of the wild-type S protein. Analysis of similar alanine substitution mutants within the leucine zipper domain of the paramyxovirus F protein has revealed similar results in that substitution of at least two heptadic leucine residues is necessary to abrogate fusion activity, although it did not exhibit a position-dependent effect, since such abrogation of fusion activity occurred for any pair of heptadic leucine residues replaced by alanine (40). In HIV gp41, the leucine zipper domain is more sensitive to alanine substitution, as single replacement was able to eliminate fusion activity (19, 37).
Since heptadic leucine residues are pivotal in contributing to the oligomeric function of a leucine zipper domain, we examined whether the loss of fusion is associated with any changes in the oligomerization of S. The sedimentation profiles of fusion-negative double-alanine-substitution mutants indicate that fusion incompetence is correlated with the loss of oligomeric forms of S. One explanation for the loss of oligomerization may be that substitution of alanine for two heptadic leucines significantly weakens the leucine zipper-driven hydrophobic interaction between individual subunits so that individual S protein molecules are not able to oligomerize. Alternatively, S proteins may still oligomerize during intracellular maturation but the oligomerization force provided by the leucine zipper domain may be weak, such that oligomeric S dissociates into individual molecules during the sucrose gradient assay. The concomitant loss of oligomerization and fusion activity suggests that S may require a proper oligomeric structure in order to be fusogenic.
A somewhat surprising result of this study is that mutant S proteins that are defective in oligomerization exhibit levels of endo H resistance and cell surface expression similar to that of the wild-type S. In a previous study of two temperature-sensitive mutants of MHV-A59 (29), the S proteins encoded by these mutants were retained in the ER and were both defective in oligomerization and sensitive to endo H treatment at the restrictive temperature. It is likely that these mutant S proteins do not fold correctly, contributing to the lack of oligomerization. The sensitivity of these mutant S proteins to endo H digestion is probably caused by the inability to be transported from the ER to the Golgi, where endo H resistance is acquired. However, the alanine substitution mutants described in this paper may be able to fold correctly and proceed from the ER to the Golgi, gain endo H resistance, and be transported to the cell surface to complete the maturation process. Consistent with our findings, Delmas and Laude (16), in their studies of the oligomerization of porcine coronavirus transmissible gastroenteritis virus S protein, reported that monomeric endo H-resistant forms of S were readily detectable in mature virions. Thus, defects in S oligomerization may not be sufficient to prevent coronavirus S proteins from completing the maturation process.
Studies of other viral glycoproteins with mutations in the leucine zipper domain indicate that the loss of fusogenicity is not always accompanied by the loss of oligomerization. Fusion-negative double-alanine-substitution mutants in the leucine zipper domain of the paramyxovirus Newcastle disease virus F protein exhibit wild-type-like sedimentation profiles in sucrose gradients (40). Among alanine substitution mutants in the leucine zipper domain of HIV gp41 that affect fusion, only some display defects in oligomerization during intracellular processing (19, 37). More recent studies reveal that the leucine zipper domain participates in the oligomerization of HIV gp41 and that this region is sufficient to confer oligomerization of a naturally monomeric protein (2, 42, 48). Another study of the HIV gp41 leucine zipper domain suggests that there may be two separate oligomerization events: during intracellular maturation and during fusion pore formation (50). The leucine zipper domain, as part of the heptad repeat region, participates in the formation of a coiled-coil structure which serves as an associative force for recruiting individual glycoproteins to form a functional fusion pore (49). Disruption of this associative force, caused by amino acid substitutions at certain positions within the leucine zipper domain, would prevent the formation of the fusion pore structure, whereas oligomerization during intracellular processing may or may not be affected by the same substitution(s). The loss of MHV-A59 S oligomerization in fusion-negative mutants may indicate that the leucine zipper domain plays a more significant role in oligomerization during intracellular processing than the domains of HIV gp41 and paramyxovirus F protein and thus is more sensitive to alanine substitution. Alternatively, the two oligomerization roles suggested for HIV gp41 and paramyxovirus F may be less clearly separated by mutagenesis in the case of the coronavirus S protein.
It is worth noting that leucine zipper domains as heptad repeat sequences may directly participate in disruption of the membrane bilayer structure along with fusion peptides (40). Heptad repeat sequences are known to form amphipathic helices that are characteristic of some natural fusogenic peptides (41). Accumulating experimental evidence appears to favor the involvement of multiple domains of viral fusion proteins in the fusion process; these domains include fusion peptides; heptad repeat sequences, including the leucine zipper domain; and transmembrane domains (7, 13). Our previous study of MHV-A59 S (28) indicates that a fusion peptide domain (designated PEP1 in Fig. 1A) may be present not as a topologically independent domain as reported for other viral glycoproteins but as part of the longer heptad repeat region of S (14). The results presented in this study support the view that multiple regions of S act together as an integrated fusion machinery to bring about membrane fusion.
ACKNOWLEDGMENTS
This work was supported by NIH grants NS-21954 and NS-30606. Zongli Luo was supported in part by NIH training grant T32 AI07324.
We thank Kathryn Holmes for the AO4 antiserum and Bernard Moss for the vTF7-3 vaccinia virus and plasmid pG1NTβGal. We thank Paul Bates, Henry Teng, and Joanna Philips for comments on the manuscript.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley-Interscience; 1989. [Google Scholar]
- 2.Bernstein H B, Tucker S P, Kar S R, McPherson S A, McPherson D T, Dubay J W, Lebowitz J, Compans R W, Hunter E. Oligomerization of the hydrophobic heptad repeat of gp41. J Virol. 1995;69:2745–2750. doi: 10.1128/jvi.69.5.2745-2750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos E C W, Heunen L, Luytjes W, Spaan W J M. Mutational analysis of the murine coronavirus spike protein: effect on cell-to-cell fusion. Virology. 1995;214:453–463. doi: 10.1006/viro.1995.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton P. Coronavirus motif. Nature. 1991;353:394. doi: 10.1038/353394a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckland R, Malvoisin E, Beauverger P, Wild F. A leucine zipper structure present in the measles virus fusion protein is not required for its tetramerization but is essential for fusion. J Gen Virol. 1992;73:1703–1707. doi: 10.1099/0022-1317-73-7-1703. [DOI] [PubMed] [Google Scholar]
- 6.Buckland R, Wild F. Leucine zipper motif extends. Nature. 1989;338:547. doi: 10.1038/338547a0. [DOI] [PubMed] [Google Scholar]
- 7.Caballero M, Carabana J, Ortego J, Fernandez-Munoz R, Celma M L. Measles virus fusion protein is palmitoylated on transmembrane-intracytoplasmic cysteine residues which participate in cell fusion. J Virol. 1998;72:8198–8204. doi: 10.1128/jvi.72.10.8198-8204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanagh D. The coronavirus surface glycoprotein. In: Siddell S G, editor. The coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 73–113. [Google Scholar]
- 9.Cavanagh D, Davis P J, Darbyshire J H, Peters R W. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or hemagglutination-inhibiting antibody, or induce chicken tracheal protection. J Gen Virol. 1986;67:1435–1442. doi: 10.1099/0022-1317-67-7-1435. [DOI] [PubMed] [Google Scholar]
- 10.Chambers P, Pringle C R, Easton A J. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J Gen Virol. 1990;71:3075–3080. doi: 10.1099/0022-1317-71-12-3075. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Surendran R, Lee J C, Matthews K S. Construction of a dimeric repressor: dissection of subunit interfaces in Lac repressor. Biochemistry. 1994;33:1234–1241. doi: 10.1021/bi00171a025. [DOI] [PubMed] [Google Scholar]
- 12.Chen S S, Lee C N, Lee W R, McIntosh K, Lee T H. Mutational analysis of the leucine zipper-like motif of the human immunodeficiency virus type 1 envelope transmembrane glycoprotein. J Virol. 1993;67:3615–3619. doi: 10.1128/jvi.67.6.3615-3619.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleverley D Z, Lenard J. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc Natl Acad Sci USA. 1998;95:3425–3430. doi: 10.1073/pnas.95.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Groot R J, Luytjes W, Horzinek M C, van der Zeijst B A, Spaan W J, Lenstra J A. Evidence for a coiled-coil structure in the spike proteins of coronaviruses. J Mol Biol. 1987;196:963–966. doi: 10.1016/0022-2836(87)90422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Groot R J, Van Leen R W, Dalderup M J, Vennema H, Horzinek M C, Spaan W J. Stably expressed FIPV peplomer protein induces cell fusion and elicits neutralizing antibodies in mice. Virology. 1989;171:493–502. doi: 10.1016/0042-6822(89)90619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delmas B, Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J Virol. 1990;64:5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delwart E L, Mosialos G, Gilmore T. Retroviral envelope glycoproteins contain a “leucine zipper”-like repeat. AIDS Res Hum Retroviruses. 1990;6:703–706. doi: 10.1089/aid.1990.6.703. [DOI] [PubMed] [Google Scholar]
- 18.Doms R W, Lamb R A, Rose J K, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 19.Dubay J W, Roberts S J, Brody B, Hunter E. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J Virol. 1992;66:4748–4756. doi: 10.1128/jvi.66.8.4748-4756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dveksler G S, Pensiero M N, Cardellichio C B, Williams R K, Jiang G S, Holmes K V, Dieffenbach C W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991;65:6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frana M F, Behnke J N, Sturman L S, Holmes K V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol. 1985;56:912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinz D W, Baase W A, Matthews B W. Folding and function of a T4 lysozyme containing 10 consecutive alanines illustrate the redundancy of information in an amino acid sequence. Proc Natl Acad Sci USA. 1992;89:3751–3755. doi: 10.1073/pnas.89.9.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 25.Kouzarides T, Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988;336:646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- 26.Lamb R A. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197:1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- 27.Landschulz W H, Johnson P F, McKnight S L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 28.Luo Z, Weiss S R. Roles in cell-to-cell fusion of two conserved hydrophobic regions in the murine coronavirus spike protein. Virology. 1998;244:483–494. doi: 10.1006/viro.1998.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luytjes W, Gerritsma H, Bos E, Spaan W. Characterization of two temperature-sensitive mutants of coronavirus mouse hepatitis virus strain A59 with maturation defects in the spike protein. J Virol. 1997;71:949–955. doi: 10.1128/jvi.71.2.949-955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luytjes W, Sturman L S, Bredenbeek P J, Charite J, van der Zeijst B A, Horzinek M C, Spaan W J. Primary structure of the glycoprotein E2 of coronavirus MHV-A59 and identification of the trypsin cleavage site. Virology. 1987;161:479–487. doi: 10.1016/0042-6822(87)90142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyu P C, Liff M I, Marky L A, Kallenbach N R. Side chain contributions to the stability of alpha-helical structure in peptides. Science. 1990;250:669–673. doi: 10.1126/science.2237416. [DOI] [PubMed] [Google Scholar]
- 32.Niemann H, Boschek B, Evans D, Rosing M, Tamura T, Klenk H D. Post-translational glycosylation of coronavirus glycoprotein E1: inhibition by monensin. EMBO J. 1982;1:1499–1504. doi: 10.1002/j.1460-2075.1982.tb01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neil K T, DeGrado W F. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990;250:646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- 35.O’Shea E K, Rutkowski R, Kim P S. Evidence that the leucine zipper is a coiled coil. Science. 1989;243:538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- 36.Poumbourios P, El Ahmar W, McPhee D A, Kemp B E. Determinants of human immunodeficiency virus type 1 envelope glycoprotein oligomeric structure. J Virol. 1995;69:1209–1218. doi: 10.1128/jvi.69.2.1209-1218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poumbourios P, Wilson K A, Center R J, El A W, Kemp B E. Human immunodeficiency virus type 1 envelope glycoprotein oligomerization requires the gp41 amphipathic alpha-helical/leucine zipper-like sequence. J Virol. 1997;71:2041–2049. doi: 10.1128/jvi.71.3.2041-2049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsdale E E, Kingsman S M, Kingsman A J. The “putative” leucine zipper region of murine leukemia virus transmembrane protein (P15e) is essential for viral infectivity. Virology. 1996;220:100–108. doi: 10.1006/viro.1996.0290. [DOI] [PubMed] [Google Scholar]
- 39.Ransone L J, Visvader J, Sassone-Corsi P, Verma I M. Fos-Jun interaction: mutational analysis of the leucine zipper domain of both proteins. Genes Dev. 1989;3:770–781. doi: 10.1101/gad.3.6.770. [DOI] [PubMed] [Google Scholar]
- 40.Reitter J N, Sergel T, Morrison T G. Mutational analysis of the leucine zipper motif in the Newcastle disease virus fusion protein. J Virol. 1995;69:5995–6004. doi: 10.1128/jvi.69.10.5995-6004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segrest J P, De L H, Dohlman J G, Brouillette C G, Anantharamaiah G M. Amphipathic helix motif: classes and properties. Proteins. 1990;8:103–117. doi: 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- 42.Shugars D C, Wild C T, Greenwell T K, Matthews T J. Biophysical characterization of recombinant proteins expressing the leucine zipper-like domain of the human immunodeficiency virus type 1 transmembrane protein gp41. J Virol. 1996;70:2982–2991. doi: 10.1128/jvi.70.5.2982-2991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spaan W, Cavanagh D, Horzinek M C. Coronaviruses: structure and genome expression. J Gen Virol. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- 44.Sturman L S, Ricard C S, Holmes K V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: activation of cell-fusing activity of virions by trypsin and separation of two different 90K cleavage fragments. J Virol. 1985;56:904–911. doi: 10.1128/jvi.56.3.904-911.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taguchi F. The S2 subunit of the murine coronavirus spike protein is not involved in receptor binding. J Virol. 1995;69:7260–7263. doi: 10.1128/jvi.69.11.7260-7263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vennema H, Heijnen L, Zijderveld A, Horzinek M C, Spaan W J. Intracellular transport of recombinant coronavirus spike proteins: implications for virus assembly. J Virol. 1990;64:339–346. doi: 10.1128/jvi.64.1.339-346.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vennema H, Rottier P J, Heijnen L, Godeke G J, Horzinek M C, Spaan W J. Biosynthesis and function of the coronavirus spike protein. Adv Exp Med Biol. 1990;276:9–19. doi: 10.1007/978-1-4684-5823-7_3. [DOI] [PubMed] [Google Scholar]
- 48.Weissenhorn W, Wharton S A, Calder L J, Earl P L, Moss B, Aliprandis E, Skehel J J, Wiley D C. The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 1996;15:1507–1514. [PMC free article] [PubMed] [Google Scholar]
- 49.White J M. Membrane fusion. Science. 1992;258:917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- 50.Wild C, Dubay J W, Greenwell T, Baird T, Jr, Oas T G, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci USA. 1994;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young J K, Hicks R P, Wright G E, Morrison T G. The role of leucine residues in the structure and function of a leucine zipper peptide inhibitor of paramyxovirus (NDV) fusion. Virology. 1998;243:21–31. doi: 10.1006/viro.1998.9044. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X J, Baase W A, Matthews B W. Toward a simplification of the protein folding problem: a stabilizing polyalanine alpha-helix engineered in T4 lysozyme. Biochemistry. 1991;30:2012–2017. doi: 10.1021/bi00222a001. [DOI] [PubMed] [Google Scholar]