Abstract
Background and Hypothesis
Structural brain alterations are well-established features of schizophrenia but they do not effectively predict disease/disease risk. Similar to polygenic risk scores in genetics, we integrated multifactorial aspects of brain structure into a summary “Neuroscore” and examined its potential as a marker of disease.
Study Design
We extracted measures from T1-weighted scans and diffusion tensor imaging (DTI) models from three studies with schizophrenia and healthy individuals. We calculated individual-level summary scores (Neuroscores) for T1-weighted and DTI measures and a combined score (Multimodal Neuroscore-MM). We assessed each score’s ability to differentiate schizophrenia cases from controls and its relationship to clinical symptomatology, intelligence quotient (IQ), and medication dosage. We assessed Neuroscore specificity by performing all analyses in a more inclusive psychosis sample and by using scores generated from MDD effect sizes.
Study Results
All Neuroscores significantly differentiated schizophrenia cases from controls (T1 d = 0.56, DTI d = 0.29, MM d = 0.64) to a greater degree than individual brain regions. Higher Neuroscores (ie, increased liability) were associated with lower IQ (T1 β = −0.26, DTI β = −0.15, MM β = −0.30). Higher T1-weighted Neuroscores were associated with higher positive and negative symptom severity (Positive β = 0.21, Negative β = 0.16); Higher Multimodal Neuroscores were associated with higher positive symptom severity (β = 0.30). SZ Neuroscores outperformed MDD Neuroscores in predicting IQ (T1: z = 3.5, q = 0.0007; MM: z = 1.8, q = 0.05).
Conclusions
Neuroscores are a step toward leveraging widespread structural brain alterations in psychosis to identify robust neurobiological markers of disease. Future studies will assess ways to improve neuroscore calculation, including developing the optimal methods to calculate neuroscores and considering disorder overlap.
Keywords: structural MRI, diffusion MRI, psychotic disorders, individual-level, neuroimaging score, IQ, symptoms
Introduction
Schizophrenia is a debilitating psychiatric illness that prevents individuals from reaching their optimal level of functioning and is associated with significant societal economic and medical burden.1,2 Gray and white matter disruptions in the brain are well-established features of individuals with schizophrenia3–6; however, we have not successfully leveraged this information to identify psychosis-specific biomarkers and/or detect those at greatest risk for developing psychosis.
Several factors contribute to our failure in using imaging findings as effective biomarkers. First, structural imaging studies often evaluate several measures across multiple regions, resulting in hundreds of tests, and strict corrections for multiple comparisons. Additionally, considering results across individual regions of interest (ROIs) makes interpretation difficult given the number of ROIs tested. This issue is exacerbated when extending analyses across modalities. Another factor is that, in people diagnosed with schizophrenia, brain alterations are subtle and distributed across the brain. As such, no single region accounts for all, or even most schizophrenia diagnoses. Last, findings across studies often differ, either due to the heterogeneous nature of the disorder and/or site-specific heterogeneity; these challenges to reproducibility contribute to difficulty in linking brain alterations to individual differences.7
One way to address these factors is to create a single summary score that combines effects from individual brain regions. This reduces the burden of multiple testing and leverages diffuse effects across the brain. This concept has been used extensively in genetics where, similarly, the contribution of individual genetic variants to psychiatric disorders is small and effects are distributed across the genome.8–10 In genetics, this is done by creating a polygenic risk score. Polygenic risk scores are calculated by summing weighted effects of single variants across the genome, where weights are determined from an independent large-scale genome-wide association study (GWAS).11 The weights are summed into a single number that represents a person’s genetic liability for disease. Polygenic risk scores have the potential to impact clinical care via identifying targeted treatments, indicating specific prognoses, and enhancing risk prediction/stratification in the general population. Polygenic risk score applications in cardiovascular disease, breast cancer, and diabetes already provide information that is clinically actionable.12
A similar method can be employed with neuroimaging data.13–15 Weights can be determined using “Big data” neuroimaging studies of schizophrenia, such as those available from the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) consortium. Via harmonized analysis pipelines of T1- and diffusion-weighted data across multiple studies, the Schizophrenia ENIGMA Working Group has identified robust structural neuroimaging profiles in schizophrenia, including widespread reductions in cortical thickness, surface area, subcortical volume,3–5 and diffuse alterations in white matter integrity, evidenced by global reduced fractional anisotropy, as well as higher radial and mean diffusivity.6 Similar to a GWAS, these studies can serve as the “discovery” sample and researchers can then apply the study weights to individual regions derived from their own data and sum the values across the brain to achieve a “Neuroscore.” This cumulative score would putatively index a person’s neuroanatomical liability for disease (or any other phenotype of interest). Similar frameworks have been used in other samples to explore the relationship between cognition and summary scores derived from resting-state functional magnetic resonance imaging (fMRI) in youth.16,17
Recently, a study using such an approach, constructed a structural MRI “morphometric risk score” and found that, in two independent samples, individuals with a schizophrenia diagnosis had elevated scores in comparison to healthy controls.15 However, regional measures were only included in an individual’s weighted Neuroscore if the measure z-score within the sample distribution was in the same direction as the ROI effect size in the discovery sample, potentially biasing results (eg, inflating the effect size when comparing clinical and healthy samples). Also, in this method, an individual’s z-score value is dependent on the sample used, making this an unstable score. Furthermore, this score only used gray matter measures and it is unknown if using white matter measures or both gray and white matter measures combined would be superior in identifying case/control status or predicting factors associated with schizophrenia (eg, IQ, clinical symptomatology, or medication). Last, the specificity of the score, with regards to diagnosis, has not yet been directly tested.
Here, we used effect sizes from ENIGMA neuroimaging studies of schizophrenia to weight individual brain regions across multiple T1 and DTI measures to create T1, DTI, and multimodal neuroimaging summary scores, or Neuroscores (T1-weighted Neuroscore, DTI Neuroscore, and Multimodal Neuroscore, respectively). Using a mega-analytic approach that included three independent studies of participants with a schizophrenia diagnosis and healthy controls, we evaluated which Schizophrenia Neuroscore was the most successful in differentiating schizophrenia cases from controls. For single modality Schizophrenia Neuroscores (T1-weighted and DTI), we examined which features (eg, thickness, fractional anisotropy) appear to be driving group differentiation. We also evaluated relationships between Schizophrenia Neuroscores and IQ, clinical symptoms, and antipsychotic medication dosage. We then addressed Neuroscore specificity in two ways. First, we assessed whether including other psychosis diagnoses reduces the effectiveness of Schizophrenia Neuroscores. We predicted this would not greatly alter our results, as disruptions in gray and white matter are largely shared between schizophrenia and other psychotic disorder diagnoses.18,19 Second, we compared the effectiveness of Schizophrenia Neuroscores to MDD Neuroscores, ie, Neuroscores constructed using effect sizes from ENIGMA studies of major depressive disorder.20–22 We predicted that Schizophrenia Neuroscores will outperform MDD Neuroscores in differentiating individuals with schizophrenia from healthy controls, as well as differentiating participants with a psychosis-spectrum diagnosis from healthy controls.
Methods
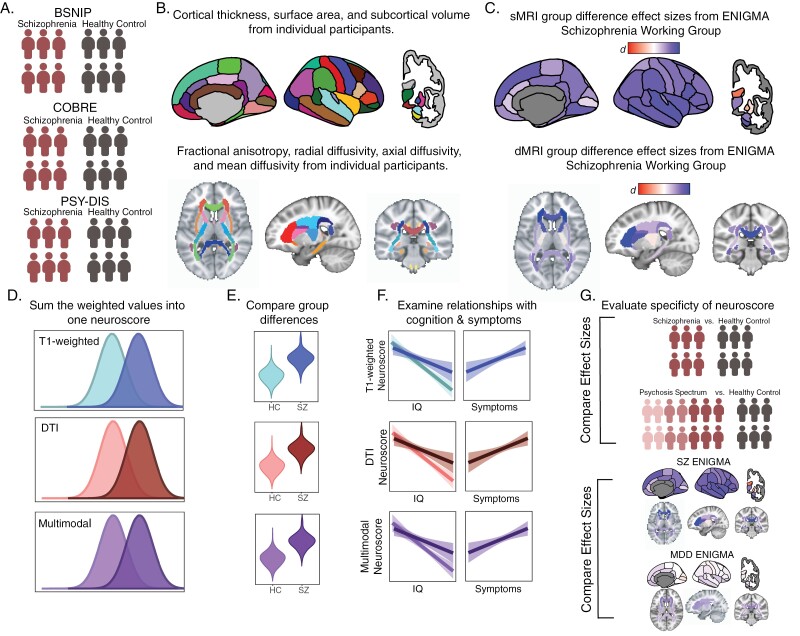
An outline of the study procedure is detailed in figure 1.
Fig. 1.
Analysis overview. (A) We started with three independent samples containing schizophrenia and healthy controls. (B) MRI measures from T1 and diffusion-weighted images were then calculated for each individual subject and residualized for age and sex. Each MRI measure was then z-scored across the whole sample. (C) Cohen’s d effect sizes were obtained from previously published meta-analyses from the ENIGMA consortium. (D) Individual’s z-scores for each ROI measure were multiplied by the ENIGMA effect size for the corresponding variable and summed across measures to calculate three Neuroscores. (E) We assessed the effectiveness of this score by testing its ability to distinguish cases from controls (F) as well as its relationship to IQ ad positive symptoms. (G) We evaluated the specificity of each Neuroscore by running all analyses (E-F) using a more inclusive psychosis sample and using ENIGMA effect sizes from a study of Major Depressive Disorder. DTI, diffusion tensor imaging; SZ, schizophrenia; MDD, major depressive disorder; sMRI, structural magnetic resonance imaging; dMRI, diffusion magnetic resonance imaging.
Participants
All studies included data from adult participants (18–65 years old) who were either healthy controls (individuals with no DSM-IV diagnosis) or individuals with a psychosis-spectrum diagnosis (see table 1 and supplementary table 1). Psychosis-spectrum diagnoses included schizophrenia, schizoaffective disorder, major depressive disorder with psychosis, bipolar disorder with psychosis, or psychotic disorder not otherwise specified. Diagnoses were obtained through a semi-structured interview conducted by a trained researcher or clinician. Within each study, participants provided written informed consent at the respective site and the local institutional review board approved the study. Study-specific details are listed below.
Table 1.
MEGA sample demographics
| T1 | DTI | Multimodal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | SZ | Psychosis | C | SZ | Psychosis | C | SZ | Psychosis | |
| N | 315 | 309 | 618 | 229 | 224 | 441 | 209 | 192 | 385 |
| Sex, M, n (%) | 158 (50%) | 216 (69%) | 353 (57%) | 122 (53%) | 161 (72%) | 263 (60%) | 114 (55%) | 136 (70%) | 227 (59%) |
| Age, yrs. (SD) | 36.8 (12.6) | 36.2 (13.0) | 37.1 (12.8) | 38 (13.0) | 37.2 (12.8) | 37.8 (12.6) | 37.3 (12.8) | 36.9 (12.7) | 37.5 (12.5) |
| Race, n (%) | |||||||||
| Caucasian | 159 (50%) | 145 (47%) | 281 (45%) | 101 (44%) | 103 (46%) | 185 (42%) | 89 (43%) | 87 (45%) | 125 (32%) |
| African American | 145 (46%) | 147 (48%) | 307 (50%) | 124 (54%) | 108 (48%) | 236 (54%) | 116 (56%) | 94 (49%) | 209 (54%) |
| Other | 11 (3%) | 17 (6%) | 30 (5%) | 4 (2%) | 13 (6%) | 20 (5%) | 4 (2%) | 11 (6%) | 18 (5%) |
| IQ | 102.9 (14.8) | 94.6 (15.7) | 95.4 (15.3) | 101.8 (15.6) | 94.4 (15.5) | 94.1 (15.3) | 102.7 (15.4) | 94.4 (15.5) | 94.3 (15.4) |
| Diagnostic breakdown | |||||||||
| SZ | NA | 309 (100%) | 309 (50%) | NA | 224 (100%) | 224 (51%) | NA | 192 (100%) | 192 (50%) |
| SAD | NA | NA | 164 (27%) | NA | NA | 136 (31%) | NA | NA | 118 (31%) |
| BPD | NA | NA | 135 (22%) | NA | NA | 72 (16%) | NA | NA | 67 (17%) |
| OTH | NA | NA | 10 (2%) | NA | NA | 9 (2%) | NA | NA | 8 (2%) |
| a Symptoms, Mean (SD) | NA | N: 15.8 (5.6) P: 15.8 (5.5) |
N: 14.4 (5.2) P: 15.4 (5.5) |
NA | N: 15.0 (5.6) P:15.2 (5.6) |
N: 14.5 (5.4) P:15.1 (5.6) |
NA | N: 15.1 (5.7) P:15.2 (5.5) |
N: 14.3 (5.5) P:15.0 (5.5) |
| CPZ Equivalents, Mean (SD) | NA | 493.4 (506.2) | 386.3 (443.4) | NA | 553.2 (632.6) | 415.8 (523.2) | NA | 515.7 (547.6) | 381.8 (455.3) |
aSymptoms were assessed via the PANSS for BSNIP and COBRE; this was not available for the PSY-DIS study. As such, symptom means for the Mega sample are averaged over BSNIP and COBRE studies only. SZ, schizophrenia; SAD, schizoaffective disorder; BPD, bipolar disorder with psychosis; OTH, other psychotic disorder; N, negative PANSS subscale; P, positive PANSS subscale.
BSNIP.
We downloaded the Bipolar-Schizophrenia Network on Intermediate Phenotypes (BSNIP)23 data set from the NIMH National Data Archives (https://nda.nih.gov/). Exclusion criteria were: known central neurological illness, a history of substance abuse (within 6 months) or dependence (within 2 years), and a positive urine toxicology or pregnancy screen. Healthy controls also had no first-degree relative with a psychotic illness.
COBRE.
We downloaded the Center of Biomedical Research Excellence (COBRE) data set24 from SchizConnect (http://schizconnect.org/).25 Exclusion criteria were: history of a neurological disorder, intellectual disability and/or severe head trauma with loss of consciousness >5 min, and history of substance abuse or dependence a year before the assessment.
Psychotic Disconnectivity (PSY-DIS)
. Participants were healthy controls and individuals with psychosis from a previously collected study focusing on connectivity disruptions in psychosis in African Americans.26,27 Exclusion criteria were a history of major medical disorders, severe head injury, IQ <70, MRI contraindications, dementia, and presence of drugs other than THC in the urine toxicology screen. Healthy controls had no first-degree relative with a psychotic disorder.
MRI Scans
In all studies, participants completed a T1-weighted MPRAGE scan and/or a diffusion-weighted MRI scan. For T1 data in the BSNIP study and DTI in the BSNIP and COBRE studies, we adjusted MRI data to account for site or scanner protocol variations using neuroComBat,3 a well-established MRI harmonization method. Previous publications have detailed the MRI parameters18,19,24,28 and are reported in supplementary tables 2–3. QC metrics (number of scans excluded for each modality and average motion during DTI for each group) are reported in supplementary table 4.
Clinical Assessments
We estimated Neuroscore relationships with IQ, clinical symptoms, and antipsychotic medication dosage. IQ was quantified using the Wechsler Abbreviated Scale of Intelligence (WASI)29 for COBRE and PSY-DIS studies and the Wechsler Wide Range of Achievement Test (WRAT)30 for the BSNIP study. Both measures estimate IQ on the same scale; therefore, like our group difference analyses, we combined the data and conducted analyses across all three studies. Symptoms were quantified with the Positive and Negative Symptom Scale (PANSS)31 for the BSNIP and COBRE studies and with the Brief Psychiatric Rating Scale (BPRS)32 for the PSY-DIS sample. As such, only BSNIP and COBRE samples were used in symptom analyses. Chlorpromazine (CPZ) mg daily dose equivalents were calculated for each sample using the Chlorpromazine R software package.33 In each sample, we identified participants who reported taking a medication that is listed in the antipsychotic class of drugs by the National Library of Medicine RxClass API (https://mor.nlm.nih.gov/RxClass/). Cases with sufficient reported information (medication name, dosage, and frequency) were converted to a CPZ equivalent using multiplicative factors identified in previous literature.34–38 CPZ equivalents were then summed for any participants who reported taking multiple medications, for a total daily dose equivalent reported in milligrams. There were very few unmedicated individuals in each sample except for the PSY-DIS sample; we chose to exclude unmedicated individuals from the CPZ analysis.
sMRI Data Processing
We visually examined all sMRI using results from the MRIQC39 output and gave each scan a score (1[unusable]-4 [excellent]). All scans with a rating of 1 were excluded. The remaining “usable” sMRI scans were processed through Freesurfer.40–42 We extracted cortical thickness and surface area from 34 lateralized ROI using the Desikan-Killanny atlas43 and volume from 18 subcortical structures. Due to availability of effect sizes from some ENIGMA studies,21 Neuroscore weights were calculated for eight bilateral subcortical ROIs; we included lateralized ROIs for thickness and surface area.
dMRI Data Processing
We processed diffusion-weighted images for each study with identical procedures using FSL (v 6.0.3).44 P We used output from eddyQC45 to determine subject exclusions. Within each study, we excluded subjects if they displayed absolute motion greater than two times the shortest voxel dimension, relative motion greater than a voxel, susceptibility distortion greater than a voxel, slice to volume motion greater than 1 standard deviation, phase-encoding outliers greater than 3%, and/or were outliers (defined as 25% or 75% + 2.5 * IQR) in the eddy current, signal to noise, or contrast to noise distributions. Images were also visually inspected at several points during the preprocessing pipeline to identify any artifacts. Preprocessed maps passing quality control procedures were fed into the Tract-Based Spatial Statistics pipeline.46 For comparability across studies, FA, MD, RD, and AD maps were projected onto the ENIGMA DTI skeleton (https://enigma.ini.usc.edu/protocols/dti-protocols/). DTI measures were extracted from the overlap between the ENIGMA DTI skeleton and the JHU White Matter Label Atlas.47 Due to availability of effect sizes from some ENIGMA studies,48 we calculated Neuroscore weights for 17 bilateral tracts and 3 sections of the corpus callosum.
Schizophrenia Neuroscore Calculation
After regressing out the effects of age and sex on each individual brain measure, we z-scored each MRI measure across the whole sample. We then calculated a Neuroscore for each individual (i), using (j) z-scored ROIs. We multiplied the Cohen’s d effect size for each ROIj by ROIij. Cohen’s d effect sizes came from ENIGMA Working group publications46,48 (figure 1C).
For each modality, we summed the weighted values from all regions to create one cumulative score, with a higher score indicating greater neuroanatomical liability for schizophrenia. To calculate the T1-weighted Neuroscore, we used 144 ROIs (68 cortical thickness measures, 68 surface area measures, 8 subcortical volume measures). To calculate the DTI Neuroscore, we used 80 ROIs (17 bilateral tracts and three sections of the corpus collosum for FA, MD, RD, and AD each). After creating the original T1-weighted Neuroscore, we recalculated Neuroscores for cortical thickness, surface area, and subcortical volume separately. Similarly, after creating the DTI Neuroscore, we recalculated separate Neuroscores for FA, MD, RD, and AD. For example, we summed the weighted values of all 20 FA tracts to create a FA Neuroscore. Finally, we created a combined “multimodal” Neuroscore using weighted features from both neuroimaging modalities. Exact measures that were used to calculate all Neuroscores are reported in supplementary table 5.
Statistical Analysis
We ran all analyses across all studies (Mega sample) and within each study. Similar to corrections within BSNIP and COBRE studies, we used neuroComBat3 to correct for dataset differences in the mega sample using age, sex, and group status in the model. We first assessed group differences in a more traditional univariate fashion, comparing individual ROI measures using multiple linear regression. For each ROI, we ran a model with group as the independent variable and the respective ROI as the dependent variable with age and sex as covariates. We conducted these supplemental analyses for others to compare their own samples with those used in this manuscript. We then residualized ROI values for age and sex and calculated Neuroscores as detailed above. To assess the Neuroscore’s ability to differentiate between diagnostic groups, we used two-sample independent t-tests (as Neuroscores were already corrected for age and sex effects). To assess relationships between Neuroscores and IQ and positive symptoms, and medication effects (ie, CPZ equivalents) we used multiple linear regression. For IQ models, we include age, sex, group, and group × score as covariates. For symptom and medication models, we included age and sex only as covariates, as these models were run exclusively in individuals with a psychosis diagnosis. Multiple testing correction was performed by accounting for the false discovery rate (FDR).49
To evaluate the specificity of our Neuroscores, we conducted all Neuroscore analyses in an expanded sample with a broader definition of psychosis, including individuals with a diagnosis of schizoaffective disorder, bipolar disorder, or MDD with psychotic features, or a not otherwise specified psychotic disorder. We then used z-tests50 to directly compare the Schizophrenia Neuroscore effect sizes obtained in the original sample (schizophrenia vs controls) to the expanded samples (psychosis-spectrum disorders vs controls) for group membership, IQ, and symptom predictions. We ran a similar set of z-tests to compare the performance of Schizophrenia Neuroscores to MDD Neuroscores which were calculated using group difference effect sizes from an ENIGMA study of Major Depressive Disorder (MDD).20–22
Results
All results discussed below are in reference to the mega analysis. Results within individual studies are outlined in the supplement. Univariate analyses for T1-weighted and DTI measures are also reported in the supplement (supplementary figures 1–2 and supplementary tables 6–7); case/control effect sizes mostly correlated with ENIGMA effect sizes used for weights in Neuroscore construction (supplementary table 8).
Select Neuroscores Significantly Differentiate Individuals With Schizophrenia From Controls
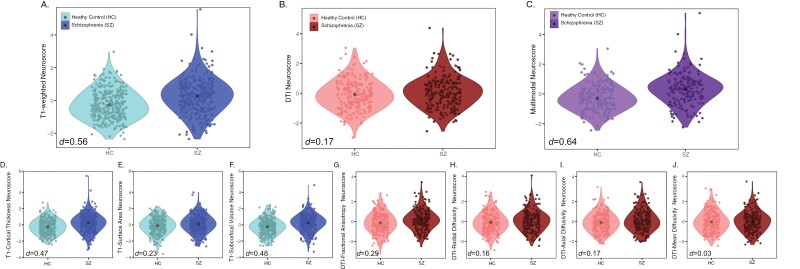
Individuals with schizophrenia had significantly higher T1-weighted, but not DTI Neuroscores, compared with controls (T1 d = 0.56, q < 0.00001; DTI d = 0.17, q = 0.07, supplementary table 9). When we examined the individual measure contributions to the T1-weighted Neuroscore, cortical thickness measures, and subcortical volumes were the strongest differentiators of case/control status, although all T1 measures significantly differentiated cases from controls (subcortical volume d = 0.48, q < 0.00001; thickness d = 0.47, q < 0.00001; surface area d = 0.23, q = 0.004, figure 2 and supplementary table 10). For DTI measures, FA was the strongest differentiator of case/control status (d = 0.29, q = 0.008); no other single DTI measure Neuroscore (RD, MD, or AD) significantly differentiated case/control status (figure 2 and supplementary table 10). As such, all further analyses using Neuroscores constructed from DTI measures are composed of FA measures only. Results for DTI and multimodal Neuroscores including all DTI measures can be found in the supplement supplementary table 9, whereas results using FA measures only can be found in supplementary table 11 and figures 3 and 4.
Fig. 2.
Neuroscore distributions. Group-wise distributions and group effect sizes (Cohen’s d) from an independent sample t-test of summary Neuroscores (A–C) and individual measure Neuroscores (D–J). Plots A and D-F show Neuroscores built with T1 measures, plots B and G-L show Neuroscores built with DTI measures, and plot C shows the multimodal Neuroscore. HC, healthy control; SZ, schizophrenia; DTI, diffusion tensor imaging.
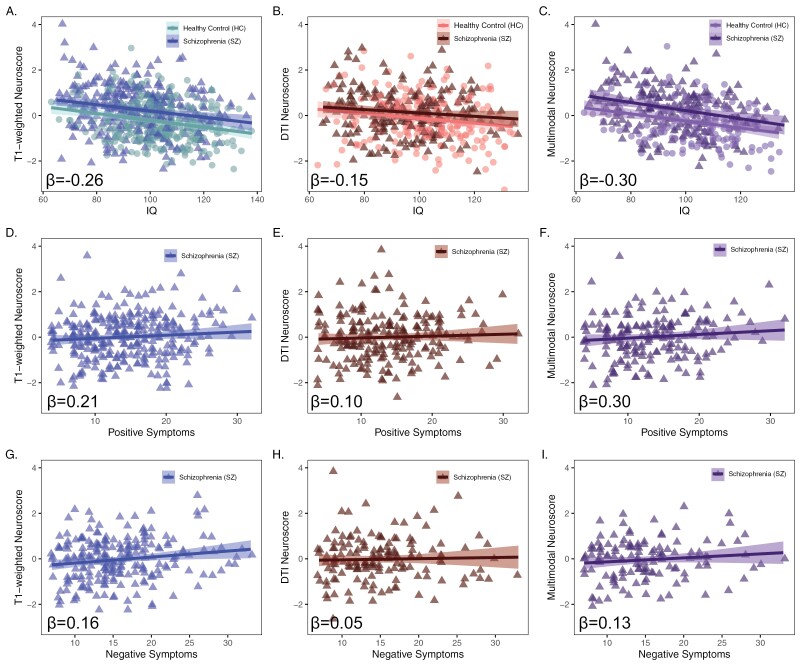
Fig. 3.
Neuroscore associations with psychosis-related factors. Associations between summary Neuroscores and IQ (A–C), positive symptoms (D–F), and negative symptoms (G–I). Betas are standardized effect sizes and represent neuroscore effects from IQ models (covarying for age, sex, and group) and from symptom models (covarying for age and sex). Plots A, D, and G show T1 Neuroscore associations, plots B, E, and H show DTI Neuroscore associations, and plots C, F, and I show multimodal Neuroscore associations. HC, healthy control; SZ, schizophrenia; DTI, diffusion tensor imaging.
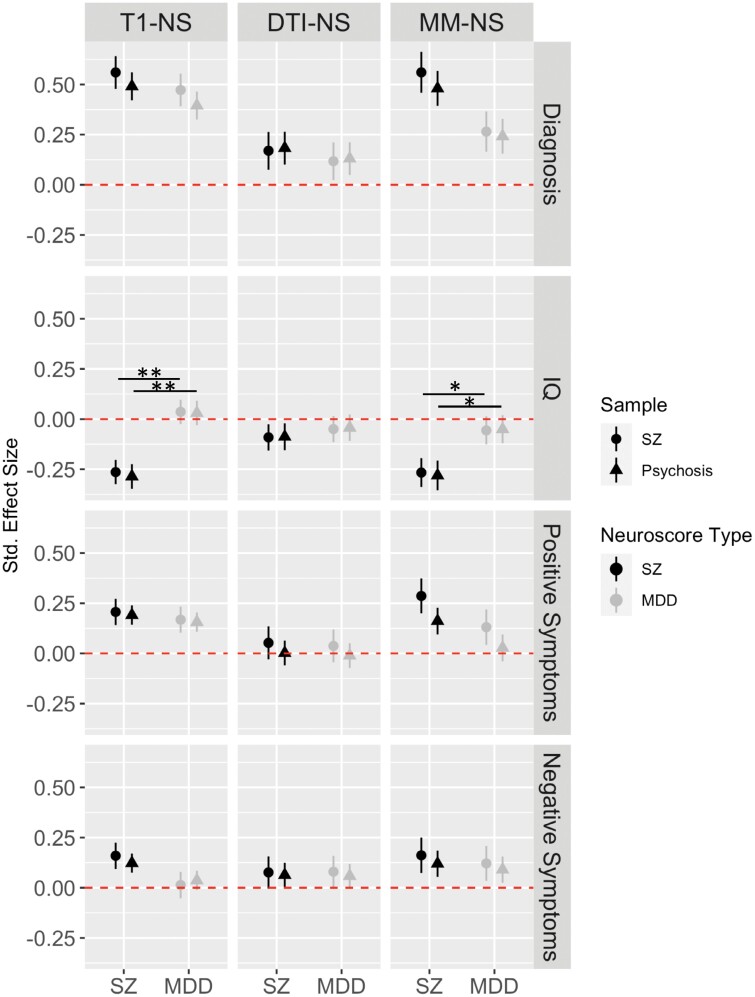
Fig. 4.
Neuroscore specificity. Plot shows standardized effect sizes for the original SZ Neuroscore analyses (dark circles) compared with analyses with a more inclusive psychosis sample (dark triangles). Standardized effect sizes are also plotted for analyses using MDD Neuroscores for both the original sample (light circles) and the more inclusive psychosis sample (light triangles). Neuroscore results were largely unchanged when including all psychosis diagnoses regardless of whether SZ or MDD Neuroscores were used. Although numerically larger, SZ Neuroscores did not significantly outperform MDD Neuroscores in case/control differentiation; effect sizes from T1 and Multimodal SZ Neuroscores, however, significantly outperformed MDD Neuroscores in predicting IQ.*FDR corrected P-value <.05; **FDR corrected P-value <.001. SZ, schizophrenia, MDD, major depressive disorder, T1-NS, T1-weighted Neuroscore, DTI-NS, DTI Neuroscore (FA only), MM-NS, multimodal Neuroscore.
Although numerically larger, T1 Neuroscores did not significantly outperform FA Neuroscores (z = −2.15, q = 0.10) (figure 2). Multimodal Neuroscores significantly differentiated schizophrenia cases and controls (d = 0.64, q < 0.00001), however, they showed no significant advantage over single modality Neuroscores (multimodal vs T1 z = −0.60, q = 0.65; multimodal vs FA z = −2.50, q = 0.07).
Higher Schizophrenia Neuroscores are Related to IQ and Symptoms
Across schizophrenia cases and controls, lower IQ was associated with significantly higher T1-weighted, FA, and Multimodal Neuroscores (figure 3A–C, supplementary table 11). There were no significant group by Neuroscore interactions in predicting IQ.
Within the patient sample, higher positive symptom scores were associated with significantly higher T1-weighted and Multimodal Neuroscores; higher negative symptoms were associated with significantly higher T1-weighted Neuroscores only (figure 3D–I, supplementary table 11). We did not detect any statistically significant relationships between schizophrenia Neuroscores and CPZ equivalents (supplementary table 11).
Schizophrenia Neuroscores Distinguish Psychosis-Spectrum Diagnoses From Controls
Schizophrenia Neuroscore group differences were largely unchanged when we included an expanded sample of individuals with a psychosis-spectrum diagnosis (figure 4, supplementary table 11). When we directly compared the Neuroscore effect sizes obtained from the original vs the expanded sample, there were no statistically significant differences between the effect sizes for case/control differentiation, IQ, or symptom predictions (supplementary table 12).
Mixed Results Regarding Specificity of Schizophrenia Neuroscores Compared With MDD Neuroscores
While effect sizes for predicting case/control status were numerically larger for schizophrenia Neuroscores, they did not significantly outperform MDD Neuroscores in the original sample (figure 4, supplementary tables 12 and 13). Schizophrenia T1 and Multimodal Neuroscores significantly outperformed MDD Neuroscores in predicting IQ (T1 z = 3.5, q = 0.0007; Multimodal z = 1.8, q = 0.05), but not positive (T1 z = 0.4, q = 0.76; Multimodal z = 0.5, q = 0.76) or negative symptoms (T1 z = 1.6, q = 0.26; Multimodal z = 0.2, q = 0.54) (supplementary tables 12 and 13). MDD Neuroscore results were unchanged when performing analyses with the expanded psychosis sample (supplementary tables 12 and 13).
Discussion
We found that neuroimaging summary scores (ie, Neuroscores) from different neuroimaging modalities (sMRI, dMRI) differentiated schizophrenia cases from controls and are related to individual differences in cognition and clinical symptomatology. In fact, case/control effect sizes were equivalent or larger for Neuroscores than those for individual ROIs, reducing analytic complexity while maximizing group differentiation. Specifically, subcortical volume, cortical thickness, surface area, and fractional anisotropy significantly contributed to the Multimodal Neuroscore, highlighting neurobiological mechanisms that may be important for understanding schizophrenia. Finally, the Schizophrenia T1 and Multimodal Neuroscores outperformed MDD Neuroscores in the ability to detect relationships with IQ. These findings represent a significant step toward leveraging the widespread nature of structural brain alterations in psychosis to identify a robust neurobiological marker of the disorder.
Our results extend an active, developing area of research using summary scores to enhance our understanding of individual differences in psychiatric disorders and behavior.14–17 We show that Schizophrenia Neuroscores are effective at distinguishing cases from controls, even when calculated without the potential bias we observed in the Lancaster et al.13 publication. We also advanced this line of research by creating a DTI Schizophrenia Neuroscore and showed that a Multimodal Neuroscore combining the two modalities was also associated with psychosis group status. Other approaches, like the Regional Vulnerability Index (RVI)14 or the Person-Based Similarity Index (PBSI)13 have also been proposed. While the RVI successfully differentiated schizophrenia cases from controls,14 the PBSI only did so for cortical thickness.13 The RVI estimates the similarity between an individual’s MRI measures and the expected pattern of impairments obtained in schizophrenia ENIGMA studies, whereas the PBSI is an average correlation of each subject’s brain measures with all other subjects in their diagnostic group.51 Future studies should compare the common and distinct features associated with other imaging summary scores and Neuroscores. Additionally, a recent study from the Adolescent Brain and Cognitive Development consortium found that “polyneuro risk scores” from resting-state fMRI data are associated with cognition16 and that these scores can account for more variance in cognition than the small variance typically accounted for in “brain-wide association studies.”7 Furthermore, in many cases (but not all, see supplementary table 14), Neuroscore group effect sizes were significantly stronger than group effect sizes obtained from global MRI measures (eg, total surface area).
In our sample, all Schizophrenia Neuroscores (T1, FA, and Multimodal) differentiated between cases and controls, as did MDD Neuroscores. Indeed, there is strong overlap between schizophrenia and MDD regarding alterations in cortical thickness and subcortical volume.52,53 We confirmed this relationship by conducting Spearman correlations of effect sizes between schizophrenia and MDD ENIGMA studies; T1-weighted effect sizes were correlated at r = 0.37 and DTI measures were correlated to a greater degree at r = 0.46. We expect that similar results would be obtained when contrasting the effectiveness of a Bipolar Disorder Neuroscore, where overlap in brain alterations with schizophrenia is commonly reported.54 Similarly, research shows substantial genetic correlations among these disorders,55,56 which could possibly contribute to overlap in our Neuroscore predictions. Similarities in neuroimaging and genetic findings across schizophrenia and MDD suggest that there is shared etiology and pathophysiology among these psychiatric disorders. Similar to work done in psychiatric genetics,57 it may be beneficial to capitalize on this covariation and develop a transdiagnostic Neuroscore to detect liability for psychiatric disorders in general. Such a score is likely more realistic given we also saw little to no difference in Neuroscore prediction in the broader psychosis sample vs the sample with schizophrenia cases only. A more integrative approach may help address heterogeneity in symptom presentation and instability in psychiatric diagnoses, particularly early in the course of psychiatric illness.58,59
Though the Multimodal Neuroscore had the largest effect size in detecting case-control differences, it was not significantly larger than those for individual modality Neuroscores. Additionally, FA Neuroscores, while not significantly different from T1-weighted or Multimodal Neuroscores, were weaker predictors of diagnosis, IQ, and symptoms. Alternate methods of constructing Neuroscores may boost Neuroscore effectiveness. For example, there are multiple methods for calculating polygenic risk scores,60 one of which involves using only variants surpassing a particular p-threshold in the discovery sample used to generate weights. Polygenic risk scores are generated at different thresholds and the risk score accounting for the most variance (largest R2) is selected. These methods could further tune Neuroscores by reducing noise as well as increasing specificity. Optimal threshold selection, however, is not possible if there is no access to individual data from the discovery sample. Furthermore, polygenic risk score methods including all variants tend to outperform those using thresholding.61 Future work should determine if this also holds true for Neuroscores by leveraging individual-level data from ENIGMA Consortiums.
We found robust associations between cognition and all Schizophrenia Neuroscores, in both individuals with a psychotic disorder diagnosis and healthy controls. Correlations were negative, with a higher Schizophrenia Neuroscore associated with lower cognition, with the Schizophrenia Neuroscore accounting for between 2% and 7% of the variance in cognition, depending on neuroimaging modality (T1: 5%, FA: 2%, MM: 7%). Given that impaired cognition is considered a hallmark feature of schizophrenia,62,63 it is important that we detected this relationship in healthy controls as well. These findings highlight the importance of using Neuroscores to map onto individual differences in cognitive ability, not just psychopathology. Our results suggest that individual differences associated with cognition are linked with summary indices that harness the complex variation of white and gray matter structure observed in schizophrenia.
We also found that higher positive and negative symptoms are related to higher T1-weighted Schizophrenia Neuroscores in individuals with a psychotic disorder. Higher Multimodal Schizophrenia Neuroscores were related to higher positive symptoms as well. Significant correlations between positive symptoms and individual brain regions are scarce. Even when significant correlations are identified with individual brain regions,64 relationships are often small, accounting for less than one percent of the variation between the two measures. In this study, the T1-weighted score accounted for approximately 4% of the variance in positive symptoms, suggesting that Neuroscores may be a more robust index than measures of individual brain regions.
Limitations
As with any study, there are limitations that must be noted. There is some concern that ComBat, the method we used to account for site and protocol, may eliminate important biological differences, particularly for diffusion-weighted imaging measures. In the future, it will be important to determine what correction techniques are best suited for this method. Given the known challenge in applying polygenic risk scores from European samples to other ancestries,65 we will also need to determine if Schizophrenia Neuroscores are equally effective across race and ethnicity. Our data suggests this may not be an issue, given the PSY-DIS sample is 100% African American and the remaining samples are predominantly Caucasian. One of the datasets included in this study (COBRE) was used in the original ENIGMA Schizophrenia working group publication to estimate cortical thickness and surface area group effect sizes.5 This could bias our findings, although the PSY-DIS and BSNIP datasets are independent replication samples for T1 Neuroscores. The DTI analysis was not affected by this overlap. It will also be important to test the reliability of this score over time, in both adult and pediatric populations. Finally, the extent to which Neuroscores from various phenotypes covary with polygenic scores of the same phenotype is an open, important future question.
Future Directions and Conclusions
Unlike genetic variation, which is largely stable across the lifespan, important changes occur in brain structure across development. Incorporating age improves prognostic accuracy of risk for a phenotype in other fields of medicine;66–68 thus, differing lifespan trajectories likely have important implications for classifying psychosis group status and/or determining those at greatest risk for developing psychosis. Recent evidence shows that incorporating adult’s deviations from a same-aged reference significantly improves cortical thickness classification accuracy in schizophrenia69 and this improvement may extend to calculation of Neuroscores in adults and high-risk youth. Furthermore, in the future, for Neuroscores to be used on a regular basis, we need to find a way to make neuroimaging more accessible. Low-field portable MRI scanners offer an unprecedented opportunity to expand access.70,71 Testing our ability to calculate the Schizophrenia Neuroscore using low-field MRI measures is an important next step we are currently pursuing. In summary, this study provides a framework for showing how Neuroscores can be leveraged to differentiate cases from control and link to individual differences in cognition and psychopathology.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Contributor Information
Amanda L Rodrigue, Department of Psychiatry, Boston Children’s Hospital, Boston, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
Rebecca A Hayes, Department of Psychiatry, Boston Children’s Hospital, Boston, MA, USA.
Emma Waite, Department of Psychiatry, Boston Children’s Hospital, Boston, MA, USA.
Mary Corcoran, Department of Psychiatry, Boston Children’s Hospital, Boston, MA, USA.
David C Glahn, Department of Psychiatry, Boston Children’s Hospital, Boston, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA; Olin Neuropsychiatry Research Center, Institute of Living, Hartford, CT, USA.
Maria Jalbrzikowski, Department of Psychiatry, Boston Children’s Hospital, Boston, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
Conflict of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by National Institute of Mental Health (MH077945 [BSNIP-1 sample Harford site], MH077852 [BSNIP-1 sample Baltimore site], MH106324 [D.G.], MH129636 [M.J.]); National Institute of Health, Center of Biomedical Research Excellence (1P20RR021938-01A2 [COBRE sample]); the Tommy Fuss Center for Neuropsychiatric Research Next Generation Award [M.J.].
References
- 1. Vigo D, Thornicroft G, Atun R.. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3(2):171–178. [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Barber RM, Bell B, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Radua J, Vieta E, Shinohara R, et al. ; ENIGMA Consortium Collaborators. Increased power by harmonizing structural MRI site differences with the ComBat batch adjustment method in ENIGMA. Neuroimage. 2020;218:116956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Erp TG, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Erp TG, Walton E, Hibar DP, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol Psychiatry. 2018;84(9):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelly S, Jahanshad N, Zalesky A, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23(5):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marek S, Tervo-Clemmens B, Calabro FJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ripke S, Neale B, Corvin A, JTR W, Farh K.. Schizophrenia working group of the psychiatric genomics: biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stahl EA, Breen G, Forstner AJ, et al. ; eQTLGen Consortium. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51(5):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pantelis C, Papadimitriou GN, Papiol S, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi SW, Mak TS-H, O’Reilly PF.. Tutorial: a guide to performing polygenic risk score analyses. Nat Protocols. 2020;15(9):2759–2772. doi: 10.1038/s41596-020-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adeyemo A, Balaconis MK, Darnes RD, et al. Responsible use of polygenic risk scores in the clinic: potential benefits, risks and gaps. Nat Med. 2021;27(11):1876–1884. [DOI] [PubMed] [Google Scholar]
- 13. Doucet GE, Lin D, Du Y, et al. Personalized estimates of morphometric similarity in bipolar disorder and schizophrenia. npj Schizophr. 2020;6(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kochunov P, Fan F, Ryan MC, et al. Translating ENIGMA schizophrenia findings using the regional vulnerability index: association with cognition, symptoms, and disease trajectory. Hum Brain Mapp. 2022;43(1):566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lancaster TM, Dimitriadis SI, Perry G, Zammit S, O’Donovan MC, Linden DE.. Morphometric analysis of structural MRI using schizophrenia meta-analytic priors distinguish patients from controls in two independent samples and in a sample of individuals with high polygenic risk. Schizophr Bull. 2021;48(2):524–532. doi: 10.1093/schbul/sbab125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Byington N, Grimsrud G, Mooney MA, et al. Polyneuro risk scores capture widely distributed connectivity patterns of cognition. Dev Cogn Neurosci. 2023;60:101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao W, Palmer CE, Thompson WK, et al. Individual differences in cognitive performance are better predicted by global rather than localized BOLD activity patterns across the cortex. Cereb Cortex. 2021;31(3):1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170(11):1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skudlarski P, Schretlen DJ, Thaker GK, et al. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry. 2013;170(8):886–898. [DOI] [PubMed] [Google Scholar]
- 20. Schmaal L, Hibar D, Sämann PG, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22(6):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21(6):806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Velzen LS, Kelly S, Isaev D, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry. 2020;25(7):1511–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G.. Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum. Schizophr Bull. 2014;40(suppl_2):S131–S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aine C, Bockholt HJ, Bustillo JR, et al. Multimodal neuroimaging in schizophrenia: description and dissemination. Neuroinformatics. 2017;15:343–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ambite JL, Tallis M, Alpert K, et al. SchizConnect: Virtual data integration in Neuroimaging. Data Integr Life Sci. 2015;9162:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koenis MM, Durnez J, Rodrigue AL, et al. Associations of cannabis use disorder with cognition, brain structure, and brain function in African Americans. Hum Brain Mapp. 2021;42(6):1727–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mollon J, Mathias SR, Knowles EE, et al. Cognitive impairment from early to middle adulthood in patients with affective and nonaffective psychotic disorders. Psychol Med. 2020;50(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodrigue AL, Mastrovito D, Esteban O, et al. Searching for imaging biomarkers of psychotic dysconnectivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(12):1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 30. Jastak SR, Wilkinson GS.. Wide Range Achievement Test: WRAT-R. Los Angeles: Western Psychological Services; 1984. [Google Scholar]
- 31. Kay SR, Fiszbein A, Opler LA.. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 32. Lukoff D, Nuechterlein K, Ventura J.. Manual for the expanded brief psychiatric rating scale. Schizophr Bull. 1986;12:594–602. [Google Scholar]
- 33. Brown E, Shah P, Kim J. chlorpromazineR: Convert Antipsychotic Doses to Chlorpromazine Equivalents. https://docs.ropensci.org/chlorpromazineR/, https://github.com/ropensci/chlorpromazineR. [Google Scholar]
- 34. Davis JM. Dose Equivalence of the Antipsychotic Drugs. Catecholamines and Schizophrenia. Elsevier; 1975:65–73. [Google Scholar]
- 35. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ.. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–693. [DOI] [PubMed] [Google Scholar]
- 36. Leucht S, Crippa A, Siafis S, Patel MX, Orsini N, Davis JM.. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry. 2020;177(4):342–353. [DOI] [PubMed] [Google Scholar]
- 37. Leucht S, Samara M, Heres S, Davis JM.. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42(suppl_1):S90–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. [DOI] [PubMed] [Google Scholar]
- 39. Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ.. MRIQC: advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One. 2017;12(9):e0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fischl B, Dale AM.. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 42. Fischl B, Sereno MI, Dale AM.. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 43. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 44. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 45. Bastiani M, Cottaar M, Fitzgibbon SP, et al. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage. 2019;184:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 47. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kelly S, Jahanshad N, Zalesky A, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2017;23:1261–1269. doi: 10.1038/mp.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57(1):289–300. [Google Scholar]
- 50. Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Cambridge:Academic Press; 1985. [Google Scholar]
- 51. Doucet GE, Moser DA, Rodrigue A, Bassett DS, Glahn DC, Frangou S.. Person-based brain morphometric similarity is heritable and correlates with biological features. Cereb Cortex (New York, N.Y.: 1991). 2019;29(2):852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Opel N, Goltermann J, Hermesdorf M, Berger K, Baune BT, Dannlowski U.. Cross-disorder analysis of brain structural abnormalities in six major psychiatric disorders: a secondary analysis of mega-and meta-analytical findings from the ENIGMA consortium. Biol Psychiatry. 2020;88(9):678–686. [DOI] [PubMed] [Google Scholar]
- 53. Radonjić NV, Hess JL, Rovira P, et al. Structural brain imaging studies offer clues about the effects of the shared genetic etiology among neuropsychiatric disorders. Mol Psychiatry. 2021;26(6):2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rimol LM, Hartberg CB, Nesvåg R, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68(1):41–50. [DOI] [PubMed] [Google Scholar]
- 55. Consortium B, Anttila V, Bulik-Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360(6395):eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smoller JW, Andreassen OA, Edenberg HJ, Faraone SV, Glatt SJ, Kendler KS.. Psychiatric genetics and the structure of psychopathology. Mol Psychiatry. 2019;24(3):409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krapohl E, Patel H, Newhouse S, et al. Multi-polygenic score approach to trait prediction. Mol Psychiatry. 2018;23(5):1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Castro-Fornieles J, Baeza I, de la Serna E, et al. Two-year diagnostic stability in early-onset first-episode psychosis. J Child Psychol Psychiatry. 2011;52(10):1089–1098. [DOI] [PubMed] [Google Scholar]
- 59. Wood AJ, Carroll AR, Shinn AK, Ongur D, Lewandowski KE.. Diagnostic stability of primary psychotic disorders in a research sample. Front Psychiatry. 2021;12:734272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ni G, Zeng J, Revez JA, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium. A comparison of ten polygenic score methods for psychiatric disorders applied across multiple cohorts. Biol Psychiatry. 2021;90(9):611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ge T, Chen C-Y, Ni Y, Feng Y-CA, Smoller JW.. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10(1):1776. doi: 10.1038/s41467-019-09718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barch DM, Ceaser A.. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Green MF, Harvey PD.. Cognition in schizophrenia: past, present, and future. Schizophr Res: Cogn. 2014;1(1):e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walton E, Hibar DP, van Erp TG, et al. ; Karolinska Schizophrenia Project Consortium (KaSP). Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA Schizophrenia consortium. Acta Psychiatr Scand. 2017;135(5):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schultz LM, Merikangas AK, Ruparel K, et al. Stability of polygenic scores across discovery genome-wide association studies. HGG Adv. 2022;3(2):100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bonner C, Bell K, Jansen J, et al. Should heart age calculators be used alongside absolute cardiovascular disease risk assessment? BMC Cardiovasc Disord. 2018;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ.. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. [DOI] [PubMed] [Google Scholar]
- 68. Wells S, Kerr A, Eadie S, Wiltshire C, Jackson R.. “Your Heart Forecast”: a new approach for describing and communicating cardiovascular risk? Heart. 2010;96(9):708–713. [DOI] [PubMed] [Google Scholar]
- 69. Rutherford S, Barkema P, Tso IF, et al. Evidence for embracing normative modeling. Elife. 2023;12:e85082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Deoni SC, Bruchhage MM, Beauchemin J, et al. Accessible pediatric neuroimaging using a low field strength MRI scanner. Neuroimage. 2021;238:118273. [DOI] [PubMed] [Google Scholar]
- 71. Sheth KN, Mazurek MH, Yuen MM, et al. Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients. JAMA Neurol. 2021;78(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.