Abstract
In Northeast China, Goubao pickle is a popular food fermented from the roots of Platycodon grandiflorum as the main material, offering a unique flavor and rich nutritional value. Platycosides in roots of P. grandiflorum may play a crucial role in determining the quality of Goubao pickle through microorganism fermentation. However, biotransfermation of platycosides has not been reviewed during fermentation. In this study, we reviewed platycosides in chemical diversity, metabolic processes in vivo, biotransformation of platycosides in vitro, and pharmacological effects. Finally, we also discussed how to improve the bioactive secondary platycosides we desire by regulating enzymes from microorganisms in the future.
Keywords: biotransformation, Goubao pickle, microorganisms, pharmacological activities, platycosides, Platycodon grandiflorum (Jacq.) A. DC
1. Introduction
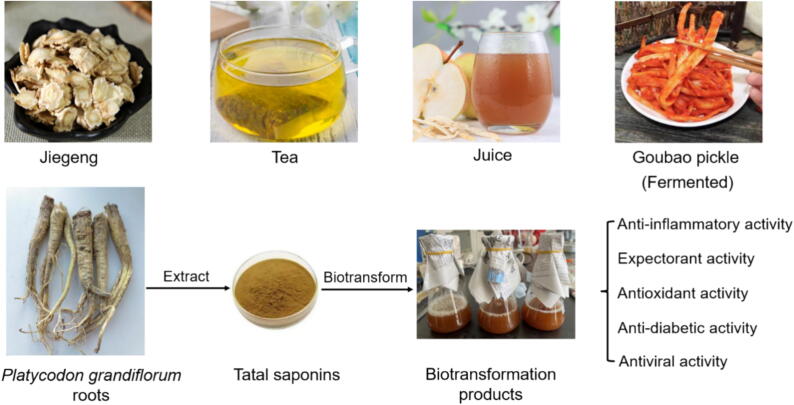
Platycodonis Radix (Jiegeng in Chinese), the dried root of Platycodon grandiflorum (Jacq.) A. DC. (Campanulaceae), is widely distributed in China, Korea, Mongolia, Japan and Russia. It was first recorded in the book Sheng Nong's Herbal Classic 2000 years ago. Platycodonis Radix is a famous edible and medicinal plant in Northeast Asia (Zhang et al., 2020, Zhang et al., 2023), which is also called Doraji in Korea, Huridunzhaga in Mongolia and Kikyo in Japan (Zhang et al., 2015). Platycodonis Radix products such as pickles, tea, cosmetics, and herbal supplements are very popular (Fig. 1). Goubao pickle is a traditional fermented pickled Platycodonis Radix made by Korean-Chinese people in Northeast China. It is favored for unique flavor and efficacy. This dish originates from North Korea and it is made from roots of burdock. Later it was replaced by Platycodonis Radix due to the similar taste and shape in 1930s, from then on Platycodonis Radix began to be called Goubao, phonetically similar to burdock in Japanese. Also P. grandiflorum roots have better smell than burdock roots. The major pharmacological active constituents of P. grandiflorum roots are oleanane-type pentacyclic triterpenoid saponins, called platycosides (Nyakudya, Jeong, Lee, & Jeong, 2014). Due to total platycosides, P. grandiflorum roots frequently taste much bitter than the pickle. If saponins are largely lost during pickling process of Goubao, bitter taste is mostly eliminated, and Platycodonis Radix losts health care function (Jin, Yu, Shan, & Wang, 2010).
Fig. 1.
Application of P. grandiflorum roots in edible medicinal plants field. Biotransformation products of P. grandiflorum roots have various biological activities.
Previous studies demonstrated that platycosides have various beneficial effects, such as apophlegmatic and anti-tussive (Ryu et al., 2014), immunomodulatory (Wang et al., 2004), anti-inflammatory (Gao et al., 2017, Lu et al., 2018, Meng et al., 2017), anti-cancer (Li et al., 2019, Yim, Hwang, Liang, & Ma, 2016), cholesterol-lowering, insulin-resistance (Yoon, Bang, Kim, & Imm, 2018), cardiovascular protection (Choi, Lee, Kim, Lee, & Ko, 2020), anti-obesity (Hwang, Hwang, Pu, Hwang, & Kim, 2019), treatment of hepatitis (Liu et al., 2020), neuro-protective effect (Zhang et al., 2021) and anti-oxidant activity (Fu, Liu, Wang, & Guan, 2009). However, the absorption rate of saponins in vivo is very low, which limits their utilization (He et al., 2019). Increasing researches showed that platycosides could be transformed into deglycosylated, dehydroxylated, acetylated and hydroxylated metabolites in vivo and in vitro (Zhang, Song, Liu, & Guo, 2022). Similar to ginsenosides, deglycosylated platycosides, obtained through the biotransformation of glycosylated saponins, showed biological effects better than their glycosylated forms (Ju, Lee, Lee, Kim, & Oh, 2021, Kang, Kim, Shin, Ko, & Oh, 2019, Shin, Kil, Lee, & Oh, 2021). Deapiosylated platycosides or secondary glycosides such as the anti-inflammatory, antioxidant activities, expectorant effects, were more active than those of the precursor glycosylated platycosides (Shin, Kil, Lee, & Oh, 2021).
Thus, we reviewed platycosides metabolites and pathways in vivo and biotransformed by enzymatichy drolysis or microorganism, which provides the scientific basis and ideas for the further development of platycodins biotransformation.
2. Major bioactive platycodins in fermented roots of P. grandiflorum
Platycosides are oleanane-type triterpene carboxylic acid 3,28-O-bisdesmosides with two side sugar chains (Fu, Dou, Shimizu, Takeda, Pei, & Chen, 2006). The main sugar groups are D-glucose, L-arabinose, L-rhamnose, D-xylose, D-apiose, and their derivatives (Zhang et al., 2015). Platycosides are typically composed of only a glucose unit with an ether linkage at C-3 position, and an ester linkage between C-28 and the sugar groups. The sites are fixed, that is, arabinose, rhamnose, xylose and apiose from inside to outside. Alkali hydrolysis can crack the ester bond at the C-28 position, while mild acid hydrolysis can remove the sugars at the C-3 position (Liu, Wei, & Zheng, 2013).
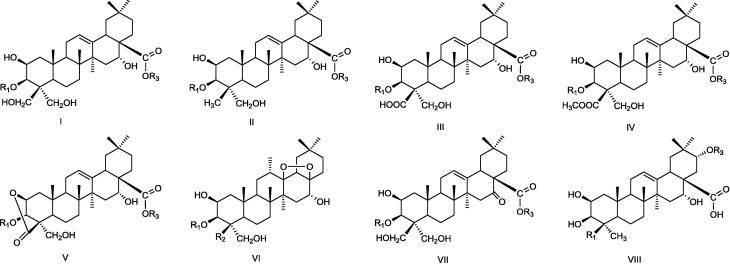
To date, as the main active components of Platycodonis Radix, 89 kinds of platycosides have been isolated belonging to eight kinds of triterpenoid saponins, including six kinds with methyl esters (Fig. 2) (Xu, Luo, Xie, Di, Guo, & Shan, 2021). Platycodin D (PD) (Fig. 3) is a chemical marker for the quality control of Platycodonis Radix in the Chinese Pharmacopeia (2020 edition) with no less than 0.1%. In functional foods, it is rare to have apiose as glycosyl group. The apiose in platycosides is usually connected to the end of the C-28 sugar chain, and PD is the first saponin containing apiose isolated from plants (Hiroshi, Kazuo, Takehiko, & Yohko, 1984). Table 1 showed the chemical structure of main saponins in fermented roots of P. grandiflorum (Ha et al., 2006, Ha & Kim, 2010, Liu, Wei, & Zheng, 2013).
Fig. 2.
Chemical structures of aglycones from P. grandiflorum roots. The parent nucleus is connected at (I) C-24 is CH2OH; (II) C-24 is CH3; (III) C-24 is COOH; (IV) C-24 is COOCH3; (V) C-24 and C-2 are connected by ester bond; (VI) C-28 and C-13 are connected by ester bond; (VII) C-24 is CH2OH, C-16 is carbonyl; (VIII) C-23 is CH3, C-24 is R1, C-21 is OR3.
Fig. 3.
Chemical structures of Platycodin D, 3-O-β-D-Glucopyranosylplatycodigenin and 3-O-β-D-Glucopyranosylplatyconic acid.
Table 1.
Chemical structures of main saponins in fermented roots of P. grandiflorum.
| Compounds | Molecula fomula | Types | R1 | R2 | R3 |
|---|---|---|---|---|---|
| Deapi-platycoside E (Platycoside G1) |
C64H104O34 | I | S1 | − | S2 |
| Platycoside E | C69H112O38 | I | S1 | − | S3 |
| Deapi-platycoside D3 | C58H94O29 | I | −Gen | − | S2 |
| Platycoside D3 | C63H102O33 | I | −Gen | − | S3 |
| Deapi-platycoside D | C52H84O24 | I | −Glc | − | S2 |
| Platycodin D | C57H92O28 | I | −Glc | − | S3 |
| Polygalacin D | C57H92O27 | II | −Glc | − | S3 |
| 3″-O-Acetyl polygalacin D | C59H94O28 | II | −Glc | − | S4 |
| Platycodin A | C59H94O29 | I | −Glc | − | S5 |
| 2″-O-Acetyl polygalacin D | C59H94O28 | II | −Glc | − | S5 |
Note: S1, −Glc(6 → 1) Glc(6 → 1)Glc; S2, −Ara(2 → 1) Rha(4 → 1)Xyl; S3, −Ara(2 → 1) Rha(4 → 1) Xyl(3 → 1)Api; S4, −Ara(2 → 1) Rha-Ac-O-3-(4 → 1) Xyl(3 → 1)Api; S5, −Ara(2 → 1) Rha-Ac-O-2-(4 → 1) Xyl(3 → 1)Api; Gen, −Glc(6 → 1) Glc.
Na, Ha, Kim, & Kim, 2008 elucidated the chemical structures of platycosides and prosapogenins from Platycodonis Radix by HPLC/MSn. The nomenclature of a general platycosides are shown in Fig. 4 (Domon & Costello, 1988).
Fig. 4.

Fragments ions of a general platycosides.
3. Metabolic process of platycosides in vivo
How do the intestinal microflora transform platycosides in the body? Shan et al. (2015) examined the differences between PD in monomer and Platycodi Radix extract (PRE) in terms of pharmacokinetic properties, intestinal absorption, and microbial metabolism. It was found that after intragastric administration of 20 mg/kg PD in rat and the same amount of PD in PRE, the maximum plasma concentration reached 44.5 ng/mL and 17.94 ng/mL at 30 min and 75 min, respectively. Drug concentration–time curve product (AUC) was (73.00 ± 24.17) h·ng/mL and (96. 06 ± 48. 51) h·ng/mL. The results indicated that the absorption degree of PD in PRE was enhanced significantly compared with that of single PD.
Analysis of platycosides metabolites from rats plasma by UPLC-Q/TOF-MS showed that the major absorbed platycosides were 3-O-β-D-glucopyranosylplatyconic acid (GPA) and 3-O-β-D-glucopyranosylplatycodigenin (GP) (Fig. 3). And GPA was likely to be the main absorbed saponin in vivo (Tang et al., 2017). In the paper by Zhang et al., a new method involving gut microbiota biotransformation, spectrum-effect relationship analysis and metabolomics analysis has been reported. And a total of 22 common microbial metabolites were tentatively identified from the mice’s gut microbiota transformations, and their potential parent platycosides were also found (Zhang et al., 2022). In Yau’s study (2022), 25 deglycosylated metabolites of platycosides after dietary consumption were profiled in rat plasma, urine and feces. Two new 3-O-β-D-glucopyranosyl platycosides (M3 and M11, Fig. 5) were unambiguously characterized. The metabolites of rats were analyzed by UHPLC-QTOF-MS, the possible deglycosylation metabolism pathway of platycosides in vivo were both intestinal bacterial metabolism and hydrolysis of ester linkage at C-28 by carboxylesterases in liver.
Fig. 5.
Chemical structures of M3 and M11.
4. Biotransformation of platycosides in vitro
Due to the difficulty of purification of metabolites in the body, to obtain saponins with high medicinal value, in recent years, researchers in and outside China have worked extensively on the transformation of platycosides. Prosapogenin is the aglycone moiety linked only with a 3-O-sugar chain (Nyakudya, Jeong, Lee, & Jeong, 2014). In addition, PD with short 28-O-glycosyl chains is called dexyl-platycodin D (dPD). Deglycosylated platycosides such as dPD are reported to have bioactivities better than glycosylated platycosides (Kang, Kim, Shin, Ko, & Oh, 2019, Shin, Kil, Lee, & Oh, 2021).
In vitro transformation methods include chemical, enzymatic hydrolysis and microbial transformation techniques. Nowadays, various methods have been applied to prepare P. grandiflorum saponins, including chemical methods such as mild acid hydrolysis, alkali treatment, biological conversion, etc (Nyakudya, Jeong, Lee, & Jeong, 2014). Biological transformation offers a shorter cycle, less pollution, and higher product purity than chemical techniques.
4.1. Enzymatic hydrolysis
Enzymatic hydrolysis has very great specificity. Referring to previous paper, enzymes with different properties act on glycosidic bonds of different configurations and compositions for the purpose of directional hydrolysis (Zhang, Chen, & Zhao, 2008). PRE contains two major PD precursors such as platycoside E and platycodin D3 which can be enzymatically converted to PD via β-D-glucosidase hydrolysis (Ahn et al., 2018). The commercial enzymes also can convert deapiosylated platycosides and platycosides.
Li et al., 2012, Li et al., 2012 reported snailase showed a strong ability to transform deapiosylated platycoside E (deapio-PE) and platycoside E (PE) into deapiosylated platycodin D (deapi-PD) and PD with 100% conversion. The biotransformation pathways were deapi-PE → deapi-PD3 → deapi-PD and PE → PD3 → PD, respectively. Ha et al. (2010) selected the enzyme with the highest ability to convert six major saponins (deapi-PE, PE, deapi-PD3, PD3, deapi-PD, and PD) and optimized conditions to obtain higher amounts of PD by HPLC analysis. The results revealed that cellulase, β-galactosidase, and β-glucosidase were able to transform PE and PD3 into PD but neither glucuronidase had any effect (Table 2). They also developed an efficient method for the isolation and purification of PD from enzymatically transformed products by preparative HSCCC.
Table 2.
The relative ratio of major saponins after enzymatic reaction.
| Enzymes | Origin | Relative ratio (%) |
||
|---|---|---|---|---|
| PE | PD3 | PD | ||
| Control (no enzyme) | No incubation | 23.8 | 14.9 | 61.3 |
| Control | Incubation | 24.0 | 14.6 | 61.5 |
| Glucosidase | Almonds (Sigma) | 14.2 | 15.5 | 70.3 |
| Glucosidase | Almonds (Fluka) | 21.2 | 14.4 | 64.4 |
| Cellulase (solid) | Trichoderma reesei | 0.0 | 0.0 | 100.0 |
| Cellulase (liquid) | Trichoderma reesei | 0.0 | 0.0 | 100.0 |
| Galactosidase | Aspergillus oryzae | 4.2 | 10.6 | 85.3 |
| Glucuronidase | Escherichia coli | 24.2 | 14.2 | 61.6 |
| Glucuronidase | Helic pomatia | 20.4 | 15.4 | 64.2 |
Laminarinase was a suitable enzyme to recognize the glycosylated platycosides by enzymatic hydrolysis; its enzymatic activity was assessed using gentiobiose and laminaritriose (Jeong, Ha, Kim, & Na, 2014). It produced deapi-PD and PD from the isolated deapi-PE and PE through the loss of two glucose units by enzymatic reaction, respectively. The researchers optimized enzymic reaction conditions to convert the PD precursors into PD with the highest productivity, in which the total PD content was as high as 48% (w/w). Caldicellulosiruptor bescii β-glucosidase converts the platycosides by four pathways including deapi-PE → deapi-PD3 → deapi-PD, PE → PD3 → PD, polygalacin D3 → polygalacin D, and 3″-O-acetyl polygalacin D3 → 3″-O-acetyl polygalacin D (Kil, Kang, Kim, Shin, & Oh, 2019). The substrate specificity of β-glycosides from C. owensensis was measured by aryl-glycosides and platycosides (Shin, Seo, Kim, Yeom, & Kim, 2019). Under the optimum reaction conditions, recombinant β-glycosides completely transformed PE into PD. The concentration and productivity were the highest reported since 2019. Shin et al. (2020) examined the enzymatic conversion of platycosides and showed that Cytolase PCL5 completely converted PE and polygalacin D3 into deapiose-xylosylated PD and deapiose-xylosylated polygalacin D, respectively.
4.2. Microbial transformation
Microbial transformation is to transform a compound into more economically valuable products via one or more enzymes produced by microbial cells, so as to complete biochemical reactions that are difficult to be achieved by conventional chemical methods. In essence, it is to catalyze exogenous substrates by enzymes produced by microorganisms themselves.
Among the numberous edible microorganisms, Aspergillus, Lactobacillus, and Bifidobacterium spp. are considered by the food industry as effective β-D-glucosidase producers (Ahn et al., 2018, Kang, Kim, Shin, Ko, & Oh, 2019). A crude enzyme extract from Aspergillus niger can modify PD to several partially degraded platycodin glycosides (Wei, Zhao, Chang, Kim, Hwang, & Ji, 2007). Researchers demonstrated the metabolites structures and determine metabolic pathway by human intestinal bacteria converted PD in PRE into PD metabolites by LC-MS3 (Ha, Na, Ha, Kim, & Kim, 2010). Lee et al. (2016b) identified a new strain of microorganism-Cyberlindnera fabianii for producing PD. It is the first non-Saccharomyces yeast that can significantly enhance PD level in P. grandiflorum roots, which is isolated from nuruk − a traditional Korean starter. Huang used Pichia kudriavzevii, Bacillus velezensis and the two mixed strains to ferment platycodon saponins. The deapi-PD and PD in 30 °C were higher in content than 37 °C; the deapi-PD increased by 16.88%, 3.83%, 22.71%, respectively with increasing temperature, so did PD by 8.86%, 11.21%, 8.55%, respectively. It showed the mixed fermentation mode was a pathway for transformation of platycosides (Huang, 2019). Korean researchers reported that platycosides content of P. grandiflorum roots fermented by lactic acid bacteria such as Leuconostoc plantarum N56-12 was higher than the non-fermented extract. The results demonstrated that the fermented had more antibacterial action against bacteria that cause bronchus illness than the non-fermented did (Lee et al., 2016a).
The crude enzyme from Rhizopus oryzae, a food-available fungus is a new fermentation microorganic strain to obtain deapiosylated platycosides (Shin, Kil, Lee, & Oh, 2021). Compared with a previous study that β-glucosidase from Dictyoglomus turgidum converted platycosides into deglucosylated ones (Kang, Kim, Shin, Ko, & Oh, 2019), Ju et al. (2021) reported that pectinase from Aspergillus aculeatus can biotansformate glycosylated platycosides into 3-O-β-D-glucopyranosyl platycosides (Fig. 6). PE and polygalacin D3 (PGD3) can be converted into deglucose-apiose-xylosylated (deGAX)-PD and deGAX-polygalacin D (PGD), respectively, by a crude enzyme from A. tubingensis. The crude enzyme was the most effective in producing deGAX platycoside at pH 5.0 and 60 °C (Shin, Kil, Kang, & Oh, 2022). The biotransformation pathway of platycosides into deglycosylations are shown in Fig. 6.
Fig. 6.
Biotransformation pathway of platycosides into deglycosylations. (A) Biotransformation pathway of platycoside E. (B) Biotransformation pathway of polygalacin D3. (C) Biotransformation pathway of platyconic acid A. (D) Biotransformation pathway of 3″-O-acetyl polygalacin D3. (E) Biotransformation pathway of platycodin A.
5. Pharmacological activities
Microbial fermentation technology has improved over the past few decades and is now a safe way to increase the amount of active ingredients in medicinal plants. Fermentation can use the natural compounds in plants to produce secondary metabolites with higher biological activities (Yu, 2015). It is recognized that there is a strong correlation between the biological actions of saponins and the chemical structure of their glycochains (Shi, Wang, Shi, & Tan, 2017). Clinically, aglycones can be used in patients, especially the ones with dysbiosis of intestinal flora under pathological conditions. After taking degradations, they can be directly absorbed by the body without intestinal microbial transformation. Compared with glucosides, secondary saponins have been widely used due to the advantages of rapid effect. Deglycosylated platycosides, the products of biotransformational platycosides, were reported to exhibit larger activity than glycosylated platycosides in numerous studies (Shin, Kil, Lee, & Oh, 2021, Yu, Li, Li, Han, & Cui, 2016, Zheng, Liu, Zheng, & Li, 2011).
5.1. Anti-inflammatory activity
Platycosides are the key compounds of anti-inflammatory activity of Platycodonis Radix. Saponins with different lengths of sugar chain possess different activities. Compared with baicalein as positive control, Kang et al evaluated platcosides bioactivities using a Lipoxygenase (LOX) inhibitory assay in vitro (Kang, Kim, Shin, Ko, & Oh, 2019). The found that Deglu PD and deglucosylated PRE showed higher anti-inflammatory effects than PE, PD3, PD, PRE and baicalein at 4 μmol/L. A another study showed that the order of anti-inflammatory activity was deapi-PD (four glycosides) > PD (five glycosides) > PE (seven glycosides) at 4 μmol/L of each sample. indicating that the anti-inflammatory effect of the same type platycosides increased with the decreasing number of glycosides (Shin, Kil, Lee, & Oh, 2021).
5.2. Expectorant activity
Platycodonis Radix is a traditional Chinese medicine commonly used in China, with the effects of relieving cough and resolving phlegm, in which platycosides are the major components responsible for antitussive and expectorant effects. Metabolomic analysis showed that the active forms of platycosides fraction may be their microbial metabolites (Zhang et al., 2022). The results demonstrated that they both exhibited the effects at the same dosage after oral administration of 3.2 g/kg crude drug for mice. A total of 11 active antitussive microbial metabolites and 12 active expectorant microbial metabolites were identified by spectrum-effect relationship analysis, including eight shared components (Zhang et al., 2022). The expectorant effects of total saponins and secondary saponins conversion with A. niger in an animal experiment were studied (Zheng, Liu, Zheng, & Li, 2011). Cough model was induced by phenol red expectoration test. The high dose (0.5 g/kg orally) and middle dose groups (0.3 g/kg orally) of total saponins and secondary saponins conversion had expectorant effect on mice induced by phenol red (P < 0.05), revealing the samples had the expectorant effect.
5.3. Antioxidant activity
Juice from Platycodonis Radix after enzymolysis treatment showed a proven significant antioxidant activity (Li, Ma, Zhao, Ding, Zhu, & Chu, 2021). At the concentration of 0.8 mg/mL, DPPH radical scavenging rate, hydroxyl radical scavenging rate and FRAP (Ferric ion reducing antioxidant power) antioxidant capacity reached 94.96%, 80.59% and 2.18 mmol/L, respectively. And the antioxidant capacity was greater than Vc at the concentration of 0–1.0 mg/L.
Li (2018) fermented PRE using No. 15 strain from the nutrient agar medium Bacillus velezensis and YPD-21 strain from YPD solid medium Pichia kudriavzevii, respectively. Studies have shown that the content of PD, total flavonoids and total phenols increased significantly in the extract at 50 mg/mL. The scavenging capacity was increased for DPPH, hydroxyl radicals and superoxide anion. And No.15 strain significantly improved the antioxidant capacity of the extract in vitro after fermentation.
Besides, the total anti-antioxidant activity (TAC) of deapiosylated platycosides was assayed at 1 mmol/L. The trolox equivalent capacity values of the platycodigenintype platycosides were in the order of deapi-PD (four glycosides) > PD (five glycosides) > PE (seven glycosides). The trolox equivalent capacity values of the deapiosylated platycosides were in the following order: deapi-PD > deapi-PA > deapi-PCA > deapi-PGD. These results indicate that deapi-PD is the most efficient antioxidant among them (Shin, Kil, Lee, & Oh, 2021).
5.4. Anti-diabetic activity
On rats with type II diabetes, the fermented PRE has been shown to exhibit hypoglycemic effects (Yu, Li, Li, Han, & Cui, 2016). The dry powder of P. grandiflorum roots was fermented by lactic acid bacteria and yeast. The product was tested for anti-diabetes in rats, the dosage using the concentrations of 0.8 g/kg·BW. Compared with unfermented dried powder, the total saponins content increased from 3.50% to 5.44%. After fermentation, the main hypoglycemic components in P. grandiflorum roots were generally improved. The fermented extract can significantly reduce the blood glucose level of diabetic animals, enhance OGTT results; increase glycogen reserves, improve the T-SOD content, and reduce the MDA level in serum. At the same concentration of the two extracts, the scavenging capacity of DPPH radical increased by 5%–7%, the scavenging capacity of ·OH radical increased by about 4%–9%.
5.5. Antiviral activity
An earlier study showed that modifying total platycodins may increase their bioactivities and antiviral activity (Xu, Mohammed, Gao, & Qin, 2006). The researchers investigated the effect on the growth of Chick Embryo Fibroblasts (CEF) cells line to study the antivirus activity. The results suggested that transformed platycodins (25 μg/mL) could prevent Infectious Bursal Disease Virus (IBDV), Avian Influenza Virus (AIV) and Newcastle Disease Virus (NDV) intruding into CEF cells by 84.7%, 63.5% and 45.5%, compared with inhibition rate of total platycodins 66.3%, 55.4% and 36.9%, respectively. They also inhibited IBDV, AIV and NDV to reproduce in CEF cell line by 77.8%, 63.5% and 41.9%, respectively, compared with the reproducing inhibition rates of total ones were 69.4%, 60.8% and 26.2%, respectively (Xu, Mohammed, Gao, & Qin, 2006).
6. Discussion
Saponins from roots of P. grandiflorum showed biological activities of anti-inflammatory, expectorant, antioxidant, liver protection, anti-diabetic and lipid-lowering (Wang, Wang, Wang, Shi, & Wu, 2022). The saponins can be biotransformed in vivo and in vitro, by which the sugar chains were reduced. Researchers have found that the shorter the sugar chains of the saponins are, the stronger the bioactivities of the saponins are, due to increament of bioavailability and cellular permeability (Li et al., 2012, Li et al., 2012, Wang et al., 2007). For example, the order of anti-inflammatory and antioxidant activities of platycosides were as follows: deapi-PD (4 glycosides) > PD (5 glycosides) > PE (7 glycosides) (Shin, Kil, Lee, & Oh, 2021). Xu et al. reported that platycosides can be increased in antivirus activities after structure modification (Xu, Mohammed, Gao, & Qin, 2006). However, less studies have been carried out in clinic application or efficiency of those secondary saponins. In addition, the metabolic fates after dietary consumption of most secondary saponins are still unclear (Yau et al., 2022). Some secondary saponins have been mixedly bioassaied but not seperately (Yu, Li, Li, Han, & Cui, 2016). Therefore, it is necessary to produce more platycosides for biological and clinic studies
Goubao snack is an example that platycosides of high bioactivities can be produced by nature fermentation. However, the platycosides greatly changes in yield and diversity duo to changes of fermentation strains and physical conditions. So it is hard to isolate the target platycosides. It is generally believed that microorganism strains are the key to produce target platycosides. Lactic acid bacteria strains are often utilized for platycoside biotransformation due to the ability of generating β-glucosidase. Therefor we proposed that target platycosides could be produced more by certain strains under certain fermentation conditions. Now we are screening the responsible strains and establishing optimal fermentation conditions.
7. Conclusion
Recent years have seen a resurgence of interest in and research into triterpenoid saponins due to their broad chemical variety (He et al., 2019). Platycodi Radix is a kind of traditional Chinese herbal medicine, rich in oleanane-type triterpenoid saponins, not only has good medicinal value, but also can be used as a food. This review summarized secondary products of saponins in P. grandiflorum roots in the biotransformation and bioactivities. It has a certain guiding significance for research on the health care effects of the Platycodi Radix ferment food − Goubao pickle, providing new ideas for large-scale industrial deglucosylated platycosides to prevent and treat chronic diseases.
CRediT authorship contribution statement
Lin Shi: Data curation, Formal analysis, Visualization, Writing – original draft. Tong Cui: Writing – review & editing. Xinyue Wang: Writing – review & editing. Rina Wu: Supervision, Writing – review & editing. Junrui Wu: Supervision, Writing – review & editing. Yanqun Wang: Writing – review & editing. Weiming Wang: Conceptualization, Data curation, Project administration, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. U20A20400) and the Scientific Research Foundation of Heilongjiang Provincial Scientific Research Institutes (No. CZKYF2021A002). Young and middle-aged graduate tutor training program of Shenyang Agricultural University (No. 2022-ZZDS-09).
Contributor Information
Rina Wu, Email: wrn6956@163.com.
Weiming Wang, Email: wangweiming475@yahoo.com.
References
- Ahn H., You H., Park M., Johnston T.V., Ku S., Ji G.E. Biocatalysis of platycoside E and platycodin D3 using fungal extracellular β-glucosidase responsible for rapid platycodin D production. International Journal of Molecular Sciences. 2018;19(9):2671. doi: 10.3390/ijms19092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Pharmacopeia Commission (2020). Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science and Technology Press.
- Choi Y.J., Lee S.J., Kim H.I., Lee H.J., Ko S.G. Platycodin D enhances LDLR expression and LDL uptake via down-regulation of IDOL mRNA in hepatic cells. Scientific Reports. 2020;10(1):19834. doi: 10.1038/s41598-020-76224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon B., Costello C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate Journal. 1988;5:397. [Google Scholar]
- Fu W.W., Dou D.Q., Shimizu N., Takeda T., Pei Y.H., Chen Y.J. Studies on the chemical constituents from the roots of Platycodon grandiflorum. Journal of Natural Medicines. 2006;60(1):68–72. [Google Scholar]
- Fu X.J., Liu H.B., Wang P., Guan H.S. A study on the antioxidant activity and tissues selective inhibition of lipid peroxidation by saponins from the roots of Platycodon grandiflorum. The American Journal of Chinese Medicine. 2009;37(5):967–975. doi: 10.1142/S0192415X09007375. [DOI] [PubMed] [Google Scholar]
- Gao W., Guo Y., Yang H. Platycodin D protects against cigarette smoke-induced lung inflammation in mice. International Immunopharmacology. 2017;47:53–58. doi: 10.1016/j.intimp.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Ha I.J., Ha Y.W., Kang M., Lee J., Park D., Kim Y.S. Enzymatic transformation of platycosides and one-step separation of platycodin D by high-speed countercurrent chromatography. Journal of Separation Science. 2010;33(13):1916–1922. doi: 10.1002/jssc.200900842. [DOI] [PubMed] [Google Scholar]
- Ha Y.W., Na Y.C., Ha I.J., Kim D.H., Kim Y.S. Liquid chromatography/mass spectrometry-based structural analysis of new platycoside metabolites transformed by human intestinal bacteria. Journal of Pharmaceutical & Biomedical Analysis. 2010;51(1):202–209. doi: 10.1016/j.jpba.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Ha Y.W., Na Y.C., Seo J.J., Kim S.N., Linhardt R.J., Kim Y.S. Qualitative and quantitative determination of ten major saponins in Platycodi Radix by high performance liquid chromatography with evaporative light scattering detection and mass spectrometry. Journal of Chromatography A. 2006;1135(1):27–35. doi: 10.1016/j.chroma.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y.W., Kim Y.S. Preparative isolation of six major saponins from Platycodi Radix by high-speed counter-current chromatography. Phytochemical Analysis. 2010;20(3):207–213. doi: 10.1002/pca.1116. [DOI] [PubMed] [Google Scholar]
- He Y., Hu Z.Y., Li A.R., Zhu Z.Z., Yang N., Ying Z.X.…Cheng S.Y. Recent advances in biotransformation of saponins. Molecules. 2019;24:2365. doi: 10.3390/molecules24132365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroshi I., Kazuo T., Takehiko T., Yohko Y. Saponins from roots of Platycodon grandiflorum. Part 2. Isolation and structure of new triterpene glycosides. Journal of the Chemical Society Perkin Transactions. 1984;I:661–668. [Google Scholar]
- Huang, C. H. (2019). Study on the biotransformation of platycodon saponins by two strains. Yanbian University. Thesis of Master Degree.
- Hwang K.A., Hwang Y.J., Pu R.I., Hwang H.J., Kim Y.J. Platycodon grandiflorum extract reduces high-fat diet-induced obesity through regulation of adipogenesis and lipogenesis pathways in mice. Journal of Medicinal Food. 2019;22(10):993–999. doi: 10.1089/jmf.2018.4370. [DOI] [PubMed] [Google Scholar]
- Jeong E.K., Ha I.J., Kim Y.S., Na Y.C. Glycosylated platycosides: Identification by enzymatic hydrolysis and structural determination by LC-MS/MS. Journal of Separation Science. 2014;37(1–2):61–68. doi: 10.1002/jssc.201300918. [DOI] [PubMed] [Google Scholar]
- Jin W.R., Yu K., Shan C.G., Wang Z.F. Dynamic change of platycodin in Platycodon grandiflorum (Jacq.) A. DC. during course of salt cure. Food and Nutrition in China. 2010;1(1):34–36. [Google Scholar]
- Ju J.H., Lee T.E., Lee J., Kim T.H., Oh D.K. Improved bioactivity of 3-O-β-D-glucopyranosyl platycosides in biotransformed Platycodon grandiflorum root extract by pectinase from Aaspergillus aculeatus. Journal of Microbiology and Biotechnology. 2021;31(6):847–854. doi: 10.4014/jmb.2102.02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.H., Kim T.H., Shin K.C., Ko Y.J., Oh D.K. Biotransformation of food-derived saponins, platycosides, into deglucosylated saponins including deglucosylated platycodin D and their anti-inflammatory activities. Journal of Agricultural & Food Chemistry. 2019;67(5):1470–1477. doi: 10.1021/acs.jafc.8b06399. [DOI] [PubMed] [Google Scholar]
- Kil T.G., Kang S.H., Kim T.H., Shin K.C., Oh D.K. Enzymatic biotransformation of balloon flower root saponins into bioactive platycodin D by deglucosylation with Caldicellulosiruptor bescii β-glucosidase. International Journal of Molecular Sciences. 2019;20(16):3854. doi: 10.3390/ijms20163854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.S., Seong B.J., Kim S.I., Jee M.G., Park S.B., Park M.H.…Kim H.H. Changes in platycoside components and antimicrobial activities of bronchus disease-inducing bacteria of fermented Platycodon grandiflorum root by lactic acid bacteria. Journal of the Korean Society of Food Science and Nutrition. 2016;45(7):1017–1025. [Google Scholar]
- Lee N.K., Nyakudya E., Jeong Y.S. Bioconversion of Platycodon grandiflorum saponins by the platycodin D-converting microorganism, yeast Cyberlindnera fabianii. Journal of Food Biochemistry. 2016;40(3):358–365. [Google Scholar]
- Li G., Ma Y.F., Zhao Y., Ding C., Zhu F., Chu L. Optimization of enzymatic hydrolysis for production of Platycodon grandiflorum juice and antioxidant activity. Food Science and Technology. 2021;46(4):64–69. [Google Scholar]
- Li, M. Z. (2018). Analysis of microbial conversion and antioxidant activity of platycodon D. Yanbian University. Thesis of Master Degree.
- Li N., Wu C.F., Xu X.Y., Liu Z.Y., Li X., Zhao Y.Q. Triterpenes possessing an unprecedented skeleton isolated from hydrolysate of total saponins from Gynostemma pentaphyllum. European Journal of Medicinal Chemistry. 2012;50:173–178. doi: 10.1016/j.ejmech.2012.01.052. [DOI] [PubMed] [Google Scholar]
- Li W., Zhao L.C., Wang Z., Zheng Y.N., Liang J., Wang H. Response surface methodology to optimize enzymatic preparation of deapio-platycodin D and platycodin D from Radix Platycodi. International Journal of Molecular Sciences. 2012;13:4089–4100. doi: 10.3390/ijms13044089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wu Y., Xia Q., Zhao Y., Deng S. Platycodon grandiflorus enhances the effect of DDP against lung cancer by down regulating PI3K/Akt signaling pathway. Biomedicine & Pharmacotherapy. 2019;120 doi: 10.1016/j.biopha.2019.109496. [DOI] [PubMed] [Google Scholar]
- Liu Q., Wei L.I., Zheng Y.N. Review on triterpenoid saponins and pharmacological activity of Platycodon grandiflorum. Journal of Jilin Agricultural University. 2013;35(2):221–228. [Google Scholar]
- Liu Y.M., Cong S., Zhuo C., Hu Y., Lei Y., Zhu L.…Cheng M. Platycodin D alleviates liver fibrosis and activation of hepatic stellate cells by regulating JNK/c-JUN signal pathway. European Journal of Pharmacology. 2020;876(5) doi: 10.1016/j.ejphar.2020.172946. [DOI] [PubMed] [Google Scholar]
- Lu Q., Yu X., Liu Y.Q., Peng L.X., Liao W., Fu Q. Platycosides P and Q, two new triterpene saponins from Platycodon grandiflorum. Journal of Asian Natural Products Research. 2018;21(5):1–7. doi: 10.1080/10286020.2018.1488835. [DOI] [PubMed] [Google Scholar]
- Meng Y.L., Wang W.M., Lv D.D., An Q.X., Lu W.H., Wang X., Tang G.X. The effect of platycodin D on the expression of cytoadherence proteins P1 and P30 in Mycoplasma pneumoniae models. Environmental Toxicology & Pharmacology. 2017;49(1):188–193. doi: 10.1016/j.etap.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Na Y.C., Ha Y.W., Kim Y.S., Kim K.J. Structural analysis of platycosides in Platycodi Radix by liquid chromatography/electrospray ionization-tandem mass spectrometry. Journal of Chromatography A. 2008;1189(1–2):467–475. doi: 10.1016/j.chroma.2007.11.085. [DOI] [PubMed] [Google Scholar]
- Nyakudya E., Jeong J.H., Lee N.K., Jeong Y.S. Platycosides from the roots of Platycodon grandiflorum and their health benefits. Preventive Nutrition and Food Science. 2014;19:59–68. doi: 10.3746/pnf.2014.19.2.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J., Lee H.J., Park S.H., Kim J., Lee D., Lee S.K.…Lee C.J. Effects of the root of Platycodon grandiflorum on airway mucin hypersecretion in vivo and platycodin D3 and deapi-platycodin on production and secretion of airway mucin in vitro. Phytomedicine. 2014;21(4):529–533. doi: 10.1016/j.phymed.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Shan J., Zou J., Xie T., An K., Wang S. Pharmacokinetics, intestinal absorption and microbial metabolism of single platycodin D in comparison to Platycodi Radix extract. Pharmacognosy Magazine. 2015;11(44):750–755. doi: 10.4103/0973-1296.165576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Wang Z.C., Shi S.M., Tan D.H. Research progress on chemical constituents in hydrolyzed products of gypenosides and their pharmacological activities. Drug Evaluation Research. 2017;40(5):711–716. [Google Scholar]
- Shin K.C., Kil T.G., Kang S.H., Oh D.K. Production of deglucose-apiose-xylosylated platycosides from glycosylated platycosides by crude enzyme from Aspergillus tubingensis. Journal of Microbiology and Biotechnology. 2022;32(4):430–436. doi: 10.4014/jmb.2112.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K.C., Kil T.G., Lee T.E., Oh D.K. Production of bioactive deapiosylated platycosides from glycosylated platycosides in balloon flower root using the crude enzyme from the food-available fungus Rhizopus oryzae. Journal of Agricultural and Food Chemistry. 2021;69(16):4766–4777. doi: 10.1021/acs.jafc.0c06756. [DOI] [PubMed] [Google Scholar]
- Shin K.C., Kim D.W., Woo H.S., Oh D.K., Kim Y.S. Conversion of glycosylated platycoside E to deapiose-xylosylated platycodin D by cytolase PCL5. International Journal of Molecular Sciences. 2020;21(4):1207. doi: 10.3390/ijms21041207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K.C., Seo M.J., Kim D.W., Yeom S.J., Kim Y.S. Characterization of β-glycosidase from Caldicellulosiruptor owensensis and its application in the production of platycodin D from balloon flower leaf. Catalysts. 2019;9:1025. [Google Scholar]
- Tang Z., Hou Y., Hu X., Liu A., Yau L., Tong T.…Bai G. Metabolite identification and pharmacokinetic study of Platycodi Radix (Jiegeng) in vivo. Rsc Advances. 2017;7(59):37459–37466. [Google Scholar]
- Wang C., Levis G.B.S., Lee E.B., Levis W.R., Lee D.W., Kim B.S.…Park E. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-α in activated RAW 264.7 cells. International Immunopharmacology. 2004;4(8):1039–1049. doi: 10.1016/j.intimp.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Wang W., Zhao Y.Q., Rayburn E.R., Hill D.L., Wang H., Zhang R.W. In vitro anti-cancer activity and structure–activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemotherapy & Pharmacology. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Wang W.M., Wang N., Shi L., Wu R.N. Research progress of Platycodon grandiflorum functionality and food development. Food Research and Development. 2022;43(18):199–207. [Google Scholar]
- Wei H.J., Zhao H.L., Chang J.H., Kim Y.S., Hwang I.K., Ji G.E. Enzymatic modification of saponins from Platycodon grandiflorum with Aspergillus niger. Journal of Agricultural and Food Chemistry. 2007;55:8908–8913. doi: 10.1021/jf0716937. [DOI] [PubMed] [Google Scholar]
- Xu W.C., Luo Z.C., Xie T., Di L.Q., Guo Q., Shan J.J. Advance in research on Platycodonis Radix and preliminary analysis of quality marker prediction. Journal of Nanjing University Traditional Chinses Medicine. 2021;37(2):294–302. [Google Scholar]
- Xu X.Q., Mohammed K., Gao P.G., Qin A.J. The anti-virus effect of transformed platycodin on IBDV, AIV and NDV. Journal of Yangzhou University (Agriculture and Life Sciences Edition) 2006;27(1):24–28. [Google Scholar]
- Yau L.F., Huang H., Tong T.T., Bai L.B., Zhu G.Y., Hou Y.…Jiang Z.H. Characterization of deglycosylated metabolites of platycosides reveals their biotransformation after oral administration. Food Chemistry. 2022;393(11) doi: 10.1016/j.foodchem.2022.133383. [DOI] [PubMed] [Google Scholar]
- Yim N., Hwang Y., Liang C., Ma J.Y. A platycoside-rich fraction from the root of Platycodon grandiflorum enhances cell death in A549 human lung carcinoma cells via mainly AMPK/mTOR/AKT signal-mediated autophagy induction. Journal of Ethnopharmacology. 2016;194(24):1060–1068. doi: 10.1016/j.jep.2016.10.078. [DOI] [PubMed] [Google Scholar]
- Yoon H.J., Bang M.H., Kim H., Imm J.Y. Improvement of palmitate-induced insulin resistance in C2C12 skeletal muscle cells using Platycodon grandiflorum seed extracts. Food Bioscience. 2018;25:61–67. [Google Scholar]
- Yu T. Yanbian University; 2015. The effect of fermentation on main hypoglycemic composition of Platycodon grandiflorum and its hypoglycemic function. Thesis of Master degree. [Google Scholar]
- Yu T., Li P.F., Li X., Han C.J., Cui C.B. Study on hypoglycemic activity of Platycodon grandiflorum alcohol extract. Food Research and Development. 2016;37(10):19–22. [Google Scholar]
- Zhang C., Wang X.H., Zhong Y.H., Zhou L., Liang J., Zeng J.X.…Yuan C.S. Active microbial metabolites study on antitussive and expectorant effects and metabolic mechanisms of platycosides fraction of Platycodonis Radix. Journal of Chromatography B. 2022;1195(15) doi: 10.1016/j.jchromb.2022.123171. [DOI] [PubMed] [Google Scholar]
- Zhang J., Song N., Liu Y., Guo J. Platycodin D inhibits β-amyloid-induced inflammation and oxidative stress in BV-2 cells via suppressing TLR4/NF-κB signaling pathway and activating Nrf2/HO-1 signaling pathway. Neurochemical Research. 2021;46(3):638–647. doi: 10.1007/s11064-020-03198-6. [DOI] [PubMed] [Google Scholar]
- Zhang L.L., Huang M.Y., Yang Y., Huang M.Q., Shi J.J., Zou L., Lu J.J. Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chemistry. 2020;327(15) doi: 10.1016/j.foodchem.2020.127029. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang Y.L., Yang D.W., Zhang C.H., Zhang N., Li M.H., Liu Y.Z. Platycodon grandifloras-An ethnopharmacological, phytochemical and pharmacological review. Journal of Ethnopharmacology. 2015;164:147–161. doi: 10.1016/j.jep.2015.01.052. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Du C.H., Zhan H.X., Shang C.L., Li R.F., Yuan S.J. Comparative and phylogeny analysis of Platycodon grandiflorus complete chloroplast genomes. Chinese Traditional and Herbal Drugs. 2023;54(15):4981–4991. [Google Scholar]
- Zhang Y.X., Chen X.Y., Zhao W.Q. Advances in studies on biotransformation of ginsenosides. Journal of Shenyang Pharmaceutical University. 2008;25(5):419–422. [Google Scholar]
- Zheng F.H., Liu W.C., Zheng Y.N., Li W. Comparation of expectorant effect of total platycosides and secondary saponin from the roots of Platycodon grandifloruma. Journal of Jilin Agricultural University. 2011;33(5):541–544. [Google Scholar]