Abstract
Ferumoxytol is an ultra-small superparamagnetic iron oxide (USPIO) particle that is FDA-approved for parenteral treatment of iron deficiency anemia in adults with chronic kidney disease. Because of the association between gadolinium-based contrast agents and nephrogenic systemic fibrosis in patients with severe chronic kidney disease, we sought to evaluate the diagnostic role of ferumoxytol-enhanced MR venography in children with chronic kidney disease. Twenty children underwent 22 high-resolution ferumoxytol-enhanced MR venography examinations at 3.0 T. High-resolution 3-D contrast-enhanced imaging was performed at a minimum of 3 time points following injection of ferumoxytol at a total dose of 4 mg/kg. Two blinded pediatric radiologists independently scored six named veins on ferumoxytol-enhanced MR venography examinations according to a three-point subjective score, where a score ≥2 was considered diagnostic. Additionally, all relevant venous structures in the included field of view were analyzed for occlusive or non-occlusive thrombosis, compression and presence of collaterals. All patients underwent ferumoxytol-enhanced MR venography successfully and without adverse event. The overall scores of the reviewing radiologists for all venous structures were 2.7–2.9. In all cases, the reviewers were confident basing their diagnosis on the ferumoxytol-enhanced MR venography findings. In 12 of 22 examinations, findings on follow-up imaging or invasive procedures were available to correlate with the findings on ferumoxytol-enhanced MR venography. There was complete concordance between the findings from follow-up imaging and invasive procedures with findings from ferumoxytol-enhanced MR venography. Ferumoxytol holds promise as a powerful alternative to gadolinium-based contrast agents for reliable, high-resolution MR venography in children with chronic kidney disease.
Keywords: Children, Chronic kidney disease, Ferumoxytol, Magnetic resonance venography, Vascular mapping
Introduction
Children with chronic kidney disease frequently require detailed vascular mapping for venous access planning, transplantation work-up and vascular patency assessment. Ultrasound is a useful first-line test, but acoustic access is variable and results are often inconclusive. Contrast-enhanced CT venography is also widely used, although less often in children because of concerns about contrast nephrotoxicity and cumulative radiation dose. Contrast-enhanced MR arteriography and venography are excellent radiation-free techniques for children [1]. However, the use of contrast-enhanced MR angiography in patients with chronic kidney disease has been negatively impacted in recent years by concerns about nephrogenic systemic fibrosis (NSF), a potentially debilitating disorder described in patients with chronic kidney disease who received certain gadolinium-based contrast agents (GBCA) [2].
Initially described as a vascular imaging agent almost two decades ago [3], ferumoxytol has been viewed in recent reports as a potential alternative to gadolinium-based contrast agents in patients with chronic kidney disease [4]. Ferumoxytol is an ultra-small, superparamagnetic iron oxide (USPIO) particle that is U.S. Food and Drug Administration (FDA)-approved for intravenous treatment of iron deficiency anemia in adults with chronic kidney disease [5]. It is marketed in the U.S. as Feraheme (AMAG Pharmaceuticals, Lexington, MA) and was the only intravenous iron supplement approved for rapid bolus intravenous injection (up to 30 mg/s) until recently. In March 2015, the FDA issued a boxed warning highlighting severe and potentially fatal anaphylactoid reactions noted in post-marketing data for ferumoxytol when administered to adults with renal failure [6]. Although our experience is limited, we are unaware of any published severe reactions to ferumoxytol associated with its use as an MRI contrast agent in children.
Ferumoxytol exerts a strong T1 shortening effect that greatly enhances the signal from blood on appropriately weighted pulse sequences. The iron particle of ferumoxytol is embedded in a dextran derivative coat, which prolongs its intravascular half-life and decreases leakage into the extravascular space. Both high T1 relaxivity and long intravascular residence time are positive attributes for a venographic imaging agent [7]. Preliminary studies in adults and children suggest ferumoxytol would be a useful vascular imaging agent in patients with chronic kidney disease [8]. However, clinical experience with ferumoxytol for high-resolution vascular imaging is limited.
We sought to evaluate ferumoxytol for high-resolution venous imaging in children with chronic kidney disease, and to evaluate image quality parameters and diagnostic performance.
Description
Informed parental consent was obtained for all prospective individual participants included in the study. The study was approved by the institutional review board and was in compliance with Health Insurance Portability and Accountability Act regulations.
Study population
Twenty pediatric patients who required detailed venographic imaging were referred for ferumoxytol-enhanced MR venography. Two patients had follow-up studies, resulting in a total of 22 consecutive ferumoxytol-enhanced MR venography examinations.
Patients ranged in age from 3 to 21 years (mean age: 10.3 years) and had a mean weight of 31.0 kg. Among the 20 study patients, 16 had hemodialysis dependent chronic kidney disease, 2 had functional renal transplants, 1 had acute kidney injury and 1 had normal renal function. None of the patients had a documented allergy to IV iron replacement products.
Ferumoxytol-enhanced MR venography patients were enrolled prospectively from June 2013 to August 2014. Ferumoxytol-enhanced MR venography was performed to determine vascular patency for a variety of indications, including vascular access planning and pre-/post-transplantation evaluation (Table 1). All 22 ferumoxytol-enhanced MR venography examinations were successfully performed between June 2013 and August 2014. In all patients, the entire vascular bed was clearly visualized and confidently evaluated from the neck to the lower limbs.
Table 1.
Study indications for fereumoxytol-enhanced magnetic resonance venography examinations
| Provided indication | # of examinations |
|---|---|
| Evaluate for new vascular access or for existing catheter dysfunction | 9 |
| Evaluate patient pre- or post-transplant | 6 |
| Evaluate for hemorrhage | 3 |
| Evaluate for deep vein thrombus | 2 |
| Other | 2 |
For 18 of the 22 ferumoxytol-enhanced MR venography examinations, patients were examined under general anesthesia with controlled ventilation. The remaining four examinations were performed without anesthesia as the patients were able to comply with breath-hold instructions. All anesthetized patients were monitored throughout the examination by pediatric anesthesiologists. Inhalational anesthesia was maintained with a mixture of oxygen and sevoflurane and rocuronium bromide was used for muscle relaxation. Positive pressure ventilation was employed using an MR-compatible machine (Fabius MRI; Drager Medical, Telford, PA) with positive end expiratory pressure as considered clinically appropriate. Physiological monitoring included continuous electrocardiogram, pulse oximetry, noninvasive blood pressure and end-tidal CO2 using an MR-compatible monitoring system (InVivo Research, Orlando, FL).
None of the patients experienced an adverse reaction to contrast agent administration, which was defined as a drop in blood pressure, an increase in heart rate or respiratory signs or symptoms. Patients who did not undergo general anesthesia were additionally monitored for nausea and vomiting. One 20-year-old woman was scheduled to undergo ferumoxytol-enhanced MR venography prior to dialysis. She became nauseated and vomited in the waiting room before the examination. She improved initially without treatment but became nauseated and claustrophobic while in the scanner some minutes after receiving a test bolus of ferumoxytol (0.05 mg/kg). The examination was stopped and performed the next day with the full dose of ferumoxytol without incident following dialysis and under anesthesia (Fig. 1).
Fig. 1.
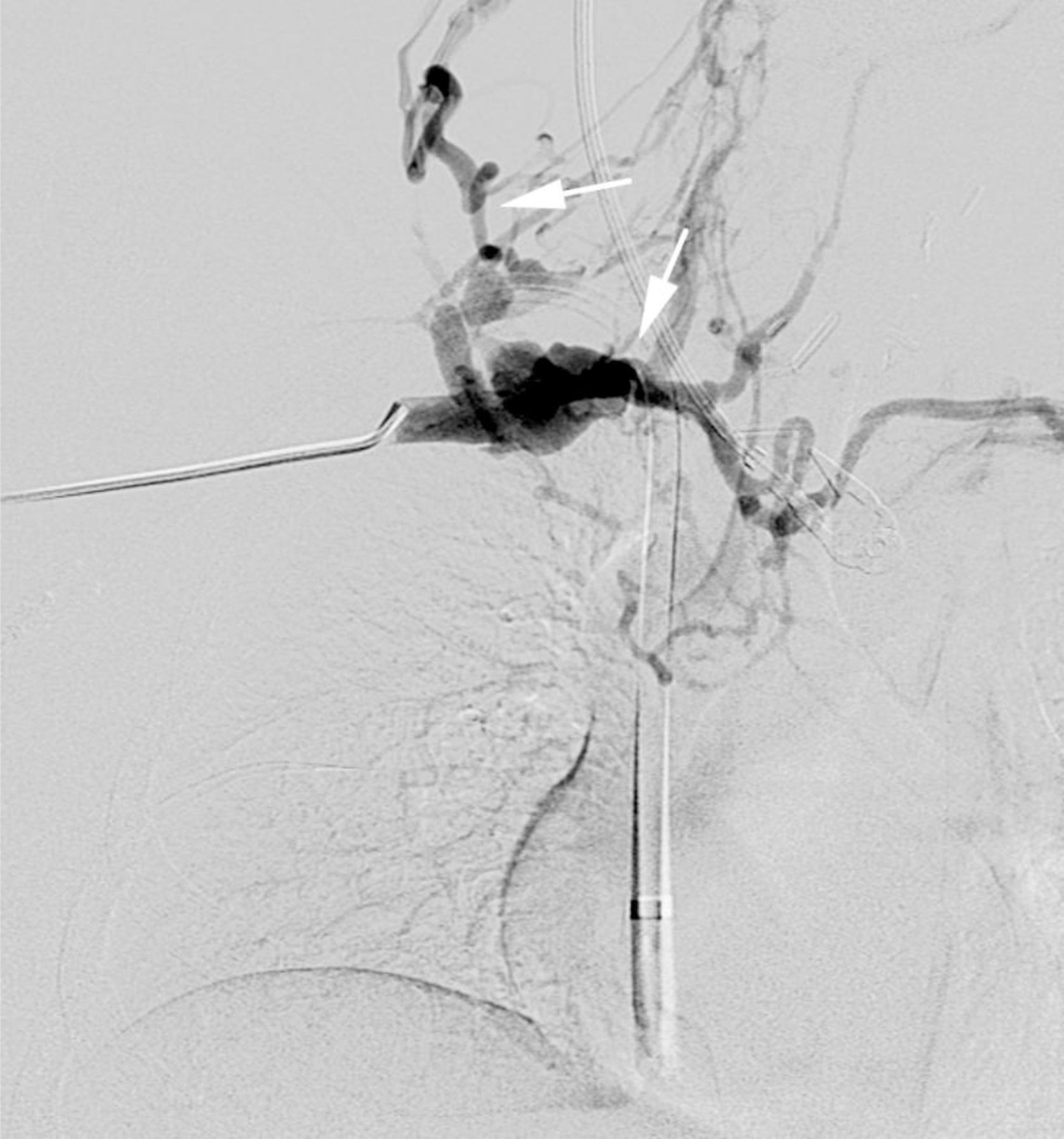
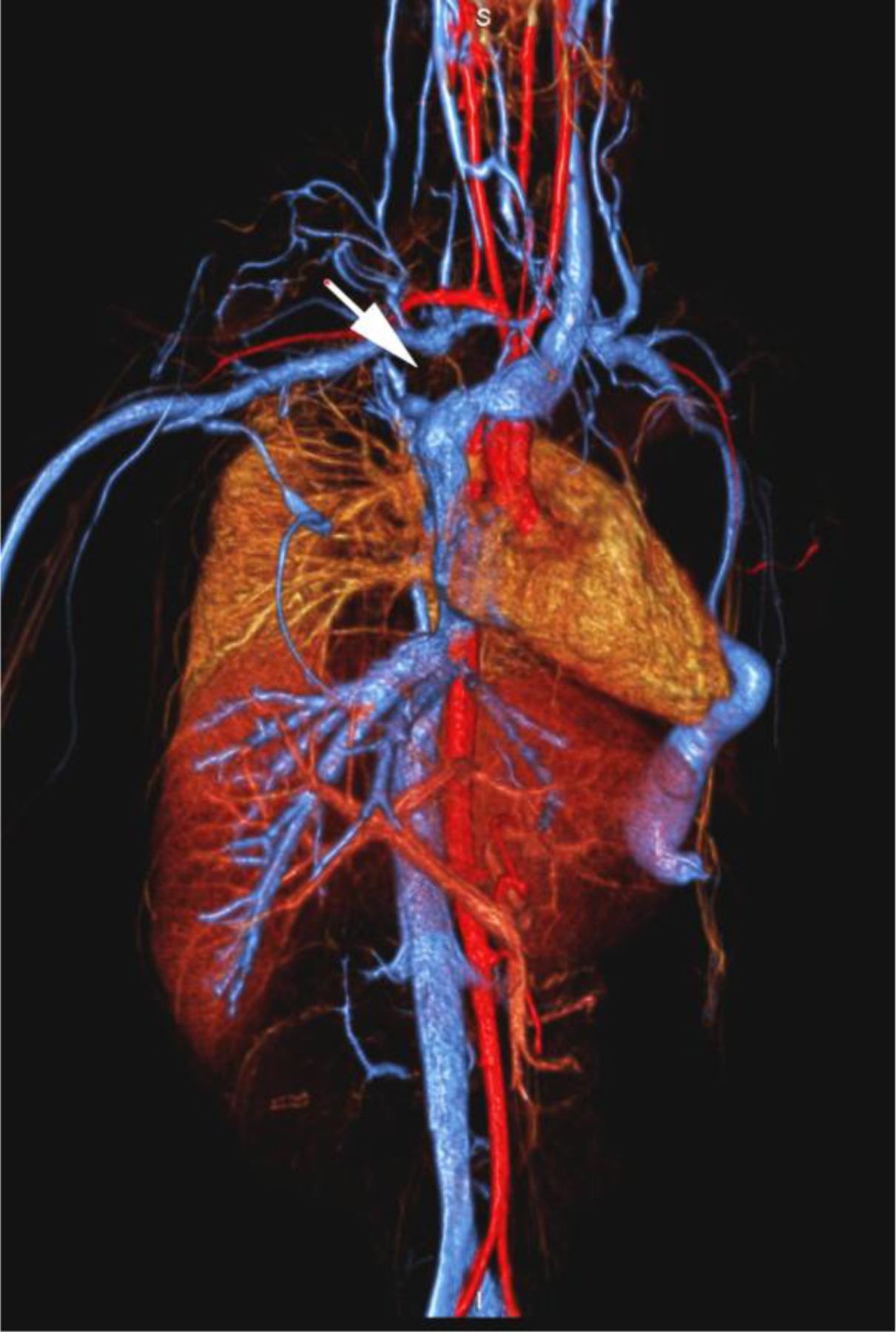
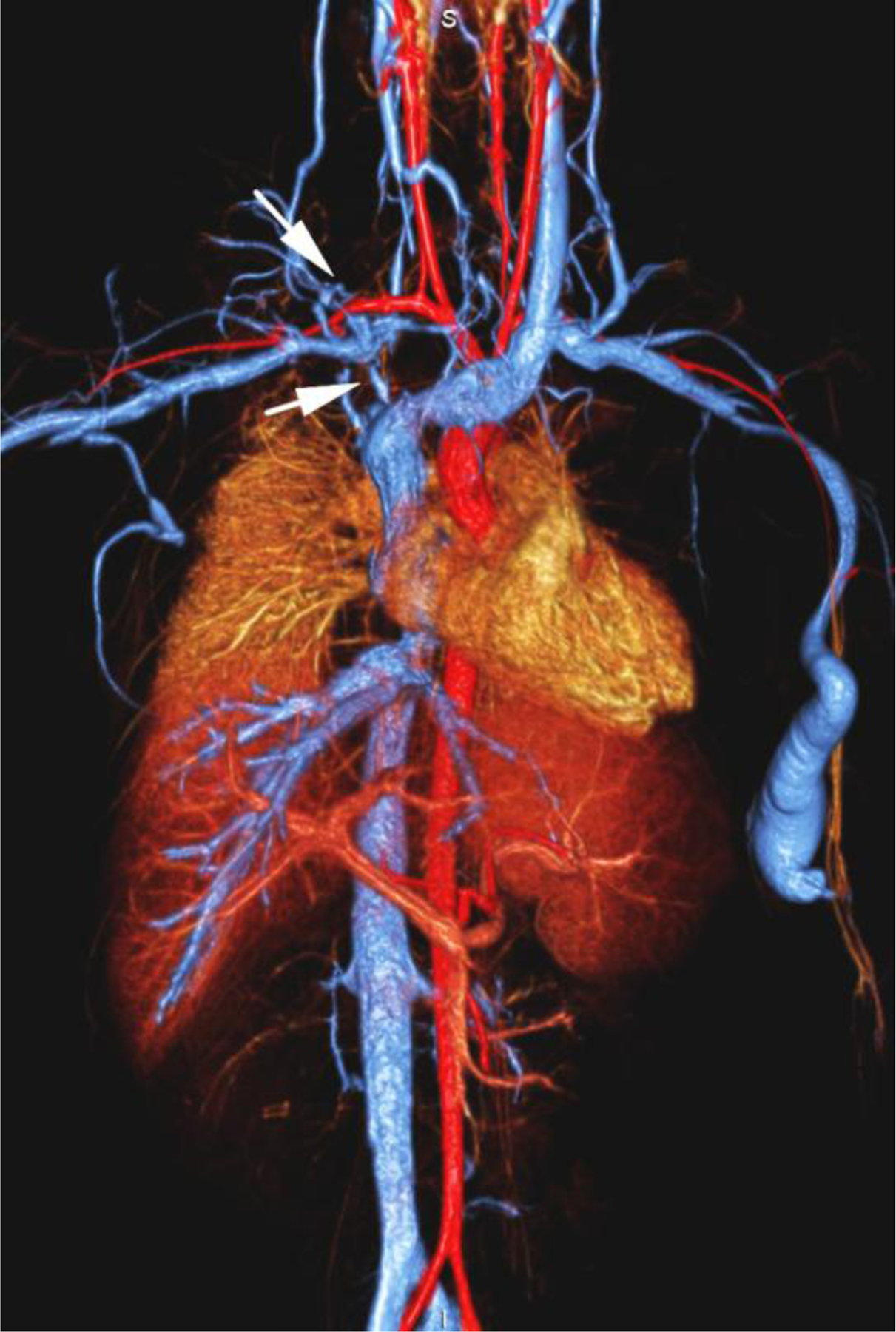
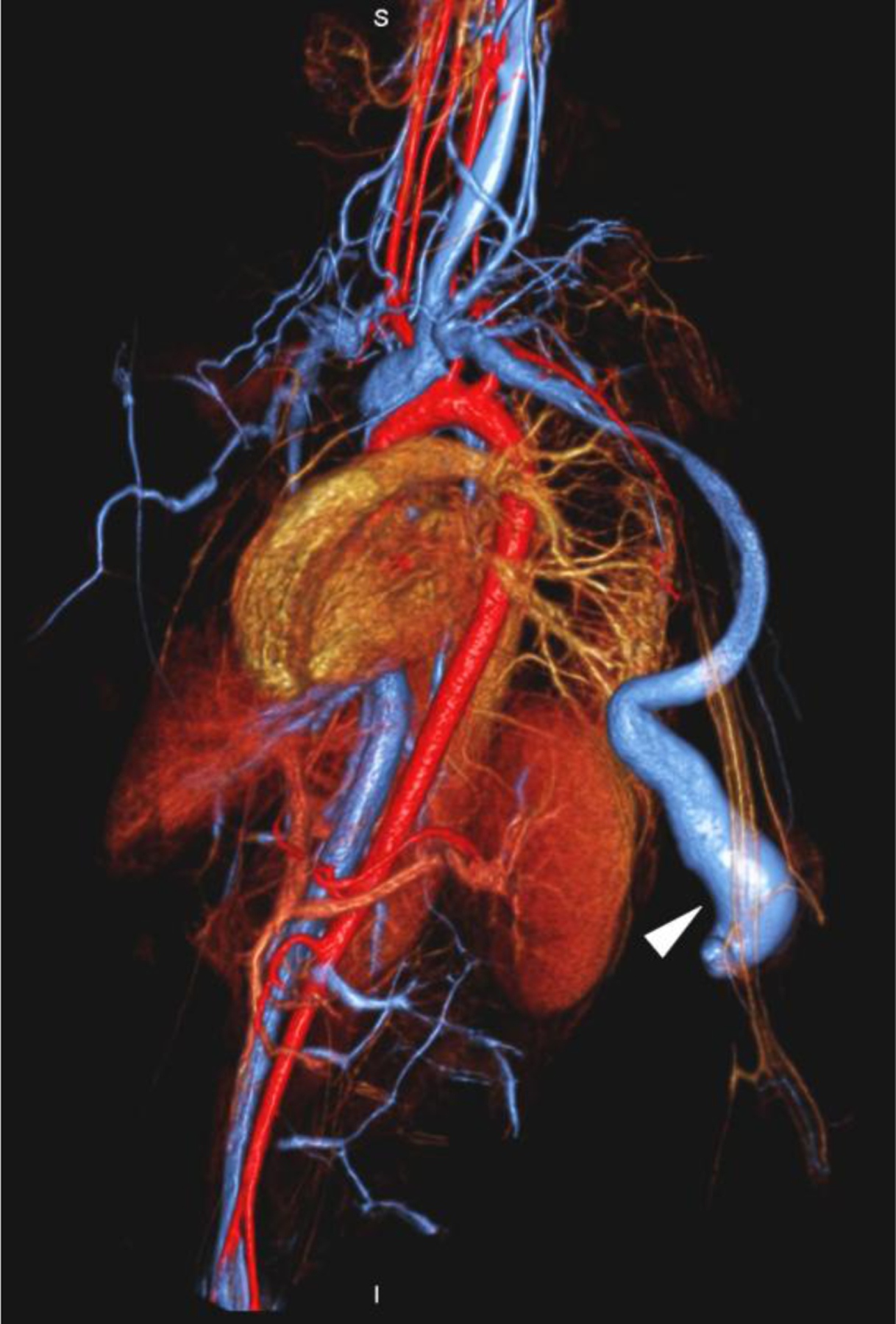
A 20-year-old dialysis-dependent woman with end-stage renal disease and new diagnosis of right upper extremity deep vein thrombus (case 10). Catheter venography shows (a) occlusion of the right brachiocephalic vein with abundant collaterals (arrows). Representative 3-D volume-rendered reconstruction images from ferumoxytol-enhanced magnetic resonance venography confirm the occluded right subclavian, internal jugular and brachiocephalic veins (arrows in b, c), and show a dilated venous limb from a contralateral dialysis fistula (arrowhead in d).
MR imaging
Ferumoxytol-enhanced MR venography examinations were acquired on a 3.0-T scanner (Magnetom TIM Trio; Siemens Medical Solutions, Malvern, PA). High-resolution images, encompassing the neck, thorax, abdomen and pelvis, were acquired following intravenous administration of 4 mg/kg of ferumoxytol. High-resolution 3-D contrast-enhanced images were acquired over a minimum of 3 time points. First pass images were acquired during the arterial phase, early steady-state images were acquired approximately 2 min after injection, and delayed phase steady-state images were acquired in the late venous phase, 20 min or more after injection.
First pass imaging was performed as previously described for gadolinium based contrast agents and was timed for arterial phase enhancement using a test bolus of ferumoxytol [9]. Depending on the result of the timing test bolus, the first pass images were acquired from 8–15 s after the start of the main ferumoxytol injection. The injection rate was changed on a patient-specific basis depending on size, based on the calculated dose of contrast, such that the infusion period was 15 s [9].
Specifically, for first pass imaging, 50% of the total contrast dose was delivered as a bolus infusion over 75% of the image acquisition time, such that the arterial concentration was approximately constant throughout most of the k-space coverage. The infusion duration was routinely 15 s, corresponding to an image acquisition window of 20 s. In order to deliver the calculated dose of 2 mg/kg during the specified time window for first pass imaging, the stock formulation of ferumoxytol (30 mg/ml) was diluted by a factor of 6–10 with normal saline and infused at a rate appropriate to patient weight. In all cases, an MR-compatible electronic injector was utilized (Spectris, Medrad, PA) with dilute ferumoxytol in the contrast syringe A and normal saline in syringe B as a chaser. Respiration was suspended during the first pass acquisition and resumed immediately thereafter. Following first pass acquisition, the remaining 2 mg/kg was infused over 1 min while the patient was ventilated normally.
Early steady-state venous phase imaging was then performed during suspended respiration using the same imaging parameters at a minimum of 2 min following ferumoxytol infusion at a total steady-state dose of 4 mg /kg.
Delayed steady-state imaging was performed during suspended respiration using the same imaging parameters at a minimum of 20 min following ferumoxytol infusion at a total steady-state dose of 4 mg/kg.
The reviewers noted that the appearance of the vessels did not change from early steady-state imaging to delayed steady-state imaging (Fig. 2). The longest interval between the arterial phase imaging and the last measurement delayed steady-state imaging was 50 min.
Fig. 2.
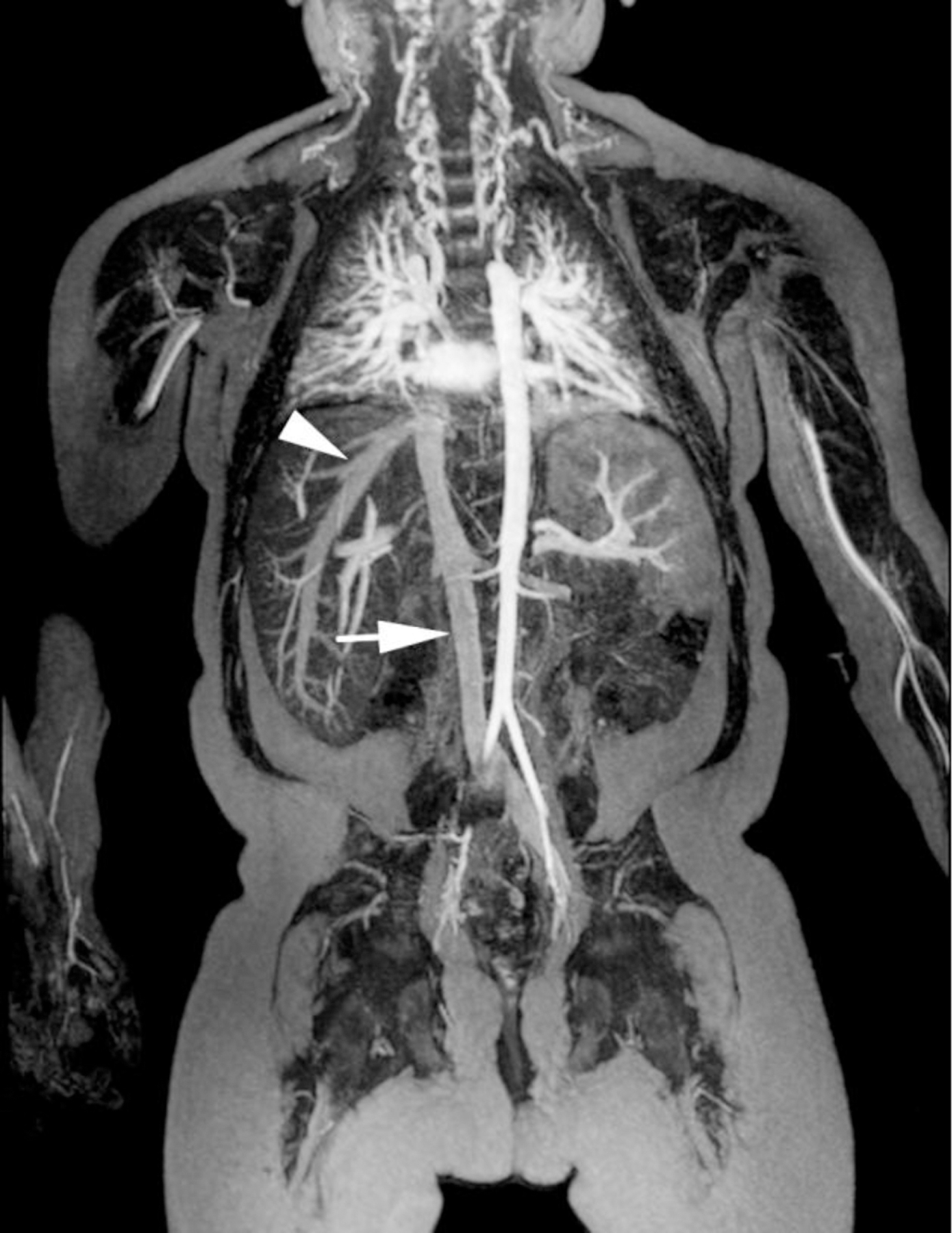
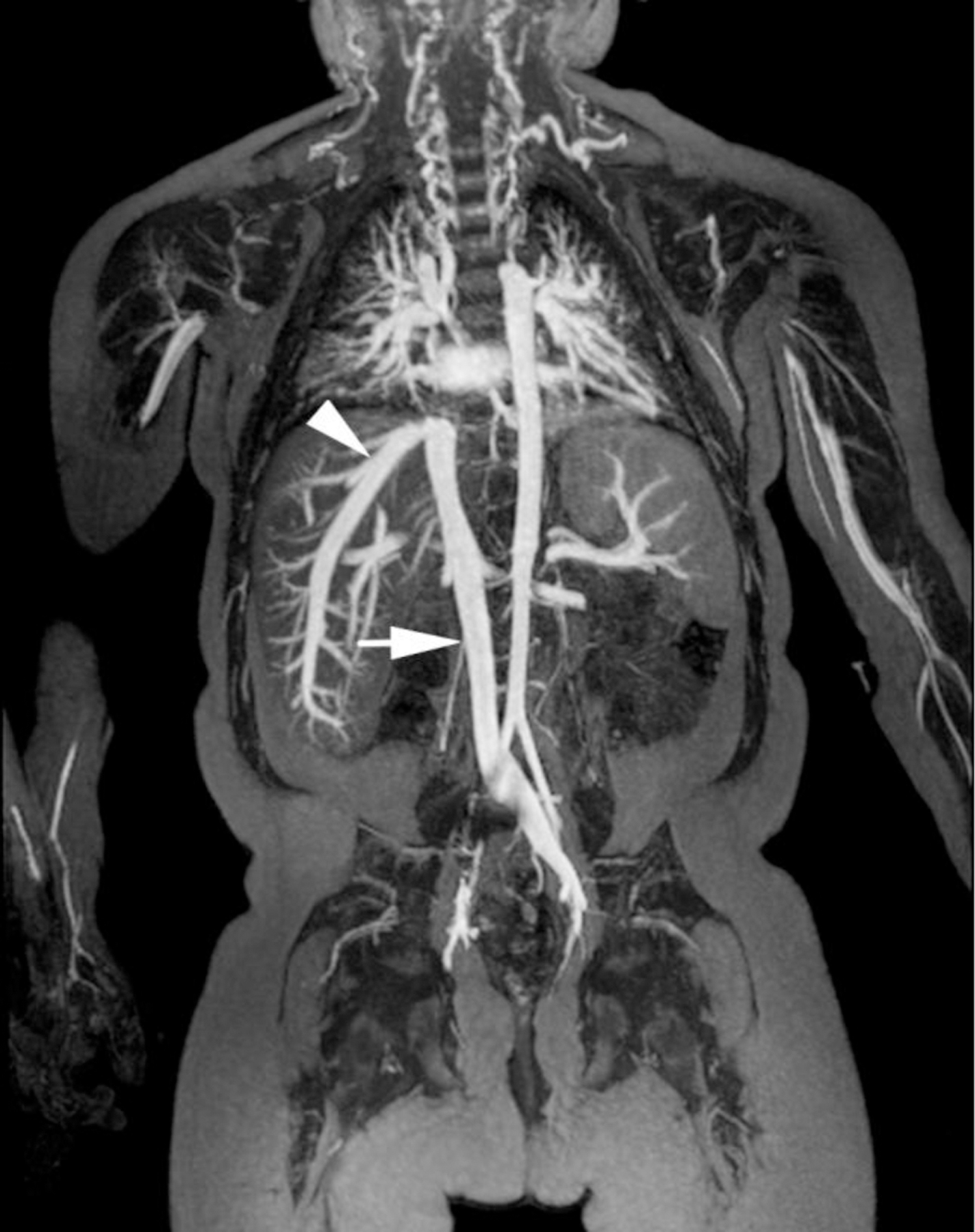
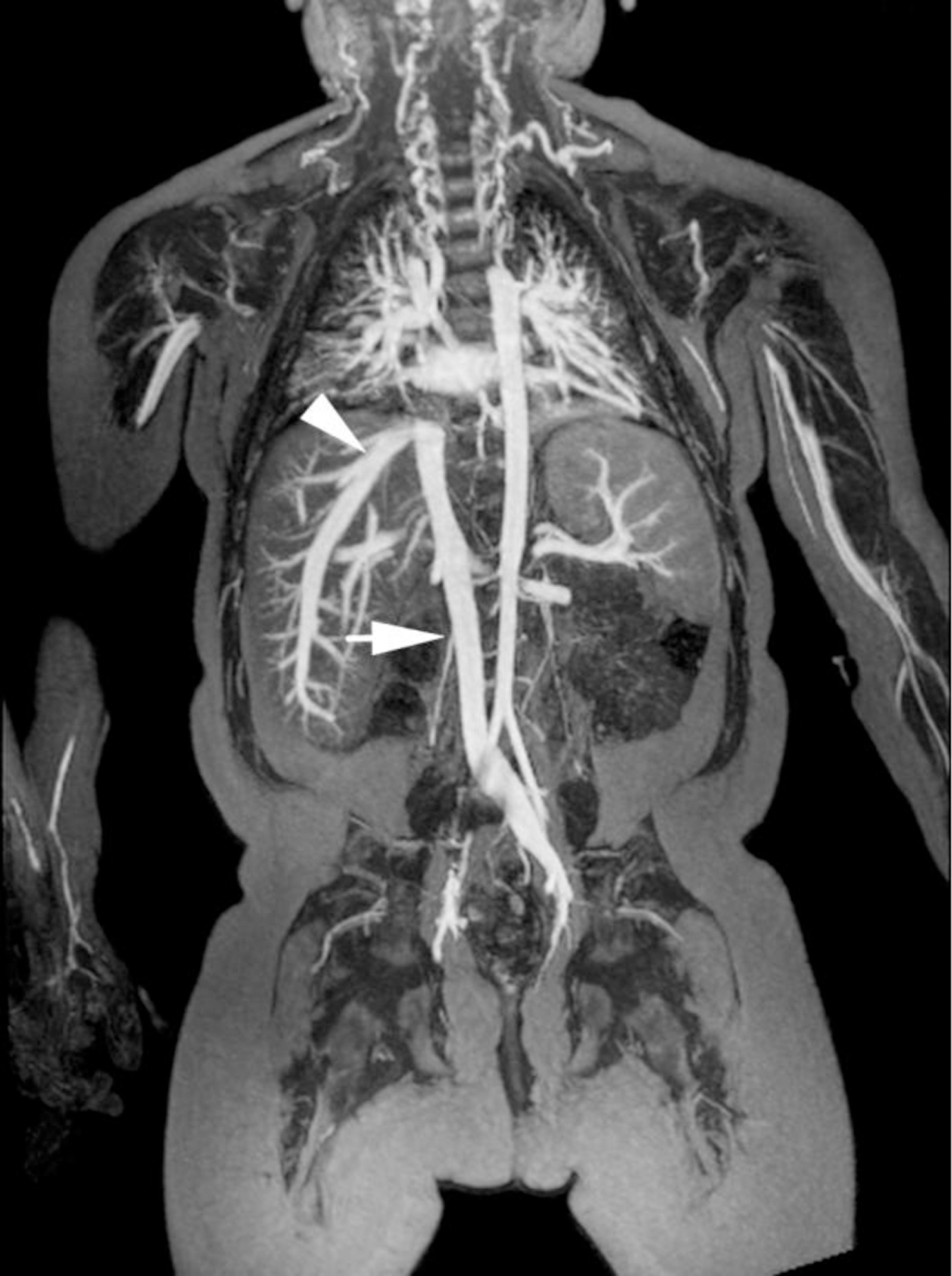
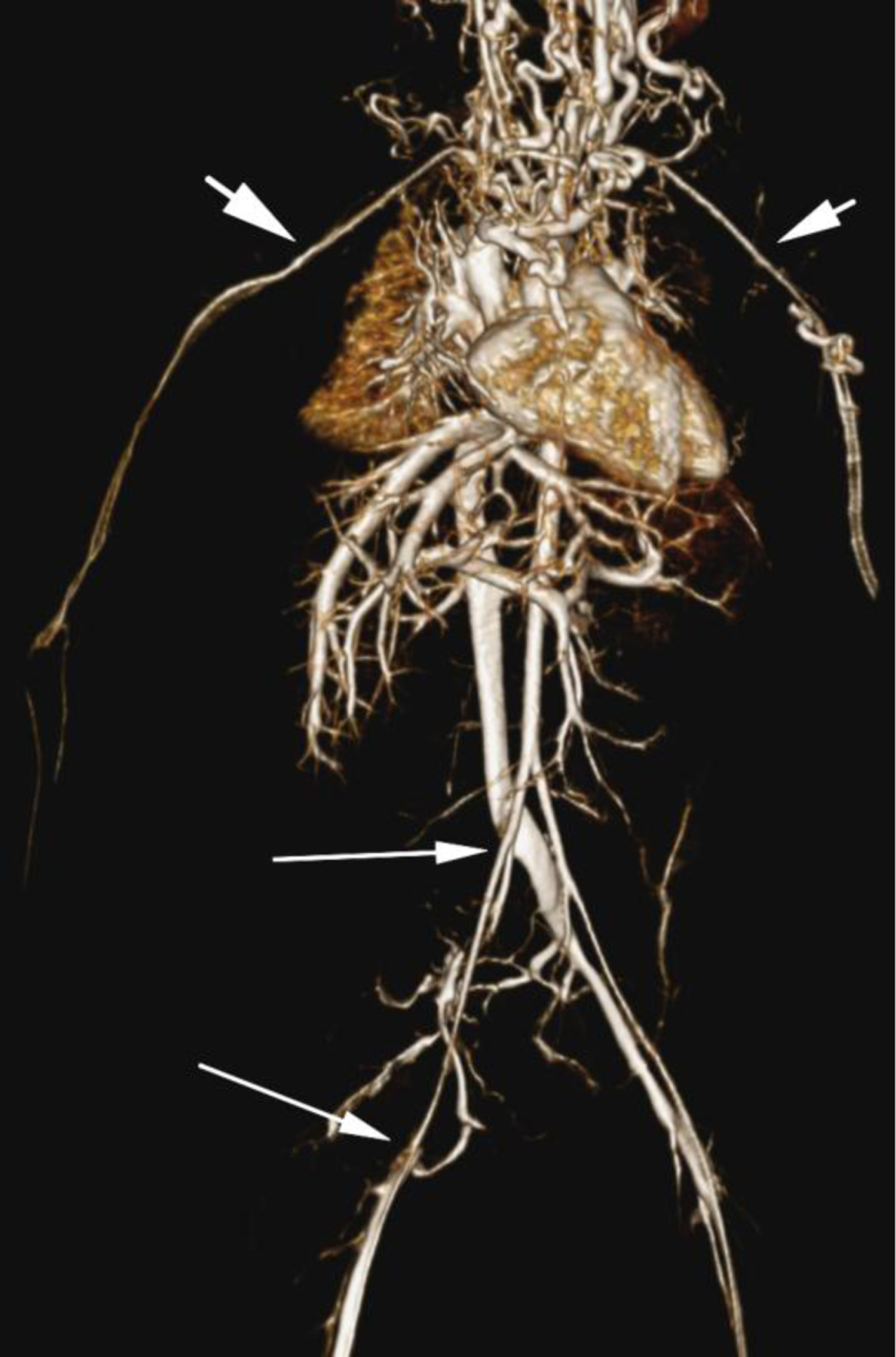
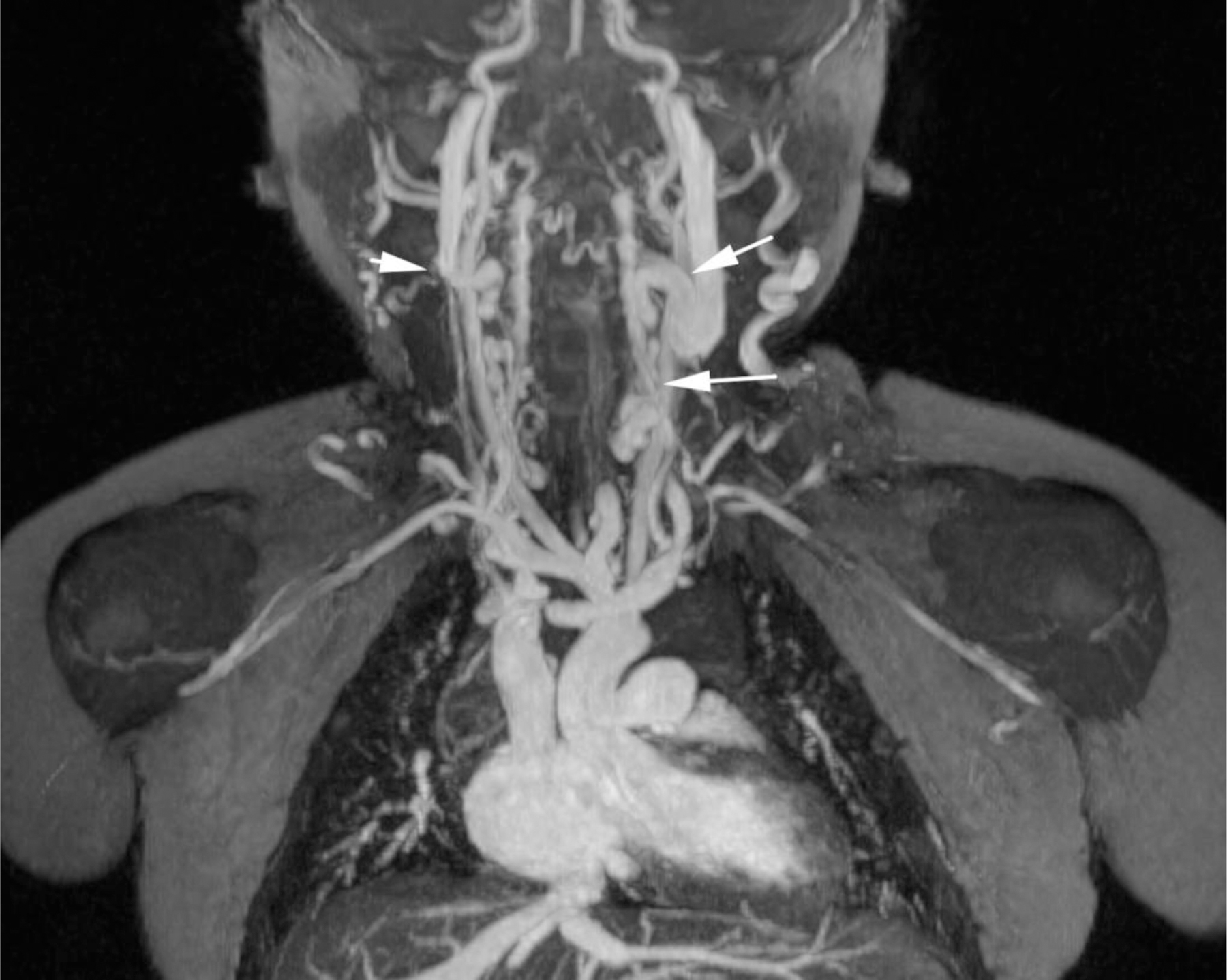
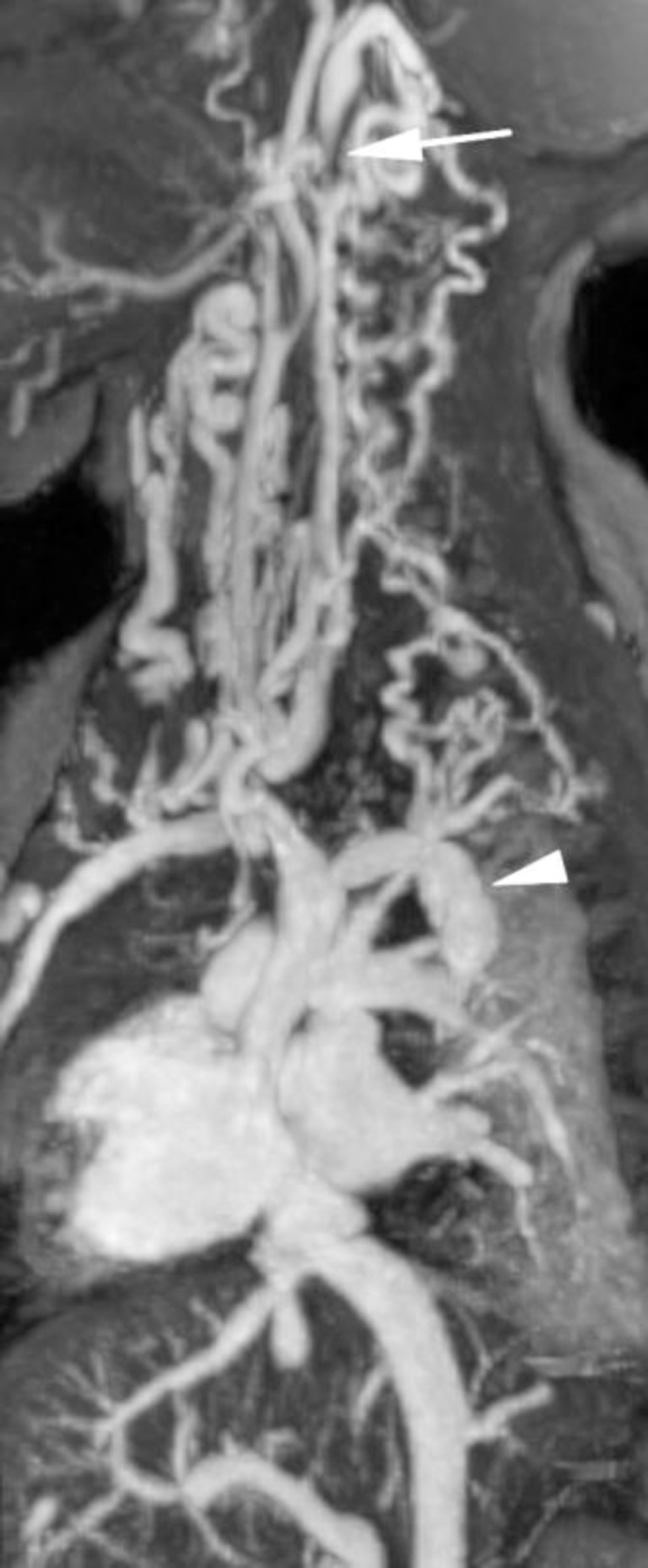
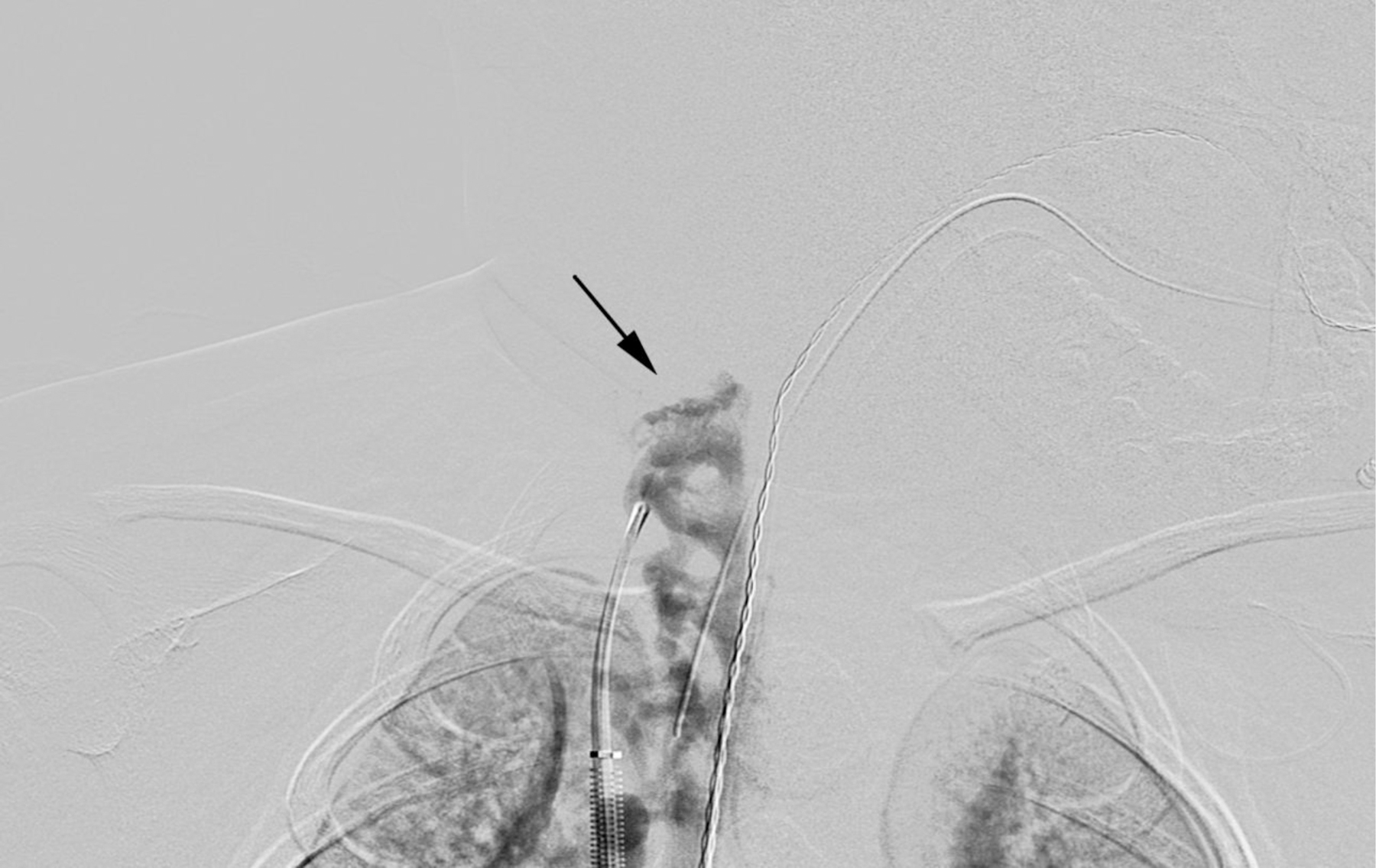
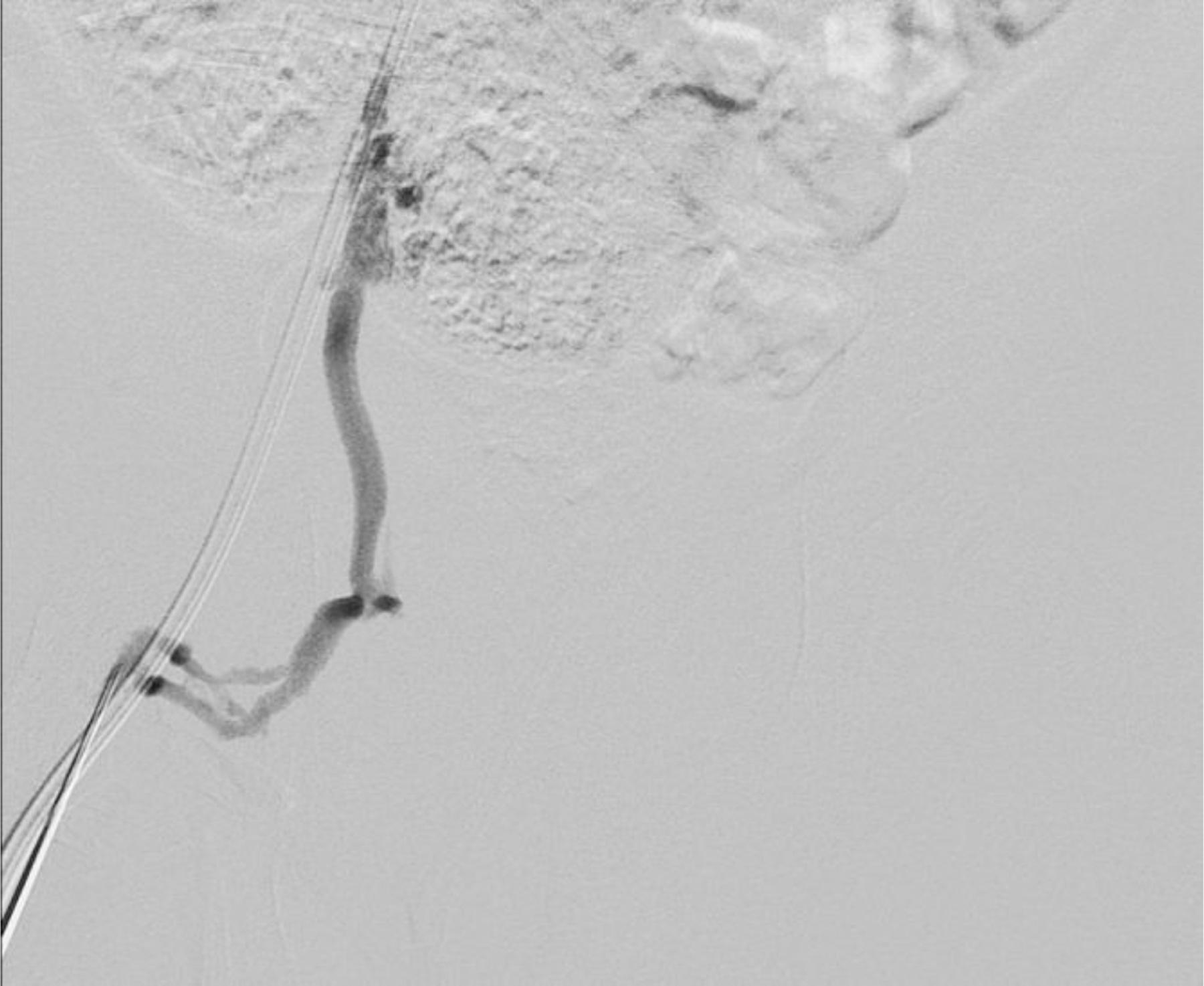
Ferumoxytol-enhanced magnetic resonance venography examination of a hemodialysis-dependent 4-year-old boy to assess for supradiaphragmatic vascular access planning (case 6). Representative coronal maximum intensity projection images (a, b, c) through the middle hepatic vein (arrowheads) and inferior vena cava (short arrows) from (a) first pass imaging, (b) early steady state 2 min later and (c) delayed steady state imaging 26 min later show stable enhancement of the venous structures in steady state. Additional 3-D reconstruction images (d) from the same ferumoxytol-enhanced MR venography demonstrate occlusion of the bilateral internal jugular, axillary (short arrows), subclavian and brachiocephalic veins as well as the right iliac vein (long arrows) with development of collateral vessels. Coned-down coronal (e) and sagittal (f) maximum intensity projection images show occlusion of the bilateral internal jugular veins with collateral formation (arrows), including an enlarged azygos vein (arrowhead). Selected images from follow-up angiography, confirmed occlusion of the right internal jugular vein (g) (arrow) and the right iliac vein (h) with collateral formation
Details of the imaging parameters are summarized in Table 2. Partition (slice) images, overlapping thin maximum intensity projection reconstructions and volume-rendered reconstructions were made available to reviewers in all cases.
Table 2.
Magnetic resonance imaging parameters for ferumoxytol-enhanced magnetic resonance venography at 3.0 T
| Magnetic resonance imaging parameters | Numeric value |
|---|---|
| TR (ms) | 2.53–3.38 |
| TE (ms) | 0.95–1.35 |
| Flip angle (degrees) | 15–25 |
| Bandwidth (Hz/pixel) | 587–797 |
| Field of view (mm) phase x frequency | 122–323x380–500 |
| Acquisition matrix | 202–373x512–640 |
| Slice thickness (mm) | 0.7–1.3 |
| In plane resolution (mm2) | (0.6–1.0)x(0.9 −1.2) |
| Acquisition time (sec) | 15.30–26.50 |
| Specific absorption rate (watts/kg) | 0.58–3.76 |
| Parallel acceleration factor | 3–4 |
Image analysis
Delayed steady-state ferumoxytol-enhanced MR venography images were presented to two board certified pediatric radiologists (S.G., R.G., with 7 and 1 years of post-fellowship experience respectively) who independently evaluated each examination. The radiologists were blinded to all patient information including name, age, gender, patient demographics and medical history. Six named vessels across a spectrum of sizes (internal jugular veins, subclavian veins, brachiocephalic veins, inferior vena cava, portal vein, hepatic veins) were scored for quality on each examination according to a three-point ordinal scale: 1=Poor, precluding confident assessment; 2=Adequate for confident assessment of stenosis or occlusion, and 3=Excellent vascular definition sufficient for evaluation of fine detail. A score greater than or equal to 2 was regarded as diagnostic quality. The examinations were anonymized and randomized; the first five evaluations were used as training cases, and the scores from these five examinations were discarded to minimize learning bias.
All ferumoxytol-enhanced MR venography examinations were scored as diagnostic or excellent (mean+/−standard deviation). The mean score for ferumoxytol-enhanced MR venography ranged from 2.7 to 2.9. Findings from the qualitative analysis are summarized in Table 3.
Table 3.
Mean qualitative scores for ferumoxytol-enhanced magnetic resonance venography image quality
| Vein | Qualitative score [Mean (±SD)] | P-value |
|---|---|---|
| Jugular | 2.9 (±0.3) | <0.01 |
| Subclavian | 2.9 (±0.2) | <0.01 |
| Brachiocephalic | 2.9 (±0.2) | <0.01 |
| Inferior vena cava | 2.9 (±0.2) | <0.01 |
| Portal | 2.9 (±0.3) | <0.01 |
| Hepatic | 2.7 (±0.3) | 0.03 |
Enhanced vessels were scored for quality on each examination according to an ordinal scale: 1=Poor, precluding confident assessment; 2=Adequate, for confident assessment of stenosis or occlusion; 3=Excellent, sufficient for evaluation of fine detail. A score greater than or equal to 2 was regarded as diagnostic quality. Two-sided p-values from AC1 calculations of inter-rater reliability are provided for each graded vessel on ferumoxytol-enhanced magnetic resonance venography
Statistical analysis
Inter-rater reliability was analyzed for each vessel using a first-order agreement coefficient (AC1) calculation and were significant (P<0.05) for all vessels. Two-sided p-value results for the inter-rater reliability are listed in Table 3.
Imaging findings
Examination quality was further evaluated by comparison of findings from ferumoxytol-enhanced MR venography to findings from other modalities and/or from subsequent procedures. The medical records of patients who underwent ferumoxytol-enhanced MR venography were examined for comparable imaging examinations using other imaging modalities or subsequent procedures such as line placement by Interventional Radiology or Pediatric Surgery. If available, catheter angiography was regarded as a gold standard reference.
Twelve examinations had follow-up imaging or procedures that included interventional angiography, CT, US and surgical line placement. For all 12 cases, ferumoxytol-enhanced MR venography was consistent with findings from follow-up imaging or procedures (Table 4). Three of these cases are provided as examples (Figs. 1–3). In all cases, ferumoxytol-enhanced MR venography was able to definitively answer the clinical questions and guide clinical decision-making, even where follow-up correlative imaging was not available (Fig. 4).
Table 4.
Comparison of findings from ferumoxytol enhanced magnetic resonance venography examinations with findings from available follow-up imaging or procedures. Two-sided p-values from AC1 calculations for inter-rater reliability are provided for each graded vessel
| Patient | FE-MRV indication | FE-MRV findings | Comparison modality | Comparison findings |
|---|---|---|---|---|
| 1 | Evaluate liver transplant vasculature in context of rejection. | Patent vasculature. Moderate narrowing of portal vein at transplant anastomosis. | 1) Doppler ultrasound 2) Venogram (IR) |
Patent vasculature with moderate narrowing at transplant anastomosis |
| 2 | Evaluate liver transplant vasculature in context of liver dysfunction. | Patent vasculature. Moderate narrowing of portal vein at transplant anastomosis. | 1) Doppler ultrasound 2) Venogram (IR) |
Patent vasculature with moderate narrowing at transplant anastomosis. |
| 3 | Evaluate vasculature for source of bleeding in context of dropping hemoglobin. | No extravasation. | CT angiogram | No extravasation. |
| 4 | Evaluate vasculature prior to renal re-transplant. | Patent renal transplant vasculature. Occlusion of bilateral IJV with extensive collaterals in L UE. | 1) Doppler ultrasound 2) HD catheter placement (peds surgery) |
1) Patent renal vasculature. 2) Occlusion of L IJV with ext collat in L UE. |
| 5 | Evaluate vasculature for vascular access. | Occlusion of the right internal jugular, subclavian, and right innominate veins. | HD catheter placement (peds surgery) | Unsuccessful attempt to place R subclavian line with eventual success using L IJV. |
| 6 | Evaluate vasculature for HD access. | Complete occlusion of the bilateral IJV. | HD catheter placement with venogram (IR). | Occlusion of R IJV. |
| 7 | Evaluate renal transplant vasculature in context of severe acute rejection. | Poor perfusion of LLQ renal transplant and abrupt termination of transplant renal artery. | Doppler ultrasound | Poor perfusion of LLQ renal transplant. Main renal artery and vein not visualized. |
| 8 | Evaluate liver in context of possible mass. | Large multiseptated mass in liver with compression of right and middle hepatic veins. | CT angiogram | Large mass with compression of right and middle hepatic veins. |
| 9 | Evaluate vasculature for source of bleeding in context of known metastatic epithelial angiosarcoma with dropping hemoglobin. | Multiple masses with a dominant porta hepatic mass. The dominant mass compresses the proximal main portal vein. | Gadobenate dimeglumine Enhanced MRI | Multiple masses with a dominant porta hepatic mass. The dominant mass compresses the proximal main portal vein. |
| 10 | Evaluate vasculature for DVT. | Occlusion of right brachiocephalic vein. | HD Catheter placement with venogram (IR) | Complete occlusion of right brachiocephalic vein |
| 11 | Evaluate liver transplant vasculature in context of rejection. | Status post liver transplant with patent vasculature. No intra- or extrahepatic biliary duct dilation. | Doppler ultrasound | Patent vasculature. No intra- or extrahepatic biliary dilatation. |
| 12 | Evaluate vasculature for vascular access | Occlusion of right internal jugular vein, right brachiocephalic vein, and adjacent right subclavian vein. Patent left internal jugular vein. | HD Catheter placement with venogram (IR) | Successful placement of HD catheter via patent left internal jugular vein. |
AC1 first-order agreement coefficient, DVT deep venous thrombosis, FE-MRV ferumoxytol-enhanced MR venography, HD hemodialysis, IJV internal jugular vein, IR interventional radiology, L left, LLQ left lower quadrant, R right, UE upper extremity
Fig. 3.
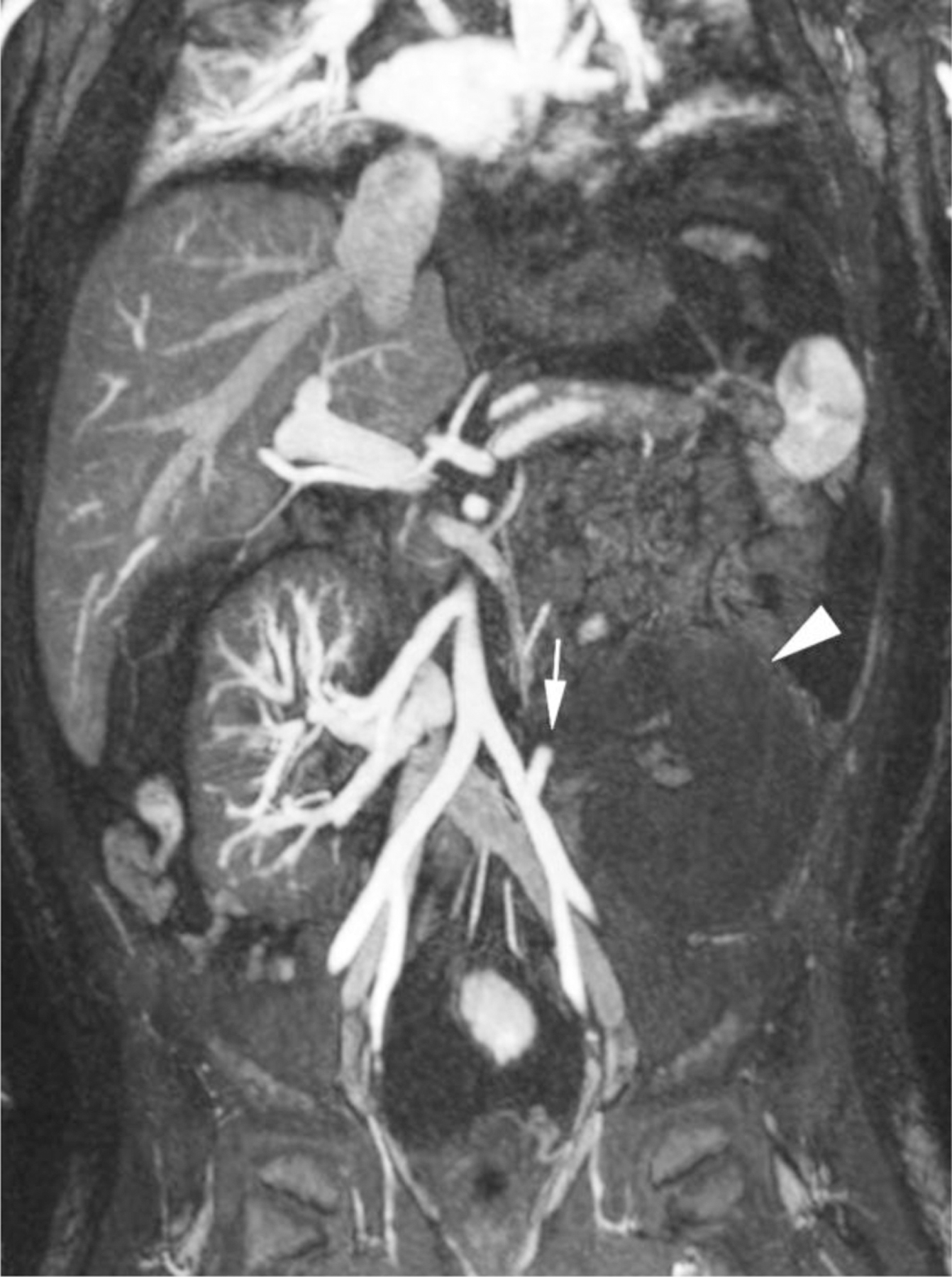
Ferumoxytol-enhanced magnetic resonance angiography in a 7-year-old boy (case 7) with two renal transplants and chronic rejection of the first graft. The boy presented with abdominal pain and suspected acute vascular compromise of the new graft. Duplex Doppler sonography showed poor perfusion of the transplant kidney and was unable to demonstrate flow in the main renal artery. Representative coronal maximum intensity projection image from emergent ferumoxytol-enhanced MR venography demonstrates abrupt occlusion of the main transplant renal artery (arrow) and absent perfusion to the graft (arrowhead). The vascular supply to the failed first graft is widely patent. The patient was taken immediately to the operating room and the infarcted transplant kidney was removed
Fig. 4.
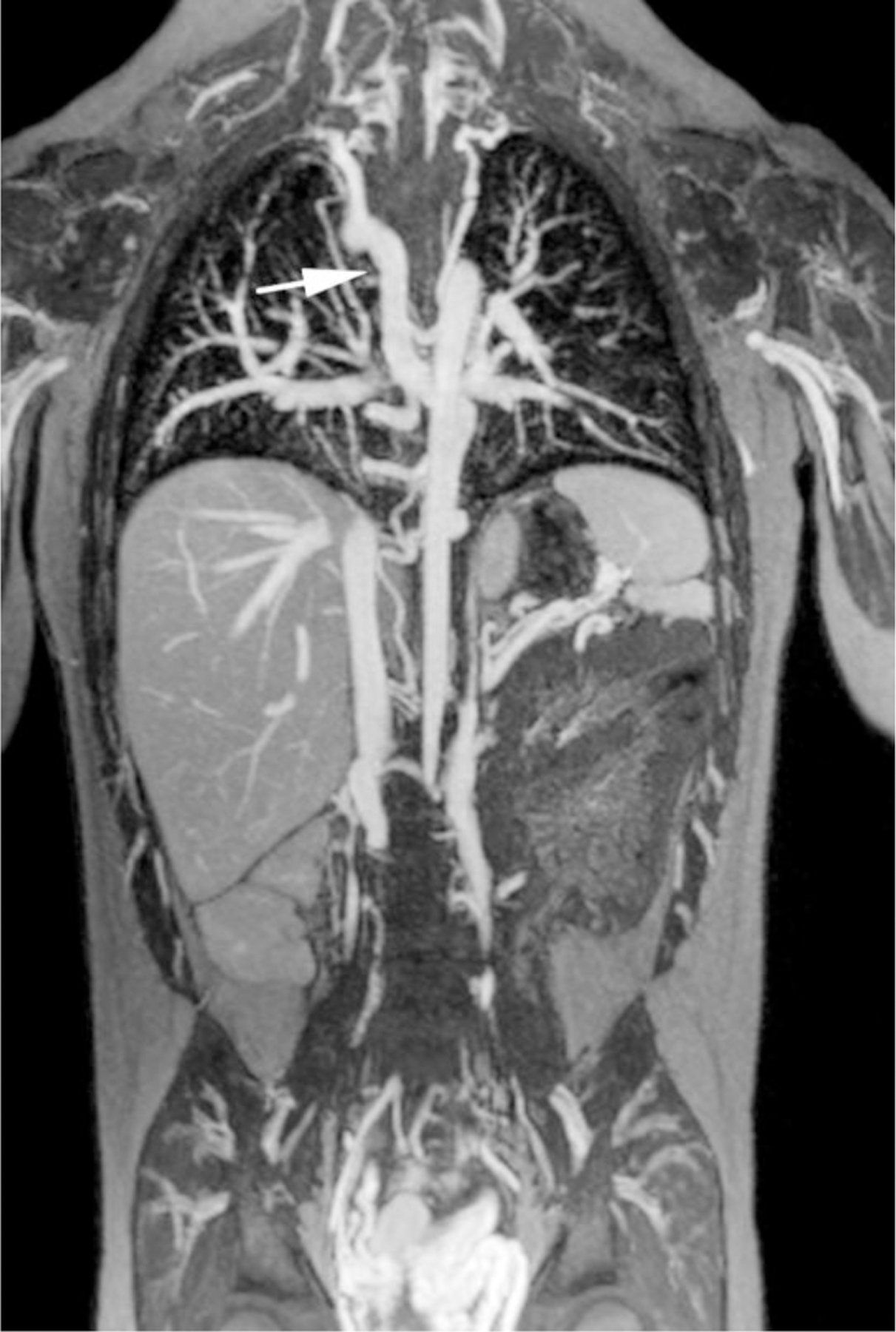
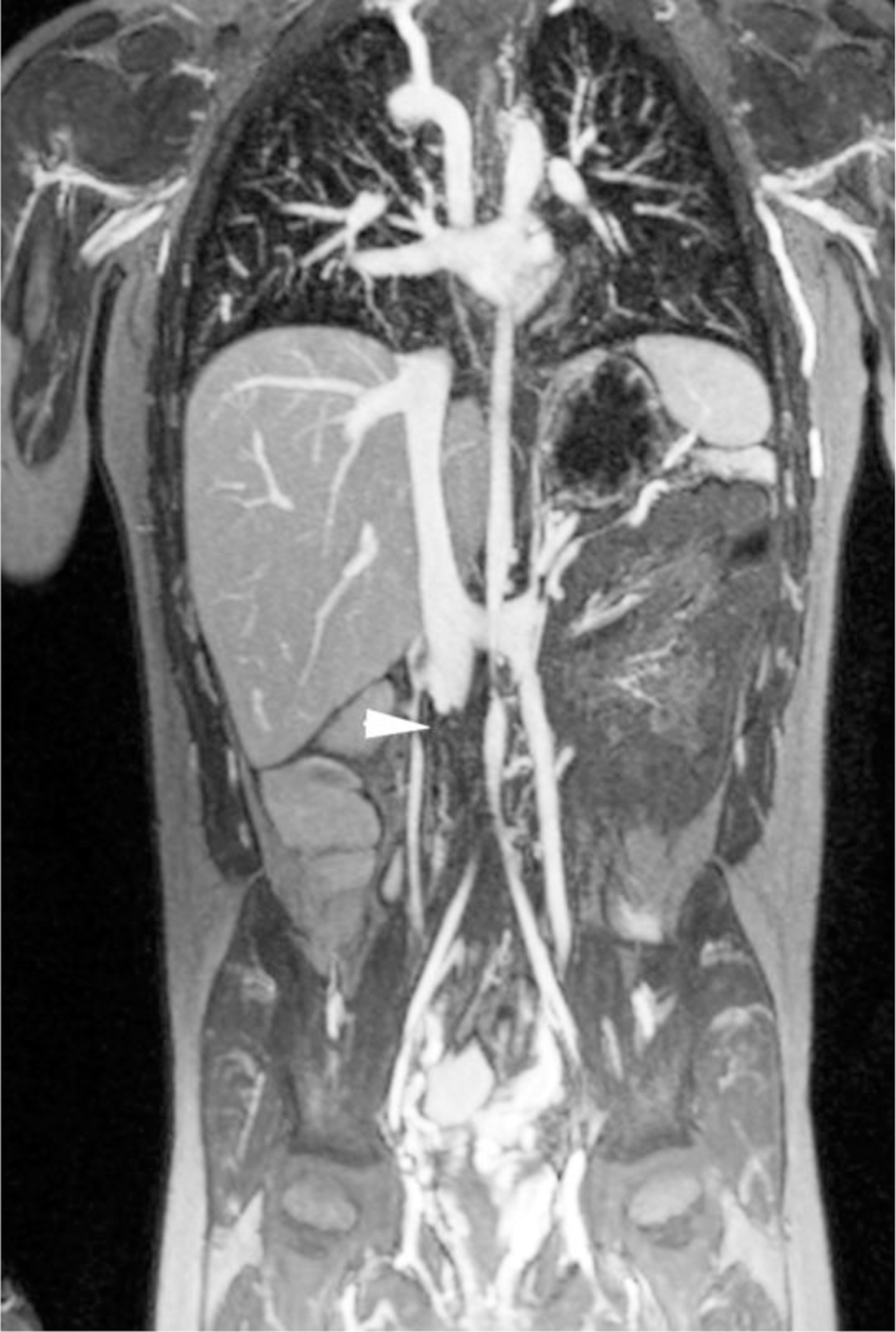
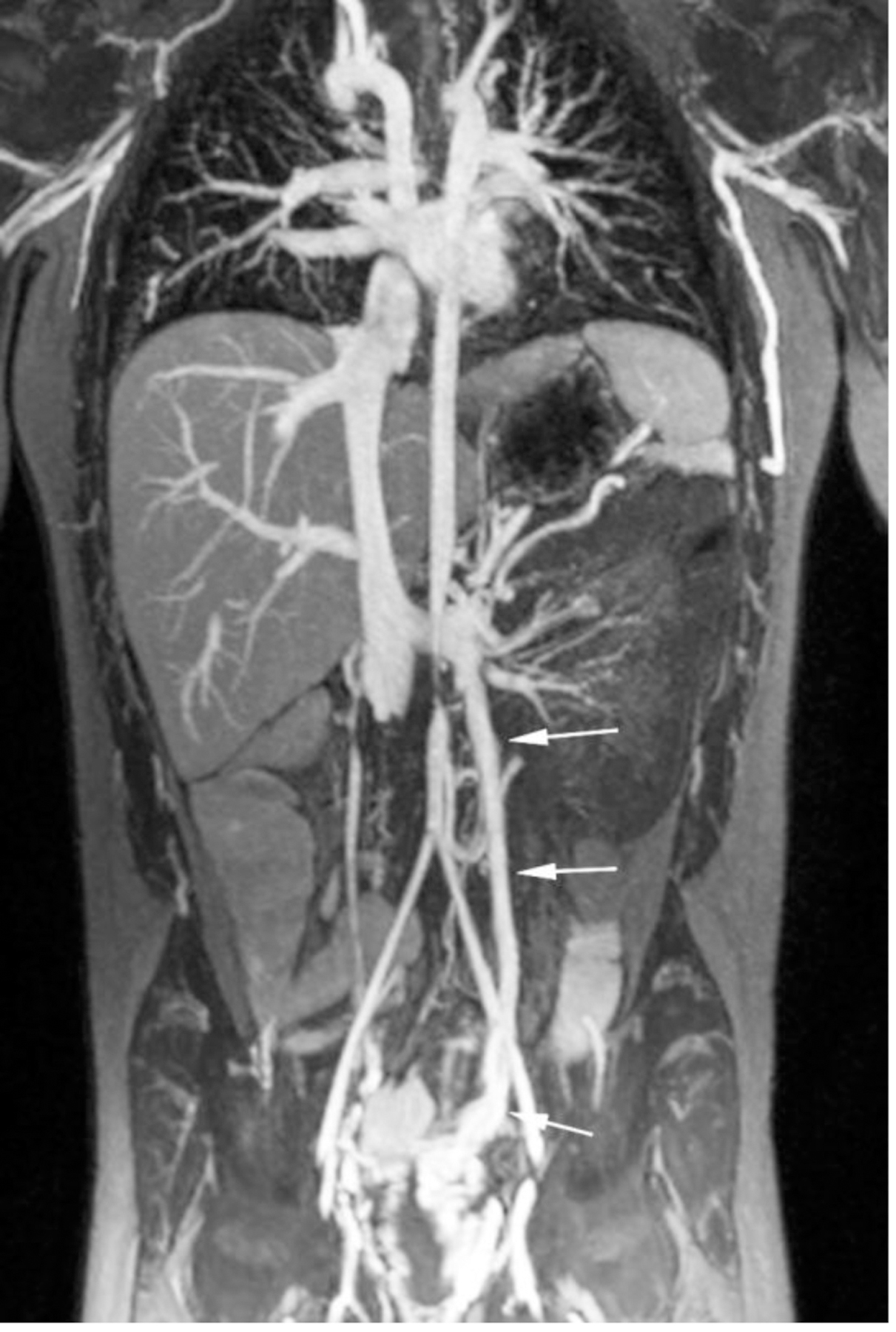
Ferumoxytol-enhanced magnetic resonance venography in a dialysis-dependent 5-year - old girl with non-functioning hemodialysis catheter and concern for line infection. Ferumoxytol-enhanced MR venography was ordered to evaluate catheter position and possible alternative sites for vascular access. Representative coronal maximum intensity projection images show (a) enlarged azygos vein (arrow), (b) occlusion of the inferior vena cava at the tip of the hemodialysis catheter (arrowhead) as well as (c) enlarged gonadal veins (arrows), which provide collateral drainage to the pelvis
Discussion
The results of our study indicate that ferumoxytol-enhanced MR venography at 3.0 T produced diagnostic, high-quality exams safely and consistently in 20 pediatric patients, with high spatial resolution and high temporal signal stability. Ferumoxytol-enhanced MR venography clearly defined significant vascular abnormalities, many of which would likely be difficult or impossible to detect by US or non-contrast techniques.
Our patients primarily consisted of renal failure and renal transplant patients. Long-term venous cannulation is frequent in this patient population, and vascular access is paramount due to high rates of thrombosis. The preponderance of clinical questions for which the ferumoxytol-enhanced MR venography examinations were performed related to definition of vascular anatomy or complication from an existing line. For these reasons, we chose to evaluate multiple vessels of varying caliber that could be consistently compared across patients, rather than focus only on the renal vasculature.
Ferumoxytol represents a potentially powerful contrast agent for vascular imaging in the pediatric patient. Children with chronic kidney disease require multiple vascular mapping studies from a young age and for indications ranging from vascular access to transplant evaluation. The advantages of contrast-enhanced MR angiography over other imaging tests in these patients had been well recognized before concerns about nephrogenic systemic fibrosis changed the diagnostic algorithm. The use of gadolinium-based contrast agents in renal failure patients is no longer routine. For the past seven years, the barriers to performing contrast-enhanced MR angiography have ranged from pretesting of renal function to full pre-procedural consultation in cases of renal impairment. Although the risk of nephrogenic systemic fibrosis can be minimized by using low doses of an appropriate gadolinium-based contrast agent, it cannot be eliminated and many clinicians remain reluctant to order MRI with gadolinium-based contrast agents. Avoidance of gadolinium-based contrast agent administration will, by general agreement, remove any risk of nephrogenic systemic fibrosis. For this reason, there has also been a resurgence of interest in non-contrast MR arteriography techniques over the past seven years.
As an alternative to gadolinium and with a very stable intravascular phase, ferumoxytol presents an immediate solution both to the issue of nephrogenic systemic fibrosis risk and of complex flow patterns. Also, the speed and simplicity of the acquisition and of the underlying pulse sequences make ferumoxytol-enhanced MR venography a practical technique, with little vulnerability to issues of poor contrast bolus timing and renal elimination.
Ferumoxytol is a USPIO particle with a high T1 relaxivity, similar to gadofosveset. Ferumoxytol is FDA-approved as an intravenous iron supplement for adults with anemia secondary to chronic kidney disease. It has been used extensively in adults with chronic kidney disease with an estimated 1.2 million administrations since 2006 (communication from AMAG Pharmaceuticals, September 2015). The reported rate of serious hypersensitivity reaction with ferumoxytol when used as an iron supplement in phase III clinical trials was 0.2%, which is similar to that reported for gadofosveset but is higher than that reported for the extracellular gadolinium-based contrast agents (0.02%) [10–12]. Of concern is a boxed warning recently issued by the FDA, highlighting the possibility of severe and potentially fatal anaphylactoid reactions noted in post-marketing data for ferumoxytol when injected rapidly in therapeutic doses to adults with renal failure [6]. The FDA warning notes serious adverse events in 79 patients who had received ferumoxytol, of whom 18 patients died. During this time period, since the approval of ferumoxytol on June 30, 2009, more than 1.2 million vials were distributed. To our best knowledge, no serious adverse events have been reported when used as an imaging agent in children. Nonetheless, caution would dictate than ferumoxytol should be infused slowly as suggested in the FDA advisory, and with close physiological monitoring. Furthermore, a history of allergy to intravenous iron products is an absolute contraindication and, as with any drug, administration should be performed only at sites with personnel and procedures to deal with acute adverse events. Since the FDA boxed warning issued in March 2015, we have been using slow intravenous infusion rather than bolus injection, also with no adverse events. Our experience has been that, once the blood pool is enhanced, the signal remains stable for hours. The stability of signal provides a wide time window for acquisition of high-quality steady-state imaging, such that the arterial and venous signal intensities are equal.
Although ferumoxytol provides excellent image quality in the vascular equilibrium phase, there are specific, unique attributes of ferumoxytol that are important to note. Ferumoxytol can persist in the blood pool for weeks and in the reticuloendothelial system for months after administration, which could cause confusion on follow-up imaging. It is important, therefore, to make explicit entry into the patients’ clinical notes and to inform patients and/or parents that this information should be made available to the interpreting radiologist reviewing subsequent MRI studies.
Our study has limitations. It represents a single-center prospective study with a relatively small number of examinations and lacks a reference standard. However, sequential enrollment helped to minimize selection bias and the routine use of anesthesia made it possible to implement a well–defined, repeatable image acquisition protocol.
Our study suggests that high-resolution MR venography with ferumoxytol in the steady state is a powerful and reliable diagnostic test in children with chronic kidney disease, providing detailed and comprehensive imaging of venous anatomy. Although no adverse effects occurred in our study, larger clinical series will be required to establish the safety profile of ferumoxytol for diagnostic use over a range of applications and patient groups.
Footnotes
This article was awarded the John Kirkpatrick Young Investigator Award at the Society for Pediatric Radiology 2014 meeting.
Compliance with ethical standards
Conflicts of interest None
References
- 1.Pagnan L, Tona G, Belgrano M et al. (2005) Direct contrast enhanced MR in the study of central venous accesses in children receiving total parenteral nutrition. Radiol Med 110:241–248 [PubMed] [Google Scholar]
- 2.Grobner T (2006) Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21:1104–1108 [DOI] [PubMed] [Google Scholar]
- 3.Anzai Y, Prince MR, Chenevert TL et al. (1997) MR angiography with an ultrasmall superparamagnetic iron oxide blood pool agent. J Magn Reson Imaging 7:209–214 [DOI] [PubMed] [Google Scholar]
- 4.Neuwelt EA, Hamilton BE, Varallyay CG et al. (2009) Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int 75:465–474. doi: 10.1038/ki.2008.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spinowitz BS, Kausz AT, Baptista J et al. (2008) Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol 19:1599–1605. doi: 10.1681/ASN.2007101156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA Drug Safety Communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (2015). U.S. Food and Drug Administration website http://www.fda.gov/Drugs/DrugSafety/ucm440138.htm Accessed May 1 2015.
- 7.Bremerich J, Bilecen D, Reimer P (2007) MR angiography with blood pool contrast agents. Eur Radiol 17:3017–3024 [DOI] [PubMed] [Google Scholar]
- 8.Ruangwattanapaisarn N, Hsiao A, Vasanawala SS (2015) Ferumoxytol as an off-label contrast agent in body 3T MR angiography: a pilot study in children. Pediatr Radiol 45:831–839. doi: 10.1007/s00247-014-3226-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen KL, Khan SN, Moriarty JM et al. (2015) High-field MR imaging in pediatric congenital heart disease: initial results. Pediatr Radiol 45:42–54. doi: 10.1007/s00247-014-3093-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung JW, Kang HR, Kim MH et al. (2012) Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 264:414–422. doi: 10.1148/radiol.12112025 [DOI] [PubMed] [Google Scholar]
- 11.Prince MR, Zhang H, Zou Z et al. (2011) Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol 196:W138–143. doi: 10.2214/AJR.10.4885 [DOI] [PubMed] [Google Scholar]
- 12.Lu M, Cohen MH, Rieves D, Pazdur R (2010), FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol 85: 315–319. doi: 10.1002/ajh.21656 [DOI] [PubMed] [Google Scholar]


