Abstract
Introduction
Rehabilitation therapies are critical for optimizing quality-of-life and daily functions for individuals living with Parkinson’s disease (PD). Thus, understanding the patterns of and under what conditions physicians make rehabilitation referrals is important for optimizing care.
Method
We analyzed data from 5020 participants (4 countries) collected from 1/3/2016 to 4/20/2018 as part of the Parkinson’s Foundation Quality Improvement Initiative (PF QII). Data were analyzed for single discipline and multidiscipline referrals to speech language pathology (SLP), physical therapy (PT), and occupational therapy (OT). Group comparisons (referred vs. not-referred) and regression procedures were implemented to determine demographic and clinical variables that were associated with an increased likelihood of rehabilitation referral.
Results
35.3% of participants were referred to rehabilitation services. Of these, 25.1% received a multidiscipline referral. There was a statistically significant effect of disease stage on both single discipline (χ2(2) = 45.1, p < 0.0001) and multidiscipline (χ2(2) = 74.2, p < 0.0001) referrals, with higher rates in later stages. Referred vs. not-referred participants differed significantly on a number of variables; however, only falls in the 6-months prior, advanced- and moderate-stage disease, older age, hospital admissions, and higher caregiver burden were associated with an increased likelihood of rehabilitation referral (adjusted odds ratios ≥ 1, Range = 1.08 to 1.62).
Conclusions
Despite evidence supporting multidiscipline and proactive rehabilitation in PD, the majority of referrals were made to a single service and may be reactions to falls or advancing disease. Data suggest there may be missed opportunities for optimizing care through proactive rehabilitation interventions.
Introduction
Physical therapy (PT), occupational therapy (OT), and speech language pathology (SLP) services are critical for addressing myriad and complex symptoms in Parkinson’s disease (PD) [1]. Understanding the patterns of and under what conditions physicians make rehabilitation referrals is important because a number of studies suggest that these services may be underutilized in PD [3–5]. Much of the extant literature reports data collected prior to the current American Academy of Neurology (AAN) quality standards and National Institute for Health and Care Excellence (NICE) guideline that support the annual assessment of rehabilitation needs in individuals with PD [5,6]. While more research is needed, there is evidence in support of multidiscipline rehabilitation approaches for individuals with PD [7–9]. However, existing utilization studies have largely focused on single discipline referrals and no published study has specifically evaluated the utilization of multidiscipline referrals. Although valuable in their scope, existing studies that use medical record and billing databases are vulnerable to misclassification errors and constrain analyses of referral predictors to a limited number of demographic variables [10]. Consequently, the clinical implementation of published rehabilitation referral guidelines, and the factors associated with the decision to refer to rehabilitation services in PD, remains unclear [11].
To bridge these gaps, the current study analyzed rehabilitation data from the Parkinson’s Foundation Quality Improvement Initiative (PF-QII) study. We used rehabilitation referral data collected during routine clinic visits in expert care centers to examine single discipline versus multiple discipline rehabilitation referral patterns for PT, OT, and SLP. To identify factors associated with physician referrals to rehabilitation, we analyzed a number of demographic variables and clinical measures in relation to rehabilitation referral patterns.
Method
The PF-QII project is an international multicenter prospective clinical study of individuals with PD designed to assess predictors of care and outcomes at expert centers (NCT01629043) [12]. At the time of this study, PF-QII was in its ninth year (June 2009 to current), with 11,448 enrolled participants from 30 Parkinson’s Foundation (PF) Centers of Excellence (i.e., expert care centers) in 5 countries. A PF Center of Excellence is a medical center with a specialized team of neurologists, movement disorder specialists, physical and occupational therapists, mental health professionals and others who demonstrate up to date practice standards regarding Parkinson’s disease (PD) medications, therapies and research. To become a PF Center of Excellence, each center must meet rigorous clinical, research, professional education and patient care criteria. The centers must set the highest standards of care for people with PD, conduct and advance research to improve the lives of people with PD, and provide patient education programs, community outreach programs and specialized PD training for healthcare professionals. As a result of these criteria, similar standards of care are maintained across all Centers of Excellence, regardless of geographical location.
Participants with PD were recruited quasi-sequentially by appointment slots without exclusion criteria. Described in earlier publications, data (i.e., demographic, clinical status, Parkinson’s care delivered, quality of life, and caregiver strain) were collected and recorded approximately annually during routine clinic visits using standardized questionnaires of quality of life, caregiver strain, physical exams, and clinical interviews [12]. All participants provided written consent and the study was approved by the ethics review boards at each study site.
De-identified PF-QII data collected between 1/3/2016 to 4/20/2018 were queried cross-sectionally across 29 centers for all participants with an unambiguous diagnosis of idiopathic PD. Data from six centers from a single European country whose cohorts included participants treated in inpatient care settings were excluded to avoid inflating rehabilitation referral numbers (Figure 1). Participants with missing Hoehn and Yahr stage data, sex, and/or age data were also excluded. (Figure 1).
Figure 1.
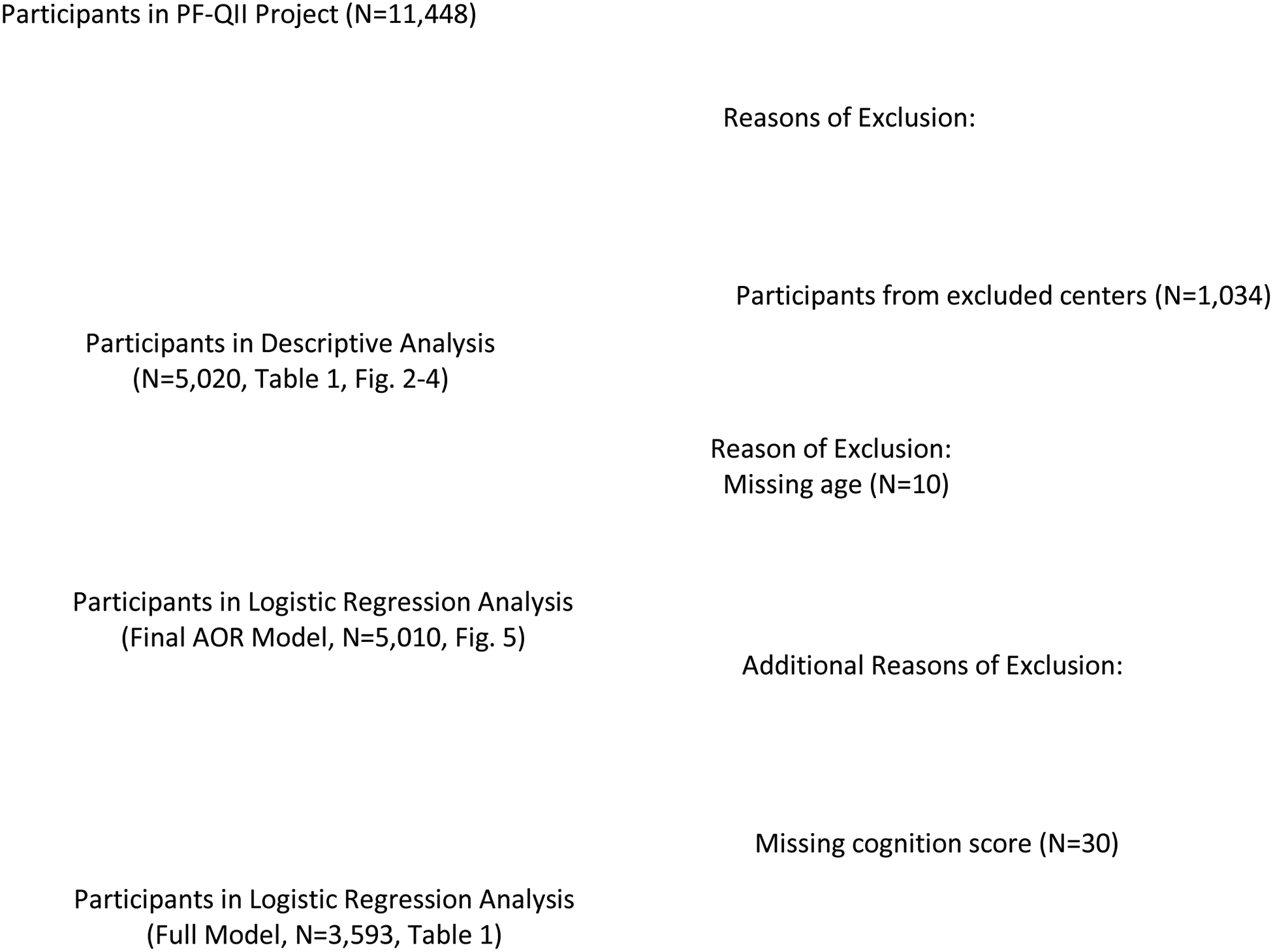
Participant Data Flow
Participants
The final PF-QII registry query yielded 5,020 outpatient records from 23 PD expert care outpatient centers in 4 countries (Supplement Table 1-1). The sample was biased toward men with 3233 (64.4%) male patients and 1787 (35.6%) female patients. Participants were categorized into one of three disease stage categories using their Hoehn and Yahr scores: ‘early’ (1–2), ‘moderate’ (2.5–3), or ‘advanced’ (3.5–5). The sample was comprised of 3088 (61.5%) early stage patients, 1399 (27.9%) patients with moderate stage disease, and 533 (10.6%) patients with advanced stage disease.
Variables Analyzed
Rehabilitation Referrals.
A rehabilitation referral was defined as a referral (e.g., written order or recommendation) made at the time of the study visit for future PT, OT, or SLP assessment and/or treatment. Referrals were categorized into non-overlapping categories as either single-discipline (referred to only one rehabilitation discipline) or multidiscipline (referred to ≥ 2 rehabilitation disciplines). Single-discipline referrals were further categorized into individual disciplines (SLP, PT, OT). For multidiscipline referrals, participants were assigned to one of four non-overlapping categories (SLP+PT; OT+PT; SLP+OT; SLP+PT+OT).
Predictors of Rehabilitation Referrals.
Demographic variables entered as predictors included age, female sex, disease duration (years from symptom onset), disease stage group based on Hoehn and Yahr scores (early, moderate, advanced), whether the participant was living in their own home, and whether they had a regular care partner. Clinical variables included whether the participant had ≥ 2 comorbid diagnoses, occurrence of falls in the 6-months prior, whether the participant had undergone deep brain stimulation (DBS) surgery for PD-related symptom management, number of hospital admissions in the 6-months prior, and number of emergency room visits in the 6-months prior. Clinical measures entered as predictors included the: Modified Caregiver Strain Index (MCSI) [13], the Parkinson’s Disease Questionnaire-39 subscale scores (mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and pain) [14], the Timed Up and Go (TUG) task score (a standard measure of mobility) [15], and a composite cognition variable comprised of the animal verbal fluency mean z-score/delayed 5-word recall mean z-score. To examine the role of caregiver strain as a predictor, participants without missing data were divided equally across three MCSI score groups (low, medium, and high strain), while those with missing data were treated as a separate category.
Statistical Analyses
We conducted descriptive analyses and univariate comparisons on baseline demographic and clinical characteristics between participants referred and not referred for any rehabilitation service using Chi-square tests and two-sample t-tests, as appropriate. Single-discipline referrals and multidiscipline referrals were compared across disease stages based on Chi-square tests. Effect sizes were evaluated using Cramer’s V. Logistic regression models were fitted for dichotomous outcome on referral (Yes/No) to assess effects of all predictors (described above) on referral status. Adjusted odds ratios (AOR) are presented for each predictor in the full model, as well as in the final model that resulted from backward selection procedures. All individual tests were 2-sided, and unless otherwise noted, p-values less than 0.05 were deemed statistically significant.
Results
Rehabilitation Referral Patterns
Referrals collapsed across disciplines.
Across centers, 35.3% of participants (n = 1771) were referred to at least one rehabilitation discipline at the time of their routine clinic visit. (Supplemental Table 1-1). The effect of center on referral rate was significant (χ2 (22) = 253.8, p < 0.0001) with percentage of referrals by center ranging from 15.3% to 66.7% (Median = 33.3%) (Supplement Table 1-1). Of the 3088 participants with early stage disease, 908 (29.4%) were referred to at least one rehabilitation discipline. Of participants with moderate stage (N = 1399) and advanced stage (N = 533) disease, 604 (43.2%) and 259 (48.6%) respectively were referred to at least one rehabilitation service. There was a significant effect of disease stage on total referrals (χ2(2) = 126.2, p < 0.0001, Cramér’s V = 0.16).
Single-discipline vs. multidiscipline referrals (Figure 2).
Figure 2.
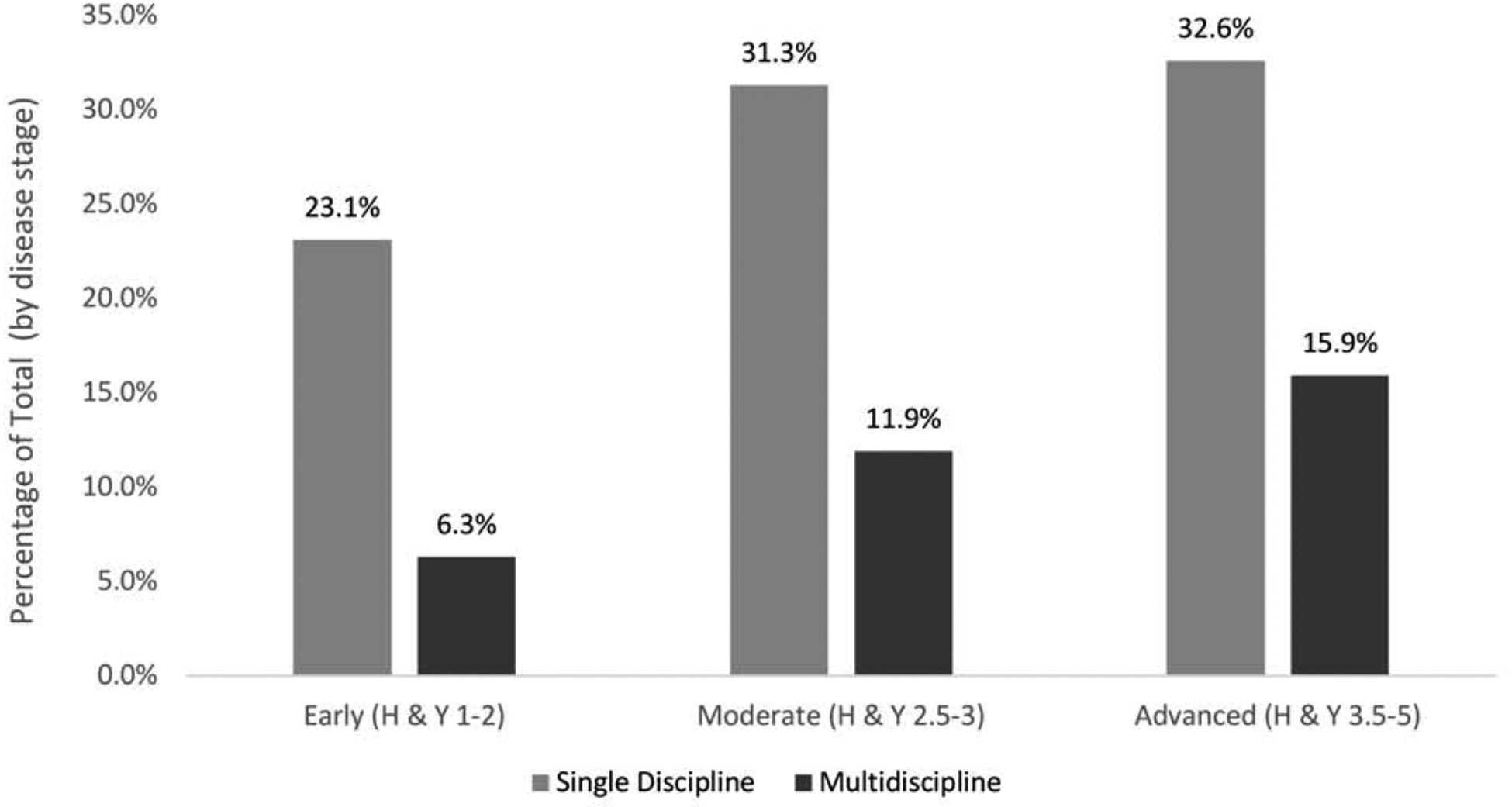
Single discipline versus multidiscipline referrals by disease stage.
Note. Percentages calculated based on total participants at each disease stage: Early = 3088; Moderate = 1399; Advanced = 533.
The first aim was to examine single-discipline vs. multidiscipline referrals in PD. Of the 1771 participants referred to rehabilitation services, 1326 (74.9%) were referred to a single discipline with the remaining 445 (25.1%) referred to more than one discipline. There was a statistically significant but modest effect of disease stage on both single-discipline (χ2(2) = 45.1, p < 0.0001, Cramér’s V = 0.09) and multidiscipline referrals (χ2(2) = 74.2, p < 0.0001, Cramér’s V = 0.12). At all disease stages, rates of single-discipline referrals were higher than multidiscipline referrals.
Single-discipline referrals (Figure 3).
Figure 3.
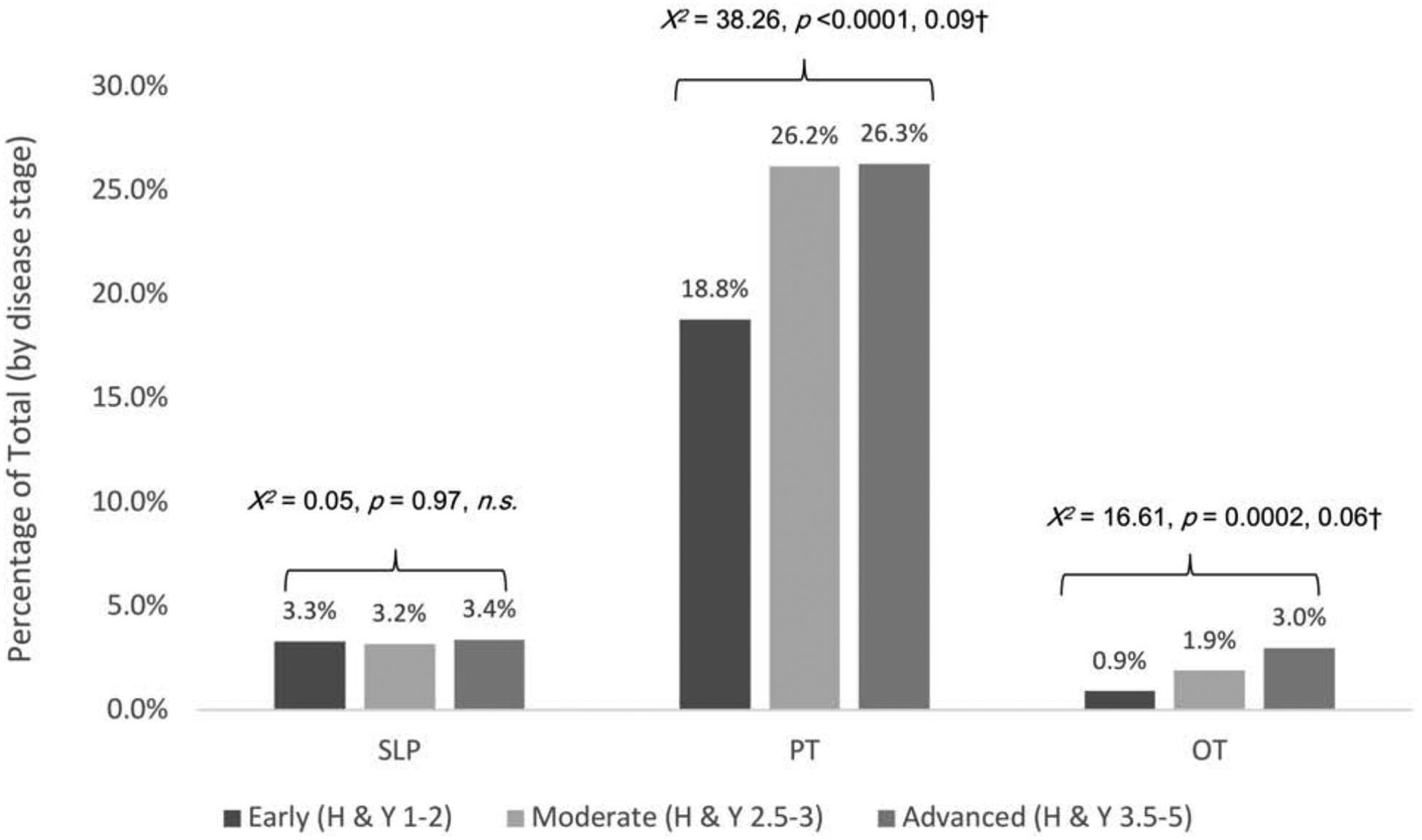
Single-discipline referrals by discipline and disease stage
Note. Percentages calculated based on total participants at each disease stage: Early = 3088; Moderate = 1399; Advanced = 533. Significant p-level corrected for multiple comparison bias = 0.016. †Effect size estimate was calculated as √χ2/(n*df), where χ2is the chi-squared test statistics, n is sample size and degree of freedom df=min (number of rows-1, number of columns-1).
We also analyzed data by individual discipline and disease stage. Data presented in Figure 3 show that referrals to PT exceeded those to either SLP or to OT at all stages of disease. There was a statistically significant but modest effect of disease stage on PT and on OT referrals, but no effect on ST referrals was observed (Figure 3).
Multidiscipline referrals (Figure 4).
Figure 4.
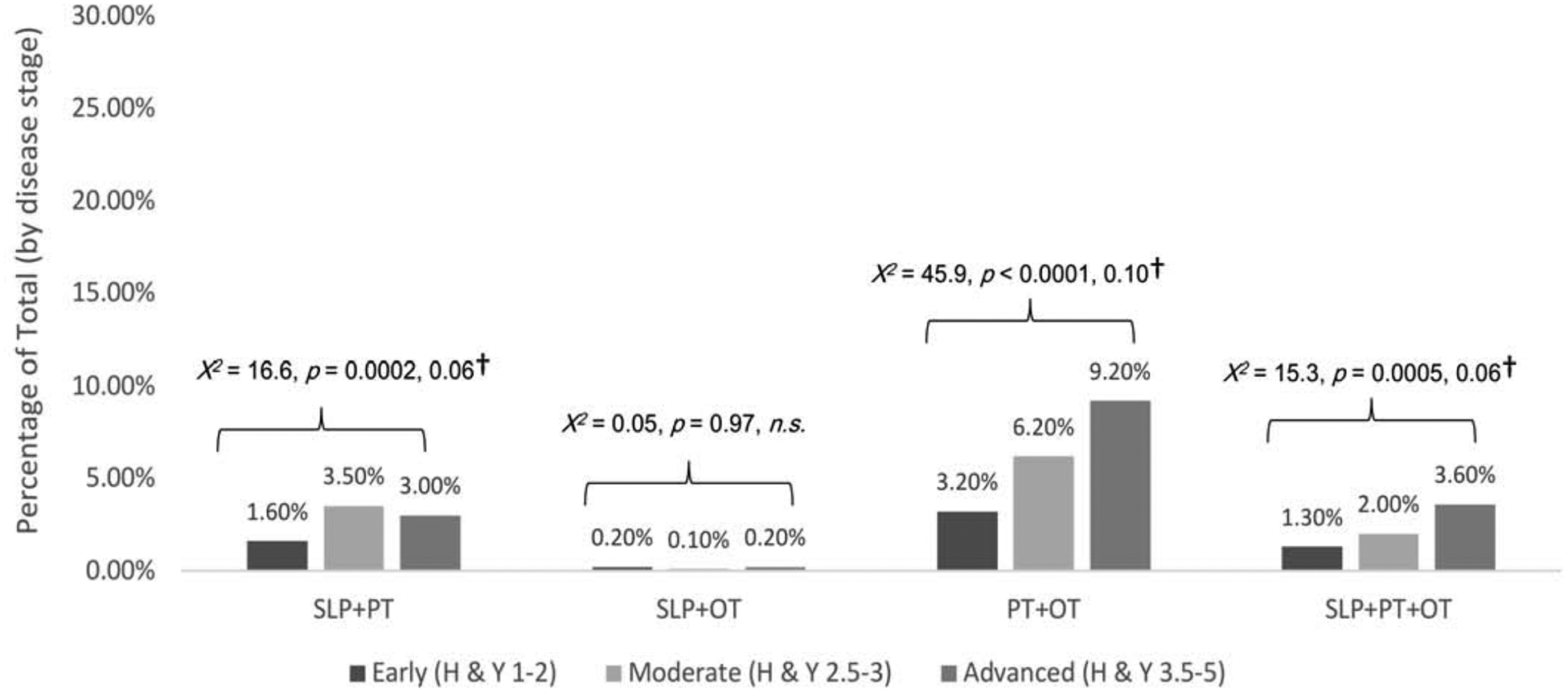
Multidiscipline referrals by disease stage
Note. Percentages calculated based on total participants at each disease stage: Early = 3088; Moderate = 1399; Advanced = 533. Significant p-level corrected for multiple comparison bias = 0.0125. n.s. = non-significant. †Effect size estimate was as √χ2/(n*df), where χ2 is the chi-squared test statistics, n is sample size and degree of freedom df=min (number of rows-1, number of columns-1).
Overall, multidiscipline referral rates were low at < 10% for all combinations of services (range 0.1% to 9.2%). Referral rates were highest for combined PT+OT services. Rates of PT+OT, SLP+PT, and SLP+PT+OT differed significantly across disease stages (Figure 3–4). Referral rates to combined SLP+OT services were not affected by disease stage.
Predictors of Rehabilitation Referrals
Demographic characteristics of participants referred and not referred for rehabilitation services are presented in Table 1. While the majority of variables differed between groups, differences were generally small in magnitude with effect sizes ranging from .05 to .36. The full model results for the logistic regression analysis, including adjusted odds ratios, are also presented in Table 1. Variables from the full model analysis that were statistically significant predictors of rehabilitation referrals were retained through backward selection procedures to identify a final model and accompanying adjusted odds ratios (Figure 5). In decreasing order, falls in the 6-months prior, advanced and moderate stage disease, a higher number of hospital admissions, older age, and higher caregiver strain increased the likelihood of a physician referral to a rehabilitation service. A detailed look at the MCSI scores by high (MCSI range 0 to 9.8), medium (MCSI range 9.9 to 24.6), and low (MCSI range 24.7 to 100) groups suggests that participants whose caregivers reported the highest caregiver strain scores were more likely to be referred to a rehabilitation service compared to those with lower caregiver strain scores. By contrast, being in the lowest caregiver strain group carried an adjusted odds ratio value < 1.0 suggesting low strain was associated with a lower likelihood of rehabilitation referral. None of the PDQ-39 subscales were significant predictors of rehabilitation referral.
Table 1.
Differences in Participants Referred vs. Not-Referred for Any Rehabilitation Service
| Referred versus Not Referred for Rehabilitation Services | Logistic Regression Results Full Model | ||||||
|---|---|---|---|---|---|---|---|
| Referred | Not Referred | Group Comparisons | |||||
| Variable | Value +/− SD or N [% Total] | Value +/− SD or N [% Total] | p-value‡ | Effect Size Estimate† | AOR | 95% CI for AOR | p-value |
| Male Sex | 1149 [64.9%] | 2084 [64.1%] | 0.62 | 0.01 | |||
| Female Sex | 622 [35.1%] | 1165 [35.9%] | 0.97 | 0.83, 1.15 | 0.76 | ||
| Age (years) | 69.8 +/− 8.7 | 67.4 +/− 9.4 | <0.0001 | 0.26 | 1.21 | 1.11, 1.31 | <0.0001 |
| Disease Duration (years) | 12.1 +/− 6.9 | 10.9 +/− 6.6 | <0.0001 | 0.16 | 0.98 | 0.97, 1.00 | 0.02 |
| Hoehn and Yahr Stage | |||||||
| Early (1–2) | 908 [51.3%] | 2180 [67.1%] | <0.0001 | 0.16 | |||
| Moderate (2.5–3) | 604 [34.1%] | 795 [24.5%] | 1.22 | 1.01, 1.47 | 0.04 | ||
| Advanced (3.5–5) | 259 [14.6%] | 274 [8.4%] | 0.92 | 0.62, 1.36 | 0.68 | ||
| PDQ-39 | |||||||
| PDQ-39 summary index | 27.9 +/− 15.8 | 23.3 +/− 15.9 | <0.0001 | ||||
| PDQ-39 Mobility | 15.9 +/− 11.6 | 11.8 +/− 11.2 | <0.0001 | 0.36 | 1.01 | 1.00, 1.02 | 0.07 |
| PDQ-39 ADL | 8.8 +/− 6.2 | 6.9 +/−5.9 | <0.0001 | 0.31 | 1.02 | 1.00, 1.04 | 0.12 |
| PDQ-39 Emotional well-being | 6.4 +/− 4.8 | 5.6 +/− 4.8 | <0.0001 | 0.16 | 0.99 | 0.97, 1.01 | 0.38 |
| PDQ-39 Stigma | 3.0 +/− 3.3 | 2.8 +/− 3.3 | 0.05 | n.s. | 1.00 | 0.97, 1.02 | 0.74 |
| PDQ-39 Social support | 1.5 +/− 2.1 | 1.3 +/− 2 | <0.0001 | 0.13 | 1.02 | 0.97, 1.07 | 0.39 |
| PDQ-39 Cognition | 4.7 +/− 3.2 | 4.0 +/− 3.1 | <0.0001 | 0.21 | 1.00 | 0.97, 1.03 | 0.95 |
| PDQ-39 Communication | 3.5 +/− 2.7 | 2.8 +/− 2.7 | <0.0001 | 0.25 | 1.02 | 0.98, 1.06 | 0.33 |
| PDQ-39 Pain | 4.0 +/− 2.7 | 3.7 +/− 2.8 | 0.01 | 0.08 | 0.97 | 0.94, 1.00 | 0.07 |
| MCSI index (%) | 22.4 +/− 15.7 | 18.3 +/− 16.0 | <0.0001 | 0.26 | |||
| MCSI Low | 221 [12.5%] | 583 [17.9%] | <0.0001 | 0.10 | 0.75 | 0.60, 0.94 | 0.01 |
| MCSI Medium | 313 [17.7%] | 495 [15.2%] | 1.02 | 0.82, 1.26 | 0.87 | ||
| MCSI High | 353 [19.9%] | 454 [14.0%] | 1.23 | 0.97, 1.56 | 0.08 | ||
| Standardized TUG score | 15.2 +/− 8.4 | 13.3 +/− 7.2 | <0.0001 | 0.24 | 1.00 | 0.99, 1.02 | 0.73 |
| Hospital admission last 6 months (number) | 0.4 +/− 0.8 | 0.3 +/− 0.7 | <0.0001 | 0.23 | 1.18 | 1.03, 1.35 | 0.01 |
| Emergency room visit last 6 months (number) | 0.6 +/− 1.1 | 0.4 +/− 0.8 | <0.0001 | 0.19 | 1.01 | 0.90, 1.12 | 0.92 |
| Falls last 6 months (yes) | 875 [49.4%] | 1059 [32.6%] | <0.0001 | 0.17 | 1.59 | 1.34, 1.88 | <0.0001 |
| DBS (yes) | 423 [23.9%] | 578 [17.8%] | <0.0001 | 0.07 | 1.20 | 0.97, 1.49 | 0.09 |
| ≥ 2 Comorbidities (yes) | 1097 [61.9%] | 1856 [57.1%] | 0.001 | 0.05 | 1.19 | 1.00, 1.41 | 0.05 |
| Presence of Care Partner (yes) | 1475 [83.3%] | 2599 [80.0%] | 0.01 | n.s. | 1.05 | 0.85, 1.29 | 0.66 |
| Lives at home (yes) | 1054 [59.8%] | 2051 [62.9%] | 0.01 | n.s. | 0.84 | 0.71, 1.00 | 0.05 |
| Average z-score (verbal fluency/delayed 5-word recall) | −0.1 +/−.8 | 0 +/− 0.8 | <0.0001 | −0.15 | 1.08 | 0.98, 1.20 | 0.30 |
Note. PDQ-39 = Parkinson’s Disease Questionnaire summary index score. MCSI = Modified Caregiver Strain Index total score. TUG = Timed up and go task. AOR = Adjusted Odds Ratio.
Significant p-level corrected for multiple comparison bias = 0.004.
Effect size estimate calculated as mean (%) difference/pooled standard deviation or Cramer’s V as appropriate. n.s. = non-significant finding. Negative effect sizes indicate higher data values/scores in the ‘not referred’ group. Effect sizes for non-significant comparisons are not reported. Percentages based on total number of participants in study, N=5020. Standard deviations noted as +/−. Percentage of total participants noted in brackets [].
Figure 5.
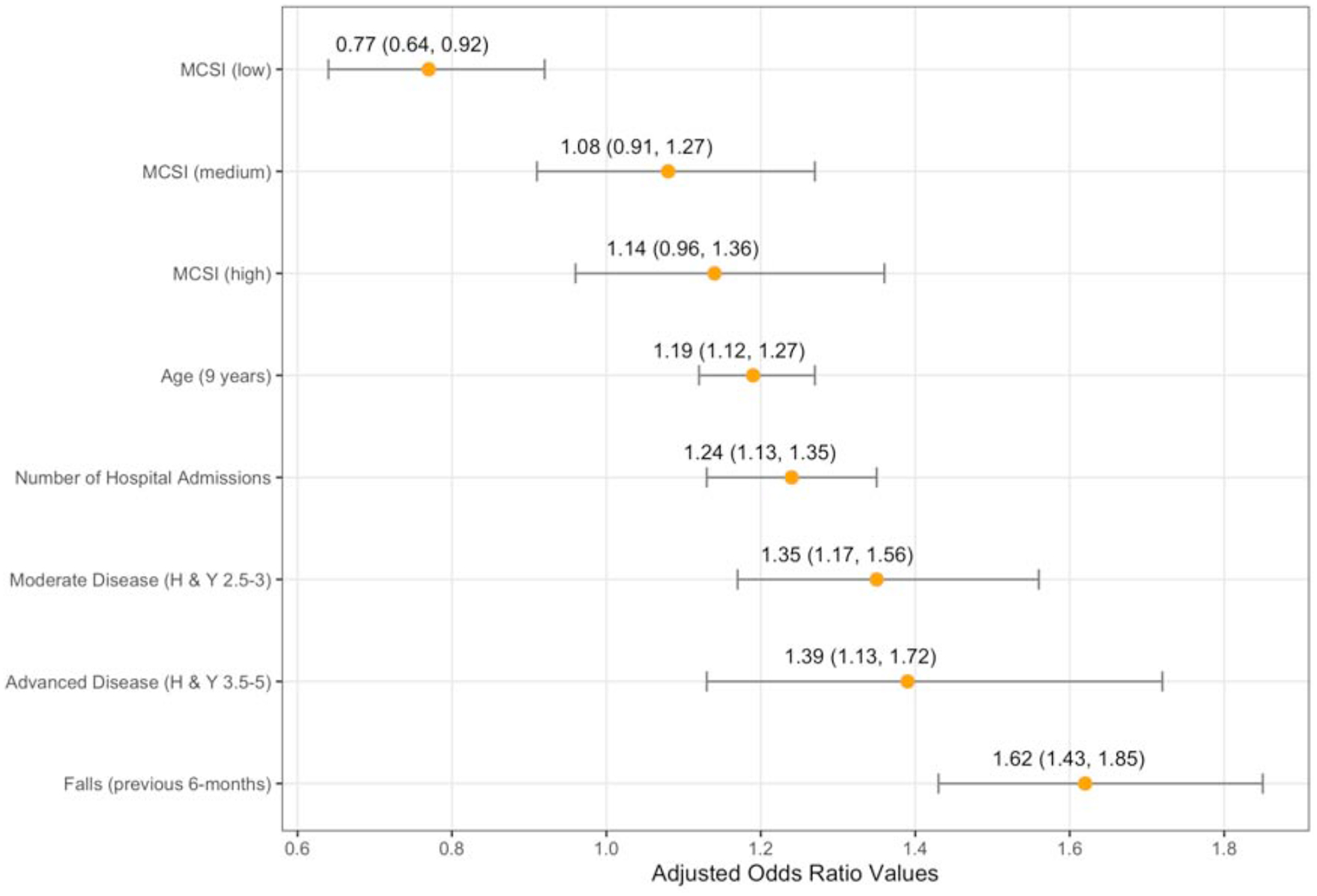
Adjusted Odds Ratios and Confidence Intervals for the Final Rehabilitation Referral Predictor Model.
Discussion
The current study is distinguished from other large datasets in three important ways: a) the use of a robust prospective study design with low risk of classification error by virtue of enrolling individuals with expert-clinician confirmed idiopathic PD, b) the examination of point-of-care referral patterns (e.g., referrals made at the time of routine follow-up visits) in clinics with similar standards of care, rehabilitation access, and management practices, and c) the sampling of data collected after the publication of updated rehabilitation quality care standards. As such, the current study offers a unique window into the implementation of rehabilitation referral best practices in PD.
Just over a third (35.3%) of participants were referred to rehabilitation services. Referrals from expert care centers were largely made to a single rehabilitation discipline, physical therapy, and thus addressed a narrow range of potential functional impairments in PD. Although a growing number of studies suggest that early proactive interventions may benefit individuals with PD [16,18], in the current study rehabilitation referrals were made reactively in response to falls, advancing disease, and hospital admissions. In fact, individuals reporting falls in the 6-months prior to their routine clinic visit were 1.62 times more likely to receive a rehabilitation referral. This suggests that in expert care centers there may be missed opportunities for proactive referrals that may improve quality of life [16,17]; optimize mobility and balance [16,18]; minimize disability [16,17]; increase neurotrophic factors [19]; slow disease-related functional decline [16]; and reduce caregiver strain/burden [17]. The observation that rehabilitation referrals follow a reactive pattern is underscored by the finding that caregiver strain, and not participant self-ratings of quality of life (in any domain), predicted whether a referral was made.
Although perhaps lower than expected, our median referral rate of 35.3% (SLP, PT, and OT combined) exceeded utilization rates from previous studies by Fullard et al. [4] using U.S. Medicare billing records (combined rate = 28.2%; 14.6% for SLP and 14.2% for PT /OT related services) and by Bryant et al. [3] using U.S. Veteran’s Administration records (combined rate = 30.3%; 10% SLP, 12.8% PT and 7.5% OT services). The higher referral rates observed in expert care centers may be due to better access to specialty care teams. Patients seen in expert centers typically have annual (at minimum) clinic appointments with a team that includes a movement disorders neurologist, a specialized clinic nurse, and in some clinics an in-house rehabilitation team. This explanation is consistent with Bryant et al. [4] who found that increased neurologist access was associated with increased utilization of rehabilitation services in PD. However, it is also possible that our referral rates were inflated by the inclusion of countries, such as the Netherlands, that have a national network of rehabilitation professionals who are connected through a platform that facilitates both education and interdisciplinary evidence-based practice in PD. In both the present study, and in previously published research, clinics in the Netherlands reported PT utilization rates > 60% in PD [1, 20]. Despite similar standards of care, the number of individuals referred for rehabilitation services in our study differed across centers. There are a number of potential reasons for this observation, including differences in reimbursement/payor systems and regional differences in rehabilitation access (e.g., rural vs. urban; medically underserved areas) in communities where clinic patients reside [4,11].
With the exception of OT, the relative distribution of referrals to individual rehabilitation disciplines in our data was similar to that from a Korean study in which authors reported rehabilitation utilization rates (for dates between 2004 and 2015) of 35–40% for PT, 16–19% for OT, and 4–6% for SLP [2]. In contrast, our cumulative referral rates at 30.4%, 8.0%, and 7.5% for PT, OT, and SLP respectively were lower than patient-reported utilization data from previous PF-QII studies [21,22]. Considering patients of similar mean disease duration, previous PF-QII studies found that 40.9%, 16.2%, and 15.4% of participants self-reported receiving PT, OT, or SLP services respectively in the 12-months prior to their clinic visit [21]. The observation that utilization rates exceed referral rates in similar data sets suggests that entry into the rehabilitation care path may occur through mechanisms outside of routine clinic visits including other rehabilitation service providers, primary care physicians, and emergency department visits. However, opportunities for rehabilitation referrals can be missed in those contexts as a result of decreased knowledge of the broad scope of rehabilitation interventions in PD, low clinician confidence in treating PD patients, and reduced care coordination between expert clinical teams and primary care clinicians [20,23–25]. This may particularly disadvantage early stage patients who have less experience with or who may be more hesitant to accept rehabilitation services [18] and also for those who primary care physicians judge are better able to self-manage care with less coordination between primary and movement disorders clinicians [24]. This is important given our finding that early stage patients are particularly vulnerable to lower rehabilitation referral rates.
Although recent guidelines and research studies endorse multidiscipline rehabilitation approaches in PD for improving quality of life and physical function [6–9], referrals to more than one service were relatively rare in our data. While multidiscipline referrals were more common in advanced disease stages, the effect of disease severity was not uniform across all combinations of multidiscipline referrals suggesting that disease severity drives multidiscipline referral patterns for some (SLP+PT; PT+OT; SLP+PT+OT) but not all (SLP+OT) multidiscipline services. Consistent with previous studies, PT referral rates were higher than referrals to either SLP or to OT at all stages of disease [1–3,21,22]. This finding is also consistent with research showing that individuals with PD who self-identify as having difficulties with activities of daily living, work-related activities, leisure activities, eating, and drooling are less likely to receive rehabilitation services than those reporting mobility difficulties [20].
Like other studies, we found positive associations between rehabilitation utilization and increased number of falls [26], disease severity [3], and older age [2–4]. We also found that increased caregiver strain was associated with an increased likelihood of rehabilitation referral, which has not been reported previously. Specifically, participants whose caregivers reported the highest level of strain were more likely to receive a referral to at least one rehabilitation service. With limited availability of validated screening tools and evidence-informed care paths, clinicians may rely on caregiver input to identify rehabilitation needs [11]. Further, these results spotlight the need for developing better tools and strategies for incorporating the perspective of the person with PD into the rehabilitation referral decision-making process. While caregiver input is critical in PD care [9], depending exclusively on patients and families to self-identify rehabilitation needs may be less optimal during clinical care transition points - owing to increased emotional burdens [27]. Additionally, caregivers’ limited knowledge of the scope of rehabilitation services and/or their perceptions of service value may bias their input to physicians [27, 28]. Interestingly, research shows that PD family caregivers’ perceptions of rehabilitation service value are highest for PT and lowest for OT [28]. This aligns with our observation that referrals were significantly higher for PT than for OT services. While purely associative, we assert that the relationship between perceived service value and rehabilitation referrals in PD may be an important consideration for future studies.
We interpret the results of this study not as a critique of clinical practice in expert care centers, but as a vociferous call for an increased investment into the expansion of best practice rehabilitation guidelines across all disease stages and healthcare funding models; the development of novel, integrated and multidisciplinary service-delivery models that can foster increased care coordination [17,18]; the rigorous validation of rehabilitation screening tools that can be applied in a variety of settings; the development of clear rehabilitation indicators to support clinical decision-making in both expert and non-expert centers [11]; and rigorous, pragmatic clinical trials that evaluate the efficacy and cost-effectiveness of rehabilitation services for individuals with PD. It is critical that we understand how investments into such programs affect healthcare utilization overall (e.g., fewer repeat hospital admissions) as well as impact the economic burden associated with caregiving. Parkinson’s Foundation expert care centers take a leadership role in setting these standards of care and advocating for essential allied health services in the communities they serve. To this point, Rafferty and colleagues recently implemented a proactive physical therapy program in early-stage PD patients in a Parkinson’s Foundation expert care center program. The program resulted in increased physician referrals to PT and in improved self-selected walking speed over a 12-month duration [18]. There is also emerging evidence that coordinated, symptom-comprehensive, multidiscipline team models may optimally achieve the new AAN quality outcome indicators in individuals with PD [30].
Our results further underscore the importance of expanding interprofessional, rehabilitation-focused knowledge translation activities to a variety of clinical settings (primary, acute, skilled nursing, home health, emergency care departments), care partners, persons with PD, and health care practitioners. ParkinsonNet (https://www.parkinsonnet.com/), which was founded in the Netherlands, is one example of how interdisciplinary experts came together to exert changes in clinical practices for people living with PD.
Over the last several years Parkinson’s disease care guidelines and clinical consensus documents have been published that provide guidance relative to rehabilitation assessment, treatment, and outcome measurement [6, 29, 31–36], including recommendations for organizing multidisciplinary care teams [37]. The European physiotherapy guidelines [32], and those co-authored by ParkinsonNet, Parkinson Foundation, and Ergotherapie Nederland in occupational therapy [31] are notable examples of professional organizations partnering with patient advocacy groups to advance PD-care standards, professional education, and thus potentially rehabilitation utilization in PD. In some cases, these guidelines have been translated into accessible web-based knowledge products that can help inform patients, families, and non-clinical communities [31–33]. Developing accessible education, training, and needs-assessment tools for elevating rehabilitation-literacy among family members and individuals with PD is of growing importance especially in today’s healthcare market where rapidly evolving policy and funding changes facilitate direct access (e.g., self-referral) to rehabilitation services. These steps will help to optimize rehabilitation referrals at multiple care path entry points.
Limitations
While the current study includes expert care centers from four countries, there is the potential for geographical bias in the overall patterns observed because the majority of centers were located in the United States. We also acknowledge that the definition of allied health services used in the current paper is limited. Social workers, recreational therapists, nurses, dieticians, among others are valuable members of the allied health team that are excluded here. The exclusion of these disciplines reflects available data and is not intended as a prioritization of services. Our data reflect referral practices within Parkinson’s Foundation expert centers and thus may be limited in their generalizability to programs outside of academic medical centers. Further, because data were collected during clinic visits, an important source of referrals ignored by the current study are those generated via telephone/email contacts with expert care center staff between clinic visits [38]. It should also be noted that whether rehabilitation referrals captured in the PF-QII dataset were actualized cannot be determined from this analysis.
In our dataset the physician’s specific rationale for referral to rehabilitation services is unknown. Moreover, the only clinical instruments and/or common bedside assessments in our data set were the TUG and a composite cognition measure. Referrals may have been made for a number of reasons including response to a physician-observed impairment, patient/caregiver-reported impairment, or may have been proactive/consultative in nature in keeping with existing practice guidelines [6, 29, 31–33]. Future studies examining more direct relationships among specific indicators, clinical assessments, physician referral, and outcomes will be important for informing screening tools and developing robust clinical care paths.
Conclusions
While studies continue to examine the optimal dosing of, targets of, and delivery models for rehabilitation interventions for individuals with PD, the current study uniquely reveals referral gaps across rehabilitation disciplines (particularly for SLP and OT) and between single discipline and multidiscipline referrals even in expert PD care centers. Moreover, these results provide evidence of a persistent pattern of problem-reactive versus symptom-proactive patterns of rehabilitation referrals in PD. Our findings also indicate that a number of common challenges for people living with PD including swallowing [39], communication [40], and activity of daily living impairments [41] may be under-addressed by rehabilitation referrals during clinic visits. Our study calls for improved clinical care based in international guidelines on early referrals, underscores the critical need for interdisciplinary training programs that raise awareness to the myriad functional impairments that can be targeted by rehabilitation services, and also highlights potential barriers that will need to be considered for optimizing allied health service access in order to achieve current clinical care standards.
Supplementary Material
Highlights.
Single discipline referrals are more common than multidiscipline referrals in PD
Rehabilitation referrals are made reactively in response to falls and advancing disease
There may be missed opportunities for proactive referrals that address quality of life/function
Speech therapy, occupational therapy, and early stage referrals are especially underutilized
Acknowledgements.
The parent study (study design, data collection, analysis and interpretation) was funded by the Parkinson’s Foundation (Parkinson’s Foundation Quality Improvement Initiative Study). Project support (study design, analysis, interpretation of data, and writing) was provided by awards to Dr. Roberts (NIDCD 1R21DC017255) and Dr. Rafferty (Foundation for Physical Therapy NIFTI2016-2018; Agency for Healthcare Research and Quality F32HS025077).
Disclosures
All authors, in addition to the PF-QII Steering Committee, approved the final manuscript submission. Authors Roberts, Rafferty, Cubillos, and Simuni conceptualized the study. Authors Wu, Miao, and Roberts completed data analyses and interpretation. Author Roberts wrote the first draft, revised all subsequent drafts, and prepared the final manuscript for submission. All authors provided intellectual and format revisions to the first draft. Authors Simuni, Cubillos, and the PF-QII study investigators were involved with the PF-QII parent study design, data collection, and oversight.
Dr. Roberts receives speaker honoraria and travel reimbursement from the Parkinson’s Foundation. Dr. Rafferty is a member of the Parkinson’s Foundation Parkinson’s Outcomes Project Steering Committee and receives accompanying honorarium and travel reimbursement. Dr. Rafferty also receives salary support from Shirley Ryan AbilityLab, a clinical affiliate of one of the enrolling study sites. Dr. Cubillos is employed by the Parkinson’s Foundation. Dr. Simuni is a founding member and Principal Investigator for the PF-QII study and also receives salary support from one of the enrolling study sites in her role as center Director. Dr. Wu received financial support from the Parkinson’s Foundation for performing statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Angela C. Roberts, Roxelyn and Richard Pepper Department of Communication Sciences and Disorders, Northwestern University, 2240 Campus Drive, Evanston, Illinois USA 60025.
Miriam R. Rafferty, Shirley Ryan AbilityLab, Northwestern University Department of Physical Medicine and Rehabilitation and Department of Psychiatry and Behavioral Science, 355 E Erie St. 19th Floor. Chicago, IL 60611..
Guanhuong Miao, Department of Biostatistics, College of Public Health & Health Professions College of Medicine, University of Florida, 2004 Mowry Rd, P.O. Box 117450, Gainesville, Florida USA32611.
Fernando Cubillos, Parkinson’s Foundation, 200 SE 1st Street, Ste 800, Miami, FL USA 33131.
Tanya Simuni, Feinberg School of Medicine, Department of Neurology, Northwestern University Parkinson’s Disease and Movement Disorders Center, Room 1900, NMH/259 E Erie, Chicago, Illinois USA 60611.
References
- [1].Nijkrake MJ, Keus SHJ, Kalf JG, Sturkenboom IHWM, Munneke M, Kappelle AC, Bloem BR, Allied health care interventions and complementary therapies in Parkinson’s disease, Parkinsonism Relat. Disord 13 (2007) S488–S494. doi: 10.1016/S1353-8020(08)70054-3. [DOI] [PubMed] [Google Scholar]
- [2].Seo HG, Park SJ, Seo J, Byun SJ, Oh B-M. Rehabilitation Therapy Utilization in Patients with Parkinson’s Disease in Korea. Parkinsons Dis. (2018) Article ID 9475415, 55/2018/9475415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bryant M, Rintala D, Lai E, Protas E, Needs for rehabilitation services in veterans with Parkinson’s disease (PD), Mov. Disord 26 (2011) S290. [Google Scholar]
- [4].Fullard ME, Thibault DP, Hill A, Fox J, Bhatti DE, Burack MA, Dahodwala N, Haberfeld E, Kern DS, Klepitskava OS, Urrea-Mendoza E, Myers P, Nutt J, Rafferty MR, Schwalb JM, Shulman LM, Willis AW, Parkinson Study Group Healthcare Outcomes and Disparities Working Group, Utilization of rehabilitation therapy services in Parkinson disease in the United States, Neurology. 89 (2017) 1162–1169. doi: 10.1212/WNL.0000000000004355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Factor SA, Bennett A, Hohler AD, Wang D, Miyasaki JM, Quality improvement in neurology: Parkinson disease update quality measurement set, Neurology. 86 (2016) 2278–2283. doi: 10.1212/WNL.0000000000002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].National Institute for Health and Care Excellence. Non-pharmacological management of motor and non-motor symptoms, in: NICE Guideline: Parkinson’s disease in Adults. National Institute for Health and Care Excellence. 2017. https://www.nice.org.uk/guidance/ng71/chapter/Recommendations#non-pharmacological-management-of-motor-and-non-motor-symptoms. (accessed June 19, 2019). [Google Scholar]
- [7].Ferrazzoli D, Ortelli P, Zivi I, Cian V, Urso E, Ghilardi MF, Maestri R, Frazzitta G, Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: a randomised controlled study, J. Neurol. Neurosurg. Psychiatry 89 (2018) 828–835. doi: 10.1136/jnnp-2017-316437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carne W, Cifu DX, Marcinko P, Baron M, Pickett T, Qutubuddin A, Calabrese V, Roberge P, Holloway K, Mutchler B, Efficacy of multidisciplinary treatment program on long-term outcomes of individuals with Parkinson’s disease, J. Rehabil. Res. Dev 42 (2005) 779–786. [DOI] [PubMed] [Google Scholar]
- [9].van der Marck MA, Kalf JG, Sturkenboom IH, Nijkrake MJ, Munneke M, Bloem BR, Multidisciplinary care for patients with Parkinson’s disease. Parkinsonism Relat Disord. Suppl 3 (2009) S219–223. [DOI] [PubMed] [Google Scholar]
- [10].Bloem, Ypinga JHL, Willis A, Canning CG, Barker RA, Munneke M, De Vries NM, Using Medical Claims Analyses to Understand Interventions for Parkinson Patients, J. Park. Dis 8 (2018) 45–58. doi: 10.3233/JPD-171277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Domingos J, Coelho M, Ferreira JJ, Referral to rehabilitation in Parkinson’s disease: who, when and to what end?, Arq. Neuropsiquiatr 71 (2013) 967–972. doi: 10.1590/0004-282X20130209. [DOI] [PubMed] [Google Scholar]
- [12].Okun MS, Siderowf A, Nutt JG, O’Conner GT, Bloem BR, Olmstead EM, Guttman M, Simuni T, Cheng E, Cohen EV, Parashos S, Marsh L, Malaty IA, Giladi N, Schmidt P, Oberdorf J, Piloting the NPF data-driven quality improvement initiative, Parkinsonism Relat. Disord 16 (2010) 517–521. doi: 10.1016/j.parkreldis.2010.06.005. [DOI] [PubMed] [Google Scholar]
- [13].Thornton M, Travis SS, Analysis of the Reliability of the Modified Caregiver Strain Index, J. Gerontol. B. Psychol. Sci. Soc. Sci 58 (2003) S127–S132. doi: 10.1093/geronb/58.2.S127. [DOI] [PubMed] [Google Scholar]
- [14].Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score, Age Ageing. 26 (1997) 353–357. [DOI] [PubMed] [Google Scholar]
- [15].Podsiadlo D, Richardson S, The timed “Up & Go”: a test of basic functional mobility for frail elderly persons, J. Am. Geriatr. Soc (1991) 142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- [16].Frazzitta G, Maestri R, Bertotti G, Riboldazzi G, Boveri N, Perini M, Uccellini D, Turla M, Comi C, Pezzoli G, Ghilardi MF, Intensive Rehabilitation Treatment in Early Parkinson’s Disease: A Randomized Pilot Study With a 2-Year Follow-up, Neurorehabil. Neural Repair 29 (2015) 123–131. doi: 10.1177/1545968314542981. [DOI] [PubMed] [Google Scholar]
- [17].Gage H, Grainger L, Ting S, Williams P, Chorley C, Carey G, Borg N, Bryan K, Castleton B, Trend P, Kaye J, Jordan J, Wade D, Specialist rehabilitation for people with Parkinson’s disease in the community: a randomised controlled trial, Health Serv. Deliv. Res (2014) 1–376. doi: 10.3310/hsdr02510. [DOI] [PubMed] [Google Scholar]
- [18].Rafferty MR, MacDonald J, Byskosh A, Sloan L, Toledo S, Marciniak C, Simuni T, Using Implementation Frameworks to Provide Proactive Physical Therapy for People with Parkinson Disease: Case Report, Phys. Ther 99 (2019) 1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Frazzitta G, Maestri R, Ghilardi MF, Riboldazzi G, Perini M, Bertotti G, Boveri N, Buttini S, Lombino FL, Uccellini D, Turla M, Pezzoli G, Comi C, Intensive Rehabilitation Increases BDNF Serum Levels in Parkinsonian Patients: A Randomized Study, Neurorehabil. Neural Repair 28 (2014) 163–168. doi: 10.1177/1545968313508474. [DOI] [PubMed] [Google Scholar]
- [20].Nijkrake MJ, Keus SHJ, Oostendorp RAB, Overeem S, Mulleners W, Bloem BR, Munneke M, Allied health care in Parkinson’s disease: Referral, consultation, and professional expertise, Mov. Disord 24 (2009) 282–286. doi: 10.1002/mds.22377. [DOI] [PubMed] [Google Scholar]
- [21].Hassan A, Wu SS, Schmidt P, Malaty IA, Dai YF, Miyasaki JM, Okun MS, What are the issues facing Parkinson’s disease patients at ten years of disease and beyond?: Data from the NPF-QII study, Parkinsonism Relat. Disord 18 (2012) S10–S14. doi: 10.1016/j.parkreldis.2012.06.014. [DOI] [PubMed] [Google Scholar]
- [22].Hassan A, Wu SS, Schmidt P, Simuni T, Giladi N, Miyasaki JM, Bloem BR, Malaty IA, Okun MS, The Profile of Long-term Parkinson’s Disease Survivors with 20 Years of Disease Duration and Beyond, J. Park. Dis 5 (2015) 313–319. doi: 10.3233/JPD-140515. [DOI] [PubMed] [Google Scholar]
- [23].Abbott LM, Naismith SL, Lewis SJG, Parkinson’s disease in general practice: Assessing knowledge, confidence and the potential role of education, J. Clin. Neurosci 18 (2011) 1044–1047. doi: 10.1016/j.jocn.2010.12.041. [DOI] [PubMed] [Google Scholar]
- [24].Plouvier AOA, Olde Hartman TC, Verhulst CEM, Bloem BR, van Weel C, Lagro-Janssen ALM, Parkinson’s disease: patient and general practitioner perspectives on the role of primary care, Fam. Pract 34 (2017) 227–233. doi: 10.1093/fampra/cmw115. [DOI] [PubMed] [Google Scholar]
- [25].Plouvier AOA, Olde Hartman TC, de Bont OA, Maandag S, Bloem BR, van Weel C, Lagro-Janssen ALM, The diagnostic pathway of Parkinson’s disease: a cross-sectional survey study of factors influencing patient dissatisfaction, BMC Fam. Pract 18 (2017). doi: 10.1186/s12875-017-0652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Keus SHJ, Bloem BR, Verbaan D, de Jonge PA, Hofman M, van Hilten BJ, Munneke M, Physiotherapy in Parkinson’s disease: Utilisation and patient satisfaction, J. Neurol 251 (2004) 680–687. doi: 10.1007/s00415-004-0402-7. [DOI] [PubMed] [Google Scholar]
- [27].Plouvier AOA, Olde Hartman TC, van Weel C, Bloem BR, Lagro-Janssen ALM, Transitions in Parkinson’s disease in primary care: protocol of a longitudinal mixed methods study, BMJ Open. 5 (2015). doi: 10.1136/bmjopen-2014-007171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lageman SK, Mickens MN, Cash TV, Lageman SK, Mickens MN, & Cash TV, Caregiver-identified needs and barriers to care in Parkinson’s disease, Geriatr Nurs. 36 (2015) 197–201. doi: 10.1016/j.gerinurse.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [29].Grimes D, Fitzpatrick M, Gordon J, Miyasaki J, Fon EA, Schlossmacher M, Suchowersky O, Rajput A, Lafontaine AL, Mestre T, Appel-Cresswell S, Kalia SK, Schoffer K, Zurowski M, Postuma RB, Udow S, Fox S, Barbeau P, Hutton B, Canadian guideline for Parkinson disease, Can. Med. Assoc. J 191 (2019) E989–E1004. 10.1503/cmaj.181504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vaughan CP, Prizer LP, Vandenberg AE, Goldstein FC, Trotti LM, Hermida AP, Factor SA, A Comprehensive Approach to Care in Parkinson’s Disease Adds Quality to the Current Gold Standard, Mov. Disord. Clin. Pract 4 (2017) 743–749. doi: 10.1002/mdc3.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sturkenboom IHWM, Thijssen MCE, Gons-van Elsacker JJ, Jansen IJH, Maasdam A, Schulten M, Vijver-Visser D, Steultjens E, Bloem B, Munneke M. Guidelines for Occupational Therapy in Parkinson’s Disease Rehabilitation. (2011). Retrieved from https://www.parkinsonnet.com/discipline/occupational-therapy/.
- [32].Keus SHJ, Munneke M, Graziano M, Paltamaa J, Pelosin E, Domingos J, Brühlmann S, Ramaswamy B, Prins J, Struiksma C, Rochester L, Nieuwboer A, Bloem B, On behalf of the Guideline Development Group. European Physiotherapy Guideline for Parkinson’s disease. the Netherlands: KNGF/ParkinsonNet; 2014. https://www.parkinsonnet.com/discipline/physiotherapy/. [Google Scholar]
- [33].Kalf H, de Swart B, Bonnier-Baars M, Hofman M, Kanters J, Kocken J, Bloem B, Munneke M, Guidelines for speech-language therapy in Parkinson’s disease. Nijmegen, The Netherlands/Miami, FL: ParkinsonNet/NPF; 2011. https://www.parkinsonnet.com/discipline/speech-and-language/. [Google Scholar]
- [34].Kim DY, Oh HM, Bok S-K, Chang WH, Choi Y, Chun MH, Han SJ, Han T-R, Jee S, Jung SH, Jung HY, Jung T-D, Kim MW, Kim EJ, Kim HS, Kim Y-H, Kim Y, Kim DY, Kim DY, Kim D-K, Ko S-H, Ko M-H, Lee JK, Lee J, Lee SJ, Lee S-G, Lim SH, Oh B-M, Paik N-J, Park KD, Park S-W, Park G-Y, Park JH, Park YG, Pyun S-B, Ryu B, Seo HG, Shin Y-I, Sohn MK, Yang SN, Don Yoo S, Yoo W-K, KSNR Clinical Consensus Statements: Rehabilitation of Patients with Parkinson’s Disease, Brain & Neurorehabilitation. 13 (2020). 10.12786/bn.2020.13.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moore JL, Potter K, Blankshain K, Kaplan SL, OʼDwyer LC, Sullivan JE, A Core Set of Outcome Measures for Adults With Neurologic Conditions Undergoing Rehabilitation, J. Neurol. Phys. Ther 42 (2018) 174–220. 10.1097/NPT.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Academy of Neurologic Physical Therapy. Parkinson’s Disease Evaluation Database to Guide Effectiveness (PD-EDGE). 2014. http://www.neuropt.org/professional-resources/neurology-section-outcome-measures-recommendations/parkinson-disease. Accessed October 27, 2017. [Google Scholar]
- [37].Radder DLM, Nonnekes J, van Nimwegen M, Eggers C, Abbruzzese G, Alves G, Browner N, Chaudhuri KR, Ebersbach G, Ferreira JJ, Fleisher JE, Fletcher P, Frazzitta G, Giladi N, Guttman M, Iansek R, Khandhar S, Klucken J, Lafontaine A-L, Marras C, Nutt J, Okun MS, Parashos SA, Munneke M, Bloem BR, Recommendations for the Organization of Multidisciplinary Clinical Care Teams in Parkinson’s Disease, J. Parkinsons. Dis 10 (2020) 1087–1098. 10.3233/JPD-202078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Roberts-South A, Hall L, Jog MS, Telehealth Management in Movement Disorder: A Retrospective Study, Can. J. Neurol. Sci. / J. Can. Des Sci. Neurol 40 (2013) 230–234. 10.1017/S0317167100013780. [DOI] [PubMed] [Google Scholar]
- [39].Suttrup I, Warnecke T, Dysphagia in Parkinson’s Disease, Dysphagia. 31 (2016) 24–32. 10.1007/s00455-015-9671-9. [DOI] [PubMed] [Google Scholar]
- [40].Schalling E, Johansson K, Hartelius L, Speech and Communication Changes Reported by People with Parkinson’s Disease, Folia Phoniatr. Logop 69 (2017) 131–141. 10.1159/000479927. [DOI] [PubMed] [Google Scholar]
- [41].Foster ER, Instrumental Activities of Daily Living Performance Among People With Parkinson’s Disease Without Dementia, Am. J. Occup. Ther 68 (2014) 353. 10.5014/ajot.2014.010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


