Abstract
Background and Aim:
The potential of plants as anthelmintics is very large, but there is still very little research conducted in the search for effective, safe, easily obtained, and affordable anthelmintic candidates. Palem putri (Veitchia merrillii) is an ornamental plant that is interesting to study because it is included in the areca nut group which is reported to have strong abilities as anthelmintics. The study aims to evaluate the anthelmintic efficacy of Veitchia merrillii against trematode worms such as Paramphistomum spp. and Fasciola hepatica.
Materials and Methods:
This research employs both in vitro and computational techniques. An anthelmintic in vitro test was carried out on Paramphistomum spp. worms at concentrations of 10%, 25%, and 40% (gr/v), assessing mortality index as the observable outcome, followed by a histopathological investigation of the deceased worms for tissue and cellular damage evaluation. Seventeen compounds from V. merrillii seeds were studied in silico for their anthelmintic activity against F. hepatica worms using the quantitative structure-activity relationship technique, molecular docking, and Lipinski’s rule analysis for orally administered medication.
Results:
About 25% and 40% extracts of V. merrillii damaged the tegument organs in the worms. Seventeen compounds in V. merrillii seed extract, on average, yielded a higher anthelmintic index on F. hepatica than praziquantel. Eleven of the 17 compounds exhibit stronger affinity than praziquantel, with routine and gallic acid being the top two ligands (∆Gbinding values: −11.65 kcal/mol and −11.07 kcal/mol, respectively). According to Lipinski’s rule analysis, only routine compounds cannot be orally administered.
Conclusion:
The seeds of V. merrilli have potential as an anthelmintic agent for Paramphistomum spp. at concentrations of 25%–40% (gr/v).
Keywords: Molecular docking, QSAR, Trematoda, Veitchia merillii
Introduction
Parasitic diseases significantly impact global health and livestock productivity. Small ruminants are susceptible to gastrointestinal infections from nematodes, trematodes, and cestodes [1]. In Indonesia, the presence of Haemonchus spp., Fasciola gigantica, and Paramphistomum spp. worms pose a significant threat to young livestock. Haemonchus placei, a type of gastrointestinal nematode, can hinder growth and lead to death in both cattle and sheep. This parasite, which feeds on blood, is responsible for numerous deaths, predominantly among the young. In addition, these gastrointestinal worms are easily resistant to commercial anthelmintics, especially in countries that have many small ruminants [2]. Paramphistomum spp. worms, including both adults and young images, have been known to cause Paramphistomiasis. The infection can progress from the rumen to the abomasum and small intestine. Ruminants are at risk from this disease [3–6]. Livestock is commonly infected by Paramphistomum cervi, Paramphistomum ichikawai, Paramphistomum gotoi, and Paramphistomum scotiae. These trematodes, including Fasciola hepatica and F. gigantica cause infections (fasciolosis) in cattle, sheep, and other ruminants. Fasciolosis causes economic losses amounting to USD 31.65 million annually. Worm infestation in the liver and gallbladder of ruminants can impede growth, decrease production, render the liver unsuitable for consumption, and potentially result in fatality. The necessary measures should be implemented to combat losses from fasciolosis resulting from infections with these two worms.
Using natural anthelmintic sources as an alternative can eliminate worms. The availability and affordability of numerous raw materials, easy financing, and lower chances of resistance make plants attractive alternatives for anthelmintic production. It is estimated that more than 20,000 plant species have been used worldwide to treat various types of diseases, most of them as antibiotics [7–9], some of which also study the anthelmintic properties of these medicinal plants. In Cameroon and Ghana, the medicinal use of Anogeissus leiocarpus, Khaya senegalensis, Euphorbia hirta, Annona senegalensis water extracts, and Parquetina nigrescens is reported for their anthelmintic properties [10]. Vidyadhar et al. [11] revealed the anthelmintic property of Enicostemma littorale. Jeyathilakan et al. [12] found that Cymbopogan nargus and Azadirachta indica were useful for treating F. gigantica infections. Nigella sativa extract and ivermectin were reported to be effective as an anthelmintic by Shalaby and El-Moghazy [13]. Pal and Pandap [14] explained that the anthelmintic potential is also found in the Cynodon dactylon plant, which is traditionally used as a cure for epilepsy, diarrhea, dysentery, cancer, coughs, wounds, hypertension, and rheumatism by people in India. Gynandropsis gynandra and Buchholzia coriaceae’s leaf and root extracts are active against F. gigantica, Tenia solium, and Pheritima pashuma trematode worms [15]. Zahir et al. [16] explained that ethyl acetate extract of Achyranthes aspera leaves, acetone and chloroform extract of Anisomeles malabarica leaves, methanol extract of Gloriosa superba flowers, and methanol extract of Ricinus communis leaves have the potential to be used in controlling parasites Paramphistomum cervi, Rhipicephalus (Boophilus) microplus, Anopheles subpictus, and Culex tritaeniorhynchus. Ethanol extract of Jatropha curcas seeds inhibits Haemoncus contortus nematodes [17], while Allium sativum and Lawsonia inermis extracts exhibit fluidal activity against F. gigantica [18]. In Indonesia, most people have utilized areca nuts as an anthelmintic medicine [19–29]. The Veitchia merrillii, also referred as its areca nut family name Arecaceae [23,30,31], is a palm species. In-home gardens, offices, parks, and urban roadsides, this plant is popularly used as an ornamental plant. Beyond its economic significance, this plant is also found to have fatty acids, including palmitic acid, oleic acid, and linoleic acid [32, 33]. Using high-performance liquid chromatography (HPLC), Vafaei [34] identified gallic acid, caffeic acid, vanillic acid, syringic acid, pyrogallol, quinic acid, and naringenin as active compounds. The last two compounds are the most frequently occurring components in the fruit of this plant. Hamzah et al. [23] have reported that this plant is effective in killing Ascaridia galli worms in vitro. This article provides information on V. merrillii’s anthelmintic properties against F. hepatica and Paramphistomum spp. trematode worms.
This study aimed to investigate the antitrematode properties of V. merrilli fruit seeds using both in vitro and in silico techniques. The in vitro mortality and histology of Paramphistomum spp. worm tegument was examined following treatment with V. merrillii ethanol extract at concentrations of 10%, 25%, and 40%. In vitro studies employing QSAR and molecular docking techniques were performed to evaluate the anthelmintic potential of 17 secondary metabolites in V. merrillii seeds against F. hepatica worms [32–34].
Materials and Methods
Ethical approval
This study was conducted in vitro using worm parasites and in silico, so ethical approval is not required.
Study period and location
The study was conducted from February to July 2023 in the Parasitology and Pharmacology Laboratory, Faculty of Veterinary Medicine, Syiah Kuala University.
Materials
The sample consisted of Veitchia merillii fruit seeds sourced from the “Blang Padang” park region of Banda Aceh, Indonesia. It was identified by Devi Syafrianti in the Biology Laboratory of Syiah Kuala University. Ethanol 70% was used to extract dried V. merrillii fruit seeds for in vitro testing on worm motility and mortality. Seventeen V. merrillii fruit seed active compounds, downloaded from PubChem and converted in pdb format, were used as ligands against thioredoxin enzyme receptor (pdb id. 2VIM) in an in silico test whose hardware specifications was a set of computers with processorCore™ i5-3230M2 Cores chip, 4 Threads@2.6GHz, 4.00 GB DDR3 1600 MHz random access memory, 2GB DDR3 Radeon HD 8670M video graphics array, supported by internet access. While software used was the Molecular Operating Environment (MOE) (V.9 2010, Chemical Computing Group, Inc., Canada) relies on various web servers including http://www.way2drug.com/PASSOnline/predict.php, http://stitch.embl.de, and https://biosig.lab.uq.edu.au/pkcsm. for support.
Methods
Ethanol extraction of V. merrillii fruits seeds
Following the method of Jiraungkoorskul et al. [35] with modifications (maceration container connected to automatic stirring tool), we weighed V. merrillii seeds to ±5 kg, dried them in the absence of sunlight, and ground them into powder using a blender. The filtrate was taken 3 times after macerating the powder with ethanol solution. In the Rotavapor® R-300 (China), a vacuum rotary evaporator, the macerate was evaporated to yield a thick, ethanol-free extract.
Worm motility and mortality test using V. merrillii extract
Paramphistomum spp. was collected from the abomasums of cattle slaughtered at the Lambaro Aceh Besar. Worms were transferred to RPMI 1640 (Sigma-Aldrich®, USA) medium for motility and mortality testing. Ten worms for each treatment were placed in separate Petri dishes, and 10% (P1), 25% (P2), and 40% (P3) of V. merrillii extract were added to each, respectively, in triplicate. Praziquantel served as the positive control (C0), while phosphate-buffered saline (PBS) acted as the negative control (C1). Every 15 min, an index score was used to determine worm motility. The worm’s body movement is assessed. A body moving completely was scored 3, partially moved, 2; alive but not moving, 1; and dead, 0. We confirmed worm death by touching them with a stir bar. A moving worm confirms its life. The worm’s death is confirmed if it remains silent.
Histopathological examination
Histopathological preparations were made from each treatment group using dead worms. The examination involves an initial rinse of worm samples with PBS, followed by fixation using 10% buffer neutral formalin, then a stopping point with 70% alcohol, and subsequent dehydration using graded alcohol concentrations (70%, 80%, 90%, and absolute). The tissue in Silol I, II, and III fluids is cleared, infiltrated with liquid paraffin, and then embedded in paraffin to form a paraffin block. 5-μm thick tissue slices were prepared using a microtome, stained with hematoxylin-eosin, and mounted on slides with Entellan® (Merck, Germany) adhesive. Observations were made and recorded as photomicrographs using the Olympus CX31 (Japan) microscope.
Literature search for secondary metabolites of V. merrillii plant seeds and download of the smile structure of these compounds through PubChem
Seventeen compounds, including gallic acid, caffeic acid, vanillic acid, syringic acid, pyrogallol, rutin, naringenin, limonene, cis-β-ocimene, allo-ocimene, linalool oxide, linalool, methyl salicylate, eucalyptol, palmitic acid, oleic acid, and linoleic acid, have been isolated from the V. merrillii seeds according to Rodríguez-Leyes et al. [32] and Vafaei [34] (Table-1).
Table-1.
| S. No. | Compounds | Canonical SMILES |
|---|---|---|
| 1 | Gallic acid | C1=C (C=C (C(=C1O) O) O) C(=O) O |
| 2 | Pyrogallol | C1=CC(=C (C(=C1) O) O) O |
| 3 | Caffeic acid | C1=CC(=C (C=C1C=CC(=O) O) O) O |
| 4 | Vanillic acid | COC1=C (C=CC(=C1) C(=O) O) O |
| 5 | Syringic acid | COC1=CC(=CC(=C1O) OC) C(=O) O |
| 6 | Rutin | CC1C (C (C (C (O1) OCC2C (C (C (C (O2) OC3=C (OC4=CC(=CC(=C4C3=O) O) O) C5=CC(=C (C=C5) O) O) O) O) O) O) O) O |
| 7 | Naringenin | C1C (OC2=CC(=CC(=C2C1=O) O) O) C3=CC=C (C=C3) O |
| 8 | Limonene | CC1=CCC (CC1) C(=C) C |
| 9 | cis-β-ocimene | CC(=CCC=C (C) C=C) C |
| 10 | Allo-ocimene | CC=C (C) C=CC=C (C) C |
| 11 | Linalool oxide | CC(=CCCC (C)(C1CO1) O) C |
| 12 | Linalool | CC(=CCCC (C)(C=C) O) C |
| 13 | Methyl salicylate | COC(=O) C1=CC=CC=C1O |
| 14 | Eucalyptol | CC1(C2CCC (O1)(CC2) C) C |
| 15 | Palmitic acid | CCCCCCCCCCCCCCCC(=O) O |
| 16 | Oleate acid | CCCCCCCCC=CCCCCCCCC(=O) O |
| 17 | Linoleic acid | CCCCCC=CCC=CCCCCCCCC(=O) O |
| Praziquantel (Control) | C1CCC (CC1) C(=O) N2CC3C4=CC=CC=C4CCN3C(=O) C2 |
Determination of the potential of 17 metabolites of V. merillii plant seed compounds as anthelmintic agents in F. hepatica worms based on Way2Drug QSAR analysis
The Prediction of Activity Spectra for Substances (PASS) web server, available at http://www.way2drug.com/PASSOnline/predict.php, was used to predict anthelmintic activity against F. hepatica using SMILES data for the test compound. The probability of Pa and Pi varies between 0.000 and to1.000. PASS predictions are interpreted within a flexible range, namely: (i) Pa > Pi values are considered to have the possibility of being active; (ii) if Pa > 0.7, the probability of being experimentally active is high; (iii) if Pa >0.5 but <0.7, there is a chance that it will be experimentally active, but the compound may be different from the known active compound; (iv) if Pa <0.5 the chance of finding activity experimentally is low, but the chance of finding new chemical entities is high [36, 37].
The potential as an anthelmintic for trematodes is an average of 17 secondary metabolites of V. merrilli fruit seeds based on the anthelmintic parameter score for F. hepatica worms shown by the way2drug webserver varying from 0 to 1, which shows the accuracy of the analysis [38]. The anthelmintic activity of certain compounds against F. hepatica was used to determine their Pa scores through QSAR analysis. The similarity of a compound’s structure increases its predictive power.
Molecular docking
Through molecular docking analysis, the thioredoxin enzyme from the worm F. hepatica serves as the target, chosen due to QSAR method findings indicative of its role in the anthelmintic mechanism. Seventeen V. merrillii seed compound structures, downloaded from PubChem as “canonical SMILES” and converted to the pdb format, were used as test ligands. The 2VIM pdb structure for thioredoxin enzyme was retrieved from www.rscb.org. The initial molecular docking process involved optimizing ligand and receptor structures through adding hydrogen atoms, partial energy, and adjusting the system energy to a minimum for maximum binding affinity. The MOE application’s site finder is used to trace the binding site of the 2VIM receptor during the docking process. A ”site” matching the docking target reported by Shukla et al. [39] was chosen. The selected test ligands are then docked. ∆Gbinding values for docking results are displayed in a table and visualized in the form of 2-dimensional image (MOE LigPlot; Chemical Computing Group, Canada).
Sequence alignment and superposition of the 3D structures
Alignment was performed to observe the differences between the sequences that make up the thioredoxin enzyme between the worm species F. hepatica and the host Bos taurus using the sequence alignment method with the help of the web server https://www.ebi.ac.uk/Tools/msa/clustalo/. Furthermore, the 3D thioredoxin enzyme structures of the two species were superimposed to observe differences in their geometric conformations.
Analysis of drug-likeness, absorption, distribution, metabolism, excretion, and toxicity of 17 metabolites of V. merrillii fruit seed compounds
Absorption, distribution, metabolism, and excretion (ADME) analysis evaluates a compound’s potential behavior within the body, encompassing ADME. A compound’s suitability for oral drug administration can be determined by Lipinski’s rule of five, which states that a molecule must have a molecular mass under 500 daltons, a LogP value below five, no more than five hydrogen donor bonds, no more than 10 hydrogen acceptor bonds, and a molar refraction between 40 and 130. ADME and compound toxicity were predicted using the pkCSM (predicting small-molecule pharmacokinetic properties using graph-based signatures) web server, as described by Pires et al. [40].
Results and Discussion
Anthelmintic treatment
The abomasum samples obtained from the Lambaro Aceh Besar slaughterhouse only found Paramphistomum spp. worms, so in vitro treatment was only carried out on this type of worm. The results show that 40% ethanol extract of V. merrillii fruit seeds has the strongest anthelmintic power compared with concentrations of 10% and 25% against Paramphistomum spp. worms. All worms died within 80 min after soaking. These results were even better than those of the positive control. The anthelmintic power parameters observed were the motility and mortality scores of Paramphistomum spp. worms after being given ethanol extract of V. merrillii fruit seeds at 30, 60, 90, and 100 min after incubation.
The negative control group demonstrated continuous movement for all Paramphistomum spp. worms for 30, 60, 90, and 100 min following incubation (score 3). 30 min after incubation, all Paramphistomum spp. worms in the control group were still active (scores 3); 60 min after incubation, four worms had entire body movement (scores 3) and six had incomplete body movement (scores 2); 90 min after incubation, four worms were alive with no movement (scores 1) and nine were died (scores 0); 100 min after incubation, one worm had no movement but was still alive (scores 1) and nine were died (scores 0).
Eight Paramphistomum spp. worms exhibited active movement (score 3) and two had partial movement (score 2) during 30 min of incubation with 10% ethanol extract from V. merrillii fruit seeds. Six tails remained alive with no movement (score 1) while one worm died (score 0) within 60 min post-incubation. All worms (score 0) ceased to move at 90 and 100 min post-incubation.
Seven Paramphistomum spp. worms, with some part of their bodies still moving, were found in the group given 25% ethanol extract, while three worms remained alive with part of their bodies active during the 30-min incubation; however, one tail was only alive with part of the worm body moving, seven tails did not move but remained alive, and two tail worms were died after 60 min; unfortunately, all worms had died by 90 and 100 min.
In the group given 40% ethanol extract of V. merrillii fruit seeds, three Paramphistomum spp. worms were found to still be actively moving throughout their bodies (score 3), and seven worms showed that they were still alive with part of the worm’s body moving (score 2) during the 30 incubation period; six worms did not move but were still alive (score 1), four worms died (score 0) during 60 min post-incubation, and all worms died (score 0) at 90 and 100 min post-incubation. The in vitro experiment results are presented in Table-2.
Table-2.
Motility of Paramphistomum spp. worms in Veitchia merrillii fruit seeds extract and control.
| Time (min) | Score | Treatment (n=10) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| C0 | C1 | P1 | P2 | P3 | ||
| 30 | 3 | 10 | 10 | 8 | 7 | 3 |
| 2 | 2 | 3 | 7 | |||
| 1 | ||||||
| 0 | ||||||
| 60 | 3 | 10 | 4 | |||
| 2 | 6 | 3 | 1 | |||
| 1 | 6 | 7 | 6 | |||
| 0 | 1 | 2 | 4 | |||
| 90 | 3 | 10 | ||||
| 2 | ||||||
| 1 | 4 | |||||
| 0 | 6 | 10 | 10 | 10 | ||
| 100 | 3 | 10 | ||||
| 2 | ||||||
| 1 | 1 | |||||
| 0 | 9 | 10 | 10 | 10 | ||
V. merrillii fruit seeds have been identified as rich sources of alkaloids, phenol-flavonoids, and tannins, according to Balqis et al. [19]. Vafaei [34] identified gallic acid, vanillic acid, kaffic acid, syringic acid, nagarin, pyrogallol, and routine flavonoids as the constituents of V. merrillii seeds using HPLC analysis. Tannins are polyphenolic compounds with astringent or protein-precipitating properties. Damaging the worm’s protein membrane with this ability leads to paralysis and death. Tannins can hinder the nutritional intake of worms by suppressing their digestive metabolism [41, 42]. Mali and Mehta [43] reported the uncoupling mechanism of oxidative phosphorylation and cuticular glycoprotein binding in A. galli worms. This mechanism may occur in F. hepatica. The root extract of Adhatoda vasica plant inhibits nerve impulses in A. galli worms, leading to paralysis [44]. Alkaloids enhance gastrointestinal contractility, amplifying peristaltic waves to expel parasites from the digestive system [44]. V. merrillii seeds’ flavonoids inhibit the development of worms and filarial parasites [10]. Lakshmi et al. [45] reported antifilarial activity of naringenin, flavone, hesperetin, rutin, naringenin, and chrysin against Brugia malayiin. Against various parasites, triterpenoids demonstrate anthelmintic properties. According to Mali and Mehta [43], extracts from Mimusops elengi Linn contain triterpenoids and saponins which lead to paralysis and death of worms. Strychnos spinosa leaves’ triterpenoids were reported by Hoet et al. [46] to inhibit Trypanosoma brucei’s development in vitro. Previously, the insecticidal bioactivity of A. indica leaf triterpenoids against Aedes aegypti mosquito larvae was proven by Siddiqui et al. [47].
Histopathological observations revealed ongoing mortality effects. The intention was to examine organ damage in Paramphistomum spp. worms caused by V. merrillii fruit seed extract administration. 25% and 40% V. merrillii seed extract-induced tegument damage in Paramphistomum spp., as evidenced by their thinned and disintegrated layers (Figure-1). The tegument layer significantly contributes with crucial enzymes such as acid and alkaline phosphatases, amino peptidase, glutathione S-transferase, acetylcholine esterase, glucose transporter, serine hydrolase, and glycolytic enzymes [48]. The tegument layer, as a sensory organ, adapts to the environment by absorbing exogenous food ingredients [49].
Figure-1.
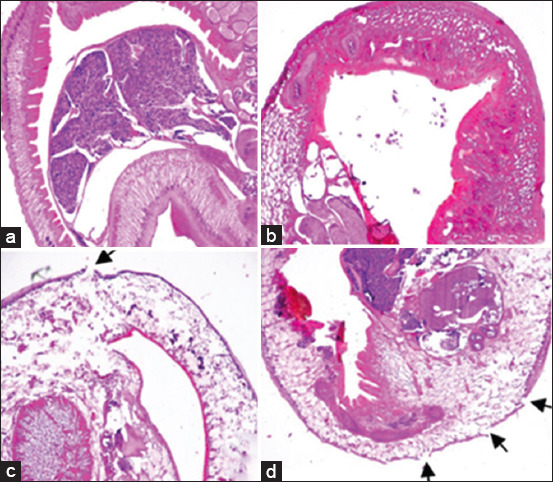
Tegument of Paramphistomum spp. (a) Negative control. (b) 10% V. merrillii extract. (c) 25% V. merrillii extract. (d) 40% V. merrillii extract. The arrows indicate a damaged tegument. V. merrillii=Veitchia merrillii.
The PASS prediction web server was used to predict the biological activity of each test compound. The main biological activity of 17 V. merrillii fruit seed compounds is enhanced by various mechanisms as revealed in Table-3. The Pa > Pi value in this study indicated the biological activity of all test compounds. The average anthelmintic Pa value, determined by anthelmintic activity, among the 17 test compounds was 0.340 (±0.123), falling within a range of 0.145–0.580. The linoleic acid compound had the greatest Pa value, while limonene had the least17 V. merrillii seed compounds exhibit stronger anthelmintic potential than praziquantel, as determined by the QSAR method.
Table-3.
Prediction scores for 17 Veitchia merrillii fruit seed compounds which are associated with anthelmintic effects on Fasciola hepatica worms.
| S. No. | Compounds | Anthelmintic | Microtubule formation inhibitor | Ca+2 channel activator | Thioredoxin inhibitor | Cholinergic antagonist | Agonist apoptosis | Ubiquinol- cytochrome- c reductase inhibitor | TP53 expression enhancer | Neurotransmitter antagonist | Caspase 3 stimulant |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Praziquantel (Control) | 0.305 | 0 | 0.125 | 0 | 0.284 | 0 | 0 | 0 | 0.417 | 0 | |
| 1 | Gallic acid | 0.356 | 0.234 | 0.612 | 0.409 | 0.573 | 0.562 | 0.915 | 0.718 | 0.64 | 0.413 |
| 2 | Pyrogallol | 0.524 | 0.23 | 0.69 | 0.864 | 0.641 | 0.775 | 0.914 | 0.744 | 0.66 | 0.507 |
| 3 | Caffeic acid | 0.277 | 0.285 | 0.521 | 0.563 | 0.482 | 0.711 | 0.843 | 0.776 | 0.523 | 0.579 |
| 4 | Vanillic acid | 0.253 | 0.309 | 0.363 | 0.488 | 0.57 | 0.512 | 0.922 | 0.713 | 0.556 | 0.64 |
| 5 | Syringic acid | 0.271 | 0.276 | 0.359 | 0.363 | 0.566 | 0.538 | 0.925 | 0.712 | 0.547 | 0.52 |
| 6 | Rutin | 0.321 | 0 | 0.292 | 0 | 0 | 0.747 | 0.512 | 0.893 | 0 | 0.893 |
| 7 | Naringenin | 0.213 | 0.237 | 0.476 | 0.578 | 0.462 | 0.709 | 0.868 | 0.822 | 0.642 | 0.557 |
| 8 | Limonene | 0.145 | 0.355 | 0.447 | 0.464 | 0.223 | 0.816 | 0.707 | 0.627 | 0.357 | 0.516 |
| 9 | cis-β-ocimene | 0.218 | 0.257 | 0.477 | 0.661 | 0.518 | 0.858 | 0.857 | 0.694 | 0.402 | 0.496 |
| 10 | Allo-ocimene | 0.289 | 0.302 | 0.518 | 0.706 | 0.232 | 0.948 | 0.958 | 0.785 | 0.456 | 0.382 |
| 11 | Linalool oxide | 0.43 | 0 | 0.341 | 0.429 | 0.195 | 0.67 | 0.782 | 0.52 | 0 | 0.713 |
| 12 | Linalool | 0.372 | 0.245 | 0.503 | 0.579 | 0.188 | 0.667 | 0.727 | 0.719 | 0.302 | 0.435 |
| 13 | Methyl salicylate | 0.561 | 0.15 | 0.341 | 0.429 | 0.233 | 0.425 | 0.67 | 0.52 | 0.572 | 0.713 |
| 14 | Eucalyptol | 0.314 | 0 | 0.22 | 0.603 | 0.174 | 0.221 | 0.735 | 0.58 | 0 | 0.296 |
| 15 | Palmitate acid | 0.23 | 0 | 0.246 | 0.769 | 0.641 | 0.342 | 0.907 | 0.74 | 0.642 | 0.355 |
| 16 | Oleate acid | 0.425 | 0.226 | 0.516 | 0.681 | 0.713 | 0.499 | 0.886 | 0.791 | 0.547 | 0.361 |
| 17 | Linoleat acid | 0.58 | 0.235 | 0.49 | 0.648 | 0.449 | 0.545 | 0.87 | 0.763 | 0.518 | 0.333 |
| Average | 0.340 | 0.197 | 0.438 | 0.543 | 0.393 | 0.620 | 0.823 | 0.709 | 0.422 | 0.512 | |
| Standard deviation | 0.123 | 0.117 | 0.128 | 0.190 | 0.207 | 0.185 | 0.115 | 0.100 | 0.235 | 0.155 | |
Score prediction ≥0.5 is marked in dark green Score prediction is between 0.3 and 0.5, it is marked in light green Score prediction ≤0.3 is marked in yellow
In Table-3, the prediction scores for the 17 metabolites’ roles in providing anthelmintic effects are categorized by three different colors. Compounds scoring above 0.5 in prediction are represented in dark green, 0.3–0.5 in light green, and below 0.3in yellow. The cholinergic and neurotransmitter antagonistic properties of this agent enhance its potential as an anthelmintic, with prediction scores of 0.301 and 0.416, respectively. Oleic acid and pyrogallol exhibited the greatest cholinergic and neurotransmitter antagonist activities, with scores of 0.713 and 0.66, respectively. In worms, this cholinergic antagonist can induce muscle paralysis and death. The two receptors identified by You et al. [50] are crucial for comprehending the mechanism of anthelmintic drugs through their effects on Schistosoma haematobium, Schistosoma mansoni, and Schistosoma japonicum. Inhibit the function of the ubiquinol-cytochrome c reductase enzyme. The QSAR prediction results show an average score of 0.807, meaning that if the Pa score is >0.7, the chance of this mechanism being experimentally proven is very high [36, 37]. In the mitochondria of eukaryotic cells, the ubiquinol-cytochrome c reductase complex functions as an electron transfer enzyme during cellular respiration. Suppressing ubiquinol-cytochrome c reductase function impairs sugar metabolism energy production.
Seventeen secondary metabolites of V. merrillii seeds, on average, demonstrated a greater ability to induce Ca+2 channels compared to praziquantel, with a score of 0.438 versus 0.125. Increased Ca+2 channel activity leads to endogenous Ca+2 release and tetanic contractions/paralysis in the worm. In the tapeworm Hymenolepis diminuta, praziquantel induces contraction and expulsion from the digestive tract by eliciting endogenous Ca+2release from cells. In schistosomes, this compound triggers muscle contractions and paralysis, as well as the creation of balloon-like structures on the surface of the tegument that serve as antigens for antibodies and are subject to phagocytosis. Seventeen secondary metabolites of V. merrillii seeds, on average, exhibit considerable potential as apoptotic agonists, with a Pa score of 0.620. The caspase-3 stimulant and TP53 expression enhancer mechanisms increased the potency of the apoptosis agonist, as indicated by Pa scores of 0.512 and 0.709. Thioredoxin enzyme activity inhibition is a reported mechanism for anthelmintic medications [39]. Seventeen secondary metabolites of V. merrillii seeds have an average Pa score of 0.543 for inhibiting thioredoxin enzyme activity (Table 3).
Molecular docking
Seventeen V. merrillii compounds’ anthelmintic potential was confirmed using molecular docking with trematode worms. Based on the thioredoxin model by Shukla et al. [39], the thioredoxin enzyme was selected as the molecular docking target. A 3D model of F. hepatica worm’s thioredoxin enzyme is accessible online at https://www.rcsb.org/structure/2VIM. Paramphistomum spp. worms and their enzyme’s amino acid sequence have not been identified yet. In silico studies were exclusively conducted on F. hepatica.
Previously, thioredoxin ligands and enzymes were prepared with minimum system energy by adding hydrogen atoms, partial charges, and conditioning the system. Next, the “binding site” area was identified using MOE software “site finder.” Given the absence of a native ligand for the downloaded thioredoxin 2VIM receptor, the molecular docking was carried out utilizing the “blind docking” technique at the reported target “binding site” [39]. ∆Gbinding (kcal/mol) indicates the energy released upon ligand-receptor interaction, while its visualization reveals the orientation and position of the inhibitor.
The results indicate that the test ligands, including the routine compound with the strongest inhibitory effect (−11.65 kcal/mol), gallic acid (−11.07 kcal/mol), caffeic acid (−9.75 kcal/mol), and eucalyptol (−5.59 kcal/mol), all demonstrate potential as thioredoxin enzyme inhibitors. The stronger the affinity between an enzyme and its ligand, the more negative the ∆Gbinding value. The strength of this affinity is directly proportional to the value of the inhibition constant (Ki), which indicates the level of the compound’s ability to inhibit the activity of the target enzyme [51, 52].
Seventeen V. merrillii compounds, based on molecular docking, exhibited strong anthelmintic potential, as evidenced by a ∆Gbinding value of −7.45 kcal/mol for praziquantel as a control. The strength of the ligand-receptor complex interaction is significantly affected by the presence of hydrogen bonds. Only those compounds with the strongest binding affinity form hydrogen bonds with thioredoxin. The docking results obtained from the complete value of ΔGbinding17 bioactive compounds are shown in Table-4 and Figure-2.
Table-4.
∆Gbinding and hydrogen bonds of 17 compounds in V. merilli fruit seeds
| Compounds | PubChem CID | ∆Gbinding(kcal/mol) | Hydrogen bond |
|---|---|---|---|
| Gallic acid | 370 | –11,0716 | Met 1(2x), Pro 49, Val 51 |
| Pyrogallol | 1057 | –9,2807 | Pro 49, Val 51, Glu 52 |
| Caffeic acid | 689043 | –9,7496 | Met 1(2x), Arg 20 |
| Vanillic acid | 8468 | –8,4007 | Asn 17, Pro 49 |
| Syringic acid | 10742 | –8,3156 | Met 1, Ala 45 |
| Rutin | 5280805 | –11,6546 | Asn 17, Ala 45(2x) |
| Naringenin | 439246 | –8,8228 | Val 51 |
| Limonene | 22311 | –5,8445 | - |
| cis-β-ocimene | 5320250 | –5,6785 | - |
| Allo-ocimene | 5368821 | –5,5986 | - |
| Linalool oxide | 102611 | –7,6026 | Arg 2, Asn 17, Glu 52 |
| Linalool | 6549 | –7,7762 | Met 1(2x) |
| Methyl salicylate | 4133 | –7,6671 | Val 51 |
| Eucalyptol | 2758 | –5,5880 | - |
| Palmitic acid | 985 | –8,8364 | Met 1(2x), Ala 45 |
| Oleic acid | 445639 | –7,3524 | Met 1(2x) |
| Linoleic acid | 5280450 | –7,4306 | Val 51 |
| Praziquantel (Control) | 370 | –7,4473 | - |
Figure-2.
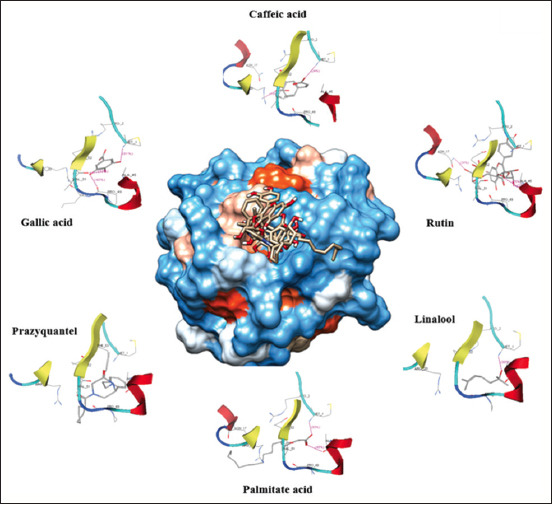
Visualization of molecular docking of five secondary metabolites of Veitchia merrillii with the strongest affinity compared with the praziquantel control for the Fasciola hepatica thioredoxin enzyme.
The molecular docking results demonstrate the efficacy of 17 V. merrilli compounds as potential anthelmintics against F. hepatica in cattle (Bos taurus). Instead of docking the F. hepatica worm’s molecule with the thioredoxin enzyme, alignment of their sequences and superposition of their 3D structures through the thioredoxin enzyme can suffice for comparison. Seventeen V. merrillii metabolites may inhibit thioredoxin enzyme in cattle as they do in F. hepatica. The thioredoxin enzyme’s crucial function in maintaining redox homeostasis, proliferation, and DNA synthesis [53–56] in cattle renders them susceptible to difficulties. According to Figure-3, sequence alignment and 3D structure superposition of thioredoxin enzyme from these two species reveal no similarities.
Figure-3.

Sequence alignment and superposition of the 3D structure of Fasciola hepatica thioredoxin (cyan) with Bos taurus (yellow).
Lipinski’s rule analysis
Lipinski’s rule applies to orally administered active compounds. Lipinski’s rule parameters, which measure the solubility and intestinal permeability of compounds in the gastrointestinal tract, serve as the foundation for estimating the oral bioavailability of active substances [57]. The test compound can meet Lipinski’s rules except for one parameter. The rules that must be met are log p ≤5, molecular weight ≤500 g/mol, hydrogen bond donor ≤5, and hydrogen bond acceptor ≤10 [58]. Table-5 presents the Lipinski parameters for 17 V. merrillii metabolite compounds.
Table-5.
Lipinsks rule parameters for 17 secondary metabolite compounds of Veitchia merrillii fruit seeds.
| Compounds | Molecular weight (<500 g/mol) | LogP (<5) | Hydrogen bond | Lipinski’s rules of five | |
|---|---|---|---|---|---|
|
| |||||
| Donor (<5) | Acceptor (<10) | ||||
| Gallic acid | 170.12 | 0.5 | 4 | 5 | Yes |
| Pyrogallol | 126.11 | 0.97 | 3 | 3 | Yes |
| Caffeic acid | 180.16 | 0.97 | 3 | 4 | Yes |
| Vanillic acid | 168.15 | 1.40 | 2 | 4 | Yes |
| Syringic acid | 198.17 | 1.54 | 2 | 5 | Yes |
| Rutin | 610.52 | 1.58 | 10 | 16 | No |
| Naringenin | 272.75 | 1.75 | 3 | 5 | Yes |
| Limonene | 136.23 | 2.72 | 0 | 0 | Yes |
| cis-β-ocimene | 136.23 | 2.80 | 0 | 0 | Yes |
| Allo-ocimene | 136.23 | 2.93 | 0 | 0 | Yes |
| Linalool oxide | 170.25 | 2.56 | 1 | 2 | Yes |
| Linalool | 154.25 | 2.70 | 1 | 1 | Yes |
| Methyl salicylate | 152.15 | 2.03 | 1 | 3 | Yes |
| Eucalyptol | 154.25 | 2.58 | 0 | 1 | Yes |
| Palmitic acid | 256.42 | 3.85 | 1 | 2 | Yes |
| Oleic acid | 282.46 | 4.27 | 1 | 2 | Yes |
| Linoleic acid | 280.45 | 4.14 | 1 | 2 | Yes |
| Praziquantel (control) | 312.41 | 3.00 | 22 | 2 | Yes |
Prediction of absorption, distribution, metabolism, and toxicity
Predicting pharmacokinetic and toxic properties through the web-based pkCSM platform is crucial to prevent costly and unnecessary drug development failures. Seventeen secondary metabolites of V. merillii seeds’ ADME and toxicity predictions are presented in Table-6. The human intestinal absorption value and Caco-2 cell permeability were the absorption predictions evaluated. Plasma protein binding (PPB), cytochrome P450 (CYP) inhibitor metabolic profile, and toxicity (AMES and hepatotoxicity tests) were assessed. The absorption, distribution, metabolism, and toxicity data are presented in Table-6. The HIA value measures the extent of intestinal absorption of an active substance in humans. A compound is categorized as being well absorbed if the % HIA value is in the range of 70%–100%, adequate in the range of 20%–70%, and poor in the range of 0%–20% [59]. Fifteen compounds exhibit desirable HIA values, and 2 of them fall within the 20%–70% range. Eucalyptol had the highest HIA value, at 96.51%, while routine had the lowest, with only 23.45%. The in vitro oral absorption of active substances was predicted using Caco-2 cell modeling. In the pkCSM model, a Caco-2 cell permeability value >0.9 × 10−6 cm/s is indicative of high permeability. There are 11 of 17 compounds with values >0.9 × 10−6 cm/s, whereas there are five compounds with values <0.9 × 10−6 cm/s, namely, gallic acid, syringic acid, caffeic acid, vanillic acid, and routine.
Table-6.
Prediction of absorption, distribution, metabolism, elimination, and toxicity of 17 secondary metabolites of Veitchia merrillii fruit seeds.
| Compounds | Absorption | Distribution | Metabolism | Carcinogenic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| HIA (%) | Caco-2 | Plasma protein binding (%) | blood brain barrier | 3A4 substrate | 2D6 substrate | 1A2 inhibitor | C19 inhibitor | 2C9 inhibitor | 2D6 inhibitor | 3A4 inhibitor | ||
|
| ||||||||||||
| Log (10−6cm/s) | ||||||||||||
| Gallic aci | 43.37 | −0.081 | 38.3 | −1.855 | No | No | No | No | No | No | No | - |
| Pyrogallol | 83.55 | 1.12 | 28.8 | −0.441 | No | No | No | No | No | No | No | - |
| Caffeic acid | 69.41 | 0.634 | 38,7 | −0.647 | No | No | No | No | No | No | No | + |
| Vanillic acid | 78.15 | 0.33 | 50.2 | −0.38 | No | No | No | No | No | No | No | - |
| Syringic acid | 73.07 | 0.495 | 39.1 | −0.191 | No | No | No | No | No | No | No | - |
| Rutin | 23.45 | −0.949 | 81.3 | −1.898 | No | No | No | No | No | No | No | - |
| Naringenin | 91.31 | 1.029 | 93.6 | −0.578 | No | No | Yes | No | No | No | No | - |
| Limonene | 95.89 | 1.401 | 61.4 | 0.732 | No | No | No | No | No | No | No | - |
| cis-β-ocimene | 94.73 | 1.406 | 23.9 | −1.848 | No | No | No | No | No | No | No | - |
| Allo-ocimene | 95.45 | 1.419 | 25.4 | 0.746 | No | No | No | No | No | No | No | + |
| Linalool oxide | 93.91 | 1.584 | 46 | 0.368 | No | No | No | No | No | No | No | - |
| Linalool | 93.16 | 1.493 | 51.6 | 0.598 | No | No | No | No | No | No | No | - |
| Methyl salicylate | 89.46 | 1.202 | 51 | −0.222 | No | No | No | No | No | No | No | - |
| Eucalyptol | 96.51 | 1.485 | 44.7 | 0.368 | No | No | No | No | No | No | No | - |
| Palmitic acid | 92 | 1.558 | 89.9 | −0.111 | No | Yes | No | No | No | No | No | - |
| Oleic acid | 91.82 | 1.563 | 94.8 | −0.168 | Yes | Yes | No | No | No | No | No | - |
| Linoleic acid | 92.33 | 1.57 | 94.6 | −0.142 | Yes | Yes | No | No | No | No | No | - |
| Praziquantel | 93.42 | 1.759 | 55.2 | 0.468 | No | Yes | No | Yes | No | No | No | - |
A PPB value above 90% indicates strong protein binding while below 90% indicates weak protein binding, enabling effective drug distribution [40]. According to Table-6, naringenin has a PPB value exceeding 90%. The blood–brain barrier (BBB) is another distribution parameter. A drug’s concentration in the brain is signified by its BBB value. Determining the drug’s capacity to permeate the BBB relies on this parameter. Molecules with values >0.3 in the pkCSM predictive model are assumed to readily cross the BBB, whereas those below −1 are poorly distributed in the brain. Allo-ocimene produces the highest BBB value of 0.746, allowing it to penetrate the BBB among the five identified compounds (Table-6).
A drug’s metabolic characteristics are assessed based on its ability to inhibit cytochrome enzymes. CYP isozymes, a superfamily accounting for significant drug elimination through metabolic biotransformation [60]. There are five main isoforms of CYP450, including CYP1A, CYP2C19, CYP2C9, CYP2D6, and CYP3A4 [40]. Pharmacokinetic-related drug interactions leading to toxic side effects or unwanted drug reactions are often caused by the inhibition of this specific isoenzyme [61]. The CYPenzyme’s main isoform may be influenced by naringin compounds, palmitic acid, oleic acid, and linoleic acid (Table-6). The safer induction/inhibition of cytochrome enzymes is estimated compared to the praziquantel control, which can induce/inhibit multiple types of cytochromes.
The AMES test assesses carcinogenic potential in the toxicity profile. The AMES test evaluates bacteria’s susceptibility to a compound’s mutagenic effects. A positive test indicates that the compound is mutagenic and, therefore, may act as a carcinogen [62]. Be cautious of the mutagenic or carcinogenic potential of the caffeic acid and allo-cimene compounds during therapy (Table-6).
Conclusion
This study found that in vitro V. merrillii seed extract killed trematode worms in Paramphistomum spp. by inducing mortality and damaging their tegument. The seeds of V. merrillii exhibit in silico anthelmintic activity against F. hepatica similar to that of praziquantel, employing multiple mechanisms, including microtubule inhibition, Ca+2 channel activation, thioredoxin inhibition, caspase three stimulation, apoptosis induction, ubiquinol-cytochrome c reductase inhibition, neurotransmitter action, and cholinergic antagonism, as verified by QSAR analysis. The thioredoxin enzyme is inhibited more effectively by most compounds in V. merrillii seeds, as shown by molecular docking studies. According to Lipinski’s rule analysis, all compounds except for rutin are suitable for oral administration. The seeds of V. merrillii show promise as an anthelmintic for trematode worms from potential plant sources.
Authors’ Contributions
FA: Conceived and designed the in vitro study. HV: Carried out in vitro tests in the parasitology laboratory. MH: Preparation of sample extracts. FF: Conceived, designed, and analyzed in silico study. WES: in vitro treatment. All authors have read, reviewed, and approved the final manuscript.
Acknowledgments
This study was funded by Universitas Syiah Kuala, Indonesia, with contract number 170/UN11/SPK/PNBP/2021.
Footnotes
This study was funded by Universitas Syiah Kuala, Indonesia, with contract number 170/UN11/SPK/PNBP/2021.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Cai W, Cheng C, Feng Q, Ma Y, Hua E, Jiang S, Hou Z, Liu D, Yang A, Cheng D, Xu J, Ta J. Prevalence and risk factors associated with gastrointestinal parasites in goats (Capra hircus) and sheep (Ovis aries) from three provinces of China. Front. Microbiol. 2023;14:1287835. doi: 10.3389/fmicb.2023.1287835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasil B.S.A.F, Nunes R.L, Bastianetto E, Drummond M.G, Carvalho D.C, Leite R.C, Molento M.B, Oliveir D.A.A. Genetic diversity patterns of Haemonchus placei and Haemonchus contortus populations isolated from domestic ruminants in Brazil. Int. J. Parasitol. 2012;42(5):469–479. doi: 10.1016/j.ijpara.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Soulsby E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th ed. Bailliére Tindall, London: 1982. [Google Scholar]
- 4.Rizwan H.M, Usman M, Naeem M.A, Farid M.U, Younus M, Sajid M.S, Tahir U.B, Luqman N, Abbas H, Ateeq M.K, Taseer M.S.A, Asi M. Prevalence of ruminant paramphistomosis and comparative histopathology of the infected rumens in Narowal District, Punjab, Pakistan. Helminthologia. 2022;59(4):377–384. doi: 10.2478/helm-2022-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martindah E, Sawitri D.H, Wardhana AH, Ekawast F. Prevalence of amphistomes and Fasciola in large ruminants reared by smallholders in Lampung and Banten Provinces, Indonesia. Vet. World. 2023;16(10):2104–2109. doi: 10.14202/vetworld.2023.2104-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meguini M.N, Righi S, Bouchekhchoukh M, Sedraoui S, Benakhl A. Investigation of flukes (Fasciola hepatica and Paramphistomum spp.) parasites of cattle in north-eastern Algeria. Ann. Parasitol. 2021;67(3):455–464. doi: 10.17420/ap6703.358. [DOI] [PubMed] [Google Scholar]
- 7.Githiori J.B, Athanasiadou S, Thamsbor S.M. Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Vet. Parasitol. 2006;139(4):308–320. doi: 10.1016/j.vetpar.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Miller S.A, Ferreira J.P, LeJeun J.T. Antimicrobial use and resistance in plant agriculture:A one health perspective. Agriculture. 2022;2(12):289. [Google Scholar]
- 9.Magryś A, Olender A, Tchórzewsk D. Antibacterial properties of Allium sativum L. against the most emerging multidrug-resistant bacteria and its synergy with antibiotics. Arch. Microbiol. 2021;203(5):2257–2268. doi: 10.1007/s00203-021-02248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndjonka D, Agyare C, Lüersen K, Djafsia B, Achukwi D, Nukenine E.N, Hensel A, Lieba E. In vitro activity of Cameroonian and Ghanaian medicinal plants on parasitic (Onchocerca ochengi) and free-living (Caenorhabditis elegans) nematodes. J. Helminthol. 2011;85(3):304–312. doi: 10.1017/S0022149X10000635. [DOI] [PubMed] [Google Scholar]
- 11.Vidyadhar S, Saidulu M, Gopal T.K, Chamundeeswari D, Rao U, Banj D. In vitro anthelmintic activity of the whole plant of Enicostemma littorale by using various extracts. Int. J. Appl. Biol. Pharm. Technol. 2010;1(3):1119–1125. [Google Scholar]
- 12.Jeyathilakan N, Murali K, Anandaraj A, Latha B.R, Basit S.A. The anthelmintic activity of essential oils of Cymbopogan nardus and Azadirachta indica on Fasciola gigantica. Tamilnadu J. Vet. Anim. Sci. 2010;6(5):204–209. [Google Scholar]
- 13.Shalaby H.A, El-Moghaz F.M. In vitro effect of Nigella sativa oil on adult Toxocara vitulorum. Pak. J. Biol. Sci. 2013;16(22):1557–1562. doi: 10.3923/pjbs.2013.1557.1562. [DOI] [PubMed] [Google Scholar]
- 14.Pal D, Panda K. Evaluation of anthelmintic activity of aerial part of Cynodon dactylon Pers. Anc. Sci. Life. 2010;30(1):12–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Ajaiyeoba E.A, Onocha P.A, Olarenwaj O.T. In vitro anthelmintic properties of Buchholzia coriaceae and Gynandropsis gynandra extracts. Pharm. Biol. 2001;39(3):217–220. [Google Scholar]
- 16.Zahir A.A, Rahuman A.A, Kamaraj C, Bagavan A, Elango G, Sangaran A, Kuma B.S. Laboratory determination of the efficacy of indigenous plant extracts for parasite control. Parasitol. Res. 2009;105(2):453–461. doi: 10.1007/s00436-009-1405-1. [DOI] [PubMed] [Google Scholar]
- 17.Monteiro M.V.B, Bevilaqua C.M.L, Morais S.M, Machado L.K.A, Camurça-Vasconcelos A.L.F, Campello C.C, Ribeiro W.L.C, Mesquit M.A. Anthelmintic activity of Jatropha curcas L. seeds on Haemonchus contortus. Vet. Parasitol. 2011;182:259–263. doi: 10.1016/j.vetpar.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Jeyathilakan N, Murali K, Anandaraj A, Basit S.A. In vitro evaluation of anthelmintic property of ethno-veterinary plant extracts against the liver fluke. Fasciola gigantica. J. Parasit. Dis. 2011;36(1):26–30. doi: 10.1007/s12639-011-0064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balqis U, Darmawi D, Maryam, Muslina, Hamzah A, Daud R, Hambal M, Rinidar, Harri A, Muttaqie M, Azha A, Eliawardan E. Motilitas Ascaridia galli dewasa dalam larutan ekstrak etanol biji palem puri (Veitchia merrillii) Agripet. 2016;16(1):9–15. [Google Scholar]
- 20.Liu P.F, Chan Y.F. The controversial roles of Areca nut:Medicine or toxin? Int. J. Mol. Sci. 2023;24(10):8996. doi: 10.3390/ijms24108996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jannah M, Machfuzh M, Sugiarto S. Potential added value of Areca nut products in aceh. J. Teknol. Ind. Pertanian. 2021;31(2):190–197. [Google Scholar]
- 22.Sudarmanto B, Akbarrizki M, Wahidah-Mubaraka W. Population of Ascaridia galli according to its predilection after being treated with Areca nut infusion and its economic analysis. J. Kedokteran Hewan. 2022;16(1):34–38. [Google Scholar]
- 23.Hamzah A, Hambal M, Balqis U, Darmawi D, Maryam M, Rosmaidar R, Athaillah F, Muttaqien M, Azhar A, Ismail I. In vitro anthelmintic activity of Veitchia merrillii nuts against Ascaridia galli. Tradit. Med. J. 2016;21(2):55–62. [Google Scholar]
- 24.Sari L.M. Antioxidant Activity of Areca Nut to Human Health:Effect on Oral Cancer Cell Lines and Immunomodulatory Activity. IntechOpen, London. 2021 [Google Scholar]
- 25.Anto E.J, Lelo A, Ilyas S, Nainggola M. Effect of Ethanol extract and ethyl acetate fraction of betel nut (Areca catechu L.) in colonic goblet cells of mice (Mus musculus) given orally infective egg of Trichuris muris. Open Access Maced. J. Med. Sci. 2022;8(B):637–642. [Google Scholar]
- 26.Murwani R, Kusumanti E, Naumov E.N. Areca catechu L. and Anredera cordifolia (Ten) Steenis supplementation reduces faecal parasites and improves caecal histopathology in laying hens. Int. J. Vet. Sci. Med. 2022;10(1):52–63. doi: 10.1080/23144599.2022.2090732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewi P, Indriyanti D.R, Herlina L, Gunawan A.S, Lusian C.E. Potency of Areca catechu flesh extract in inhibiting soft rot fungi of melons and bananas. J. Teknol. 2022;84(4):133–138. [Google Scholar]
- 28.Asrianto A, Asrori A, Sahli I.T, Hartati R, Kurniawan F.B, Purwat R. Bioaktivitas ekstrak etanol biji pinang (Arecha catechu L.) terhadap Staphylococcus aureus dan Escherichia coli:Bioactivity of betel nut (Arecha catechu L.) ethanol extract against Staphylococcus aureus and Escherichia coli. J. Sains Kesehatan. 2021;3(6):839–845. [Google Scholar]
- 29.Yayuk A, Burhanuddin B, Hakim A, Loka I.N, Muti'a M. Chemical content in the Sembeq traditional rituals of the Lombok Community. J. Pijar Mipa. 2021;16(4):531–534. [Google Scholar]
- 30.Latifah S, Valentino N, Sari D, Sar B.S.A. Species composition, and diversity of Mataram University green open space, West Nusa Tenggara. IOP Conf. Ser. Earth Environ. Sci. 2021;891:012026. [Google Scholar]
- 31.Chen D.J, Landis J.B, Wang H.X, Sun Q.H, Wang Q, Wan H.F. Plastome structure, phylogenomic analyses and molecular dating of Arecaceae. Front. Plant Sci. 2022;13:960588. doi: 10.3389/fpls.2022.960588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Leyes E.A, Vicente-Murillo R, González-Canavaciolo V.L, Sierra-Pérez R.C, Marrero-Delange D, Leiva-Sánche Á.T. Content of grass acids in the lipid fraction of Arecaceae three-fuirt crops in Cuba [Contenido de ácidos grasos en la fracción lipídica de frutos de tres Arecaceae cultivadas en Cuba] Rev. Cenic Cien. QuíMicas. 2013;44:23–28. [Google Scholar]
- 33.Iyasele J.U, Uadia J.O, Akhigbe I.U, Jacob J.N, Ogbeid O.K. Physico-chemical properties, chemical composition and antimicrobial activity of Adonidia merrillii kernel seed oil. Trop. J. Nat. Prod. Res. 2022;6(4):599–605. [Google Scholar]
- 34.Vafaei A. Antioxidant and Cytotoxicity Activities of Veitchia merrillii Fruits. The 4th World Congress on Biotechnology, 23-25 Raleigh, North Carolina, USA. 2013 [Google Scholar]
- 35.Jiraungkoorskul W, Sahaphong S, Tansatit T, Kangwanrangsan N, Pipatshukia S. Eurytrema pancreaticum:The in vitro effect of praziquantel and triclabendazole on the adult fluke. Exp. Parasitol. 2005;111(3):172–177. doi: 10.1016/j.exppara.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Anzali S, Barnickel G, Cezanne B, Krug M, Filimonov D, Poroiko V. Discriminating between drugs and nondrugs by prediction of activity spectra for substances (PASS) J. Med. Chem. 2001;44(15):2432–2437. doi: 10.1021/jm0010670. [DOI] [PubMed] [Google Scholar]
- 37.Ramadhan D.S.F, Fakih T.M, Arfa A. Activity prediction of bioactive compounds contained in Etlingera elatior against the SARS-CoV-2 main protease:An in silico approach. Borneo J. Pharm. 2020;3(4):232–245. [Google Scholar]
- 38.Filimonov D.A, Lagunin A.A, Gloriozova T.A, Rudik A.V, Druzhilovskii D.S, Pogodin P.V, Poroiko V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Comp. 2014;50:444–457. [Google Scholar]
- 39.Shukla R, Shukla H, Kalita P, Sonkar A, Pandey T, Singh D.B, Kumar A, Tripath T. Identification of potential inhibitors of Fasciola gigantica thioredoxin1:Computational screening, molecular dynamics simulation, and binding free energy studies. J. Biomol. Struct. Dyn. 2018;36(8):2147–2162. doi: 10.1080/07391102.2017.1344141. [DOI] [PubMed] [Google Scholar]
- 40.Pires D.E, Blundell T.L, Asche D.B. pkCSM:Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015;58(9):4066–4072. doi: 10.1021/acs.jmedchem.5b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chafton L.A. The Effect of a Condensed Tannin-Containing Forage, Sericea lespedeza, on Existing and Challenging Infections of Haemonchus contortus in Sheep. Thesis. The Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College. 2006 [Google Scholar]
- 42.Bachaya H.A, Iqbal Z, Khan M.N, Shindu Z.D, Jabba A. Anthelmintic activity of Ziziphus nummularia (bark) and Acacia nilotica (fruit) against Trichostrongylid nematodes of sheep. J. Ethnopharmacol. 2009;123(2):325–329. doi: 10.1016/j.jep.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 43.Mali R.G, Meht A.A. A review on anthelmintics plants. Nat. Prod. Radiance. 2008;7(5):466–475. [Google Scholar]
- 44.Lateef M, Iqbal Z, Khan M.N, Akhtar M.S, Jabba A. Anthelmintic activity of Adhatoda vesica roots. Int. J. Agric. Biol. 2003;5(1):86–90. [Google Scholar]
- 45.Lakshmi V, Joseph S.K, Srivastava S, Verma S.K, Sahoo M.K, Dube V, Mishra S.K, Murth P.K. Antifilarial activity in vitro and in vivo of some flavonoids tested against Brugia malayi. Acta Trop. 2010;116(2):127–133. doi: 10.1016/j.actatropica.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Hoet S, Pieters L, Muccioli G.G, Habib-Jiwan J.L, Opperdoes F.R, Quetin-Leclerc J. Antitrypanosomal activity of triterpenoids and sterols from the leaves of Strychnos spinosa and related compounds. J. Nat. Prod. 2007;70(8):1360–1363. doi: 10.1021/np070038q. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqui B.S, Afshan F, Ghiasuddin G, Faizi S, Naqvi S.N.H, Tari R.M. Two insecticidal tetranortriterpenoids from Azadirachta indica. Phytochemistry. 2000;53(3):371–376. doi: 10.1016/s0031-9422(99)00548-8. [DOI] [PubMed] [Google Scholar]
- 48.Mansour T.E, Mansou J.M. Target Kemoterapi pada Parasit. Cambridge University Press, UK. 2002:192–297. [Google Scholar]
- 49.Smyth J.D, Halto D.W. Fisiologi Trematoda. Cambridge University Press, Inggris, UK. 1983 [Google Scholar]
- 50.You H, Liu C, Du X, McManu D.P. Acetylcholinesterase and nicotinic acetylcholine receptors in schistosomes and other parasitic helminths. Molecules. 2017;22(9):1550. doi: 10.3390/molecules22091550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trott O, Olso A.J. AutoDock vina:Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoichet B.K, McGovern S.L, Wei B, Irwi J.J. Lead discovery using molecular docking. Curr. Opin. Chem. Biol. 2002;6(4):439–446. doi: 10.1016/s1367-5931(02)00339-3. [DOI] [PubMed] [Google Scholar]
- 53.Oberacker T, Kraft L, Schanz M, Latus J, Schricke S. The importance of thioredoxin-1 in health and disease. Antioxidants (Basel) 2023;12(5):1078. doi: 10.3390/antiox12051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muri J, Heer S, Matsushita M, Pohlmeier L, Tortola L, Fuhrer T, Conrad M, Zamboni N, Kisielow J, Kop M. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. Nat. Commun. 2018;9(1):1851. doi: 10.1038/s41467-018-04274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasan AA, Kalinina E, Tatarskiy V, Shti A. The thioredoxin system of mammalian cells and its modulators. Biomedicines. 2022;10(7):1757. doi: 10.3390/biomedicines10071757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kansal H, Chopra V, Garg K, Sharm S. Role of thioredoxin in chronic obstructive pulmonary disease (COPD):A promising future target. Respir. Res. 2023;24(1):295. doi: 10.1186/s12931-023-02574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramachandran B, Kesavan S, Rajkuma T. Molecular modeling and docking of small molecule inhibitors against NEK2. Bioinformation. 2016;12(2):62–68. doi: 10.6026/97320630012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lipinski C.A. Lead- and drug-like compounds:The rule-of-five revolution. Drug Discov. Today. Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Cheng J, Palva A.M, de Vos W.M, Satokar R. Contribution of the intestinal microbiota to human health:From birth to 100 years of age. Curr. Top. Microbiol. Immunol. 2013;358:323–346. doi: 10.1007/82_2011_189. [DOI] [PubMed] [Google Scholar]
- 60.Bibi Z. Role of cytochrome P450 in drug interactions. Nutr. Metab. (Lond) 2008;5:27. doi: 10.1186/1743-7075-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Kirchmair J, Göller A.H, Lang D, Kunze J, Testa B, Wilson I.D, Glen R.C, Schneide G. Predicting drug metabolism:Experiment and/or computation? Nat. Rev. Drug Discov. 2015;14(6):387–404. doi: 10.1038/nrd4581. [DOI] [PubMed] [Google Scholar]
- 62.Mortelmans K, Zeige E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000;455((1–2)):29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]


