Abstract
Background and Aim:
These three parks in North-east Thailand, Kosamphi Forest Park, Kumphawapi Monkey Garden, and Dong Ling Don Chao Pu Park, are internationally recognized for sheltering long-tailed macaques. Overfeeding by tourists and locals significantly increases the frequency of human-macaque encounters. Being close to each other raises the chances of contracting gastrointestinal (GI) parasites. This study was designed to estimate the prevalence and range of GI parasite infections in long-tailed macaques among the three major natural habitats.
Materials and Methods:
Three hundred fecal samples were collected from long-tailed macaques, with 100 samples from each of the three study sites. The samples underwent Formalin-ethyl acetate concentration technique examination. Parasites were identified based on their morphology and size as observed under a light microscope.
Results:
About 66.67% of the population had GI parasitic infection. Balantidium coli had the highest occurrence (41.66%), whereas Strongyloides spp. (24.33%), Trichuris spp. (18.33%), Entamoeba coli (10.33%), and Endolimax nana (2.33%) followed next in prevalence. A higher rate of single infections (41%) was reported compared to mixed infections (25.66%). At Dong Ling Don Chao Pu Park, the prevalence rate of B. coli in long-tailed macaques was 70%, markedly higher than those reported at the other two study sites. In these areas, the first known case of B. coli infection occurred in long-tailed macaques. In the Kumphawapi Monkey Garden, the prevalence of Strongyloides spp. and Trichuris spp. infections was significantly greater (45% and 28%, respectively) compared to the other two areas.
Conclusion:
In northeast Thailand, long-tailed macaques are predominantly infected with B. coli, causing GI protozoal infections. In this primate population of the region, Strongyloides and Trichuris species are common helminths. This study offers new knowledge on parasitic loads in Thai long-tailed macaques, essential for devising effective One Health approaches to prevent and manage zoonotic diseases.
Keywords: Dong Ling Don Chao Pu Park, gastrointestinal parasite, Kosamphi Forest Park, Kumphawapi Monkey Garden, long-tailed macaques
Introduction
The long-tailed macaque (Macaca fascicularis), also referred to as the crab-eating macaque or cynomolgus macaque, is the predominant species of nonhuman primates (NHPs) found throughout mainland southeast Asia and its numerous islands [1, 2]. In Thailand, they are abundant and documented at 91 sites nationwide, including notable locations such as Kosamphi Forest Park in the Kosum Phisai district of Maha Sarakham province, Kumphawapi Monkey Garden in the Kumphawapi district of Udon Thani province, and Dong Ling Don Chao Pu Park in the Phana district of Amnat Charoen province, all situated in the northeastern part of the country [3–5]. Over the past decade, macaques have shifted their habitat to temple grounds and park areas near human settlements due to altered foraging behavior caused by human intervention. These macaques may pose a risk for zoonotic transmission of animal parasites to humans. Long-tailed macaques may transmit GI parasites harmful to humans. These parasites include several species, including Strongyloides spp., Trichuris spp., Hookworm, Ascaris spp., Trichostrongylus spp., Oesophagostomum spp., Enterobius spp., Taenia spp., and Hymenolepis diminuta. Balantidium coli, Entamoeba coli, Entamoeba histolytica, Endolimax nana, Giardia lamblia, Blastocystis hominis, and Cryptosporidium spp. have been reported as protozoan parasites in macaques [5–15]. These GI parasites can be transmitted through a fecal-oral route or by skin penetration.
In Thailand, numerous studies have identified the major gastrointestinal (GI) parasites of long-tailed macaques as Strongyloides spp., Trichuris spp., and hookworms [5–11]. Ascaris spp. [5, 7–8] have low prevalence rates. Enterobius spp. [9] and H. diminuta [11] were found to have similarly low prevalence rates as Ascaris spp. The North-east and eastern regions of the country hold the current data on GI helminth infection in long-tailed macaques. The prevalence of GI helminths in macaques from other regions of Thailand is not well documented. More research is required to determine the extent of their occurrence nationwide. Protozoan species such as E. coli, E. histolytica, G. lamblia, B. hominis, Cryptosporidium spp., and E. nana have been detected at San Phra Kan shrine and Phra Prang Sam Yod temple in central Thailand, and Khao Sam Muk in eastern Thailand [7, 12]. E. coli is the most commonly found among them [7]. In northeast Thailand, unlike other regions in the country, there are no reports of protozoan infections in macaques.
In Maha Sarakham province’s Kosamphi Forest Park, Udon Thani province’s Kumphawapi Monkey Garden, and Amnat Charoen province’s Dong Ling Don Chao Pu Park lie are three popular tourist destinations. Despite several publications on GI parasites, no reports exist on protozoan infections among long-tailed macaques at Kosamphi Forest Park [5–6, 8–10]. Updating the current status of GI parasite infections in macaques at Kosamphi Forest Park is essential for improving control strategies. GI parasite prevalence in long-tailed macaques at the Kumphawapi Monkey Garden remains unknown [10]. No previous reports have documented the prevalence of GI parasites in long-tailed macaques at Dong Ling Don Chao Pu Park.
This study estimated the prevalence and diversity of GI parasite infections in long-tailed macaques from three major habitats in North-east Thailand. This study provides a unique understanding of the parasitic loads of long-tailed macaques in northeastern Thailand, crucial for devising effective One Health strategies to prevent and control zoonotic diseases.
Materials and Methods
Ethical approval
The Mahidol University-Institute Animal Care and Use Committee (MU-IACUC) in Nakhon Pathom, Thailand, supervised and approved this research protocol under Approval Letter Number F02-64-007. All samples were collected without causing harm to the long-tailed macaques.
Study period and location
The study was conducted from March to April 2022 in three well-known locations that hosted long-tailed macaques in North-east Thailand. Dong Ling Don Chao Pu Park in Phana district, Amnat Charoen province; Kosamphi Forest Park in Kosum Phisai district, Maha Sarakham province; and Kumphawapi Monkey Garden in Kumphawapi district, Udon Thani province. The geographical locations of the sampling points are illustrated in Figure-1.
Figure-1.
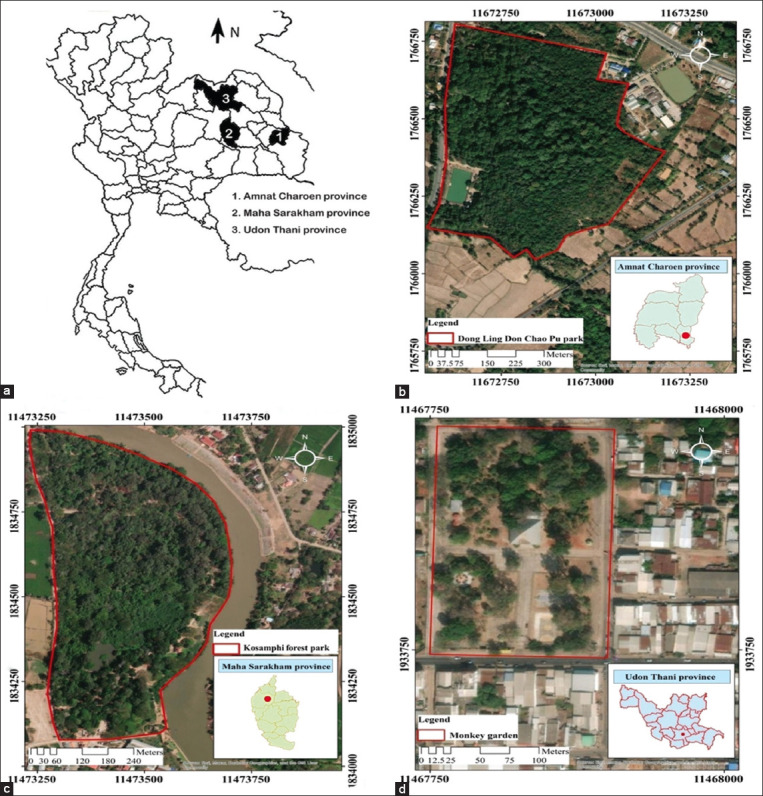
(a) The study area encompasses three provinces in northeastern Thailand. The geographical locations of sampling points include: (b) Dong Ling Don Chao Pu Park in Phana district, Amnat Charoen province; (c) Kosamphi Forest Park in Kosum Phisai district, Maha Sarakham province; (d) Kumphawapi Monkey Garden in Kumphawapi district, Udon Thani province. A red line delineates the habitat range of macaques [Source: Figure-1 (a): edited form: https://commons.wikimedia.org/wiki/File:BlankMap-Thailand-provinces.svg. Figure-1(b), (c), and (d): https://www.qgis.org/en/site/].
Fecal sample collection
Fecal samples were obtained from 300 long-tailed macaques, with 100 samples collected from each of the following locations. Dong Ling Don Chao Pu Park, Kosamphi Forest Park, and Kumphawapi Monkey Garden. Feces were collected from the ground promptly after defecation, packed in a stool container, and kept in foam boxes during transportation to the laboratory.
Formalin-ethyl acetate concentration technique (FECT) and fecal examination
Two grams of fecal matter was dissolved in 15 mL of 0.85% normal saline solution, and the mixture was thoroughly vortexed to dissolve the feces. The filtrate was collected in a 15 mL centrifuge tube after filtering the resulting mixture through wet gauze in a funnel. The tube was centrifuged at 500× g for 5 min. After discarding the supernatant, 7 mL of 10% formalin was added to the pellet. The mixture was evenly blended and let sit at room temperature for 5 min. 3 mL of ethyl acetate was added and evenly blended in. The supernatant was discarded after centrifuging the mixture once more at 500× g for 5 min. The cytopipette was employed to blend the residual sediment completely. 1% iodine solution was added to a drop of this mixture on a glass slide. The slide was examined under a microscope (Olympus, Japan) with a coverslip in place. Parasites were identified by their unique morphological traits, discernible under a light microscope [16, 17].
Statistical analysis
Each GI parasite species’ prevalence was determined. Fisher’s exact test was employed to assess the statistical significance of varying parasite prevalence among long-tailed macaques at three study sites. With a confidence level of 95% and statistical significance of p < 0.05, the analyses were conducted.
Results
Two hundred out of the 300 analyzed fecal samples (66.67%) tested positive for GI parasites. Protozoan infection prevalence was higher, with 136 (45.33%) samples, compared with helminth infection, which accounted for 106 (35.33%) samples. Infection prevalence was highest (81%) at Dong Ling Don Chao Pu Park and significantly higher than at Kosamphi Forest Park (31%) and Kumphawapi Monkey Garden (24%). In Kumphawapi Monkey Garden, the helminth infection prevalence rate was significantly higher (61%) compared to that in Dong Ling Don Chao Pu Park (29%) and Kosamphi Forest Park (16%) (Table-1).
Table-1.
Prevalence of GI parasite infection among long-tailed macaques across three study locations.
| Study locations | Number of examined | Type of GI parasite identified | ||
|---|---|---|---|---|
|
| ||||
| Number of positive samples (%) | ||||
|
| ||||
| Protozoa | Helminth | Absence | ||
| Dong Ling Don Chao Pu Park | 100 | 81 (81)* | 29 (29) | 12 (12) |
| Kosamphi Forest Park | 100 | 31 (31) | 16 (16) | 57 (57) |
| Kumphawapi Monkey Garden | 100 | 24 (24) | 61 (61)** | 31 (31) |
| Total | 300 | 136 (45.33)# | 106 (35.33) | 100 (33.33) |
Significant difference between Dong Ling Don Chao Pu Park and each of the other two locations.
Significant difference between Kumphawapi Monkey Garden and each of the other two locations.
Significant difference among type of GI parasite infections. GI=Gastrointestinal
As shown in Table-2, five distinct GI parasites were identified in long-tailed macaques. The Figure-2 illustrates three protozoa species; B. coli, E. nana, and E. coli, as well as two helminth species, Strongyloides spp. and Trichuris spp. The prevalence rates were highest for B. coli (41.66%), followed by Strongyloides spp. (24.33%), Trichuris spp. (18.33%), E. coli (10.33%), and E. nana (2.33%). In Dong Ling Don Chao Pu Park, the B. coli prevalence rate was markedly higher (70%) than in Kumphawapi Monkey Garden (24%) and Kosamphi Forest Park (31%). In the Kumphawapi Monkey Garden, the prevalence of Strongyloides spp. (45%) and Trichuris spp. (28%) infections was notably higher than in the other two monkey sites. In this research, single infections occurred more frequently than mixed infections. About 41% had the highest infection status for single infections, while double, triple, and multiple infections accounted for 21%, 4.33%, and 0.33%, respectively (Table-3).
Table-2.
Prevalence of GI parasite species identified among long-tailed macaques across three study locations.
| Study locations | Number of examined | Species of GI parasite identified | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Number of positive samples (%) | ||||||
|
| ||||||
| Trichuris spp. | Strongyloides spp. | Balantidium coli | Entamoeba coli | Endolimax nana | ||
| Dong Ling Don Chao Pu Park | 100 | 17 (17) | 20 (20) | 70 (70)* | 28 (28) | 7 (7) |
| Kosamphi Forest Park | 100 | 10 (10) | 8 (8) | 31 (31) | 0 (0.0) | 0 (0.0) |
| Kumphawapi Monkey Garden | 100 | 28 (28)# | 45 (45)** | 24 (24) | 3 (3) | 0 (0.0) |
| Total | 300 | 55 (18.33) | 73 (24.33) | 125 (41.66) | 31 (10.33) | 7 (2.33) |
Significant difference between Dong Ling Don Chao Pu Park and each of the other two locations.
Significant difference between Kumphawapi Monkey Garden and each of the other two locations.
Significant difference between Kumphawapi Monkey Garden and Kosamphi Forest Park. GI=Gastrointestinal
Figure-2.
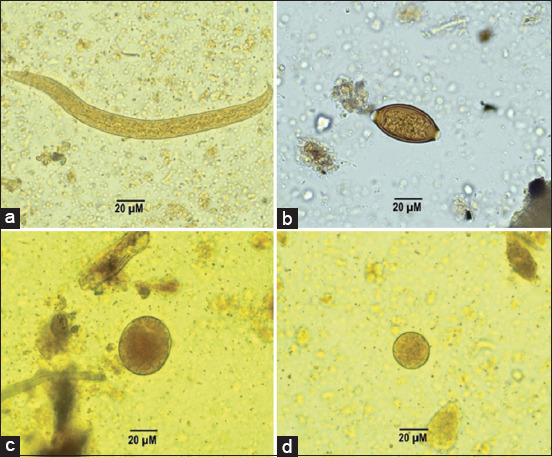
Gastrointestinal parasite identified in fecal samples of long-tailed macaques (a) Strongyloides spp., (b) Trichuris spp., (c) Balantidium coli, and (d) Entamoeba coli.
Table-3.
Infection status of GI parasite among long-tailed macaques across three study locations.
| Study locations | Number of examined | Infection status (%) | |||
|---|---|---|---|---|---|
|
| |||||
| Single infection | 2 | 3 | ≥4 | ||
| Dong Ling Don Chao Pu Park | 100 | 44 (44) | 34 (34)* | 9 (9) | 1 (1) |
| Kosamphi Forest Park | 100 | 38 (38) | 5 (5) | 0 (0.0) | 0 (0.0) |
| Kumphawapi Monkey Garden | 100 | 41 (41) | 24 (24)* | 4 (4) | 0 (0.0) |
| Total | 300 | 123 (41) | 63 (21) | 13 (4.33) | 1 (0.33) |
Significant difference with Kosamphi Forest Park. GI=Gastrointestinal
Discussion
GI parasites, such as helminths and protozoans, have been extensively documented in wild animals [18], and are particularly prevalent among NHPs [19–23]. In this study, the long-tailed macaques in Thailand displayed a prevalence rate of 66.67% (200/300) for GI parasites. In one Thai study [7], a prevalence of 84.1% was reported, while another study in a different location [6] found a prevalence of 62.69%. In Baluran National Park, East Java, Indonesia, and Barangay Sumile, Butuan City, Philippines, studies reported GI parasite prevalences of 80.97% [13] and 100% [24], respectively.
At the three study sites, protozoan infections occurred more frequently (45.33%) than helminthic infections (35.33%). In Dong Ling Don Chao Pu Park, the highest prevalence of protozoal infection (81%) was observed, while the prevalence of helminth infection was 29%. About 70% of protozoa in this study were B. coli, 28% were E. coli, and 7% were E. nana. Our study corroborates the 84.7% prevalence of B. coli in long-tailed macaques from non-human primate breeding facilities in Rio de Janeiro, Brazil [25]. However, our findings contrast with those of a previous study in eastern Thailand, where a high prevalence of protozoa in long-tailed macaques (73%) was documented; notably, E. coli was identified as the predominant species in that study, with a prevalence rate of 58.7%, whereas B. coli was not observed [7]. Similarly, a study conducted in Baluran National Park, Situbondo, East Java, Indonesia, reported that the highest prevalence of protozoa infection in such macaques was 89%, with Entamoeba spp. also identified as the predominant protozoa species (53%), whereas B. coli was not detected [13]. In Barangay Sumile, Butuan City, Philippines, Eimeria spp. infections were the most common, affecting 76.47% of long-tailed macaques [24]. In northeast Thailand, long-tailed macaques were infected predominantly with B. coli among GI protozoa. B. coli is known to parasitize various animals, including swine, ostriches, humans, and NHPs, thus presenting a potential for zoonotic transmission [26, 27]. Asymptomatic B. coli infections are common, but individuals with pre-existing health issues can suffer prolonged diarrhea, abdominal discomfort, and in rare cases, bowel perforation [28]. 20% of long-tailed macaques at Dong Ling Don Chao Pu Park were infected with Strongyloides spp., while 17% carried Trichuris spp. infection.
About 61% of cases at the Kumphawapi Monkey Garden had helminth infections, compared to 24% with protozoa infections. In this location, the most common helminth identified was Strongyloides spp. (45%), matching the 46.26% documented by Thanchonang et al. [10]. The first occurrences of Trichuris spp. and B. coli were noted with prevalence rates of 28% and 24%, respectively.
About 16% of long-tailed macaques in Kosamphi Forest Park had helminth infections. About 10% and 8% of the identified helminths were accounted for Trichuris spp. and Strongyloides spp., respectively. In contrast to the two recent studies [8, 9], our findings suggest a lower prevalence and a reduced number of GI parasite species. Damrongsukij et al. [8] found that 35.11% of the population had parasite infections, among which Trichuris spp. accounted for 22.90%. The prevalence rates for Strongyloides spp., hookworm, and Ascaris spp. were 15.27%, 4.58%, and 1.53%, respectively. 31% of the population in this area was reported to have parasite infections, with Strongyloides species and Trichuris species being the predominant types [9]. This study reveals a 31% prevalence of B. coli infection in long-tailed macaques, representing the initial documented instance in this region.
These findings underscore long-tailed macaques as critical reservoirs for GI parasites capable of transmitting to humans. Therefore, controlling and preventing the spread of these parasitic diseases among macaques and their transmission to humans is crucial. Effective control, prevention, or eradication of zoonotic diseases nowadays relies on a One Health approach - a synergistic strategy involving experts from human health, animal health, and environmental health sectors [29]. By implementing long-term preventive measures, this approach can mitigate the incidence of contagious zoonotic diseases in the future.
Conclusion
The long-tailed macaque habitat in northeast Thailand predominantly hosts B. coli, which causes GI protozoal infections. These macaques in this region were predominantly infected with Strongyloides and Trichuris species. In the surveyed locations, five zoonotic GI parasites; Strongyloides spp., Trichuris spp., B. coli, E. coli, and E. nana were present in macaques and pose a risk for parasitic infections among locals and tourists. To prevent the spread of parasitic diseases in macaques, we must implement prevention and control programs, enhance sanitation, and educate the public. Effective management of public health issues requires collaboration among public health authorities, physicians, veterinarians, park staff, and environmental health personnel. The need for research on GI parasites in nearby human communities persists.
Authors’ Contributions
IP and JJ: Devised and designed the experiment, conducted data analysis, interpreted the results, and contributed to drafting and revising the manuscript. IP, NP, and JB: Performed fieldwork and laboratory experiments. TT: Supervised the laboratory work and data analysis. All authors have read, reviewed, and approved the final manuscript.
Acknowledgments
This research project was supported by Mahidol University. The authors wish to thank the Department of Public Health, Amnatcharoen Campus, Mahidol University, Thailand, and the Biomedical Science Research Unit, Mahasarakham University, Thailand, for providing the instruments and facilities used during the study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published map and institutional affiliation.
References
- 1.Introduced Species Summary Project:Crab-eating Macaque (Macaca fascicularis) [Retrieved on 27-01-2024]. Available from: https://www.columbia.edu/itc/cerc/danoff-burg/invasion_bio/inv_spp_summ/macaca_fascicularis.htm .
- 2.Fuentes A, Gumert M.D, Jones-Enge L. Monkeys on the Edge:Ecology and Management of Long-Tailed Macaques and their Interface with Humans. Cambridge University Press, Cambridge. 2011 [Google Scholar]
- 3.Malaivijitnond S, Hamad Y. Current situation and status of long-tailed macaques (Macaca fascicularis) in Thailand. Nat. Hist. J. Chulalorgkorn Univ. 2008;8(2):185–204. [Google Scholar]
- 4.Roos C, Boonratana R, Supriatna J, Fellowes J.R, Groves C.P, Nash S.D, Rylands A.B, Mittermeie R.A. An updated taxonomy and conservation status review of Asian primates. Asian Primates J. 2014;4(1):2–38. [Google Scholar]
- 5.Schurer J.M, Ramirez V, Kyes P, Tanee T, Patarapadungkit N, Thamsenanupap P, Trufan S, Gran E.T, Garland-Lewis G, Kelley S, Nueaiton H, Kye R.C, Rabinowitz P. Long-tailed macaques (Macaca fascicularis) in urban landscapes:Gastrointestinal parasitism and barriers for healthy coexistence in Northeast Thailand. Am. J. Trop. Med. Hyg. 2019;100(2):357–364. doi: 10.4269/ajtmh.18-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pumipuntu N. Detection for potentially zoonotic gastrointestinal parasites in long-tailed macaques, dogs and cattle at Kosamphi Forest Park, Maha Sarakham. Vet. Integr. Sci. 2018;16(2):69–77. [Google Scholar]
- 7.Buppan P, Kosuwin R, Chartkul M, Tachan R, Losantea K, Khamse S. Prevalence of intestinal helminthic infection in population of long-tailed macaques (Macaca fascicularis) from Khao Sam Muk, Chonburi Province. J. Mahanakorn Vet. Med. 2017;12(2):47–55. [Google Scholar]
- 8.Damrongsukij P, Doemlim P, Kusolsongkhrokul R, Tanee T, Petcharat P, Siriporn B, Piratae S, Pumipunt N. One health approach of melioidosis and gastrointestinal parasitic infections from Macaca fascicularis to human at Kosumpee Forest Park, Maha Sarakham, Thailand. Infect. Drug Resist. 2021;15(14):2213–2223. doi: 10.2147/IDR.S299797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangkawattana S, Sereerak P, Upontain S, Tangkawattana P, Srip B. Investigation of possible alternate animal reservoir hosts of Opisthorchis viverrini. Acta Trop. 2021;217:105850. doi: 10.1016/j.actatropica.2021.105850. [DOI] [PubMed] [Google Scholar]
- 10.Thanchomnang T, Intapan P.M, Sanpool O, Rodpai R, Sadaow L, Phosuk I, Somboonpatarakun C, Laymanivong S, Tourtip S, Maleewon W. First molecular identification of Strongyloides fuelleborni in long-tailed macaques in Thailand and Lao People's Democratic Republic reveals considerable genetic diversity. J. Helminthol. 2019;93(5):608–615. doi: 10.1017/S0022149X18000512. [DOI] [PubMed] [Google Scholar]
- 11.Sricharern W, Inpankaew T, Kaewmongkol S, Jarudecha T, Inthon N. Molecular identification of Trichuris trichiura and Hymenolepis diminuta in long-tailed macaques (Macaca fascicularis) in Lopburi, Thailand. Vet. World. 2021;14(4):884–888. doi: 10.14202/vetworld.2021.884-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sricharern W, Inpankaew T, Keawmongkol S, Supanam J, Stich R.W, Jittapalapon S. Molecular detection and prevalence of Giardia duodenalis and Cryptosporidium spp. among long-tailed macaques (Macaca fascicularis) in Thailand. Infect. Genet. Evol. 2016;40:310–314. doi: 10.1016/j.meegid.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Kurniawati D.A, Suwanti L.T, Lastuti N.D.R, Kusdarto S, Suprihati E, Mufasirin M, Pratiw A. Zoonotic potential of gastrointestinal parasite in long-tailed Macaque Macaca fascicularis at Baluran National Park, Situbondo, East Java, Indonesia. Aceh. J. Anim. Sci. 2020;5(1):47–56. [Google Scholar]
- 14.Kumar S, Kumara H.N, Velankar A.D, Mishra P.S, Pal A, Sundararaj P, Singh M, Vinot S. Prevalence of gastrointestinal parasites in the Nicobar long-tailed macaque (Macaca fascicularis umbrosus) on the Nicobar Group of Islands, India. Curr. Sci. 2022;122(10):1199–1208. [Google Scholar]
- 15.Fauziyah N.N, Lastuti N.D.R, Damayanti R, Koesdarto S, Suprihati E, Witaningru A.M. Identification and the prevalence of gastrointestinal endoparasite in long-tailed macaque (Macaca fascicularis) in Wonorejo and Gunung Anyar Mangrove Eco-Tourism Surabaya. Vet. Bio. Clin. J. 2023;5(1):35–39. [Google Scholar]
- 16.Flynn R.J. Parasites of Laboratory Animals. The Iowa State University Press, Ames, IO. 1973 [Google Scholar]
- 17.Hasegawa H, Chapman C.A, Huffma M.A. Useful Diagnostic References and Images of Protozoans, Helminths, and Nematodes Commonly Found in Wild Primates. Cambridge University Press, Cambridge. 2009 [Google Scholar]
- 18.Sprenger L.K, Yoshitani U.Y, Buzatti A, Molent M.B. Occurrence of gastrointestinal parasites in wild animals in State of Paraná, Brazil. An. Acad. Bras. Ciênc. 2018;90(1):231–238. doi: 10.1590/0001-3765201720150030. [DOI] [PubMed] [Google Scholar]
- 19.Adhikari A, Koju N.P, Maharjan B, Khanal L, Upreti M, Kye R.C. Gastro-intestinal parasites of urban rhesus macaques (Macaca mulatta) in the Kathmandu Valley, Nepal. Int. J. Parasitol. Parasites Wildl. 2023;14(22):175–183. doi: 10.1016/j.ijppaw.2023.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sangpeng J, Eamudomkarn C, Hongsrichan N, Artchayasawat A, Chaisongkram C, Ponsrila K, Kimkamkaew S, Laoprom N, Boonmars T, Sithithaworn P, Pitaksakulra O. Prevalence of gastrointestinal parasites in captive mammals at Khon Kaen Zoo, Thailand. Vet. World. 2023;16(12):2416–2424. doi: 10.14202/vetworld.2023.2416-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X, Fan Z, Li S, Yi H. A review on zoonotic pathogens associated with non-human primates:Understanding the potential threats to humans. Microorganisms. 2022;11(2):246. doi: 10.3390/microorganisms11020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tandan S, Kshetri S, Paudel S, Dhakal P, Kyes R.C, Khana L. Prevalence of gastrointestinal helminth parasites in rhesus macaques and local residents in the central mid-hills of Nepal. Helminthologia. 2023;60(4):327–335. doi: 10.2478/helm-2023-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Illia G, Jouliá R.B, Citon L, Oklander L, Kowalewsk M. parasites and other infectious agents in non-human primates of Argentina. Curr. Trop. Med. Rep. 2022;9(4):267–277. doi: 10.1007/s40475-022-00277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estaño L.A, Baloria H.T, Gamalinda E.F, Rosa J.J. Determination of enteroparasites in long-tailed macaques (Macaca fascicularis) of Barangay Sumile, Butuan City, Philippines. Asian J. Biol. Life Sci. 2022;11(3):751–766. [Google Scholar]
- 25.da Silva Barbosa A, Pissinatti A, Dib L.V, de Siqueira M.P, Cardozo M., Fonseca A.B, de Barros Oliveira A, da Silva F.A, Uchôa C.M, Bastos O.M, Amendoeir M.R. Balantidium coli and other gastrointestinal parasites in captives non-human primates of the Rio de Janeiro, Brazil. J. Med. Primatol. 2015;44(1):18–26. doi: 10.1111/jmp.12140. [DOI] [PubMed] [Google Scholar]
- 26.Nakauchi K. The prevalence of Balantidium coli infection in fifty-six mammalian species. J. Vet. Med. Sci. 1999;61(1):63–65. doi: 10.1292/jvms.61.63. [DOI] [PubMed] [Google Scholar]
- 27.Giarratana F, Nalbone L, Napoli E, Lanzo V, Panebianc A. Prevalence of Balantidium coli (Malmsten, 1857) infection in swine reared in South Italy:A widespread neglected zoonosis. Vet. World. 2021;14(4):1044–1049. doi: 10.14202/vetworld.2021.1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponce-Gordo F, García-Rodrígue J.J. Balantioides coli. Res. Vet. Sci. 2021;135:424–431. doi: 10.1016/j.rvsc.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Thompson R.A. Parasite zoonoses and wildlife:one health, spillover and human activity. Int. J. Parasitol. 2013;43(12–13):1079–1088. doi: 10.1016/j.ijpara.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


