Abstract
Background and Aim:
Artemisia annua (AA), used as a growth promoter in poultry, lowers feed costs and enhances economic efficiency. This study aimed to assess the impact of varying AA concentrations on broiler chicken growth, gene expression, and profitability.
Materials and Methods:
Two hundred 1-day-old male Cobb chicks were randomly allocated into four treatment groups, each containing five replicates and 10 birds. The experimental groups consisted of G1 (basal diet), G2 (basal diet with 0.3% AA), G3 (basal diet with 0.6% AA), and G4 (basal diet with 0.9% AA). The birds had continuous access to feed and water throughout the study. The experiment lasted for 42 days. We measured the growth performance (Feed intake, Life weight), carcass traits (weight after slaughter, dressed carcass, heart, gizzard, spleen, giblet and thymus weight), liver and spleen antioxidants (CAT, GSH, SOD), and gene expression of anti-inflammatory and immune- related genes.
Results:
The primary findings revealed that the addition of 0.6% AA had a positive impact (p < 0.05) on all investigated variables compared with the control and other groups. Dietary supplementation with 0.6% AA led to increased breast, giblet, skeleton, and total yield, and net return compared with the control group. Supplementation with AA exhibited antioxidant, anti-inflammatory, and immunological effects through improved levels of antioxidant superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) in tissue homogenates of the liver and spleen. It also upregulated the relative messenger RNA levels of anti-inflammatory interleukin (IL)-10, SOD, CAT, and GSH-Px, whereas IL-1β and tumor necrosis factor-alpha were downregulated.
Conclusion:
The study found that AA is a promising replacement for antibiotics in poultry farming as a growth promoter for chickens. 0.6% AA in the broiler diet yielded the best results, striking a balance between superior performance and robust economic benefits.
Keywords: Artemisia annua, broiler, carcass traits, economics, immunity, mRNA gene expression
Introduction
Artemisia annua (AA) is a promising feed additive for increasing live weight in broiler chickens. Studies show that AA supplementation in broiler chickens, due to the presence of artemisinin and flavonoids, can enhance nutrient absorption, digestion, and metabolism, resulting in increased weight gain [1, 2]. These compounds possess anticancer and antimicrobial properties against antibiotic-resistant pathogens and stimulate the immune system. AA’s antioxidant properties, including free radical scavenging, emerge from its richness in antioxidant compounds such as phenolics and flavonoid complexes. These antioxidant molecules work together to neutralize harmful free radicals [3, 4].
By 2050, poultry meat will surpass other meats in consumption, with demand doubling current levels. Poultry plays a crucial role and is a vital source of high-quality protein and essential nutrients for underprivileged populations in developing countries, stretching beyond affordability [5, 6]. The poultry industry is shifting toward incorporating antibiotic substitutes, such as phytogenic feed additives, in broiler feed to modulate chicken gut microorganisms, enhance productivity, and positively impact feed intake (FI) and digestion efficiency [7]. Additives significantly enhance feed efficiency, growth, and disease resistance by balancing intestinal microbiota, fortifying intestinal integrity, and hindering pathogens, resulting in an optimized immune system. Assessing the herb’s impact on the immune system and its economic worth is crucial for a complete appraisal of its benefits [8]. The antimicrobial and antioxidant properties of AA enhance the health and well-being of broiler chickens. For maximum benefits and immune response enhancement with minimal adverse effects, the optimal dosage, duration, and formulation of AA supplementation should be carefully considered [6, 9, 10].
The antioxidative and anti-inflammatory properties of AA in animals lead to improved intestinal configurations in heat-stressed broilers, enhancing their immunity and antioxidant capacity [3, 11]. Overproduction of reactive oxygen species (ROS) can cause extensive damage to DNA, proteins, and lipids. Cellular antioxidant defense systems, including superoxide dismutase (SOD), glutathione peroxidase GSH-Px catalase (CAT), and reduced glutathione (GSH), have evolved to protect cellular components against ROS-induced damage [12].
Wan et al. [11] reported enhanced activities of CAT and SOD in serum and liver under thermoneutral conditions due to AA supplementation in broiler diets. The antioxidant capacity of broilers is enhanced by AA’s phenolic and flavonoid content [13]. A decrease in malondialdehyde (MDA) levels in both serum and liver samples indicates enhanced protection against oxidative stress. 1 g/kg AA in the diet improved immune and antioxidant functions of heat-stressed broilers. Antioxidant enzymes such as SOD, CAT, and GSH-Px in the liver were enhanced while MDA accumulation was reduced, indicating a decrease in oxidative stress [14, 15]. Carefully considering the optimal dosage, duration, and formulation of AA supplementation is crucial for maximizing benefits while minimizing adverse effects.
This study investigated the impact of different AA concentrations (0.3%, 0.6%, and 0.9%) on broiler growth performance and gene expression. In addition, we focused on the primary antioxidant enzyme system in hepatic tissue, including SOD, glutathione peroxidase (GPX), and CAT, synergistic with the action of some immune-related genes in splenic tissue and interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-10 in broiler chicken.
Materials and Methods
Ethical approval
All procedures were performed after obtaining ethical approval from the Animals Care and Use Committee Research Ethics Board, Faculty of Veterinary Medicine, Benha University, under ethical number BUFVTM-28-10-22.
Study period and location
The study was conducted from September 9 to October 21, 2022. The study was conducted at the Center of Experimental Animal Research, located within the Faculty of Veterinary Medicine at Benha University, Egypt.
Birds and their diets
The study used two hundred 1-day-old male Cobb-500 broiler chicks from local hatcheries. All chick populations were maintained under identical hygienic and managerial conditions during the study. The chicks went through transportation, individual weighing, wing banding and were housed in well-ventilated litter floor pens. The chicks were kept in their pens. Each had dimensions of 50 cm × 100 cm × 100 cm. 23 h of light and 1 h of darkness were continuously rotated throughout the study to mimic natural daylight patterns.
During the 6-week experiment, the nutritional needs of growing chicks were met through a personalized feeding regimen. The diet followed the Cobb-500 broilers management guide’s nutritional guidelines, using corn-soybean meal basal formulations (Accessible at: https://www.cobb-vantress.com/assets/5a88f2e793/Broiler-Performance). During the 6-week experiment, the chicks were fed pelleted diets tailored to their age. During the first 2 weeks, chicks were given a starter diet. From the 3rd to 6th week, they received a grower and finisher diet. The chemical compositions of each basal diet’s components are listed in Table-1.
Table-1.
Ingredients and the calculated chemical composition of the basal diets used during different growth stages of broiler birds.
| Items | Starter diet | Grower diet | Finisher diet |
|---|---|---|---|
| Yellow corn | 54.67 | 58.28 | 62.62 |
| Soybean meal, 46% | 36 | 33.8 | 28.9 |
| Vegetable oil | 2.5 | 3.5 | 4.5 |
| Corn gluten meal, 60% | 2 | 0 | 0 |
| Di calcium phosphate | 1.7 | 1.45 | 1.33 |
| Limestone | 1.45 | 1.35 | 1.2 |
| L-Lysine | 0.33 | 0.29 | 0.23 |
| Sodium chloride | 0.32 | 0.3 | 0.3 |
| Vitamin and mineral premix1 | 0.3 | 0.3 | 0.3 |
| DL-Methionine | 0.28 | 0.27 | 0.23 |
| Sodium bicarbonate | 0.19 | 0.17 | 0.17 |
| Anti-coccidian | 0.05 | 0.05 | 0.05 |
| Anti-mycotoxin | 0.05 | 0.05 | 0.05 |
| Anti-clostridia | 0.03 | 0.03 | 0.03 |
| L-Threonine | 0.03 | 0.04 | 0 |
| Energy enzyme | 0.02 | 0.04 | 0.01 |
| Lysomax | 0.01 | 0.01 | 0.01 |
| Phytase enzyme | 0.01 | 0.01 | 0.01 |
| Protease B | 0.01 | 0.01 | 0.01 |
| Choline chloride | 0.05 | 0.05 | 0.05 |
| Calculated composition | |||
| Crude protein% | 23.02 | 21.03 | 19.03 |
| MEn kcal/kg | 3053.85 | 3152.05 | 3224.10 |
| Crude fiber% | 2.27 | 2.25 | 3.13 |
| Lysine % | 1.35 | 1.25 | 1.09 |
| Methionine% | 0.63 | 0.59 | 0.54 |
| Methionine+cysteine% | 1.02 | 0.95 | 0.86 |
| Threonine % | 0.94 | 0.88 | 0.77 |
| Calcium % | 1.05 | 0.95 | 0.85 |
| Available phosphorus% | 0.50 | 0.45 | 0.42 |
1Each 3 kg contained: Vit. A 12,000,000 IU, Vit. D3 2,000,000 IU, Vit. E 10,000 mg, Vit. K3 2000 mg, Vit. B 11,000 mg, Vit. B2 5000 mg, Vit. B6 1500 mg, Vit. B12 10 mg, Biotin 50 mg, Pantothenic acid 10,000 mg, Nicotinic acid 30,000 mg, Folic acid 1000 mg, Manganese 60,000 mg, Zinc 50,000 mg, Iron 30,000 mg, Copper 10,000 mg, Iodine 1000 mg, Selenium 100 mg, Cobalt 100 mg, carrier (CaCo3) added to make the total 3 kg, MEn=Metabolizable energy
Preparation of AA powder
In June, leaves from undamaged AA trees with consistent green color were harvested from a single location to ensure uniform soil micronutrient levels. Tree was identified by Talaat Khedr El-Rayes (author). During the day, the leaves were turned regularly as they were air-dried to inhibit fungal growth. After 5 days of drying, the leaves were ground into a fine powder using a 0.15-mm sieve. The leaf meal was stored in airtight high-density polyethylene bags at room temperature until use.
11.4% moisture content was identified in the AA’s chemical makeup. 8.3% of the primary macromolecules consisted of carbohydrates, while 24.37 mg/100 g were proteins. The sample comprised 14.2% dietary fiber and 7.5% ash. 6.07% was the moderate fat content. Phytate (140.4 mg/100 g), total tannins (0.61 mg/100 g), and tocopherol (2.74 mg/100 g) were all identified in the analysis.
Experimental design
Two hundred of broiler chicks were distributed randomly into four groups, each containing five replicate pens. Ten birds per pen received the basal diet (G1), while dietary supplements of 0.3% AA (G2), 0.6% AA (G3), and 0.9% AA (G4) were added to the respective groups’ food.
Assessment of growth indices
Body weight was determined following the methodology outlined by Shehata et al. [16]. FI was determined by subtracting the weight of the feed refused from the initially offered feed weight [17, 18].
Evaluation of carcass traits
In the 6th week of rearing, 10 birds were randomly chosen from each group. Birds were weighed alive first before being slaughtered with a knife. After bleeding, the birds were then defeathered, including the removal of their heads and legs. The weights of different carcass components, such as the dressed carcass, heart, gizzard, spleen, liver, thymus, and intestine, were measured after manual evisceration. The combined weight of the heart, liver, spleen, gizzard, and thymus (giblets) was computed according to Azam et al.’s methodology [19]. The weight (g) of the dressed carcass, excluding giblets and the neck, was recorded 2 h after refrigeration. The weights (g) for the wings, leg quarters, breasts, and frame were recorded after the carcasses were deboned. According to Park et al. [20], the collective measurement of chicken parts, including wings, leg quarters, breasts, frame, head, and legs is referred to as chicken skeletons.
Assessment of SOD, CAT, and GSH levels in liver and spleen homogenates
The supernatant was removed from the homogenate following the manufacturer’s instructions (Sigma-Aldrich, USA). 0.1 g tissue samples were homogenized in 10% solution and centrifuged at 1000× g, 4°C, for 10 min. Using bio-diagnostic commercial kits (Sigma-Aldrich), we measured quantitatively the levels of CAT, GSH, and SOD in both spleen and liver homogenates. Each kit’s calorimetric measurements were carefully followed.
Polymerase chain reaction (PCR)-based quantitation of messenger RNA (mRNA) transcripts
In triplicate reverse transcription PCR (RT-PCR) experiments, gene expression levels of SOD, CAT, and glutathione peroxidase (GPX) were measured in liver samples from each experimental group. Triplicate splenic tissue samples from each experimental group underwent total RNA extraction using TRIzol Reagent (Thermo Fisher Scientific, USA), following the manufacturer’s directions. The RNA was converted to cDNA using Applied Biosystems kits (USA) and then analyzed by quantitative PCR (qPCR) in the 7500 Fast system with SYBR Green master mix (Thermo Fisher Scientific). The qPCR assay measured the amounts of mRNA for IL-1β, TNF-α, and IL-10 cytokines. In the 20 μL reaction, the programmed sequence consisted of an initial stage of 95°C for 30 s, followed by 40 cycles each with steps at 95°C for 5 s, 60°C for 30 s, and 72°C for 20 s. The 5´-3´ primer sequences for qRT-PCR analysis are given in Table-2 [21–25]. Transformed gene expression levels were calculated by the 2-∆∆Ct formula using normalization to β-actin, as reported by Livak and Schmittgen [26].
Table-2.
Primer sequences (5´-3´) of candidate genes in qReal time PCR.
| Gene | Primer (5-3) oligonucleotides | Accession No. | Reference |
|---|---|---|---|
| SOD | F: CGGGCCAGTAAAGGTTACTGGAA R: TGTTGTCTCCAAATTCATGCACATG | NM_205064.1 | [21] |
| CAT | F: ACTGGTGCTGGCAACCC R: ACGTGGCCCAACTGTCAT | NM_001031215 | |
| GPX | F: CAAAGTTGCGGTCAGTGGA R: AGAGTCCCAGGCCTTTACTACTTTC | NM_001163245.1 | [22] |
| IL 1B | F: TGCCTGCAGAAGAAGCCTCG R: GACGGGCTCAAAAACCTCCT | NM_204524.1 | [23] |
| TNF-α | F: CGCTCAGAACGACGTCAA R: GTCGTCCACACCAACGAG | MF000729.1 | [24] |
| IL10 | F: CAGACCAGCACCAGTCATCA R: TCCCGTTCTCATCCATCTTCTC | NM_001004414.2 | [23] |
| β-actin | F: ACCTGAGCGCAAGTACTCTGTCT R: CATCGTACTCCTGCTTGCTGAT | NM_205518.1 | [25] |
PCR=Polymerase chain reaction, SOD=Superoxide dismutase, CAT=Catalase, IL=Interleukin, TNF-α=Tumor necrosis factor-α
Evaluation of economic efficiency
To assess economic efficiency, a comprehensive evaluation of production costs and revenue was conducted. The total production cost (TC) consists of both variable and fixed expenses. The expenses related to feed consumption, veterinary services, labor, chick purchase, utilities, and litter, as described by Al-Khalaifah et al. [27], were classified as total variable costs (TVCs). Instead, rent for buildings and equipment made up the total fixed costs. The total feed cost was determined by multiplying the total FI per bird by the cost per kilogram [28, 29]. The total revenue (TR) calculation included breast meat, leg quarters, giblets, skeletons, and litter returns. Net profit (NP) was determined as the difference between TR and TCs, according to Mohammed et al. [30].
This study assessed economic efficiency using the benefit–cost ratio (BCR) (TR/TC, TR/TVC, NP/TC, and NP/TVC) as per method described by Shehata et al. [29].
Statistical analysis
Statistical analysis of the results was performed using IBM Statistical Package for the Social Sciences Version 22.0 (IBM Corp., NY, USA) [31]. A one-way analysis of variance was used to establish differences between the treatment groups. To identify the effects of varying levels of AA on different independent variables, contrast analysis was carried out between the control group, AA 0.3%, AA 0.6%, and AA 0.9% experimental groups. At a 5% significance level, Tukey’s test was utilized to identify disparities among the means of the experimental groups. The results are presented as the mean ± pooled standard error.
Results
Growth performance and carcass traits
The data in Table-3 indicate the effects of introducing various degrees of AA into broiler diets on factors such as FI, live weight, carcass attributes, and internal organ development throughout the experiment. Including AA in broiler diets led to significantly heavier spleen and giblets (p < 0.05). Birds given AA supplementation showed greater spleen and giblet weights without significant differences in FI, final live weight, or carcass cuts.
Table-3.
Effect of diet supplemented with Artemisia annua (%−1 diet) on life weight, some carcass traits and some internal organs of broiler chickens (Mean±standard error).
| Item | Control | Artemisia annua (%−1 diet) | Pooled SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 0.3 | 0.6 | 0.9 | T | C versus 3% T | C versus 6% T | C versus 9% T | |||
| Feed intake | 3404.17 | 3461.17 | 3485.17 | 3463.33 | 20.79 | NS | 0.77 | 0.53 | 0.75 |
| Life weight | 2159.74 | 2161.50 | 2161.67 | 2163.00 | 30.92 | NS | 1 | 1 | 1 |
| Weight after slaughter | 2106.67 | 2115.83 | 2123.33 | 2109.17 | 3.65 | NS | 1 | 0.99 | 0.99 |
| Weight after defeathering | 53.33 | 58.33 | 58.33 | 81.67 | 3.65 | NS | 0.96 | 0.96 | 0.06 |
| Weight after leg and head | 1950.00 | 1945.83 | 1967.50 | 1925.00 | 28.801 | NS | 1 | 0.99 | 0.99 |
| Dressed carcass | 1386.05 | 1388.58 | 1414.38 | 1390.73 | 28.52 | NS | 0.97 | 0.72 | 0.75 |
| Heart weight | 9.67 | 9.5 | 10 | 9.5 | 0.13 | NS | 0.97 | 0.81 | 0.97 |
| Liver weight | 50.17 | 53.83 | 57.33 | 57.33 | 0.75 | NS | 0.34 | 0.02 | 0.02 |
| Gizzard before | 74.17 | 75.00 | 76.67 | 79.17 | 2.10 | NS | 1 | 0.97 | 0.84 |
| Gizzard after | 54.67 | 57.5 | 62.5 | 58.83 | 1.35 | NS | 0.88 | 0.21 | 0.70 |
| Spleen weight | 3.33b | 4.13a | 3.80ab | 3.95a | 0.11 | * | 0.07 | 0.43 | 0.21 |
| Bursa weight | 1.80 | 2.17 | 2.03 | 2.10 | 0.06 | NS | 0.13 | 0.47 | 0.26 |
| Thymus weight | 2.65 | 2.62 | 2.45 | 2.55 | 0.06 | NS | 1.00 | 0.62 | 0.93 |
| Giblet weight | 122.28b | 129.75b | 138.12ab | 134.27a | 1.87 | * | 0.51 | 0.03 | 0.14 |
| Intestine weight | 96.67 | 86.67 | 80.00 | 75.00 | 2.91 | NS | 0.63 | 0.21 | 0.07 |
| Breast without bone | 486.33 | 515.00 | 508.33 | 506.67 | 12.89 | NS | 0.59 | 0.70 | 072 |
| Leg quarters | 601.67 | 613.33 | 606.67 | 591.67 | 15.84 | NS | 0.99 | 0.99 | 0.99 |
| All skeleton weight | 393.33 | 383.33 | 405.83 | 379.17 | 13.33 | NS | 0.79 | 0.74 | 0.71 |
1Means within a row with different superscripts (a, b) significantly differ (*p < 0.05), NS=Not significant, BW=Body weight, SEM=Standard error of the mean, C=Control, and T=Treatment
Metabolism and gene expression
The data presented in Tables-4 and 5 reveal a significant impact (p < 0.05) of AA supplementation on antioxidant enzyme levels in the liver and spleen tissues of broiler chickens. In the liver, a dose-dependent response was observed, with the highest AA concentrations (0.6% and 0.9%) leading to significantly (p < 0.05) elevated levels of CAT, GSH, and SOD compared with the control and 0.3% AA-supplemented groups. Notably, a similar pattern was observed in the spleen, with all AA-supplemented groups (0.3%, 0.6%, 0.9%) exhibiting significantly (p < 0.05) higher levels of CAT, GSH, and SOD than the control. The expression of liver antioxidant-related genes, the antioxidant activity resulting from dietary AA supplementation is evident in the improved activity of antioxidant enzymes. This enhancement is reflected in substantially higher gene expression levels of CAT, SOD, and GPx with increasing supplementation doses, particularly evident in the 0.6% AA supplemented group (Figure-1). In the 0.3%, 0.6%, and 0.9% AA-supplemented groups, both a linear decrease in pro-inflammatory IL-1β and TNF-α expression (p < 0.01) and an increase in anti-inflammatory IL-10 expression compared with the control group were observed (Figure-2).
Table-4.
Effect of diet supplemented with Artemisia annua (%−1 diet) on liver antioxidant of broiler chickens (mean±standard error).
| Item | Control | Artemisia annua (%−1 diet) | Pooled SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 0.3 | 0.6 | 0.9 | T | C versus 3% T | C versus 6% T | C versus 9% T | |||
| Liver antioxidant | |||||||||
| CAT (U/g) | 1030.25c | 1252.25c | 1355b | 1626.00a | *** | >0.0001 | 0.000 | 0.000 | 0.000 |
| GSH (mg/g) | 46.52c | 55.98c | 64b | 83.00a | *** | >0.0001 | 0.000 | 0.000 | 0.000 |
| SOD (U/mg) | 745.07c | 752.25b | 933.04a | 933.5a | *** | >0.0001 | 0.000 | 0.000 | 0.000 |
1Means within a row with different superscripts (a, b, c) significantly differ (*p < 0.05, **p < 0.01,
p < 0.001), NS=Not significant, CAT=Catalase, GSH=Glutathione, SOD=Superoxide dismutase, SEM=Standard error of the mean, C=Control, and T=Treatment
Table-5.
Effect of diet supplemented with Artemisia annua (%−1 diet) on spleen antioxidant of broiler chickens (mean±standard error).
| Item | Control | Artemisia annua (%−1 diet) | Pooled SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 0.3 | 0.6 | 0.9 | T | C versus 3% T | C versus 6% T | C versus 9% T | |||
| Spleen antioxidant | |||||||||
| CAT (U/g) | 368.25c | 378.66b | 378.66b | 383.25a | *** | >0.0001 | 0.000 | 0.000 | 0.000 |
| GSH (mg/g) | 55.57c | 64.4475c | 72.25b | 88.50a | *** | >0.0001 | 0.000 | 0.000 | 0.000 |
| SOD (U/mg) | 745.07c | 752.25b | 933.04a | 933.5a | *** | >0.0001 | 0.000 | 0.000 | 0.000 |
1Means within a row with different superscripts (a, b, c) significantly differ (*p < 0.05, **p < 0.01,
p < 0.001), NS=Not significant, SOD=Superoxide dismutase, CAT=Catalase, GSH=Glutathione, SEM=Standard error of the mean, C=Control, and T=Treatment
Figure-1.
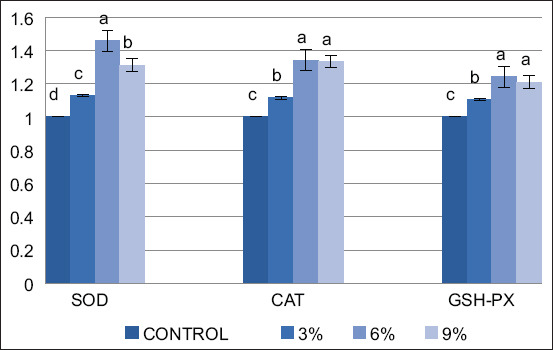
Effect of Artemisia annua on the expression of antioxidant-related genes in the liver of broilers, SOD=Total superoxide dismutase, CAT=Catalase, GSH-Px=glutathione peroxidase at 42 days of age with concentration, 3, 6 and 9% A. annua in diet. Each value is exhibited as the mean and the standard error of mean (n = 3), different superscript letters (a, b, and c) point to significant differences among experimental groups (p < 0.01), while the same letter means no significant difference among groups.
Figure-2.
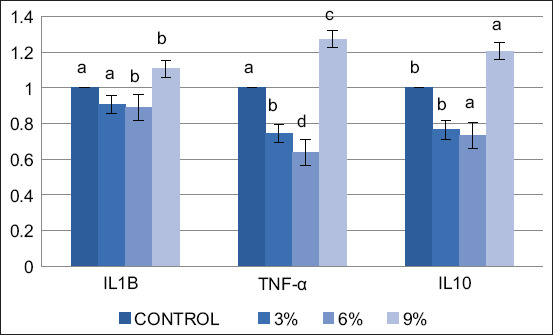
Effect of Artemisia annua on the expression of anti-inflammatory and immune-related genes, interleukin 1β, tumor necrosis factor-α, and interleukin 10 in the spleen of broilers at 42 days of age with concentration, 3, 6, and 9% A. annua in diet. Each value is displayed as the mean and the standard error of mean (n = 3), different superscript letters (a, b, c, and d) reveal significant differences between experimental groups (p < 0.01), while the same letter means no significant difference among groups.
Economic efficiency
Economic parameters and efficiency indices varied during the experimental period based on the different levels of AA are presented in Table-6. The control and AA-supplemented groups had similar TVCs, TCs, TR, and net returns at the three doses (0.3%, 0.6%, 0.9%). In the 0.6% and 0.3% AA-supplemented groups, TR and NR values were higher. The 0.6% AA group showed significantly higher values for TR/TVC, BCR, NP/TC, and NP/TVC compared to the other groups (0.3%, 0.9%, and control). In contrast, lower sales of broiler cuts and TR were seen in the control group, with the 0.9%, 0.3%, and 0.6% AA-supplemented groups exhibited less decline.
Table-6.
Effect of diet supplemented with Artemisia annua (%−1 diet) on the economic parameters and economic efficiency measures during the experimental period of broiler chickens.
| Item | Control | Artemisia annua (%-1 diet) | Pooled SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 0.3 | 0.6 | 0.9 | T | C versus 3% T | C versus 6% T | C versus 9% T | |||
| Chick price | 6.5 | 6.5 | 6.5 | 6.5 | 0.00 | – | – | – | – |
| Drug cost | 1.4 | 1.4 | 1.4 | 1.4 | 0.00 | – | – | – | – |
| Vaccine cost | 1.1 | 1.1 | 1.1 | 1.1 | 0.00 | – | – | – | – |
| Disinfectant cost | 0.3 | 0.3 | 0.3 | 0.3 | 0.00 | – | – | – | – |
| TVM | 2.8 | 2.8 | 2.8 | 2.8 | 0.00 | – | – | – | – |
| Water and Electricity | 0.15 | 0.15 | 0.15 | 0.15 | 0.00 | – | – | – | – |
| Equipment | 0.15 | 0.15 | 0.15 | 0.15 | 0.00 | – | – | – | – |
| Labor | 1.6 | 1.6 | 1.6 | 1.6 | 0.00 | – | – | – | – |
| Litter cost | 1.35 | 1.35 | 1.35 | 1.35 | 0.00 | – | – | – | – |
| Building | 2.1 | 2.1 | 2.1 | 2.1 | 0.00 | – | – | – | – |
| TFC | 2.25 | 2.25 | 2.25 | 2.25 | 0.00 | – | – | – | – |
| Feed intake | 3404.17 | 3461.17 | 3485.17 | 3463.33 | 20.79 | NS | 0.77 | 0.53 | 0.75 |
| Feed cost | 29.14 | 29.65 | 29.86 | 29.69 | 0.18 | NS | 0.75 | 0.50 | 0.70 |
| TVC | 41.54 | 42.05 | 42.26 | 42.09 | 0.17 | NS | 0.75 | 0.50 | 0.70 |
| Total cost | 43.79 | 44.30 | 44.51 | 44.34 | 0.17 | NS | 0.75 | 0.50 | 0.70 |
| Litter return | 0.55 | 0.55 | 0.55 | 0.55 | 0.00 | – | – | – | – |
| Breast return | 47.78 | 49.56 | 51.45 | 49.35 | 0.95 | NS | 0.51 | 0.19 | 0.56 |
| Leg quarters return | 18.05 | 18.4 | 18.2 | 17.75 | 0.47 | NS | 0.79 | 0.91 | 0.82 |
| Giblet return | 3.67 | 3.89 | 4.14 | 4.03 | 0.05 | NS | 0.16 | 0.16 | 0.16 |
| Skeleton return | 3.93 | 3.83 | 4.06 | 3.79 | 0.13 | NS | 0.79 | 0.74 | 0.71 |
| Return broiler cuts | 73.43 | 75.69 | 77.85 | 74.92 | 1.40 | NS | 0.57 | 0.28 | 0.71 |
| TR | 73.98 | 76.23 | 78.40 | 75.47 | 1.40 | NS | 0.58 | 0.28 | 0.71 |
| NR | 30.18 | 31.93 | 33.89 | 31.13 | 1.41 | NS | 0.66 | 0.61 | 0.82 |
| Economic efficiency measures | |||||||||
| TR-TC (BCR) | 1.69 | 1.72 | 1.76 | 1.70 | 0.03 | NS | 0.75 | 0.45 | 0.90 |
| TR/TVC | 1.78 | 1.81 | 1.86 | 1.79 | 0.04 | NS | 0.76 | 0.47 | 0.91 |
| NR/TC | 0.69 | 0.72 | 0.76 | 0.70 | 0.03 | NS | 0.75 | 0.45 | 0.90 |
| NR/TVC | 0.69 | 0.72 | 0.76 | 0.70 | 0.03 | NS | 0.76 | 0.47 | 0.90 |
TVM=Total veterinary management, TFC=Total feed cost, TVC=Total variable cost, TC=Total costs, TR=Total return, NR=Net return, kg=Kilogram, BCR=Benefit cost ratio, SEM=Standard error of the mean, C=Control, and T=Treatment
Discussion
In poultry feeding, prioritizing gut health to achieve optimal feed conversion ratio (FCR) and growth performance is crucial, especially in the absence of growth-promoting antibiotics. Incorporating various additives into poultry feed results in improved weight gains and a lower FCR for each bird.
We examined the influence of varying AA amounts on final weight, FI, and carcass yield in broiler chickens. According to Wan et al. [11] and Panaite et al. [8], AA had a minimal impact on broiler performance. While FI, final live weight, and carcass cuts showed no significant differences, the spleen and giblet weights increased remarkably in the AA-supplemented groups. The documented enhancements in spleen and giblet weights in broilers supplemented with AA extract [32] correspond to our findings. Saracila et al. [33] did not find significant differences in carcass cuts between AA-supplemented and control groups. The varied results of AA supplementation on FI, live weight, and carcass traits among studies can be explained by differences in AA dosage and duration and the basal diet’s composition. Environmental factors and management practices significantly affect the observed outcomes, as suggested by Saracila et al. [34]. Our study found no significant difference in dressed carcass weight, internal organs, breast, leg quarters, and overall skeleton weights when broiler diets were supplemented with varying levels of AA. A substantial rise in spleen and giblet weights (p ≤ 0.05) occurred, in line with Al-Shuwaili et al.’s findings [35]. These findings concur with those of Wan et al. [11] and Park and Kim [36], who demonstrated that oil extracts could exert stimulatory effects on the digestive system of poultry, enhance liver function, and enhance the activity of pancreatic digestive enzymes.
Oxidative stress in poultry, caused by moldy feed, an inadequate feeding environment, and an imbalance in intestinal flora [37], negatively impact the quality of broiler meat. Recognizing the potential harmful effects of oxidative stress on broiler meat, it is imperative to incorporate dietary antioxidants to shield poultry meat from the damage inflicted by free radicals, especially given its high polyunsaturated fatty acid content [38]. Antioxidant enzymes such as SOD, GSH-Px, and CAT work together in poultry to preserve optimal redox balance. This equilibrium significantly influences the diverse processes of cell signaling, gene expression, stress modulation, and homeostasis preservation [39]. Antioxidant defense’s first line is formed by SOD, a metalloenzyme made up of proteins and metal cofactors. It catalyzes the conversion of harmful superoxide radicals into less damaging oxygen and hydrogen peroxide. This hydrogen peroxide is further neutralized by the combined efforts of GSH-Px and CAT, ultimately breaking it down into harmless water and oxygen [40]. The levels of antioxidant enzymes (SOD, GSH-Px, and CAT) in the liver and spleen supernatants were measured to evaluate the antioxidant power of AA. The control group had significantly (p < 0.05) lower levels of CAT, GSH, and SOD than the AA-supplemented groups. AA supplementation at 0.6% and 0.9% doses significantly (p < 0.05) increased target antioxidant enzyme levels compared to the control group. The liver gene expression data confirmed a significant, dose-dependent increase in SOD, CAT, and glutathione peroxidase levels by day 42 (p < 0.01). Cherian et al. [41] and Gholamrezaie [42] confirmed the notable antioxidant and immune-enhancing effects of adding AA to broiler diets. In addition, previous studies by Guo et al. [43] and Shi et al. [2] demonstrated increased antioxidant enzyme activity in various body compartments of broilers fed AA. Notably, the optimal dosage and duration of AA supplementation were crucial in influencing the observed effects on antioxidant capacity [14].
Inflammation and oxidative stress are linked. As AA levels in the diet increased from 0.3% to 0.6%, mRNA expression of IL-1β and TNF-α in the spleen of broilers decreased. The downregulation in the 0.6% AA group was most notable, suggesting superior anti-inflammatory action in these broilers. According to Hunt et al. [44] and Niu et al. [45], AA inhibits the generation of inflammatory cytokines. The expression of the anti-inflammatory IL-10 gene in the spleen was significantly increased in the groups receiving 0.6% and 0.9% AA supplementation compared to the control group (p ≤ 0.01). According to Fu et al. [46], AA can modify the immune response through the toll-like receptor 4/nuclear factor κB (NF-κB) signaling pathway. In addition, the anti-inflammatory and antioxidant potential of broilers was enhanced with dietary AA intake, as indicated by decreased mRNA expression of pro-inflammatory genes triggered by lipopolysaccharides [47–49]. The impact of Artemisia on TNF-α gene expression shows its anti-inflammatory potential in broilers [50]. Inhibiting the inflammatory cascade, AA modulates mitogen-activated protein kinase and decreases NF-κB activity [51].
The immunomodulatory function of AA in broilers is aided by its antioxidant and anti-inflammatory effects, derived from its flavonoid and phenolic content [9, 52, 53]. Li et al. [54] demonstrated that artemisinin extracted from AA suppresses IL-6 and IL-1β production, exhibiting anti-inflammatory attributes. TNF-α, a multitasking cytokine instrumental to host immunity and inflammatory responses in both acute and chronic stages, is identified as TNF-α. In addition, as reported by Song et al. [47], Artemisia was found to mitigate intestinal inflammation in heat-stressed broilers, leading to an increase in IL-10 production. IL-10, a potent and multifaceted anti-inflammatory cytokine, is renowned for its ability to inhibit the production of major pro-inflammatory cytokines. It also promotes upregulation of the humoral immune response and mitigates cell-mediated immune reactions, which are actions exerted by both innate and adaptive immune cells.
This study probed the economic consequences of AA supplementation for broilers. 0.6% AA intake resulted in the greatest breast return, giblet return, skeleton return, total return, and net return among the groups, including the control. 0.6% AA supplementation boosted the immune system, leading to the group’s economic advantage and profitability. AA supplementation results in increased profitability, immune system strengthening, and improved growth performance [11, 25].
Conclusion
0.6% AA diet supplementation resulted in greater overall yield and profit than the control group. The addition of AA to the diet led to increased expression of CAT, SOD, and GSH-Px in the liver and spleen. Dietary AA upregulates IL-10, CAT, SOD, and GSH-Px mRNAs while downregulating IL-1β and TNF-α, ultimately reducing inflammation and enhancing immunological responses through antioxidant properties. AA, at an optimal dose of 0.6%, acts as a growth promoter and antibiotic alternative in chickens.
Data Availability
The supplementary data can be available from the corresponding author on a request.
Authors’ Contributions
MM, SHB, and SFS: Conceptualization and methodology. SFS, SHB, MMME, MM, TKE, AE, and DAA: Investigation and data curation and writing – original draft preparation and editing. All authors have read, reviewed, and approved the final manuscript.
Acknowledgments
The authors are thankful to Benha University for providing the necessary facilities for the study. The authors did not receive any funds for this study.
Footnotes
The authors did not receive any funds for this study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Yang S, Zhang J, Jiang Y, Xu Y.Q, Jin X, Yan S.M, Sh BL. Effects of Artemisia argyi flavonoids on growth performance and immune function in broilers challenged with lipopolysaccharide. Anim. Biosci. 2021;34(7):1169–1180. doi: 10.5713/ab.20.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi L, Guo Y, Cheng Y, Xing Y, Guo S, Zhang L, Xu Y, Jin X, Yan S, Sh B. An Artemisia ordosica extract:Effects on growth performance, immune, and inflammatory response in lipopolysaccharide-challenged broilers. Front. Vet. Sci. 2022;9:980690. doi: 10.3389/fvets.2022.980690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brisibe E.A, Umoren U.E, Owai P.U, Brisib F. Dietary inclusion of dried Artemisia annua leaves for management of coccidiosis and growth enhancement in chickens. Afr. J. Biotechnol. 2008;7(22):4083–4092. [Google Scholar]
- 4.Younas U, Iqbal S, Bashir R, Sajjad N, Saeed Z, Pervaiz M, Hassan F, Ali F, Ibrahim S, Batool F, Hussain I, Iqba M. An eco-friendly approach for the extraction of antioxidant components from Artemisia annua leaves using response surface methodology. Pol. J. Environ. Stud. 2021;30(5):4827–4833. [Google Scholar]
- 5.Coroian M, Pop L.M, Popa V, Friss Z, Oprea O, Kalmár Z, Pintea A, Borşan S.D, Mircean V, Lobonţiu I, Militaru D, Vârban R, Györk A. Efficacy of Artemisia annua against coccidiosis in broiler chickens:A field trial. Microorganisms. 2022;10(11):2277. doi: 10.3390/microorganisms10112277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du H, Xing Y, Jin X, Yan S, Sh B. Effects of Artemisia ordosica polysaccharide on growth performance and antioxidant capacity in broilers. J. Appl. Anim. Res. 2023;51(1):92–101. [Google Scholar]
- 7.Engida D.T, Ayele M, Waktole H, Tamir B, Regassa F, Tuf T.B. Effects of phytogenic feed additives on body weight gain and gut bacterial load in broiler chickens. World Vet. J. 2023;13(1):205–213. [Google Scholar]
- 8.Panaite T.D, Criste R.D, Vlaicu V.P, Saracila M, Tabuc C, Olteanu M, Turc R.P, Buleandră M. Influence of Artemisia annua on broiler performance and intestinal microflora. Braz. J. Poult. Sci. 2019;21(4):1–10. [Google Scholar]
- 9.Attia Y.A, Abd El-Hamid A.E, Abdallah A.A, Berikaa M.A, El-Gandy M.F, Sahin K, Abou-Shehem B.M. Effect of betaine, vitamin C and vitamin E on egg quality, hatchability, and markers of liver and renal functions in dual-purpose breeding hens exposed to chronic heat stress [Einfluss von betain, vitamin C und vitamin E auf die eiqualität, den bruterfolg und ausgewählte indikatoren der leber- und nierenfunktion bei unter hitzestress gehaltenen legehennen des zweinutzungstyps] Europ. Poult. Sci. 2018;82:1–12. [Google Scholar]
- 10.Zhou L, Li H, Hou G, Hu C, Ji F, Peng W, Zhou H, Wan D. Effects of blended microbial feed additives on performance, meat quality, gut microbiota and metabolism of broilers. Front. Nutr. 2022;9:1026599. doi: 10.3389/fnut.2022.1026599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan X.L, Song Z.H, Niu Y, Cheng K, Zhang J.F, Ahmad H, Zhang L.L, Wan T. Evaluation of enzymatically treated Artemisia annua L. on growth performance, meat quality, and oxidative stability of breast and thigh muscles in broilers. Poult. Sci. 2017;96(4):844–850. doi: 10.3382/ps/pew307. [DOI] [PubMed] [Google Scholar]
- 12.Omara T, Kiprop A.K, Ramkat R.C, Cherutoi J, Kagoya S, Moraa Nyangena D, Azeze Tebo T, Nteziyaremye P, Nyambura Karanja L, Jepchirchir A, Maiyo A, Jematia Kiptui B, Mbabazi I, Kiwanuka Nakiguli C, Nakabuye B.V, Chepkemoi Kosk M. Medicinal plants used in traditional management of cancer in Uganda:A review of ethnobotanical surveys, phytochemistry, and anticancer studies. Evid. Based Complement. Alternat. Med. 2020;2020:3529081. doi: 10.1155/2020/3529081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang S.J, Schmiech M, Hafner S, Paetz C, Steinborn C, Huber R, El Gaafary M, Werner K, Schmidt C.Q, Syrovets T, Simme T. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomedicine. 2019;62:152962. doi: 10.1016/j.phymed.2019.152962. [DOI] [PubMed] [Google Scholar]
- 14.Baghban-Kanani P, Hosseintabar-Ghasemabad B, Azimi-Youvalari S, Seidavi A, Ragni M, Laudadio V, Tufarell V. Effects of using Artemisia annua leaves, probiotic blend, and organic acids on performance, egg quality, blood biochemistry, and antioxidant status of laying hens. J. Poult. Sci. 2019;56(2):120–127. doi: 10.2141/jpsa.0180050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaab H.T, Hameed S.S, Sahi A.M. The effect of Artemisia on immune response and productive performance against Newcastle disease in broiler chickens. J. World Poult. Res. 2022;12(1):22–30. [Google Scholar]
- 16.Shehata S.F, Baloza S.H, Elsokary M.M.M, Hashem N.M, Khawanda M.M. Effect of stocking density and vitamin E or zinc supplementation on growth, physiology, gene expression, and economic efficiency of growing broiler chicks. Trop. Anim. Health Prod. 2022;54(6):403. doi: 10.1007/s11250-022-03382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goo D, Kim J.H, Par G.H, Delos Reye J.B, Kil D.Y. Effect of stocking density and dietary tryptophan on growth performance and intestinal barrier function in broiler chickens. Poult. Sci. 2019;98(10):4504–4508. doi: 10.3382/ps/pez215. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed L.S, Sallam E.A, Edris S.N, Khalifa O.A, Soliman M.M, Shehat S.F. Growth performance economic efficienc meat quality, and gene expression in two broiler breeds fed different levels of tomato pomace. Vet. Res. Commun. 2021a;45(4):381–397. doi: 10.1007/s11259-021-09819-x. [DOI] [PubMed] [Google Scholar]
- 19.Azam A, Shehata S, Mohammed L, Salla E. Comparative study on the hygienic, production and economic indices of Japanese quails reared on floor and cage systems. Benha Vet. Med. J. 2021;41(1):8–12. [Google Scholar]
- 20.Park S.Y, Byeon D.S, Kim G.W, Ki H.Y. Carcass and retail meat cuts quality properties of broiler chicken meat based on the slaughter age. J. Anim. Sci. Technol. 2021;63(1):180–190. doi: 10.5187/jast.2021.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdo S.E, El-Kassas S, El-Nahas A.F, Mahmou S. Modulatory effect of monochromatic blue light on heat stress response in commercial broilers. Oxid. Med. Cell. Longev. 2017;2017:1351945. doi: 10.1155/2017/1351945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Kassas S, El-Naggar K, Abdo S.E, Abdo W, Kirrella A.A.K, El-Mehaseeb I, Abu El-Mag M. Dietary supplementation with copper oxide nanoparticles ameliorates chronic heat stress in broiler chickens. Anim. Prod. Sci. 2019;60(2):254–268. [Google Scholar]
- 23.Adu-Asiamah P, Zhang Y, Amoah K, Leng Q.Y, Zheng J.H, Yang H, Zhang W.L, Zhan L. Evaluation of physiological and molecular responses to acute heat stress in two chicken breeds. Animal. 2021;15(2):100106. doi: 10.1016/j.animal.2020.100106. [DOI] [PubMed] [Google Scholar]
- 24.Hao X, Li S, Li J, Yang Y, Qin A, Shan S. An anti-tumor vaccine against Marek's disease virus induces differential activation and memory response of gd T cells and CD8 T cells in chickens. Front. Immunol. 2021;12:645426. doi: 10.3389/fimmu.2021.645426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirrella A.A, Abdo S.E, El-Naggar K, Soliman M.M, Aboelenin S.M, Dawood M.A.O, Sale A.A. Use of corn silk meal in broiler diet:Effect on growth performance, blood biochemistry, immunological responses, and growth-related gene expression. Animals (Basel) 2021;11(4):1170. doi: 10.3390/ani11041170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak K.J, Schmittge T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Al-Khalaifah H.S, Shahin S.E, Omar A.E, Mohammed H.A, Mahmoud H.I, Ibrahi D. Effects of graded levels of microbial fermented or enzymatically treated dried brewer's grains on growth, digestive and nutrient transporter genes expression and cost-effectiveness in broiler chickens. BMC Vet. Res. 2020;16(1):424. doi: 10.1186/s12917-020-02603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tareen M.H, Wagan R, Siyal F.A, Babazadeh D, Bhutto Z.A, Arain M.A, Saee M. Effect of various levels of date palm kernel on growth performance of broilers. Vet. World. 2017;10(2):227–232. doi: 10.14202/vetworld.2017.227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shehata S.F, Sallam E.A, Azam A.E, Soliman M.M, Mohamme L.S. Effect of different dietary inclusion levels of mulberry leaves on productive traits, economic indices, and immunity of white and brown Japanese quail. Alex. J. Vet. Sci. 2021;70(2):63–75. [Google Scholar]
- 30.Mohammed L.S, Sallam E.A, El Basuni S.S, Eldiarby A.S, Soliman M.M, Aboelenin S.M, Shehat S.F. Ameliorative effect of neem leaf and pomegranate peel extracts in coccidial infections in New Zealand and v-line rabbits:Performance intestinal healt oocyst shedding carcass trait and effect on economic measures. Animals (Basel) 2021b;11(8):2441. doi: 10.3390/ani11082441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IBM. SPSS Statistics for Windows, Version 22.0. SPSS22. IBM, Armonk NY, USA. 2013 [Google Scholar]
- 32.Guo S, Ma J, Xing Y, Shi L, Zhang L, Xu Y, Jin X, Yan S, Sh B.G. Artemisia annua L. Aqueous extract promotes intestine immunity and antioxidant function in broilers. Front. Vet. Sci. 2022;9:934021. doi: 10.3389/fvets.2022.934021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saracila M, Criste R.D, Panaite T.D, Vlaicu P.A, Tabuc C, Turcu R.P, Oltean M. Artemisia annua as phytogenic feed additive in the diet of broilers (14-35 Days) reared under heat stress (32ºC) Braz. J. Poult. Sci. 2018;20(4):825–832. [Google Scholar]
- 34.Saracila M, Panaite T.D, Mironeasa S, Unte A.E. Dietary supplementation of some antioxidants as attenuators of heat stress on chicken meat characteristics. Agriculture. 2021;11(7):638. [Google Scholar]
- 35.Al-Shuwaili M.A, Ibrhim I.E, Naqi Al-Bayat M.T. Effect of dietary herbal plants supplement in turkey diet on performance and some blood biochemical parameters. Glob. J. Biosci. Biotechnol. 2014;4(2):153–157. [Google Scholar]
- 36.Park S.Y, Ki H.Y. Effects of marketing ages on the physicochemical properties and sensory aspects of cured broiler chicken breast meat. Foods. 2021;10(9):2152. doi: 10.3390/foods10092152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Xing T, Li J, Zhang L, Jiang Y, Ga F. Hydrogen peroxide-induced oxidative stress impairs redox status and damages aerobic metabolism of breast muscle in broilers. Poult. Sci. 2021;100(2):918–925. doi: 10.1016/j.psj.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan B.L, Norhaizan M.E, Lie W.P.P. Nutrients and oxidative stress:Friend or Foe? Oxid. Med. Cell. Longev. 2018;2018:9719584. doi: 10.1155/2018/9719584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abd El-Hack M.E, El-Saadony M.T, Saad A.M, Salem H.M, Ashry N.M, Abo Ghanima M.M, Shukry M, Swelum A.A, Taha A.E, El-Tahan A.M, AbuQamar S.F, El-Tarabil K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition:A comprehensive review. Poult. Sci. 2022;101(2):101584. doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surai F.P. Antioxidant systems in poultry biology:Superoxide dismutase. J. Anim. Res. Nutr. 2016;1(1):8. [Google Scholar]
- 41.Cherian G, Orr A, Burke I.C, Pa W. Feeding Artemisia annua alters digesta pH and muscle lipid oxidation products in broiler chickens. Poult. Sci. 2013;92(4):1085–1090. doi: 10.3382/ps.2012-02752. [DOI] [PubMed] [Google Scholar]
- 42.Gholamrezaie Sani L, Mohammadi M, Jalali Sendi J, Abolghasemi S.A, Roostaie Ali Meh M. Extract and leaf powder effect of Artemisia annua on performance, cellular and humoral immunity in broilers. Iran J. Vet. Res. 2013;14(1):15–20. [Google Scholar]
- 43.Guo S, Ma J, Xing Y, Xu Y, Jin X, Yan S, Sh B. Artemisia annua L. Aqueous extract as an alternative to antibiotics improving growth performance and antioxidant function in broilers. Ital. J. Anim. Sci. 2020;19(1):399–409. [Google Scholar]
- 44.Hunt S, Yoshida M, Davis C.E, Greenhill N.S, Davis P.F. An extract of the medicinal plant Artemisia annua modulates production of inflammatory markers in activated neutrophils. J. Inflamm. Res. 2015;8:9–14. doi: 10.2147/JIR.S75484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niu Y, Zhao Y, He J, Yun Y, Shi Y, Zhang L, Wan T. Effect of diet supplemented with enzymatically treated Artemisia annua L. on intestinal digestive function and immunity in weaned pigs. Ital. J. Anim. Sci. 2020;19(1):1171–1180. [Google Scholar]
- 46.Fu C, Yu P, Manyuan W, Qi F. Phytochemical analysis and geographic assessment of flavonoids, coumarins and sesquiterpenes in Artemisia annua L. based on HPLC-DAD quantification and LC-ESI-QTOF-MS/MS confirmation. Food Chem. 2020;312:126070. doi: 10.1016/j.foodchem.2019.126070. [DOI] [PubMed] [Google Scholar]
- 47.Song Z, Cheng K, Zhang L, Wan T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J. Therm. Biol. 2017;69:184–190. doi: 10.1016/j.jtherbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Xing Y.Y, Zheng Y.K, Yang S, Zhang L.H, Guo S.W, Shi L.L, Xu Y.Q, Jin X, Yan S.M, Sh B.L. Artemisia ordosica polysaccharide alleviated lipopolysaccharide-induced oxidative stress of broilers via Nrf2/Keap1 and TLR4/NF-kB pathway. Ecotoxicol. Environ. Saf. 2021;223:112566. doi: 10.1016/j.ecoenv.2021.112566. [DOI] [PubMed] [Google Scholar]
- 49.Xing Y, Zheng Y, Yang S, Zhang L, Guo S, Shi L, Xu Y, Jin X, Yan S, Sh B. Artemisia ordosica polysaccharide ameliorated LPS-induced growth inhibition and intestinal injury in broilers through enhancing immune-regulation and antioxidant capacity. J. Nutr. Biochem. 2023;115:109284. doi: 10.1016/j.jnutbio.2023.109284. [DOI] [PubMed] [Google Scholar]
- 50.Abate G, Zhang L, Pucci M, Morbini G, Sweeney E.M, Maccarinelli G, Ribaudo G, Gianoncelli A, Uberti D, Memo M, Lucini L, Mastin A. Phytochemical analysis and anti-inflammatory activity of different ethanolic phyto-extracts of Artemisia annua L. Biomolecules. 2021;11(7):975. doi: 10.3390/biom11070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shinyuy L.M, Loe G.E, Jansen O, Mamede L, Ledoux A, Noukimi S.F, Abenwie S.N, Ghogomu S.M, Souopgui J, Robert A, Demeyer K, Frederic M. Secondary metabolites isolated from Artemisia afra and Artemisia annua and their anti-malarial, anti-inflammatory and immunomodulating properties-pharmacokinetics and pharmacodynamics:A review. Metabolites. 2023;13(5):613. doi: 10.3390/metabo13050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan X.L, Niu Y, Zheng X.C, Huang Q, Su W.P, Zhang J.F, Zhang L.L, Wan T. Antioxidant capacities of Artemisia annua L. Leaves and enzymatically treated Artemisia annua L. In vitro and in broilers. Anim. Feed Sci. Technol. 2016;221:27–34. [Google Scholar]
- 53.Habibian M, Sadeghi G, Karim A. Comparative effects of powder, aqueous and methanolic extracts of purslane (Portulaca oleracea L.) on growth performance, antioxidant status, abdominal fat deposition and plasma lipids in broiler chickens. Anim. Prod. Sci. 2019;59(1):89–100. [Google Scholar]
- 54.Li T, Chen H, Wei N, Mei X, Zhang S, Liu D.L, Gao Y, Bai S.F, Liu X.G, Zho Y.X. Anti-inflammatory and immunomodulatory mechanisms of artemisinin on contact hypersensitivity. Int. Immunopharmacol. 2012;12(1):144–150. doi: 10.1016/j.intimp.2011.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The supplementary data can be available from the corresponding author on a request.


