Abstract
Objective:
Aortic dissection, a rare but serious condition, requires timely diagnosis and treatment.
Case report:
A case report involving a 33-year-old female with Stanford type B aortic dissection at 32 + 3 weeks gestational age highlights the importance of being alert to the symptoms and signs of this condition, particularly in patients with hypertension or a history of connective tissue disorders. The case report suggests a delivery first strategy followed by TEVAR procedure as the preferred approach for managing aortic dissection in pregnancy. This approach can alleviate pressure on the aorta, reduce the risk of rupture, and provide time for stabilization and preparation for the TEVAR procedure.
Conclusion:
The case report emphasizes the criticality of recognizing and treating aortic dissection in pregnant patients promptly, given its potential life-threatening impact on both mother and fetus.
Keywords: Aortic dissection, hypertension in pregnancy, thoracic endovascular aortic repair
Introduction
The incidence of aortic dissection (AD) is significantly higher in pregnant women (14.5 per million) compared to non-pregnant women (1.24 per million).1,2 This increased risk is due to various physiological changes during pregnancy, such as an increase in blood volume, cardiac output, and heart rate, as well as hormonal changes that lead to structural modifications in the aortic wall, resulting in increased diameter and compliance. These changes peak in the third trimester and postpartum period, and approximately 50% of pregnancy-related AD cases occur during the third trimester and 33% during the postpartum period. 2
This case report presents a pregnant woman with Stanford type B AD, compromised by false lumon complicated with left renal artery and left external iliac artery dissection.
Recent reports suggest delivery around 30 weeks gestation as a threshold for considering delivery-first versus aorta repair-first approaches in managing AD during pregnancy.3,4
Case Report
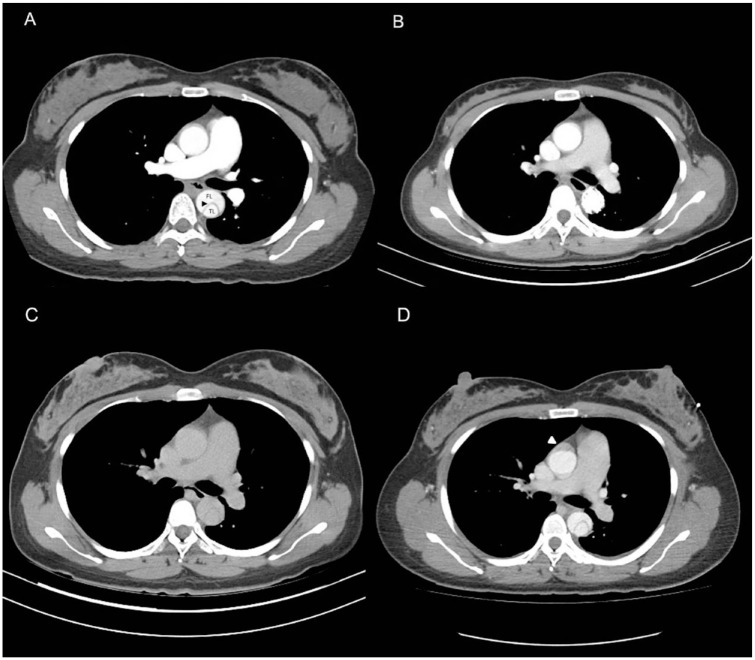
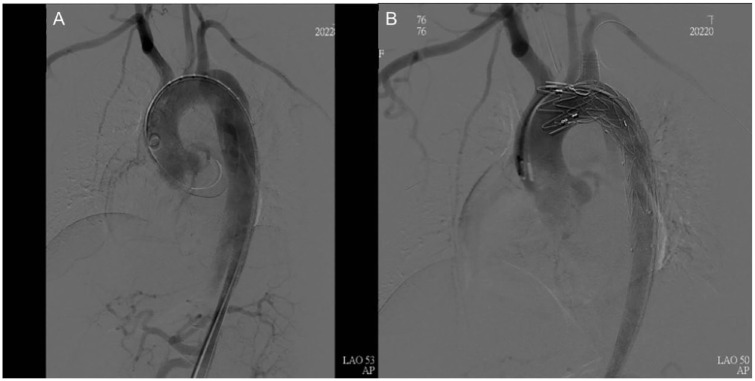
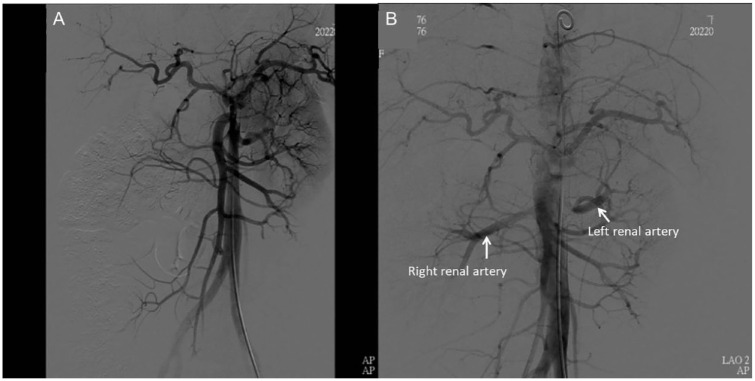
A 33-year-old pregnant woman, standing 165 cm tall and weighing 75 kg (gravida 1, para 0 and 32 + 3 weeks gestation), with no known history of aortopathy or connective tissue disorders like Marfan syndrome, presented to the emergency room complaining of severe back pain radiating to her chest and shortness of breath, making it difficult for her to lie down. Her NST (Non-Stress Test) was reactive with no regular contraction, and her physical exam revealed weak pulsation over her left lower leg. Laboratory results were normal, and an EKG (Electrocardiogram) indicated no evidence of myocardial infarction. Given her history of gestational hypertension and typical symptoms of AD, contrast-enhanced computed tomography (CT) was ordered, which confirmed an acute Stanford type B AD (Figure 1A). She was admitted to the intensive care unit for strict blood pressure control, but her blood pressure remained high despite maximal intravenous antihypertensive medication. The following day, a physical exam showed relatively decreased pulsation over the left femoral artery. AD progression with the left iliac artery compromise was suspected. A CT aortography confirmed progression of AD, with a new intramural hematoma over the ascending aorta and dissection of the left common and external iliac arteries. The CT aortography images revealed the new intramural hematoma over the ascending aorta (Figure 1C and D). Under the concern of fetal circulation compromise, an emergent cesarean section was arranged with a cardiovascular team standby, and spinal anesthesia was chosen to lower blood pressure. Spinal anesthesia is often preferred for Cesarean sections when conditions permit. It is generally safer than general anesthesia as it avoids potential complications related to airway management and the effects of general anesthetics on the mother and fetus. It allows the mother to be awake and alert during the delivery, facilitating immediate bonding with the newborn. Additionally, it typically provides effective and reliable pain control. The cesarean section was smooth, and the neonate was taken by the pediatric team. The patient was transferred back to the intensive care unit for postoperative care. Her blood pressure stabilized under intravenous antihypertensive medication after the cesarean section, and thoracic endovascular aortic repair (TEVAR) was arranged after 1 week to seal the intimal tear and prevent aneurysmal changes. The TEVAR involved creating a fenestration on a stent graft (Valiant Captivia, Medtronic Vascular, Santa Rosa, Calif) for a longer landing zone and to preserve the left subclavian artery. The fenestrated stent graft was deployed under fluoroscopy, and a Viabahn (W. L. Gore & Associates, Flagstaff, Ariz) was inserted into the fenestration as a bridging endograft (Figure 2). The blood flow of the false lumen decreased, and the perfusion of the right renal artery and left iliac artery were restored (Figure 3). She was transferred to a general ward on postoperative day 5 and discharged on postoperative day 10. A 1-month follow-up CT scan showed no progression of AD or endoleak of the stent graft. A CT scan taken 9 months after the procedure showed a healed thoracic aorta (Figure 1B). The baby, born with an Apgar score of 6 at 1 min and 8 at 5 min, is growing normally 19 months after birth, as observed during a physical check-up.
Figure 1.
(A) CT images of the configuration of the true lumen (TL) and false lumen (FL) in a thoracic aortic dissection one day before TEVAR. The beak sign(arrowheads) demonstrate represents the dissection flap. (B) The healed thoracic aorta 9 months after TEVAR. (C) and (D) A new intramural hematoma over the ascending aorta.
Figure 2.
(A) The aortic arch before TEVAR. (B) The aortic arch after Zone 2 landing with left subclavian artery fenestration.
Figure 3.
(A) The right renal artery compromised by the false lumen before TEVAR. (B) It demonstrates that after TEVAR, the blood flow to the right renal artery was restored.
Informed consent was obtained from the patient before conducting the study. The patient voluntarily participated in this study and gave permission for this case study to be published.
Discussion
Although a rare condition, AD is a catastrophic event that causes significant maternal and fetal mortality rates of 12% and 28%, 3 respectively, despite its incidence rate of approximately 0.0004%. 4 Risk factors leading to AD can be multifactorial, including pregnancy-related hemodynamic stresses and hormonal effects. Estrogen, for example, suppresses the synthesis of collagen and elastin, 5 causing weakness of the aortic wall. 6 Pregnancy-induced hypertension, such as in the case mentioned, also contributes to AD attacks because high blood pressure can cause the arterial wall to deteriorate. Additionally, the presence of hereditary connective tissue disorders such as Marfan syndrome, Loeys-Dietz, Bicuspid aortic valve, and Ehlers-Danlos syndrome all increase the risk of AD during pregnancy. 7
A large case review by Fattori et al 8 examined 6711 patients with Stanford type B AD who underwent medical treatment, open surgery, and TEVAR. For acute type B AD patients, within the first 2 weeks, the pooled early mortality rates for the 3 respective treatments were found to be 6.4% for medical treatment, 17.5% for open surgery, and 10.2% for TEVAR. Common risk factors for early mortality included malperfusion syndrome and unstable hemodynamic status. Malperfusion accounted for approximately 20% of the complications of type B AD. Signs of malperfusion included abdominal pain (61%), lower extremity weakness (27%), a pulseless lower extremity (24%), as well as abnormal lactate, renal, and liver functions.
According to 2022 American College of Cardiology Joint Committee (ACC)/American Heart Association (AHA) guideline for the diagnosis and management of aortic disease, 9 TEVAR is advantageous compared to open surgery for acute complicated type B AD in pregnant patients. The minimally invasive approach avoids a major open operation and complications like hemorrhage, allowing faster recovery and stabilization postpartum. The timing and coordination of TEVAR can be tailored to hemodynamic changes after delivery. In cases of malperfusion, TEVAR can help quickly restore perfusion of end organs like the kidneys which is critical in these young patients. TEVAR has been adopted as routine procedure for type B AD patients, but further studies are still needed for pregnant procedure.
Throughout the whole discussion, the management of aortic dissection (AD) during pregnancy, focusing on the timing and strategy of delivery in relation to gestational age and the condition of the aorta. It highlights a delivery-first strategy as recommended for AD in pregnancy at ⩾30 weeks’ gestation, based on several references. 3 This approach is favored due to the benefits of removing hemodynamic stresses of pregnancy, which enables better blood pressure control and planning for operative interventions on the aorta. In this case, the patient maintained relatively stable blood pressure during her pregnancy, experiencing only mild hypertension (140/90 mmHg). Her medical history revealed hypertension that began 3 days prior with a blood pressure reading of 150/100 mmHg. She experienced 2 severe episodes of hypertension, 1 at the time of her aortic dissection and another 3 days after her cesarean section, with both incidents recording a blood pressure of 192/113 mmHg. Her urine protein levels remained negative throughout. Controlling blood pressure is critical to reducing stress on the aorta and preventing further dissection or complications. The discussion acknowledges the risks associated with prioritizing aorta repair first, especially the risk of mortality due to potential rupture or progression of the dissection during surgery.
There is also a mention of the limited data available on managing AD with delivery at <30 weeks’ gestation, noting that some small case series have recommended prioritizing maternal stabilization and aortic repair over early preterm delivery. However, for the case described, involving a patient at 32 + 3 weeks gestation, the delivery-first approach is consistent with other reports that suggest around 30 weeks as a threshold for considering delivery in the context of AD.
Furthermore, the management of AD in pregnancy, particularly regarding the timing and strategy of delivery, remains a challenging clinical scenario. The delivery-first strategy for gestations ⩾30 weeks is supported by the literature, but each case requires individualized consideration, especially in earlier gestations or when complicating factors arise. Further research is needed to refine management guidelines and improve outcomes for both mothers and their babies.
The emphasis on prompt diagnosis and management to optimize outcomes for both the mother and fetus underscores the critical nature of this condition during pregnancy. The delivery-first strategy, feasible when fetal viability allows, is presented as a means to facilitate these goals.
Delivery should be performed in the operation room with angiography equipment and a cardiopulmonary pump standby. A multidisciplinary team should be on site, including an obstetrician, a cardiovascular surgeon, anesthesiologists, and pediatricians. If the aorta ruptures during delivery, cardiovascular surgeons should take over immediately to perform TEVAR or open repair.
In conclusion, adequate investigation, prompt diagnosis, and timely management of AD are crucial to reduce the mortality and morbidity of both the mother and the fetus. 10
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contribution: CMH and CYL are the primary contributors, mainly responsible for the conception and drafting of the case report. YTC provided conclusive guidance regarding the management of the patient and assisted in the creation of this case report. The conception and design of the study were carried out by CMH and CYL. HCW and SYS were responsible for data acquisition, while data analysis and interpretation were conducted by CYL and YTC. The manuscript was drafted by CYL, HCW, and SYS, with critical revisions for important intellectual content provided by CMH, CHW, and YTC. All authors, including CMH, CHW, HCW, YTC, SYS, and CYL, agreed with the manuscript results and conclusions, jointly developed the structure and arguments for the paper, and reviewed and approved the final manuscript.
References
- 1. Nasiell J, Lindqvist PG. Aortic dissection in pregnancy: the incidence of a life-threatening disease. Eur J Obstet Gynecol Reprod Biol. 2010;149:120-121. [DOI] [PubMed] [Google Scholar]
- 2. Poniedzialek-Czajkowska E, Sadowska A, Mierzynski R, Leszczynska-Gorzelak B. Aortic dissection during pregnancy - obstetric perspective. Ginekol Pol. 2019;90:346-350. [DOI] [PubMed] [Google Scholar]
- 3. Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003;76:309-314. [DOI] [PubMed] [Google Scholar]
- 4. Sawlani N, Shroff A, Vidovich MI. Aortic dissection and mortality associated with pregnancy in the United States. J Am Coll Cardiol. 2015;65:1600-1601. [DOI] [PubMed] [Google Scholar]
- 5. Dubey RK, Gillespie DG, Mi Z, Rosselli M, Keller PJ, Jackson EK. Estradiol inhibits smooth muscle cell growth in part by activating the cAMP-adenosine pathway. Hypertension. 2000;35(1 Pt 2):262-266. [DOI] [PubMed] [Google Scholar]
- 6. Manalo-Estrella P, Barker AE. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol. 1967;83:336-341. [PubMed] [Google Scholar]
- 7. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165-3241. [DOI] [PubMed] [Google Scholar]
- 8. Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61:1661-1678. [DOI] [PubMed] [Google Scholar]
- 9. Isselbacher EM, Preventza O, Hamilton Black J, 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: A report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:e334-e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuan SM. Aortic dissection during pregnancy: a difficult clinical scenario. Clin Cardiol. 2013;36:576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]