Abstract
We previously showed that envelope (gp160)-based vaccines, used in a live recombinant virus priming and subunit protein boosting regimen, protected macaques against intravenous and intrarectal challenges with the homologous simian immunodeficiency virus SIVmne clone E11S. However, the breadth of protection appears to be limited, since the vaccines were only partially effective against intravenous challenge by the uncloned SIVmne. To examine factors that could affect the breadth and the efficacy of this immunization approach, we studied (i) the effect of priming by recombinant vaccinia virus; (ii) the role of surface antigen gp130; and (iii) the role of core antigens (Gag and Pol) in eliciting protective immunity. Results indicate that (i) priming with recombinant vaccinia virus was more effective than subunit antigen in eliciting protective responses; (ii) while both gp130 and gp160 elicited similar levels of SIV-specific antibodies, gp130 was not as effective as gp160 in protection, indicating a possible role for the transmembrane protein in presenting functionally important epitopes; and (iii) although animals immunized with core antigens failed to generate any neutralizing antibody and were infected upon challenge, their virus load was 50- to 100-fold lower than that of the controls, suggesting the importance of cellular immunity or other core-specific immune responses in controlling acute infection. Complete protection against intravenous infection by the pathogenic uncloned SIVmne was achieved by immunization with both the envelope and the core antigens. These results indicate that immune responses to both antigens may contribute to protection and thus argue for the inclusion of multiple antigens in recombinant vaccine designs.
The envelope antigen of human immunodeficiency virus type 1 (HIV-1) is a major determinant for virus infectivity, cellular tropism, cytopathicity, and in vivo pathogenicity and is the primary target for virus neutralizing antibodies (14, 98). Most of the early efforts in AIDS vaccine development have therefore focused on the envelope glycoproteins as the target antigen, especially the surface antigen gp120 (22, 36, 56). The efficacy of this approach has been demonstrated primarily in chimpanzees (8, 9, 12, 13, 24, 30, 58) and more recently in macaques, using chimeric simian-human immunodeficiency viruses (11, 46, 55, 64, 91). Protection has been attributed to neutralizing antibodies, particularly those directed to the V3 hypervariable region of gp120 (8, 26, 89). However, antisera from gp120-immunized vaccinees failed to neutralize primary HIV-1 isolates (35, 59), and the potential efficacy of such vaccines in humans has been debated (10, 19).
We and others have used simian immunodeficiency viruses (SIV) as a model to gain insights into the correlates of protection and the requirements for an efficacious vaccine against primate lentiviruses. Recombinant subunit envelope vaccines alone have shown little or no efficacy (61, 67). Various degrees of success have been reported by investigators using a combination immunization strategy in which a poxvirus-vectored vaccine was used for priming and subunit immunogens for boosting (1, 3, 21, 29, 38, 42, 45, 70). We recently reported that while such a combination immunization regimen resulted in complete protection against a pathogenic cloned virus, E11S, only partial protection was achieved against the uncloned virus, SIVmne (76). The breadth of protection by recombinant gp160 vaccines thus appears restricted. Given the hypervariable nature of the envelope antigen, it remains important to explore approaches that will broaden the protective immunity of envelope-based vaccines.
The core antigens of HIV-1 are attractive targets for vaccine development not only because they are relatively conserved but also because they contain major determinants for recognition by cytotoxic T lymphocytes (CTL) (15, 16, 60, 71), which are believed to play an important role in controlling virus infection (52, 83, 84). However, the protective role of immune responses to the core antigens has not been directly demonstrated. Emini et al. (25) failed to protect chimpanzees against HIV-1 IIIB infection with a Gag p55 antigen produced in yeast cells. Similarly, a peptide vaccine containing a conserved CTL epitope of SIV failed to protect macaques against SIVmne E11S, despite the presence of a robust CTL response (100). Core antigens have also been used in combination with envelope antigens in vaccine studies. However, results have been variable, ranging from a complete lack of protection (21) to variable degrees of partial protection with reduction of viral load and prolongation of survival (1, 3, 38). The potential role of immune responses to the core antigens are difficult to discern from these studies in part because of a lack of direct comparisons and in part because of the divergent nature of the challenge models and immunization regimens used.
In the present study, we sought to determine the role of the envelope and the core antigens in eliciting protective responses by the combination immunization approach. Specifically, we examined the effect of priming by recombinant vaccinia virus, the role of the surface antigen gp130 versus whole envelope protein gp160, and the role of core antigens, alone or in combination with envelope antigens, in eliciting protective immunity against SIV infection. Our results indicate that immune responses to both antigens may contribute to protection and that protection is feasible with recombinant vaccines against a primate lentivirus of moderate complexity and pathogenicity.
MATERIALS AND METHODS
Immunogens and immunization regimen.
The following recombinant viruses were used for primary immunizations: v-SE5, which expresses the native full-length gp160 antigen of SIVmne cl 8 from the vaccinia virus 7.5K promoter (44); v-SE6, which expresses only the surface antigen gp130 from the 7.5K promoter; v-SG11, which contains the entire gag-pol coding region of SIVmne under the control of vaccinia virus H6 (40K) promoter; and v-SGE14, which contains both the full-length env and the gag-pol constructs in v-SE5 and v-SG11 under the control of their respective promoters. All recombinant viruses were constructed by using a plaque-purified New York City Board of Health strain of vaccinia virus (v-NY) (43) as the vector. Immunizations with recombinant viruses were performed by skin scarification at approximately 108 PFU per dose. All subunit and particle vaccines were produced by African green monkey kidney (BSC-40) cells infected with recombinant vaccinia virus expressing the corresponding antigens (gp160, gp130, Gag-Pol, or Gag-Pol-Env) under the control of the late vaccinia virus 11K promoter. Full-length SIVmne envelope glycoprotein rgp160 molecules produced by this system are glycosylated and are capable of binding to recombinant human CD4. They have an apparent molecular mass of 160 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and are oligomeric (mostly trimeric or tetrameric) by size exclusion chromatography (data not shown). The rgp130 molecules are CD4-binding monomeric glycoproteins secreted from infected cells. Each dose contained approximately 100 μg of gp160 or gp130, corresponding to 200 and 110 μg of total proteins, respectively (51). The Gag-Pol and Gag-Pol-Env antigens expressed by v-SG11 and v-SGE14, respectively, are processed and assembled in particle forms similar to those described for HIV-1 antigens (33, 34, 41). Each dose of Gag-Pol particles contained 500-μg equivalents of p27 antigens. Each dose of Gag-Pol-Env particles contained 500-μg equivalents of p27 antigens, plus approximately 30 μg of envelope proteins copurified with the core particles (pseudovirions). All subunit and particle immunogens were formulated in incomplete Freund’s adjuvant and were administered intramuscularly. Methods for the construction, preparation, and characterization of SIVmne recombinant viruses and subunit immunogens are similar to those previously described for the HIV-1 counterparts (34, 39, 42, 51) and will be described in further detail elsewhere (47). Cynomolgus macaques (Macaca fascicularis; n = 4/group) were immunized at 0, 3, 18, and 24 months as described in Table 1.
TABLE 1.
Immunization regimen
| Group | Immunization
|
Macaque | |
|---|---|---|---|
| Primary | Booster(s)a | ||
| A | rgp160 | rgp160 | 91286 |
| 91292 | |||
| 91268 | |||
| 91262 | |||
| B | v-SE5 (gp160) | rgp160 | J90304 |
| 91272 | |||
| 91250 | |||
| 91263 | |||
| C | v-SE6 (gp130) | rgp130 | 91255 |
| 91254 | |||
| 91244 | |||
| 91293 | |||
| D | v-SG11 (Gag-Pol) | Gag-Pol particles | 91245 |
| 91278 | |||
| 91288 | |||
| 91253 | |||
| E | v-SE5 + v-SG11b | Gag-Pol particles + rgp160c | 91251 |
| 91281 | |||
| 91248 | |||
| 91283 | |||
| F | v-SGE14 (Gag-Pol-gp160)d | Gag-Pol-Env particlese | 91287 |
| 91291 | |||
| 91282 | |||
| 91247 | |||
| Control | 91077 | ||
| 91078 | |||
| M92387 | |||
| 93057 | |||
Booster immunizations were performed by intramuscular injections at 3, 18, and 24 months after the primary immunization.
Animals received equal doses (108 PFU) of each recombinant virus administered separately by skin scarification at opposite sites along the midline of the back.
Inoculum was formulated as a mixture of core particles (containing 500-μg equivalents of p27 antigen) and rgp160 (100 μg).
Inoculum contained a single recombinant virus (108 PFU) expressing the gag-pol genes under the control of vaccinia 40K promoter and the full-length env gene under the 7.5K promoter.
Immunogen was produced from recombinant vaccinia virus-infected cells and was composed of both the core (Gag-Pol) and the envelope antigens in a mature virus-like particle structure (pseudovirions). Each inoculum contained a 500-μg equivalent of p27 antigens and approximately 30 μg of envelope proteins.
Challenge virus and conditions.
SIVmne was isolated from a pigtailed macaque (M. nemestrina) with lymphoma and was propagated on HuT78 cells (4). E11S is a single-cell clone of SIVmne-infected HuT78 cells that produced large amounts of envelope glycoproteins (6). Animals were challenged by intravenous injection at 4 weeks after the last immunization. Challenge with SIVmne clone E11S was performed with 10 to 100 animal infectious doses (AID) of virus grown on macaque (M. fascicularis) peripheral blood mononuclear cells (PBMC). The animals in groups B, E, and F (Table 1) that were completely protected against E11S challenge were boosted again at 8 to 9 months after the challenge and were rechallenged 4 weeks later with uncloned SIVmne grown on HuT78 cells at 2 to 20 AID. Blood samples were collected on the day of challenge, at 2, 4, 6, and 8 weeks after each challenge, and monthly thereafter. Plasma and serum samples were collected and stored until used at −70 and −20°C, respectively. Lymph node biopsy specimens were obtained at indicated times after challenge and were frozen at −70°C for DNA analysis or fixed for histological examinations. Animals were housed in the Washington Regional Primate Research Center and were under the care of licensed veterinarians. Euthanasia was performed on the basis of the following criteria: (i) AIDS; (ii) termination of experiment; or (iii) unrelated cause. Euthanasia is considered to be AIDS related if the animal exhibits CD4+ cell depletion (≤200 cells/mm3) in the peripheral blood and two or more of the following conditions: wasting, unsupportable diarrhea, opportunistic infections, proliferative diseases (e.g., lymphoma), and abnormal hematology (e.g., anemia or thrombocytopenia).
Virus isolation.
PBMC were isolated over Histopaque-1077 (Sigma Chemical Co., St. Louis, Mo.) as described previously (5, 7). Briefly, 4 × 106 PBMC were cocultivated with 5 × 106 AA-2CL5 cells, and cultures were maintained for 8 to 9 weeks. Virus was detected by reverse transcriptase assays performed as described previously (7). A positive value means positive results in reverse transcriptase assays, and a negative value means no reverse transcriptase activity detected after 8 to 9 weeks of cocultivation.
Serum neutralization assays.
Neutralizing antibodies against uncloned SIVmne and SIVmne clone E11S were measured in CEM-x174 cells by methods similar to those described by Montefiori et al. (62). The uncloned SIVmne used for neutralization studies was grown on HuT78 cells and was identical to the challenge stock but prepared at different times. The E11S clone used for neutralization assays was grown on macaque PBMC after the initial isolation and propagation in HuT78 cells (4). It was derived from the same stock as the challenge virus but grown on PBMC from a different M. fascicularis. Neutralization of a related but heterologous virus, SIVmac251, was measured in HuT78 cells as described by Langlois et al. (54). Twofold serum dilutions (heat inactivated at 56°C for 30 min) were done in 96-well plates. The neutralization titer is expressed as the reciprocal serum dilution that inhibits 50% of SIVmne-induced cytopathic effect in CEMx174 cells or 90% syncytium formation by SIVmac251-infected HuT78 cells.
ELISA.
SIV-specific antibodies were measured by enzyme-linked immunoabsorbent assay (ELISA) as described previously (40) except that sucrose gradient-purified and disrupted whole SIVmne clone E11S virion was used as antigen in ELISA. Endpoint titers were determined as the reciprocal of the highest serum dilution that resulted in an optical density reading threefold greater than that obtained with negative control sera.
Nested PCR analysis.
PBMC were isolated from EDTA-treated blood by Hypaque-Ficoll gradient centrifugation, and nucleic acid was extracted by means of standard techniques. One microgram of total nucleic acid was used as a template for a two-step amplification by PCR using a nested set of oligonucleotides primers specific for the envelope regions. The conditions for the first and second rounds of amplification were as described elsewhere (44). The final amplified fragment was approximately 642 bp. Amplified products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining. Results for a subset of samples were also confirmed by PCR with primers from long terminal repeat (LTR)-gag regions.
Semiquantitative PCR analysis of proviral DNA load.
Proviral DNA in PBMC was measured by PCR with the use of radiolabeled primer incorporation for quantification (75) and was expressed as copies of proviral genome detected per million PBMC. Briefly, 1 μg of DNA from each sample was amplified in a PCR mixture that contained 0.2 μM each primer, 200 μM each of four deoxynucleoside triphosphates, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 1.0 U of Taq polymerase (Perkin-Elmer Cetus, Branchburg, N.J.) in a volume of 50 μl. The reaction mixture was subjected to 30 cycles of denaturation for 1 min at 94°C, annealing for 2 min at 60°C, and elongation for 3 min at 70°C. The oligonucleotide primers used were derived from the nucleotide sequence of SIVmne (GenBank accession no. M32741 [37]). They consist of a primer pair specific for the envelope region, env10 (nucleotide 7191 to 7211 [sense]) and env12 (7541 to 7561 [antisense]), and a pair of primers specific for the LTR-gag region, S1 (228 to 251 [sense]) and S8 (536 to 559 [antisense]). The amplified products for the env and LTR-gag sequences are 370 and 330 bp, respectively. One oligonucleotide of each complementary pair was 5′ end labeled with [32P]ATP by the use of polynucleotide kinase (New England Biolabs, Inc., Beverly, Mass.). The 32P-labeled PCR products obtained by amplification were analyzed by electrophoresis on 8% nondenaturing polyacrylamide gels and quantified by autoradiography with phosphorimager analysis (75). Quantification of SIV DNA was determined with a standard curve generated by known quantities of a plasmid clone of E11S.
RT-QC (quantitative competitive)-PCR determination of plasma viral RNA.
Plasma viral RNA was prepared as described by Watson et al. (97). The viral RNA samples were serially diluted in a 96-well PCR microplate into a reaction buffer containing a fixed copy number of a competitor RNA containing an internal deletion. The template and the competitor were subjected to reverse transcription (RT) followed by PCR. The primers used are from the SIVmne gag sequence: 5′ primer (5G) from nucleotides 675 to 698 (AAAGCCTGTTGGAGAACAAAGAAG); 3′ primer (3Diii) from nucleotides 993 to 1011 (AATTTTACCCAGGCATTTA). The internal RNA control contains a deletion of 82 bp which enables the discrimination between products amplified from the viral (336-bp) and the control (254-bp) templates. The conditions for the RT reaction and PCR were as described by Watson et al. (97).
Lymphocyte subset analysis.
Cell surface immunofluorescence was quantified by use of a FACScan flow cytometer and Lysis II software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Lymphocyte subsets (CD4, CD8, CD2, and CD20) of whole heparinized blood samples were evaluated by conventional methods.
Statistical analysis.
Differences in proportions were tested by Fisher’s exact test. Differences in the mean peak proviral load for control animals and immunized animals were tested by using the t test. Repeated-measures analysis of variance (ANOVA) models with weeks as a fixed within-subject factor and group as a fixed between-subject factor were used to test for differences in viral load between the control group and the immunized groups in the first 20 weeks of infection. To determine if protection correlated with SIV-specific antibody responses, bivariate ANOVA models with protection status and group as fixed effects were used (the interaction between group and protection status was tested but was not significant in any of the models). If the global hypothesis test testing whether all groups were the same was rejected, all pairwise differences were tested by the Bonferroni method to adjust for multiple comparisons. The median time to AIDS for immunized and control animals was compared by using the Mann-Whitney U test.
RESULTS
Immunization regimen and outcome of challenge with clone E11S.
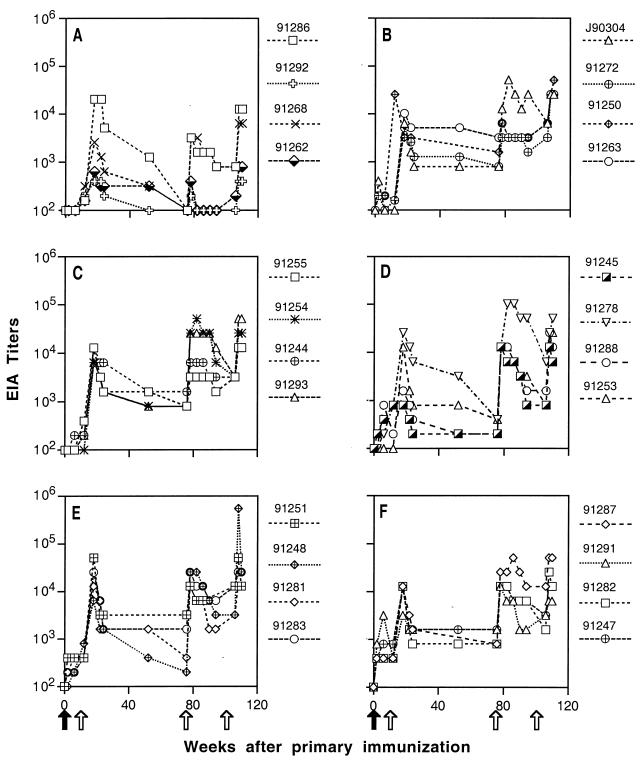
Twenty-four juvenile cynomolgus macaques (M. fascicularis) were divided into six groups and were immunized at 0, 3, 18, and 24 months as described in Table 1. Except for animals in group A, which were immunized with subunit recombinant gp160 (rgp160) only, all animals received primary immunizations of recombinant vaccinia virus(es) followed by booster immunizations with recombinant subunit or particle vaccines. Most animals developed low levels of SIV-specific antibody responses after the primary immunization (Fig. 1). After the first booster immunization, all animals showed a significant increase in SIV-specific antibodies (Fig. 1). Although antibody titers declined after the booster immunization, they generally did not fall below the baseline (1:100). The exceptions, however, were animals immunized with subunit rgp160 only (group A). SIV antibody titers in these animals decreased to baseline level by the time of the second booster immunization (Fig. 1A). Even after the second boost, two of four animals in this group failed to generate a sustained antibody response. In contrast, all animals primed with recombinant vaccinia virus(es) showed recall responses to boosts and retained low to moderate levels of antibody titer between boosts (Fig. 1).
FIG. 1.
ELISA analysis of SIV-specific antibody responses in immunized macaques prior to challenge. Dilutions of macaque sera collected at the indicated times were incubated with disrupted, sucrose gradient-purified SIVmne virions proteins on microtiter plates. Endpoint ELISA (EIA) titers were defined as the reciprocal of the highest dilution that gave an optical absorbance value at least threefold higher than the average values obtained with SIV-negative macaque sera. Panels A through F correspond to experimental groups A through F (Table 1). Positions of the arrows indicate the times of primary (solid arrows) and booster (open arrows) immunizations.
Four weeks after the third boost, all immunized animals, together with four naive controls, were challenged intravenously with the homologous pathogenic virus clone E11S grown on macaque PBMC. Infection was monitored by virus isolation by coculture, nested PCR analysis, and measurements of anamnestic response and seroconversion to nonvaccine antigens. Results of virus isolation and PCR analysis are summarized in Table 2.
TABLE 2.
Virus isolation and nested PCR analysis of PBMC and lymph node cells from macaques after intravenous challenge with clone E11S
| Groupa | Macaque | Results of analysisb at indicated wk postchallenge
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 12 | 16 | 20 | 28 | 36 | 44 | 60 | 99 | 148 | 183 | 200 | |||
| A | 91286 | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 91292 | +/+ | +/+ | −/+ | +/+ | −/− | +/+/+ | −/+ | −/+ | −/− | −/− | −/+ | −/− | −/+ | E | ||
| 91268 | +/+ | −/+ | −/− | −/− | +/+ | −/+/+ | −/+ | −/− | −/+ | −/− | −/− | −/− | −/− | E | ||
| 91262 | +/+ | +/+ | +/+ | −/− | −/− | +/+/+ | −/+ | +/+ | +/+ | +/+ | +/+ | A | ||||
| B | J90304 | −/− | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | R | ||||||
| 91272 | −/+ | +/− | −/− | −/− | −/−/+ | −/−/+ | −/− | +/− | −/+ | +/+ | A | |||||
| 91250 | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | R | |||||||
| 91263 | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | R | |||||||
| C | 91255 | +/+ | +/+ | −/+ | +/− | −/− | −/−/+ | −/− | −/+ | −/− | −/+ | −/+ | +/+ | +/+ | E | |
| 91254 | +/+ | +/+ | −/− | +/− | −/− | −/−/+ | −/− | −/− | −/− | −/− | −/+ | −/− | −/− | E | ||
| 91244 | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |||
| 91293 | +/+ | +/+ | −/+ | −/+ | NT/−/+ | −/+ | −/− | −/+ | −/− | −/− | −/− | −/− | −/− | E | ||
| 2 | 4 | 8 | 12 | 16 | 20 | 28 | 36 | 45 | 66 | 99 | 150 | 173 | 183 | 220 | ||
| D | 91245 | +/+ | +/+ | −/+ | +/+ | +/+ | −/+/+ | −/+ | −/− | −/+ | −/− | −/+ | −/− | −/− | −/− | E |
| 91278 | −/? | −/− | −/− | −/−/− | −/− | −/−/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 91288 | +/+ | −/+ | −/− | −/−/+ | −/+ | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 91253 | +/+ | −/+ | −/+ | −/+ | −/+/+ | −/− | −/+ | −/− | −/− | −/− | −/− | −/− | −/− | −/− | E | |
| E | 91251 | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | R | ||||||
| 91281 | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | R | |||||||
| 91248 | +/+ | +/+ | +/+ | −/+/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | A | ||
| 91283 | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | R | |||||||
| F | 91287 | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | −/− | R | ||||||
| 91291 | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | R | |||||||
| 91282 | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | −/− | R | |||||||
| 91247 | −/− | −/− | −/− | −/− | −/−/− | −/− | −/− | −/− | R | |||||||
| Control | 91077 | +/+ | +/+ | +/+ | −/+ | −/+/+ | −/+ | −/+ | −/+ | −/− | −/+ | −/− | −/− | −/− | −/+ | E |
| 91078 | +/+ | +/+ | +/+ | −/+ | −/+ | −/+/+ | −/+ | −/+ | +/+ | −/+ | −/+ | −/− | −/− | −/− | E | |
| M92387 | +/+ | +/+ | +/+ | +/+/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | A | |||
| 93057 | +/+ | +/+ | +/+ | +/+ | +/+/+ | −/+ | −/+ | −/+ | −/+ | −/+ | −/+ | −/− | −/− | NT/− | E | |
See Table 1 and Materials and Methods for details of the immunization regimen.
Presented as positive (+) or negative (−) in the following assays: virus isolation from PBMC/PCR analysis of PBMC DNA/PCR analysis of lymph node DNA. When only two results are indicated, they refer to those from the first two assays. The cause of death is denoted as AIDS-related euthanasia (A) or elective euthanasia (E). R, reassigned to be rechallenged with uncloned SIVmne; nt, not tested; ?, results inconclusive.
Among animals that received just the envelope immunogens, the only group that showed significant protection were those that received rgp160 in a recombinant vaccinia virus priming and subunit protein boosts (group B). Three of the four animals in this group were completely protected (Table 2). One animal (macaque 91272) was transiently virus positive by PCR analysis and PBMC coculture, respectively, at weeks 2 and 4 after challenge. Compared with the cumulative data for 15 control animals from three separate experiments (76), immunized animals in group B showed significant protection (3 of 4 immunized animals versus 1 of 15 controls; P = 0.02) (Table 3).
TABLE 3.
Summary of results after E11S challenge
| Groupa | Protection against E11S challenge
|
|||
|---|---|---|---|---|
| No. protected/total | Pb | Mean peak proviral loadc (log10 copies/106 PBMC) | P | |
| A | 1/4 | 0.4 | 2.97 | 0.07 |
| B | 3/4d | 0.02 | ||
| C | 1/4 | 0.4 | 2.33 | 0.004 |
| D | 0/4e | 1.0 | 2.42 | 0.001 |
| E | 3/4 | 0.02 | ||
| F | 4/4 | 0.001 | ||
| Control | 0/4 | 4.04 | ||
| Cumulative controlsf | 1/15 | |||
See Table 1 and Materials and Methods for details of the immunization regimen.
Calculated by using data from cumulative controls. If data from concurrent controls are used, only results from group F after E11S challenge are statistically significant (P = 0.03).
At 2 weeks after challenge.
The infected animal in this group (macaque 91272) was virus positive by nested PCR and virus isolation from PBMC only at weeks 2 and 4, respectively, after challenge. However, its virus load increased significantly about 1 year after infection, and the animal eventually developed AIDS (76).
One of the infected animals (macaque 91278) was virus positive only by semiquantitative PCR analysis of PBMC collected at weeks 2 after challenge.
Including 15 animals infected intravenously with E11S in three separate experiments (76).
In contrast, immunization with subunit rgp160 alone (group A) or with the surface antigen rgp130 in a prime-and-boost regimen (group C) was much less effective. Three of four animals in each group became infected after challenge (Table 2). The proportion of uninfected animals in each group failed to reach statistical significance (1 of 4 in the experimental groups versus 1 of 15 in the cumulative controls; P = 0.4) (Table 3). However, some degree of protection was apparent in these animals since the quantity and the duration of detectable proviral DNA in their PBMC were considerably less than those of naive controls (Fig. 2). The mean peak proviral loads (log10 copies/106 PBMC) at 2 weeks after challenge were 4.04 for the control animals and 2.97 and 2.33 for the immunized animals in groups A and C, respectively (Table 3). This difference was highly significant for animals in group C (P = 0.004) but marginally so for those in group A (P = 0.07). To test whether animals in groups A and C had lower viral loads in primary infection (through week 20), we performed repeated-measures ANOVA on proviral loads as described in Materials and Methods. Animals in both group A and group C showed significant reduction of viral load during primary infection compared to the controls (P for groups A and C are 0.005 and 0.001, respectively). Thus, immunization with gp130 in a prime-and-boost regimen, or even with subunit rgp160 alone, resulted in some degree of protection.
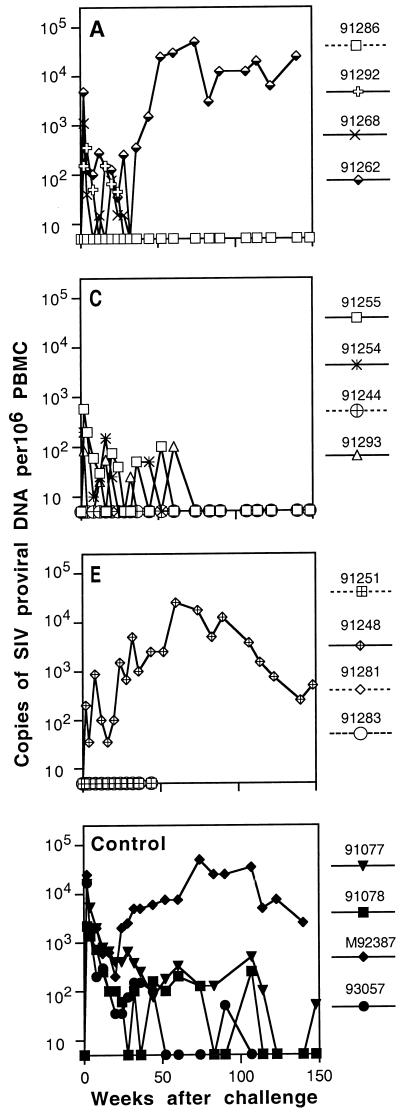
FIG. 2.
PBMC provirus load in macaques after intravenous challenge with E11S. Proviral load was determined by PCR analysis with radiolabeled primer incorporation as described in Materials and Methods. Proviral DNA was measured by using an external E11S DNA standard of known quantity. Panels A through F correspond to experimental groups A through F (Table 1). Solid lines indicate infected animals.
All four animals in group D that received only the core antigens (Gag-Pol) became infected after challenge (Table 2). However, they all showed significantly reduced viral load compared to the controls as early as 2 weeks after challenge (mean viral load of log10 2.42 copies/106 PBMC for group D versus 4.04 for the controls; P = 0.001) (Fig. 2). The duration of detectable proviral DNA in their PBMC was also reduced. One animal (macaque 91278) was virus positive by PCR analysis only at week 2 after challenge. Proviral loads in primary infection (through week 20) were also significantly lower for group D animals than for the control animals (P < 0.001). These results indicate that although immune responses to the core antigens were not protective, they facilitated the clearance of virus-infected cells and the control of infection.
Animals immunized with both envelope and core antigens (groups E and F) showed high degrees of protection. Three of the four animals in group E and all four animals in group F were completely protected against E11S challenge (Tables 2 and 3; Fig. 2E and F). Compared to the data for the cumulative controls, the P values were 0.02 and 0.001 for groups E and F respectively. This result, together with those from animals immunized with Gag-Pol particles only (group D), reaffirms the important role of immune responses against the envelope antigens in protection against the homologous virus.
SIV-specific antibody responses in animals challenged with clone E11S.
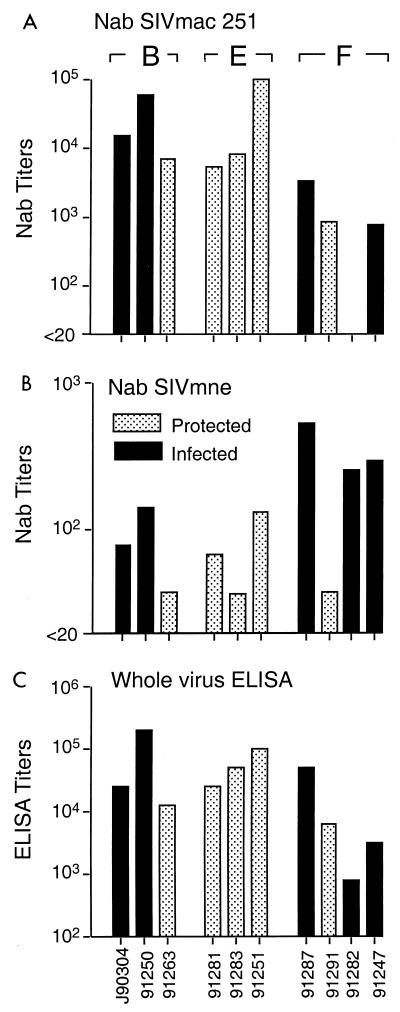
To determine if protection correlated with SIV-specific antibody responses, we analyzed by ELISA and virus neutralization assays the sera collected from immunized animals on the day of challenge. With the exception of two animals in group A (macaques 91292 and 91262), all immunized animals generated moderate levels of SIV-specific antibody response as measured by whole-virus ELISA (Fig. 3C). Among animals immunized with envelope antigens, the mean ELISA titer for animals in group A (log10 3.39) was significantly lower than that for animals in groups B (log10 4.45) and C (log10 4.37) (P = 0.05 in both cases), indicating the potential advantage of primary immunization with recombinant vaccinia virus. However, there is no apparent correlation between the titer of SIV-specific antibodies on the day of challenge and protection.
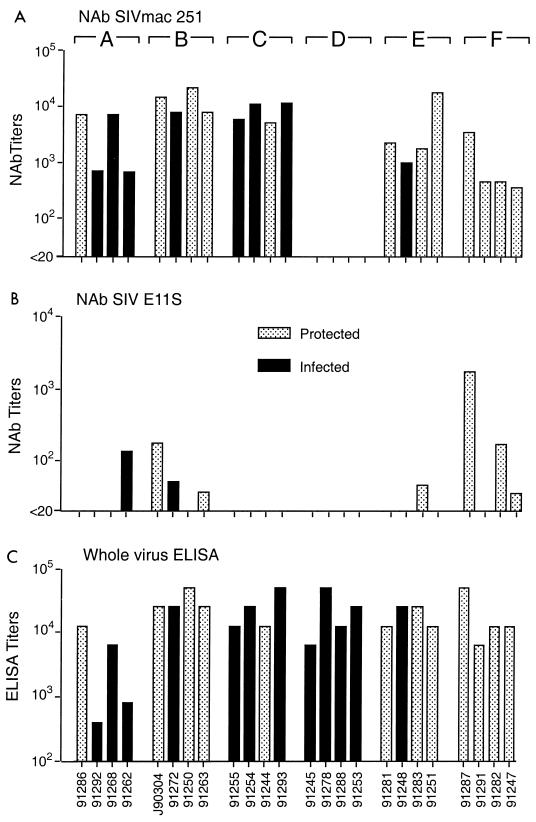
FIG. 3.
SIV-specific antibody responses in immunized macaques on day of challenge with E11S. Sera collected on the day of challenge were analyzed for neutralizing antibody (NAb) activities against the homologous challenge virus E11S or a heterologous virus, SIVmac251. Neutralizing titers are expressed as the reciprocal serum dilutions that results in >90% reduction of syncytium formation by SIVmac251 on HuT78 cells (A) or >50% reduction of cytopathicity of E11S-infected CEMx174 cells (B). Serum reactivity with disrupted SIVmne E11S virion proteins was analyzed by ELISA, and the results are expressed as endpoint titers (C). Designations of experimental groups (A through F) are indicated at the top.
Serum neutralization activities were measured by two different assays. Only a minority of animals had detectable levels of neutralizing antibody against the homologous virus on the day of challenge (Fig. 3B). However, all animals immunized with envelope antigens alone, or with both envelope and core antigens, showed low to moderate levels of neutralizing activities against a related but heterologous virus, SIVmac251 (Fig. 3A). As expected, no neutralizing antibody was detected in animals immunized with core antigens alone. Again, there was no correlation between protection and serum neutralizing activity as measured by either assay.
Protection against uncloned SIVmne.
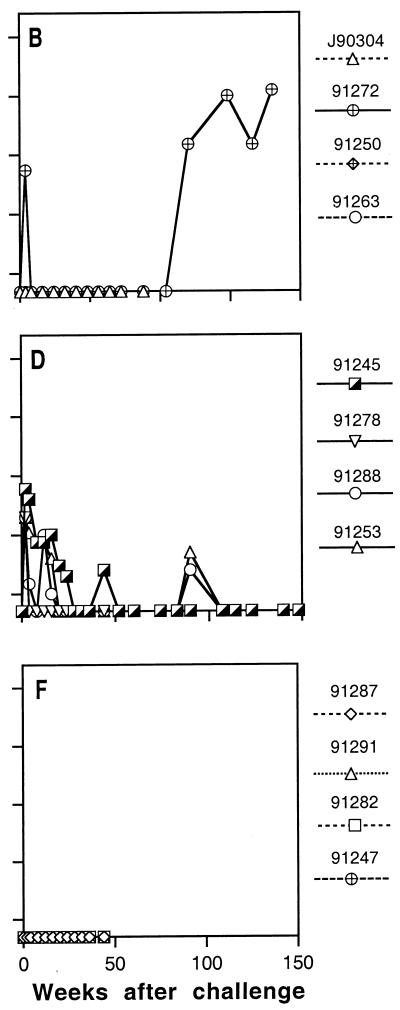
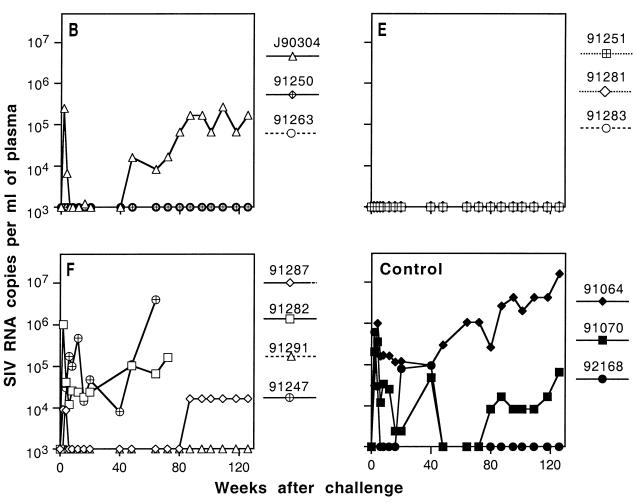
To examine the breadth of protective immunity, we boosted 10 animals in groups B, E, and F that were completely protected against E11S and rechallenged them with uncloned SIVmne. These animals were virus negative at all times for 8 to 9 months after the E11S challenge by all criteria tested (virus isolation, nested PCR analysis of PBMC and lymph node cells, anamnestic response, and, for group B animals, seroconversion to nonvaccine antigens). As expected from our previous studies (76), only partial protection against the uncloned SIVmne was achieved by immunization with rgp160 alone with the prime-and-boost regimen (group B). One animal was protected (macaque 91263), one was transiently virus positive (macaque 91250), and one was persistently infected (macaque J90304) (Table 4). Concordant results were obtained by virus isolation and nested PCR analyses of PBMC and lymph node cells (Table 4), by QC-PCR analyses of viral load in PBMC (data not shown) and plasma (Fig. 4), and by ELISA of SIV-specific serum antibody responses after challenge (Fig. 5). In contrast, all three animals in group E, which were immunized by both gp160 and core antigens, were completely protected against the uncloned virus challenge (3 of 3 immunized animals versus 0 of 10 cumulative controls; P = 0.003) (Table 5). Interestingly, protection was observed in only one of four animals in group F, which also received rgp160 and core antigens, not as a mixture of immunogens (as in group E) but as a single recombinant virus for priming and pseudovirion for boosting. Again, concordant results were obtained from all analyses performed to determine protection or infection in these animals (Table 4; Fig. 4 and 5).
TABLE 4.
Virus isolation and nested PCR analysis of PBMC and lymph node cells from macaques after intravenous challenge with uncloned SIVmne
| Groupa | Macaque | Results of analysisb at indicated wk postchallenge
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 6 | 8 | 12 | 16 | 20 | 32 | 48 | 56 | 64 | 87 | 126 | 136 | 162 | 220 | ||
| B | J90304 | +/+ | +/+/+ | +/+ | −/+ | +/+ | −/+ | +/+ | +/+ | +/+ | +/+ | +/+ | −/+ | +/+ | −/+ | A |
| 91250 | +/− | −/+/+ | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 91263 | −/− | −/−/− | −/− | −/− | −/− | −/? | −/− | −/− | ? | −/− | −/− | −/− | −/− | −/− | ||
| E | 91251 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| 91281 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 91283 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | ||
| F | 91287 | +/+ | −/+ | −/− | −/+ | −/+ | −/− | +/+ | −/+ | +/+ | +/+ | +/+ | A | |||
| 91291 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 91282 | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | A | |||||
| 91247 | −/− | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | A | ||||||
| Control | 91064 | +/+ | +/+/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | A | ||
| 91070 | +/+ | +/+ | NT/+/+ | +/+ | +/+ | +/+ | −/+ | +/+ | +/+ | +/+ | +/+ | +/NT | +/+ | A | ||
| 92168 | +/+ | +/+ | +/+/+ | −/+ | −/+ | −/+ | +/+ | −/+ | −/+ | −/+ | −/− | −/− | +/+ | −/− | E | |
FIG. 4.
Plasma viral load in macaques after uncloned SIVmne challenge. Plasma viral load was determined by RT-QC-PCR using an internally controlled template as described in Materials and Methods. Panels B, E, and F correspond to experimental groups B, E, and F. Solid lines indicate infected animals.
FIG. 5.
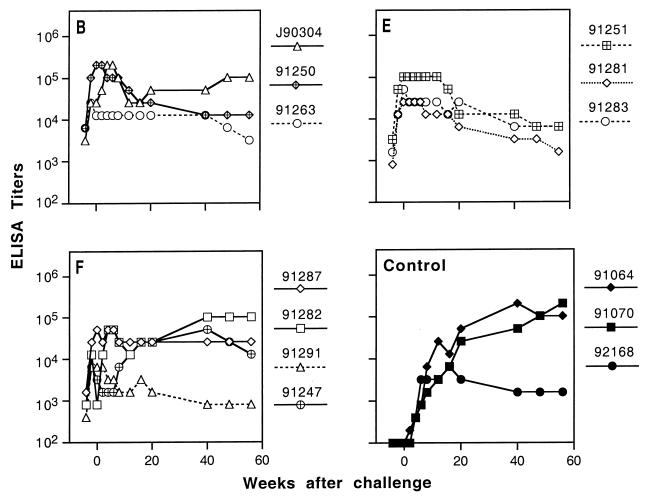
ELISA analysis of SIV-specific antibodies in macaques after uncloned SIVmne challenge. Animals in experimental groups B, E, and F (represented by panels B, E, and F) that were protected against E11S challenge were held for 8 to 9 months to confirm their virus-negative status. They were then boosted again and challenged 4 weeks later with uncloned SIVmne (week 0 on the abscissa). Endpoint titers were defined as the reciprocal of the highest dilution that gave an optical absorbance value at least threefold higher than the average values obtained with SIV-negative macaque sera. Solid lines indicate infected animals.
TABLE 5.
Summary of results after uncloned SIVmne challenge
| Groupa | Protection against uncloned SIVmne challenge
|
|
|---|---|---|
| No. protected/total | Pb | |
| B | 1/3c | 0.2 |
| E | 3/3 | 0.003 |
| F | 1/4 | 0.3 |
| Control | 0/3 | |
| Cumulative controlsd | 1/10 | |
See Table 1 and Materials and Methods for details of the immunization regimen.
Calculated by using cumulative controls.
One of the two infected animals, macaque 91250, was virus positive only by virus isolation at week 2 after challenge and by nested PCR at week 6.
Including 10 animals infected intravenously with uncloned SIVmne in three separate experiments (76).
SIV-specific antibody responses in animals challenged with uncloned SIVmne.
After the first challenge by E11S virus, titers of SIV-specific antibodies in all the protected (i.e., uninfected) animals declined approximately 10-fold over 6 to 7 months and then stabilized (data not shown). A final booster immunization was given 4 weeks prior to the uncloned virus challenge, resulting in recall responses in most of the animals. On the day of challenge, all but one animal (macaque 91282) had SIV-specific antibody titers comparable to those prior to the E11S challenge (compare Fig. 6C and 3C). With the exception of the same macaque 91282, comparable titers of neutralizing antibodies against SIVmac251 were also detected (Fig. 6A and 3A). All animals also showed low to moderate levels of serum neutralizing antibodies against the challenge virus, uncloned SIVmne (Fig. 6B). However, we failed to observe any correlation between protection and the titer of SIV-specific antibodies as determined by any of these three assays. After uncloned SIVmne challenge, SIV-specific antibody titers declined in all protected animals and one transiently virus-positive animal (macaque 91250), whereas titers increased in all persistently infected animals, as in the infected control animals (Fig. 5).
FIG. 6.
SIV-specific antibody responses in immunized macaques on day of challenge with uncloned SIVmne. Sera collected on the day of rechallenge were analyzed for neutralizing antibody (NAb) activities against the homologous challenge virus uncloned SIVmne or a heterologous virus, SIVmac251. Neutralizing titers are expressed as the reciprocal serum dilutions that results in >90% reduction of syncytium formation by SIVmac251 on HuT78 cells (A) or >50% reduction of cytopathicity of uncloned SIVmne-infected CEMx174 cells (B). Serum reactivity with disrupted SIVmne E11S virion proteins was analyzed by ELISA, and the results are expressed as endpoint titers (C). Designations of experimental groups (B, E, and F) are indicated at the top.
Clinical outcome of infection.
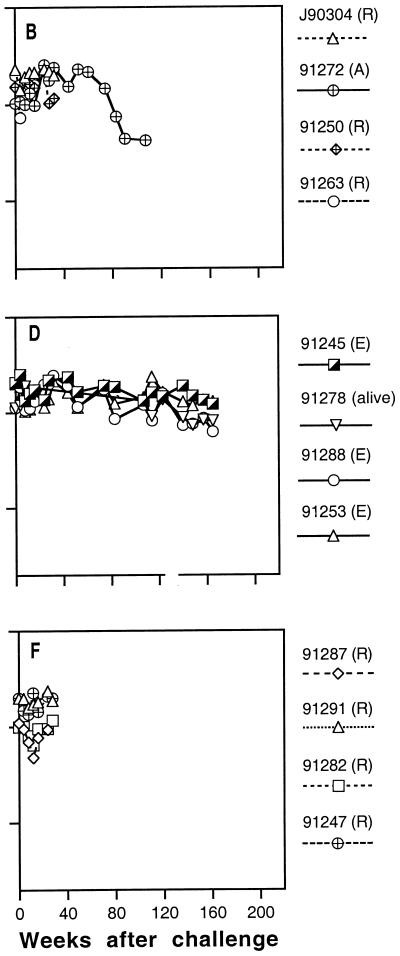
Infected animals were maintained for more than 3 years after challenge to determine the clinical outcome of infection and the effects of immunization. Animals were monitored periodically for lymphocyte subsets, hematology, blood chemistry, body weight, opportunistic infections, and proliferative diseases. Figures 7 and 8 summarize their peripheral blood CD4+ cell levels and survival time after challenge.
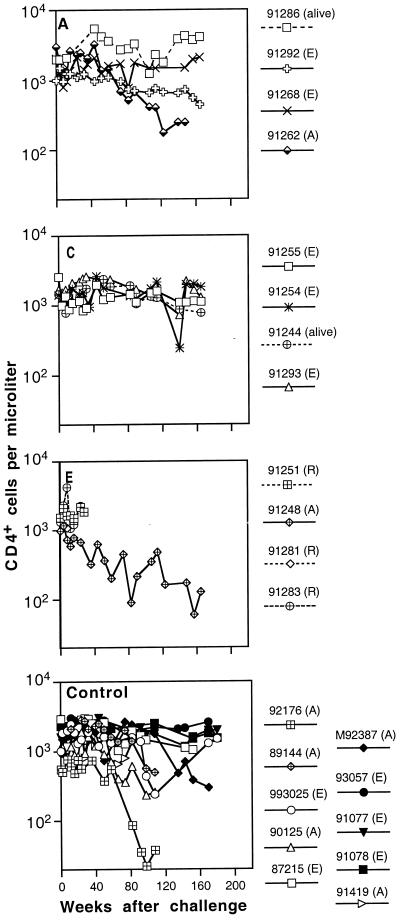
FIG. 7.
Peripheral blood CD4+ T-lymphocyte numbers in immunized and control macaques infected with SIVmne E11S. (A), animals sacrificed because of AIDS; (E), animals euthanized at the end of the experiment; (R), animals reassigned for challenge with uncloned SIVmne. The last datum point for each animal represents the time of death or reassignment. Panels A through F represent data from individual animals groups A through F (Table 1). Cumulative data from 15 naive control animals infected with E11S (Control) were obtained from three separate experiments previously described (76). Data for concurrent controls are indicated by solid symbols; solid lines indicate infected animals.
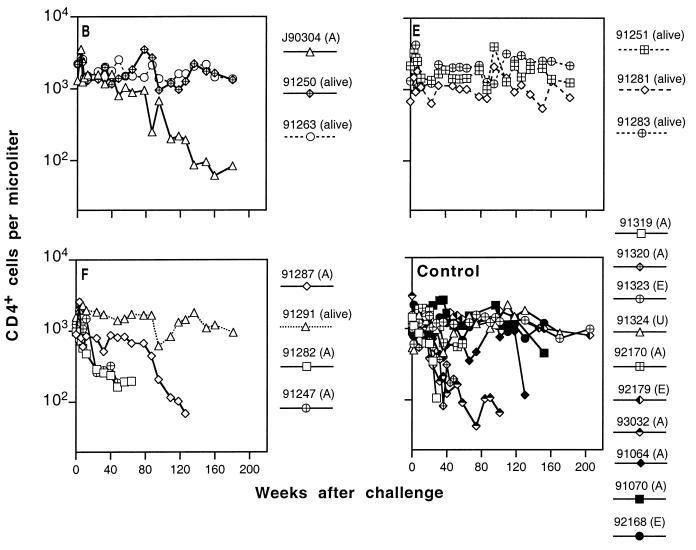
FIG. 8.
Peripheral blood CD4+ T-lymphocyte numbers in immunized and control macaques infected with uncloned SIVmne. Designations (A) and (E) are as described in the legend to Fig. 7; the animal that died of causes unrelated to AIDS is labeled (U). The last datum point for each animal represents the time of death or termination of the experiment. Panels B, E, and F represent data from individual animals in groups B, E, and F (Table 1). Cumulative data from 10 naive control animals infected with uncloned SIVmne (Control) are obtained from three separate experiments previously described (76). Data for concurrent controls are indicated by solid symbols; solid lines indicate infected animals.
To help conserve animals, we only used a small number of control animals in this study. We therefore included in our analysis cumulative data from three studies (76) in which control animals were similarly infected with E11S or uncloned SIVmne. As reported previously, 50 to 60% of naive control M. fascicularis infected with either E11S or uncloned SIVmne developed AIDS-like diseases within 3 years after infection (Fig. 7 and 8, Control). However, CD4+ cell depletion occurs more rapidly in some of the animals infected with the uncloned virus (Fig. 8) than in those infected with E11S (Fig. 7).
Prior immunization did not appear to affect significantly the clinical course of infection. There was no statistically significant difference between controls and each group of immunized and infected animals in their rate of CD4+ cell decline and progression to AIDS. Among 12 immunized animals infected with E11S, 3 were euthanatized due to AIDS between 1.9 to 3.5 years after challenge (Fig. 7A to F). In comparison, 5 of 10 naive controls infected with the same virus were euthanatized within the same period (P = 0.4). Among animals infected with the uncloned virus, 4 of 5 of the immunized animals developed AIDS (Fig. 8B, E, and F), compared with 6 of 10 of the naive controls (P = 0.6). The median survival times for immunized and control macaques that developed AIDS after infection with uncloned SIVmne were 64 and 90.5 weeks, respectively (Fig. 8, P = 0.7).
All immunized animals that were completely protected against virus challenge showed no sign of infection throughout the study period (8 to 9 months for animals used for rechallenge and up to 3.5 years for the rest) (Tables 2 and 4). Their clinical status and CD4+ cell levels remained normal during this time (Fig. 7 and 8).
DISCUSSION
We have shown that vaccine efficacy is dependent on the immunization regimen as well as the nature of the immunogens. To date, immunization with recombinant subunit vaccines containing either the envelope or the core antigens has not resulted in complete protection against pathogenic primate lentiviruses (3, 21, 29, 38, 70, 76). By incorporating both immunogens in a prime-and-boost immunization strategy, we achieved complete protection against intravenous infection by an uncloned virus of moderate pathogenicity, SIVmne.
We previously reported that immunization with the envelope antigen rgp160 in a prime-and-boost regimen resulted in complete protection against intravenous and intrarectal challenge by a homologous cloned virus SIVmne E11S (44, 76, 77). In this study, we examined factors that potentially affect the breadth and the efficacy of this immunization approach. Our findings indicate that priming with recombinant vaccinia virus played a key role in potentiating the protective efficacy of envelope-based vaccines. The mechanism by which this was achieved remains unclear. However, one or more known properties of vaccinia virus infection may have contributed. These include endogenous expression of antigens resulting in presentation by the major histocompatibility complex class I molecules and the generation of CTL responses (20, 88, 101), in situ presentation of native antigenic structures (33, 39, 41), prolonged and low-level expression of antigens, and possible adjuvant effects due to the cumulative local and systemic responses to vaccinia virus infection (66). Since the initial success with the prime-and-boost strategy, a number of investigators have used other viral vectors as well as naked DNA for priming immune responses (1, 3, 38, 55, 70, 80). It would be of interest to compare these approaches to gain further insight on the requirements for efficient priming. It also remains to be shown whether subunit envelope vaccines alone, perhaps with improved adjuvants and immunization regimen, could prime protective responses as well as live viruses.
Our results also showed that immunization with the full-length envelope antigen rgp160 was superior to the surface antigen rgp130, thus indicating a potentially important role for the transmembrane protein (TM). It is possible that immune responses directed to TM itself may contribute to protection. Multiple antigenic sites, including immunodominant epitopes, have been identified in the TM of HIV-1 (27, 31, 72, 99) and SIV (90, 92). They serve as targets for antibody-dependent cellular cytoxicity (57, 94), virus neutralization (68, 69, 93), complement-binding antibody (63, 81, 82), lymphoproliferative (87), and CTL (48, 49, 84, 85, 95) responses. Although their roles in vivo have yet to be defined, one or more of these responses may participate in protection. Alternatively, TM may help present functionally important epitopes in the surface antigen by maintaining proper oligomeric structure (17, 23, 78). Recent studies revealed important functional and antigenic differences between oligomeric and monomeric forms of HIV-1 envelope proteins (14, 65, 79, 86). It is possible that such differences contribute in part to the apparent disparity in the protective efficacy of rgp130 and rgp160. It is of interest that partial protection was observed in macaques immunized with virion-derived oligomeric, but not monomeric, forms of gp130 (73). In that study, better clinical outcome was correlated with high neutralizing antibodies and long-lasting reactivity to the TM after challenge (74). Finally, the full-length and functionally competent envelope glycoprotein expressed in the context of recombinant vaccinia virus priming may expose epitopes important for cell fusion and virus infectivity. LaCasse et al. recently described a “fusion-competent” vaccine that was capable of eliciting broadly neutralizing antibodies against primary HIV-1 isolates (53). It is of interest to determine if one or more of these responses may contribute to the protective immunity elicited by the native full-length envelope vaccines in the prime-and-boost regimen described here.
Immunization with core antigens alone failed to protect against the homologous cloned virus E11S. However, even in the absence of any neutralizing (or env-specific) antibodies, immunized macaques showed significantly reduced viral load compared to controls as early as 2 weeks after infection. The duration of detectable proviral DNA in their PBMC was also reduced, with one animal (macaque 91278) showing only transient infection. These results indicate that immune responses to the core antigens may not protect against infection but may help to control infection, perhaps by facilitating the clearance of virus-infected cells. The mechanism by which this is achieved has yet to be determined. Kent et al. (50) examined a subset of animals in this study and found SIV-specific lymphoproliferative responses in eight of eight animals prior to challenge but no CTL response. CTL response was detected after challenge in a number of animals, including one, macaque 91278, that was immunized with core antigens alone and showed only transient viremia after infection. Thus, the rapid onset and persistent CTL responses induced after infection may play an important role in controlling infection after challenge. It should be noted, however, that CTL responses to a single Gag epitope failed to protect against the same E11S challenge virus (100). Viral load was not determined in that study. It is possible that effective CTL responses to multiple targets, primed by immunization and expanded after exposure, reduce viral load sufficiently, resulting in transient or inapparent infection and thus partial if not complete protection.
Immunization with both envelope and core antigens was highly protective. This was most apparent in animals immunized with these antigens as separate immunogens (group E). Significant protection was observed in these animals against not only the homologous virus E11S but also the uncloned virus upon rechallenge. We reported earlier that immunization with rgp160 alone by the prime-and-boost regimen has only limited efficacy against an intravenous infection by the uncloned virus, especially the non-E11S-type (or variant-type) viruses present in the challenge stock (76). Therefore, it is likely that immune responses to the core antigens may have contributed to controlling infection by both the E11S- and variant-type viruses. This is consistent with the highly conserved nature of the core antigens. Alternatively, prior exposure to the E11S virus, even without resulting in an apparent infection, may have contributed to the protection in group E animals against the uncloned virus upon rechallenge. This possibility cannot be excluded without a direct comparative study. However, it should be noted that we found no sign of infection in these animals by multiple and stringent assays over a period of 8 to 9 months after E11S challenge. Furthermore, complete protection was not achieved against intravenous infection by the uncloned SIVmne in animals immunized only with rgp160, including those previously challenged by and protected from E11S (76). Similarly, three of four animals in group F of the present study became infected by the uncloned virus, even though they were previously protected against E11S. Therefore, complete protection against the uncloned virus observed in animals in group E could not be attributed solely to the potential effects of prior exposure to E11S. Nevertheless, a challenge study with a newly immunized cohort will be needed to confirm the findings of the present study.
It is not clear why protection against the uncloned virus was much less efficient in group F animals than in those in group E, since they were all immunized with both envelope and core antigens, albeit presented in different forms. Several factors could have contributed. First, the quantity of envelope antigens present in the Gag-Pol-Env (pseudovirion) preparations was considerably less (∼3-fold) than that in the mixed immunogens used in group E. Second, the envelope antigens used for groups E and F may have very different compositions and conformations. The surface protein could have shed from the pseudovirion preparations, or its conformation could have been altered, during formulation. The suboptimal responses to the envelope antigens in the group F animals, together with responses to the core antigens, may have been sufficient to protect against E11S infection but not against the uncloned virus. Finally, the lack of protection may also be due to poor responses in individual animals in group F. Recall responses to the last booster immunization in macaques 91282 and 91247 were so short-lived that their antibody titers as determined by one or more assays were significantly lower at the time of rechallenge compared to the E11S challenge. Our results therefore demonstrate that although multicomponent particle vaccines, such as the psuedovirions described here, may have advantages over subunit immunogens, significant problems need to be resolved before such potentials can be realized.
Results from this study have several important implications for the current efforts in HIV-1 vaccine development. First, findings from our present and previous (44, 76, 77) studies demonstrate the feasibility of immunization with recombinant vaccines against a primate lentivirus of moderate complexity and pathogenicity. This is significant in view of the recent findings concerning the safety of live attenuated virus approaches (2, 18, 32). Second, results from the present study (i.e., the comparison between immunization with subunit rgp160 alone versus priming with live recombinant virus and boosting with gp160) point to the advantages of a combination approach such as the prime-and-boost regimen described here. Large-scale clinical trials were recently initiated to evaluate the potential efficacy of subunit vaccines based solely on HIV-1 surface antigens (28, 96). While the efficacy of any vaccine can be demonstrated only by properly designed clinical trials, our studies indicate that immunization with subunit immunogens alone, especially with monomeric surface antigens, may not be the most effective approach. Finally, our results indicate that complex immune mechanisms targeted to multiple antigens may contribute to protection. These results indicate the potential advantage for the inclusion of multiple antigens in a combination immunization strategy for the design of recombinant vaccines against AIDS.
ACKNOWLEDGMENTS
We thank Randy Nolte, LaRene Kuller, and Tom Beck for assistance with veterinary studies, Susan Gallinger, Robin Watanabe, Kia Kornas, Lynda Misher, Walter Knott, Richard Hill, Andre Marozsan, and Anthony Scarzello for expert technical assistance, Bryan Kennedy for flow cytometry analysis, Gail Sylva for help in preparing immunogens, Bruce Travis and Andy Watson for advice on QC-PCR analyses, and Marjorie Domenowske and Andrea Clarke for manuscript preparation.
This work was supported in part by NIH grants AI26503, RR00166, and AI85343 and NIH contract AI65302.
REFERENCES
- 1.Abimiku A G, Franchini G, Tartaglia J, Aldrich K, Myagkikh M, Markham P D, Chong P, Klein M, Kieny M P, Paoletti E, Gallo R C, Robert-Guroff M. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat Med. 1995;1:321–329. doi: 10.1038/nm0495-321. [DOI] [PubMed] [Google Scholar]
- 2.Baba T W, Liska V, Kimani A H, Ray N B, Dailey P J, Penninck D, Bronson R, Greene M F, McClure H M, Martin L N, Ruprecht R M. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- 3.Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo R C, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against highly pathogenic simian immunodeficiency virus SIVmac251 dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benveniste R E, Arthur L O, Tsai C C, Sowder R, Copeland T D, Henderson L E, Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986;60:483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benveniste R E, Raben D, Hill R, Knott W, Drummond J E, Arthur L O, Jahrling P B, Morton W R, Henderson L E, Heidecker G. Molecular characterization and comparison of simian immunodeficiency virus isolates from macaques, mangabeys, and African green monkeys. J Med Primatol. 1989;18:287–303. [PubMed] [Google Scholar]
- 6.Benveniste R E, Hill R W, Eron L J, Csaikl U M, Knott W B, Henderson L E, Sowder R C, Nagashima K, Gonda M A. Characterization of clones of HIV-1 infected HuT78 cells defective in gag gene processing and of SIV clones producing large amounts of envelope glycoprotein. J Med Primatol. 1990;19:351–366. [PubMed] [Google Scholar]
- 7.Benveniste R E, Kuller L, Rodman S T, Hu S-L, Morton W R. Long-term protection of macaques against high-dose type D retrovirus challenge after immunization with recombinant vaccinia virus expressing envelope glycoproteins. J Med Primatol. 1993;22:74–79. [PubMed] [Google Scholar]
- 8.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 9.Berman P W, Murthy K K, Wrin T, Vennari J C, Cobb E K, Eastman D J, Champe M, Nakamura G R, Davison D, Powell M F, Bussiere J, Francis D P, Matthews T, Gregory T J, Obijeski J F. Protection of MN-rgp120-immunized chimpanzees from heterologous infection with a primary isolate of human immunodeficiency virus type 1. J Infect Dis. 1996;173:52–59. doi: 10.1093/infdis/173.1.52. [DOI] [PubMed] [Google Scholar]
- 10.Berman P W, Gray A M, Wrin T, Vennari J C, Eastman D J, Nakamura G R, Francis D P, Gorse G, Schwartz D H. Genetic and immunologic characterization of viruses infecting MN-rgp120-vaccinated volunteers. J Infect Dis. 1997;176:384–397. doi: 10.1086/514055. [DOI] [PubMed] [Google Scholar]
- 11.Boyer J D, Wang B, Ugen K E, Agadjanyan M, Javadian A, Frost P, Dang K, Carrano R A, Ciccarelli R, Coney L, Williams W V, Weiner D B. In vivo protective anti-HIV immune responses in non-human primates through DNA immunization. J Med Primatol. 1996;25:242–250. doi: 10.1111/j.1600-0684.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 12.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 13.Bruck C, Thiriart C, Fabry L, Francotte M, Pala P, Van-Opstal O, Culp J, Rosenberg M, De Wilde M, Heidt P, Heeney J. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzees. Vaccine. 1994;12:1141–1148. doi: 10.1016/0264-410x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 14.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11(Suppl. A):S87–S98. [PubMed] [Google Scholar]
- 15.Buseyne F, McChesney M, Porrot F, Kovarik S, Guy B, Riviere Y. Gag-specific cytotoxic T lymphocytes from human immunodeficiency virus type 1-infected individuals: Gag epitopes are clustered in three regions of the p24gag protein. J Virol. 1993;67:694–702. doi: 10.1128/jvi.67.2.694-702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao H, Kanki P, Sankale J L, Dieng-Sarr A, Mazzara G P, Kalams S A, Korber B, Mboup S, Walker B D. Cytotoxic T-lymphocyte cross-reactivity among different human immunodeficiency virus type 1 clades: implications for vaccine development. J Virol. 1997;71:8615–8623. doi: 10.1128/jvi.71.11.8615-8623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. Weakened SIV vaccine still kills. Science. 1997;278:24–25. doi: 10.1126/science.278.5335.24. [DOI] [PubMed] [Google Scholar]
- 19.Connor R I, Kober B T, Graham B S, Hahn B H, Ho D D, Walker B D, Neumann A U, Vermund S H, Mestecky J, Jackson S, Fenamore E, Cao Y, Gao F, Kalams S, Kunstman K J, McDonald D, McWilliams N, Trkola A, Moore J P, Wolinsky S M. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooney E L, McElrath M J, Corey L, Hu S-L, Collier A C, Arditti D, Hoffman M, Coombs R W, Smith G E, Greenberg P D. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci USA. 1993;90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel M D, Mazzara G P, Simon M A, Sehgal P K, Kodama T, Panicali D L, Desrosiers R C. High-titer immune responses elicited by recombinant vaccinia virus priming and particle boosting are ineffective in preventing virulent SIV infection. AIDS Res Hum Retroviruses. 1994;10:839–851. doi: 10.1089/aid.1994.10.839. [DOI] [PubMed] [Google Scholar]
- 22.Dolin R. Human studies in the development of human immunodeficiency virus vaccines. J Infect Dis. 1995;172:1175–1183. doi: 10.1093/infdis/172.5.1175. [DOI] [PubMed] [Google Scholar]
- 23.Earl P L, Moss B. Mutational analysis of the assembly domain of the HIV-1 envelope glycoprotein. AIDS Res Hum Retroviruses. 1993;9:589–594. doi: 10.1089/aid.1993.9.589. [DOI] [PubMed] [Google Scholar]
- 24.el-Amad Z, Murthy K K, Higgins K, Cobb E K, Haigwood N L, Levy J A, Steimer K S. Resistance of chimpanzees immunized with recombinant gp120SF2 to challenge by HIV-1SF2. AIDS. 1995;9:1313–1322. doi: 10.1097/00002030-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Emini E A, Schleif W A, Quintero J C, Conard P G, Eichberg J W, Vlasuk G P, Lehman E D, Polokoff M A, Schaeffer T F, Schultz L D, Hofmann K J, Lewis J A, Larson V M. Yeast-expressed p55 precursor core protein of human immunodeficiency virus type 1 does not elicit protective immunity in chimpanzees. AIDS Res Hum Retroviruses. 1990;6:1247–1250. doi: 10.1089/aid.1990.6.1247. [DOI] [PubMed] [Google Scholar]
- 26.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Matsushita S, Cobb K E, Jett C M, Eichberg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 27.Evans L A, Thomson-Honnebier G, Steimer K, Paoletti E, Perkus M E, Hollander H, Levy J A. Antibody-dependent cellular cytotoxicity is directed against both the gp120 and gp41 envelope proteins of HIV. AIDS. 1989;3:273–276. doi: 10.1097/00002030-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Francis D P, Gregory T, McElrath M J, Belshe R B, Gorse G J, Migasena S, Kitayaporn D, Pitisuttitham P, Matthews T, Schwartz D H, Berman P W. Advancing AIDSVAX to phase 3. Safety, immunogenicity, and plans for phase 3. AIDS Res Hum Retroviruses. 1998;14(Suppl. 3):S325–S331. [PubMed] [Google Scholar]
- 29.Giavedoni L D, Planelles V, Haigwood N L, Ahmad S, Kluge J D, Marthas M L, Gardner M B, Luciw P A, Yilma T D. Immune response of rhesus macaques to recombinant simian immunodeficiency virus gp130 does not protect from challenge infection. J Virol. 1993;67:577–583. doi: 10.1128/jvi.67.1.577-583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girard M, Kieny M P, Pinter A, Barré-Sinoussi F, Nara P, Kolbe H, Kusumi K, Chaput A, Reinhart T, Muchmore E, Ronco J, Kaczoreck M, Gomard E, Gluckman J C, Fultz P. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gnann J W, Jr, Nelson J A, Oldstone M B. Fine mapping of an immunodominant domain in the transmembrane glycoprotein of human immunodeficiency virus. J Virol. 1987;61:2639–2641. doi: 10.1128/jvi.61.8.2639-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenough T C, Sullivan J L, Desrosiers R C. Declining CD4 T-cell counts in a person infected with nef-deleted HIV-1. N Engl J Med. 1999;340:236–237. doi: 10.1056/NEJM199901213400314. [DOI] [PubMed] [Google Scholar]
- 33.Haffar O, Garrigues J, Travis B M, Moran P A, Zarling J M, Hu S-L. Assembly of nonreplicating human immunodeficiency virus type 1 particles in a recombinant vaccinia virus expression system. J Virol. 1990;64:2653–2659. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haffar O K, Smithgall M D, Moran P A, Travis B M, Zarling J M, Hu S-L. HIV-specific humoral and cellular immunity in rabbits vaccinated with recombinant human immunodeficiency virus-like gag-env particles. Virology. 1991;183:487–495. doi: 10.1016/0042-6822(91)90978-k. [DOI] [PubMed] [Google Scholar]
- 35.Hanson C V. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS Res Hum Retroviruses. 1994;10:645–648. doi: 10.1089/aid.1994.10.645. [DOI] [PubMed] [Google Scholar]
- 36.Haynes B F. HIV vaccines: where we are and where we are going. Lancet. 1996;348:933–937. doi: 10.1016/S0140-6736(96)09339-7. [DOI] [PubMed] [Google Scholar]
- 37.Heidecker G, Muñoz H, Lloyd P, Hodge D, Ruscetti F W, Morton W R, Hu S-L, Benveniste R E. Macaques infected with cloned simian immunodeficiency virus show recurring nef gene alterations. Virology. 1998;249:260–274. doi: 10.1006/viro.1998.9325. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak Jr M, Elkins W R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu S-L, Kosowski S G, Dalrymple J M. Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature. 1986;320:537–540. doi: 10.1038/320537a0. [DOI] [PubMed] [Google Scholar]
- 40.Hu S-L, Zarling J M, Chinn J, Travis B M, Moran P A, Sias J, Kuller L, Morton W R, Heidecker G, Benveniste R E. Protection of macaques against simian AIDS by immunization with a recombinant vaccinia virus expressing the envelope glycoprotein of simian type D retrovirus. Proc Natl Acad Sci USA. 1989;86:7213–7217. doi: 10.1073/pnas.86.18.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu S-L, Travis B M, Garrigues J, Sridhar P, Zarling J M, Eichberg J W, Alpers C E. Processing, assembly and immunogenicity of human immunodeficiency virus (HIV-1) core antigens expressed by recombinant vaccinia virus. Virology. 1990;179:321–329. doi: 10.1016/0042-6822(90)90300-g. [DOI] [PubMed] [Google Scholar]
- 42.Hu S-L, Klaniecki J, Dykers T, Sridhar P, Travis B M. Neutralizing antibodies against HIV-1 BRU and SF2 isolates generated in mice immunized with recombinant vaccinia virus expressing HIV-1 (BRU) envelope glycoproteins and boosted with homologous gp160. AIDS Res Hum Retroviruses. 1991;7:615–620. doi: 10.1089/aid.1991.7.615. [DOI] [PubMed] [Google Scholar]
- 43.Hu S-L, Travis B, Stallard V, Abrams K, Misher L, Moran P, Zarling J M, Langlois A J, Kuller L, Morton W R, Benveniste R. Immune responses to SIVmne envelope glycoproteins protect macaques from homologous SIV infection. AIDS Res Hum Retroviruses. 1992;8:1489–1494. doi: 10.1089/aid.1992.8.1489. [DOI] [PubMed] [Google Scholar]
- 44.Hu S-L, Abrams K, Barber G N, Moran P, Zarling J M, Langlois A J, Kuller L, Morton W R, Benveniste R E. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 45.Hu S-L, Stallard V, Abrams K, Barber G N, Kuller L, Langlois A J, Morton W R, Benveniste R E. Protection of vaccinia-primed macaques against SIVmne infection by combination immunization with recombinant vaccinia virus and SIVmne gp160. J Med Primatol. 1993;22:92–99. [PubMed] [Google Scholar]
- 46.Hu S-L, Klaniecki J, Travis B M, Wrey T, Pennathur S, Montefiori D C, Thompson J L, Agy M B, Kuller L, Morton W R. Immunization with HIV-1 gp160 by the “prime and boost” regimen protects macaques against SHIV HXBc2 challenge. In: Brown F, Burton D, Doherty P, Mekalanos J, Norrby E, editors. Vaccines 97. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. pp. 291–298. [Google Scholar]
- 47.Hu, S.-L. Unpublished data.
- 48.Johnson R P, Trocha A, Buchanan T M, Walker B D. Identification of overlapping HLA class I-restricted cytotoxic T cell epitopes in a conserved region of the human immunodeficiency virus type 1 envelope glycoprotein: definition of minimum epitopes and analysis of the effects of sequence variation. J Exp Med. 1992;175:961–971. doi: 10.1084/jem.175.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalams S A, Johnson R P, Dynan M J, Hartman K E, Harrer T, Harrer E, Trocha A K, Blattner W A, Buchbinder S P, Walker B D. T cell receptor usage and fine specificity of human immunodeficiency virus 1-specific cytotoxic T lymphocyte clones: analysis of quasispecies recognition reveals a dominant response directed against a minor in vivo variant. J Exp Med. 1996;183:1669–1679. doi: 10.1084/jem.183.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kent S J, Hu S-L, Corey L, Morton W R, Greenberg P D. Detection of simian immunodeficiency virus (SIV)-specific CD8+ T cells in macaques protected from SIV challenge by prior SIV subunit vaccination. J Virol. 1996;70:4941–4947. doi: 10.1128/jvi.70.8.4941-4947.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klaniecki J, Dykers T, Travis B, Scmitt R, Wain M, Watson A, Sridhar P, McClure J, Morein B, Ulrich J T, Hu S-L, Lewis J. Cross-neutralizing antibodies in rabbits immunized with HIV-1 gp160 purified from simian cells infected with a recombinant vaccinia virus. AIDS Res Hum Retroviruses. 1991;7:791–798. doi: 10.1089/aid.1991.7.791. [DOI] [PubMed] [Google Scholar]
- 52.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaCasse R A, Follis K E, Trahey M, Scarborough J D, Littman D R, Nunberg J H. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283:357–362. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 54.Langlois A J, Weinhold K J, Matthews T J, Bolognesi D P. In vitro assay for detecting neutralizing and fusion-inhibiting antibodies to SIVmac251. AIDS Res Hum Retroviruses. 1991;7:713–720. doi: 10.1089/aid.1991.7.713. [DOI] [PubMed] [Google Scholar]
- 55.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M E, Lekutis C, Alroy M, Freed D C, Lord C I, Handt L K, Liu M A, Shiver J W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 57.Loomis-Price L D, Cox J H, Mascola J R, VanCott T C, Michael N L, Fouts T R, Redfield R R, Robb M L, Wahren B, Sheppard H W, Birx D L. Correlation between humoral responses to human immunodeficiency virus type 1 envelope and disease progression in early-stage infection. J Infect Dis. 1998;178:1306–1316. doi: 10.1086/314436. [DOI] [PubMed] [Google Scholar]
- 58.Lubeck M D, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, Alipanah S, Murthy S C, Chanda P K, Nigida S M, Jr, Markham P D, Zolla-Pazner S, Steimer K, Wade M, Reitz Jr M S, Arthur L O, Mizutani S, Davis A, Hung P P, Gallo R C, Eichberg J, Robert-Guroff M. Long term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med. 1997;3:651–658. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- 59.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 60.McAdam S, Kaleebu P, Krausa P, Goulder P, French N, Collin B, Blanchard T, Whitworth J, McMichael A, Gotch F. Cross-clade recognition of p55 by cytotoxic T lymphocytes in HIV-1 infection. AIDS. 1998;12:571–579. doi: 10.1097/00002030-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Mills K H G, Page M, Chan W L, Kitchin P, Stott E J, Taffs L F, Jones W, Rose J, Ling C, Silvera P, Corcoran T, Flanagan B, Burny A, Bex F, Delchambre M, Van Opstal O, Fabry L, Thiriart C, Delers A, DeWilde M, Bruck C. Protection against SIV infection in macaques by immunization with inactivated virus from the BK28 molecular clone, but not with BK28-derived recombinant env and gag proteins. J Med Primatol. 1992;21:50–58. [PubMed] [Google Scholar]
- 62.Montefiori D C, Robinson W E, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montefiori D C. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Semin Immunopathol. 1997;18:371–390. doi: 10.1007/BF00813504. [DOI] [PubMed] [Google Scholar]
- 64.Mooij P, van der Kolk M, Bogers W M, ten Haaft P J, Van Der Meide P, Almond N, Stott J, Deschamps M, Labbe D, Momin P, Voss G, Von Hoegen P, Bruck C, Heeney J L. A clinically relevant HIV-1 subunit vaccine protects rhesus macaques from in vivo passaged simian-human immunodeficiency virus infection. AIDS. 1998;12:F15–F22. doi: 10.1097/00002030-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991;252:1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- 67.Mossman S P, Bex F, Berglund P, Arthos J, O’Neil S P, Riley D, Maul D H, Bruck C, Momin P, Burny A, Fultz P N, Mullins J I, Liljestrom P, Hoover E A. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki forest virus gp160 vaccine and by gp120 subunit vaccine. J Virol. 1996;70:1953–1960. doi: 10.1128/jvi.70.3.1953-1960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Myagkikh M, Alipanah S, Markham P D, Tartaglia J, Paoletti E, Gallo R C, Franchini G, Robert-Guroff M. Multiple immunizations with attenuated poxvirus HIV type 2 recombinants and subunit boosts required for protection of rhesus macaques. AIDS Res Hum Retroviruses. 1996;12:985–992. doi: 10.1089/aid.1996.12.985. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura Y, Kameoka M, Tobiume M, Kaya M, Ohki K, Yamada T, Ikuta K. A chain section containing epitopes for cytotoxic T, B and helper T cells within a highly conserved region found in the human immunodeficiency virus type 1 Gag protein. Vaccine. 1997;15:489–496. doi: 10.1016/s0264-410x(96)00224-1. [DOI] [PubMed] [Google Scholar]
- 72.Oldstone M B, Tishon A, Lewicki H, Dyson H J, Feher V A, Assa-Munt N, Wright P E. Mapping the anatomy of the immunodominant domain of the human immunodeficiency virus gp41 transmembrane protein: peptide conformation analysis using monoclonal antibodies and proton nuclear magnetic resonance spectroscopy. J Virol. 1991;65:1727–1734. doi: 10.1128/jvi.65.4.1727-1734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petry H, Stahl-Henning C, Dittmer U, Jones D, Farrar G, Watchter H, Nisslein T, Jurkiewicz E, Hunsmann G, Luke W. A subunit vaccine consisting of gp130 oligomers but not gp130 monomers protects rhesus macaques against productive infection with SIV mac32H. AIDS. 1998;12:329–330. [PubMed] [Google Scholar]
- 74.Petry H, Dittmer U, Jones D, Farrar G, Wachter H, Fuchs D, Nisslein T, Jurkiewicz E, Hunsmann G, Stahl-Henning C, Luke W. Prechallenge high neutralizing antibodies and long-lasting immune reactivity to gp41 correlate with protection of rhesus monkeys against productive simian immunodeficiency virus infection or disease development. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:441–450. doi: 10.1097/00042560-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 75.Polacino P S, Liang H A, Firpo E J, Clark E. T-cell activation influences initial DNA synthesis of simian immunodeficiency virus in resting T lymphocytes from macaques. J Virol. 1993;67:7008–7016. doi: 10.1128/jvi.67.12.7008-7016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polacino P, Stallard V, Klaniecki J E, Montefiori D C, Langlois A J, Richardson B A, Overbaugh J, Morton W R, Benveniste R E, Hu S-L. Limited breadth of the protective immunity elicited by simian immunodeficiency virus SIVmne gp160 vaccines in a combination immunization regimen. J Virol. 1999;73:618–630. doi: 10.1128/jvi.73.1.618-630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Polacino P, Stallard V, Montefiori D C, Brown C R, Richardson B A, Morton W R, Benveniste R E, Hu S-L. Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant SIVmne gp160 vaccines. J Virol. 1999;73:3134–3146. doi: 10.1128/jvi.73.4.3134-3146.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poumbourios P, Wilson K A, Center R J, El Ahmar W, Kemp B E. Human immunodeficiency virus type 1 envelope glycoprotein oligomerization requires the gp41 amphipathic alpha-helical/leucine zipper-like sequence. J Virol. 1997;71:2041–2049. doi: 10.1128/jvi.71.3.2041-2049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richardson T M, Jr, Stryjewski B L, Broder C C, Hoxie J A, Mascola J R, Earl P L, Doms R W. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J Virol. 1996;70:753–762. doi: 10.1128/jvi.70.2.753-762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robert-Guroff M, Kaur H, Patterson L J, Leno M, Conley A J, McKenna P M, Markham P D, Richardson E, Aldrich K, Arora K, Murty L, Carter L, Zolla-Pazner S, Sinangil F. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J Virol. 1998;72:10275–10280. doi: 10.1128/jvi.72.12.10275-10280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robinson W E, Jr, Kawamura T, Lake D, Masuho Y, Mitchell W M, Hersh E M. Antibodies to the primary immunodominant domain of human immunodeficiency virus type 1 (HIV-1) glycoprotein gp41 enhance HIV-1 infection in vitro. J Virol. 1990;64:5301–5305. doi: 10.1128/jvi.64.11.5301-5305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson W E, Jr, Gorny M W, Xu J-Y, Mitchell W M, Zolla-Pazner S. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol. 1991;65:4169–4176. doi: 10.1128/jvi.65.8.4169-4176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rowland-Jones S, Tan R, McMichael A. Role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 84.Safrit J T, Andrews C A, Zhu T, Ho D D, Koup R A. Characterization of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope. J Exp Med. 1994;179:463–472. doi: 10.1084/jem.179.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Safrit J T, Koup R A. The immunology of primary HIV infection: which immune responses control HIV replication? Curr Opin Immunol. 1995;7:456–461. doi: 10.1016/0952-7915(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 86.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schrier R D, Gnann J W, Jr, Langlois A J, Shriver K, Nelson J A, Oldstone M B. B- and T-lymphocyte responses to an immunodominant epitope of human immunodeficiency virus. J Virol. 1988;62:2531–2536. doi: 10.1128/jvi.62.8.2531-2536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen L, Chen Z W, Miller M D, Stallard V, Mazzara G P, Panicali D L, Letvin N L. Recombinant virus vaccine-induced SIV-specific CD8+ cytotoxic T lymphocytes. Science. 1991;252:440–443. doi: 10.1126/science.1708168. [DOI] [PubMed] [Google Scholar]
- 89.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 90.Siegel F, Norley S, Hartung S, Kurth R. B-cell epitope mapping of the entire SIVagm envelope glycoprotein including fine mapping of immunogenic regions. J Acquir Immune Defic Syndr. 1992;5:583–590. [PubMed] [Google Scholar]
- 91.Stott E J, Almond N, Kent K, Walker B, Hull R, Rose J, Silvera P, Sangster R, Corcoran T, Lines J, Silvera K, Luciw P, Murphy-Corb M, Momin P, Bruck C. Evaluation of a candidate human immunodeficiency virus type 1 (HIV-1) vaccine in macaques: effect of vaccination with HIV-1 gp120 on subsequent challenge with heterologous simian immunodeficiency virus-HIV-1 chimeric virus. J Gen Virol. 1998;79:423–432. doi: 10.1099/0022-1317-79-3-423. [DOI] [PubMed] [Google Scholar]
- 92.Tanchou V, Sgro-Serpente P, Durand H, Aubertin A M, Dormont D, Venet A, Benarous R. B-cell continuous epitopes of the SIVmac-251 envelope protein in experimentally infected macaques. Res Virol. 1995;146:19–32. doi: 10.1016/0923-2516(96)80586-3. [DOI] [PubMed] [Google Scholar]
- 93.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tyler D S, Stanley S D, Zolla-Pazner S, Gorny M K, Shadduck P P, Langlois A J, Matthews T J, Bolognesi D P, Palker T J, Weinhold K J. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J Immunol. 1990;145:3276–3282. [PubMed] [Google Scholar]
- 95.Voss G, Letvin N L. Definition of human immunodeficiency virus type 1 gp120 and gp41 cytotoxic T-lymphocyte epitopes and their restricting major histocompatibility complex class I alleles in simian-human immunodeficiency virus-infected rhesus monkeys. J Virol. 1996;70:7335–7340. doi: 10.1128/jvi.70.10.7335-7340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wadman M. NIH institute to work with trial of AIDS vaccine, despite concerns. Nature. 1998;394:818. doi: 10.1038/29604. [DOI] [PubMed] [Google Scholar]
- 97.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P R, Hu S-L, Haigwood N L. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 99.Xu J Y, Gorny M K, Palker T, Karwowska S, Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol. 1991;65:4832–4838. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yasutomi Y, Koenig S, Woods R M, Madsen J, Wassef N M, Alving C R, Klein H J, Nolan T E, Boots L J, Kessler J A. A vaccine-elicited, single viral epitope-specific cytotoxic T lymphocyte response does not protect against intravenous, cell-free simian immunodeficiency virus challenge. J Virol. 1995;69:2279–2284. doi: 10.1128/jvi.69.4.2279-2284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zarling J M, Eichberg J W, Moran P A, McClure J, Sridhar P, Hu S-L. Proliferative and cytotoxic T cells to AIDS virus glycoproteins in chimpanzees immunized with a recombinant vaccinia virus expressing AIDS virus envelope glycoproteins. J Immunol. 1987;139:988–990. [PubMed] [Google Scholar]