Abstract
Background
The perfect repair of damaged skin has always been a constant goal for scientists; however, the repair and reconstruction of skin is still a major problem and challenge in injury and burns medicine. Human amniotic membrane (hAM), with its good mechanical properties and anti‐inflammatory, antioxidant and antimicrobial benefits, containing growth factors that promote wound healing, has evolved over the last few decades from simple skin sheets to high‐tech dressings, such as being made into nanocomposites, hydrogels, powders, and electrostatically spun scaffolds. This paper aims to explore the historical development, applications, trends, and research hotspots of hAM in wound healing.
Methods
We examined 2660 publications indexed in the Web of Science Core Collection (WoSCC) from January 1, 1975 to July 12, 2023. Utilizing bibliometric methods, we employed VOSviewer, CiteSpace, and R‐bibliometrix to characterize general information, identify development trends, and highlight research hotspots. Subsequently, we identified a collection of high‐quality English articles focusing on the roles of human amniotic epithelial stem cells (hAESCs), human amniotic mesenchymal stem cells (hAMSCs), and amniotic membrane (AM) scaffolds in regenerative medicine and tissue engineering.
Results
Bibliometric analysis identified Udice–French Research Universities as the most productive affiliation and Tseng S.C.G. as the most prolific author. Keyword analysis, historical direct quotations network, and thematic analysis helped us review the historical and major themes in this field. Our examination included the knowledge structure, global status, trends, and research hotspots regarding the application of hAM in wound healing. Our findings indicate that contemporary research emphasizes the preparation and application of products derived from hAM. Notably, both hAM and the cells isolated from it – hADSCs and hAESCs are prominent and promising areas of research in regenerative medicine and tissue engineering.
Conclusion
This research delivers a comprehensive understanding of the knowledge frameworks, global dynamics, emerging patterns, and primary research foci in the realm of hAM applications for wound healing. The field is rapidly evolving, and our findings offer valuable insights for researchers. Future research outcomes are anticipated to be applied in clinical practice, enhancing methods for disease prevention, diagnosis, and treatment.
Keywords: amniotic membrane, bibliometrics, tissue engineering, wound healing
1. INTRODUCTION
The human amniotic membrane (hAM) is derived from the inner layer of the placentas. 1 It is very strong and elastic, which allows it to resist the gradual elongation of the embryo, and both internal and external trauma. AM is a semi‐transparent membrane with a thickness ranging from 0.02 to 0.05 mm. The hAM consists of five layers. 2 The amniotic epithelial layer contains an enormous number of human amniotic epithelial stem cells (hAESCs), which are known for their remarkable paracrine function and stem cell characteristics. These cells have been studied in several areas of wound healing, especially in diabetic wounds. 3 The basement membrane layer consists mainly of reticular fibers, with abundant amounts of glycoproteins and collagen fibers which form and maintain its structural and functional integrity. 4 The amniotic basement membrane has been shown to be crucial for ocular surface reconstruction in various studies, probably due to the similarity of its composition to the corneal basement membrane. 5 The compact layer is soft in texture and can be changed with increasing tension. 6 The fibroblast layer is the main site of origin of human amniotic mesenchymal stem cells (hAMSCs), and it is also the thickest layer of the five‐layer structure. The outermost layer, the sponge layer, contains a collagen fibril network composed mainly of type III collagen, which facilitates the preservation of the natural ECM structure. 7 The sponge layer is loosely attached to the chorion so that the hAM can be easily and bluntly detached.
In addition, hAM is inexpensive and easily accessible for most patients. The process of obtaining it is straightforward and virtually unrestricted, eliminating the need for establishing large‐scale tissue banks. 8 Secondly, hAM has no blood supply of its own, which underpins the widespread use of hAM as a dressing to promote wound healing, while its necessary nutrients are usually sourced from the surrounding environment, which ensures that hAM grafts in wounds do not transmit blood‐borne diseases. 9 Thirdly, hAM has many properties as a natural wound healing dressing, such as anti‐adhesion, strong antimicrobial properties, pain relief, promotion of epithelialization, and non‐immunogenicity. 10 Wound healing has always been a complex and dynamic problem to be solved perfectly, and it is closely related to the processes of cell proliferation, inflammation, and scar reconstruction after injury. hAM contains growth factors, interleukins (IL), and tissue inhibitors of metalloproteinases (TIMPs), and has been shown the potential to positively influence unique key physiological processes closely related to wound healing. 11 The hAM has inspired many researchers to use it as a biomaterial dressing due to its proprietary histological structure and properties. They not only conducted more in‐depth research into the properties of the hAM, but also made it more suitable for certain specific wounds by altering its structure. In summary, based on these characteristics, the hAM can promote more perfect wound healing.
For more than 100 years, hAM has been used to contribute wound healing. It was used in a highly pioneering way by Davis in 1910 for wound management. Subsequently, it was found to be a useful discovery of surgical dressings. 7 A growing interest in hAM has emerged since the 1960s, and many scholars have found structural similarities between hAM and the corneal basement membrane. As a result, hAM has been used to restore the surface structure and function of the eyes, including the treatment of glaucoma, cellular carriers of cornea‐deficient‐related diseases, and conjunctival reconstruction. 12 , 13 , 14 , 15 Until now, hAM has been widely studied and applied in ocular reconstruction. 16 Subsequently, other scientists have explored the possibility of using hAM to effectively reconstruct the oral cavity, 17 bladder, 18 and cervix 19 ; repair urothelial tissue 20 ; tympanoplasty 21 ; arthroplasty 22 ; and so forth. In addition, the resolution of difficult‐to‐heal wounds is a world‐standard problem, and hAM has been used experimentally to treat such conditions. 23 Different types of hAM products have also been successfully developed and applied for the healing of chronic cutaneous wounds, including diabetes, 24 venous leg ulcers, 25 burns 26 , and various disorders of the ocular surface. 12 Until now, hAM has been recognized as a good material for wound healing and has a variety of clinical applications covering ocular surface reconstruction, dermal applications, regenerative medicine, and tissue engineering.
Although it has been studied for a long period, the effectiveness on wound healing varies greatly due to differences in storage methods and delivery, as well as subregional differences in hAM. In addition, in recent years, emerging technologies and materials have made it possible to be utilized as an excellent resource for regenerative medicine and tissue engineering. To get a fuller historical trajectory of hAM's development and the different effects it has had on wound healing in the 21st century by combining with new materials and technologies, we have conducted the following review.
2. METHODS
2.1. Data source and search strategy
For identifying the application of hAM in wound healing in the past few decades, the Web of Science Core Collection (WoSCC) was utilized to comprehensively search for publications in this field. And to eliminate deviations resulting from the update of the database, we conducted and completed the retrieval on July 12, 2023. The retrieval strategy was ((TS = Amnion) OR (TS = Amniotic Membrane) OR (TS = Amnia)) AND ((TS = Wound Healing) OR (TS = Regenerat∗) OR (TS = Tissue Engineer∗) OR (TS = Repair)). All time points were reserved, but only research articles and reviews were retrieved. We downloaded information for 2660 relevant articles as TXT files. Subsequently, all downloaded records were imported into bibliometric tools for statistical analysis and visualization. Based on research trends and hotspots, we found several high‐quality articles in English focusing on the roles played by hAESCs, hAMSCs, and hAM scaffolds in regenerative medicine and tissue engineering, respectively.
2.2. Statistical analysis
R version 4.2.0 was used to analyze and visualize the literature. 27 The modeling and visualization process, based on Java applications, is implemented in CiteSpace 28 and VOSviewer, 29 and by visualizing the salient trends and key points in the network, we can discuss the practical implications of this work to identify some of the challenges and opportunities for future research. We use a series of bibliometric indicators to conduct overview analysis including country analysis, affiliation analysis, author analysis, source analysis, article analysis, historical direct citation analysis, reference analysis, keyword analysis, and thematic analysis to assess and summarize the developing trends and hotspots in the applications of hAM in wound healing research field. For the inferential analysis, Braford's Law 30 was used to identify the core cluster of sources. In the authors' analysis, Lotka's Law 31 can determine the core cluster of sources. In addition, the H Index can assess the importance, significance, and wider impact of authors' cumulative research contributions. 32 Finally, synthesizing the results of article analysis, especially keyword analysis and thematic analysis, combined with historical citation networks, we analyzed and summarized the trends and hot spots related to hAM in wound healing. Afterward, some high‐quality literature was identified and discussed based on relevant trends and hotspots.
3. RESULTS
3.1. hAM is attracting increasing interest in promoting wound healing since 2002
In the current search, we ultimately required 2660 documents from 830 sources by 10801 authors with 75537 references from WoSCC from January 1, 1975 to July 12, 2023. According to Figure 1, although the number of publications has sometimes risen and sometimes fallen, the overall trend has continued to increase distinctly. The amount of production before 2000 shows no obvious change until 2001, when the number of publications increased to more than 25. Since then, researchers have become increasingly interested in the study of hAM. The explosive growth began in 2002 and peaked in 2020, suggesting that hAM has clinical significance and development potential. U.S. Food and Drug Administration approval of processed hAM for ocular surface reconstruction may be responsible for this outbreak, 33 and after that there has been an increasing number of developments regarding commercial‐derived products of hAM. 34 Additionally, the average annual citations for hAM wound healing from 1975 to 2023 are displayed in Figure S1(A) and we can see from the graph that the number of citations reaches its highest in 1989 and 2001, respectively. Besides, a three‐field plot presented an overview of key references cited by various key authors with multiple keywords in Figure S1(B).
FIGURE 1.
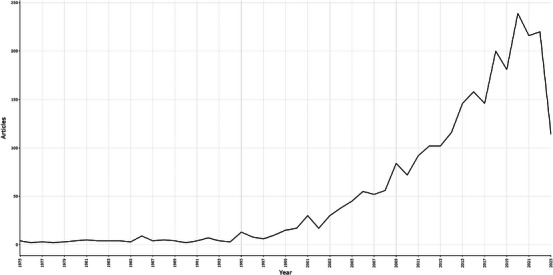
An annual number of publications on human amniotic membrane (hAM) in wound healing globally from 1975 to 2023. The number of papers in this area peaked in 2020.
3.2. In hAM for wound healing research, the U.S., and China have the most attention
Based on Table 1, the top 20 countries most prolific for hAM applications in trauma are the United States (n = 605, 22.7%), China (n = 378, 14.2%), IRAN (n = 164, 6.2%), and Germany (n = 164, 6.2%). However, although the USA had both the highest single‐country publications (SCP, N = 496) and multiple‐country publications (MCPs, N = 91), the ratio of MCPs (15.5%) was quite low, much lower than United Kingdom (40.6%). In addition, the countries’ collaboration map is portrayed in (Figure 2A). In the red line, two countries are collaborating. The darker the color, the more documents are published in the country. As we can see from the map, international partnerships are strongest in the United States. Thus, the USA was recognized to be the most productive and cooperative country in the study of the applications of hAM in wound healing. In (Figure 2B), on this histogram, we can see the top 20 countries/regions with the most collaborations, and the number of multi‐country publications (MCPs) and single‐country publications (SCPs) based on the country of the author. Additionally, as was shown in Figure S2(A), which illustrated the top twenty most cited countries, USA (N = 21981, China (N = 7574), Japan (N = 6275), the United Kingdom (N = 5225), and Germany (N = 4640) rank in the top five. The influence of the United States was clear, cited three times more often than China (ranked 2). In addition, the total citations (TCs) and the average article citations (AACs) of the top 20 most cited countries were demonstrated clearly. The USA and China have remained steadily in the top two positions in TCs, but the average article citations lag far behind, especially China, which has dropped to 17th place (n = 20.0), and the AAC of the USA is also only in the seventh place (n = 36.3). In contrast, Singapore ranked 15th in total cited countries but first in the AAC, which represents the high quality of articles from Singapore.
TABLE 1.
Top 20 countries for hAM research in wound healing.
| Rank | Country | Publications | Proportion of publications (%) | SCP | MCP | Proportion of MCP (%) |
|---|---|---|---|---|---|---|
| 1 | USA | 605 | 22.74% | 508 | 97 | 22.74% |
| 2 | CHINA | 378 | 14.21% | 340 | 38 | 14.21% |
| 3 | IRAN | 164 | 6.17% | 127 | 37 | 6.17% |
| 4 | JAPAN | 133 | 5.00% | 107 | 26 | 5.00% |
| 5 | INDIA | 132 | 4.96% | 117 | 15 | 4.96% |
| 6 | ITALY | 125 | 4.70% | 93 | 32 | 4.70% |
| 7 | UNITED KINGDOM | 125 | 4.70% | 77 | 48 | 4.70% |
| 8 | GERMANY | 115 | 4.32% | 86 | 29 | 4.32% |
| 9 | KOREA | 101 | 3.80% | 88 | 13 | 3.80% |
| 10 | AUSTRALIA | 65 | 2.44% | 49 | 16 | 2.44% |
| 11 | FRANCE | 65 | 2.44% | 53 | 12 | 2.44% |
| 12 | TURKEY | 65 | 2.44% | 61 | 4 | 2.44% |
| 13 | SPAIN | 58 | 2.18% | 48 | 10 | 2.18% |
| 14 | BRAZIL | 57 | 2.14% | 45 | 12 | 2.14% |
| 15 | CANADA | 33 | 1.24% | 27 | 6 | 1.24% |
| 16 | SWITZERLAND | 33 | 1.24% | 17 | 16 | 1.24% |
| 17 | AUSTRIA | 30 | 1.13% | 21 | 9 | 1.13% |
| 18 | POLAND | 28 | 1.05% | 24 | 4 | 1.05% |
| 19 | MALAYSIA | 27 | 1.02% | 23 | 4 | 1.02% |
| 20 | INDONESIA | 22 | 0.83% | 20 | 2 | 0.83% |
Abbreviations: hAM, human amniotic membrane; MCP, multiple country publications; SCP, single country publications.
FIGURE 2.
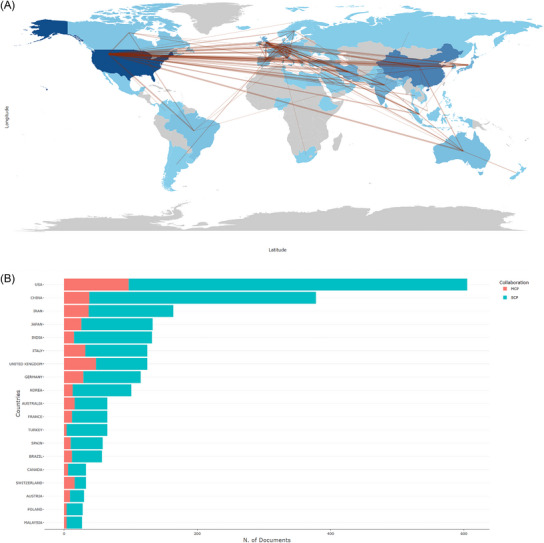
Collaboration and production of countries/regions. The USA is the leading countries. (A) Countries/Regions collaboration and production world map for the field of hAM in wound healing research. The blue color intensity represents proportionally the number of publications. The red lines refer to the collaborations between countries/regions, and the thickness of the lines is proportional to the strength of global collaborations. (B) Top 20 countries/regions collaboration histogram in the field of hAM in wound healing research. hAM, human amniotic membrane; MCP, multiple‐country publications; SCP, single‐country publications.
3.3. Udice–French Research University had the most achievements in the field of hAM in wound healing
In Figure S2(B), the 20 most relevant affiliations are shown. Udice–French Research University leads the way in terms of publications (N = 124), followed by Shahid Beheshti University of Medical Sciences (N = 88), University College London (N = 87), Harvard University (N = 82), and University of London (N = 79).
3.4. Tseng S.C.G. contributed a predominant influence to the research related to hAM in wound healing
The discovery of influential and prolific authors can help researchers learn more about active research teams, gain a more intuitive overview of developments in this research field, and establish direct collaborations with potential research teams. Totally, 10801 authors contributed to the research related to hAM repair trauma since 1975. The top 20 most productive authors for this study were demonstrated in Figure S3(A). We discovered that Tseng S.C.G. had the highest publications (N = 52), followed by Parolini O. (N = 29), Kinoshita S. (N = 23), Nakamura T. (N = 23) and Yoshida T. (N = 21). Figure S3(B) shows the authors' local citations, which showed that Tseng S.C.G. (N = 997), Niknejad H. (N = 448), Kim J.C. (N = 421), Parolini O. (N = 386), and Selfalian H.A.M. (N = 382) ranked the top five. The h‐index is used to evaluate the impact level of an author, visualized in Figure S3(C). We discovered that Tseng S.C.G. has the highest H‐index (N = 33), well ahead of the second place. According to Lotka's law (Figure S3D), only 20 percent of authors published more than one article in the field. Figure S3(E) shows the production of the most productive authors from 1995 to 2023. Generally speaking, author Tseng S.C.G., who ranked 1st with 52 publications, also had the highest LCs (n = 997) and H‐index (n = 33). His research spans from 1995 to 2020, with six articles published in 2001. However, the most locally cited publication of Tseng S.C.G. was in 1995, but before 1995, research on hAM had stagnated for many years. Using transplanted hAM as an effective scaffold for the restoration of ocular surface function in a rabbit model, Tseng S.C.G. et al. provide valuable insight for future research. 35 So, it is reasonable to assume that Tseng S.C.G. contributed a predominant influence to the research related to hAM in wound healing. 36 , 37 , 38
3.5. The journal of CORNEA is an important access to acquire cutting‐edge information on hAM for wound healing research
The top 20 most productive sources for the research related to hAM repair trauma were demonstrated in Figure S4(A), which showed that CORNEA ranked first with 67 publications, followed by INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE (N = 46) and WOUNDS‐A COMPENDIUM OF CLINICAL RESEARCH AND PRACT (N = 39). Moreover, Figure S4(B) presents various sources, INVESTIGATIVE OPHTHALMOLOGY&VISUAL SCIENCE had the highest LCs (N = 4750), followed by BIOMATERIALS (N = 3349) and CORNEA (N = 3194). In addition, the top 20 sources with the highest H‐index are depicted in Figure S4(C). According to Bradford's law, we finally obtained 32 core sources as shown in (Figure 3A), which demonstrates that one‐third of the articles in this field come from 3.85 percent of the total sources. Ultimately, the top five most productive journals’ production growth over time was demonstrated in (Figure 3B), which clearly showed the cumulating trend of occurrences of these journals. CORNEA has been leading the publications in this field since 1994, compared to others. Based on the above data, we determined that the percentage of hAM in wound healing is highest in ophthalmology.
FIGURE 3.
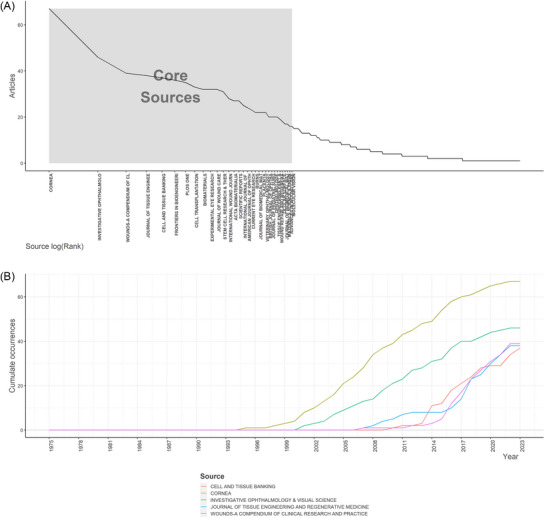
Source analysis suggests that CORNEA is the most impact journal. (A) Source analysis by Bradford's law for the field of human amniotic membrane (hAM) in wound healing research. (B) Top five most relevant sources’ dynamic growth curves over time for the field of hAM in wound healing.
3.6. Relationship between highly influential literature clusters and historical evolution
Table 2 and Figure S5(A) show the most relevant articles in this field globally, while Table 3 and Figure S5(B) present the citation status of local articles. According to the Normalized TCs, we found that the article published in PHYSIOLOGICAL by Gerald Gimpl in 2001 had the highest GCs (N = 2141) with the second highest GCs per year (N = 93.09). 39 Then the article by Robert G. Frykberg from Advances in Wound Care in 2015 ranked 2nd with 1032 GCs and first with 114.67 TCs per year. 40 This article highlights the urgent need to develop new treatments for chronic wounds. Following closely the article from the Journal of Wound Repair and Regeneration by Gregory S. Schultz in 2009 41 acquired 726 TCs and 48.40 TCs per year. It discusses the interaction and therapeutic mechanisms of extracellular matrix and growth factors in difficult‐to‐heal wounds in detail. These crucial articles provided a relatively definite molecular mechanism and laid the substantial foundation for subsequent extensive research and application of hAM in this field. Additionally, in (Figure 4A), all the papers were coupled into red and blue clusters measured by references in a four‐quadrant diagram.
TABLE 2.
The 20 most popular articles on hAM in wound healing research all around the world.
| Rank | Title | Author | Journal | Year | TCs | TC per Year |
|---|---|---|---|---|---|---|
| 1 | The oxytocin receptor system: structure, function, and regulation | GIMPL G | PHYSIOL REV | 2001 | 2141 | 93.09 |
| 2 | Challenges in the Treatment of Chronic Wounds. | FRYKBERG RG | ADV WOUND CARE | 2015 | 1032 | 114.67 |
| 3 | Interactions between extracellular matrix and growth factors in wound healing | SCHULTZ GS | WOUND REPAIR REGEN | 2009 | 726 | 48.40 |
| 4 | Whole‐organ tissue engineering: decellularization and recellularization of three‐dimensional matrix scaffolds | BADYLAK SF | ANNU REV BIOMED ENG | 2011 | 690 | 53.08 |
| 5 | Advancing biomaterials of human origin for tissue engineering | CHEN FM | PROG POLYM SCI | 2016 | 610 | 76.25 |
| 6 | Antigenicity and Immunogenicity of Collagen | LYNN AK | J BIOMED MATER RES B | 2004 | 529 | 26.45 |
| 7 | Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. | KIM JC | CORNEA | 1995 | 526 | 18.14 |
| 8 | Properties of the amniotic membrane for potential use in tissue engineering | NIKNEJAD H | EUR CELLS MATER | 2008 | 525 | 32.81 |
| 9 | Concise Review: Role of Mesenchymal Stem Cells in Wound Repair | MAXSON S | STEM CELL TRANSL MED | 2012 | 524 | 43.67 |
| 10 | Corneal transplantation | TAN DTH | LANCET | 2012 | 507 | 42.25 |
| 11 | Growth factor mRNA and protein in preserved human amniotic membrane | KOIZUMI N | CURR EYE RES | 2000 | 473 | 19.71 |
| 12 | Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature‐responsive cell culture surface. | NISHIDA K | TRANSPLANTATION | 2004 | 447 | 22.35 |
| 13 | Suppression of transforming growth factor‐beta isoforms, TGF‐beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix | TSENG SCG | J CELL PHYSIOL | 1999 | 395 | 15.80 |
| 14 | Identification and characterization of limbal stem cells. | SCHLOTZER‐SCHREHARDT U | EXP EYE RES | 2005 | 358 | 18.84 |
| 15 | Biomechanics and wound healing in the cornea | DUPPS WJ | EXP EYE RES | 2006 | 353 | 19.61 |
| 16 | Advances in Skin Regeneration Using Tissue Engineering | Komal Vig | Int J Mol Sci | 2017 | 350 | 50.00 |
| 17 | Hyaluronic Acid in Inflammation and Tissue Regeneration | Małgorzata Litwiniuk | WOUNDS | 2016 | 316 | 39.50 |
| 18 | Stem cells: a revolution in therapeutics‐recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. | MIMEAULT M | CLIN PHARMACOL THER | 2007 | 316 | 18.59 |
| 19 | Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. | SHIMAZAKI J | OPHTHALMOLOGY | 1997 | 315 | 11.67 |
| 20 | Term Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitro | ALVIANO F | BMC DEV BIOL | 2007 | 308 | 18.12 |
Abbreviation: TCs, total citations.
TABLE 3.
Top 20 most locally cited articles.
| Rank | Title | Journal | Author | Year | LCs | GCs | LCs/GCs Ratio (%) |
|---|---|---|---|---|---|---|---|
| 1 | Properties of the amniotic membrane for potential use in tissue engineering | EUR CELLS MATER | NIKNEJAD H | 2008 | 306 | 525 | 58.29% |
| 2 | Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas | CORNEA | KIM JC | 1995 | 186 | 526 | 35.36% |
| 3 | The potential of amniotic membrane/amnion‐derived cells for regeneration of various tissues | J PHARMACOL SCI | TODA A | 2007 | 118 | 252 | 46.83% |
| 4 | Amniotic membrane transplantation for ocular surface reconstruction | BRIT J OPHTHALMOL | AZUARA‐BLANCO A | 1999 | 116 | 288 | 40.28% |
| 5 | Amniotic membrane: from structure and functions to clinical applications | CELL TISSUE RES | MAMEDE AC | 2012 | 115 | 224 | 51.34% |
| 6 | Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn | EXP EYE RES | KIM JS | 2000 | 109 | 252 | 43.25% |
| 7 | Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing | INT WOUND J | KOOB TJ | 2013 | 107 | 190 | 56.32% |
| 8 | A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers | INT WOUND J | ZELEN CM | 2013 | 104 | 165 | 63.03% |
| 9 | Preservation, sterilization and de‐epithelialization of human amniotic membrane for use in ocular surface reconstruction | BIOMATERIALS | RIAU AK | 2010 | 97 | 153 | 63.40% |
| 10 | Production of an acellular amniotic membrane matrix for use in tissue engineering | TISSUE ENG | WILSHAW SP | 2006 | 95 | 176 | 53.98% |
| 11 | Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns | OPHTHALMOLOGY | SHIMAZAKI J | 1997 | 92 | 315 | 29.21% |
| 12 | Suppression of interleukin 1 and interleukin 1 in human limbal epithelial cells cultured on the amniotic membrane stromal matrix | BRIT J OPHTHALMOL | SOLOMON A | 2001 | 87 | 212 | 41.04% |
| 13 | Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting‐a review. | CELL TISSUE BANK | JIRSOVA K | 2017 | 84 | 164 | 51.22% |
| 14 | Useofamnioticmembranetransplantationinthetreatmentof venous legulcers | WOUND REPAIR REGEN | MERMET I | 2007 | 83 | 133 | 62.41% |
| 15 | Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration | J BIOMED MATER RES B | KOOB TJ | 2014 | 82 | 133 | 61.65% |
| 16 | Amniotic membrane transplantation in the human eye | DTSCH ARZTEBL INT | MELLER D | 2011 | 75 | 138 | 54.35% |
| 17 | Antibacterial properties of human amniotic membranes | PLACENTA | TALMI YP | 1991 | 73 | 170 | 42.94% |
| 18 | Ex Vivo Expansion of Limbal Epithelial Stem Cells: Amniotic Membrane Serving as a Stem Cell Niche | SURV OPHTHALMOL | GRUETERICH M | 2003 | 73 | 206 | 35.44% |
| 19 | Human amnion as an adjunct in wound healing. | LANCET | FAULK WP | 1980 | 66 | 153 | 43.14% |
| 20 | Human amniotic membrane: a versatile wound dressing | CAN MED ASSOC J | GRUSS JS | 1978 | 56 | 108 | 58.33% |
Abbreviations: GCs, global citations; LCs, local citations.
FIGURE 4.
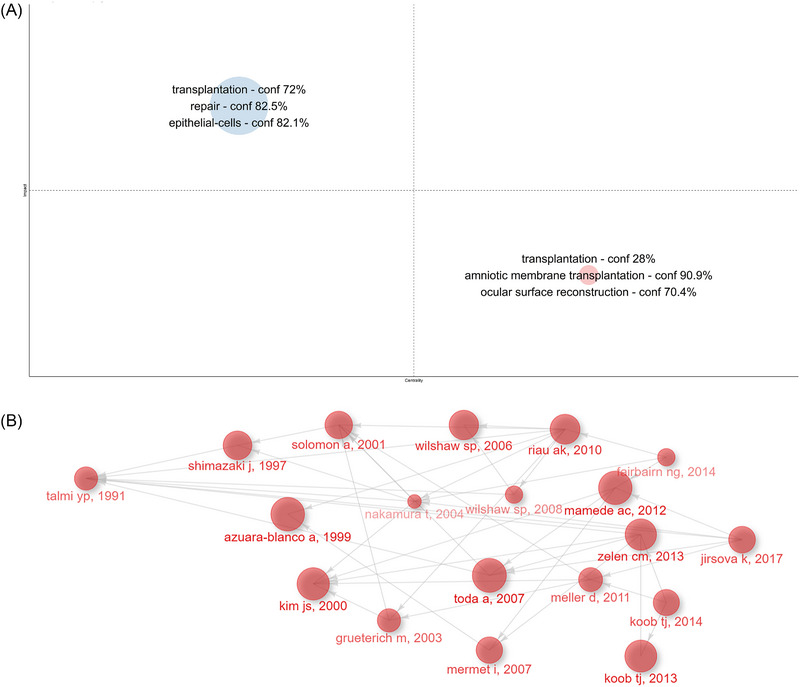
According to document Analysis, human amniotic membrane (hAM)‐derived stem cells are the central research to be focused in these papers. (A) Clusters by documents coupling. (B) Historical direct citation network for the field of hAM in wound healing.
The blue cluster had the highest impact in this field, which includes the main keywords “transplantation,” “repair,” and “epithelial‐cells.” In this cluster, the representative was the comprehensive review published by Quan‐Wen Liu et al. (2021), which not only reviews the properties of hAM‐derived stem cells(hADSCs) as promising cells for cell therapy but also focuses on the therapeutic applications and advances of hADSCs in preclinical studies and clinical trials. 42 In addition, researchers have paid much attention to the application of hADSCs in regenerative medicine as these cells not only possess endogenous mechanisms that can activate tissue regeneration through paracrine secretion but also possess the potential to trans‐differentiate into cardiomyocytes, 43 hepatocytes, 39 endothelial cells, 44 neuronal cells. 45
The Red Cluster had the best centrality. A systematic review of preparation techniques and methods for ocular surface reconstruction using hAM and the impact on bioactivity was conducted by Andri K. Riau et al. (2010). 47 Subsequently, the hAM has been increasingly explored for applications in ophthalmology.
In (Figure 4B), the historiography displayed the historical direct citation network, which included 16 articles from Figure S5(B). In the historical direct citation network, the connection between elements was represented by the connecting line, and the contribution degree of each factor was represented by the size of the circle, which provided us with an important reference for reviewing the developing trends and hotspots for the research related to hAM in wound healing.
3.7. Current status of research in highly cited literature related to hAM for wound healing
Locally cited references in the top 20 for the research related to hAM repair trauma were illustrated in Figure S6(A), and the reference publication year spectroscopy was displayed in Figure S6(B). The most locally cited reference was by Hassan Niknejad in 2008 from the Journal of European Cell Materials with 305 local citations 7 , followed by Yanxia Hao in 2000 from the Journal of Cornea with 227 citations 48 and the article by Noriko Koizumi in 2000 from the Journal of Current Eye Research with 224 citations. 49 Noriko Koizumi et al. determined the mRNA expression of various growth factors in preserved hAM by RT‐PCR and the protein concentration of seven growth factors in decellularized amnion by ELISAs to analyze the different effects of amniotic epithelium and amniotic matrix on corneal re‐epithelialization and the different functions for ocular surface reconstruction. Yanxia Hao et al. demonstrated that hAESCs and hAMSCs express a variety of anti‐inflammatory proteins and anti‐angiogenic proteins, which confirms the anti‐angiogenic and anti‐inflammatory effects of the hAM. Hassan Niknejad et al. discussed the various excellent properties of hAM in detail, summarized and prospected its potential use in tissue engineering.
3.8. Examination of high‐frequency keywords and three significant hotspots through co‐occurrence analysis
With their finely tuned ability to encapsulate a certain article topic, keywords can give readers a thorough picture of the body of literature. Therefore, to demonstrate the fundamental challenges in a subject and to pinpoint possible research hot spots in the chosen topic, high‐frequency keywords were selected from current publications using bibliometric approaches. In a word cloud (Figure S7A) and a tree map (Figure S7B), we show the related keywords. (Figure 5A) shows the top 10 most frequent keywords, which were “transplantation” (n = 514), “amniotic membrane” (n = 466), “repair” (n = 351), “in‐vitro” (n = 296), “differentiation” (n = 271), “stem‐cells” (n = 267) “expression” (n = 266), “amniotic membrane transplantation” (n = 221), “management” (n = 216), and “reconstruction” (n = 213). What's more, their growth of occurrences employed by word dynamics analysis was shown in Figure S7(C), which revealed a steady upward trend for all keywords in the top 10. Moreover, trend topics analysis presented the occurrence trend of the top 46 keywords in (Figure 5B), which suggested that “drug delivery”, “ophthalmology”, “proteomic analysis”, “hydrogel”, “exosomes”, “decellularization”, “delivery”, “outcomes” and “endothelial growth‐factor” were the most popular topics in the last 3 years. More importantly, the keyword co‐occurrence network was depicted in (Figure 5C), which categorized keywords into two clusters as follows:
FIGURE 5.
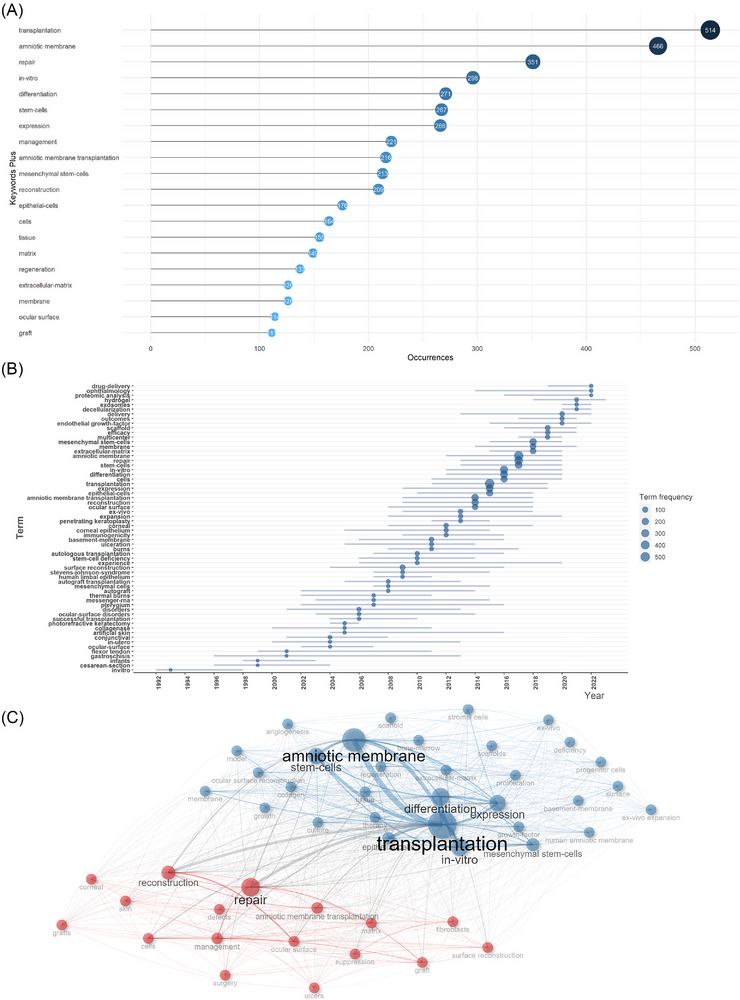
“transplantation”, “amniotic membrane”, and “repair” are the top three keywords. (A) Top 10 most frequent keywords for the field of human amniotic membrane (hAM) in wound healing. (B) Trend topics analysis showing the occurrences trend of the top 40 keywords for the field of hAM in wound healing. (C) Keywords co‐occurrence network for the field of hAM in wound healing.
Cluster 1 (blue): The most important keywords in this cluster include “amniotic membrane”, “transplantation”, “stem‐cells”, “expression”, and “differentiation”. These keywords exhibit research on amniotic stem cells for differentiation and expression in vitro models.
Cluster 2 (red): “repair”, “reconstruction”, “amniotic membrane transplantation”, “matrix”, and “management”. Those keywords depicted the reconstructive role of the matrix of the amniotic membrane in transplantation and repair
3.9. Thematic analyses of trends
Generally speaking, the theme and trend topics are indicative of the changing research foci and evolutionary trends in recent years. A thematic map demonstrated four quadrants of themes about the research of hAM. The results of the analysis show that the five keyword clusters are distributed in a coordinate system and are displayed in different locations based on their centrality and density. There are two clusters just located on the coordinate axes. As shown in Figure 6, the X‐axis (centrality) indicates the strength of linkage between a topic and other topics, and the Y‐axis (density) measures the strength of the connections between different knowledge units within a topic.
FIGURE 6.
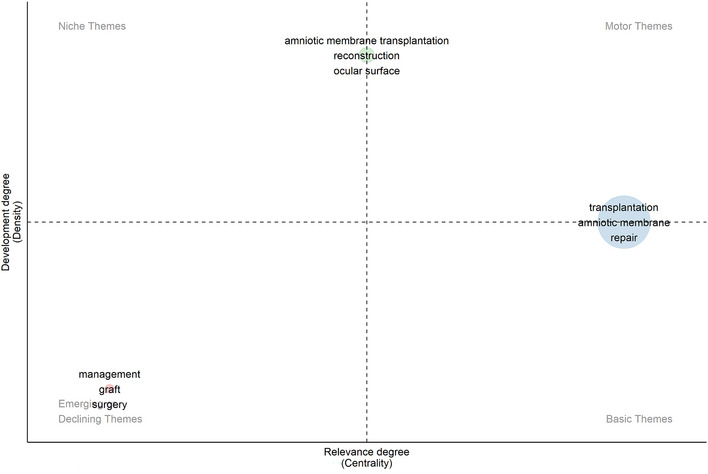
Recent shifts and trends in research focus: A thematic analysis map. The map displays four quadrants of themes including motor themes, niche themes, emerging or declining themes, and basic themes for the field of human amniotic membrane (hAM) in wound healing.
As we can see, “amniotic membrane transplantation” “reconstruction” and “ocular surface” were noted as a cluster, which achieves a high density, so it is easy to notice that the research of hAM transplantation applied to reconstruct ocular surface is extensive. On this topic, a recent noteworthy study by Minglei Zhao et al. demonstrated that transplantation of stem cells isolated from peripheral blood mononuclear cells onto the hAM sheet significantly restored corneal damage due to corneal limbal stem cell deficiency, they provided a new strategy. 50 The cluster includes the keywords “transplantation” “amniotic membrane” and “repair” which are of high centrality. It indicates that the relevant themes are close to the core themes. This theme discussed the mechanisms and roles of novel grafts incorporating hAM in a variety of traumatic injuries. For example, Kamakshi Bankoti found in 2020 that reactive oxygen species‐modified bioactive hydrogels loaded with hAM‐derived stem cells had significant pro‐epithelialization effects in chronically wounded skin such as diabetes. 51 Emerging or declining themes (left lower quadrant): “management” “graft” and “surgery” were recognized to have both little relevance and development in this field.
Moreover, Figure S8(A), which depicted the development of top topics from 1975 to 2023 in this research area and showed a thematic evolution with three cutting points in this field in 2000, 2010, and 2017. The conceptual structure map through MCA is displayed in Figure S8(B). In this figure, the top 50 keywords were distributed in the coordinate system. Furthermore, their more specific hierarchical clustering was revealed, supplemented in the topic dendrogram map in Figure S8(C).
4. DISCUSSION
HAM possesses a variety of excellent properties and is rich in bioactive factors, making it a logical choice to promote wound healing. 52 To investigate the mechanism, historical development, and application of hAM in promoting wound healing, we conducted a comprehensive bibliometric analysis to explore the developmental trends and hotspots of scientific research on hAM for wound healing. The search results showed 2532 relevant publications. The results show that the annual publications scales and cited frequencies have almost been steadily on the rise these years, which indicates that the research in this field is arousing increasing attention. In this research area, the USA is the most prolific and cited country. In addition, the USA, China, and Japan had the strongest collaboration relationship with other countries. Tseng S.C.G. is considered to be the most influential author who has made outstanding contributions to research in the treatment of corneal diseases by applying hAM to reduce pain, inflammation and promote healing. CORNEA, INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE, PLOS ONE were the three most published journals in this field. Subsequently, by integrating the results of the historical citation network, the keyword analysis, and the thematic analysis, we summarized and reviewed the knowledge structure, global status, trends, and research hotspots of research in hAM for wound healing. We then summarized the three hotspots as follows and we identified some high‐quality articles in English focusing on the roles played by hAESCs, hAMSCs, and hAM scaffolds in regenerative medicine and tissue engineering, respectively. Relevant information is shown in Table 4.
TABLE 4.
High‐quality articles focus on hAESCs, hAMSCs, and hAM scaffolds in regenerative medicine and tissue engineering.
| Component | Year | Author | Journal | Country | Target |
|---|---|---|---|---|---|
| hAESCs | 2018 | Bin Zhao et al. | Stem Cells International | China | Local injection of exosomes derived from hAESCs into skin wounds accelerates wound healing by stimulating the proliferation and migration of fibroblasts. |
| hAESCs | 2020 | Pei Wei et al. | Burns & Trauma | China | Exosomes derived from hAESCs promote diabetic wound healing through the PI3K‐AKT‐mTOR pathway. |
| hAESCs | 2017 | Bin Zhao et al. | Journal of Molecular Histology | China | Exosomes derived from hAESCs inhibit scar formation and improve wound healing. |
| hAMSCs | 2019 | Jing‐Yuan Li et al. | Stem Cell Research & Therapy | China | HAMSCs and their paracrine factors inhibit skin cell apoptosis through activation of the PI3K/AKT signalling pathway to promote wound healing. |
| hAMSCs | 2020 | Dan He et al. | Aging (Albany NY) | China | hAMSCs and their secreted factors interact with keratinocytes to promote wound healing through activation of the c‐Jun N‐terminal kinase (JNK) signalling pathway. |
| hAMSCs | 2022 | Seong‐Ho Han et al. | International Journal of Molecular Sciences | Korea | Improvement of inflammatory regulation and angiogenic properties of hAMSCs by gene editing and testing in vivo for its efficacy. |
| hAM scaffold | 2021 | Hamid Reza Aghayan | Drug Delivery and Translational Research | Iran | Inoculation of different mesenchymal stem cells on decellularized hAM scaffolds for wound treatment helps to restore the epidermal layer back to its normal thickness and regeneration of skin appendages. |
| hAM scaffold | 2022 | Ebrahim Mirzadegan | International Immunopharmacology | Iran | Inoculation of transdermal stem cells on a bilayer scaffold composed of hAM and filipin proteins promotes healing of diabetic wounds. |
| hAM scaffold | 2023 | Davood Nasiry | Tissue and Cell | Iran | Microporous three‐dimensional decellularized hAM scaffolds contribute to diabetic wound healing. |
| hAM scaffold | 2019 | Sean V Murphy | Stem Cells Medicine | USA | hAM hydrogel and hAM powder accelerate wound healing in a full thickness. |
Abbreviation: hAESCs, human amniotic epithelial stem cells; hAMSCs, human amniotic mesenchymal stem cells; hAM, human amniotic membrane.
4.1. HAESCs in regenerative medicine
Stem cells (SCs) therapy is becoming a popular area of research due to its powerful effects on tissue regeneration. 53 HAESCs are derived from the epiblast layers of amnion after 8 days of fertilization and have a very high capacity for induced differentiation. 54 hAESCs not only have powerful proliferation and differentiation capacity and excellent paracrine function but also have many unique and excellent properties. Over the past two decades, hAESCs have gained much attention as emerging seed cells in the field of wound healing.
hAESCs have many good properties. (1) hAESCs have stem cell‐like properties. Firstly, hAESCs express surface markers of embryonic stem cells, suggesting that it also possess some degree of self‐renewal and pluripotent properties, including stage‐specific embryonic antigens‐3 and, TRA1‐60 and TRA1‐80 as well as important transcription factors that maintain stem cell pluripotency and self‐renewal capacities such as Nanog. 39 , 55 (2) The construction of a serum‐free culture environment for hAESCs makes it more feasible to be used for basic medical research and clinical applications. 56 Initially, hAESCs were pioneered by Akle et al. for isolation and culture from full‐term placenta. 57 Over a long period, detailed protocols for in vitro isolation and culture of hAESCs have been described in multiple studies by multiple investigators. 39 , 43 , 58 These protocols optimized the conditions for hAESCs to maintain their proliferative, differentiation capacities and preserve the pluripotency characteristic in vitro, which encouraged the development of hAESCs as seed cells in the wound healing area, especially in transplantation. (3) hAESCs can exhibit extremely high differentiation plasticity. Several studies have shown that hAESCs can differentiate into ectoderm, mesoderm, and endoderm after being treated in different methods in vitro. 59 A study demonstrated that exposure to air‐to‐liquid boundary could prompt differentiation of hAESCs into epidermal cells. 60 Studies have also shown that hAESCs can mediate corneal epithelial reconstitution by differentiating into corneal epithelial‐like cells upon induction and stimulation. 61 , 62 The powerful differentiation of hAESCs helps them fit different wounds and form necessary cells, enhancing tissue regeneration. (4) Non‐tumorigenicity of hAESCs. Since hAESCs lack telomerase activity, they do not proliferate indefinitely in vivo, which makes the risk of tumourigenesis zero. This lays the foundation for subsequent basic research and clinical trials with large numbers of skin grafts. In addition, studies by Niknejad have found that AESCs have some antitumor effects by inducing apoptosis, stimulating cell cycle arrest, inhibiting angiogenesis, and expressing cytotoxic factors to target the tumor and inhibit his growth. 63 , 64 (5) Besides their encouraging results in wound healing models, the immunosuppressive activity and anti‐inflammatory properties of hAESCs have gained power in inflammation‐associated diseases. Transplanted hAESCs modulate the inflammatory state by inducing macrophage differentiation towards the M2 phenotype, thereby modulating the healing of diabetic ulcers and other chronic clinical wounds. 3 In addition, key antigens involved in the immune rejection response were expressed at relatively low levels in hAESCs, 65 which makes it less difficult to be used as a skin substitute afterward. (6) Abundant sources and lack of ethical concerns make it a relative alternative to the clinical cell transplantation method.
The migration and functioning of transplanted hAESCs towards the injured tissue are considered to be one of the main repair mechanisms for wound healing. It has been reported that hAESC regulates signaling networks altering the proliferation and migration of keratinocytes to promote wound healing. 66 However, limited research reported the long‐term integration of transplanted hAECs within target organs. There are many obstacles in the process of cell transplantation, such as low survival and limited repair capacity. As of now, much research evidence suggests that the large therapeutic effect of hAESCs is retained in their derived conditioned medium (CM) and extracellular vesicles (ECVs), which is directly related to the fact that hAESCs have a potent paracrine effect. 67 The activation of endogenous mechanisms that promote tissue regeneration through the secretion of these bioactive substances provides a beneficial microenvironment for cell survival during wound healing. Studies have shown that hAESCs secrete a large amount of biologically active substances that are essential in skin and tissue healing. 68 , 69 , 70 Following that, in a rat full‐thickness skin defects model, it is of great importance to find inhibitory effect of hAESCs‐Exosome on scar formation. 71 In addition, hAESCs‐Exosome have been considered to serve as a new window in the promotion of diabetic wound healing. 72 hAESCs‐CM were shown to inhibit the senescence process of keratinocytes and fibroblasts by suppressing the RAGE/P21 signal pathway. In addition, intradermal injection of transplanted hAESCs into diabetic wounds promotes re‐epithelialization and granulation tissue formation. However, these cells cannot survive long periods in the wounds, which reinforces that HAESCs‐Exosome are expected to replace hAECs in regenerative medicine to perform the biological functions of stem cell therapy and be applied as new therapeutic vectors for clinical research. 39 Not only like that, research in vivo by Wei Pei et al. hAESCs‐ Exosome were shown to promote angiogenesis and fibroblast function in full‐thickness skin wounds of diabetic (db/db) mice. However, the progress of wound healing performed worse when the PI3K‐AKT‐mTOR pathway was blocked, which provides a new strategy for the treatment of difficult‐to‐heal wounds in diabetes. 73 To determine which molecules in hAESCs‐Exos mediate wound healing, scientists applied purified hAESC‐Exos to skin wounds to explore the effects. They found that hAESCs‐Exos‐derived microRNAs improve wound healing and induce well‐reorganized collagen fibers after that. 74 In addition, it has been shown that hAESCs undergo epithelial‐mesenchymal transition (EMT) during proliferation and migration and the mesenchymal‐epithelial transition (MET) progresses at the damage site to promote wound healing. 75 Figure 7 visualizes the properties and mechanisms of hAESCs in wound healing.
FIGURE 7.
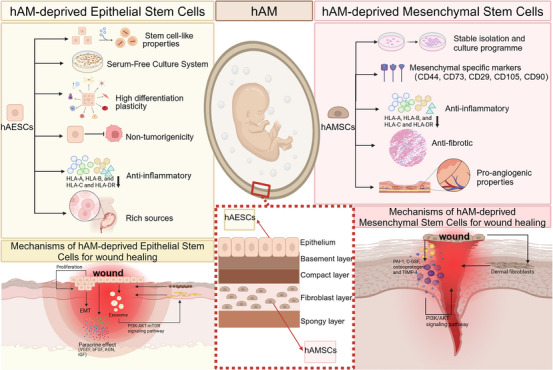
Characterization and mechanism of amniotic epithelial stem cells and amniotic mesenchymal stem cells in wound healing.
The role of hAESCs and their CM in promoting wound healing has been demonstrated in a multitude of basic experiments, but may be compromised by improper methods of preservation and delivery to a certain degree. However, the emergence of several new technology and materials related to hAESCs are gradually attracting great interest to ameliorate this dilemma. A fabrication system and strategy of hAESCs and while Wharton's jelly derived mesenchymal stem cells‐laden alginate/gelatin composite hydrogels through 3D bioprinting provides promising potential for future skin engineering. 76 Overall, hAESCs have great potential for application in clinical cell therapy, but there are still plenty of details that need to be modified. Not much research has been done on materials that bind to hAESCs and are applied to wound healing. Additionally, clinical trials on hAESCs should be conducted more often in the future to determine safety, effectiveness, and utility.
4.2. hAMSCs in regenerative medicine
Different from hAESCs, the hAMSCs are found in the amniotic mesoderm, deeper than hAESCs. But similarly, hAMSCs are also the most studied for their excellent characteristic, which will be the focus of this part. Although preclinical trials and clinical researches of hAMSCs in the field of wound healing are not well established, it remains a worthwhile goal.
hAMSCs possess a number of advantages. (1) The hAMSCs are genetically stable, and many studies have shown that isolation and culture protocols are relatively mature and suitable for mass production. 39 (2) HAMSCs are positive for the mesenchymal‐specific markers including CD44, CD73, CD29, CD105, and CD90, which showed high differentiation potential. 39 Like hAESCs, they also express Oct4 and SSEA‐4. 42 (3) HAESCs have low immunogenicity. 77 By inhibiting the proliferation of different immune cell subsets, inflammatory cytokine production, the ability of hAMSCs and their CM to acquire high immunomodulatory functions to suppress inflammatory conditions has been widely demonstrated. 39 (4) Anti‐inflammatory and anti‐fibrotic properties. Amniocytes have also been shown to modulate anti‐inflammatory and wound healing‐promoting M2 macrophages, by facilitating the transition of wounds from the inflammatory phase to the tissue repair phase. 78 In vivo research showed that hAMSCs have anti‐inflammatory and tissue remodeling properties and are effective in corneal wound healing by significantly inhibiting inflammatory cell infiltrate and decreasing corneal myofibroblast differentiation. 79 (5) Pro‐angiogenic properties. hAMSCs‐CM significantly promotes the proliferative capacity of the vascular endothelium compared to hAESCs‐CM. 68 In addition, hAMSCs secreted a large number of angiogenic factors, as determined by ELISA kits, which proved therapeutic significance for chronic wounds. 39 Separately, it was demonstrated that the pro‐angiogenic effect of hAMSC‐CM on HUVECs was dependent on circ‐100290 via the miR‐449a/eNOS and miR‐449a/VEGFA axes or the circ‐ABCB10/miR‐29b‐3p/VEGFA pathway. 80 , 81 In promoting diabetic wound healing, hAMSC‐Exos offers a promising strategy by promoting angiogenesis and secretion‐associated exosomal lncRNAs. 39
Comparing the paracrine activity of hAMSCs with umbilical cord derived mesenchymal stem cells (UCMSCs) and adult adipose tissue‐derived mesenchymal stem cells (ADMSCs), hAMSCs had a sizable pro‐angiogenic generative effect and produced high levels of TGF‐β. 82 These paracrine effects are highly relevant to the four processes of wound healing. A group of cytokines secreted from hAMSCs (including PAI‐1, C‐GSF, osteoprotegerin and TIMP‐1) may contribute to amelioration of heat stress‐induced skin injury through activation of the PI3K/AKT signal pathway in vitro. 39 Activated stem cells are crucial in the wound healing process as they coordinate cells for repair responses, including keratinocytes, fibroblasts, vascular endothelial cells, etc. hAMSCs‐CM is enriched with molecules that regulate wound healing, including lysyl oxidase‐2 (LOXL2), which promotes wound healing by interacting with keratinocytes through activation of the c‐Jun N‐terminal kinase (JNK) signaling pathway. 39 Another study found that human dermal fibroblasts treated with human amniotic stem cell‐CM had been enhanced proliferation and migration capacity. 83 Improvement of inflammatory regulation and angiogenic properties of hAMSCs by gene editing and testing in vivo for its efficacy. 39 , 84 Figure 7 visualizes the properties and mechanisms of hAMSCs in wound healing. On July 3, 2022, five clinical trials of “hAMSCs” were listed in the Clinical Trials Database (ClinicalTrials.gov), and although lacked studies of their application to wound healing, the results of these clinical trials will provide strong evidence for the subsequent research in wound healing, once the safety and efficacy of hAMSCs have been established.
In order to better preserve and enhance the therapeutic properties of hAMSCs, several emerging construction and materials combined with hAMSCs are coming into view in the field of promoting wound healing. A novel double‐layered tissue‐engineered skin established by hAMSCs and hAESCs achieves satisfactory repair results when applied to athymic mice as a skin equivalent. 85 Tuca et al. found that hAMSCs in combination with collagen/elastin scaffolds (Matriderm) applied to local wounds promoted wound healing in vivo. 86 Subsequently, another research compared the suitability of Matriderm as carriers for hAMSCs with electrostatically spun poly (ε‐caprolactone)/poly (l‐propylene cross‐lactone) (PCL/PLA) scaffolds, which showed better cell adhesion and certain anti‐shrinkage properties. 87 Overall, the prospects for the use of hAMSCs are quite attractive, but the mechanism and clinical research of hAMSCs are not sufficiently investigated. From the existing studies, hAMSCs‐CM is powerful, which provides a new idea for hAMSCs to exert the biological function in regenerative medicine afterward.
4.3. hAM as an appropriate scaffold for tissue engineering
The components of TE primarily include cells, scaffolds, and growth factors or biomolecules to induce tissue growth. 88 hAM is enriched with extracellular matrix and growth factors that provide an environment for various cells' growth and proliferation, and its basement membrane and dense layers contain collagens III, IV, and V as well as other glycoproteins such as laminin and fibronectin, which facilitate the formation of specialized ECM. 33 Secondly, hAM is also extremely biocompatible and has a certain degree of biodegradability and fluidity. These benefits make it ideally suited to act as scaffolds for tissue engineering, acting as a vehicle to deliver stem cells, fibroblasts or other cells into the local tissue environment for repair and regeneration. 89
Conventional and natural hAM has gradually failed to meet the needs of people due to the content of growth factors and modulators of ECM remodeling, the area of the hAM, and the mode of delivery between donors. 90 Glycerol‐preserved, frozen, or air‐dried hAM may completely eliminate its cytokine content. Jana Dragúňová et al. developed a method using polyester mesh for deep freezing (cryopreservation) of hAM and demonstrated that this technique facilitates the culture of keratin‐forming cells in vitro. 91 However, refrigerated transport is costly and difficult to realize in less economically developed areas. Therefore, the development of tissue analogs using innovative techniques or combining amniotic scaffolds with different types of cells to support the regeneration of damaged tissue is a promising aspect in the field of tissue engineering. It has been proved that transplantation of adipose MSCs and placental MSCs into decellularized‐hAM has been shown to help promote skin regeneration in vivo. 92 Another clinical trial demonstrated that inoculation of dermal fibroblasts and Wharton's Jelly Mesenchymal Stem Cells on decellularized‐hAM scaffolds accelerated the restoration of chronic wounds in diabetic. 93 These studies represent a good transition from basic experiments to clinical applications of decellularized scaffolds. In addition, Shi‐zhao Ji et al. described the preparation of 3D micronized hAM as a novel natural microcarrier, which not only facilitates the rapid expansion of epidermal stem cells, but also enables the construction of dermal scaffolds carrying epidermal stem cells for the repair of full‐layer skin defects. 94
Another promising orientation in healing is exosomes rather than stem cells on hAM scaffold dressings. The results of the research show that exosome‐conjugated hAM scaffolds significantly improve then diabetic skin wounds. 95
In addition, due to the constant changes and improvements in science and technology, new high‐tech materials are being developed and applied. Materials such as hydrogels, electrostatic spinning, and new polymers have been combined with hAM for wound healing applications. Hydrogels are used as scaffolds because they have a hydrophilic polymeric mesh structure, which compensates for the weak mechanical strength of the hAM and maximizes wound exposure to the beneficial substances within it. 96 The hAM matrix was powdered and injected into a mucoadhesive hydrogel solution and later lyophilized by chemical cross‐linking to create a bioengineered microporous three‐dimensional (3D) hAM scaffold, which was applied to the wound to help promote healing of ischemic diabetic wounds. 97 Furthermore, in another research, the hAM collagen matrix was decellularized and combined with methacrylic anhydride (MA) and then photo‐crosslinked with methacrylic gels to form GelMA‐dHAMMA composite hydrogels, which are not only have the porous structure of a two‐component polymer network, but also contribute to the expression of a‐smooth muscle actin (a‐SMA), and are reasonably considered to be a promising material for skin tissue engineering. 98 Electrostatic spinning is a biotechnology that can mimic natural extracellular matrices and able to generate nanometer diameter fibrous polymer scaffolds, such as collagen (500 nm) and elastin (400 nm), which promotes wound healing, by using high applied voltages. 99 Moghaddam et al. developed an electrospun skin scaffold based on hAM using fibroin nanofibers and CM11 peptide which is a kind of antimicrobial peptide, and it exhibited better power of wound healing in the mouse full‐thickness wound model. 100 In another pre‐clinical study, by combining a bilayer scaffold consisting of hAM and electrospun silk protein nanofibers, and equipped with menstrual blood‐derived mesenchymal stem cells, the evaluation showed that this skin substitute material could modulate impaired chronic wound healing, offering a new solution to the current situation of difficult healing of diabetic ulcers. 101 Additionally, the combination of hAM electrostatic spinning scaffolds and a new polymer(polycaprolactone) is also a promising candidate for the bioengineering of wound healing. 102
3D printing technology is becoming increasingly powerful and used in the medical field, making it a valuable area of research that can be identified in the future. 3D printing technology is a favorable option for controlling the morphology and appearance of scaffolds through layer‐by‐layer deposition of polymers. 88 Dehghani et al. fabricated specific 3D printed membranes for conjunctival reconstruction using a mixture of gelatin, elastin, and sodium hyaluronate. The 3D membrane comparison with the commonly used hAM transplantation in terms of surgical management and clinical applicability, has a lower pro‐inflammatory response and anti‐scarring effect in a male albino rabbit model. 103 In another clinical study, polylactic acid (PLA) amniotic fornix rings (AFR) were fabricated by 3D printing technology for patient ocular surface reconstruction, significantly outperforming traditional hAM grafts in terms of operating time and cost‐effectiveness, with no significant side effects produced. 104 However, as far as the current research is concerned, the technology of direct 3D printing of hAM has not yet been established, which may be a direction that scientists need to work on in the future.
hAM can also be utilized as a delivery system and reservoir of cells and drugs. The potential of the amnion as an effective drug delivery system was first reported by Kim et al. 105 They removed the corneal epithelial layer in a New Zealand white rabbit model and compared it with the hAM graft group and found that the concentration of ofloxacin was significantly higher in the corneas with hAM coverage. Since then, there has been an increasing amount of research on Ham in this area. Yelchuri et al. found that thick and thin hAM have different ability to encapsulate the drug (moxifloxacin), and histological evidence suggests that the phenomenon is due to the different thickness of the amniotic stroma layer that helps them to trap more of the drug. 106 In an in vitro research study, sustained release of voriconazole was observed for up to 5 weeks when voriconazole was incubated with hAM. This provides strong evidence that voriconazole‐impregnated hAM may be considered as a tool for sustained delivery in fungal keratitis. 107 These discussions help provide clues to the role of hAM as a sustained‐release drug delivery system for wound healing.
Research on hAM in promoting tissue regeneration and wound healing has led to far‐reaching potential for tissue engineering. To better demonstrate the functionality of hAM in tissue engineering, we have created Figure 8. However, the mechanical properties of hAM limit his application. Some approaches to improve scaffold properties have been combined with hAM, but clinical studies are far from sufficient. Therefore, multicenter, randomized controlled clinical trials are necessary to determine hAM's safety and efficacy.
FIGURE 8.
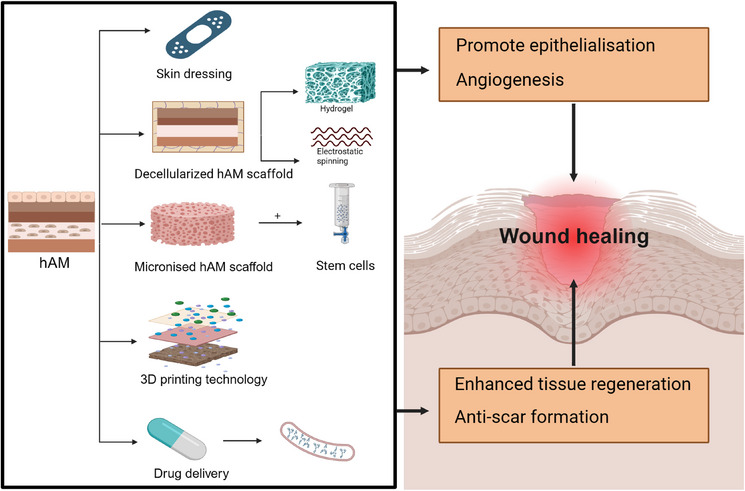
Human amniotic membrane (hAM) scaffolds in tissue engineering for wound healing.
At present, the research on hAM in tissue‐engineered skin has made initial progress, but the construction of artificial skin in the true sense has not yet been completed. There are still many defects in the research process. First, although amniotic stem cells have been studied in wound healing and they have been shown to be excellent seed cells, it is a challenge to preserve these cells. Improper preservation can alter their critical properties. Second, hAM tissue‐engineered dressings contain a variety of cells, growth factors and scaffolding structures, and the mechanisms of their interactions have not been fully elucidated, especially the mechanisms related to binding with stem cells to induce skin tissue regeneration still need more investigation. Third, amniotic tissue‐engineered skin is costly to manufacture and is notoriously difficult to scale up for clinical use. Its efficacy in different types of wounds has not yet been rigorously demonstrated, and multi‐centric, random controlled clinical studies need to be conducted in the future. Although there are still a lot of problems in the current technology, performance, and promotion of application, with the continuous development and improvement of science and technology, management and regulations, the prospect of regenerative medicine and tissue engineering application of hAM will be broader.
5. CONCLUSION
In this study, to explore the research on hAM in the field of wound healing, bibliometric analysis was used to summarize and visualize the global situation, research hotspots, and trends. In addition to identifying the most productive and influential authors, countries, institutions, and core journals in the field, we focused on three hot and trending research topics, including hAESCs in regenerative medicine, hAMSCs in regenerative medicine, and hAM as suitable scaffolds for tissue engineering. Here, developments and advances in the integration of hAM with regenerative medicine and tissue engineering for wound healing have been more comprehensively demonstrated. This research field is developing rapidly, and we contribute to providing a valuable reference for scientific researchers. More research achievements will be hopefully applied to clinical practice to expand the method of diseases’ diseases prevention, diagnosis and treatment.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
The study was approved by the Ethics Committee of the First Affiliated Hospital of Naval Medical University.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank the Web of Science (WOS, http://www.webofknowledge.com) team for allowing us to use their data. This study was supported in part by the National Natural Science Foundation of China (81930057, 81971836), CAMS Innovation Fund for Medical Sciences (2019‐I2M‐5‐076), Clinical Key Discipline Project of Shanghai; Shanghai Top Priority Research Center Project (2023ZZ02013); the Excellent Academic Leader Project of Shanghai Science and Technology Committee (23XD1425000); Deep Blue Talent Project of Naval Medical University, 234 Academic Climbing Programme of Changhai hospital and Achievements Supportive Fund (2018‐CGPZ‐B03); Postdoctoral Fellowship Program of CPSF, Shanghai Rising‐Star Program (Sailing Special Program) (No. 23YF1458400). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Guo X, Zhang W, Lu J, et al. Amniotic miracle: Investigating the unique development and applications of amniotic membrane in wound healing. Skin Res Technol. 2024;30:e13860. 10.1111/srt.13860
Xinya Guo, Wei Zhang, and Jianyu Lu are co‐first authors and have contributed equally to this work.
Contributor Information
Shichu Xiao, Email: shizhaoji2022@163.com.
Runzhi Huang, Email: huang2022@163.com.
Shizhao Ji, Email: huangzhuoxiao4@hotmail.com.
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available on the Web of Science (WOS, http://www.webofknowledge.com).
REFERENCES
- 1. Koob TJ, Lim JJ, Massee M, et al. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B: Appl Biomater. 2014;102(6):1353–1362. [DOI] [PubMed] [Google Scholar]
- 2. Bourne GL. The microscopic anatomy of the human amnion and chorion. Am J Obstetr Gynecol. 1960;79(6):1070–1073. [DOI] [PubMed] [Google Scholar]
- 3. Zheng Y, Zheng S, Fan X, et al. Amniotic epithelial cells accelerate diabetic wound healing by modulating inflammation and promoting neovascularization. Stem Cells Int. 2018;2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mamede AC, Carvalho MJ, Abrantes AM, et al. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447‐458. [DOI] [PubMed] [Google Scholar]
- 5. Kc S, Jj M, Cs F. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001;12(4):269‐281. [DOI] [PubMed] [Google Scholar]
- 6. Favaron P, Carvalho R, Borghesi J, et al. The amniotic membrane: development and potential applications—a review. Reprod Domestic Anim. 2015;50(6):881‐892. [DOI] [PubMed] [Google Scholar]
- 7.Department of Nanomedicine and Tissue Engineering, Shaheed Beheshti Medical University, Tehran I, Niknejad H, Peirovi H, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater. 2008;7:88‐99. [DOI] [PubMed] [Google Scholar]
- 8. Wilshaw SP, Kearney JN, Fisher J, et al. Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng. 2006;12(8):2117‐2129. [DOI] [PubMed] [Google Scholar]
- 9. Walkden A. Amniotic membrane transplantation in ophthalmology: an updated perspective. Clin Ophthalmol. 2020;14:2057‐2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azuara‐Blanco A, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Brit J Ophthalmol. 1999;83(4):399‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koob TJ, Rennert R, Zabek N, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10(5):493‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meller D, Pauklin M, Thomasen H, et al. Amniotic membrane transplantation in the human eye. Deutsches Ärzteblatt Int. 2011;108(14):243‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sangwan V, Burman S, Tejwani S, et al. Amniotic membrane transplantation: a review of current indications in the management of ophthalmic disorders. Indian J Ophthalmol. 2007;55(4):251. [DOI] [PubMed] [Google Scholar]
- 15. Sheha H, Liang L, Li J, et al. Sutureless amniotic membrane transplantation for severe bacterial keratitis. Cornea. 2009;28(10):1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tseng SCG, Prabhasawat P, Lee SH. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997;124(6):765‐774. [DOI] [PubMed] [Google Scholar]
- 17. Odet S, Louvrier A, Meyer C, et al. Surgical application of human amniotic membrane and amnion‐chorion membrane in the oral cavity and efficacy evaluation: corollary with ophthalmological and wound healing experiences. Front Bioeng Biotechnol. 2021;9:685128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adamowicz J, Pokrywczyńska M, Tworkiewicz J, et al. New amniotic membrane based biocomposite for future application in reconstructive urology. PLoS ONE. 2016;11(1):e0146012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mhaskar R. Amniotic membrane for cervical reconstruction. Int J Gynecol Obstetr. 2005;90(2):123‐127. [DOI] [PubMed] [Google Scholar]
- 20. Adamowicz J, Van Breda S, Tyloch D, et al. Application of amniotic membrane in reconstructive urology; the promising biomaterial worth further investigation. Expert Opin Biol Ther. 2019;19(1):9–24. [DOI] [PubMed] [Google Scholar]
- 21. Shojaku H, Takakura H, Okabe M, et al. Effect of hyperdry amniotic membrane patches attached over the bony surface of mastoid cavities in canal wall down tympanoplasty: effect of Hyperdry Amniotic Membrane Patches. Laryngoscope. 2011;121(9):1953–1957. [DOI] [PubMed] [Google Scholar]
- 22. Horn A, Saller J, Cuttica DJ, et al. Utility of dehydrated human amniotic membrane (DHAM) in total ankle arthroplasty. Foot Ankle Int. 2020;41(5):513‐520. [DOI] [PubMed] [Google Scholar]
- 23. Fairbairn NG, Randolph MA, Redmond RW. The clinical applications of human amnion in plastic surgery. J Plast Reconstruct Aesth Surg. 2014;67(5):662–675. [DOI] [PubMed] [Google Scholar]
- 24. Zelen CM, Serena TE, Denoziere G, et al. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mermet I, Pottier N, Sainthillier JM, et al. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regener. 2007;15(4):459‐464. [DOI] [PubMed] [Google Scholar]
- 26. DCunha A, Jehangir S, Rebekah G, et al. Human amniotic membrane vs collagen in the treatment of superficial second‐degree burns in children. Wounds: compendium Clin Res Pract. 2022;34(5):135‐140. [PubMed] [Google Scholar]
- 27. Aria M, Cuccurullo C. bibliometrix: an R‐tool for comprehensive science mapping analysis. J Informetr. 2017;11(4):959‐975. [Google Scholar]
- 28. Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inform Sci Technol. 2006;57(3):359‐377. [Google Scholar]
- 29. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brookes BC. Bradford's Law and the Bibliography of Science. Nature. 1969;224:953. [DOI] [PubMed] [Google Scholar]
- 31. Pao ML. Lotka's law: a testing procedure? 16.
- 32. Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci USA. 2005;102(46):16569‐16572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arrizabalaga JH, Nollert MU. Human amniotic membrane: a versatile scaffold for tissue engineering. ACS Biomater Sci Eng. 2018;4(7):2226‐2236. [DOI] [PubMed] [Google Scholar]
- 34. Dua HS, Gomes JAP, King AJ, et al. The amniotic membrane in ophthalmology. Survey Ophthalmol. 2004;49(1):51‐77. [DOI] [PubMed] [Google Scholar]
- 35. Kim JC, Tseng SCG. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14(5):473‐484. [PubMed] [Google Scholar]
- 36. Zhu YT, Li F, Zhang Y, et al. HC‐HA/PTX3 purified from human amniotic membrane reverts human corneal fibroblasts and myofibroblasts to keratocytes by activating BMP signaling. Invest Opthalmol Visual Sci. 2020;61(5):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He H, Zhang S, Tighe S, et al. Immobilized heavy chain‐hyaluronic acid polarizes lipopolysaccharide‐activated macrophages toward M2 phenotype. J Biol Chem. 2013;288(36):25792–25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shay E, Kheirkhah A, Liang L, et al. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens‐Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol. 2009, 54(6):686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629‐683. [DOI] [PubMed] [Google Scholar]
- 40. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regener. 2009;17(2):153‐162. [DOI] [PubMed] [Google Scholar]
- 42. Liu QW, Huang QM, Wu HY, et al. Characteristics and therapeutic potential of human amnion‐derived stem cells. Int J Mol Sci. 2021;22(2):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evron A, Goldman S, Shalev E. Human amniotic epithelial cells cultured in substitute serum medium maintain their stem cell characteristics for up to four passages. Int J Stem Cells. 2011;4(2):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perin L, Sedrakyan S, Da Sacco S, et al. Characterization of human amniotic fluid stem cells and their pluripotential capability. Meth Cell Biol. 2008;86:85‐99. [DOI] [PubMed] [Google Scholar]
- 45. García‐Castro IL, García‐López G, Ávila‐González D, et al. Markers of pluripotency in human amniotic epithelial cells and their differentiation to progenitor of cortical neurons. PLoS ONE. 2015;10(12):e0146082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Q, Lai D. Application of human amniotic epithelial cells in regenerative medicine: a systematic review. Stem Cell Res Therapy. 2020;11(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riau AK, Beuerman RW, Lim LS, et al. Preservation, sterilization and de‐epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials. 2010;31(2):216–225. [DOI] [PubMed] [Google Scholar]
- 48. Hao Y, Ma DHK, Hwang DG, et al. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348‐352. [DOI] [PubMed] [Google Scholar]
- 49. Koizumi NJ, Inatomi TJ, Sotozono CJ, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20(3):173‐177. [PubMed] [Google Scholar]
- 50. Zhao M, Zhang H, Zhen D, et al. Corneal recovery following rabbit peripheral blood mononuclear cell‐amniotic membrane transplantation with antivascular endothelial growth factor in limbal stem cell deficiency rabbits. Tissue Eng C Meth. 2020;26(10):541‐552. [DOI] [PubMed] [Google Scholar]
- 51. Bankoti K, Rameshbabu AP, Datta S, et al. Carbon nanodot decorated acellular dermal matrix hydrogel augments chronic wound closure. J Mater Chem B. 2020;8(40):9277–9294. [DOI] [PubMed] [Google Scholar]
- 52. Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting—a review. Cell Tissue Banking. 2017;18(2):193–204. [DOI] [PubMed] [Google Scholar]
- 53. Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, et al. Skin tissue engineering: wound healing based on stem‐cell‐based therapeutic strategies. Stem Cell Res Ther. 2019;10(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ghamari SH, Abbasi‐Kangevari M, Tayebi T, et al. The bottlenecks in translating placenta‐derived amniotic epithelial and mesenchymal stromal cells into the clinic: current discrepancies in marker reports. Front Bioeng Biotechnol. 2020;8:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miki T, Mitamura K, Ross MA, et al. Identification of stem cell marker‐positive cells by immunofluorescence in term human amnion. J Reprod Immunol. 2007;75(2):91–96. [DOI] [PubMed] [Google Scholar]
- 56. Yang PJ, Yuan WX, Liu J, et al. Biological characterization of human amniotic epithelial cells in a serum‐free system and their safety evaluation. Acta Pharmacol Sin. 2018;39(8):1305–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Akle CA, Welsh KI, Adinolfi M, et al. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;318(8254):1003‐1005. [DOI] [PubMed] [Google Scholar]
- 58. Gramignoli R, Srinivasan RC, Kannisto K, et al. Isolation of human amnion epithelial cells according to current good manufacturing procedures. Curr Protocols Stem Cell Biol. 2016;37:1E.10.1‐1E.10.13. [DOI] [PubMed] [Google Scholar]
- 59. Miki T, Strom SC. Amnion‐derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133‐141. [DOI] [PubMed] [Google Scholar]
- 60. Fatimah SS, Chua K, Tan GC, et al. Organotypic culture of human amnion cells in air‐liquid interface as a potential substitute for skin regeneration. Cytotherapy. 2013;15(8):1030‐1041. [DOI] [PubMed] [Google Scholar]
- 61. Yao M, Chen J, Yang XX, et al. Differentiation of human amniotic epithelial cells into corneal epithelial‐like cells in vitro. Int J Ophthalmol. 2013;6(5):564‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu X, Chen J, Zhou Q, et al. [Differentiation of human amniotic epithelial cells (HAECs) into corneal epithelial cells induced by co‐culture of HAECs and corneal epithelial cells in vitro]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chin J Cell Mol Immunol. 2017;33(4):508‐512. [PubMed] [Google Scholar]
- 63. Niknejad H, Khayat‐Khoei M, Peirovi H, et al. Human amniotic epithelial cells induce apoptosis of cancer cells: a new anti‐tumor therapeutic strategy. Cytotherapy. 2014;16(1):33–40. [DOI] [PubMed] [Google Scholar]
- 64. Niknejad H, Yazdanpanah G, Ahmadiani A. Induction of apoptosis, stimulation of cell‐cycle arrest and inhibition of angiogenesis make human amnion‐derived cells promising sources for cell therapy of cancer. Cell Tissue Res. 2016;363(3):599–608. [DOI] [PubMed] [Google Scholar]
- 65. Wassmer CH, Berishvili E. Immunomodulatory properties of amniotic membrane derivatives and their potential in regenerative medicine. Curr Diab Rep. 2020;20(8):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhao B, Liu JQ, Zheng Z, et al. Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways. Cell Tissue Res. 2016;365(1):85–99. [DOI] [PubMed] [Google Scholar]
- 67. Fathi I, Miki T. Human amniotic epithelial cells secretome: components, bioactivity, and challenges. Front Med. 2021;8:763141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu Q, Fang T, Lang H, et al. Comparison of the proliferation, migration and angiogenic properties of human amniotic epithelial and mesenchymal stem cells and their effects on endothelial cells. Int J Mol Med. 2017;39(4):918‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jeschke MG, van Baar ME, Choudhry MA, et al. Burn injury. Nature Rev Disease Primers. 2020;6(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Song YS, Joo HW, Park IH, et al. Transplanted human amniotic epithelial cells secrete paracrine proangiogenic cytokines in rat model of myocardial infarction. Cell Transplant. 2015;24(10):2055‐2064. [DOI] [PubMed] [Google Scholar]
- 71. Zhao B, Zhang Y, Han S, et al. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J Mol Histol. 2017;48(2):121‐132. [DOI] [PubMed] [Google Scholar]
- 72. Feng J, Yao Y, Wang Q, et al. Exosomes: potential key players towards novel therapeutic options in diabetic wounds. Biomed Pharmacother. 2023;166:115297. [DOI] [PubMed] [Google Scholar]
- 73. Wei P, Zhong C, Yang X, et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K‐AKT‐mTOR‐mediated promotion in angiogenesis and fibroblast function. Burns Trauma. 2020;8:tkaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhao B, Li X, Shi X, et al. Exosomal microRNAs derived from human amniotic epithelial cells accelerate wound healing by promoting the proliferation and migration of fibroblasts. Stem Cells Int. 2018;2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Richardson L, Menon R. Proliferative, migratory, and transition properties reveal metastate of human amnion cells. Am J Pathol. 2018;188(9):2004–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu P, Shen H, Zhi Y, et al. 3D bioprinting and in vitro study of bilayered membranous construct with human cells‐laden alginate/gelatin composite hydrogels. Coll Surf B: Biointerfaces. 2019;181:1026‐1034. [DOI] [PubMed] [Google Scholar]
- 77. Umezawa A, Hasegawa A, Inoue M, et al. Amnion‐derived cells as a reliable resource for next‐generation regenerative medicine. Placenta. 2019;84:50‐56. [DOI] [PubMed] [Google Scholar]
- 78. Magatti M, Vertua E, De Munari S, et al. Human amnion favours tissue repair by inducing the M1‐to‐M2 switch and enhancing M2 macrophage features. J Tissue Eng Regener Med. 2017;11(10):2895–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Navas A, Magaña‐Guerrero FS, Domínguez‐López A, et al. Anti‐inflammatory and anti‐fibrotic effects of human amniotic membrane mesenchymal stem cells and their potential in corneal repair. Stem Cells Transl Med. 2018;7(12):906‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tang Z, Wu X, Hu L, et al. Circ‐100290 positively regulates angiogenesis induced by conditioned medium of human amnion‐derived mesenchymal stem cells through miR‐449a/eNOS and miR‐449a/VEGFA axes. Int J Biol Sci. 2020;16(12):2131–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tang Z, Tan J, Yuan X, et al. Circular RNA‐ABCB10 promotes angiogenesis induced by conditioned medium from human amnion‐derived mesenchymal stem cells via the microRNA‐29b‐3p/vascular endothelial growth factor A axis. Experim Therap Med. 2020;20(3): 2021‐2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dabrowski FA, Burdzinska A, Kulesza A, et al. Comparison of the paracrine activity of mesenchymal stem cells derived from human umbilical cord, amniotic membrane and adipose tissue. J Obstetr Gynaecol Res. 2017;43(11):1758‐1768. [DOI] [PubMed] [Google Scholar]
- 83. Pan C, Lang H, Zhang T, et al. Conditioned medium derived from human amniotic stem cells delays H2O2‑induced premature senescence in human dermal fibroblasts. Int J Mol Med. 2019;44(5):1629–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xiao S, Huang G, Wei Z, et al. IL‐10 gene‐modified human amniotic mesenchymal stem cells augment regenerative wound healing by multiple synergistic effects. Stem Cells Int. 2019;2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li H, Chu Y, Zhang Z, et al. Construction of bilayered tissue‐engineered skin with human amniotic mesenchymal cells and human amniotic epithelial cells. Artif Organs. 2012;36(10):911‐919. [DOI] [PubMed] [Google Scholar]
- 86. Tuca AC, Ertl J, Hingerl K, et al. Comparison of matrigel and matriderm as a carrier for human amnion‐derived mesenchymal stem cells in wound healing. Placenta. 2016;48:99‐103. [DOI] [PubMed] [Google Scholar]
- 87. Vonbrunn E, Mueller M, Pichlsberger M, et al. Electrospun PCL/PLA Scaffolds are more suitable carriers of placental mesenchymal stromal cells than collagen/elastin scaffolds and prevent wound contraction in a mouse model of wound healing. Front Bioeng Biotechnol. 2020;8:604123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Leal‐Marin S, Kern T, Hofmann N, et al. Human amniotic membrane: a review on tissue engineering, application, and storage. J Biomed Mater Res B: Appl Biomater. 2021;109(8):1198‐1215. [DOI] [PubMed] [Google Scholar]
- 89. Chen P, Lu M, Wang T, et al. Human amniotic membrane as a delivery vehicle for stem cell‐based therapies. Life Sci. 2021;272:119157. [DOI] [PubMed] [Google Scholar]
- 90. Litwiniuk M, Radowicka M, Krejner A, et al. Amount and distribution of selected biologically active factors in amniotic membrane depends on the part of amnion and mode of childbirth. Can we predict properties of amnion dressing? A proof‐of‐concept study. Central‐Eur J Immunol. 2018;43(1):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dragúňová J, Kabát P, Cucorová V, et al. Deep frozen amniotic membrane used as a scaffold and/or carrier for different cell types. Cell Tissue Bank. 2019;20(1):35–48. [DOI] [PubMed] [Google Scholar]
- 92. Aghayan HR, Hosseini MS, Gholami M, et al. Mesenchymal stem cells’ seeded amniotic membrane as a tissue‐engineered dressing for wound healing. Drug Deliv Transl Res. 2022;12(3):538‐549. [DOI] [PubMed] [Google Scholar]
- 93. Hashemi SS, Mohammadi AA, Moshirabadi K, et al. Effect of dermal fibroblasts and mesenchymal stem cells seeded on an amniotic membrane scaffold in skin regeneration: a case series. J Cosmetic Dermatol. 2021;20(12):4040–4047. [DOI] [PubMed] [Google Scholar]
- 94. zhao Ji S, chu Xiao S, fei Luo P, et al. An epidermal stem cells niche microenvironment created by engineered human amniotic membrane. Biomaterials. 2011;32(31):7801‐7811. [DOI] [PubMed] [Google Scholar]
- 95. Xiao S, Xiao C, Miao Y, et al. Human acellular amniotic membrane incorporating exosomes from adipose‐derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Therapy. 2021;12(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Grey C. Tissue engineering scaffold fabrication and processing techniques to improve cellular infiltration. Theses & Dissertations. 2014. [Google Scholar]
- 97. Nasiry D, Khalatbary AR, Abdollahifar MA, et al. Correction to: engraftment of bioengineered three‑dimensional scaffold from human amniotic membrane‑derived extracellular matrix accelerates ischemic diabetic wound healing. Archiv Dermatol Res. 2022;314(7):719. [DOI] [PubMed] [Google Scholar]
- 98. Zhang Q, Chang C, Qian C, et al. Photo‐crosslinkable amniotic membrane hydrogel for skin defect healing. Acta Biomater. 2021;125:197–207. [DOI] [PubMed] [Google Scholar]
- 99. Feltz KP, Kalaf EAG, Chen C, et al. A review of electrospinning manipulation techniques to direct fiber deposition and maximize pore size. Electrospinning. 2017;1(1):46‐61. [Google Scholar]
- 100. Moosazadeh Moghaddam M, Farhadie B, Mirnejad R, et al. Evaluation of an antibacterial peptide‐loaded amniotic membrane/silk fibroin electrospun nanofiber in wound healing. Int Wound J. 2023;20(9):3443‐3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mirzadegan E, Golshahi H, Saffarian Z, et al. The remarkable effect of menstrual blood stem cells seeded on bilayer scaffold composed of amniotic membrane and silk fibroin aiming to promote wound healing in diabetic mice. Int Immunopharmacol. 2022;102:108404. [DOI] [PubMed] [Google Scholar]
- 102. Lotfi Z, Khakbiz M, Davari N, et al. Fabrication and multiscale modeling of polycaprolactone/amniotic membrane electrospun nanofiber scaffolds for wound healing. Artif Organs. 2023;47(8):1267‐1284. [DOI] [PubMed] [Google Scholar]
- 103. Dehghani S, Rasoulianboroujeni M, Ghasemi H, et al. 3D‐Printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: an in vitro & in vivo study. Biomaterials. 2018;174:95‐112. [DOI] [PubMed] [Google Scholar]
- 104. Zhang N, Liu R, Liu X, et al. Personalized 3D‐printed amniotic fornical ring for ocular surface reconstruction. Int J Bioprint. 2023;9(3):713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim HS, Sah WJ, Kim YJ, et al. Amniotic membrane, tear film, corneal, and aqueous levels of ofloxacin in rabbit eyes after amniotic membrane transplantation. Cornea. 2001;20(6):628‐634. [DOI] [PubMed] [Google Scholar]
- 106. Yelchuri ML, Madhavi B, Gohil N, et al. In vitro evaluation of the drug reservoir function of human amniotic membrane using moxifloxacin as a model drug. Cornea. 2017;36(5):594‐599. [DOI] [PubMed] [Google Scholar]
- 107. Hazarika M, Prajna NV, Senthilkumari S. Drug reservoir function of voriconazole impregnated human amniotic membrane: an in vitro study. Indian J Ophthalmol. 2021;69(5):1068‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The datasets generated and/or analyzed during the current study are available on the Web of Science (WOS, http://www.webofknowledge.com).


