Abstract
Beyond their role as brain immune cells, microglia act as metabolic sensors in response to changes in nutrient availability, thus playing a role in energy homeostasis. This review highlights the evidence and challenges of studying the role of microglia in metabolism regulation.
Keywords: diet-induced obesity, glial, hypothalamus, metabolism
Introduction
In the central nervous system (CNS), the hypothalamus plays a key role in sensing and responding to peripheral metabolic fluctuation to maintain whole body energy homeostasis. Much evidence has shown the crucial role of microglia in this energy homeostasis process (1–5). Microglia exert immune and metabolic functions by serving as brain-resident immune cells with natural macrophage characteristics. Unlike peripheral macrophages, microglia originate from the yolk sac (6) and have a slower turnover rate. Although microglia activation is necessary for neuronal homeostasis, prolonged microglia activation has been linked to neurodegeneration and obesity. Upon high-fat diet (HFD) exposure, microglia activation is predominantly observed in the arcuate nucleus (ARC) of the hypothalamus, the area in which first-order neurons sensing peripheral metabolic signals are located (7, 8). Several studies have implied the causative role of hypothalamic microglia activation in the development of obesity. Here, we review and discuss the current understanding on the role of microglia in metabolism, as well as the limitations in microglia research. Furthermore, we discuss potential approaches and directions that might help advance the understanding of the metabolic role of microglia in regulating energy homeostasis.
Microglia, Central Players in Hypothalamic Function
Microglial cells, as the resident macrophages of the CNS, play an essential role in responding to injuries and infections. Additionally, and somehow similar to peripheral macrophages (e.g., adipose tissue-resident macrophages), microglial cells can sense and be activated by dietary molecules such as lipids (9, 10) and carbohydrates (11–13) as well as hormones such as leptin (12, 13), ghrelin (14), adiponectin (APN) (15–17), and glucagon-like peptide 1 (GLP-1) (18–20). Indeed, microglia cells express transporters for fuel substrates such as glucose, fatty acids, and amino acids (21) and receptors for hormones (12–20).
The traditional characterization of microglial M1 and M2 polarization status, a process by which microglial cells produce distinct functional phenotypes as a reaction to specific stimuli and signals, does not fully capture the complex and diverse function of microglia. Recent advances in single-cell RNA sequencing (scRNA-seq) and other high-throughput technologies have implicated the heterogeneous and diverse activation states of microglia (22, 23). Microglia activation status might be dictated by factors including the local microenvironment, the type and severity of the injury, and the dynamic interplay with other brain cells. As a result, microglia might acquire distinct molecular signatures and functional properties depending on their own unique anatomical environment (21, 24).
Although microglia are present across the entire CNS, their presence in the hypothalamus has been shown to be essential in regulating numerous aspects of whole body metabolism. In particular, hypercaloric diets are known to induce profound changes in microglia within the hypothalamic ARC. Because of its anatomical position in close proximity with the median eminence, which lacks the blood-brain barrier (BBB), the ARC has a central role in sensing nutrient changes and regulating metabolism. Here, microglia act as the first immune and metabolic-sensing population among other microglia in the brain parenchyma. In support of this, a study found that microglia in the circumventricular organs (CVOs) are in active status under normal physiological conditions (25). In the ARC, two essential neuronal populations regulating metabolic responses to nutrients are located. Here, Neuropeptide Y and Agouti-related protein (NPY/AgRP)-expressing neurons exert an orexigenic function, i.e., increase food intake, whereas proopiomelanocortin (POMC) neurons are known to be anorexigenic, i.e., decrease food intake. Not surprisingly, studies have observed a very active and complex interaction between these neurons and microglia in the ARC (see below for details).
Effects of Microglia Manipulation on Whole Body Metabolism
Genetic Models of Microglia Signaling Alterations
CX3CR1, also known as the fractalkine receptor, is a G protein-coupled receptor responsible for leukocyte migration, retention, and adhesion. It is expressed on monocytes, dendritic cells, and microglia. CX3CL1/fractalkine, the ligand of CX3CR1, is expressed and released by neurons (26). An early study on CX3CR1 signaling reported that although Cx3cr1-deficient mice had no changes in body weight, food intake, and energy expenditure on both chow diet and HFD, they showed improved glucose tolerance and insulin sensitivity on HFD compared with HFD-fed control mice (27). Despite this first evidence of possible beneficial effects of Cx3cr1 deficiency, several studies have demonstrated the deleterious outcomes caused by the loss of CX3CR1 signaling (28–32). A recent study showed that Cx3cr1-deficient mice have increased food intake compared with wild-type mice when fed on a standard chow diet (33). Furthermore, Cx3cr1-deficient mice have impaired glucose tolerance and insulin resistance and increased hepatic steatosis when fed on a HFD. Similarly, leptin-deficient (ob/ob) mice with Cx3cr1 deletion (Cx3cr1KO/ob) display increased insulin resistance, hepatic steatosis, and accumulation of macrophages in adipose tissue compared to ob/ob mice with intact CX3CR1 signaling. Interestingly, however, Cx3cr1KO/ob mice undergo less weight gain than ob/ob mice up to 8 wk of age, whereas no differences were observed during the subsequent 12 wk (34). In addition, female male-like Cx3cr1-knockout mice fed on HFD displayed a malelike phenotype with a higher body weight gain (35) compared with female diet-induced obesity (DIO) control mice. Indeed, the lower susceptibility to DIO shown by female versus male mice could be attributed, at least in part, to higher levels of CX3CR1-CX3CL1 observed in female mice exposed to HFD.
Cx3cr1-deficient mice have also shown increased levels of proinflammatory cytokines, such as IL-6 and TNFα, and the deposition of extracellular matrix (EM) in the dentate gyrus (DG). An anxiolytic-like and depressive-like phenotype is exhibited by Cx3cr1-deficient mice (35), as well as a significant reduction in oligodendrocyte progenitor cell (OPC) engulfment, an increased number of oligodendrocytes, and a reduced myelin thickness (36). Studies have shown that Cx3cr1 deficiency in mice can cause an exaggerated response to lipopolysaccharide (LPS) stimuli in CNS, with consequent microglial neurotoxicity and advanced neuronal death (37). Thus, these data suggest that microglial CX3CR1 signaling may play a regulatory role by preventing overactivation via CX3CL1 released by neurons. Therefore, microglia lacking CX3CR1 signaling by maintaining an activated status may alter neuronal activity in response to changes in metabolic signals.
CX3CR1 has been also shown to be involved in glucose homeostasis. In a hypoglycemic condition model, fasted Cx3cr1-deficient mice treated with insulin developed insulin resistance. Furthermore, when injected with the glycolytic inhibitor 2-deoxy-d-glucose (2-DG) in fed condition, Cx3cr1-deficient mice displayed increased counterregulatory responses, showing higher glucose levels compared with control mice, thus suggesting a suppressive role of microglial CX3CR1 signaling in counterregulatory responses to hypoglycemia (38).
In addition to Cx3cr1 deficiency, the actions of its ligand, CX3CL1, in metabolism regulation have also been assessed. Intracerebroventricular (icv) CX3CL1 administration suppressed food intake both physiologically and after stimulation of food intake by NPY or ghrelin (33, 39). Consistently, overexpression of Cx3cl1 in the medial basal hypothalamus reduced body weight gain and food intake, with improved leptin sensitivity in a DIO model (39, 40). Overall, CX3CR1-CX3CL1 signaling plays a role in metabolism regulation by reducing the inflammatory activity of microglia. Whether most neurons can produce CX3CL1 at a similar level or certain neuronal populations are responsible for most of CX3CL1 production is not fully understood. In this context, the effects of CX3CL1 on CX3CR1-expressing microglia could be taken as an advantage to study microglia subpopulations. Cx3cl1 deletion in specific neuronal populations, which mostly affects neighboring microglia activity, may be a potential approach for regional microglia studies. It is important to consider that CX3CR1 is not only expressed in microglia but also highly expressed in peripheral monocytes. Consequently, Cx3cr1 deletion might have complex effects in both the central and peripheral systems. Thus, to define the physiological role of CX3CR1 in microglia, a conditional knockout approach is necessary to exclude effects from the loss of CX3CR1 signaling in the periphery.
To define microglia functions, the tamoxifen-inducible Cx3cr1CreER system has been widely used to alter genetic expression specifically in microglial cells (Table 1) (41–44). Indeed, although Cx3Cr1 is expressed in both microglia and peripheral macrophages, the latter has a considerably faster turnover. Thus, peripheral macrophages carrying the modification upon tamoxifen injection will be quickly replenished without any long-lasting Cre-dependent effects, whereas microglia cells expressing Cre recombinase enzyme will persist, thus causing microglia-specific genetic modifications. By application of this Cx3cr1CreER model, IKKβ and A20, essential components of the NF-κB pathway, were deleted specifically in microglia to assess the effects of inflammatory response on obesity development. Mice lacking A20, a negative NF-κB regulator, showed spontaneous microglia activation, resulting in an obese phenotype in mice fed on a standard chow diet (7). Inversely, HFD-fed mice with specific microglial deletion of IKKβ, an essential cofactor for NF-κB activation, maintained less microglia activation, had reduced body weight and food intake, and were overall protected against DIO. Similarly, microglial deletion of uncoupling protein 2 (Ucp2), a mitochondrial protein regulated by fatty acid metabolism and shown to play a role as transporter in the malate-aspartate shuttle (45–47), resulted in milder inflammatory response to HFD (8). This change in the inflammatory response was due to attenuated mitochondria function in microglia during HFD, as Ucp2-deleted primary microglia were unable to utilize fatty acids. As a result, microglia Ucp2-deficient mice were protected from DIO (8). In support of a role for lipid metabolism in the regulation of microglial function (8), microglial lipoprotein lipase (LPL)-deficient mice display increased dysmorphic mitochondria and impaired systemic lipid homeostasis with increased body weight and POMC neuronal loss (48). Altogether these studies indicate that reducing microglia inflammatory response to HFD has beneficial effects conferring protection to DIO (8).
Table 1.
Microglia-specific gene-deleted animal models using Cx3cr1CreERT system
| Gene | Diet | Microglia Activity | Neuronal Activity | Metabolic Phenotype | Other Features | Reference |
|---|---|---|---|---|---|---|
| IKKβ | 42% fat/ 60% fat |
Reduced inflammatory response | NA | Resistant to DIO | Reduced myeloid cell recruitment into the MBH | (7) |
| A20 | CD | Enhanced inflammatory response | Reduced neuronal leptin sensitivity | Obese phenotype | Increased myeloid cell recruitment into the MBH | (7) |
| Ucp2 | 45% fat | Reduced inflammatory response | Preserved leptin sensitivity of POMC neurons | Resistant to DIO | Defective fatty acid utilization in microglia | (8) |
| EP4 | 60% fat | Reduced phagocytotic activity; no changes in inflammatory response | Preserved POMC neuronal projections to the PVN | Resistant to DIO | Reduced cellular contacts between POMC neurons and microglia | (49) |
| Bmal1 | 60% fat | Increased phagocytic activity | Increased number of POMC neurons | Resistant to DIO | Improved long-term memory | (50) |
| LPL | 58% fat | Reduced phagocytolic activity; increased mitochondrial dysmorphologies | Reduced number of POMC neurons; increased mitochondrial dysmorphologies | Susceptible to DIO | Dysmorphology of the Golgi apparatus in microglia | (48) |
ARC, arcuate nucleus; CD, chow diet; DIO, diet-induced obesity; MBH, mediobasal hypothalamus; NA, not assessed; POMC, proopiomelanocortin; PVN, paraventricular nucleus of the hypothalamus.
Likewise, attenuation of microglia phagocytotic activity has been shown to have similar benefits in reducing susceptibility to DIO. Microglial EP4 (prostaglandin PGE2 receptor)-deficient mice displayed reduced phagocytotic activity with no changes in proinflammatory cytokines, such as TNFα and IL-1β, during HFD. Nevertheless, microglial EP4 deletion markedly reduced weight gain and food intake and prevented HFD-induced obesity with enhanced insulin sensitivity (49). This resistance to DIO was associated with increased POMC and α-melanocortin stimulating hormone (α-MSH) projections onto hypothalamic paraventricular nucleus (PVN) neurons compared with control mice on HFD. However, and contrary to the consistent protective effects from DIO shown by reducing microglial inflammatory responses (7, 39, 40), the effects of attenuated phagocytotic activity in microglia on metabolism have not been consistent. For instance, mice with deletion of microglial Bmal1 (Basic Helix-Loop-Helix ARNT Like 1), a key clock gene, have increased microglia phagocytotic activity in HFD but reduced energy intake and attenuated body weight gain with increased POMC neuronal numbers compared with HFD control mice (50). Furthermore, microglia Bmal1-deficient mice displayed improved long-term memory compared with control mice. Interestingly, however, deficiency of phagocytosis-associated genes has demonstrated beneficial effects on neurodegeneration (51), thus suggesting that microglial phagocytotic activity may have a distinct role across brain regions and under different pathological conditions. Future studies are warranted to determine the role of microglial phagocytosis in brain functions.
Finally, although the loxP-inducible CreER system is a well-established genetic tool, “leaky Cre” activity, i.e., recombination occurring without tamoxifen induction, has been reported (52, 53). Because of that, Cre might accumulate in the nucleus of the long-living microglia and spontaneously induce recombination (53). Nevertheless, the inducible Cx3Cr1CreER system is the most used animal model for studying microglia, whereas additional models such as Sall1CreER, P2ry12CreER, HexbCreER, and Tmem119CreER mice have been recently developed and future studies will address the utility of these models for microglia study (54–57).
Pharmacological Models of Microglia Depletion
Microglia depletion through pharmacological means has been applied as a useful approach for understanding microglia function in the brain. Microglia development, proliferation, and survival require numerous molecular factors (58, 59). A main molecule for microglia survival is colony-stimulating factor 1 receptor (CSF1R), which is highly expressed in myeloid lineage cells, including monocytes and macrophages in the periphery, and microglia in the brain (60–62). Csf1r-depleted mice lack microglia (52), similar to mice depleted of either of its two ligands, CSF1 (63, 64) and IL-34 (65, 66). This indicates that CSF1R signaling is essential for microglia survival. Interestingly, it was observed that intraperitoneal (ip) administration of CSF1- or IL-34-blocking antibody disrupts CSF1R signaling differentially, causing depletion of microglia in gray and white matter, respectively. This might be due to the differential expression pattern (67) of CSF1 and IL-34, since CSF1 is expressed by astrocytes, oligodendrocytes, and microglia whereas IL-34 is predominantly expressed by neurons (68, 69).
Since several studies have shown the need for CSF1R signaling for microglia survival, pharmacological CSF1R targeting approaches have been applied to deplete microglia populations to address the physiological role of microglia. PLX3397, also known as pexidartinib, was approved in 2019 by the US Food and Drug Administration for the treatment of symptomatic tenosynovial giant cell tumor (TGCT). PLX3397 is a multikinase inhibitor drug (70). Similarly, another CSF1R inhibitor, PLX5622, has been shown to have more specific CSF1R inhibitory effects, without affecting c-Kit, an essential factor for hematopoietic cell survival and development (71, 72). Both of these inhibitors can permeate through the BBB and are available as dietary formulation for chronic administration, making them particularly suitable for animal studies. PLX3397-induced microglia depletion in a chronic demyelination model has been shown to ameliorate the disease progression by increasing remyelinated oligodendrocytes (73), whereas it increased apical dendritic spine density with reduction of neuronal death after traumatic brain injury (TBI) (74). Similarly, PLX5622-driven microglia repopulation in the aged brain restores the expression of genes associated with neuronal and synaptic functions to levels comparable to those of young mice, with improvement of cognitive functions (75). Chronic administration of PLX5622 maintained a small number of microglia (∼30%) and improved learning and memory in an aged 3xTg-AD mouse model of Alzheimer’s disease (AD) (76). After brain injury, microglia repopulated by PLX5622 treatment promoted brain recovery, reduced lesion-induced inflammation (77), and recovered motor and cognitive deficits in an IL-6-dependent manner (78, 79). In the PLX5622-treated 5XFAD AD mouse model, repopulated microglia displayed homeostatic genetic signatures and this was associated with a slower progression of the disease (80, 81). The beneficial effects of inhibitors may be due to the removal of detrimental microglia and the repopulation of healthy microglia. Indeed, because of the self-renewing ability of microglia, withdrawal of inhibitors after microglia depletion causes microglia to repopulate promptly and reach full repopulation within 3 wk. Consistently, an early study showed that healthy adult mice had inflammatory gene profiles similar to the original resident microglia after withdrawal of PLX3397-containing chow diet (82). Thus, microglia depletion and following repopulation might achieve a replacement with healthy microglia and the reestablishment of homeostatic condition even in pathological settings.
Unfortunately, although several studies have been performed assessing the effects of pharmacological microglial depletion on neurodegenerative diseases, limited studies have addressed the effects of microglia depletion and repopulation after inhibitor treatment on whole body metabolism. For example, Elmore and collaborators (75) found that 2 wk after microglia depletion by PLX5622 adult mice with fully repopulated microglia show increased body weight. However, when embryonic microglia depletion was achieved by administering PLX5622 to pregnant dams starting at embryonic day (E)3.5, the body weight of PLX5622-exposed male pups was lower than that of control pups up to postnatal day (P)15, with reduced number of POMC neurons. However, it significantly increased from P15 to P21, and at 6 wk of age PLX5622-exposed male mice did not show differences in body weight, although female mice were heavier than control mice (83). Thus, these results suggest that the changes in body weight observed in the early phase after microglia depletion could be due to the severe environmental changes induced by microglia cell death rather than microglia absence, as no body weight changes were observed by long-term monitoring of adult mice fed on chow diet (7). However, when exposed to HFD, PLX5622-treated adult mice showed reduced body weight and food intake with enhanced leptin sensitivity (7, 10).
Despite limited evidence of the effects of microglia depletion and repopulation, many studies have proposed the beneficial effects of repopulating microglia as a therapeutic strategy to treat pathological brain conditions (72, 84). The homeostatic signature gained on repopulated microglia after depletion might have a beneficial effect on dysregulated metabolic conditions, although its long-term efficiency has not been validated. To define the potential beneficial effects of repopulating microglia on metabolism, future studies assessing the effect of microglia depletion at different time points in obese or diabetic mice are warranted. Furthermore, gene expression profiling between disease-associated activated microglia and repopulated microglia at different time points is also needed to determine whether repopulated microglia remain healthy and whether, with time, they may become detrimental, worsening the pathological conditions.
Genetic Models of Microglial Depletion
Besides pharmacological methods, genetic approaches have also been used for microglia depletion. For microglia-specific depletion purposes, tamoxifen-dependent inducible Cx3cr1CreER mice have been crossed with cre-dependent diphtheria toxin (DT) receptor (DTR)-expressing transgenic mice. Upon DT administration, significant microglia depletion has been shown to occur after just 1 day and full repopulation after 2 wk, seemingly faster than the inhibitor treatment (41, 43). Tamoxifen administration resulted in microglia-specific DTR expression, as the short-lived peripheral monocytes were replenished by bone marrow-derived nonmutated progenitors (42). An early study showed that DT-mediated microglia depletion had no effect on body weight and cytokine expression in young mice (P30) after 1 wk of microglia deletion and did not alter the number of peripheral monocytes and peripheral tissue-resident macrophages (43). However, microglia depletion in young mice (at P19, P30, and P60) altered neuronal spine remodeling, leading to deficits in learning and memory (43).
In the Cx3cr1-DTR rat model, DT-mediated microglia depletion resulted in reduced body weight, locomotive activity, and energy expenditure accompanied by hypophagia. These metabolic changes did not correlate with the changes in hormone signals and neuronal activation, since microglia-depleted Cx3cr1-DTR rats had increased ghrelin, reduced leptin, enhanced NPY/AgRP neuronal projection onto the PVN, and a decreased number of POMC neurons (85). These contradictory results could be attributed to microglial cell death. In support of this, microglia-depleted Cx3cr1-DTR rats display febrile symptoms (86), and some studies have shown that DT-mediated microglia depletion induces astrogliosis and a cytokine storm (87), as accompanied by altered circadian rhythm-related gene expression (86).
Some studies, however, investigated the effects of long-term microglia loss without repopulation and observed changes in body weight and food intake. Although microglia-driven factors were missing, altered neuronal activity was observed. Further evaluation of the activity of specific neuronal populations, especially in the hypothalamus, is needed to clarify the effects of chronic microglia depletion.
Although microglia depletion is a promising approach to define its role in metabolism regulation, it lacks regional specificity. Many transcriptome studies have established the concept of microglia heterogeneity. However, regional depletion might be a challenging approach for microglia study. A recent study induced striatum-specific microglia depletion of ∼50%, by using Drd1aCre and Drd2Cre mice with deleted Il-34 in either D1 or D2 neurons (88). On the other hand, inducible ablation of Csf1 in NestinCre mice resulted in the loss of microglia predominantly in the white matter of the striatum (88). According to this study, neuron-specific Cre mice can be used for local microglia depletion. For instance, Il-34 deficiency in POMC, AgRP, or Sf1 neurons could be used to alter local microglia and thus to study their role in energy and glucose homeostasis. Similarly, Cx3cl1 could also be considered as a target to attenuate microglia activity. Additionally, direct and regional gene manipulation on microglia could be also achieved by the adeno-associated virus (AAV) strategy. Some reports have shown the success of AAV-mediated gene delivery into microglia (89, 90). However, AAV-mediated gene delivery potentially induces damage in the brain parenchyma, thus affecting microglia activation. Therefore, more studies are needed to determine whether the AAV strategy is a safe and appropriate approach to study microglia.
Hypercaloric Diet-Induced Microglia Activation
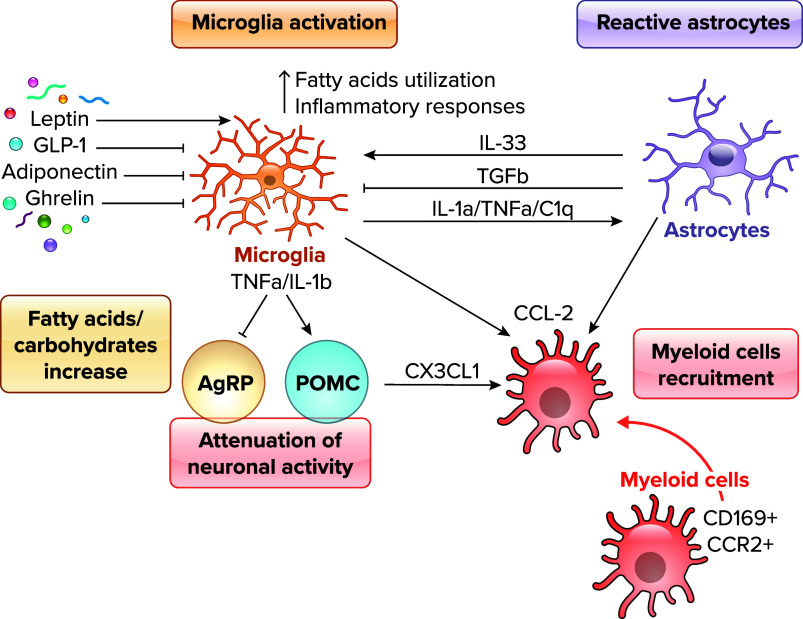
Hypothalamic microglia quickly respond and get activated after HFD consumption (FIGURE 1). Previous studies have shown that chronic hypothalamic microglia activation is associated with obesity development in both mice and humans (1–4, 10). In rodents, HFD drives microglia morphological changes into amoeboid shapes with enlarged cell bodies and thickened processes indicating microglia physiological switch from rest to activated state (5). Similar to what is observed in mouse models, in humans evidence has also suggested a correlation between obesity and hypothalamic microglia activation (3, 4). Microglial activation is observed in many areas of the brain in response to excessive dietary fats (91, 92) but predominantly in the ARC area (7, 8). HFD-induced microglial alteration is primarily a consequence of HFD consumption itself rather than the consequence of the obese phenotype. Indeed, genetically obese mice, such as mice with either leptin, leptin receptor, or melanocortin 4 receptor (MC4R) deficiency, fed on a standard chow diet do not present hypothalamic microglia activation (1). In addition, specific types of fatty acids seem to induce greater microglia activation than others, as suggested by the observation that when dietary saturated fatty acids (SFAs) are replaced by similar amounts of unsaturated fatty acids (UFAs) hypothalamic inflammation does not occur (88). In addition, Gao and collaborators (11) have shown that low-carbohydrate high-fat (LCHF) diet failed to induce microglia activation while inducing activation of astrocytes. Notably, LCHF-fed mice showed lower body weight gain compared with mice fed high-carbohydrate high-fat (HCHF) diet (11). Thus, this observation suggests that both carbohydrates and fat are necessary for microglia activation.
FIGURE 1.
Hypothalamic microglia activation during high-fat diet (HFD) Microglia response to changes in dietary molecules and hormones [leptin, glucagon-like peptide 1 (GLP-1), adiponectin, ghrelin]. The increase of fatty acids in HFD feeding switches microglia fuel utilization toward fatty acids and enhances inflammatory responses in the hypothalamus. Cytokines released from microglia can affect neuronal activity (TNFa, IL-1b) as well as astrocytic activity (IL-1a, TNFa, C1q). Activated neurons, astrocytes, and microglia recruit CD169+ and CCR2+ peripheral myeloid cells in the hypothalamus via release of chemokies such as CX3CL1 and CCL2. AgRP, Agouti-related protein; POMC, proopiomelanocortin.
At early time points from the start of HFD administration, microglia activation is accompanied by changes in cytokine expression and release. Cytokines themselves are known modulators of food intake and neuronal activation. Intracerebroventricular administration of cytokines such as TNFα, IL-1β, and IL-6 suppress food intake in rodents (93–97). In contrast, central administration of IL-4, known as an anti-inflammatory cytokine, promotes hypothalamic inflammation and increases weight gain during high-fat feeding (98). Suppressive effects on food intake by proinflammatory cytokines such as TNFα and IL-1β raise the question of whether the early inflammatory response to HFD is protective or causative for obesity development. Microglia inflammatory responses have been shown to occur very quickly, beginning as early as 1 day after HFD exposure. Within the first day of HFD feeding, the level of TNFα, IL-1β, and IL-6 mRNA significantly increased at 3 h and returned to baseline level after 6 h of HFD feeding (99). These cytokine levels were increased again at 4 wk of HFD (3), whereas at 8 wk of HFD feeding, when mice show significantly greater body weight, fat mass, and hormone resistance, the levels of TNFα, IL-1β, and IL-6 mRNA were again comparable to those observed in mice fed on a standard chow diet (8). Collectively, a strong inflammatory response has not necessarily been seen along with obesity development. With this view, the inflammatory activity in the early time points of HFD exposure may be neuroprotective rather than detrimental. In addition, the similar levels of cytokines between HFD and standard chow diet observed at 8 wk of HFD feeding suggest that the reduction in cytokine levels during HFD may cause the metabolic phenotype. Long-term HFD may reduce the expression of microglia-specific sensing genes, including P2ry12, Selplg, Slc2a5, and Trem2, and consequent reduction of CD68, TMEM119, and P2RY12 protein levels in the ARC microglia (4, 7). Interestingly, microglia in chronic HFD-exposed mice can react normally to LPS stimulation (7). Furthermore, long-term HFD-induced ARC microglial activation can also be reversed by switching to a standard chow diet (100), suggesting that HFD-induced hypothalamic microglia activation may differ from microglia activation observed in pathological conditions, such as Alzheimer’s disease, where its activation seems to be irreversible.
After a few weeks of HFD, CD169+ and CCR2+ peripheral myeloid cells are recruited to the mediobasal hypothalamus (MBH; FIGURE 1), possibly through CX3CL1/CX3CR1 and CCL2/CCR2 axes, and contribute to obesity susceptibility (7, 101–103). HFD feeding induces BBB leakage in the hypothalamic area (102), in which infiltrated macrophages can further disturb the BBB integrity and increase lipid flux. These lipids, in turn, are taken up by microglial cells for clearance. The lipid homeostasis mediated by microglial cells has been shown to be important for whole body energy homeostasis. Low-density lipoprotein receptor (LDLR)-deficient mice showed enhanced hypothalamic microglia activation on HFD compared with control mice (104) due to impaired LDL clearance and disturbed cholesterol homeostasis, indicating that microglial lipid metabolism is critical to maintain healthy metabolic condition. HFD feeding forces microglia to use lipids rather than glucose or glutamine (105). Interestingly, limiting fatty acid utilization by microglia is beneficial for DIO (FIGURE 1). For instance, primary Ucp2-deleted microglia cells lose preference for palmitate. As a result, microglia Ucp2-deficient mice displayed mild inflammatory response to HFD and resistance to DIO (8), thus suggesting that altering lipid metabolism in microglia may represent a potential target for obesity prevention.
HFD not only affects fuel preference but can also disturb the circadian rhythm regulating microglia activation (105). Under normal physiological conditions, microglia activation can be observed during the night period, when rodents show active feeding behavior (106). Although microglia-specific clock gene deletion has been shown to disturb their circadian rhythm but have no effect on microglia morphology, microglia Bmal1-deficient mice showed protection from DIO (50). Interestingly, whole body depletion of Rev-erbα, a Bmal1 repressor, caused spontaneous microglia activation during the day period (107), indicating that microglia activation during the night period seems dependent on circadian rhythm.
The observed upregulation of cathepsin S (CatS) and P2RY12, two proteins known to increase with microglia activation at the beginning of the dark period, suggests a possible mechanism for changes in microglia activation during HFD. Cortical microglia can be activated by adenosine triphosphate (ATP) released from active cortical neurons via P2RY12 and secrete CatS to the synaptic sites of neighboring neurons. CatS degrades surrounding extracellular matrixes (ECMs), including the perineuronal nets (PNNs) that regulate the maturation and elimination of neuronal dendritic spines (108), which has been shown to restrict neuronal plasticity in the ARC. Indeed, a previous report found that the ARC is surrounded by PNN-like structures that are critical for AgRP neuronal maturation (109). As observed in the prefrontal cortex, HFD reduced the PNN intensity (110), an event that could also occur in the ARC. Therefore, on HFD microglia might influence ARC neuronal activity through PNN alteration via CatS.
Microglia Communication in Metabolism Regulation
Microglia and Hormones
Several metabolic hormones have been shown to affect microglia activation (FIGURE 1) (4–12).
Leptin, a cytokine secreted by adipocytes, signals to the brain to regulate food intake. Chronic HFD feeding induces hyperleptinemia due to increased fat mass. In vitro studies performed on primary hypothalamic microglia have shown that leptin treatment has proinflammatory effects by increasing TNFα and IL-1β expression (111), possibly as a consequence of JNK-STAT3 pathway activation. However, leptin treatment did not affect TNFα-induced cytokine levels in hypothalamic slice explants (112). In vivo, mice with selective deletion of leptin receptor in myeloid cells displayed increased body weight and increased food intake without alteration of glucose homeostasis when fed on a standard chow diet (13). Interestingly, these mice showed dystrophic microglial morphology with reduced branches in the PVN but not in the ARC. Furthermore, CD68 expression was also reduced in the PVN microglia. Within the ARC, although no changes in microglia morphology and activation could be observed, the number of POMC neurons and α-MSH levels were reduced, with no changes observed in the AgRP levels (13). Overall, whereas in vitro leptin acts as a proinflammatory signal in microglia, in vivo leptin is required for microglia homeostasis and plays a role in formation of the hypothalamic circuits regulating metabolism.
In addition to leptin, the gut hormone ghrelin also modulates microglia inflammation. Although it exerts an orexigenic activity, ghrelin acts as an anti-inflammatory hormone. Evidence has shown that 1 day of HFD feeding is sufficient to induce an increased number of macrophages/microglia in the nodose ganglion and in the hypothalamus. Interestingly, subcutaneous administration of ghrelin before HFD feeding can prevent this increase in microglia, indicating that ghrelin has an anti-inflammatory role (14).
Glucagon-like peptide 1 (GLP-1) is a hormone secreted by intestinal enteroendocrine L cells (113). In the brain, GLP-1-producing neurons are present in the nucleus of the solitary tract (NTS) (114). GLP-1 receptor is expressed on microglia as well as in neurons (18, 19). In BV-2 microglia cells, GLP-1 directly suppresses the expression of proinflammatory cytokines (i.e., TNFα, IL-6, and IL-1β) (19). In line with this, a GLP-1 agonist (liraglutide) decreased the microgliosis in the MBH of HFD-fed mice (20). However, although these data suggest a role for GLP-1 as an anti-inflammatory molecule in microglia, a microglia GLP-1R-deficient animal model has not yet been evaluated.
APN, an adipokine (115), enhances insulin sensitivity through the activation of AMP-activated protein kinase (AMPK) in peripheral tissues (116). Additionally, APN crosses the BBB and acts through two APN receptors (ADIPOR1 and ADIPOR2) expressed in neurons, microglia, and astrocytes (117). Systemic APN administration suppresses microglia activation induced by HFD, whereas it has no effects on body weight gain (15). On the other hand, central APN administration has been shown to decrease body weight by enhancing energy expenditure and increasing the number of cFos-positive neurons in the PVN (16). APN-deficient mice were protected from DIO with decreased food intake and increased energy expenditure. This antiobesity phenotype might be due to a reduction in NPY and an increase in POMC levels in APN-deficient mice (17). Although much evidence points at a direct effect of APN on microglia, microglia-specific receptor-deficient mouse models have not been studied so far.
Microglia-Neuron Communication
Extensive evidence has shown that a prolonged hypercaloric diet induces chronic microglia activation leading to hypothalamic neuronal dysfunction, including loss of synapses (118), impaired responsiveness to hormones (119–121), and altered intracellular organelle function (8, 122–124), causing obesity and diabetes development. One of the critical factors regulating microglia-neuron interaction in this context is represented by the release of cytokines. Microglia activation induces the upregulation of pathways related to cytokine signaling in hypothalamic neurons (119, 125–129). Multiple studies have evaluated the direct effect of cytokines on neuronal activity through electrophysiological approaches. In vitro incubation of hypothalamic brain slice of POMCeGFP mice with TNF-α led to an increased POMC neuronal firing rate, increased intracellular ATP production, and mitochondrial fusion processes (130). In agreement with this, Chaves and collaborators (131) showed that both TNFα and IL-1β acutely inhibited neuronal activity of 35–42% of AgRP neurons, whereas ∼11% of POMC neurons were activated by TNFα, thus supporting the suppressive effect of TNFα and IL-1β on food intake through the direct modulation of ARC neuronal activity. However, this study also showed that IL-6 had no effect on AgRP or POMC neuronal activity (131), data that are in disagreement with a previous report showing the antiobesity effects of IL-6 by increasing energy expenditure and that Il-6-deficient mice developed mature-onset obesity (132). Consistent with these data, central Il-6Rα-deficient mice showed increased food intake and reduced energy expenditure (133). Future experiments studying microglia-specific cytokine-deficient mice are needed to clarify these contradictory data.
In addition to cytokines, altered phagocytotic activity can lead to impaired neuronal activity (134, 135) by altering synaptic plasticity (118). HFD has been shown to alter the synaptic reorganization of hypothalamic POMC and AgRP neurons (118), and these synaptic changes are associated with increased astrocytic activity. Although microglia-mediated effects on synaptic reorganization have not been directly investigated, indirect evidence supports the role of microglia on synaptic reorganization. For instance, altered phagocytotic activity has been reported in animal models of obesity (49, 50), and the number of POMC neurons in direct contact with microglia is increased in DIO mice (130). In addition, our laboratory has also reported that in a genetic mouse model in which HFD-induced microglial activation was attenuated astrogliosis was also reduced, resulting in a significant increase in POMC neuronal activation and leptin sensitivity that were associated with a change in the synaptic input organization onto POMC neurons (8). Whether the synaptic reorganization was due to the direct effect of microglia on neurons or through their indirect effect via astrocytes needs to be assessed.
Finally, neuron-microglia communication may also occur through neurotransmitters such as glutamate. For example, when neurons release glutamate, the brain primary excitatory neurotransmitter, astrocytes uptake glutamate from the extracellular space via GLT-1 and convert it to glutamine before releasing it back into the extracellular space for neuronal uptake. However, glutamate release can also induce microglia activation and induce release of TNFα (136, 137). In turn, TNFα stimulates microglia to release glutamate, suggesting that neurotransmitter-mediated cross talk between neurons and microglia may be a regulatory module controlling their reciprocal activity (138).
Microglia-Astrocyte Communication
Microglia and astrocytes are known to communicate between themselves as well as with neurons to maintain brain homeostasis. Since the bidirectional communication between astrocytes and neurons was initially emphasized in the concept of the tripartite synapse (139), the multipartite synapse including microglia, astrocytes, and neurons was further presented as an integral element for fine-tuned communication (140). This intercellular communication occurs through the release of a variety of molecules, including cytokines, chemokines, ATP, and growth factors (FIGURE 1).
Together with tanycytes, specialized ependymal cells that line the third ventricle and allow the transfer of signals from the cerebrospinal fluid to the CNS, hypothalamic astrocytes have been reported to play a critical role in regulating nutrient and hormone sensing within the CNS. Astrocytes can sense and transport glucose via gap junctions, the astroglial glucose transporter (GLUT)-1, and insulin receptors (IRs) (141, 142), thus suggesting a role for these glial cells in regulating systemic glucose metabolism. Indeed, astrocyte-specific IR depletion has been shown to reduce glucose-driven POMC neuronal activation. This indicates that insulin signaling in hypothalamic astrocytes requires glucose-induced activation of POMC neurons (142). Likewise, astrocyte-specific leptin receptor diminished inhibitory effects of leptin on food intake (143). Emerging evidence indicates that glial cells including microglia, astrocytes, and tanycytes integrate peripheral information and support neurons in response to systemic metabolic states. Therefore, microglia-astrocyte cross talk has been suggested to play a role in regulating whole body energy homeostasis.
As discussed above, activated microglia secrete multiple proinflammatory molecules, such as TNF-α and IL-1α, which can drive secondary inflammatory responses from astrocytes (144). Additionally, other factors originating from microglia such as nitric oxide (NO), type I IFN, C1q, and VEGF-B can also modulate astrocyte proliferation and differentiation (145). Likewise, astrocyte-driven molecules can enhance or inhibit microglial activity. Astrocytic transforming growth factor (TGF)-β, orosomucoid-2 (ORM2), and glial cell line-derived neurotrophic factor (GDNF) all exert an anti-inflammatory effect by downregulating microglial activation (146–148). On the other hand, astrocytic LCN2 enhances microglial activity under inflammatory and pathological conditions (149). Finally, astrocyte-derived chemokines such as CCL2 and CXCL10 recruit activated microglia and promote microglial phagocytosis (150, 151).
Activated microglia induce astrocytosis. However, whether these events have a detrimental or beneficial effect seems to be determined by the inflammatory conditions. In this regard, Liddelow and collaborators (144) performed genomics analysis of reactive astrocytes induced by ischemic stroke and LPS stimulation. They showed that reactive astrocytes induced by LPS administration fail to promote neuronal survival outgrowth, synaptogenesis, and phagocytosis. Instead, they induce the death of neurons and oligodendrocytes, whereas reactive astrocytes in ischemia displayed a molecularly protective phenotype. Thus, two states of activated astrocytes have been defined, A1 being detrimental and A2 being protective. Interestingly, LPS failed to induce A1 reactive astrocytes in Csf1r−/− mice (which lack microglia), indicating the necessity of microglia in inducing the A1 astrocytic phenotype (144). Indeed, it was determined that IL-1α, TNFα, and C1q produced by activated microglia are essential factors to induce A1 reactive astrocytes (144).
To better unravel the communication between microglia and astrocytes, in vitro coculture experiments have been performed. Although the results of these studies (145, 146) suggest that the interaction between microglia and astrocyte may play a role as an autoregulatory loop to prevent an excessive inflammatory response in the brain, none of these studies assessed their role in vivo in metabolism regulation.
In conclusion, the activity of astrocytes is determined by their ability to respond to factors released by activated microglia during inflammatory conditions. In turn, microglia activation is also dependent on the inflammatory conditions and their localization within the brain. For example, the excess of nutrients induces a strong microglial inflammatory response in the hypothalamic ARC that, in turn, drives a molecularly distinct subtype of reactive astrocytes in the hypothalamus (FIGURE 1). Future studies focusing on the molecular signature of ARC microglia and astrocytes in response to nutrient availability and under different inflammatory conditions will help to define the roles of these cells and their communication, thus providing new targets in the prevention of metabolic disorders.
Hypothalamic regulation of metabolism is a complex and fine-tuned process that results from the cross talk between neurons, astrocytes, and microglia. In addition to this, peripheral macrophages and CNS-resident macrophages [CAMs; choroid plexus macrophages (cpMϕs), perivascular macrophages (pvMϕs), and meningeal macrophages (mMϕs)] can infiltrate the brain parenchyma and contribute to the regulation of metabolism. Indeed, excessive calorie intake through diet promotes the infiltration of macrophages, adding an additional level of complexity in defining brain microglia function, due to their shared myeloid hallmarks. Thus, in future studies it will be critical to discriminate the role of different microglial populations in the regulation of whole body metabolism. In line with this, Kim et al. (152) have recently developed mouse models in which the parenchymal microglia and CAMs could be distinguished based on the distinct expression pattern of Sal1 and Lyve1. Whereas Sal1 expression is restricted to microglia, Lyve1 is barely expressed in microglia and abundant in perivascular macrophages. Using the transgenic “split Cre” system, in which when two pieces of Cre enzymes named NCre and CCre are coexpressed they reconstitute a functional Cre able to cause recombination, they have successfully discriminated these two populations and characterized their translatome. Therefore, future studies using these models, Sall1ncre:Cx3cr1ccre and Lyve1ncre:Cx3cr1ccre mice (152), might offer a useful tool to discriminate the metabolic roles of microglia and CAMs in the metabolic dysfunction.
Conclusions
Given the heterogeneity of brain microglia, it has been challenging to define their functions in whole body metabolism. Several advanced technologies such as scRNA-seq, single-nucleus RNA-seq, and multiplexed mass cytometry (CyTOF) have been applied and have successfully identified subtypes of microglia. This is particularly important, since different subtypes of microglia reacting to different stimuli may have distinct properties and physiological functions, and thus produce diverse outcomes.
For example, a unique subset of microglia known as DAM has been shown to be associated with AD pathology and is conserved in mice and humans (153, 154). Like DAM, an HFD-driven unique subset of microglia may exist in the ARC. Comprehensive transcriptomic analysis at the single-cell level of ARC microglia at different stages of diet-induced obesity might provide clues to understanding the unique metabolic characteristics of ARC microglia. Furthermore, it may also address the issue of regional specificity. The success of AAV-mediated gene delivery into microglia is very promising, although presenting limitations such as the potential side effects of microglial activation due to AAV injection.
In conclusion, further studies focusing on study of distinct microglial subpopulations are needed to advance our understanding on the role of microglia in metabolism regulation and the tight association between metabolic and neurodegenerative disorders.
Acknowledgments
The authors thank Lauren Peralta and Gavin Thomas White for editing the manuscript.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases R01 DK120321, DK131717, and DK136079 (to S.D.).
S. Diano is an editor of Physiology and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
J.D.K., F.C., and S.D. drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
References
- 1. Gao Y, Ottaway N, Schriever SC, Legutko B, García-Cáceres C, de la Fuente E, Mergen C, Bour S, Thaler JP, Seeley RJ, Filosa J, Stern JE, Perez-Tilve D, Schwartz MW, Tschöp MH, Yi CX. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62: 17–25, 2014. doi: 10.1002/glia.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schur EA, Melhorn SJ, Oh SK, Lacy JM, Berkseth KE, Guyenet SJ, Sonnen JA, Tyagi V, Rosalynn M, De Leon B, Webb MF, Gonsalves ZT, Fligner CL, Schwartz MW, Maravilla KR. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring) 23: 2142–2148, 2015. doi: 10.1002/oby.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol 132: 361–375, 2016. doi: 10.1007/s00401-016-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18: 225–242, 2018. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- 6. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845, 2010. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, Gerritse I, Fasnacht R, Barres BA, Thaler JP, Koliwad SK. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab 26: 185–197.e3, 2017. doi: 10.1016/j.cmet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JD, Yoon NA, Jin S, Diano S. Microglial UCP2 mediates inflammation and obesity induced by high-fat feeding. Cell Metab 30: 952–962.e5, 2019. doi: 10.1016/j.cmet.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leyrolle Q, Layé S, Nadjar A. Direct and indirect effects of lipids on microglia function. Neurosci Lett 708: 134348, 2019. doi: 10.1016/j.neulet.2019.134348. [DOI] [PubMed] [Google Scholar]
- 10. Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9: 2124–2138, 2014. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W, Guzmán-Ruiz M, Layritz C, Legutko B, Zinser E, García-Cáceres C, Buijs RM, Woods SC, Kalsbeek A, Seeley RJ, Nawroth PP, Bidlingmaier M, Tschöp MH, Yi CX. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab 6: 897–908, 2017. doi: 10.1016/j.molmet.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pohl J, Woodside B, Luheshi GN. Leptin modulates the late fever response to LPS in diet-induced obese animals. Brain Behav Immun 42: 41–47, 2014. doi: 10.1016/j.bbi.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 13. Gao Y, Vidal-Itriago A, Milanova I, Korpel NL, Kalsbeek MJ, Tom RZ, Kalsbeek A, Hofmann SM, Yi CX. Deficiency of leptin receptor in myeloid cells disrupts hypothalamic metabolic circuits and causes body weight increase. Mol Metab 7: 155–160, 2018. doi: 10.1016/j.molmet.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waise TM, Toshinai K, Naznin F, NamKoong C, Md Moin AS, Sakoda H, Nakazato M. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun 464: 1157–1162, 2015. doi: 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- 15. Lee H, Tu TH, Park BS, Yang S, Kim JG. Adiponectin reverses the hypothalamic microglial inflammation during short-term exposure to fat-rich diet. Int J Mol Sci 20: 5738, 2019. doi: 10.3390/ijms20225738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med 10: 524–529, 2004. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 17. Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6: 55–68, 2007. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 18. Spielman LJ, Gibson DL, Klegeris A. Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. Eur J Cell Biol 96: 240–253, 2017. doi: 10.1016/j.ejcb.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 19. Yoon G, Kim YK, Song J. Glucagon-like peptide-1 suppresses neuroinflammation and improves neural structure. Pharmacol Res 152: 104615, 2020. doi: 10.1016/j.phrs.2019.104615. [DOI] [PubMed] [Google Scholar]
- 20. Barreto-Vianna AR, Aguila MB, Mandarim-de-Lacerda CA. Effects of liraglutide in hypothalamic arcuate nucleus of obese mice. Obesity (Silver Spring) 24: 626–633, 2016. doi: 10.1002/oby.21387. [DOI] [PubMed] [Google Scholar]
- 21. Kalsbeek MJ, Mulder L, Yi CX. Microglia energy metabolism in metabolic disorder. Mol Cell Endocrinol 438: 27–35, 2016. doi: 10.1016/j.mce.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 22. Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, Marsh SE, Saunders A, Macosko E, Ginhoux F, Chen J, Franklin RJ, Piao X, McCarroll SA, Stevens B. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50: 253–271.e6, 2019. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mendiola AS, Ryu JK, Bardehle S, Meyer-Franke A, Ang KK, Wilson C, Baeten KM, Hanspers K, Merlini M, Thomas S, Petersen MA, Williams A, Thomas R, Rafalski VA, Meza-Acevedo R, Tognatta R, Yan Z, Pfaff SJ, Machado MR, Bedard C, Rios Coronado PE, Jiang X, Wang J, Pleiss MA, Green AJ, Zamvil SS, Pico AR, Bruneau BG, Arkin MR, Akassoglou K. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat Immunol 21: 513–524, 2020. doi: 10.1038/s41590-020-0654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci 19: 504–516, 2016. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyata S. Glial functions in the blood-brain communication at the circumventricular organs. Front Neurosci 16: 991779, 2022. doi: 10.3389/fnins.2022.991779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA 95: 10896–10901, 1998. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah R, O’Neill SM, Hinkle C, Caughey J, Stephan S, Lynch E, Bermingham K, Lynch G, Ahima RS, Reilly MP. Metabolic effects of CX3CR1 deficiency in diet-induced obese mice. PLoS One 10: e0138317, 2015. doi: 10.1371/journal.pone.0138317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDermott DH, Colla JS, Kleeberger CA, Plankey M, Rosenberg PS, Smith ED, Zimmerman PA, Combadière C, Leitman SF, Kaslow RA, Goedert JJ, Berger EA, O’Brien TR, Murphy PM. Genetic polymorphism in CX3CR1 and risk of HIV disease. Science 290: 2031, 2000. doi: 10.1126/science.290.5499.2031a. [DOI] [PubMed] [Google Scholar]
- 29. von Vietinghoff S, Kurts C. Regulation and function of CX3CR1 and its ligand CX3CL1 in kidney disease. Cell Tissue Res 385: 335–344, 2021. doi: 10.1007/s00441-021-03473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pawelec P, Ziemka-Nalecz M, Sypecka J, Zalewska T. The impact of the CX3CL1/CX3CR1 axis in neurological disorders. Cells 9: 2277, 2020. doi: 10.3390/cells9102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopez-Lopez A, Gamez J, Syriani E, Morales M, Salvado M, Rodríguez MJ, Mahy N, Vidal-Taboada JM. CX3CR1 is a modifying gene of survival and progression in amyotrophic lateral sclerosis. PLoS One 9: e96528, 2014. doi: 10.1371/journal.pone.0096528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rivas-Fuentes S, Salgado-Aguayo A, Arratia-Quijada J, Gorocica-Rosete P. Regulation and biological functions of the CX3CL1-CX3CR1 axis and its relevance in solid cancer: a mini-review. J Cancer 12: 571–583, 2021. doi: 10.7150/jca.47022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawamura N, Katsuura G, Yamada-Goto N, Nakama R, Kambe Y, Miyata A, Furuyashiki T, Narumiya S, Ogawa Y, Inui A. Brain fractalkine-CX3CR1 signalling is anti-obesity system as anorexigenic and anti-inflammatory actions in diet-induced obese mice. Sci Rep 12: 12604, 2022. doi: 10.1038/s41598-022-16944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nagashimada M, Sawamoto K, Ni Y, Kitade H, Nagata N, Xu L, Kobori M, Mukaida N, Yamashita T, Kaneko S, Ota T. CX3CL1-CX3CR1 signaling deficiency exacerbates obesity-induced inflammation and insulin resistance in male mice. Endocrinology 162: bqab064, 2021. doi: 10.1210/endocr/bqab064. [DOI] [PubMed] [Google Scholar]
- 35. Bolós M, Perea JR, Terreros-Roncal J, Pallas-Bazarra N, Jurado-Arjona J, Ávila J, Llorens-Martín M. Absence of microglial CX3CR1 impairs the synaptic integration of adult-born hippocampal granule neurons. Brain Behav Immun 68: 76–89, 2018. doi: 10.1016/j.bbi.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 36. Nemes-Baran AD, White DR, DeSilva TM. Fractalkine-dependent microglial pruning of viable oligodendrocyte progenitor cells regulates myelination. Cell Rep 32: 108047, 2020. doi: 10.1016/j.celrep.2020.108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9: 917–924, 2006. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 38. Winkler Z, Kuti D, Polyák Á, Juhász B, Gulyás K, Lénárt N, Dénes Á, Ferenczi S, Kovács KJ. Hypoglycemia-activated hypothalamic microglia impairs glucose counterregulatory responses. Sci Rep 9: 6224, 2019. doi: 10.1038/s41598-019-42728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dorfman MD, Krull JE, Douglass JD, Fasnacht R, Lara-Lince F, Meek TH, Shi X, Damian V, Nguyen HT, Matsen ME, Morton GJ, Thaler JP. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat Commun 8: 14556, 2017. doi: 10.1038/ncomms14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Banerjee J, Dorfman MD, Fasnacht R, Douglass JD, Wyse-Jackson AC, Barria A, Thaler JP. CX3CL1 action on microglia protects from diet-induced obesity by restoring POMC neuronal excitability and melanocortin system activity impaired by high-fat diet feeding. Int J Mol Sci 23: 6380, 2022. doi: 10.3390/ijms23126380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bruttger J, Karram K, Wörtge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, Mack M, Pinteaux E, Müller W, Zipp F, Binder H, Bopp T, Prinz M, Jung S, Waisman A. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 43: 92–106, 2015. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 42. Goldmann T, Wieghofer P, Müller PF, Wolf Y, Varol D, Yona S, Brendecke SM, Kierdorf K, Staszewski O, Datta M, Luedde T, Heikenwalder M, Jung S, Prinz M. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci 16: 1618–1626, 2013. doi: 10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- 43. Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155: 1596–1609, 2013. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tay TL, Mai D, Dautzenberg J, Fernández-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grün D, Ronneberger O, Prinz M. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci 20: 793–803, 2017. doi: 10.1038/nn.4547. [DOI] [PubMed] [Google Scholar]
- 45. Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschöp MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454: 846–851, 2008. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D, Paradies E, Scarcia P, Palmieri F, Bouillaud F, Fiermonte G. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci U S A 111: 960–965, 2014. doi: 10.1073/pnas.1317400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoon NA, Jin S, Kim JD, Liu ZW, Sun Q, Cardone R, Kibbey R, Diano S. UCP2-dependent redox sensing in POMC neurons regulates feeding. Cell Rep 41: 111894, 2022. doi: 10.1016/j.celrep.2022.111894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao Y, Vidal-Itriago A, Kalsbeek MJ, Layritz C, García-Cáceres C, Tom RZ, Eichmann TO, Vaz FM, Houtkooper RH, van der Wel N, Verhoeven AJ, Yan J, Kalsbeek A, Eckel RH, Hofmann SM, Yi CX. Lipoprotein lipase maintains microglial innate immunity in obesity. Cell Rep 20: 3034–3042, 2017. doi: 10.1016/j.celrep.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 49. Niraula A, Fasnacht RD, Ness KM, Frey JM, Cuschieri SA, Dorfman MD, Thaler JP. Prostaglandin PGE2 receptor EP4 regulates microglial phagocytosis and increases susceptibility to diet-induced obesity. Diabetes 72: 233–244, 2023. doi: 10.2337/db21-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang XL, Kooijman S, Gao Y, Tzeplaeff L, Cosquer B, Milanova I, Wolff SE, Korpel N, Champy MF, Petit-Demoulière B, Goncalves Da Cruz I, Sorg-Guss T, Rensen PC, Cassel JC, Kalsbeek A, Boutillier AL, Yi CX. Microglia-specific knock-down of Bmal1 improves memory and protects mice from high fat diet-induced obesity. Mol Psychiatry 26: 6336–6349, 2021. doi: 10.1038/s41380-021-01169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Butler CA, Popescu AS, Kitchener EJ, Allendorf DH, Puigdellívol M, Brown GC. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J Neurochem 158: 621–639, 2021. doi: 10.1111/jnc.15327. [DOI] [PubMed] [Google Scholar]
- 52. Chappell-Maor L, Kolesnikov M, Kim JS, Shemer A, Haimon Z, Grozovski J, Boura-Halfon S, Masuda T, Prinz M, Jung S. Comparative analysis of CreER transgenic mice for the study of brain macrophages: a case study. Eur J Immunol 50: 353–362, 2020. doi: 10.1002/eji.201948342. [DOI] [PubMed] [Google Scholar]
- 53. Van Hove H, Antunes AR, De Vlaminck K, Scheyltjens I, Van Ginderachter JA, Movahedi K. Identifying the variables that drive tamoxifen-independent CreERT2 recombination: implications for microglial fate mapping and gene deletions. Eur J Immunol 50: 459–463, 2020. doi: 10.1002/eji.201948162. [DOI] [PubMed] [Google Scholar]
- 54. Buttgereit A, Lelios I, Yu X, Vrohlings M, Krakoski NR, Gautier EL, Nishinakamura R, Becher B, Greter M. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol 17: 1397–1406, 2016. doi: 10.1038/ni.3585. [DOI] [PubMed] [Google Scholar]
- 55. McKinsey GL, Lizama CO, Keown-Lang AE, Niu A, Santander N, Larpthaveesarp A, Chee E, Gonzalez FF, Arnold TD. A new genetic strategy for targeting microglia in development and disease. eLife 9: e54590, 2020. doi: 10.7554/eLife.54590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Masuda T, Amann L, Sankowski R, Staszewski O, Lenz M, D Errico P, Snaidero N, Costa Jordão MJ, Böttcher C, Kierdorf K, Jung S, Priller J, Misgeld T, Vlachos A, Meyer-Luehmann M, Knobeloch KP, Prinz M. Novel Hexb-based tools for studying microglia in the CNS. Nat Immunol 21: 802–815, 2020. doi: 10.1038/s41590-020-0707-4. [DOI] [PubMed] [Google Scholar]
- 57. Kaiser T, Feng G. Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro 6: ENEURO.0448-18.2019, 2019. doi: 10.1523/ENEURO.0448-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci 17: 131–143, 2014. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol 32: 367–402, 2014. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patel S, Player MR. Colony-stimulating factor-1 receptor inhibitors for the treatment of cancer and inflammatory disease. Curr Top Med Chem 9: 599–610, 2009. doi: 10.2174/156802609789007327. [DOI] [PubMed] [Google Scholar]
- 61. Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, Mehler MF, Stanley ER. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol 367: 100–113, 2012. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One 6: e26317, 2011. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wegiel J, Wiśniewski HM, Dziewiatkowski J, Tarnawski M, Kozielski R, Trenkner E, Wiktor-Jedrzejczak W. Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice. Brain Res 804: 135–139, 1998. doi: 10.1016/S0006-8993(98)00618-0. [DOI] [PubMed] [Google Scholar]
- 64. Kondo Y, Lemere CA, Seabrook TJ. Osteopetrotic (op/op) mice have reduced microglia, no Abeta deposition, and no changes in dopaminergic neurons. J Neuroinflammation 4: 31, 2007. doi: 10.1186/1742-2094-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kündig TM, Frei K, Ginhoux F, Merad M, Becher B. Stroma-derived interleukin-34 controls the development and maintenance of Langerhans cells and the maintenance of microglia. Immunity 37: 1050–1060, 2012. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13: 753–760, 2012. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Easley-Neal C, Foreman O, Sharma N, Zarrin AA, Weimer RM. CSF1R ligands IL-34 and CSF1 are differentially required for microglia development and maintenance in white and gray matter brain regions. Front Immunol 10: 2199, 2019. doi: 10.3389/fimmu.2019.02199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347: 1138–1142, 2015. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 69. Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lamb YN. Pexidartinib: first approval. Drugs 79: 1805–1812, 2019. doi: 10.1007/s40265-019-01210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med 373: 428–437, 2015. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 72. Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, Cloughesy TF, Marimuthu A, Haidar S, Perry A, Huse J, Phillips J, West BL, Nolop KB, Hsu HH, Ligon KL, Molinaro AM, Prados M. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol 18: 557–564, 2016. doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tahmasebi F, Barati S, Kashani IR. Effect of CSF1R inhibitor on glial cells population and remyelination in the cuprizone model. Neuropeptides 89: 102179, 2021. doi: 10.1016/j.npep.2021.102179. [DOI] [PubMed] [Google Scholar]
- 74. Wang CF, Zhao CC, Liu WL, Huang XJ, Deng YF, Jiang JY, Li WP. Depletion of microglia attenuates dendritic spine loss and neuronal apoptosis in the acute stage of moderate traumatic brain injury in mice. J Neurotrauma 37: 43–54, 2020. doi: 10.1089/neu.2019.6460. [DOI] [PubMed] [Google Scholar]
- 75. Elmore MR, Hohsfield LA, Kramár EA, Soreq L, Lee RJ, Pham ST, Najafi AR, Spangenberg EE, Wood MA, West BL, Green KN. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 17: e12832, 2018. doi: 10.1111/acel.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dagher NN, Najafi AR, Kayala KM, Elmore MR, White TE, Medeiros R, West BL, Green KN. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J Neuroinflammation 12: 139, 2015. doi: 10.1186/s12974-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rice RA, Pham J, Lee RJ, Najafi AR, West BL, Green KN. Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia 65: 931–944, 2017. doi: 10.1002/glia.23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Willis EF, MacDonald KP, Nguyen QH, Garrido AL, Gillespie ER, Harley SB, Bartlett PF, Schroder WA, Yates AG, Anthony DC, Rose-John S, Ruitenberg MJ, Vukovic J. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell 180: 833–846.e16, 2020. doi: 10.1016/j.cell.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 79. Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB, Szeto GL, Wu J, Stoica BA, Faden AI, Loane DJ. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci 40: 2960–2974, 2020. doi: 10.1523/JNEUROSCI.2402-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Casali BT, MacPherson KP, Reed-Geaghan EG, Landreth GE. Microglia depletion rapidly and reversibly alters amyloid pathology by modification of plaque compaction and morphologies. Neurobiol Dis 142: 104956, 2020. doi: 10.1016/j.nbd.2020.104956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gratuze M, Chen Y, Parhizkar S, Jain N, Strickland MR, Serrano JR, Colonna M, Ulrich JD, Holtzman DM. Activated microglia mitigate Aβ-associated tau seeding and spreading. J Exp Med 218: e20210542, 2021. doi: 10.1084/jem.20210542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Elmore MR, Lee RJ, West BL, Green KN. Characterizing newly repopulated microglia in the adult mouse: impacts on animal behavior, cell morphology, and neuroinflammation. PLoS One 10: e0122912, 2015. doi: 10.1371/journal.pone.0122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rosin JM, Vora SR, Kurrasch DM. Depletion of embryonic microglia using the CSF1R inhibitor PLX5622 has adverse sex-specific effects on mice, including accelerated weight gain, hyperactivity and anxiolytic-like behaviour. Brain Behav Immun 73: 682–697, 2018. doi: 10.1016/j.bbi.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 84. Cartier N, Lewis CA, Zhang R, Rossi FM. The role of microglia in human disease: therapeutic tool or target? Acta Neuropathol 128: 363–380, 2014. doi: 10.1007/s00401-014-1330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. De Luca SN, Sominsky L, Soch A, Wang H, Ziko I, Rank MM, Spencer SJ. Conditional microglial depletion in rats leads to reversible anorexia and weight loss by disrupting gustatory circuitry. Brain Behav Immun 77: 77–91, 2019. doi: 10.1016/j.bbi.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 86. Sominsky L, Dangel T, Malik S, De Luca SN, Singewald N, Spencer SJ. Microglial ablation in rats disrupts the circadian system. FASEB J 35: e21195, 2021. doi: 10.1096/fj.202001555RR. [DOI] [PubMed] [Google Scholar]
- 87. Green KN, Crapser JD, Hohsfield LA. To kill a microglia: a case for CSF1R inhibitors. Trends Immunol 41: 771–784, 2020. doi: 10.1016/j.it.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A, Hwang P, Chan AT, Graves SM, Uweru JO, Ledderose C, Kutlu MG, Wheeler MA, Kahan A, Ishikawa M, Wang YC, Loh YE, Jiang JX, Surmeier DJ, Robson SC, Junger WG, Sebra R, Calipari ES, Kenny PJ, Eyo UB, Colonna M, Quintana FJ, Wake H, Gradinaru V, Schaefer A. Negative feedback control of neuronal activity by microglia. Nature 586: 417–423, 2020. doi: 10.1038/s41586-020-2777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lin R, Zhou Y, Yan T, Wang R, Li H, Wu Z, Zhang X, Zhou X, Zhao F, Zhang L, Li Y, Luo M. Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat Methods 19: 976–985, 2022. doi: 10.1038/s41592-022-01547-7. [DOI] [PubMed] [Google Scholar]
- 90. Okada Y, Hosoi N, Matsuzaki Y, Fukai Y, Hiraga A, Nakai J, Nitta K, Shinohara Y, Konno A, Hirai H. Development of microglia-targeting adeno-associated viral vectors as tools to study microglial behavior in vivo. Commun Biol 5: 1224, 2022. doi: 10.1038/s42003-022-04200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci 34: 2618–2631, 2014. doi: 10.1523/JNEUROSCI.4200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Spencer SJ, D’Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging 58: 88–101, 2017. doi: 10.1016/j.neurobiolaging.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Plata-Salamán CR, Oomura Y, Kai Y. Tumor necrosis factor and interleukin-1 beta: suppression of food intake by direct action in the central nervous system. Brain Res 448: 106–114, 1988. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- 94. Sonti G, Ilyin SE, Plata-Salamán CR. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am J Physiol Regul Integr Comp Physiol 270: R1394–R1402, 1996. doi: 10.1152/ajpregu.1996.270.6.R1394. [DOI] [PubMed] [Google Scholar]
- 95. Nelson KP, Marks NL, Heyen JR, Johnson RW. Behavior of adult and aged mice before and after central injection of interleukin-1beta. Physiol Behav 66: 673–679, 1999. doi: 10.1016/S0031-9384(98)00339-4. [DOI] [PubMed] [Google Scholar]
- 96. Plata-Salamán CR, Sonti G, Borkoski JP, Wilson CD, French-Mullen JM. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav 60: 867–875, 1996. doi: 10.1016/0031-9384(96)00148-5. [DOI] [PubMed] [Google Scholar]
- 97. Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson JO. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun 293: 560–565, 2002. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 98. Oh-I S, Thaler JP, Ogimoto K, Wisse BE, Morton GJ, Schwartz MW. Central administration of interleukin-4 exacerbates hypothalamic inflammation and weight gain during high-fat feeding. Am J Physiol Endocrinol Metab 299: E47–E53, 2010. doi: 10.1152/ajpendo.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cansell C, Stobbe K, Sanchez C, Le Thuc O, Mosser CA, Ben-Fradj S, Leredde J, Lebeaupin C, Debayle D, Fleuriot L, Brau F, Devaux N, Benani A, Audinat E, Blondeau N, Nahon JL, Rovère C. Dietary fat exacerbates postprandial hypothalamic inflammation involving glial fibrillary acidic protein-positive cells and microglia in male mice. Glia 69: 42–60, 2021. doi: 10.1002/glia.23882. [DOI] [PubMed] [Google Scholar]
- 100. Berkseth KE, Guyenet SJ, Melhorn SJ, Lee D, Thaler JP, Schur EA, Schwartz MW. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology 155: 2858–2867, 2014. doi: 10.1210/en.2014-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Morari J, Anhe GF, Nascimento LF, de Moura RF, Razolli D, Solon C, Guadagnini D, Souza G, Mattos AH, Tobar N, Ramos CD, Pascoal VD, Saad MJ, Lopes-Cendes I, Moraes JC, Velloso LA. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes 63: 3770–3784, 2014. doi: 10.2337/db13-1495. [DOI] [PubMed] [Google Scholar]
- 102. Lee CH, Kim HJ, Lee YS, Kang GM, Lim HS, Lee SH, Song DK, Kwon O, Hwang I, Son M, Byun K, Sung YH, Kim S, Kim JB, Choi EY, Kim YB, Kim K, Kweon MN, Sohn JW, Kim MS. Hypothalamic macrophage inducible nitric oxide synthase mediates obesity-associated hypothalamic inflammation. Cell Rep 25: 934–946.e5, 2018. doi: 10.1016/j.celrep.2018.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29: 313–326, 2009. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yi CX, Al-Massadi O, Donelan E, Lehti M, Weber J, Ress C, Trivedi C, Müller TD, Woods SC, Hofmann SM. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol Behav 106: 485–490, 2012. doi: 10.1016/j.physbeh.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 105. Milanova IV, Kalsbeek MJ, Wang XL, Korpel NL, Stenvers DJ, Wolff SE, de Goede P, Heijboer AC, Fliers E, la Fleur SE, Kalsbeek A, Yi CX. Diet-induced obesity disturbs microglial immunometabolism in a time-of-day manner. Front Endocrinol (Lausanne) 10: 424, 2019. doi: 10.3389/fendo.2019.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Guzmán‐Ruiz MA, Guerrero‐Vargas NN, Lagunes‐Cruz A, González‐González S, García‐Aviles JE, Hurtado‐Alvarado G, Mendez‐Hernández R, Chavarría‐Krauser A, Morin JP, Arriaga‐Avila V, Buijs RM, Guevara‐Guzmán R. Circadian modulation of microglial physiological processes and immune responses. Glia 71: 155–167, 2023. doi: 10.1002/glia.24261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, Hayes ME, Cedeño MR, Nadarajah CJ, Ezerskiy LA, Colonna M, Zhang J, Bauer AQ, Burris TP, Musiek ES. Circadian clock protein Rev-erbα regulates neuroinflammation. Proc Natl Acad Sci USA 116: 5102–5107, 2019. doi: 10.1073/pnas.1812405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nakanishi H, Ni J, Nonaka S, Hayashi Y. Microglial circadian clock regulation of microglial structural complexity, dendritic spine density and inflammatory response. Neurochem Int 142: 104905, 2021. doi: 10.1016/j.neuint.2020.104905. [DOI] [PubMed] [Google Scholar]
- 109. Mirzadeh Z, Alonge KM, Cabrales E, Herranz-Pérez V, Scarlett JM, Brown JM, Hassouna R, Matsen ME, Nguyen HT, Garcia-Verdugo JM, Zeltser LM, Schwartz MW. Perineuronal net formation during the critical period for neuronal maturation in the hypothalamic arcuate nucleus. Nat Metab 1: 212–221, 2019. doi: 10.1038/s42255-018-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dingess PM, Harkness JH, Slaker M, Zhang Z, Wulff SS, Sorg BA, Brown TE. Consumption of a high-fat diet alters perineuronal nets in the prefrontal cortex. Neural Plast 2018: 2108373, 2018. doi: 10.1155/2018/2108373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lafrance V, Inoue W, Kan B, Luheshi GN. Leptin modulates cell morphology and cytokine release in microglia. Brain Behav Immun 24: 358–365, 2010. doi: 10.1016/j.bbi.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 112. Tsunekawa T, Banno R, Mizoguchi A, Sugiyama M, Tominaga T, Onoue T, Hagiwara D, Ito Y, Iwama S, Goto M, Suga H, Sugimura Y, Arima H. Deficiency of PTP1B attenuates hypothalamic inflammation via activation of the JAK2-STAT3 pathway in microglia. EBioMedicine 16: 172–183, 2017. doi: 10.1016/j.ebiom.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Drucker DJ. The biology of incretin hormones. Cell Metab 3: 153–165, 2006. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 114. Holt MK, Richards JE, Cook DR, Brierley DI, Williams DL, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes 68: 21–33, 2019. doi: 10.2337/db18-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270: 26746–26749, 1995. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 116. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 117. Guillod-Maximin E, Roy AF, Vacher CM, Aubourg A, Bailleux V, Lorsignol A, Pénicaud L, Parquet M, Taouis M. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol 200: 93–105, 2009. doi: 10.1677/JOE-08-0348. [DOI] [PubMed] [Google Scholar]
- 118. Horvath TL, Sarman B, García-Cáceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Brönneke HS, Levin BE, Diano S, Cowley MA, Tschöp MH. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA 107: 14875–14880, 2010. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Quarta C, Fioramonti X, Cota D. POMC neurons dysfunction in diet-induced metabolic disease: hallmark or mechanism of disease? Neuroscience 447: 3–14, 2020. doi: 10.1016/j.neuroscience.2019.09.031. [DOI] [PubMed] [Google Scholar]
- 120. Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 121. Olofsson LE, Unger EK, Cheung CC, Xu AW. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc Natl Acad Sci USA 110: E697–E706, 2013. doi: 10.1073/pnas.1218284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9: 35–51, 2009. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 123. Cavadas C, Aveleira CA, Souza GF, Velloso LA. The pathophysiology of defective proteostasis in the hypothalamus—from obesity to ageing. Nat Rev Endocrinol 12: 723–733, 2016. doi: 10.1038/nrendo.2016.107. [DOI] [PubMed] [Google Scholar]
- 124. Ramírez S, Claret M. Hypothalamic ER stress: a bridge between leptin resistance and obesity. FEBS Lett 589: 1678–1687, 2015. doi: 10.1016/j.febslet.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 125. Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121: 1858–1870, 2011. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kleinridders A, Schenten D, Könner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Brüning JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 10: 249–259, 2009. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 29: 359–370, 2009. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146: 4192–4199, 2005. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 130. Yi CX, Walter M, Gao Y, Pitra S, Legutko B, Kälin S, Layritz C, García-Cáceres C, Bielohuby M, Bidlingmaier M, Woods SC, Ghanem A, Conzelmann KK, Stern JE, Jastroch M, Tschöp MH. TNFα drives mitochondrial stress in POMC neurons in obesity. Nat Commun 8: 15143, 2017. doi: 10.1038/ncomms15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chaves FM, Mansano NS, Frazão R, Donato J. Tumor necrosis factor α and interleukin-1β acutely inhibit AgRP neurons in the arcuate nucleus of the hypothalamus. Int J Mol Sci 21: 8928, 2020. doi: 10.3390/ijms21238928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79, 2002. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 133. McNeilly AD, Yianakas A, Gallagher JG, Tarlton J, Ashford ML, McCrimmon RJ. Central deficiency of IL-6Ra in mice impairs glucose-stimulated insulin secretion. Mol Metab 61: 101488, 2022. doi: 10.1016/j.molmet.2022.101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature 468: 223–231, 2010. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bessis A, Béchade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia 55: 233–238, 2007. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 136. Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 89: 337–343, 2004. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 137. Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci 20: 251–258, 2000. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Thomas AG, O’Driscoll CM, Bressler J, Kaufmann W, Rojas CJ, Slusher BS. Small molecule glutaminase inhibitors block glutamate release from stimulated microglia. Biochem Biophys Res Commun 443: 32–36, 2014. doi: 10.1016/j.bbrc.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22: 208–215, 1999. doi: 10.1016/S0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 140. Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev 98: 239–389, 2018. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chari M, Yang CS, Lam CK, Lee K, Mighiu P, Kokorovic A, Cheung GW, Lai TY, Wang PY, Lam TK. Glucose transporter-1 in the hypothalamic glial cells mediates glucose sensing to regulate glucose production in vivo. Diabetes 60: 1901–1906, 2011. doi: 10.2337/db11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. García-Cáceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi CX, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Alemán I, Kahn CR, Götz M, Horvath TL, Tschöp MH. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166: 867–880, 2016. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wang Y, Hsuchou H, He Y, Kastin AJ, Pan W. Role of astrocytes in leptin signaling. J Mol Neurosci 56: 829–839, 2015. doi: 10.1007/s12031-015-0518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481–487, 2017. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Jha MK, Jo M, Kim JH, Suk K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist 25: 227–240, 2019. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- 146. Norden DM, Fenn AM, Dugan A, Godbout JP. TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia 62: 881–895, 2014. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jo M, Kim JH, Song GJ, Seo M, Hwang EM, Suk K. Astrocytic orosomucoid-2 modulates microglial activation and neuroinflammation. J Neurosci 37: 2878–2894, 2017. doi: 10.1523/JNEUROSCI.2534-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rocha SM, Cristovão AC, Campos FL, Fonseca CP, Baltazar G. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol Dis 47: 407–415, 2012. doi: 10.1016/j.nbd.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 149. Kim JH, Ko PW, Lee HW, Jeong JY, Lee MG, Kim JH, Lee WH, Yu R, Oh WJ, Suk K. Astrocyte-derived lipocalin-2 mediates hippocampal damage and cognitive deficits in experimental models of vascular dementia. Glia 65: 1471–1490, 2017. doi: 10.1002/glia.23174. [DOI] [PubMed] [Google Scholar]
- 150. Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol 112: 195–204, 2006. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- 151. Skripuletz T, Hackstette D, Bauer K, Gudi V, Pul R, Voss E, Berger K, Kipp M, Baumgärtner W, Stangel M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 136: 147–167, 2013. doi: 10.1093/brain/aws262. [DOI] [PubMed] [Google Scholar]
- 152. Kim JS, Kolesnikov M, Peled-Hajaj S, Scheyltjens I, Xia Y, Trzebanski S, Haimon Z, Shemer A, Lubart A, Van Hove H, Chappell-Maor L, Boura-Halfon S, Movahedi K, Blinder P, Jung S. A binary Cre transgenic approach dissects microglia and CNS border-associated macrophages. Immunity 54: 176–190.e7, 2021. doi: 10.1016/j.immuni.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 153. Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169: 1276–1290.e17, 2017. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 154. Xu J, Farsad HL, Hou Y, Barclay K, Lopez BA, Yamada S, Saliu IO, Shi Y, Knight WC, Bateman RJ, Benzinger TL, Yi JJ, Li Q, Wang T, Perlmutter JS, Morris JC, Zhao G. Human striatal glia differentially contribute to AD- and PD-specific neurodegeneration. Nat Aging 3: 346–365, 2023. doi: 10.1038/s43587-023-00363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar, Scheiwe C, Nessler S, Kunz P, van Loo G, Coenen VA, Reinacher PC, Michel A, Sure U, Gold R, Grün D, Priller J, Stadelmann C, Prinz M. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566: 388–392, 2019. doi: 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 156. Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One 7: e30571, 2012. doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]