Abstract
Human immunodeficiency virus type 1 (HIV-1) infection of CD4+ lymphocytes and macrophages involves interaction of the surface subunit of the envelope protein (gp120) with coreceptors. Isolates have been found with specific tropism for macrophages and/or T-cell lines, through the utilization of chemokine receptor CCR5 (R5) or CXCR4 (X4). The third hypervariable loop (V3 loop) of gp120 is the major determinant of tropism. Using chimeric envelopes between HXB2 (X4) and ADA (R5), we found that the C-terminal half of the V3 loop was sufficient to confer on HXB2 the ability to infect CCR5-expressing cells. A sequence motif was identified at positions 289 to 292 allowing 30% of wild-type levels of infection, whereas full activity was achieved with the conversion of Lys to Glu at position 287 in addition to the above motif. Moreover, V3 loops from either SF2 (X4R5) or SF162 (R5) also allowed infection of CCR5-expressing cells, supporting the importance of V3 loops in influencing CCR5 utilization. The effects of amino acid changes at position 287 on the level of infection via CCR5 showed that negatively charged residues (Glu and Asp) were optimal for efficient interaction whereas only bulky hydrophobic residues drastically reduced infection. In addition, sequences at the N terminus of the V3 loop independently modulated the level of infection via CCR5. This study also examined the susceptibility of chimeric envelopes to neutralization by anticoreceptor antibodies and suggested the presence of differential interaction between the chimeric envelopes and CCR5. These findings highlight the critical residues in the V3 loop that mediate HIV-1 infection.
Human immunodeficiency virus type 1 (HIV-1) primarily infects human CD4-positive T cells and macrophages (3, 21, 36, 44), although other cells may also be infected (4, 23, 32, 39). Infection by HIV-1 involves interaction of the surface subunit of the viral envelope protein (gp120) with CD4 and coreceptors, which are members of the chemokine receptor family (2, 12, 14, 15, 17, 18, 20, 31, 38, 42). Isolates exhibiting specific tropism for macrophages or T-cell lines primarily utilize chemokine receptors CCR5 (R5) or CXCR4 (X4), respectively (2, 6, 20, 49, 54). The coreceptor expression patterns on different cells types regulate viral entry (8, 16, 22). The current nomenclature favors classification of viruses according to the primary coreceptor that they utilize (5), e.g., R5- or X4-tropic strains. HIV-1 strains that infect macrophages are primarily R5-tropic, T-cell-line-adapted isolates are X4-tropic, and isolates capable of infecting both macrophages and T-cell lines can use both coreceptors and are considered X4R5-tropic.
The third hypervariable loop (V3) of the HIV-1 gp120 has been identified as the major determinant of cellular tropism (36, 45, 48, 53) and coreceptor specificity (7, 13, 25, 46, 47, 54). It is not clear, however, which region or residues within this stretch of the 35- to 37-amino-acid loop are responsible for the observed phenotype. By using chimeric envelopes between viral isolates of different tropism, this study elucidates the contribution of amino acid residues in the V3 loop involved in determining CCR5 tropism. In addition, the effects on the interaction between the envelope protein and CCR5 coreceptor of amino acid substitutions in the V3 loop were examined. Finally, we propose that a conserved secondary structure within the V3 loop, most probably an α-helix, is required for interaction with CCR5.
MATERIALS AND METHODS
Antibodies and chemokines.
12G5, a monoclonal antibody (MAb) that specifically recognizes CXCR4 (19), was a gift from James Hoxie. The CCR5 MAbs 2D7 and 5C7 were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (provided by LeukoSite, Inc.). MAb 2D7 specifically recognizes the second extracellular loop of CCR5. It blocked the binding of MIP-1α, MIP-1β, and RANTES to interleukin-2-stimulated CD3 blast T cells and CCR5-expressing L1.2 cells (55) and inhibited the chemotaxis and Ca2+ influx of CCR5-expressing cells and primary lymphocytes. This antibody also inhibited infection of CCR5-expressing cells by R5-tropic and dually tropic HIV-1. MAb 5C7 (56), whose epitope mapped to the N terminus of CCR5, does not possess this neutralizing activity. The CD4 antibody OKT4 was purchased from Ortho Diagnostic Systems (Raritan, N.J.). Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) antibody, used as the secondary antibody in flow cytometry analysis, was purchased from Teknika-Capel (Organon Teknika Corp., West Chester, Pa.). Recombinant human RANTES, MIP-1α, and MIP-1β were purchased from R&D Systems, Inc. (Minneapolis, Minn.).
Cell lines.
293T cells were maintained in Dulbecco’s modified Eagle’s medium (GibcoBRL, Grand Island, N.Y.) supplemented with 10% fetal bovine serum, 4 mM l-glutamine, 1 mM sodium pyruvate (WUMS Tissue Culture Support Center), and 100 U of penicillin per ml and 100 μg of streptomycin per ml (complete DMEM). The HeLa-CD4-LTR–β-gal (MAGI) cell line was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (provided by Michael Emerman) (28), maintained in complete DMEM supplemented with 200 μg of Geneticin (GibcoBRL) per ml and 100 μg of hygromycin (Boehringer Mannheim, Indianapolis, Ind.) per ml (MAGI medium). MAGI-CCR5 cells were generated by transfecting MAGI cells with a CCR5 expression construct (a gift from Stephen Peiper). Transfectants were selected with MAGI medium plus 0.25 μg of puromycin (Sigma) per ml (MAGI-CCR5 medium), and a single clone was selected based upon susceptibility to infection by a panel of CCR5-tropic viruses.
Detection of cell surface antigens.
The surface expression of CD4, CXCR4, and CCR5 on both MAGI and MAGI-CCR5 cells was analyzed by flow cytometry analysis. The cells were incubated at 4°C for 30 min with OKT4, 12G5, or 2D7 MAb in phosphate-buffered saline (PBS) containing 5% fetal bovine serum and 0.02% sodium azide. The cells were washed with PBS and further incubated with FITC-conjugated goat anti-mouse IgG antibody in the same manner as above. The cells were washed again with PBS and fixed in 1% paraformaldehyde in PBS. In all experiments, cells stained with FITC-conjugated secondary antibody alone were used as controls. The stained cells were applied to a FACSCalibur flow cytometer (Beckton-Dickinson, San Jose, Calif.). Flow cytometry results were analyzed with Cell Quest software (Becton-Dickinson).
Chimeric virus constructs.
All viruses used in this study were generated in the background of pNLHX, an X4-tropic virus. The details of HXB2/ADA V3 chimeric virus construction are described below. Briefly, a HXB2 envelope subclone was first created to facilitate the generation of V3 chimeras. This subclone, p402 (Fig. 1), contained deletions of Glu and Arg residues near the tip of the V3 loop, an in-frame deletion in the C-terminal portion of the V3 loop, and a newly created unique AatII site as a result of the deletion. The chimeric V3 loops were then constructed by inserting double-stranded DNA oligonucleotides corresponding to the sequences of the chimeras into the AatII site. The SalI-BamHI fragment from these subclones then replaced the equivalent region of pNL4/3 (1) to generate full-length chimeric viruses with different V3 sequences in an CXCR4 viral backbone. In a similar manner, K287 site-directed mutants were constructed by using oligonucleotides with random changes at codon 287.
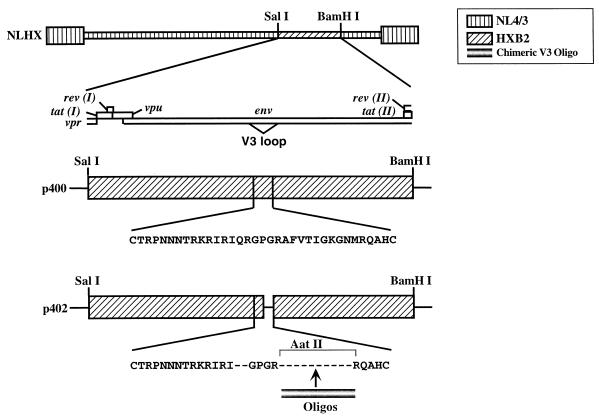
FIG. 1.
Construction of HXB2/ADA chimeras. p400 consisted of the SalI-BamHI (nucleotides 5366 to 8052) fragment of HXB2 in pSP64. The diagram above p400 indicates the open reading frames which this fragment contains. The letters under p400 indicate the HXB2 V3 loop amino acid sequence. p402, a derivative of p400, contains deletions of QR residues as well as the C-terminal portion of the HXB2 V3 loop. A unique AatII site, resulting from the deletion, is used for cloning of DNA oligonucleotides. The SalI-BamHI fragments of chimeric viruses were inserted into pNL4/3 (1) to create full-length molecular clones.
Viruses containing the V3 loops of HIV-1SF2, HIV-1SF162, or chimeric V3 loops between the two isolates were constructed in the same proviral backbone as the HXB2/ADA chimeras. To generate the SF2V3 molecular clone, the V3 loop of HIV-1SF2 was first amplified by PCR. Both 5′ and 3′ amplification primers contain unique restriction sites for the subsequent cloning process. This V3 loop fragment then replaced that of pNLHX (52) to create a full-length molecular clone with the V3 loop of HIV-1SF2.
A different HXB2 envelope subclone was created for the construction of the SF162V3 molecular clone. This subclone contained N-terminal sequences of the SF162 V3 loop, an in-frame deletion in the C-terminal portion of the V3 loop, and a newly created unique AatII site as a result of the deletion. A double-stranded DNA oligonucleotide corresponding to the sequences of the C terminus of HIV-1SF162 was inserted into the AatII site. The full-length molecular clone was constructed as described for the HXB2/ADA chimeras.
The viruses containing chimeric V3 loop sequences between SF2 and SF162 were constructed in the same manner as the HXB2/ADA V3 chimeras. Another HXB2 envelope subclone was created. This subclone contained N-terminal sequences of the SF2 V3 loop, an in-frame deletion in the C-terminal portion of the V3 loop, and a newly created unique AatII site as a result of the deletion. Constructions of both envelope subclones and full-length molecular clones were as described above.
The V3 loop coding sequences of all full-length chimeric viruses and site-directed mutants were confirmed by dideoxynucleotide-mediated chain termination sequencing (43). The sequences of the oligonucleotide inserts are available from the authors upon request.
Viral stock generation.
All viral stocks were prepared by transfecting 293T cells. Transfection mixtures containing full-length proviral DNA and Lipofectamine (GibcoBRL, Gaithersburg, Md.) were incubated with cells overnight. The mixtures were aspirated on the next day, and fresh medium was added back. Supernatants from the transfected cells were harvested on day 4 posttransfection. Following a 1,000 × g centrifugation step to discard cellular debris, the supernatants were passed through 0.22-μm-pore-size filters, aliquoted in a small volume (0.5 to 1 ml), and stored frozen (−80°C). Viral titers were determined by p24 antigen enzyme-linked immunosorbent assay (Coulter, Miami, Fla.).
Infection assay.
Infections of MAGI and MAGI-CCR5 cells were carried out by a previously described method (28) with minor modifications. Briefly, cells were seeded in 96-well plates at 7 × 103 cells/well. Medium was removed on the following day, and 2.5 ng of p24 equivalent of diluted virus plus DEAE-dextran (20 μg/ml; Sigma) was added to each well. The virus was allowed to adsorb for 2 h, and then medium was added into each well without the removal of the inoculum. Infection by each virus was performed in two or more replicate wells for each experiment. Cells were assayed for infection by staining for β-galactosidase expression at 40 to 48 h postinfection. Culture medium was removed, and fixing solution (1% formaldehyde and 0.2% glutaraldehyde in PBS) was added to each well. The monolayers were fixed at room temperature for 5 min and washed twice with PBS, and 50 μl of X-Gal staining solution (4 mM potassium ferrocyanide, 4 mM potassium ferricyanide, 2 mM magnesium chloride, and 0.4 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside [X-Gal] per ml in PBS) was added to each well. The number of blue-stained cells was counted and expressed as percentage of the wild-type virus infection level.
Antibody inhibition of infection.
Antibody inhibition assays were performed by a modified MAGI-CCR5 infection method. Cells were seeded in 96-well plates. On the following day, medium was removed and the indicated antibodies at various concentrations (in a 50-μl volume) were added. Cells were incubated with antibodies for 1 h at 37°C, and then 2.5 ng of p24 equivalent of diluted virus (in 50 μl) plus DEAE-dextran (final concentration, 20 μg/ml) was added without the removal of antibodies. MAGI-CCR5 medium (100 μl) was added to each well at the end of adsorption period. At 40 to 48 h postinfection, the cells were assayed for infection with chlorphenol red–β-d-galactopyranoside (CPRG; Boehringer Mannheim, Indianapolis, Ind.) (35). Briefly, Nonidet P-40 detergent (Sigma) was added to each well to a final concentration of 0.5% to lyse the cells. A freeze-thaw step was performed to ensure complete lysis of cells. Then 50 μl of lysate (one-quarter of the total cell lysate) from each well was added to an equal volume of 2× CPRG solution (10 mM CPRG, 120 mM Na2HPO4 · 7H2O, 80 mM NaH2PO4 · H2O, 20 mM KCl, 2 mM MgSO4 · 7H2O, 100 mM β-mercaptoethanol), and the reaction mixture was incubated for 45 min at room temperature. The amount of substrate hydrolysis, which reflected the level of infection, was measured in an enzyme-linked immunosorbent assay reader (BioTek Instrument, Inc., Burlington, Vt.) at an optical density of 590 nm. Infection levels were normalized to that seen in the absence of antibody for the particular virus, which was arbitrarily designated 100%. Any decrease in the level of infection was converted to the percentage of inhibition.
Chemokine inhibition of infection.
Inhibition of infection by β-chemokines was performed in a similar manner to the antibody inhibition experiments. MAGI-CCR5 cells were preincubated with 50 μl of a mixture of recombinant human RANTES, MIP-1α, and MIP-1β at the indicated concentrations of each for 1 h at 37°C. At the end of the preincubation period, 2.5 ng of p24 equivalent of diluted virus (in 50 μl) plus DEAE-dextran (final concentration, 20 μg/ml) was added without the removal of chemokines. Infected cells were assayed for the expression of the reporter gene at 40 to 48 h postinfection.
RESULTS
Construction of MAGI-CCR5 cells.
To study the viral determinants for CCR5 utilization, we constructed a reporter cell line stably expressing the CCR5 coreceptor on the surface. HeLa-CD4-LTR–β-gal (MAGI) cells express both human CD4 and CXCR4 coreceptor on the surface (Fig. 2A) and contain a reporter gene (β-gal) driven by the HIV-1 long terminal repeat. Upon infection, HIV-1 Tat drives the expression of the reporter gene, and the infected cells were stained for expression of β-galactosidase. Cells stably transfected with a CCR5 expression plasmid were selected (MAGI-CCR5). This cell line expressed CCR5, as well as CXCR4, on its surface as detected by flow cytometry (Fig. 2A). It was infectible by both T-cell-line-adapted and macrophage-tropic HIV-1, NLHX and NLHXADAgg, respectively, while MAGI cells could be infected only by NLHX (Fig. 2B). In addition, infections of MAGI-CCR5 were inhibited by neutralizing antibodies to the respective coreceptor (data not shown).
FIG. 2.
Characterization of the MAGI-CCR5 cell line. (A) Surface expression of human CD4, CXCR4, and CCR5 on MAGI-CCR5 and parental MAGI cells. The dotted line indicates background staining with secondary antibody alone. The solid line represents staining with indicated primary antibody. Cells were lifted from tissue culture plates with 5.3 mM EDTA in cold PBS and surface stained with corresponding MAbs as described in Materials and Methods. (B) Infection profiles of MAGI and MAGI-CCR5 cells. NLHX (53), a T-cell-line-tropic molecular clone containing the envelope sequence of HXB2, can infect both cell types. NLHXADAgg (53), a macrophage-tropic molecular clone containing the V3-loop sequence of ADA, can infect only MAGI-CCR5 cells. The level of infection is shown as the average number of blue foci per 6-mm well. Mock infections consistently gave <10 blue foci/well for both cell lines. Error bars indicate the sample standard deviations of the mean values obtained from triplicate samples. Representative data from three experiments are presented here.
Both MAGI and MAGI-CCR5 cells were used to assay the infectivity of the viruses in the subsequent experiments. From the results of the neutralizing-antibody inhibition experiments with anti-CXCR4 and CCR5 antibodies, 12G5 and 2D7, respectively, we were able to infer that the viruses which infected both MAGI and MAGI-CCR5 cells to similar levels used CXCR4 as coreceptor while those infecting only MAGI-CCR5 entered via CCR5.
Viral determinants for CCR5 map to the C terminus of the V3 loop of HIV-1ADA.
HIV-1HXB2 and HIV-1ADA are two well-characterized molecular clones. HXB2 is T-cell-line tropic and uses CXCR4 as its coreceptor. ADA, a macrophage-tropic virus, uses CCR5 as its primary coreceptor. Our previous reports showed that chimeras containing the sequences from the V3 loop of ADA could confer on HXB2 the ability to infect primary human macrophages (24). Using these chimeric clones in infection of MAGI and MAGI-CCR5 cells, we found that the V3 loop of ADA also conferred the ability to use CCR5 as a coreceptor (ADAV3) (Table 1). Substitution of the C-terminal half of the ADA V3 loop was also sufficient to confer CCR5 utilization (V3B).
TABLE 1.
Viral determinants for CCR5 utilization map to the C-terminal half of the V3 loop
| Virus | V3 loop sequencea | Activity (105 cpm/ml) in PBMCb | No. of blue focic on:
|
|
|---|---|---|---|---|
| MAGI cells | MAGI-CCR5 cells | |||
| 263 277 283 287 294 297 | ||||
| | | | | | | | ||||
| NLHX | CTRPNNNTRKRIRIQRGPGRAFVTIGKI—GNMRQAHC | 109 | 113 ± 34 | 115 ± 16 |
| ADAV3 | ..........S.H.——......Y.T.E.I.DI..... | 396 | 0 ± 0 | 202 ± 3 |
| V3B | ..............——......Y.T.E.I.DI..... | 535 | 0 ± 0 | 213 ± 3 |
| ΔQR | ..............——..................... | 187 | 48 ± 7 | 48 ± 3 |
| 196 | ..............——..........E.......... | 217 | 7 ± 1 | 5 ± 1 |
| 198 | ..............——..........D.......... | 216 | 16 ± 3 | 11 ± 1 |
Numbers above the sequences indicate the positions of amino acid residues with respect to the ADA envelope (52). Dots indicate identical sequence to NLHX; dashes indicate sequence gaps.
Peak reverse transcriptase activity. Results are from reference 24.
Values represent average number of blue foci per nanogram of virus ± standard deviation of triplicate wells.
The C-terminal half of HXB2 V3 loop differs from that of ADA at eight positions (amino acid residues 283, 285, 287, 290, 291, Gln and Arg [QR] insertion, and residue 289 deletion). Deletion of the QR residues alone or additional substitutions of Lys at position 287 (K287) to Glu or Asp resulted in viral clones that infected both MAGI and MAGI-CCR5 cells to similar levels, indicating that these changes did not confer the ability to use CCR5 as their coreceptor (Table 1). However, deletion of the QR residues is required for efficient interaction with CCR5, because a viral mutant containing the QR residues in the context of the V3B viral clone did not efficiently infect either MAGI-CCR5 or the parental MAGI cells (data not shown). These results suggested that (i) the deletion of QR residues in addition to changes at position 287 were not involved in CCR5 utilization or (ii) the changes at these two locations are necessary but not sufficient for CCR5 utilization.
Construction of HXB2/ADA chimeras.
We constructed a series of chimeras between HXB2 and ADA to identify residues within the V3 loop required for CCR5 utilization. These chimeric viruses were generated in the backbone of pNLHX, an X4-tropic virus (Fig. 2B). To facilitate the creation of V3 loops with small changes, a series of steps were taken to create a suitable subcloning vector. First, the SalI-BamHI (nucleotides 5366 to 8052) fragment of HXB2 was inserted into pSP64 (p400; Fig. 1). A unique AatII restriction site was engineered into the V3 loop by oligonucleotide substitutions, resulting in an in-frame deletion in the C-terminal region of the V3 loop. This plasmid, designated p402, contained deletions of the QR residues adjacent to the tip of V3 loop and was used for the creation of chimeric V3. Double-stranded DNA oligonucleotides corresponding to the sequences of the HXB2/ADA chimeras were inserted into the AatII site. The SalI-BamHI fragments from the subclones were then substituted into pNL4/3 to generate full-length molecular clones (Fig. 1).
Ile 288 and Ile 292 (II motif) can confer CCR5 utilization.
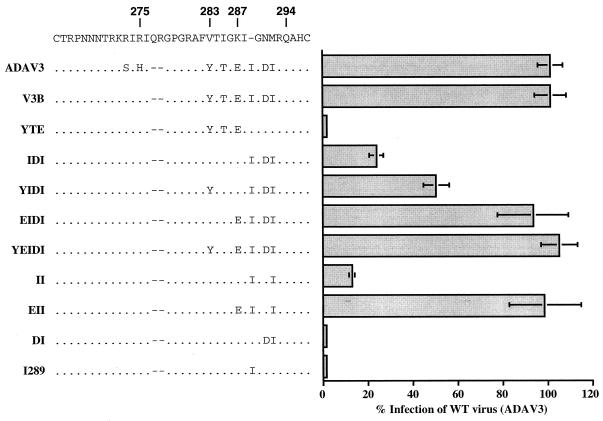
To study the critical residues within the V3 loop required for CCR5 utilization, we infected both MAGI and MAGI-CCR5 cells with HXB2/ADA chimeric viruses. None of the chimeras were able to infect MAGI cells, and infections of MAGI-CCR5 cells were not inhibited by 12G5 antibody, suggesting that they were unable to use CXCR4 as their coreceptor (data not shown). The chimera containing YTE residues from ADA (positions 283, 285, and 287) did not support the infection of MAGI-CCR5 cells (clone YTE) (Fig. 3). This mutant also lost the ability to infect both MAGI cells and primary human peripheral blood mononuclear cells (PBMC) (data not shown). Substitutions of three amino acid residues (positions 289, 291, and 292) near the C terminus of the V3 loop into that of HXB2 (clone IDI) resulted in a low level of infection, about 25% of that of the wild-type virus (clone ADAV3). While additional substitution of Tyr at position 283 (clone YIDI) increased the level of infection (50%), substitution of Glu at position 287 with the IDI changes (clone EIDI) restored the activity to the wild-type level. Substitutions at both positions (283 and 287) in addition to the IDI sequences (clone YEIDI) resulted in a virus that retained the wild-type activity. These results, together with those previously described (Table 1), demonstrated that the IDI sequences at the C terminus of the V3 loop were necessary and sufficient to confer upon an X4-tropic virus the ability to use CCR5 as a coreceptor. However, Glu at position 287 greatly increased the ability of the virus to utilize CCR5.
FIG. 3.
Mapping the minimal determinants in ADA V3 loop for CCR5 tropism. Clone names and V3 loop sequences of each chimera are shown on the left. Also shown at the top of the figure are the V3-loop sequences of the HXB2 (CXCR4-tropic) virus for comparison with the chimeras. The parental virus carrying this V3 loop (NLHX) was able to infect MAGI-CCR5 cells, albeit via CXCR4; therefore, its activity in infecting MAGI-CCR5 cells is not presented here. Numbers above the sequences indicate the positions of amino acid residues according to the ADA envelope (52). Infections were performed as described in Materials and Methods. The levels of infection were determined by counting the number of blue foci per 6-mm well and normalized to the percentage of infection of wild-type (WT) virus, clone ADAV3. ADAV3 virus consistently showed 200 to 600 blue foci/well between experiments, while mock infection gave <10 blue foci/well. Results are presented as the average result from multiple experiments with two or more wells/experiment. Error bars indicate the sample standard deviations of the mean values obtained from all experiments.
To further characterize the minimal residues that were critical for CCR5 utilization, we generated mutants containing fewer substitutions between residues 287 and 292 of the V3 loop. These mutants permitted the identification of the minimal domain within the V3 loop that was critical for CCR5 utilization. Mutants carrying either the DI291-2 substitution (clone DI) or insertion of Ile at position 289 (clone I289) did not show positive infection of MAGI-CCR5 cells (Fig. 3). When inoculated at 50-fold-higher titer, these two clones infected both MAGI-CCR5 and the parental MAGI cells to similar levels, suggesting that they still retain the ability to utilize CXCR4 as a coreceptor (data not shown). Similar to the IDI chimera, a mutant clone containing only the Ile 289 and Ile 292 substitutions (clone II) can infect MAGI-CCR5 cells to a low level (15%). An additional change of K287 to Glu (clone EII) again restored the level of infection to the wild type level (Fig. 3). These results indicated that both Ile 289 and Ile 292, near the C terminus of the V3 loop, were critical for CCR5 utilization. Furthermore, these data suggested that the amino acid residue at position 287 plays an important role in regulating the level of infection via CCR5.
Effects of residue 287 changes in MAGI-CCR5 infection.
We observed that the K287E substitution greatly enhances entry into CCR5-expressing cells (clones EIDI and EII [Fig. 3]). To further investigate the effects of amino acid changes at position 287 in CCR5 utilization, a set of mutants with site-directed mutations at this position were generated (Fig. 4) in the context of the IDI viral clone. K287 site-directed mutants were constructed in a similar manner to the HXB2/ADA chimeras, using oligonucleotides with sequences of codon 287 randomized to give 32 possible combinations encoding 18 different amino acids.
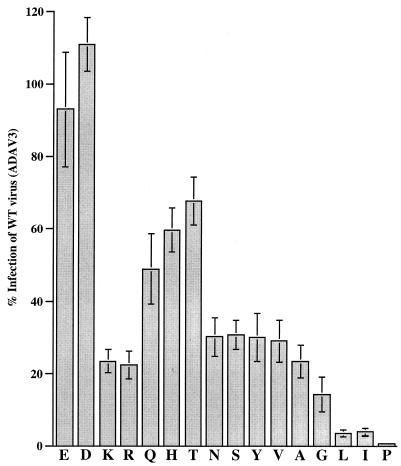
FIG. 4.
Effects of K287 mutations on CCR5 utilization. Each letter on the abscissa denotes a K287 mutant with the respective mutation. Viral clone E is EIDI and K is IDI from Fig. 3. The assays were performed as described in Materials and Methods. Results are presented as in Fig. 3. WT, wild type.
Similar to the results with EIDI clone, a change of K287 to another negatively changed residue, Asp, restored the infection to wild-type levels. The mutant containing the Arg change, another positively charged residue, was infectious to the same level as IDI clone. Although changes to Gln, His, and Thr increased the level of infection of MAGI-CCR5 cells to 50 to 70%, none of these substitutions were able to achieve the wild-type level of infection. Changes to Asn, Ser, and Tyr resulted in viruses that retained the low-infectivity phenotype seen with clone IDI (22 to 30% of the infectivity of clone ADAV3). Mutants with hydrophobic Val and Ala residues infected to a similar level to the IDI clone, whereas Gly substitution lowered the ability to use CCR5 to about 15% of the wild-type level. Interestingly, substitution with amino acids with large hydrophobic side chains, Leu or Ile, drastically reduced the ability of the virus to infect via CCR5 to 3 to 5% of the wild-type level. These viruses were functional, since they were able to infect MAGI-CCR5 to a level 10-fold higher than the background level when inoculated at higher viral titer (data not shown). These two clones also infected primary human, PBMC albeit to lower level (data not shown). In contrast, the Pro substitution abolished the ability of the virus to infect MAGI-CCR5 cells (Fig. 4) and primary human PBMC (data not shown), suggesting that this change resulted in a nonfunctional virus.
N-terminal sequences in the V3 loop of HIV-1SF2 and of HIV-1SF162 also modulate the level of CCR5 utilization.
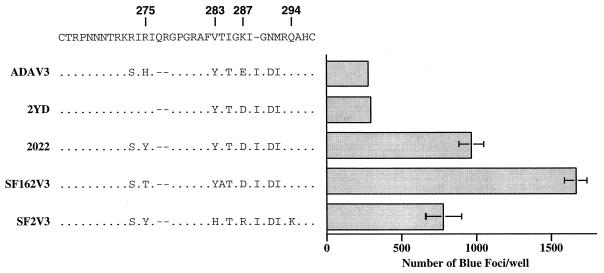
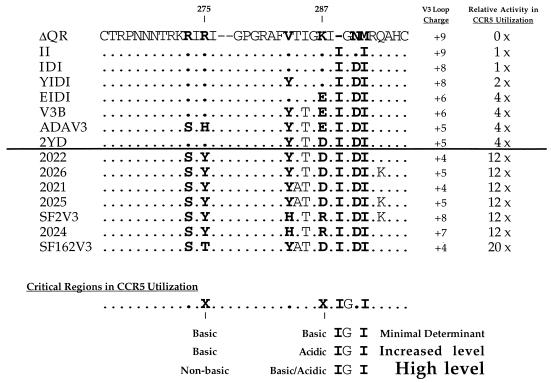
HIV-1SF162, a macrophage-tropic virus, and HIV-1SF2, a T-cell-line-tropic virus, both use CCR5 as their coreceptor (10). To study the involvement of the V3 loop in CCR5 utilization, we also constructed chimeric viruses containing the V3-loop sequences of these two strains in the pNLHX proviral backbone. Chimeras SF2V3 and SF162V3 infected MAGI-CCR5 cells efficiently (Fig. 5) but not the parental MAGI cells (data not shown). Moreover, infection of MAGI-CCR5 cells by these two viruses was not inhibited by the 12G5 antibody to CXCR4 (data not shown). This result demonstrated that, similar to ADA, the CCR5 determinants of SF2 and SF162 also map to the V3 loop. Interestingly, we observed that the levels of MAGI-CCR5 infection by these viruses were consistently at least threefold higher than that of ADAV3 (Fig. 5). Similar results were obtained with other clones containing chimeric V3-loop sequences between SF2 and SF162 (clones 2021, 2022, 2024, 2025, and 2026 [Fig. 5 and 6]). None of these chimeras were able to infect the parental MAGI cells. We speculated that sequences at the N terminus of the V3 loop might modulate the level of CCR5 utilization independently of amino acid position 287. To assess this possibility, we changed residues 273 and 275 of clone 2022 to those found in HXB2 and HXB2/ADA chimeras. This virus (clone 2YD) infected MAGI-CCR5 to a similar level to ADAV3 (Fig. 5). These data suggest that amino acid residues at the N terminus of the V3 loop also play a role in modulating CCR5 utilization. Interestingly, a positively charged amino acid at residue 287 in this V3 loop context did not reduce the ability of a virus to utilize CCR5 as its coreceptor (clones SF2V3 and 2024).
FIG. 5.
Modulation of N-terminal residues in CCR5 utilization. These assays were performed as described in Materials and Methods. The level of infection is shown as the average number of blue foci per 6-mm well. Error bars indicate the sample standard deviations of the mean values obtained from triplicate samples. Representative data from multiple experiments are presented here.
FIG. 6.
Alignments of chimeras and their relative levels of activity in CCR5 utilization. Amino acid residues are numbered according to the scheme used for the ADA envelope (52). The two numbers denote positions which play an important role in modulating the level of CCR5 utilization. The overall charges of the V3 loops are also shown.
Chimeric envelopes may interact differently with CCR5.
The panel of HXB2/ADA chimeric viruses infected MAGI-CCR5 but not the parental MAGI cells. The inability of these viruses to infect MAGI cells in the absence of CCR5 suggested that these viruses used CCR5 but not CXCR4 as their coreceptors. To further support our observation, we examined inhibition of infection of MAGI-CCR5 cells by these viruses in the presence of neutralizing antibodies to CCR5. MAb 2D7 specifically recognizes the second extracellular loop of CCR5. It is able to neutralize several bioactivities of MIP-1α, MIP-1β, and RANTES (55). MAb 5C7, on the other hand, binds to the N-terminal extracellular domain of CCR5 but does not have any neutralizing activity.
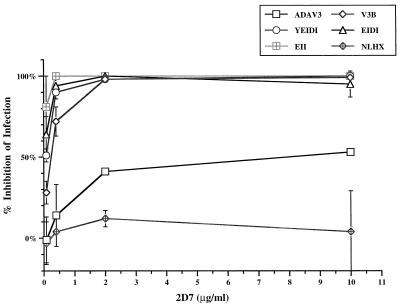
We tested the ability of MAb 2D7 to inhibit infection of MAGI-CCR5 cell by viral clones ADAV3, V3B, YEIDI, EIDI, and EII. All of these viruses infected MAGI-CCR5 cells to the same level (Fig. 3). Viral clone NLHX, the parental X4-tropic virus, was not inhibited by this antibody, even at 10 μg/ml (Fig. 7). All of the viruses analyzed were inhibited by 2D7, supporting our hypothesis that these viruses enter via CCR5. However, viral clone ADAV3 was inhibited to 50% by 10 μg of 2D7 per ml, whereas chimeric viruses with smaller substitutions in the V3 loop (clones V3B, YEIDI, EIDI, and EII) were more sensitive to neutralization by this antibody and was inhibited to greater than 60% when cells were preincubated with 0.4 μg of 2D7 per ml. The nonneutralizing MAb, 5C7, did not inhibit infections by any virus at 10 μg/ml (data not shown). These results indicated that although these viruses could infect MAGI-CCR5 efficiently, they exhibited differences in susceptibility to neutralization by an anticoreceptor antibody.
FIG. 7.
Inhibition of MAGI-CCR5 infection by anti-CCR5 antibody. Inhibition of MAb 2D7. The x axis indicates the concentrations of 2D7 antibody during the preincubation period. These assays were performed as described in Materials and Methods. The percentage of inhibition was calculated by subtracting the percentage of infection from 100. Error bars indicate the sample standard deviations of the mean values obtained from duplicate samples. Representative data from multiple experiments are presented here.
Human RANTES, MIP-1α, and MIP-1β are the natural ligands for CCR5. We also examined the ability of these β-chemokines to inhibit the infection of MAGI-CCR5 cells by the above-mentioned chimeras. Similar to the results with 2D7 inhibition, V3B, YEIDI, EIDI, and EII chimeras were sensitive to inhibition by the combination of recombinant human RANTES, MIP-1α, and MIP-1β whereas infection of MAGI-CCR5 cells by ADAV3 was not inhibited at concentrations of each of these chemokines up to 500 ng/ml (data not shown).
DISCUSSION
HIV-1 isolates display different tropism for various cell types. Since the discovery of the coreceptors for both HIV and simian immunodeficiency virus, it became evident that the coreceptors expressed on the cell surface play an important role in HIV-1 entry. The ability of the HIV-1 envelope protein to interact with a specific coreceptor determines the capability of a virus to infect these cells. Our study identified determinants in the HIV-1 envelope protein involved in CCR5 coreceptor utilization.
There is substantial evidence indicating that the V3 loop of the HIV-1 envelope protein plays a major role in influencing the tropism of HIV (36, 45, 48, 53) as well as the ability of the envelope protein to interact with the coreceptor (7, 13, 25, 46, 47, 54). HIV-1 isolates ADA, SF2, and SF162 use CCR5 as their primary coreceptor (10, 14). In this study, we showed that viruses carrying the V3 loop of these isolates were able to infect CCR5-expressing cells (Table 1 and Fig. 5). Viruses entered MAGI-CCR5 cells through a specific interaction with the CCR5 coreceptor, and infection was inhibited by a neutralizing antibody against CCR5, 2D7 (Fig. 7). Our data is in agreement with published results indicating that the V3 loop of R5-tropic HIV-1 is necessary and sufficient to confer the use of CCR5 as the coreceptor (12, 13, 46, 51, 58).
We analyzed the residues within this domain that were critical for CCR5 utilization by chimeric viruses between isolates with known coreceptor usage. HIV-1ADA is an R5-tropic virus, and its V3 loop sequence differs at 10 positions from that of HIV-1HXB2, an X4-tropic virus. Results from our study suggested that changes in three different areas of the HXB2 V3 loop are important for HIV-1 to infect via CCR5; these include (i) substitution of Ile 292, (ii) insertion of Ile 289, and (iii) deletion of QR residues immediately N-terminal of the GPGR sequences.
We aligned the V3-loop sequences of the HIV-1 chimeras and compared their relative ability to infect MAGI-CCR5 cells (Fig. 6). Both viral clones II and IDI exhibited low levels of CCR5 utilization, while other ADA chimeras (clones YIDI, EIDI, V3B, and ADAV3) had two- to fourfold-higher activities. Chimeric viruses between SF2 and SF162 achieved 12- to 20-fold-higher activity for CCR5 utilization. All of the chimeras that infected MAGI-CCR5 cells contained an Ile at position 289, a Gly at position 290, and an Ile at position 292. We concluded that, in the context of the HXB2 V3 loop, the presence of the IGXI motif was critical for envelope interaction with CCR5 coreceptor. The minimal determinant consisting of the above motif with basic residues at positions 275 (Arg) and 287 (Lys) (clone II) allowed low-level entry of HIV-1 via CCR5, while an acidic residue at position 287 (clone EII) allowed infectivity similar to that seen with clone ADAV3 (Fig. 6).
Our results obtained with K287 site-directed mutants demonstrated the contribution of different residues to modulation of the level of infectivity. A negatively charged residue (Glu or Asp) at position 287 was favorable for optimal utilization of CCR5 (Fig. 4). Amino acids with different characteristics were still acceptable at this position for infection via CCR5. Large hydrophobic amino acids (Leu and Ile), however, interfered with the ability of the envelope protein to utilize CCR5 as the coreceptor (Fig. 4), whereas Pro abolished the proper functioning of the envelope protein. Interestingly, several reports have suggested that residue 287 plays a role in influencing the ability of a virus to infect different cell types (11, 45, 53). We also observed that amino acids at the N terminus of the V3 loop modulated the level of CCR5 utilization. With a nonbasic residue at position 275 (Tyr or Thr [Fig. 6]), infection via CCR5 was efficient and the residues at position 287 did not affect the activity of these envelopes.
The overall charges of the V3 loop have been suggested to influence both cellular and coreceptor tropism of HIV-1 (26, 27). We also examined the effects of the V3 loop charges on CCR5 utilization (Fig. 6). The V3-loop charges of CCR5-tropic chimeras range from +4 to +9. On the other hand, the overall V3-loop charges of NLHX, ΔQR, I289, and DI are +10, +9, +9, and +8, respectively. Although the majority of CCR5-tropic chimeras contain fewer positively charged amino acids, we did not observe any correlation between V3-loop charges and coreceptor selectivity. The charges of this region did not influence the level of CCR5 utilization either.
Results obtained with these two sets of chimeric viruses suggested that two regions in the V3 loop could modulate the efficiency of CCR5 utilization. Amino acids at these two positions could independently increase the affinity of envelope proteins for CCR5 by directly interacting with the coreceptor. Alternatively, both positions could be interacting with the core of gp120, and this interaction might be important for the events following CD4 receptor binding. Amino acid changes at these positions may have influenced conformational changes within the envelope protein subsequent to CD4 binding, resulting in the different levels of infection seen with K287 mutants. A third possible mechanism could be attributed to cross talk between the two sides of the V3 loop. Efficient interaction between these two regions translated to efficient interaction of the V3 loop with either the core region of gp120 or CCR5 and led to optimal infection of CCR5-expressing cells.
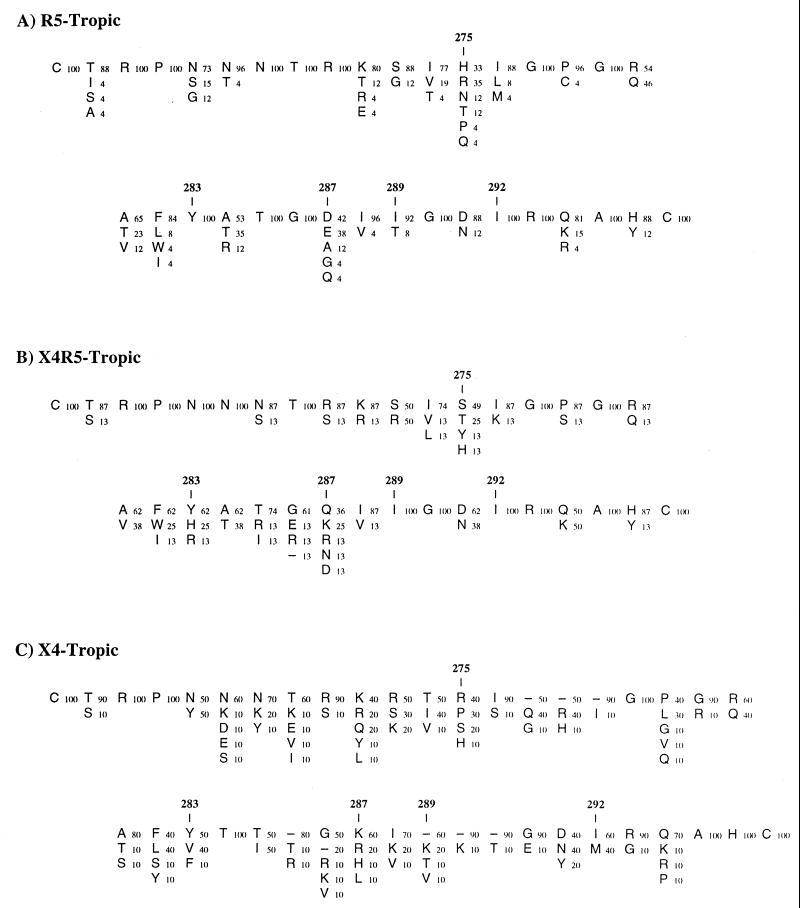
We aligned the V3-loop sequences of HIV-1 strains with known coreceptor utilization and subdivided them into three groups (Fig. 8). Our findings on critical residues for CCR5 utilization were in agreement with these consensus sequences resulting from the sequence alignments. Over 90% of the R5-tropic and 100% of the dually (X4R5) tropic isolates contained an Ile residue at position 289 (Fig. 8A and B). Ile 292 was also conserved in both R5-tropic and dually tropic viruses. The V3 loops of these two groups have consistent length, with no additional sequence insertions immediately N-terminal of the GPGR crown of the V3 loop, in contrast to X4-tropic isolates (Fig. 8C). Taken together, our data suggest that there are several stringent requirements in the V3 loop for CCR5 utilization. First, the conservation of sequences at the very C terminus of the V3 loop indicates that this may determine the specificity for interaction with CCR5. Second, it may also indicate that a certain secondary structure is present in this region. In fact, solution nuclear magnetic resonance structure data of synthetic cyclic V3-loop peptides of two distinct sequences suggested the presence of an α-helix in the region corresponding to the minimal determinants that we had identified (9, 33). Third, conservation of the V3-loop length implies that the presentation of such a structure in the V3 loop, or the interaction of the V3 loop with other regions of the envelope protein, is critical. The sequences of X4-tropic envelopes do not match these requirements (Fig. 8C). We speculate that the conserved sequences in the C-terminal portion of the V3 loop form an α-helical structure that is important for CCR5 utilization.
FIG. 8.
Summary of the V3 loop sequences from viruses with known coreceptor usage. (A) R5-tropic viruses, assembled from 26 sequences. (B) X4R5 dual tropic viruses, assembled from 8 sequences; (C) X4-tropic viruses, assembled from 10 sequences. Coreceptor utilization of the viruses is described in the HIV Database (34). The V3-loop sequences of each virus were retrieved from a National Center for Biotechnology Information GenBank search (34a). Each letter denotes the amino acid sequence at that position, while the number next to it indicates the percentage at which the amino acid residue has appeared at that position among each group of sequences. The amino acid residues most commonly found at each position are displayed at the top. Dashes indicate sequence gaps.
Using different chimeric envelopes, Speck et al. (46) reported that CCR5 utilization is influenced by the amino acid at position 292. While they ignored the contribution of the amino acid at position 289, Val was already present at this position in their X4-tropic clone. Although our results demonstrated the importance of Ile 289 for CCR5 tropism, Val, another hydrophobic residue, may be functionally equivalent. They also showed that the Ser-to-His change at position 275 was sufficient to confer CCR5 utilization. We were not able to investigate the effect of amino acid changes at the N terminus of the V3 loop alone in the context of the HXB2/ADA chimera, since changes in this region resulted in a nonfunctional virus (24). Based on the results of alanine-scanning mutagenesis, Wang et al. (50) identified other residues in the V3 loop to be important for infection via CCR5. They did not observe any effect in CCR5 utilization when either Ile 289 or Ile 292 was mutated to Ala, another hydrophobic residue. It is possible that these conservative changes still allow the V3 loop to efficiently interact with CCR5 or that the individual alanine mutation alone is not sufficient to show the importance of these two residues. Our study employed a gain-of-function analysis to show that these residues are important for CCR5 utilization. The V3-loop sequence alignment of molecular clones and primary isolates (Fig. 8) further strengthens our conclusions.
Current models of HIV entry suggest that conformational changes occur in the envelope protein following binding to the principal receptor, CD4, leading to exposure of the V3 loop for subsequent interaction with the coreceptor and the eventual fusion between viral and cellular membranes. Rizzuto et al. (40) and Wyatt et al. (57) reported that mutations affecting the interaction of the HIV-1 envelope with CCR5 were located near the base of the V3 loop in the conserved region of the envelope protein. Their finding further supports our claim that the V3 loop plays a major role in tropism determinant. Mutations in the envelope may affect the presentation of the V3 loop upon CD4 binding. On the other hand, these residues may form the CCR5-binding site together with the V3 loop. Changes either in the V3 loop or conserved regions at the base of the V3 loop could affect the interaction of the envelope protein with the coreceptor, CCR5.
Studies involving chimeric coreceptors revealed distinct requirements of different envelope proteins. Ross et al. (41) showed that the ADA envelope can interact with chimeric coreceptors containing extracellular loops 1 or 2 or the N terminus of human CCR5 in a murine CCR5 backbone. In our study, we showed that a neutralizing antibody against the second extracellular loop of human CCR5 had only a marginal effect in neutralizing infection by a virus carrying the V3 loop of ADA virus (Fig. 7). In contrast, many chimeric viruses with smaller ADA V3 loop substitutions (clones V3B, YEIDI, EIDI, and EII), which infect CCR5-expressing cells efficiently, are easily neutralized by 2D7 antibody. These chimeras are also sensitive to neutralization with mixtures of recombinant human RANTES, MIP-1α, and MIP-1β (data not shown). The results from both 2D7 antibody and β-chemokine inhibition experiments suggest that although many viruses infected MAGI-CCR5 cells to similar levels, there are differences among these viruses. Several explanations could account for this finding. First, the envelope with the ADA V3 loop may require lower levels of CCR5 on the cell surface to achieve high levels of infection. Therefore, neutralizing antibodies, which limit the numbers of available coreceptors, do not effectively block infection by viruses carrying such envelope proteins. The chimeric envelopes, on the other hand, may require higher threshold levels for efficient infection. The differences in the threshold requirement may be masked by the high level of CCR5 expression in the MAGI-CCR5 cell lines used in this study but are revealed by the antibody inhibition study. The effects of CD4 and coreceptor surface concentration on infection have been demonstrated for both X4- and R5-tropic HIV-1 (29, 37). Second, similar to the parental ADA envelope, the envelope with ADA V3 loop may interact with various loops of CCR5 while others interact primarily with the second extracellular loop of the coreceptor. Antibodies blocking this interaction could easily lead to a dramatic decrease in infection. Chimeric coreceptors would be useful to verify the latter hypothesis.
Different HIV-1 strains use coreceptors other than CXCR4 and CCR5 to gain access to cells (15, 25, 31, 42). The V3 loop is also believed to play a role in influencing the utilization of one of these coreceptors. Hoffman et al. (25) reported that CCR3 utilization by HIV-1ADA requires both the V3 and V1/2 loops of the ADA envelope. All of the HIV-1 coreceptors identified thus far belong to the family of seven-transmembrane domain, G-protein-coupled proteins with similar membrane topology. Recent reports suggested that the interactions between different envelopes and their respective coreceptors involve a common mechanism (30, 40, 57). We speculate that the critical regions involved in CCR5 utilization might also affect infection via other coreceptors. Without the crystal structure of the envelope protein interacting with the coreceptor, it is difficult to pinpoint the mechanism. Our study with chimeric envelopes and anticoreceptor antibodies elucidated the regions of the V3 loop involved in interacting with CCR5.
ACKNOWLEDGMENTS
We thank Suzanne Pontow, Maria Pirounaki, and Frosso Voulgaropoulou for advice and helpful discussions in the preparation of the manuscript. We also thank James Hoxie for generously providing 12G5 antibody and Stephen Peiper for providing the CCR5 expression construct. A number of reagents used in this study were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This work was supported by Public Health Service (PHS) grant AI24745.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Ashorn P, Berger E, Moss J. Human immunodeficiency virus envelope glycoprotein/CD4-mediated fusion of nonprimate cells with human cells. J Virol. 1990;64:2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayehunie S, Groves R W, Bruzzese A, Ruprecht R M, Kupper T S, Langhoff E. Acutely infected Langerhans cells are more efficient than T cells in disseminating HIV Type 1 to activated T cells following a short cell-cell contact. AIDS Res Hum Retroviruses. 1995;11:877–884. doi: 10.1089/aid.1995.11.877. [DOI] [PubMed] [Google Scholar]
- 5.Berger E A, Doms R W, Fenyo E-M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. HIV-1 phenotypes classified by co-receptor usage. Nature. 1997;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 6.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieniasz P D, Fridell R A, Aramori I, Ferguson S S G, Caron M G, Cullen B R. HIV-1 induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catasti P, Bradbury E M, Gupta G. Structure and polymorphism of HIV third variable loops. J Biol Chem. 1996;271:8236–8242. doi: 10.1074/jbc.271.14.8236. [DOI] [PubMed] [Google Scholar]
- 10.Cheng-Mayer C, Liu R, Landau N R, Stamatatos L. Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 13.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 14.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 15.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 16.Di Marzio P, Tse J, Landau N R. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res Hum Retroviruses. 1998;14:129–138. doi: 10.1089/aid.1998.14.129. [DOI] [PubMed] [Google Scholar]
- 17.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 18.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 19.Endres M J, Clapham P R, Marsh M, Ahuja M, Davis-Truner J, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by Fusin/CXCR-4. Cell. 1996;87:745–765. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Broder C C, Kenedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane domain, G-protein coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 21.Gartner S, Markovits P, Markovitz D, Kaplan M, Gallo R, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 22.Ghorpade A, Xia M Q, Hyman B T, Persidsky Y, Nukuna A, Che B P M, Limoges J, Gendelman H E, Mackay C R. Role of the β-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J Virol. 1998;72:3351–3361. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harouse J M, González-Scarano F. Infection of SK-N-MC cells, a CD4-negative neuroblastoma cell line, with primary human immunodeficiency virus type 1 isolates. J Virol. 1996;70:7290–7294. doi: 10.1128/jvi.70.10.7290-7294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henkel T, Westervelt P, Ratner L. HIV-1 V3 envelope sequences required for macrophage infection. AIDS. 1995;9:399–401. [PubMed] [Google Scholar]
- 25.Hoffman T L, Stephens E B, Narayan O, Doms R W. HIV type 1 envelope determinants for use of the CCR2b, CCR3, STRL33 and APJ coreceptors. Proc Natl Acad Sci USA. 1998;95:11360–11365. doi: 10.1073/pnas.95.19.11360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang S. HIV-1-coreceptors binding. Nat Med. 1997;3:367–368. doi: 10.1038/nm0497-367. [DOI] [PubMed] [Google Scholar]
- 27.Kim F M, Kolson D L, Balliet J W, Srinivasan A, Collman R G. V3-independent determinants of macrophage tropism in a primary human immunodeficiency virus type 1 isolate. J Virol. 1995;69:1755–1761. doi: 10.1128/jvi.69.3.1755-1761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozak S L, Platt E J, Madani N, Ferro F E, Jr, Peden K, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moses A V, Stenglein S G, Strussenberg J G, Wehrly K, Chesebro B, Nelson J A. Sequences regulating tropism of human immunodeficiency virus type 1 for brain capillary endothelial cells map to a unique region on the viral genome. J Virol. 1996;70:3401–3406. doi: 10.1128/jvi.70.6.3401-3406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers G, Korber B T, Foley B T, Jeang K-T, Mellors J W, Wain-Hobson S, editors. Human retroviruses and AIDS 1996: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.M: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1996. [Google Scholar]
- 34.Myers G, Korber B T, Foley B T, Jeang K-T, Mellors J W, Wain-Hobson S, editors. Human retroviruses and AIDS 1997: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.M: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1997. [Google Scholar]
- 34a.National Center for Biotechnology Information. Release version 106. Sequences. [Online.] http://www.ncbi.nlm.nih.gov/Entrez/. [April 1999, last date accessed.]
- 35.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien W A, Koyanagi Y, Namazie A, Zhao J-Q, Diagne A, Idler K, Zack J A, Chen I S Y. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 37.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophage isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pleskoff O, Tréboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 39.Pope M, Frankel S s, Mascola J R, Trkola A, Isdel F, Brix D L, Burke D S, Ho D D, Moore J P. Human immunodeficiency virus type 1 strains of subtypes B and E replicate in cutaneous dendritic cell-T cell mixtures without displaying subtype-specific tropism. J Virol. 1997;71:8001–8007. doi: 10.1128/jvi.71.10.8001-8007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzuto C D, Wyatt R, Hernández-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 41.Ross T M, Bieniaz P D, Cullen B R. Multiple residues contribute to the inability of murine CCR-5 to function as a coreceptor for macrophage-tropic human immunodeficiency virus type 1 isolates. J Virol. 1998;72:1918–1924. doi: 10.1128/jvi.72.3.1918-1924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuitemaker H, Kootstra N, de Goede R, de Wolf F, Miedema F, Tersmette M. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable at all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991;65:356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shioda T, Levy J A, Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991;349:167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- 46.Speck R F, Wherly K, Platt E J, Atchison R E, Charo I F, Kabat D, Chesebro B, Goldsmith M A. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 48.Trujillo J R, Wang W K, Lee T H, Essex M. Identification of the envelope V3 loop as a determinant of a CD4-negative neuronal cell tropism for HIV-1. Virology. 1996;217:613–617. doi: 10.1006/viro.1996.0158. [DOI] [PubMed] [Google Scholar]
- 49.Tuttle D L, Harrison J K, Anders C, Sleasman J W, Goodenow M M. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol. 1998;72:4962–4969. doi: 10.1128/jvi.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W-K, Dudek T, Essex M, Lee T-H. Hypervariable region 3 residues of HIV type 1 gp120 involved in CCR5 coreceptor utilization: therapeutic and prophylactic implications. Proc Natl Acad Sci USA. 1999;96:4558–4562. doi: 10.1073/pnas.96.8.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W-K, Dudek T, Zhao Y-J, Brumblay H G, Essex M, Lee T-H. CCR5 coreceptor utilization involves a highly conserved arginine residue of HIV type 1 gp120. Proc Natl Acad Sci USA. 1998;95:5740–5745. doi: 10.1073/pnas.95.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westervelt P, Gendelman H E, Ratner L. Identification of a determinant within the HIV-1 surface envelope glycoprotein critical for productive infection of cultured primary monocytes. Proc Natl Acad Sci USA. 1991;88:3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westervelt P, Trowbridge D B, Epstein L G, Blumberg B M, Li Y, Hahn B H, Shaw G, Price R, Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992;66:2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 55.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore J P, Mackay C R. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodrosky J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 58.Xiao L, Owen S M, Goldman I, Lal A A, deJong J J, Goudsmit J, Lal R B. CCR5 coreceptor usage of non-syncytium-inducing primary HIV-1 is independent of phylogenetically distinct global HIV-1 isolates: delineation of consensus motif in the V3 domain that predicts CCR-5 usage. Virology. 1998;240:83–92. doi: 10.1006/viro.1997.8924. [DOI] [PubMed] [Google Scholar]