Abstract
Polycythemia vera (PV) is a Philadelphia chromosome-negative myeloproliferative neoplasm characterized by clonal erythrocytosis. A phase 2 study reported that ropeginterferon alfa-2b is a well-tolerated and effective treatment for PV in Japanese patients. This post hoc analysis of the phase 2 data further evaluated outcomes in patients at low risk of thrombosis (low-risk PV). Among 20 patients with low-risk PV, 60.0% (12/20) and 85.0% (17/20) achieved < 45% hematocrit by weeks 24 and 52, respectively. The proportion of responders with complete hematologic response (CHR) was 60.0% (12/20) at week 52, and the median time to response was 11.9 months. The mean JAK2 V617F allele burden decreased from 75.8% at baseline to 53.7% at week 52. No patient experienced thrombosis or bleeding episodes. All patients experienced treatment-emergent adverse events (TEAEs) related to ropeginterferon alfa-2b, but no grade ≥ 3 TEAEs or deaths related to ropeginterferon alfa-2b occurred, and no new safety concerns arose. This analysis indicated that ropeginterferon alfa-2b may be an effective treatment option for Japanese patients with low-risk PV.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12185-024-03804-1.
Keywords: Hematologic response, JAK2 V617F allele burden, Low-risk, Polycythemia vera, Ropeginterferon alfa-2b
Introduction
Patients with polycythemia vera (PV) have an overproduction of erythrocytes, and lowering hematocrit (HCT) levels < 45% is recommended to reduce the risk of thrombotic events (TEs) and cardiovascular death [1]. The National Comprehensive Cancer Network and the European LeukemiaNet recommend cytoreductive therapy (CRT) for patients with PV (including those at low risk of thrombosis [low-risk PV], e.g., patients < 60 years old without previous TEs) who have either poor hematologic control, symptomatic PV, splenomegaly, frequent phlebotomies or are intolerant to phlebotomy [2, 3]. Currently, Japanese guidelines only recommend phlebotomy and the use of low-dose aspirin for low-risk PV [4]. However, evidence suggests that persistently elevated hematologic parameters, including HCT, white blood cell (WBC) and platelet (PLT) counts, and higher JAK2 V617F allele burden can increase the risk of TEs even in patients with low-risk PV [5–7]. Thus, patients with low-risk PV require proper management of hematologic parameters and JAK2 V617F allele burden.
Ropeginterferon alfa-2b is a novel, site-selective, monopegylated recombinant human interferon alfa-2b that is a well-tolerated and effective treatment for PV [8, 9]. The LOW-PV study reported that ropeginterferon alfa-2b is safe and effective in patients with low-risk PV [10]. However, no such evidence has been reported in Japanese patients. This analysis of a phase 2 study evaluated safety and efficacy of ropeginterferon alfa-2b in Japanese patients with low-risk PV.
Materials and methods
This was a post hoc analysis of a phase 2, open-label, multicenter, single-arm study in Japanese PV patients [9]. Patients (aged ≥ 20 years and with a diagnosis of PV [11, 12]) received subcutaneous ropeginterferon alfa-2b every 2 weeks for 12 months, starting at 100 µg (50 µg for patients already receiving CRT) and up to a maximum dose of 500 µg [9].
This analysis evaluated data from patients with low-risk PV (age < 60 years and no history of thrombosis) [4]. The key inclusion criteria are shown in Supplemental Table 1.
HCT, WBCs, PLTs, and JAK2 V617F allele burden were analyzed. The complete hematologic response (CHR) maintenance rate was defined as a HCT value < 45%, WBC count ≤ 10 × 109/L, and PLT count ≤ 400 × 109/L. Treatment-emergent adverse events (TEAEs) and their severity according to the Common Terminology Criteria for Adverse Events version 5.0 were assessed. SAS version 9.4 or higher (SAS Institute Inc., Cary, NC, USA) was used to generate figures and analyze data. The study was conducted in compliance with the ethical principles originating with the Declaration of Helsinki and all other relevant guidelines and requirements.
Results
Twenty patients with low-risk PV were analyzed (median age: 49.5 years; Table 1). Median HCT, WBC count, and PLT count were 48.1%, 16.7 × 109/L, and 798 × 109/L, respectively. Poor baseline hematologic control was observed: 17/20 (85.0%) patients had a HCT ≥ 45%, 12/20 (60.0%) had a WBC count ≥ 15.0 × 109/L, and 6/20 (30.0%) had a platelet count ≥ 1,000 × 109/L. One patient was negative for the JAK2 V617F variant; the mean JAK2 V617F allele burden was 75.8% in the remaining 19 patients. Eight (40%) patients had received hydroxyurea treatment.
Table 1.
Baseline demographics of patients with low-risk polycythemia vera
| Baseline characteristic | N = 20 |
|---|---|
| Age, years | 49.5 (26–59) |
| Female sex | 11 (55.0) |
| Hematocrit, % | 48.1 (40.5–55.4) |
| White blood cell count, 109/L | 16.7 (6.1–33.4) |
| Platelet count, 109/L | 798 (356–1781) |
| Prior hydroxyurea treatment | 8 (40.0) |
| Aspirin use | 14 (70.0) |
| JAK2 V617F mutation | 19 (95.0) |
| JAK2 V617F allele burden, % | 75.8 ± 19.9 |
Data are median (range), n (%), or mean ± standard deviation
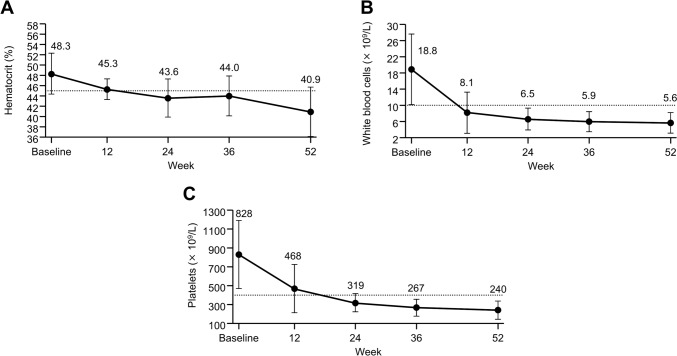
HCT, WBC count, and PLT count decreased over the treatment period (Fig. 1). Most patients had < 45% HCT at weeks 24 (60.0% [12/20]) and 52 (85.0% [17/20]). The proportions of patients requiring phlebotomy were 35.0% (7/20) at baseline and 5.0% (1/20) at week 52 (Supplemental Fig. 1).
Fig. 1.
Laboratory values. A Hematocrit. B White blood cell count. C Platelet count. Data are mean (standard deviation). The dotted line indicates the target value (hematocrit: 45%, platelets: 400 × 109/L, white blood cells: 10 × 109/L)
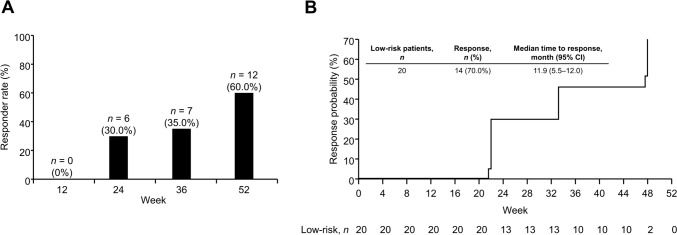
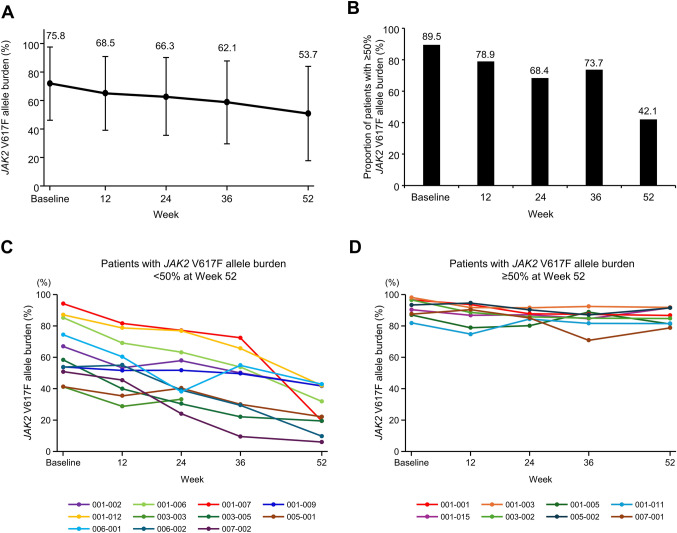
The proportions of patients with CHR were 30.0% (6/20) and 60.0% (12/20) at weeks 24 and 52, respectively; median time to response was 11.9 months (Fig. 2). The mean JAK2 V617F allele burden decreased from 75.8% at baseline to 53.7% at week 52 (Fig. 3A). The proportion of patients with ≥ 50% variant allele frequency decreased from 89.5% (17/19) at baseline to 42.1% (8/19) at week 52 (Fig. 3B), although two patients had a < 50% of variant allele at baseline. Changes in JAK2 V617F allele burden over time according to patients with < 50% or ≥ 50% allele burden at week 52 are shown in Fig. 3C and D, respectively. The patients who achieved < 50% allele burden at week 52 (11/19 cases, including 2 patients with allele burden < 50% at baseline) had a mean age of 50 years and mean allele burden of 64.4% at baseline; while those who did not achieve < 50% allele burden at week 52 (8/19 cases) had a mean age of 48 years and mean allele burden of 91.5% at baseline. Furthermore, the proportion of patients with CHR at week 52 was 100% (10/10 cases; one case could not be determined at week 52 due to discontinuation, but CHR was not achieved at end of treatment) in patients who achieved < 50% allele burden at week 52 and 37.5% (3/8 cases) in those who did not. The mean dose of ropeginterferon alfa-2b at week 52 was 350.0 μg in patients who achieved < 50% allele burden and 462.5 μg in those who did not.
Fig. 2.
Complete hematologic response. A Proportion of responders with low-risk polycythemia vera over time. B Time to response. CI confidence interval
Fig. 3.
Molecular response. A JAK2 V617F allele burden. B Proportion of patients with ≥ 50% JAK2 V617F allele burden. C Change in JAK2 V617F allele burden over time for patients with < 50% allele burden at week 52. One patient (003–003) discontinued, whose data at week 24 reflect the end of treatment. D Change in JAK2 V617F allele burden over time for patients with ≥ 50% allele burden at week 52. N = 19; data are mean (standard deviation) or percentage. One patient who was negative for JAK2 V617F at baseline was excluded
TEAEs related to ropeginterferon alfa-2b occurred in all patients; however, no deaths or grade ≥ 3 TEAEs related to ropeginterferon alfa-2b occurred (Supplemental Table 2). The most common TEAEs observed in the low-risk group but not in the high-risk group were alanine aminotransferase increased (n = 6, 30.0%), aspartate aminotransferase increased (n = 5, 25.0%), and diarrhea (n = 5, 25.0%). One patient discontinued treatment because of grade 1 silent thyroiditis related to ropeginterferon alfa-2b [13]. One serious TEAE (gastroenteritis) occurred; this was not related to ropeginterferon alfa-2b.
Discussion
This post hoc analysis of a phase 2 study found that ropeginterferon alfa-2b elicited a reduction in HCT values, WBC counts, PLT counts, and JAK2 V617F allele burden in Japanese patients with low-risk PV.
As patients with low-risk PV for whom the standard treatment in Japan is difficult to apply (including patients for whom CRT is recommended because of disease-related signs and symptoms) were enrolled in the original phase 2 study [2, 3, 9], the patients in this analysis exceeded target hematological levels at baseline, and exhibited HCT: 48.3%, WBC: 18.8 × 109/L, and PLT: 828 × 109/L. Lowering HCT levels < 45% is recommended to prevent TEs [4], and elevated WBC and PLT counts are also risk factors for TEs [5, 6]. At 52 weeks, the mean values reached the target value (HCT: 40.9%, WBC: 5.6 × 109/L, PLT: 240 × 109/L), and no TEs occurred, indicating the usefulness of ropeginterferon alfa-2b in low-risk patients with PV.
In the LOW-PV study (which compared standard treatment [phlebotomy and low-dose aspirin] versus standard treatment plus ropeginterferon alfa-2b for low-risk PV), the percentage of responders (HCT < 45% and no disease progression) at 12 months was 81% in the ropeginterferon alfa-2b group [10]. Furthermore, there were fewer mean phlebotomies per patient-year in the ropeginterferon alfa-2b group (2.9) than the standard treatment group (4.2). The percentage of patients who achieved HCT < 45% in our study (85%) was similar to that of LOW-PV. Similarly, fewer patients required phlebotomies following ropeginterferon alfa-2b treatment, indicating consistent outcomes in the Japanese population.
In this analysis, the mean JAK2 V617F allele burden notably decreased, and the proportion of patients with a ≥ 50% variant allele frequency decreased from 89.5% at baseline to 42.1% following 52 weeks of treatment. Furthermore, no cases experienced myelofibrosis transformation. A previous study found that patients with higher JAK2 V617F burden (> 50% variant allele frequency) have a higher myelofibrosis transformation rate than patients with < 50% frequency [14]. Therefore, the decreased JAK2 V617F allele burden following ropeginterferon alfa-2b treatment may have contributed to a reduced myelofibrosis transformation risk. Younger patients and those with low allele burden are reported to be more likely to have a molecular response to ropeginterferon alfa-2b [8]. Similarly, patients who achieved < 50% allele burden at week 52 had a lower baseline allele burden compared to those who did not (64.4% vs 91.5%) in this study. Additionally, the proportion of patients with CHR at week 52 was 100% (10/10 cases; one case could not be determined due to discontinuation) in patients who achieved < 50% allele burden at week 52. These results suggest that an allele reduction is associated with the CHR achievement. However, longer studies with larger populations are needed to confirm these results and to determine which patients are more likely to achieve a molecular response in Japanese patients with low-risk PV.
Although TEAEs related to ropeginterferon alfa-2b occurred in all patients, no treatment-related grade ≥ 3 TEAEs occurred. As in the original phase 2 study, the most common TEAE was alopecia [9]. One patient who discontinued treatment because of silent thyroiditis related to ropeginterferon alfa-2b had no history of thyroid dysfunction and was positive for anti-thyroid peroxidase antibodies and negative for anti-thyroglobulin antibodies at baseline [13]. Therefore, it is advisable to monitor thyroid function and thyroid antibodies both prior to and following ropeginterferon alfa-2b initiation.
Our results indicate that ropeginterferon alfa-2b can effectively reduce HCT, WBC counts, PLT counts, and JAK2 V617F allele burden, reducing phlebotomy requirements, in Japanese patients with low-risk PV. Although other details (including clinical symptoms, duration, and phlebotomy frequency) were not recorded, and despite a relatively small sample size and short duration, our results support ropeginterferon alfa-2b as a treatment option for patients with low-risk PV who do not respond adequately to phlebotomy and low-dose aspirin.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Hannah Read, PhD, of Edanz (www.edanz.com) for medical writing services, which were funded by PharmaEssentia Japan KK, in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022).
Author contributions
KS designed the study, wrote the protocol, and interpreted the data. AQ and NK designed the study, wrote the protocol, and analyzed and interpreted the data. KK supervised the study, contributed to clinical data collection, and interpreted the data. All authors contributed to drafting and reviewing the manuscript, and all authors approved the final version of the manuscript.
Funding
Open access funding provided by University of Miyazaki. This research was supported by PharmaEssentia Corporation.
Data availability
The data are available from PharmaEssentia upon reasonable request.
Declarations
Conflict of interest
KS received research funding from PharmaEssentia Corporation, PharmaEssentia Japan KK, Chugai Pharmaceutical Co., Ltd., AbbVie GK, Kyowa Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Shionogi & Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Eisai Co., Ltd., Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., and Taisho Pharmaceutical Co., Ltd; funding for medical writing and article processing charges from PharmaEssentia Japan KK; and honoraria from Novartis Pharma KK and Takeda Pharmaceutical Co., Ltd. AQ is the chief medical officer of PharmaEssentia. NK is a board member of and received funding for medical writing and article processing charges from PharmaEssentia Japan KK; research funding from PharmaEssentia Corporation, Takeda Pharmaceutical Co., Ltd., and Meiji Seika Pharma Co., Ltd.; grants from Otsuka Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Sumitomo Pharma Co., Ltd., PharmaEssentia Japan KK, Chugai Pharmaceutical Co., Ltd., and Perseus Proteomics Inc.; consulting fees from Japan Tobacco Inc., Torii Pharmaceutical Co., Ltd., and PharmaEssentia Japan KK; and honoraria from Novartis Pharma KK and Takeda Pharmaceutical Co., Ltd. KK received research funding from PharmaEssentia Corporation; funding for medical writing and article processing charges from PharmaEssentia Japan KK; and honoraria from Novartis Pharma KK, Takeda Pharmaceutical Co., Ltd., PharmaEssentia Japan KK, Sanofi KK, and AbbVie GK.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, CYTO-PV Collaborative Group, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33. 10.1056/NEJMoa1208500 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN clinical practice guidelines in Oncology: Myeloproliferative neoplasms. Version 1. 2024. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1477. Accessed 20 Jan 2024. [DOI] [PubMed]
- 3.Marchetti M, Vannucchi AM, Griesshammer M, Harrison C, Koschmieder S, Gisslinger H, et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022;9:e301–11. 10.1016/S2352-3026(22)00046-1 [DOI] [PubMed] [Google Scholar]
- 4.Shimoda K, Takahashi N, Kirito K, Iriyama N, Kawaguchi T, Kizaki M. JSH practical guidelines for hematological malignancies, 2018: I Leukemia-4 Chronic myeloid leukemia (CML)/myeloproliferative neoplasms (MPN). Int J Hematol. 2020;112:268–91. 10.1007/s12185-020-02964-0 [DOI] [PubMed] [Google Scholar]
- 5.Gerds AT, Mesa RA, Burke JM, Grunwald MR, Stein BL, Squier P, et al. Association between elevated white blood cell counts and thrombotic events in polycythemia vera: analysis from REVEAL. Blood. 2023. 10.1182/blood.2023020232. 10.1182/blood.2023020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimoda K, Yasunaga H, Sugimoto Y, Uenaka H, Dochi T, Jun G. The association between blood cell counts and thrombotic events in Japanese patients with polycythemia vera: a retrospective database study. Blood. 2023;142(Supplement 1):3191. 10.1182/blood-2023-182706 [DOI] [Google Scholar]
- 7.Moliterno AR, Kaizer H, Reeves BN. JAK2 V617F allele burden in polycythemia vera: burden of proof. Blood. 2023;141:1934–42. 10.1182/blood.2022017697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, PROUD-PV Study Group, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7:e196–208. 10.1016/S2352-3026(19)30236-4 [DOI] [PubMed] [Google Scholar]
- 9.Edahiro Y, Ohishi K, Gotoh A, Takenaka K, Shibayama H, Shimizu T, et al. Efficacy and safety of ropeginterferon alfa-2b in Japanese patients with polycythemia vera: an open-label, single-arm, phase 2 study. Int J Hematol. 2022;116:215–27. 10.1007/s12185-022-03341-9 [DOI] [PubMed] [Google Scholar]
- 10.Barbui T, Vannucchi AM, De Stefano V, Carobbio A, Ghirardi A, Carioli G, et al. Ropeginterferon versus standard therapy for low-risk patients with polycythemia vera. NEJM Evid. 2023;2:1. 10.1056/EVIDoa2200335 [DOI] [PubMed] [Google Scholar]
- 11.Teferi A, Vardiman JW. Classification and diagnosis of myelo-proliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. 10.1038/sj.leu.2404955 [DOI] [PubMed] [Google Scholar]
- 12.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 13.Kirito K. Silent thyroiditis associated with ropeginterferon alfa-2b in a patient with polycythemia vera. Intern Med. 2024;63:843–6. 10.2169/internalmedicine.2171-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triguero A, Pedraza A, Pérez-Encinas M, Mata-Vázquez MI, Vélez P, Fox L, On behalf of the MPN Spanish Group (GEMFIN), et al. Low-risk polycythemia vera treated with phlebotomies: clinical characteristics, hematologic control and complications in 453 patients from the Spanish Registry of Polycythemia Vera. Ann Hematol. 2022;101:2231–9. 10.1007/s00277-022-04963-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from PharmaEssentia upon reasonable request.