Abstract
Introduction
Preterm infants experience tremendous early life pain/stress during their neonatal intensive care unit (NICU) hospitalization, which impacts their neurodevelopmental outcomes. Mitochondrial function/dysfunction may interface between perinatal stress events and neurodevelopment. Nevertheless, the specific proteins or pathways linking mitochondrial functions to pain-induced neurodevelopmental outcomes in infants remain unidentified. Our study aims to investigate the associations among pain/stress, proteins associated with mitochondrial function/dysfunction, and neurobehavioral responses in preterm infants.
Methods
We conducted a prospective cohort study, enrolling 33 preterm infants between September 2017 and July 2022 at two affiliated NICUs located in Hartford and Farmington, CT. NICU Network Neurobehavioral Scale (NNNS) datasets were evaluated to explore potential association with neurobehavioral outcomes. The daily pain/stress experienced by infant’s during their NICU stay was documented. At 36–38 weeks post-menstrual age (PMA), neurobehavioral outcomes were evaluated using the NNNS and buccal swabs were collected for further analysis. Mass spectrometry-based proteomics was conducted on epithelial cells obtained from buccal swabs to evaluate protein expression level. Lasso statistical methods were conducted to study the association between protein abundance and infants’ NNNS summary scores. Multiple linear regression and Gene Ontology (GO) enrichment analyses were performed to examine how clinical characteristics and neurodevelopmental outcomes may be associated with protein levels and underlying molecular pathways.
Results
During NICU hospitalization, preterm premature rupture of membrane (PPROM) was negatively associated with neurobehavioral outcomes. The protein functions including leptin receptor binding activity, glutathione disulfide oxidoreductase activity and response to oxidative stress, lipid metabolism, and phosphate and proton transmembrane transporter activity were negatively associated with neurobehavioral outcomes; in contrast, cytoskeletal regulation, epithelial barrier, and protection function were found to be associated with the optimal neurodevelopmental outcomes. In addition, mitochondrial function-associated proteins including SPRR2A, PAIP1, S100A3, MT-CO2, PiC, GLRX, PHB2, and BNIPL-2 demonstrated positive association with favorable neurodevelopmental outcomes, while proteins of ABLIM1, UNC45A, keratins, MUC1, and CYB5B showed positive association with adverse neurodevelopmental outcomes.
Conclusion
Mitochondrial function-related proteins were observed to be associated with early life pain/stress and neurodevelopmental outcomes in infants. Large-scale studies with longitudinal datasets are warranted. Buccal proteins could be used to predict potential neurobehavioral outcomes.
Keywords: Preterm infants, Mass spectrometry, Mitochondrial dysfunction, Pain/stress
Introduction
Growing evidence has shown that preterm infants are vulnerable to pain and stress in their early life, impacting infant neurodevelopmental outcomes and persisting to adulthood [1–3]. The underlying mechanisms responsible for pain-induced white matter volume decrease and brain structure alteration included low pain threshold and disrupted axonal development [4, 5]. In addition, mitochondrial dysfunctions are related to neuropathic pain [6] and neurodegenerative diseases such as Parkinson’s disease [7], Alzheimer’s disease [8], Huntington’s disease [9], Friedreich’s ataxia [10], and Charcot-Marie-Tooth disease [11]. However, the potential role that mitochondrial dysfunctions play in neonatal pain/stress and neurodevelopmental outcomes remains poorly understood.
After birth, the newborn’s brain development requires enhanced mitochondrial energy generation [12]. However, stress, pain, and inflammation-induced reactive oxygen species (ROS) production in preterm infants induce mitochondrial damage and interrupt the adequate energy supply [13]. Additionally, the combination of overproduced ROS and inadequate antioxidant capacity may damage mitochondrial structure and further disturb mitochondrial function in normal metabolism (e.g., oxygen consumption, oxidative phosphorylation, ATP production, calcium buffering) and induce neurodevelopmental disorders [14, 15]. Several studies have indicated that genes/proteins, including mitochondrially encoded cytochrome c oxidase II (MT-CO2) [16], phosphate carrier [17], and keratins [18], play a role in regulating mitochondrial functions in the context of pain/stress-induced clinical behavioral disorders or skin disease. However, the specific proteins or pathways associated with mitochondrial functions that contribute to pain-induced neurodevelopmental outcomes in infants are still unknown.
Our study identified and quantified expression changes of proteins related to mitochondrial function/dysfunction, which may serve as a link between pain/stress and neurodevelopment in early life. We employed mass spectrometry (MS)-based proteomics, a method with the capability to identify potential biomarkers indicating both favorable and nonfavorable neurobehavioral outcomes [19]. This approach aids in enhancing our comprehensive understanding of the molecular mechanisms underlying mitochondrial function/dysfunction and neurodevelopment in infants who have experienced early life pain/stress.
Methods
Design
A prospective cohort study was designed to examine the associations between infant medical characteristics, pain/stress experience during the NICU stay, mitochondrial function/dysfunction-related proteins, and neurobehavioral outcomes. The study was approved by the Institutional Review Board at Connecticut Children’s Medical Center.
Participants
Subjects were cared for at two affiliated NICUs in Hartford and Farmington, CT and enrolled between September 2017 and July 2022. Inclusion criteria were (1) preterm infants with gestational age (GA) at the birth of 28–32 weeks, (2) parents who were 18 years and older to give consent, and (3) parents who were English speaking. Exclusion criteria were infants who have (1) known congenital or chromosomal abnormalities; (2) intraventricular hemorrhage (grades III and IV); (3) undergone surgical procedures; and (4) intrauterine exposure to illicit substances. Our study included infants born both vaginally and via cesarean, and thus, the delivery mode was not considered as inclusion or exclusion criteria. The exclusion criteria were based on confounding factors that could contribute to pain/stress experience and adverse neurodevelopmental outcomes. Study subjects underwent routine NICU care as determined by the managing clinical service and informed written consent was obtained from a parent on behalf of their infant.
Demographic and Medical Characteristics
Infants’ clinical data including demographics, mode of delivery, exposure to PPROM [20], treatment with antibiotics before the first 3 days, birth GA, birth weight, birth body length, birth head circumference, severity of illness measured using the Scale of Neonatal Acute Physiology with Perinatal Extension-II (SNAPPE II) [21], and the length of NICU hospital stay were collected and electronically documented to the REDCap [22] by the research nurses.
Pain/Stress Measures
Early life painful/stressful experiences during NICU hospitalization were measured using the modified NICU Infant Stressor Scale (NISS), which includes 47 acute and 23 chronic procedures and interventions [1]. During the first 28 days of the infant’s NICU stay, daily acute and chronic NISS data were collected from the electronic health record by the research nurses and documented in the REDCap [3].
Acute and chronic painful/stressful procedures were then categorized into five domains (1 = not painful/stressful; 2 = a little; 3 = moderate; 4 = very; and 5 = extremely painful/stressful). Weighted daily frequencies of acute pain and hours of chronic pain were calculated. Average weighted daily pain/stress for the first 28 days after birth was calculated by adding daily frequencies (for acute) and hours (for chronic) of pain/stress events over 4 weeks, times with levels of each event, and then divided by 28 days. Level 3 and below were considered standard NICU care, and levels 4 and 5 were considered “very” and “extreme” pain/stress events, respectively [23].
Neurodevelopmental Measures
Infants’ neurodevelopmental outcomes were measured using the NICU Neonatal Neurobehavioral Scale (NNNS) when they reached a post-menstrual age of 36–38 weeks [24]. The NNNS includes 115 items categorized into 13 summary scores. In this study, we focused on four summary scores based on previous findings [1, 25, 26]: stress/abstinence (NSTRESS), need for handling (NHANDLE), self-regulation (NREGULATION), and quality of movement (NQMOVE).
The NSTRESS summary score quantifies the signs of neonatal abstinence and stress in high-risk preterm infants. The NHANDLE summary score quantifies to what extent the handling request for the infants to keep an alert state. The NREGULATION summary score quantifies the infants’ ability to follow the auditory and visual orientation. The NQMOVE summary score measures the ability of infants’ maturity and modulation of the movement of limbs. Lower scores on NSTRESS and NHANDLE scales and higher scores on NREGULATION and NQMOVE scales indicate better neurobehavioral outcomes [1, 25, 26]. In our study, we considered NSTRESS and NHANDLE scales as nonfavorable NNNS scales, while NREGULATION and NQMOVE scales were considered as favorable NNNS scales.
Buccal Swab Collection
Infant buccal swabs were collected at 36–38 weeks post-menstrual age using the Gentra Puregene Buccal Cell Kit (Qiagen, #158845). A soft-tipped sterile swab was run 10 times on the interior of each cheek to sample the infant’s buccal epithelial tissues at least 30 min after a feeding or oral procedure [27]. Samples were immediately frozen at −80°C until they were processed and analyzed. The following steps were followed:
Buccal protein preparation for proteomic analysis.
Proteomic analysis by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry.
Statistical analysis.
Proteomic Gene Ontology (GO) analysis (all these processes have been explained in detail in eMethods in the online supplement; for all online suppl. material, see https://doi.org/10.1159/000536509).
Results
Infant Demographic and Medical Characteristics
During the study period, thirty-three preterm infants satisfied inclusion criteria were enrolled. Among the infants, the majority were males (54.6%), were white (81.8%), were delivered via C-sections (63.6%), were not exposed to PPROM (69.7%), and were not treated with antibiotics during the first 3 days of hospitalization (72.7%). The mean birth GA was 29.3 weeks (SD = 2.6), the mean SNAPPEII score was 14.0 (SD = 13.7), and the length of hospital stay was 74.1 days (SD = 38.2) (Table 1). There was no statistically significant difference between genders in terms of infants’ demographic and medical characteristics.
Table 1.
Infant demographic characteristics (N = 33)
| Variables | Total (N = 33) | Female (n = 15) | Male (n = 18) | p value |
|---|---|---|---|---|
| Race | ||||
| White | 27 (81.8) | 13 (86.7) | 14 (77.8) | 0.799 |
| Black | 4 (12.1) | 1 (6.7) | 3 (16.7) | |
| Other | 2 (6.1) | 1 (6.7) | 1 (5.6) | |
| Delivery | ||||
| C-section | 21 (63.6) | 10 (66.7) | 11 (61.1) | 1 |
| Vaginal | 12 (36.4) | 5 (33.3) | 7 (38.9) | |
| PPROM | ||||
| Yes | 10 (30.3) | 6 (40.0) | 4 (22.2) | 0.448 |
| No | 23 (69.7) | 9 (60.0) | 14 (77.8) | |
| Antibiotic used first 3 days | ||||
| Yes | 24 (72.7) | 11 (73.3) | 13 (72.2) | 0.295 |
| No | 7 (21.2) | 2 (13.3) | 5 (27.8) | |
| Unknown | 2 (6.1) | 2 (13.3) | 0 (0) | |
| Antibiotic used after 3 days | ||||
| Yes | 6 (18.2) | 3 (20.0) | 3 (16.7) | 0.279 |
| No | 25 (75.8) | 10 (66.7) | 15 (83.3) | |
| Unknown | 2 (6.1) | 2 (13.3) | 0 (0) | |
| Mean (SD) | Mean (SD) | Mean (SD) | p value | |
|---|---|---|---|---|
| Birth GA, weeks | 29.3 (2.6) | 28.8 (2.4) | 29.7 (2.8) | 0.147 |
| Birth weight, g | 1,240 (405) | 1,090 (334) | 1,360 (424) | 0.043 |
| Birth body length, cm | 37.9 (4.5) | 36.5 (3.7) | 39.0 (4.9) | 0.0886 |
| Birth HC, cm | 26.2 (2.7) | 25.5 (2.7) | 26.9 (2.6) | 0.103 |
| SNAPEII | 14.0 (13.6) | 15.5 (10.9) | 12.9 (15.6) | 0.3 |
| Hospital stay, days | 74.1 (38.2) | 77.1 (31.8) | 71.6 (43.5) | 0.437 |
| Acute pain | ||||
| Unweighted | 21.5 (4.2) | 20.9 (4.1) | 22.1 (4.4) | 0.255 |
| Weighted | 66.6 (12.9) | 64.6 (12.1) | 68.4 (13.6) | 0.259 |
| Chronic pain | ||||
| Unweighted | 52.6 (16.6) | 54.4 (13.2) | 51.0 (19.4) | 0.527 |
| Weighted | 120.8 (43.9) | 124 (35.0) | 118.4 (51.0) | 0.731 |
PPROM, preterm premature rupture of membranes; HC, head circumference; GA, gestational age; SNAPEII, Score for Neonatal Acute Physiology with Perinatal Extension-II.
Infant Daily Painful/Stressful Experience
Preterm infants experienced an average of 21.5 (SD = 4.2) acute painful/stressful events, and the daily weighted acute NISS score was 66.6 (SD = 12.9) during their first 28 days in the NICU. They also experienced daily cumulated chronic pain/stress of 52.6 (SD = 16.6) hours, and the weighted chronic NISS score was 120.8 (SD = 43.9).
Neurobehavioral Outcomes
Due to irritability exhibited by certain preterm infants during the NNNS exam, preventing them from completing the entire test, the final complete datasets for all four NNNS subscales comprised 18 out of the 33 infants. Of these, the average and range of NNNS summary scores of NSTRESS, NHANDLE, NREGULATION, and NQMOVE were 0.2 (SD = 0.1, 0.1–0.4), 0.4 (SD = 0.2, 0.1–0.9), 4.8 (SD = 0.8, 3.4–6.3), and 4.2 (SD = 0.6, 3–5.3), respectively.
Proteomic Dataset Filtering and Lasso Analysis of the Association of NNNS Scores with Protein (Application Programming Interface)
From all 33 subjects, totally 2,107 buccal proteins were identified by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry in our study after filtering to a 1% FDR at the protein and peptide-spectrum match levels plus 2 peptide/proteins minimum. After removing 56 nonhuman source proteins that were identified using the default MaxQuant contaminants database including Taxon ID 10090 for Mus musculus, Taxon ID 9825 for Sus scrofa (pig trypsin), and Taxon ID 9913 for Bos taurus, 2,051 proteins belonging to Homo sapiens taxonomy were identified and further analyzed.
After performing Lasso [28], we found a total of 52 proteins that explained 99% of the variation in NSTRESS, NHANDLE, NREGULATION, and NQMOVE summary scores, respectively, when controlling clinical covariates (see Table 2). Table 2 shows the proteins that are associated with four NNNS scales. The absolute value of the coefficients can be considered as the importance of the proteins. The greater the absolute value (magnitude, irrespective of the sign) of the coefficients for specific proteins, indicates their stronger correlation with the NNNS subscale scores. In contrast, a smaller absolute value implies a weaker relationship between proteins and NNNS subscale scores. The proteins that correlated with more than one NNNS subscale are also highlighted in Table 2. The internal consistency of proteins that are related to favorable (NREGULATION and NQMOVE) or nonfavorable (NSTRESS and NHANDLE) NNNS summary scores was observed in our findings. Among these identified proteins, SPRR2A was positively correlated with NHANDLE summary scores but negatively correlated with NREGULATION summary scores (SPRR2A↑, NHANDLE↑/NREGULATION↓). S100A3 was positively correlated with NSTRESS and NHANDLE summary scores, but negatively correlated with NQMOVE summary scores (S100A3↑, NSTRESS↑+ NHANDLE↑/NQMOVE↓). PAIP1, UNC45A, and ABLIM1 were all positively correlated with NREGULATION scores, while UNC45A and ABLIM1 were negatively correlated with NSTRESS scores, and PAIP1 was negatively correlated with NHANDLE scores (UNC45A↑ and ABLIM1↑, NREGULATION↑/NSTRESS↓; PAIP1↑, NREGULATION↑/NHANDLE↓).
Table 2.
LASSO regression model with the proteomic screening identified proteins associated with each NNNS subscale
| Protein/Gene Abb. | Protein | Accession # | Coefficient |
|---|---|---|---|
| a. NSTRESS | |||
| PSMC4 | 26S proteasome regulatory subunit 6B | Q15008 | 0.262 |
| S100a3* | Protein S100-A3 | P33764 | 0.225 |
| ApoA4 | Apolipoprotein A-IV | P06727 | 0.190 |
| RPL30 | 60S ribosomal protein L30 | P62888 | 0.049 |
| BnipL-2 | Bcl-2/adenovirus E1B 19 kDa-interacting protein 2-like protein (fragment) | A2A492 (+1) | 0.028 |
| RPL21 | 60S ribosomal protein L21 | P46778 | 0.016 |
| UBE2V2 | Ubiquitin-conjugating enzyme E2 variant 2 | Q15819 | 0.016 |
| AGT (1–10) | Angiotensin 1–10 | A0A7P0T8D1 (+1) | 0.011 |
| SAMHD1 | Deoxynucleoside triphosphate triphosphohydrolase SAMHD1 | A0A2R8YCS7 (+1) | 0.008 |
| GPD1L | Glycerol-3-phosphate dehydrogenase 1-like protein | Q8N335 | 0.001 |
| UNC45A* | Protein unc-45 homolog A | A0A1W2PNX8 (+1) | −0.003 |
| TAB182 | 182 kDa tankyrase-1-binding protein | Q9C0C2 | −0.008 |
| KRT6B | Keratin, type II cytoskeletal 6B | CON__P04259 | −0.089798 |
| Shroom3 | Protein Shroom3 | A0A2R8Y5P9 (+1) | −0.090 |
| KRT74 | Keratin, type II cytoskeletal 74 | CON__Q7RTS7 (+1) | −0.091263 |
| KRT33B | Keratin, type I cuticular HA3-II (hair keratin, type I HA3-II) | CON__Q14525 | −0.12757 |
| ABLIM1* | Actin-binding LIM protein 1 | A0A0A0MRL6 (+1) | −0.355 |
| Protein/Gene Abb. | Protein | Accession # | Coefficient |
|---|---|---|---|
| b. NHANDLE | |||
| SPRR2A* | Small proline-rich protein 2A | P35326 | 0.169 |
| Cluster of SPRRs | Cluster of small proline-rich protein | P22532 [3] | 0.168 |
| CYB5B | Cytochrome b5 type B | D6RFH4 (+2) | 0.155 |
| THBS1 | Thrombospondin-1 | P07996 | 0.152 |
| RAB9A | Ras-related protein Rab-9A | P51151 | 0.125 |
| LSM2 | U6 snRNA-associated Sm-like protein LSm2 | Q9Y333 | 0.059 |
| HsRFK | Riboflavin kinase | Q969G6 | 0.058 |
| DNAJA2 | DnaJ homolog subfamily A member 2 | O60884 | 0.054 |
| S100A3* | Protein S100-A3 | P33764 | 0.017 |
| GLOD4 | Glyoxalase domain-containing protein 4 | F6TLX2 (+1) | 0.010 |
| LMAN2 | Vesicular integral-membrane protein VIP36 | Q12907 | 0.007 |
| SOD1 | Superoxide dismutase [Cu-Zn] | P00441 | 0.002 |
| NAXE | NAD(P)H-hydrate epimerase | Q8NCW5 | 4.60E−05 |
| MUC1 | Mucin-1 | Q8WXI7 | −0.056 |
| PAIP1* | Polyadenylate-binding protein-interacting protein 1 | D6REB4 (+1) | −0.329 |
| Protein/Gene Abb. | Protein | Accession # | Coefficient |
|---|---|---|---|
| c. NREGULATION | |||
| UNC45A* | Protein unc-45 homolog A | A0A1W2PNX8 (+1) | 0.207 |
| ABLIM1* | Actin-binding LIM protein 1 | A0A0A0MRL6 (+1) | 0.103 |
| IARS1 | Isoleucyl-tRNA synthetase | A0A0A0MSX9 (+6) | 0.102 |
| KRT36 | Keratin, type I cuticular HA6 (Hair keratin, type I HA6) | CON__O76013 | 0.028 |
| DHK-MTPene | 1,2-Dihydroxy-3-keto-5-methylthiopentene dioxygenase | Q9BV57 | 0.006 |
| PAIP1* | Polyadenylate-binding protein-interacting protein 1 | D6REB4 (+1) | 5.90E−05 |
| PiC | Phosphate carrier protein, mitochondrial | F8VVM2 | −0.014 |
| Glrx | Glutaredoxin-1 | P35754 | −0.031 |
| MT-CO2 | Cytochrome c oxidase subunit 2 | P00403 | −0.050 |
| SPRR2D | Small proline-rich protein 2D | P22532 | −0.096 |
| PHB2 | Prohibitin-2 | Q99623 | −0.109 |
| SNX2 | Sorting nexin-2 | O60749 | −0.141 |
| SPRR2A* | Small proline-rich protein 2A | P35326 | −0.245 |
| NGAL | Neutrophil gelatinase-associated lipocalin | P80188 | −0.255 |
| Protein/Gene Abb. | Protein | Accession # | Coefficient |
|---|---|---|---|
| d. NQMOVE | |||
| UNC45A* | Protein unc-45 homolog A | A0A1W2PNX8 (+1) | 0.137 |
| GBP7 | Guanylate-binding protein 7 (fragment) | A0A3B3IRS3 (+1) | 0.115 |
| KRT6C | Keratin, type II cytoskeletal 6C | CON__P48668 | 0.065 |
| I3L427 | I3L427 | I3L427-DECOY | 0.063 |
| Clca4a | Calcium-activated chloride channel regulator 4 | Q14CN2 | 0.003 |
| IGHV3-49 | Immunoglobulin heavy variable 3–49 | A0A0A0MS15 | −0.001 |
| IGHA1 | Immunoglobulin heavy constant alpha 1 | P01876 | −0.007 |
| S100A3* | Protein S100-A3 | P33764 | −0.065 |
| HSP90AB4P | Putative heat-shock protein HSP 90-beta 4 | Q58FF6 | −0.071 |
| Progranulin | Progranulin | P28799 | −0.084 |
| UBA52 | Ubiquitin-60S ribosomal protein L40 | P62987 | −0.199 |
| RBM3 | RNA-binding protein 3 | P98179 | −0.232 |
| SDR9C7 | Short-chain dehydrogenase/reductase family 9C member 7 | Q8NEX9 | −0.282 |
APIs, average precursor intensities.
*Used here for proteins which showed a significant correlation with at least two NNNS subscales.
+/− sign in front of the correlation means that proteins’ APIs are either positively or negatively correlated with NNNS summary scores.
Regression Analysis of Medical Characteristics Modeled by NNNS Subscales and Principal Component Analysis
All proteins are included in the principal component analysis for dimension reduction. Each principal component (PC) is a linear combination of all proteins that could represent a certain feature or characteristic among the whole proteomic dataset. Each protein could have different coefficients in different PCs. To assess the effects of clinical covariates on NNNS subscales, the multiple linear regression modeled was used to fit each NNNS subscale by 6 covariates, including 3 most contributing PCs for each NNNS summary score extracted from the PC analysis. Three PCs explained about 45% of the variance of proteins. Chronic pain is eliminated based on variable selection using Akaike information criterion [29]. PPROM was positively associated with NSTRESS (p < 0.05), but negatively associated with NREGULATION (p < 0.01). By contrast, birth GA, SNAPPEII, acute pain, and sex were not significantly associated with any NNNS subscale (see Table 3). After performing Holm-Bonferroni adjustment, PPROM (adjusted p < 0.05) was found to be significantly associated with NREGULATION.
Table 3.
Linear regression analysis of NNNS subscales modeled by clinical covariates and 3 most contributing principle components
| Covariates | NSTRESS | NHANDLE | NREGULATION | NQMOVE |
|---|---|---|---|---|
| Birth GA | 0.0884 | −0.1977 | −0.1174 | 0.0748 |
| SNAPPEII | 0.1467 | −0.0062 | −0.3339 | −0.1095 |
| PPROM | 1.7613* | 1.2453 | −1.7042** | −1.0850 |
| Acute pain | −0.0838 | −0.0126 | −0.0691 | 0.1711 |
| Sex | 0.6367 | 0.3591 | 0.0753 | −0.5119 |
GA, gestational age; PPROM, preterm premature rupture of membrane; SNAPPEII, Score for Neonatal Acute Physiology with Perinatal Extension-II. The significant findings are highlighted: Linear regression model identified that PPROM was significantly associated with NNNS subscale scores. Specifically, PPROM showed a positive association with NSTRESS subscale scores (p < 0.05) and a negative association with NREGULATION subscale scores (p < 0.01).
*p < 0.05.
**p < 0.01.
***p < 0.001. (After p value was adjusted for multiple comparison using Holm-Bonferroni correction, PPROM (adjusted p < 0.05) remains significantly correlated with NREGULATION).
GO Enrichment of Pathways and Functions of Proteins Associated with NNNS Subscales
Each of 52 proteins that explained the majority of variation in our NNNS subscales was mapped to these enrichments of GO terms. The functional annotations were categorized as cellular components, biological processes, and molecular functions. For analysis, we subdivided our correlation dataset into four groups. Group 1 contains proteins with application programming interfaces (APIs) that were positively associated with NSTRESS and NHANDLE summary scores. Group 2 contains proteins with APIs that were negatively associated with NSTRESS and NHANDLE scores. Group 3 contains proteins with APIs that were positively associated with NREGULATION and NQMOVE scores. Group 4 contains proteins with APIs negatively associated with NREGULATION and NNQMOVE scores. Note that the increased APIs in groups 1 and 4 indicated poor neurobehavioral outcomes; conversely, increased APIs in groups 2 and 3 indicated better neurobehavioral outcomes.
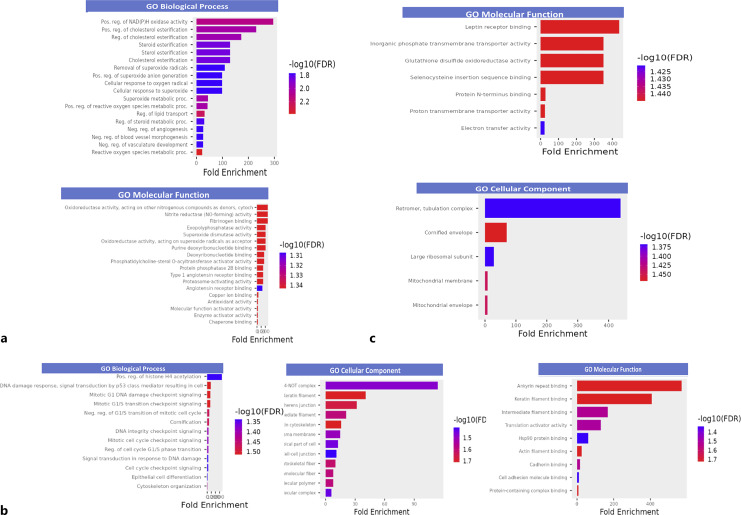
Proteins with APIs that positively correlated with NSTRESS and NHANDLE in group 1 showed significant enrichment in biological processes and molecular functions including protein binding activity (angiotensin receptor binding, copper ion binding, etc.), oxidoreductase activity (NAD(H) oxidase activity, superoxide metabolic process, etc.), cholesterol esterification regulation and lipid transport, proteasome-activating activity, and vasculature development (Fig. 1a). In group 2, proteins with APIs that negatively associate with NSTRESS and NHANDLE scores demonstrated significant enrichment in biological processes and molecular functions including cytoskeleton organization, epithelial cell differentiation, cornification, intracellular signal transduction, translation activator activity, and Hsp90 proteins binding. The significant enrichment of cellular components included cell-cell junction, actin cytoskeleton, keratin filament, adherens junction, and cytoskeleton (Fig. 1b). There was no significant enrichment of proteins in group 3 for proteins with APIs that positively correlated with NREGULATION and NQMOVE. In group 4, proteins with APIs that negatively correlated with NREGULATION and NQMOVE resulted in significant enrichment of molecular function including protein binding (leptin receptor binding, protein N-terminus binding, selenocysteine insertion sequence binding), glutathione disulfide oxidoreductase activity, and transmembrane transporter (inorganic phosphate transmembrane transporter activity and proton transmembrane transporter activity). The significant enrichment of cellular components included the mitochondrial envelope, mitochondrial membrane, cornified envelope, and large ribosomal subunit (Fig. 1c).
Fig. 1.
GO term enrichment analysis of proteins associated with neurodevelopmental outcomes. Significantly enriched GO terms was selected (p < 0.05) and the decreasing order of fold enrichment and colored by -log10 (FDR) were applied in each figure for molecular function, cellular component, and biological process. a The proteins positively correlated with NSTRESS and NHANDLE. b The proteins negatively correlated with NSTRESS and NHANDLE. c The proteins negatively correlated with NREGULATION and NQMOVE. Pain/stress, mitochondrial dysfunction, and neurodevelopment.
Discussion
To our knowledge, this is the first study analyzing the association among early life pain/stress, epithelial tissue proteins related to mitochondrial function/dysfunction, and neurobehavioral outcomes in preterm infants. A total of 52 proteins were identified whose expression levels were significantly associated with signs of stress and abstinence (NSTRESS), handling requirement to reorient to the examination (NHANDLE), physical, motor, and attention response to the examination (NREGULATION), and the smooth movement during the examination (NQMOVE). These correlations provide molecular-level mechanistic insights into how early life experiences (quantified by PPROM) may translate into neurobehavioral outcomes. We identified three distinct orders of 52 proteins as the top 3 PCs for each NNNS subscale, elucidating the varied importance of each protein in the association with neurobehavioral outcomes (see online suppl. Table 1). In addition, this set of proteins has been found to be relevant to processes and pathways related to mitochondrial function such as glutathione disulfide oxidoreductase activity, phosphate and proton transmembrane transporter activity, and response to oxidative stress [30, 31]. Here, we describe some of the significant protein types identified with respect to mitochondrial physiology.
We identified a positive correlation between PPROM and stress and abstinence responses, aligning with findings from other studies that infants exposed to PPROM are at a heightened risk of neurodevelopmental deficits at 2 years of age [32]. Our study also revealed a link between PPROM and unfavorable neurobehavioral patterns, including immature movements such as jitteriness, startles, and tremors, along with challenges in soothing during examinations. Lorthe et al. reported an association between PPROM and a high incidence of infants’ mortality and morbidity [33–35]. Adverse outcomes in preterm infants exposed to PPROM may stem from an increased risk of infections or other complications, such as hypoglycemia-induced perinatal brain injury in infants affected by PPROM [36, 37]. Furthermore, previous study highlights the significant role of overproduced ROS, intracellular calcium ion (Ca2+), and mitochondrial DNA during the inflammation process of PPROM, leading to mitochondrial dysfunction, in the complications associated with PPROM and the elevated mortality rates in preterm infants [38–40]. While our observations did not reveal a direct association between antibiotic use in the NICU and neurobehavioral outcomes, the provision of a prophylactic antibiotic regimen in the NICU was found to be positively associated with health outcomes in preterm infants [41].
Our study did not find significant associations of acute pain with any NNNS summary scores. This may be because the weighted acute pain scores, including the level 2 and level 3 pain events, which are the routine NICU care such as mouth care, position change, and diaper change, could be provided more frequently to infants when they get more mature, which may impact neurodevelopmental outcomes differently according to different GA [3]. In addition, our study did not observe associations between birth GA, SNAPPEII, and sex with any NNNS subscales. This lack of association may be attributed to the limited sample size. Our future study should contemplate the incorporation of a larger infant sample and the implementation of longitudinal studies for a more comprehensive investigation.
We also observed the associations between 52 buccal protein API values and four NNNS summary scores. The leading GO molecular functions of 52 buccal proteins most associated with optimal neurobehavioral outcomes (group 2) were majorly related to cellular signal cascade [42], cytoskeleton [43], and skin development [44]. Conversely, GO protein functions, including leptin receptor binding [45], glutathione disulfide oxidoreductase activity [46], phosphate and proton transmembrane transporter activity [47], response to oxidative stress [46], transmembrane transporter activity [48], regulation of lipid metabolism [49], and vascular development [50], were associated with nonoptimal neurobehavioral outcomes (groups 1 and 4). While numerous genes and proteins mentioned above along with their respective functions have been associated with neurodevelopment to some extent, there exists a research gap in identifying a specific group of proteins and their biological functions that collaborate to contribute to early life neurodevelopment. Our study uncovered this group of proteins, and they could potentially be incorporated into the existing set of promising noninvasive biomarkers for predicting infants’ mitochondrial function and neurobehavioral outcomes in a clinical setting. However, further studies are warranted to confirm our current findings.
Our novel discovery of buccal proteomic fingerprint of the impact of early life pain/stress on infants’ neurobehavioral outcomes not only provides a better understanding of molecular mechanisms of the association between early life experience and NNNS summary scores but also points out the possible target proteins and functions that can be used as predictors for infants’ neurodevelopmental screening (see Table 3). Preterm infants experience increased stress exposure related to ROS overload [51], inadequate antioxidant storage [52], inadequate ATP production [53], and fast postnatal catch-up growth [54], which may induce mitochondrial dysfunction through regulating mitochondrial function-related proteins [51]. The normal mitochondria generate sufficient ATP [55] and moderate level ROS [56] as a by-product, which upregulates proteins positively related to neurodevelopment. Furthermore, the excessive ROS leak from dysfunctional mitochondria [57] and upregulated proteins is negatively related to neurodevelopmental outcomes [58]. Our study observed that the diverse array of proteins, including SNX2, GLRX, THBS1, NAXE, AGT, SOD1, Shroom3, SPRR2A, S100A3, PAIP1, UNC45A, ABLIM1, MUC1, keratins, MT-CO2, phosphate carrier, GLRX, CYB5B, PHB2, and BNIPL-2, are capable of playing a role in the stress-mitochondrial function/dysfunction-neurobehavioral axis.
Limitation
Our study attempted to address the association between mitochondrial function-relevant proteins and neurodevelopmental outcomes by performing untargeted proteomic analysis in buccal swabs of preterm infants. However, our study had a limited sample size recruited from two affiliated NICU sites. This constraint not only hinders the attainment of a comprehensive representation of the overall preterm infant population but also restricts the feasibility of conducting analyses with a greater number of subgroups such as different levels of pain/stress groups, potentially yielding less precise results. Additionally, missing NNNS data for some participants from the study could potentially impact the robustness of our findings, and the caution is advised in interpreting the results. Furthermore, our study solely focused on the differential expression of a limited epithelial proteome, which is not representative of gene expression and regulation across tissue types and all organs. This study does not inform any aspects of possible gene mutations. Lastly, a lack of extensive posttranslational modification (PTM) analysis limits our understanding of how differential PTM in the midst of pain/stress can affect protein expression changes and the overall biochemical infant response. Therefore, future genetic, deep proteome, and PTM studies would greatly expand our preliminary findings and compose a more accurate and holistic picture of the infant’s response to pain and stress in early development. In addition, validation our current conclusions should be pursued through large-scale analysis examining the association between early life pain/stress, proteins, and neurodevelopmental outcomes in preterm infants. Finally, longitudinal follow-ups on neurodevelopmental outcomes are warranted to confirm the enduring effects of clinical experience.
Conclusion and Clinical Implication
A history of PPROM was associated with unfavorable neurodevelopment in preterm infants. Consequently, it is advisable to administer an early prophylactic antibiotic regimen to preterm infants to mitigate the adverse health outcomes linked to PPROM exposure in early life. Additionally, buccal proteins related to mitochondrial functions were identified as being associated with the neurodevelopmental outcomes in preterm infants. These buccal proteins could serve as potential clinical screening biomarkers for predicting the neurodevelopmental outcomes in newborns.
Statement of Ethics
The study protocol was reviewed and approved by the Institutional Review Board of Connecticut Children’s Medical Center (CCMC) and the University of Connecticut (UConn), and the protocol number (16-001). The written informed consent was obtained from parents of participants.
Conflict of Interest Statement
There are no conflicts of interest of funding for each author. There are no declared conflicts of other interests for any of this study’s authors.
Funding Sources
This study was supported by a grant from NIH/NINR (Grant No. F31NR019940, PI: Tingting Zhao), NIH/NCATS (Grant No. TL1 TR001864, PI: Tingting Zhao), and ENRS Council for the Advancement of Nursing Science Dissertation Award (PI: Tingting Zhao). The study was also supported by a grant from NIH/NINR (Grant No. 1R01NR016928-01A1, PI: Xiaomei Cong).
Author Contributions
All authors met the authorship requirements. Substantial contributions to conception and experimental design, manuscript review, and summarize the key findings: all authors. Funding acquisition: Tingting Zhao and Xiaomei Cong. Drafting and revising the manuscript: Tingting Zhao, Xiaolin Chang, Subrata Biswas, Jeremy Balsbaugh, Nathan Alder, and Xiaomei Cong. Thoroughly reviewed and critiqued the manuscript for revision: Jennifer Liddle, Ming-hui Chen, and Adam Matson. Final approval of the version to be published: Tingting Zhao, Xiaolin Chang, Subrata Biswas, Jeremy Balsbaugh, Jennifer Liddle, Ming-hui Chen, Adam Matson, Nathan Alder, and Xiaomei Cong.
Funding Statement
This study was supported by a grant from NIH/NINR (Grant No. F31NR019940, PI: Tingting Zhao), NIH/NCATS (Grant No. TL1 TR001864, PI: Tingting Zhao), and ENRS Council for the Advancement of Nursing Science Dissertation Award (PI: Tingting Zhao). The study was also supported by a grant from NIH/NINR (Grant No. 1R01NR016928-01A1, PI: Xiaomei Cong).
Data Availability Statement
Data are available upon request from the first and corresponding authors. All data generated or analyzed for this study are included in this article and its online supplementary material.
Supplementary Material.
Supplementary Material.
Supplementary Material.
References
- 1. Cong X, Wu J, Vittner D, Xu W, Hussain N, Galvin S, et al. The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Hum Dev. 2017;108:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker SM, Melbourne A, O'Reilly H, Beckmann J, Eaton-Rosen Z, Ourselin S, et al. Somatosensory function and pain in extremely preterm young adults from the UK EPICure cohort: sex-dependent differences and impact of neonatal surgery. Br J Anaesth. 2018;121(3):623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao T, Griffith T, Zhang Y, Li H, Hussain N, Lester B, et al. Early-life factors associated with neurobehavioral outcomes in preterm infants during NICU hospitalization. Pediatr Res. 2022;92(6):1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duerden EG, Grunau RE, Guo T, Foong J, Pearson A, Au-Young S, et al. Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. J Neurosci. 2018;38(4):878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McPherson C, Miller SP, El-Dib M, Massaro AN, Inder TE. The influence of pain, agitation, and their management on the immature brain. Pediatr Res. 2020;88(2):168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang KL, Li SJ, Pu XY, Wu FF, Liu H, Wang RQ, et al. Targeted up-regulation of Drp1 in dorsal horn attenuates neuropathic pain hypersensitivity by increasing mitochondrial fission [published correction appears in Redox Biol. 2022;50:102230. Redox Biol. 2022;49:102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiménez-Delgado A, Ortiz GG, Delgado-Lara DL, González-Usigli HA, González-Ortiz LJ, Cid-Hernández M, et al. Effect of melatonin administration on mitochondrial activity and oxidative stress markers in patients with Parkinson’s disease. Oxid Med Cell Longev. 2021;2021:5577541 Published 2021 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mancuso M, Filosto M, Bosetti F, Ceravolo R, Rocchi A, Tognoni G, et al. Decreased platelet cytochrome c oxidase activity is accompanied by increased blood lactate concentration during exercise in patients with Alzheimer disease. Exp Neurol. 2003;182(2):421–6. [DOI] [PubMed] [Google Scholar]
- 9. van Diemen MPJ, Hart EP, Abbruscato A, Mead L, van Beelen I, Bergheanu SC, et al. Safety, pharmacokinetics and pharmacodynamics of SBT-020 in patients with early stage Huntington’s disease, a 2-part study. Br J Clin Pharmacol. 2021;87(5):2290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark E, Johnson J, Dong YN, Mercado-Ayon E, Warren N, Zhai M, et al. Role of frataxin protein deficiency and metabolic dysfunction in Friedreich ataxia, an autosomal recessive mitochondrial disease. Neuronal Signal. 2018;2(4):NS20180060 Published 2018 Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cantarero L, Juárez-Escoto E, Civera-Tregón A, Rodríguez-Sanz M, Roldán M, Benítez R, et al. Mitochondria-lysosome membrane contacts are defective in GDAP1-related Charcot-Marie-Tooth disease. Hum Mol Genet. 2021;29(22):3589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fame RM, Lehtinen MK. Mitochondria in early forebrain development: from neurulation to mid-corticogenesis. Front Cell Dev Biol. 2021;9:780207 Published 2021 Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Angelova PR, Abramov AY. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 2018;592(5):692–702. [DOI] [PubMed] [Google Scholar]
- 14. Perrone S, Tataranno LM, Stazzoni G, Ramenghi L, Buonocore G. Brain susceptibility to oxidative stress in the perinatal period. J Matern Fetal Neonatal Med. 2015;28(Suppl 1):2291–5. [DOI] [PubMed] [Google Scholar]
- 15. Lee JW, Davis JM. Future applications of antioxidants in premature infants. Curr Opin Pediatr. 2011;23(2):161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lambertini L, Chen J, Nomura Y. Mitochondrial gene expression profiles are associated with maternal psychosocial stress in pregnancy and infant temperament. PloS One. 2015;10(9):e0138929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Veluchamy A, Hébert HL, van Zuydam NR, Pearson ER, Campbell A, Hayward C, et al. Association of genetic variant at chromosome 12q23.1 with neuropathic pain susceptibility. JAMA Netw Open. 2021;4(12):e2136560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steen K, Chen D, Wang F, Majumdar R, Chen S, Kumar S, et al. A role for keratins in supporting mitochondrial organization and function in skin keratinocytes. Mol Biol Cell. 2020;31(11):1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geyer PE, Holdt LM, Teupser D, Mann M. Revisiting biomarker discovery by plasma proteomics. Mol Syst Biol. 2017;13(9):942 Published 2017 Sep. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanke K, Hartz A, Manz M, Bendiks M, Heitmann F, Orlikowsky T, et al. Preterm prelabor rupture of membranes and outcome of very-low-birth-weight infants in the German Neonatal Network. PLoS One. 2015;10(4):e0122564 Published 2015 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. [DOI] [PubMed] [Google Scholar]
- 22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stokowski LA. Quantifying neonatal stress in the NICU. Adv Neonatal Care. 2009;9(5):205. [DOI] [PubMed] [Google Scholar]
- 24. Lester BM, Tronick EZ, Brazelton TB. The neonatal intensive care unit Network neurobehavioral scale procedures. Pediatrics. 2004;113(Suppl ment_2):641–67. [PubMed] [Google Scholar]
- 25. Kelly CE, Thompson DK, Cheong JL, Chen J, Olsen JE, Eeles AL, et al. Brain structure and neurological and behavioural functioning in infants born preterm. Dev Med Child Neurol. 2019;61(7):820–31. [DOI] [PubMed] [Google Scholar]
- 26. Montirosso R, Del Prete A, Bellù R, Tronick E, Borgatti R; Neonatal Adequate Care for Quality of Life NEO-ACQUA Study Group . Level of NICU quality of developmental care and neurobehavioral performance in very preterm infants. Pediatrics. 2012;129(5):e1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Casavant SG, Chen J, Xu W, Lainwala S, Matson A, Chen MH, et al. Multi-omics analysis on neurodevelopment in preterm neonates: a protocol paper. Nurs Res. 2021;70(6):462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tibshirani R. Regression shrinkage and selection via the lasso. J Roy Stat Soc B. 1996;58(1):267–88. [Google Scholar]
- 29. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–23. [Google Scholar]
- 30. Choi HJ, Chen TX, Hou MJ, Song JH, Li P, Liu CF, et al. Protection against glutathione depletion-associated oxidative neuronal death by neurotransmitters norepinephrine and dopamine: protein disulfide isomerase as a mechanistic target for neuroprotection. Acta Pharmacol Sin. 2022;43(10):2527–41 Epub 2022 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med. 2013;34(2–3):465–84. [DOI] [PubMed] [Google Scholar]
- 32. Spinillo A, Capuzzo E, Stronati M, Ometto A, Orcesi S, Fazzi E. Effect of preterm premature rupture of membranes on neurodevelopmental outcome: follow up at two years of age. Br J Obstet Gynaecol. 1995;102(11):882–7. [DOI] [PubMed] [Google Scholar]
- 33. Lorthe E, Torchin H, Delorme P, Ancel PY, Marchand-Martin L, Foix-L'Hélias L, et al. Preterm premature rupture of membranes at 22-25 weeks’ gestation: perinatal and 2-year outcomes within a national population-based study (EPIPAGE-2). Am J Obstet Gynecol. 2018;219(3):298.e1–4. [DOI] [PubMed] [Google Scholar]
- 34. Drassinower D, Friedman AM, Običan SG, Levin H, Gyamfi-Bannerman C. Prolonged latency of preterm prelabour rupture of membranes and neurodevelopmental outcomes: a secondary analysis. BJOG. 2016;123(10):1629–35. [DOI] [PubMed] [Google Scholar]
- 35. Drassinower D, Friedman AM, Običan SG, Levin H, Gyamfi-Bannerman C. Prolonged latency of preterm premature rupture of membranes and risk of cerebral palsy. J Matern Fetal Neonatal Med. 2016;29(17):2748–52. [DOI] [PubMed] [Google Scholar]
- 36. van der Heyden JL, Willekes C, van Baar AL, van Wassenaer-Leemhuis AG, Pajkrt E, Oudijk MA, et al. Behavioural and neurodevelopmental outcome of 2-year-old children after preterm premature rupture of membranes: follow-up of a randomised clinical trial comparing induction of labour and expectant management. Eur J Obstet Gynecol Reprod Biol. 2015;194:17–23. [DOI] [PubMed] [Google Scholar]
- 37. Armstrong-Wells J, Donnelly M, Post MD, Manco-Johnson MJ, Winn VD, Sébire G. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am J Obstet Gynecol. 2015;212(2):212.e1–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang X, He X, Wei L, He Y, Li Y, Wang Y, et al. Nuclear erythroid 2-related factor 2 protects against reactive oxygen species -induced preterm premature rupture of membranes through regulation of mitochondria. Biol Reprod. 2023;109(3):330–9. [DOI] [PubMed] [Google Scholar]
- 39. Vishnyakova PA, Tarasova NV, Volodina MA, Tsvirkun DV, Sukhanova IA, Kurchakova TA, et al. Gestation age-associated dynamics of mitochondrial calcium uniporter subunits expression in feto-maternal complex at term and preterm delivery. Sci Rep. 2019;9(1):5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kacerovsky M, Vlkova B, Musilova I, Andrys C, Pliskova L, Zemlickova H, et al. Amniotic fluid cell-free DNA in preterm prelabor rupture of membranes. Prenat Diagn. 2018;38(13):1086–95. [DOI] [PubMed] [Google Scholar]
- 41. Wolf MF, Sgayer I, Miron D, Krencel A, Sheffer VF, Idriss SS, et al. A novel extended prophylactic antibiotic regimen in preterm pre-labor rupture of membranes: a randomized trial. Int J Infect Dis. 2020;96:254–9. [DOI] [PubMed] [Google Scholar]
- 42. Wang L, Zhou K, Fu Z, Yu D, Huang H, Zang X, et al. Brain development and akt signaling: the crossroads of signaling pathway and neurodevelopmental diseases. J Mol Neurosci. 2017;61(3):379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kojic M, Wainwright B. The many faces of elongator in neurodevelopment and disease. Front Mol Neurosci. 2016;9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jameson C, Boulton KA, Silove N, Nanan R, Guastella AJ. Ectodermal origins of the skin-brain axis: a novel model for the developing brain, inflammation, and neurodevelopmental conditions. Mol Psychiatry. 2023;28(1):108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Glendining KA, Higgins MBA, Fisher LC, Jasoni CL. Maternal obesity modulates sexually dimorphic epigenetic regulation and expression of leptin receptor in offspring hippocampus. Brain Behav Immun. 2020;88:151–60. [DOI] [PubMed] [Google Scholar]
- 46. Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4(6):1247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med. 2013;34(2–3):465–84. [DOI] [PubMed] [Google Scholar]
- 48. Moral-Sanz J, Lewis SA, MacMillan S, Ross FA, Thomson A, Viollet B, et al. The LKB1-AMPK-α1 signaling pathway triggers hypoxic pulmonary vasoconstriction downstream of mitochondria. Sci Signal. 2018;11(550):eaau0296. [DOI] [PubMed] [Google Scholar]
- 49. Bruce KD, Zsombok A, Eckel RH. Lipid processing in the brain: a key regulator of systemic metabolism. Front Endocrinol. 2017;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vogenstahl J, Parrilla M, Acker-Palmer A, Segarra M. Vascular regulation of developmental neurogenesis. Front Cell Dev Biol. 2022;10:890852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao T, Alder NN, Starkweather AR, Chen MH, Matson AP, Xu W, et al. Associations of mitochondrial function, stress, and neurodevelopmental outcomes in early life: a systematic review. Dev Neurosci. 2022;44(6):438–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moore TA, Ahmad IM, Zimmerman MC. Oxidative stress and preterm birth: an integrative review. Biol Res Nurs. 2018;20(5):497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu YY, Liu Y, Cui L, Wu WB, Quinn MJ, Menon R, et al. Hypoxic effects on the mitochondrial content and functions of the placenta in fetal growth restriction. Placenta. 2021;114:100–7. [DOI] [PubMed] [Google Scholar]
- 54. Singhal A. Long-term adverse effects of early growth acceleration or catch-up growth. Ann Nutr Metab. 2017;70(3):236–40. [DOI] [PubMed] [Google Scholar]
- 55. Roger AJ, Muñoz-Gómez SA, Kamikawa R. The origin and diversification of mitochondria. Curr Biol. 2017;27(21):R1177–92. [DOI] [PubMed] [Google Scholar]
- 56. Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360(1):201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ionescu-Tucker A, Cotman CW. Emerging roles of oxidative stress in brain aging and Alzheimer's disease. Neurobiol Aging. 2021;107:86–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the first and corresponding authors. All data generated or analyzed for this study are included in this article and its online supplementary material.