Abstract
Chemokine receptors pivotal for human immunodeficiency virus type 1 (HIV-1) infection in lymphocytes and macrophages (CCR3, CCR5, and CXCR4) are expressed on neural cells (microglia, astrocytes, and/or neurons). It is these cells which are damaged during progressive HIV-1 infection of the central nervous system. We theorize that viral coreceptors could effect neural cell damage during HIV-1-associated dementia (HAD) without simultaneously affecting viral replication. To these ends, we studied the ability of diverse viral strains to affect intracellular signaling and apoptosis of neurons, astrocytes, and monocyte-derived macrophages. Inhibition of cyclic AMP, activation of inositol 1,4,5-trisphosphate, and apoptosis were induced by diverse HIV-1 strains, principally in neurons. Virions from T-cell-tropic (T-tropic) strains (MN, IIIB, and Lai) produced the most significant alterations in signaling of neurons and astrocytes. The HIV-1 envelope glycoprotein, gp120, induced markedly less neural damage than purified virions. Macrophage-tropic (M-tropic) strains (ADA, JR-FL, Bal, MS-CSF, and DJV) produced the least neural damage, while 89.6, a dual-tropic HIV-1 strain, elicited intermediate neural cell damage. All T-tropic strain-mediated neuronal impairments were blocked by the CXCR4 antibody, 12G5. In contrast, the M-tropic strains were only partially blocked by 12G5. CXCR4-mediated neuronal apoptosis was confirmed in pure populations of rat cerebellar granule neurons and was blocked by HA1004, an inhibitor of calcium/calmodulin-dependent protein kinase II, protein kinase A, and protein kinase C. Taken together, these results suggest that progeny HIV-1 virions can influence neuronal signal transduction and apoptosis. This process occurs, in part, through CXCR4 and is independent of CD4 binding. T-tropic viruses that traffic in and out of the brain during progressive HIV-1 disease may play an important role in HAD neuropathogenesis.
Human immunodeficiency virus type 1 (HIV-1) dementia (HAD) is a common complication of the late stage(s) of viral infection, affecting nearly 20% and 50% of infected adults and children, respectively. The pathological consequences of HAD are highly variable but often include brain atrophy, reactive astrocytosis, formation of microglial nodules and multinucleated giant cells, perivascular inflammation, neuronal loss, and alterations in blood-brain barrier (BBB) permeability producing myelin pallor (19, 25). Apoptosis of neurons, astrocytes, and endothelial cells has been demonstrated (48, 54). Interestingly, the best correlate for disease is the number of immune system-activated mononuclear phagocytes (MPs; brain macrophages and microglia), not the levels of virus in brain tissue. Indeed, MP secretory products, produced as a consequence of cell activation, predict the progression of cognitive, motor, and/or behavioral dysfunctions in HAD (19, 20). The MP neurotoxic factors include both viral (HIV-1 gp120 [8], gp41 [1], and Tat [49]) and cellular products such as arachidonic acid and its metabolites, platelet-activating factor, proinflammatory cytokines (for example, tumor necrosis factor alpha [TNF-α] and interleukin-1β [IL-1β]), quinolinic acid, NTox, oxygen free radicals, nitric oxide (NO), excitatory amino acids, and others (reviewed in references 19 and 20). Clearly, how HIV-1 infects MPs and affects immune system activation remains a most critical unanswered question in viral neuropathogenesis.
It is now well accepted that HIV-1 productively infects the brain MPs (most notably the perivascular macrophages) while maintaining only a restricted infection in select numbers of astrocytes and endothelial cells (20, 26, 45). MP infection occurs through CD4 and, in part, through CCR5 (19, 23, 29, 42, 63). HIV-1 entry into astrocytes and endothelial cells is CD4 independent (27, 48). Overall, viral infection in the brain is continued through macrophage recruitment, perhaps mediated through the production of chemokines. Chemokines are produced in large quantities in both astrocytes and microglia and affect both the transendothelial migration of macrophages into the brain and viral infection. For example, macrophage-inhibitory protein 1α (MIP-1α), MIP-1β, RANTES, and macrophage chemotactic protein 1 are produced by HIV-1-infected and immune-activated MPs and astrocytes in laboratory assays and are present in affected brain tissue (38, 41, 55).
Macrophage-tropic (M-tropic) HIV-1 strains use chemokine receptors CCR5 and CCR3 for infection (2, 13, 15, 23, 29), whereas T-cell-tropic (T-tropic) strains use CXCR4 (17). Importantly, several of these chemokine receptors are expressed in neural cells. CXCR4, CCR5, and CCR3 are on macrophages and microglia (23, 29, 43, 67), while astrocytes and neurons express CCR3 and/or CXCR4 (19, 43, 53, 67, 71). Although HIV-1 cannot readily infect cells that lack CD4, the engagement of chemokines or virus with a chemokine receptor could elicit intracellular signaling events that lead to cell damage. For example, our previous work and that of others has shown that CXCR4 can effect neuronal apoptosis by binding to its ligand, stromal-cell-derived factor 1α (SDF-1α) (31, 32, 71). SDF-1α is secreted by astrocytes (71) and can induce intracellular signaling and affect cell function in human neurons (31, 32, 71, 72).
One idea for how HIV-1 damages the brain during HAD is that progeny virions, released from infected MPs, produce neural damage by binding to CXCR4. Differences in the abilities of viral strains to bind CXCR4 may lead to differential outcomes with regard to neuronal signaling and apoptosis. This hypothesis is supported by reports showing that the viral envelope can bind chemokine receptors independent of CD4 binding and induce intracellular signaling events (14, 34, 71, 72). Although the HIV-1 strains that infect MPs are M-tropic (CCR5 dependent) (16, 40, 60, 64, 66), these strains have not been shown to cause brain injury (60, 64). M-tropic viruses that use CCR5 are present throughout the disease course, while T-tropic viruses that use CXCR4 emerge later in the course of infection, during the time period in which HAD is most common (7, 13, 64). Perhaps T-tropic viruses that penetrate the brain transiently during the later stages of disease are the most pathogenic in eliciting brain cell injury and/or apoptosis.
To these ends, we studied the role of progeny virions from M-tropic, T-tropic, and neurotropic viral strains for eliciting neural injury. These viruses, recovered from lymphocytes or monocytes, were previously shown to effect neural apoptosis. Alpha- and beta-chemokines and progeny virions were compared for their ability to effect intracellular signaling. SDF-1α induced neuronal but not astrocyte or monocyte-derived macrophage (MDM) apoptosis. Virions recovered from T-tropic strains (MN, IIIB, and Lai) produced the most significant alterations in neuronal and astrocyte signaling and apoptosis. M-tropic strains (ADA, JR-FL, Bal, MS-CSF, and DJV) produced the least neural cell damage, while 89.6, a dual-tropic HIV-1 strain, elicited intermediate damage. The intracellular signaling events that lead to neuronal apoptosis were found to be multifaceted. These results, in toto, suggest that the high levels of T-tropic viruses found during advancing disease may cross the BBB from the periphery into the brain and affect neuronal function and viability. This may occur independently of concomitant increases in viral replication within the brain. It may also help explain the importance of both cellular (as demonstrated elsewhere [72]) and viral factors secreted from macrophages in causing neural cell destruction. Most importantly, these findings may help explain how some patients, with high peripheral blood but low brain viral loads, can develop HAD. Here, T-tropic viruses may penetrate through a disrupted BBB and affect neural function directly, without ongoing viral replication in brain macrophages and microglia.
MATERIALS AND METHODS
Isolation and culture of primary monocytes.
Human monocytes were recovered from peripheral blood mononuclear cells of HIV- and hepatitis B virus-seronegative donors after leukopheresis and then purified by countercurrent centrifugal elutriation (21). Monocytes were >98% pure by HAM56 and CD68 staining. Monocytes were cultured as adherent monolayers (3.3 × 106 cells/well in a 48-mm-diameter plastic culture plate) in Dulbecco’s modified Eagle medium (DMEM; Sigma Chemical Co., St. Louis, Mo.) with 10% heat-inactivated pooled human serum, 50 μg of gentamicin and/or 10 μg of ciprofloxacin (Sigma)/ml, and 1,000-U/ml highly purified recombinant human macrophage colony-stimulating factor (a generous gift from Genetics Institute, Inc., Cambridge, Mass.). Identification of chemokine receptors (CCR5, CCR3, and CXCR4) was performed by double immunocytochemical staining with monoclonal antibodies (MAbs) to CCR5 (3A9; supplied by LeukoSite Inc., Cambridge, Mass.), CCR3 (7B11; supplied by LeukoSite Inc.), and CXCR4 (12G5; a generous gift from James Hoxie), as well as an antibody to a macrophage antigen (HAM56). Expression of CCR5, CCR3, and CXCR4 was detected on the cell membranes and in the cytoplasm of HAM56-positive MDM. All tissue reagents were screened and found to be negative for endotoxin (<10 pg/ml; Associates of Cape Cod, Inc., Woods Hole, Mass.) and mycoplasma (Gen-Probe II; Gen-Probe Inc., San Diego, Calif.) contamination.
HIV-1 infection of monocytes and purification of virions.
Monocytes and lymphocytes were prepared from peripheral blood mononuclear cells of normal donors (HIV-1- and -2-seronegative subjects) by leukopheresis and centrifugal elutriation. Seven days after being plated, MDM were infected with the M-tropic viral strains (ADA, MS-CSF, Bal, JR-FL, SF-162, and DJV) or the dual-tropic viral strain 89.6. Phytohemagglutinin- and IL-2-stimulated peripheral blood lymphocytes (lymphoblasts) were infected with T-tropic strains (Lai, MN, and IIIB). HIV-1 replication was measured by determination of reverse transcriptase (RT) activity in culture supernatants (37). Under these conditions, peak viral replication occurred at 7 days following HIV-1 inoculation (data not shown). Seven days after HIV-1 infection, select culture supernatants from virus-infected MDM and lymphocytes were collected over a period of 1 to 3 weeks. Supernatant samples were pooled, clarified, and then concentrated (10-fold) by ultracentrifugation for 2 h at 50,000 × g and 4°C. Concentrated viral stocks were further washed, clarified, and concentrated (40-fold) by centrifugation for 2 h at 14,000 × g and 4°C. The pelleted virions were resuspended in neurobasal medium. RT activity was measured in triplicate samples of concentrated virus for sample recovery determination. Virions with similar RT values were prepared and used for determination of neuronal signal transduction and apoptosis. All virions were obtained from the National Institutes of Health (NIH) AIDS Research Reagent Program except ADA and MS-CSF (22). MS-CSF was isolated from a HAD patient in our center whose dementia was reversed following antiretroviral therapy (22).
Isolation of human neurons.
Human fetal brain tissue was obtained from the products of elective abortions (13 to 16 weeks’ gestation) performed in full compliance with University of Nebraska Medical Center and NIH ethical guidelines. Human neuronal cultures were prepared as previously described (57) with minor modifications. Briefly, the brain tissue was mechanically dissociated and incubated with 0.25% trypsin for 30 min, neutralized with 10% fetal bovine serum (FBS), further dissociated by trituration, washed, and cultured on poly-d-lysine-coated plates in neurobasal medium containing 0.5 mM glutamine, 25 μM glutamate, 50 μg of penicillin/ml, and 50 μg of streptomycin/ml and supplemented with B27 (Life Technologies) and 5% horse serum. Cells were plated onto poly-d-lysine-coated six-well plates (Becton Dickinson, Franklin Lakes, N.J.) at a density of 106/well for extraction of RNA and analysis of phosphatidylinositol (PI) hydrolysis, in 24-well plates at a density of 5 × 105/well for analysis of cyclic AMP (cAMP) levels and apoptosis, in eight-well chamber slides at a density of 8 × 104/well for neuronal staining, in 96-well plates at a density of 2 × 104/well for enzyme-linked immunosorbent assay (ELISA; neuronal cell quantitation), and in 12-well plates (5 × 105/well) with glass inserts for intracellular calcium determinations. Five days following cell culture, 5-fluorodeoxyuridine was added to the neural cultures at a concentration of 10 μg/ml to inhibit proliferation of dividing (contaminating) astrocytes and/or fibroblasts. The purity of the cells was assessed by using antibodies produced against neuron-specific microtubule-associated protein 2 (MAP-2) (Boehringer Mannheim Corp., Indianapolis, Ind.) and glial fibrillary acidic protein (GFAP) (Dako Corp., Carpinteria, Calif.) for identification of neurons and astrocytes, respectively. Antibody staining for CD68 showed that microglia comprised <2% of the neural preparations. Antibodies to neurofilament (NF) (polyclonal; Chemicon International Inc., Temecula, Calif.) were used to confirm the neuronal purity. At 2 weeks following cell cultivation, >70% of cells were MAP-2 immunopositive.
Isolation of human fetal astrocytes.
Human fetal brain tissue (14 to 20 weeks’ gestation) was procured by following the ethical guidelines of the University of Nebraska Medical Center and NIH (see above). Tissue was washed with Hanks’ balanced salt solution lacking Ca2+ and Mg2+ and was dissociated mechanically. The dissociated tissue was resuspended in DMEM-F12 supplemented with 10% FBS, 250 μg of Fungizone/ml, and 50 μg each of penicillin, streptomycin, and neomycin/ml. The tissue was subsequently passed through a Nitex bag (pore size, 250 μm). The resulting single-cell suspension was centrifuged at 1,500 rpm for 10 min (Mistral 3,000 I centrifuge; Sanyo, Itasca, Ill.) and resuspended in fresh medium. The cells were then centrifuged at 750 rpm. The pelleted cells were counted and seeded at a density of 2 × 107/150-cm2 flask. The cells were cultured for 7 days, and the floating debris was removed. The adherent monolayers of astrocytes were washed once with phosphate-buffered saline (PBS) and then treated with trypsin-EDTA for 3 min. The detached astrocytes were resuspended in medium and centrifuged at 1,500 rpm for 10 min. The cells were cultured as adherent monolayers in 150-cm2 flasks for an additional 7 days and then trypsinized. The procedure was repeated twice to yield highly pure astrocytes. Adherent monolayers were treated with trypsin, and cells were cultured in Costar plates at the following densities: 106/well in 6-well plates for extraction of RNA and analysis of PI hydrolysis, 2.5 × 105/well in 24-well plates for analysis of cAMP levels and apoptosis, and 5 × 105/well in 12-well plates with glass inserts for analysis of intracellular calcium.
Rat cerebellar granule neuronal cultures.
Seven-day-old Sprague Dawley rats were sacrificed and cerebellar brain tissue was harvested according to the guidelines established by the Animal Welfare Act (1987) and NIH policies. Briefly, cerebellum tissue was collected and washed in cold PBS containing trypsin at 0.25 mg/ml and 0.1% DNase (about a 10-ml volume per cerebellum), then minced into 2-mm pieces and triturated with a fire-polished pipette; this was followed by incubation for 20 min at 37°C (28). The tissue was filtered through a nylon mesh, and the resulting cell suspension was loaded over a two-step Percoll gradient and centrifuged at 500 × g and 4°C for 15 min to remove the glia. The neurons were collected, washed twice in sterile medium without serum, and then resuspended in fresh DMEM-F12 medium (Sigma) with 10% horse serum. Cells were gently triturated and plated at a density of 2 × 105/12-mm-diameter glass coverslip precoated with poly-l-lysine (70,000 to 150,000 molecular weight; Sigma) in 24-well culture dishes or at a density of 3 × 106 in poly-l-lysine-coated 100-mm-diameter culture dishes. After 1 to 2 days in culture, 5-fluorodeoxyuridine and uridine were added to the cultures at 20 and 50 μg/ml, respectively, to eliminate proliferative cells (astrocytes), and the purity of the resulting neuronal population was verified by immunocytochemical staining for neuronal markers. Neurons were cultured up to 7 days at 37°C in a humidified atmosphere containing 5% CO2; the medium (serum-free DMEM-F12) was replaced every 3 days.
Immunocytochemical detection of neural cells.
Neural cells were plated on glass coverslips in 24-well culture plates (or 8-well chamber slides) to assess cell purity. After removal of the culture medium, the cells were fixed with methanol-acetone (1:1) for 10 min at −20°C. The cells were incubated with antibodies against the neuron- and astrocyte-specific antigens MAP-2 and GFAP, respectively, for 1 h. The cells were then washed with PBS and incubated for 1 h with a fluorescein isothiocyanate-labeled anti-mouse immunoglobulin G (IgG) F(ab′)2 fragment (Boehringer Mannheim Corp.) for MAP-2 detection or a rhodamine-conjugated anti-rabbit IgG F(ab′)2 fragment (Boehringer Mannheim Corp.) for GFAP detection. Histocytochemical preparations were examined with a Nikon Microphot-FXA microscope. Neuronal purity was confirmed with antibodies to NF or neuron-specific enolase. Identification of chemokine receptors (CCR5, CCR3, and CXCR4) was performed by double immunocytochemical staining with antibodies for CCR5 (3A9), CCR3 (7B11), CXCR4 (12G5), and NF and/or GFAP. Representative cell samples were stained with antibody to CD68 (Dako Corp.) and/or 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate (DiI)-labeled acetylated low-density-lipoprotein antibodies (Biomedical Technologies Inc., Stoughton, Mass.) to detect microglia. Astrocyte cultures were nearly homogeneous (>95% GFAP immunoreactive). CCR3 and CXCR4 were readily detected on a subset of astrocytes (∼20%). Human fetal brain explant cultures were immunocytochemically identified as neurons (>70%), astrocytes (<30%), and microglia (<2%). Immunocytochemical staining of the human fetal brain cells for oligodendrocytes (antigalactocerebroside antibodies; Boehringer Mannheim Corp.) and for microvascular endothelial cells (factor VIII antibodies; Dako Corp.) was negative. CXCR4 was detected on neuronal cell membranes. The proportion of neurons expressing CXCR4 was 20 to 50% (data not shown).
cAMP assay.
The assay for cAMP accumulation was performed as described previously, with minor modifications (36). Neuronal cells plated in 24-well plates were washed twice with prewarmed serum-free DMEM containing 20 mM HEPES, pH 7.4, and then loaded with 5 μCi of [3H]adenine (NEN Life Science Products, Boston, Mass.) in 0.5 ml/well at 37°C for 90 to 120 min. Intracellular [3H]cAMP was extracted overnight with 1 ml of ice-cold 5% trichloroacetic acid containing 1 mM unlabeled cAMP (as an internal control). [3H]cAMP was separated from the tritiated nucleotides by sequential ion-exchange chromatography over Dowex and alumina columns (Sigma). The ATP and cAMP fractions (3 ml each) were collected in scintillation vials to which 14 ml of Econo-Safe (Research Products International Corp., Mount Prospect, Ill.) was added per vial. The radioactivity of each sample was determined by liquid scintillation spectroscopy. Values are expressed as the percentage of conversion of [3H]ATP to [3H]cAMP. Because forskolin (FSK) directly activates adenyl cyclase, it was used as a positive control in these assays. No detectable changes in cAMP production were observed for any of the chemokines, virions, or HIV-1 gp120 tested in the absence of FSK. Therefore, all experiments were performed in the presence of FSK. Pertussis toxin (PTX) was used to deactivate Gi/Go proteins. This was performed by utilizing PTX (100 ng/ml) for 12 h prior to the performance of the assays in which deactivation of Gi/Go was required. The CXCR4 MAb, 12G5, was kindly provided by James A. Hoxie (University of Pennsylvania) or purchased from PharMingen (San Diego, Calif.). The recombinant soluble CD4 was obtained from the NIH AIDS Research Reagent Program.
PI hydrolysis assay.
Neuronal cells grown on six-well plates were labeled for 18 to 24 h with 2 μCi of [3H]inositol (Amersham, Arlington Heights, Ill.) in 1 ml of inositol-free high-glucose DMEM supplemented with 5% FBS. After being labeled, cells were rinsed once with DMEM plus 20 mM HEPES, pH 7.4 (DMEM-HEPES), and then stimulated for 20 min with various concentrations of agents in DMEM-HEPES containing 10 mM LiCl. Labeled compounds were then extracted from the cells with methanol, and chloroform and water were added as described elsewhere (73). Inositol phosphates in the resulting aqueous phase were separated on Dowex 1-X8 (formate; Sigma) columns. Total inositol phosphates were eluted with 8 ml of 1 M ammonium formate–0.1 M formic acid. The radioactivity in a 3-ml portion of the eluate (fraction a) and a 0.375-ml portion of the organic phase containing the inositol phospholipids (fraction b) was determined by liquid scintillation counting. The percentage of conversion of inositol phospholipids to inositol phosphates was then calculated with the formula a/(a + b) × 100. Replicate cells were pretreated with PTX to deactivate Gi/Go proteins (as described above for cAMP) as a specificity control for this assay.
Calcium measurements.
Cells cultured on glass coverslips were loaded with 10 μM Fura II-AM (Molecular Probes, Inc., Eugene, Oreg.) for 30 min at 37°C in Ringer’s solution of the following composition: 148 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1.6 mM Na2HPO4, 1.5 mM CaCl2, and 5 mM d-glucose. After being loaded, cells were washed twice and then incubated again for 20 min in Ringer’s solution to allow for intracellular dye cleavage. The coverslips were placed in the chamber, and Fura II was excited at wavelengths of 350 and 380 nm, using a PTI Deltascan system as previously described (73). The values for Ca2+ were calculated as follows: [Ca2+] = Kd[(R − Rmin)/(Rmax − R)] × (380min/380max), where Rmin and Rmax are the fluorescence ratios in the absence (with 3 mM EGTA) and presence of saturating Ca2+ (3 mM), respectively, and Kd = 224 nM.
In situ detection of apoptotic neurons by TUNEL staining.
Human and/or rat neuronal cell cultures were exposed to different virions or chemokines in serum-free neurobasal medium supplemented with B27 (Life Technologies). After 4 days, apoptotic cells were stained by an in situ terminal deoxynucleotidyltransferase-mediated digoxigenin-dUTP nick end labeling (TUNEL) assay method (Oncor, Gaithersburg, Md., or Trevigen, Gaithersburg, Md.) as described elsewhere (54). Briefly, neurons stained by the TUNEL assay were fixed in 4% paraformaldehyde, rinsed with PBS, postfixed with a 100% ethanol–acetic acid solution (2:1), and rinsed again with PBS. Neurons were pretreated with 2% H2O2 to quench endogenous peroxidase prior to the addition of terminal deoxynucleotidyltransferase. Anti-digoxigenin-peroxidase was then added, and it reacted catalytically with 0.05% diaminobenzidine–PBS. TUNEL-stained neurons in 15 randomly selected fields were then counted. Each field of at least 100 neurons was examined for the relative numbers of positively stained and negatively stained cells. For double staining of neuronal markers, replicate cultures were immunostained with antibodies against MAP-2 (Boehringer Mannheim Corp.) before performance of TUNEL staining.
Image analysis.
TUNEL-positive neurons and total unstained neurons were counted by acquiring TIFF images from immunostained culture fields, using an Olympus IX-70 microscope. TIFF images were acquired randomly from 20×-magnified fields. Using a macro program to identify labeled and unlabeled neurons, computerized morphometry (Image ProPlus; Media Cybernetics) was performed to obtain the number of TUNEL-positive as well as the total neurons per 20×-magnified field. A minimum of 10 fields were counted for each treatment condition.
ELISAs for cellular apoptosis.
Human or rat neural cell or MDM cultures were exposed to different virions or chemokines in serum-free neurobasal medium supplemented with B27 (Life Technologies). After 4 days, mono- and oligonucleosomes in the cytoplasm of apoptotic cells were detected by ELISA performed in accordance with the instructions of the manufacturer, Boehringer Mannheim Corp. Briefly, neurons were treated for 4 days with lysis buffer (Boehringer Mannheim Corp.), and the lysates were spun down at 1,000 × g for 5 min. The released mono- and oligonucleosomes of apoptotic cells in the supernatant were carefully removed. The supernatant was added to a 96-well ELISA plate fixed with antihistone antibody on the wall of the microtiter plate module. After the wells were washed three times, anti-DNA-peroxidase, which reacts with the DNA part of the nucleoside from apoptotic cells, was added. After removal of unbound peroxidase, the amount of peroxidase retained in the immunocomplex was determined photometrically with a peroxidase substrate, ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)]. For each condition, triplicate samples were used, and data are presented in terms of the percent increase or decrease in cell number compared with the number obtained from replicate cultures in regular culture medium. Each treatment was repeated at least three times with cells from three individual donors. The MAb to gp120, gp41 (gp160), was obtained from the NIH AIDS Research Reagent Program.
Investigations of the signal transduction pathways for neuronal apoptosis.
The investigated drugs inhibitory for the signal transduction pathways included those that inhibited and/or stimulated cAMP, inositol 1,4,5-trisphosphate (IP3), protein kinase A (PKA), protein kinase C (PKC), Ca2+ release, and/or mitogen-activated protein (MAP) kinase. For studies of cAMP signaling, the RP isomer of 8-bromo-cAMP (RP-8-Br-cAMP; a PKA inhibitor) and 8-bromo-cAMP (a PKA activator) were employed. For studies of IP3, xectospongin C (X-C; a reversible membrane-permeating inhibitor of IP3-mediated Ca2+ release with a 50% inhibitory concentration [IC50] of 358 nM) was used. To assay the role of PKC, bisindolylmaleimide I (a selective PKC inhibitor; Ki = 10 nM) and RO-31-8425 (a calcium-independent PKC inhibitor) were employed. Bisindolylmaleimide acts as a competitive inhibitor for the PKC ATP-binding site and shows a high selectivity for the PKC α, βI, βII, γ, δ, and ɛ isozymes. For MAP kinase, PD169316 (a potent, cell-permeating, and selective p38 MAP kinase inhibitor; IC50 = 89 nM) and PD98059 (a selective and cell-permeating inhibitor of MAP kinase) were employed. In addition, SKF-86002 (a bicyclic imidazole cytokine-suppressive anti-inflammatory drug that inhibits osmotic stress and UV-induced apoptosis through the blockade of p38 MAP kinase activation as well as lipopolysaccharide-stimulated IL-1 and TNF-α production; IC50 = 1 μM) was used. Finally, HA1004, an inhibitor of calcium/calmodulin-dependent protein kinase II (CaMK-II; Ki = 13 μM), PKC (Ki = 40 μM), PKA (Ki = 2.3 μM), protein kinase G (Ki = 1.3 μM), and myosin light chain kinase (Ki = 150 μM), was utilized to assess multiple signaling pathways simultaneously. HA1004 is also an intracellular Ca2+ antagonist. All signal transduction inhibitors were purchased from Calbiochem (La Jolla, Calif.).
Statistical tests.
Data were analyzed as means ± standard deviations of the means (SD). The data were evaluated statistically by the analysis of variance followed by either Fisher’s least significant difference test for multiple comparisons or Student’s t test for paired observations.
RESULTS
Chemokine receptors expressed on MDM, astrocytes, and neurons.
In previous studies, we tested the expression of chemokine receptors on MDM, astrocytes, and neurons (23, 72). Morphological and immunocytochemical characterization of MDM, astrocytes, and neurons showed that each of these cell types expressed chemokine receptors. CXCR4 antigen expression was common to all cell types. Both CCR3 and CCR5 were expressed on MDM, and CCR3 was expressed on astrocytes (data not shown) (reported in references 23 and 72).
Neural cell signaling.
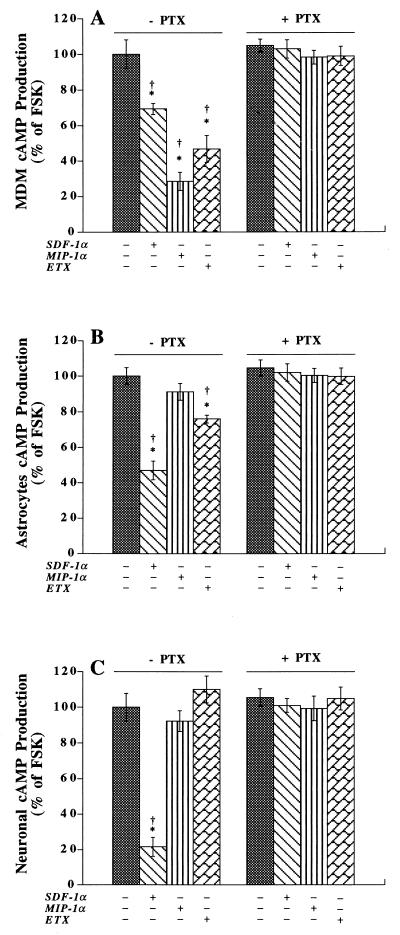
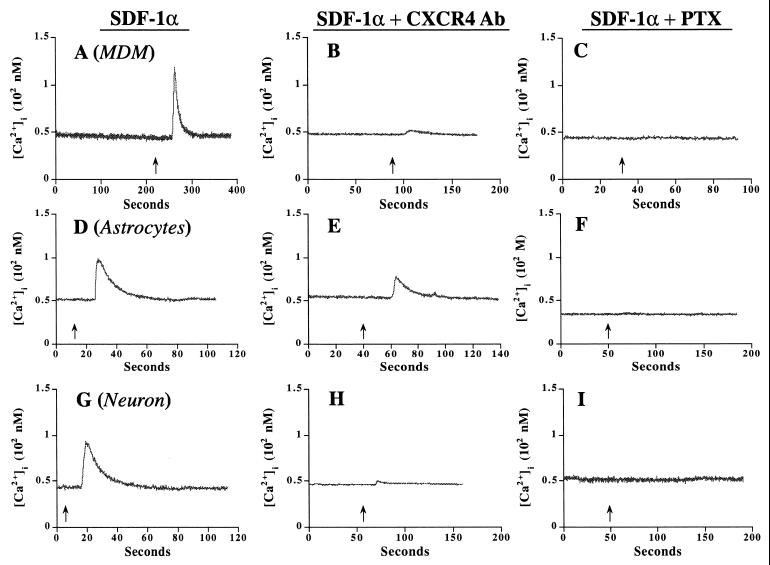
MDM, astrocytes, and neurons may be infected and/or otherwise damaged by HIV-1. Cell damage could occur through infection or by binding of virus to specific neural receptors, thereby eliciting alterations to cell signaling and apoptosis. To investigate the latter, we assayed diverse strains of HIV-1 for their ability to affect neural cell signaling through chemokine receptor binding. The first step in proving that the individual chemokine receptors expressed on MDM, astrocytes, and neurons were functional involved the assay of their respective ligands’ abilities to induce alterations in signal transduction. Each of the three cell types (MDM, astrocytes, and neurons) was tested in this manner. Since CCR5, CCR3, and CXCR4 belong to the G-protein-coupled receptor family, inhibition of FSK-stimulated cAMP production and of IP3 or intracellular calcium production was determined following exposure of cells to chemokines. MIP-1α (0.5 μg/ml; for CCR5), eotaxin (ETX; 0.5 μg/ml; for CCR3), and SDF-1α (0.5 μg/ml; for CXCR4) used individually had no effect on cAMP production in MDM, astrocytes, or neurons (data not shown). However, all three chemokines, when used at identical concentrations with FSK (30 μM), inhibited cAMP production in MDM. The response was PTX sensitive (100 ng/ml; 12-h pretreatment) (Fig. 1A). In astrocytes, ETX and SDF-1α inhibited FSK-stimulated cAMP production (Fig. 1B). No effects were observed for MIP-1α. The astrocyte response to ETX and SDF-1α was also PTX sensitive (Fig. 1B). In neurons, only SDF-1α elicited an FSK-stimulated cAMP response (Fig. 1C). The reduction in cAMP levels observed in neurons exposed to SDF-1α was >50% and was abolished by PTX (Fig. 1C). To substantiate and extend these observations, we performed calcium imaging of each of the three cell types following chemokine exposure. Importantly, SDF-1α increased the levels of intracellular calcium in all cell types (Fig. 2A, D, and G). The response was blocked by pretreatment with the CXCR4 antibody, 12G5 (10 μg/ml) (Fig. 2B, E, and H), or PTX (100 ng/ml; 12-h pretreatment) (Fig. 2C, F, and I). These results suggested that CCR5, CCR3, and CXCR4 are functional in MDM and that CCR3 and CXCR4 are active in astrocytes. In contrast, neurons primarily express CXCR4.
FIG. 1.
Intracellular signal transduction pathways of CCR5, CCR3, and CXCR4 in MDM, astrocytes, and neurons. MDM (A), astrocytes (B), and neurons (C) were treated with or without PTX (100 ng/ml for 12 h) and then stimulated with FSK (30 μM) with or without chemokines (0.5 μg/ml) for 10 min. Intracellular cAMP was measured as outlined in Materials and Methods. FSK induced a 10- to 20-fold augmentation of cAMP production. The experiments are representative of three separate assays, each performed in triplicate. Data are expressed in terms of the percent change in the intracellular cAMP level in comparison to the level attained with FSK alone and are expressed as means ± SD. ∗, P < 0.01 for cAMP levels versus those attained with FSK alone; †, P < 0.01 for the difference between chemokine-treated cells with and without PTX pretreatment.
FIG. 2.
Intracellular calcium levels are affected by SDF-1α in MDM, astrocytes, and neurons. SDF-1α (200 nM) was applied on MDM (A), astrocytes (D), and neurons (G), and intracellular calcium was measured as outlined in the text. In replicate cultures, MDM (B), astrocytes (E), and neurons (H) were pretreated with CXCR4 antibody 12G5 (10 μg/ml) for 1 h and then stimulated with SDF-1α (200 nM) in the presence of 12G5 (10 μg/ml). (C, F, and I) Parallel samples were pretreated with PTX (100 ng/ml, 12 h) and then stimulated with SDF-1α (200 nM). The experiments are representative of three replicate assays performed independently three times.
Chemokines and apoptosis in MDM, astrocytes, and neurons.
Our previous studies showed that SDF-1α mediated neuronal apoptosis through CXCR4 (72). What remained uncertain was whether SDF-1α and other chemokines could also induce apoptosis in MDM and astrocytes. Thus, MIP-1α, macrophage chemotactic protein 1 (for CCR2b), RANTES (for CCR3 and CCR5), ETX, and SDF-1α were tested for their ability to induce apoptosis in each of the three cell types. Each of the chemokines (at 0.5 μg/ml) was individually added to cultures of MDM, astrocytes, and neurons in B27-supplemented neurobasal medium for 4 days. Cellular apoptosis was tested by the apoptosis ELISA (see Materials and Methods). None of the chemokines tested induced apoptosis in astrocytes or MDM (data not shown). However, SDF-1α induced neuronal apoptosis (data not shown), which was blocked by 12G5, a CXCR4 antibody. The specificity of the SDF-1α response of neurons was confirmed by utilizing rat cerebellar granule neurons (a 95% pure neuronal cell system) (Table 1).
TABLE 1.
Apoptosis induced by SDF-1α in rodent cerebellar granule neuron cultures
| Condition | % of TUNEL-positive neurons per 20× fielda |
|---|---|
| Untreated | 7.4 ± 2.1 |
| 12G5 (10 μg/ml) | 8.1 ± 2.2 |
| SDF-1α (0.5 μg/ml) | 17.8 ± 2.4∗ |
| SDF-1α + 12G5 | 9.1 ± 1.5† |
Data are percentages of total neurons that have undergone apoptosis as stated in Materials and Methods. Data are expressed as means ± SD. ∗, P < 0.005 compared to control; †, P < 0.005 compared to cells treated with SDF-1α alone.
HIV-1 virions induce MDM and astrocyte apoptosis.
We next tested whether similar results could be obtained for progeny virus and whether there was strain variation evident in the experimental outcome. Importantly, we investigated the effects that each of the viral strains had on apoptosis of MDM, astrocytes, and neurons. To address these issues, we tested purified virions recovered from T-tropic, M-tropic, and neurotropic strains for their ability to affect signal transduction and apoptosis in primary human MDM, astrocytes, and neurons. Progeny virions from the M-tropic and/or neurotropic (ADA, Bal, JR-FL, SF-162, DJV, and MS-CSF) and the dual-tropic (89.6) viral strains were recovered from infected MDM. The T-tropic strains IIIB, MN, and Lai were obtained from phytohemagglutinin-stimulated peripheral blood lymphocytes (lymphoblasts). HIV-1 replication in culture supernatant fluids was determined by measuring the RT activity. Culture fluids were collected 1 to 3 weeks following HIV-1 inoculation of MDM or lymphoblasts. Supernatant samples were pooled, clarified, and then concentrated (10-fold) by ultracentrifugation for 2 h at 5 × 104 × g and 4°C. Concentrated viral stocks were further washed, clarified, and concentrated (40-fold) by centrifugation for 2 h at 1.4 × 104 × g and 4°C. RT activity was determined in triplicate samples of concentrated virus for sample recovery determination (37). Numbers of progeny virions were normalized based on RT levels to ensure standardization among samples.
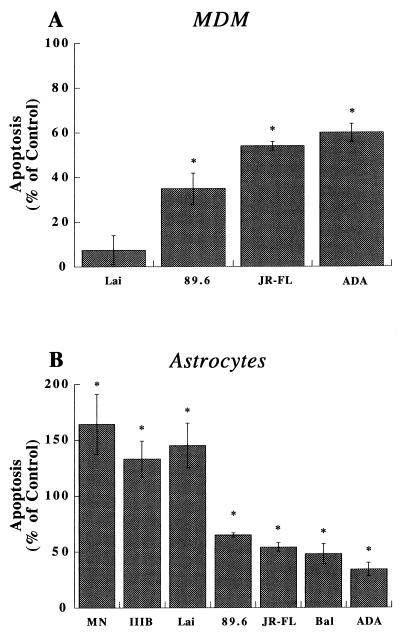
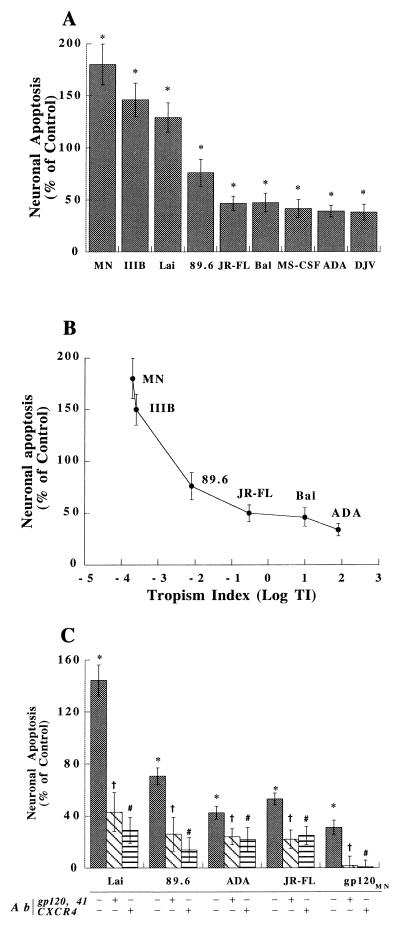
T-tropic, M-tropic, and dual-tropic progeny HIV-1 virions were used to inoculate MDM and astrocyte cultures. Cellular apoptosis was tested after 4 days with the apoptosis ELISA system. Apoptosis was observed in MDM after their exposure to M-tropic, dual-tropic, or neurotropic virions. ADA, JR-FL, and 89.6 (Fig. 3A), but not Lai, induced MDM apoptosis. In contrast, astrocytes showed high levels of apoptosis when exposed to T-tropic virions (Fig. 3B). Indeed, MN, IIIB, and Lai induced the highest levels of apoptosis in astrocytes. Such responses were lower with JR-FL, ADA, Bal, and 89.6 (Fig. 3B).
FIG. 3.
Virion-induced apoptosis in MDM and astrocytes. A panel of progeny virions from T-tropic (MN, Lai, and IIB), dual-tropic (89.6), and M-tropic (ADA, JR-FL, and Bal) strains were placed onto MDM (A) and astrocytes (B) for 4 days. The amount of virus was normalized by performing RT assays. Apoptosis was measured by ELISA, utilizing antihistone and anti-DNA antibodies (see Materials and Methods). The data are expressed in terms of the percent change in apoptosis levels in comparison to those of control cells treated with culture medium alone; the control value is 0%. The experiments represent the average of three replicate assays, performed three times, using MDM and astrocytes from three different donors. Data are expressed as means ± SD. ∗, P < 0.01 for the difference between virion-treated and control cells.
Virion-mediated chemokine receptor signaling in MDM and astrocytes.
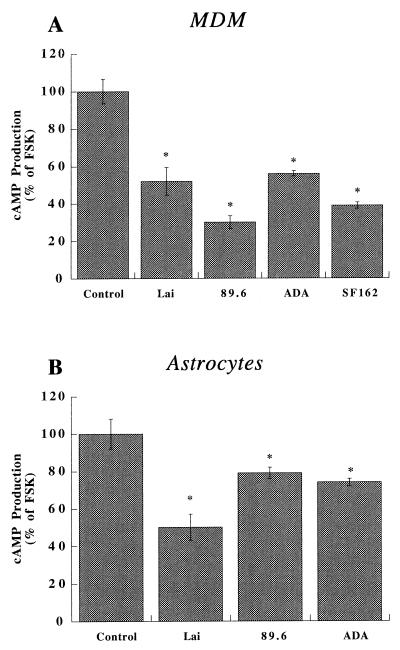
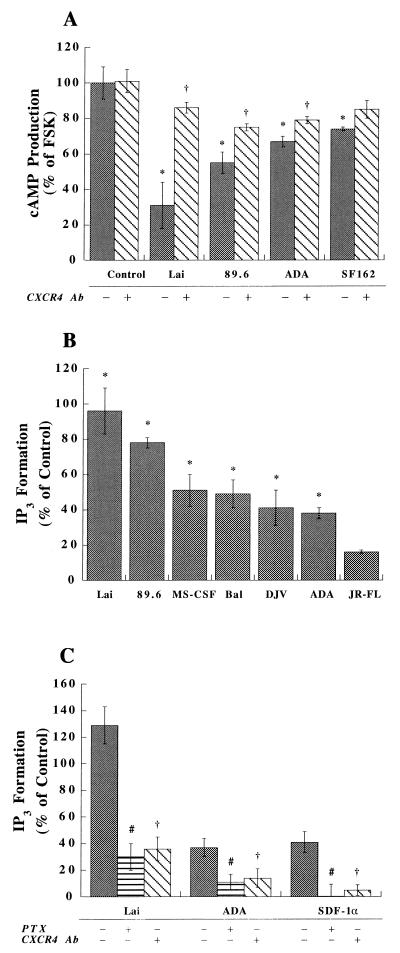
Our data showed that progeny HIV-1 virions induce MDM and astrocyte apoptosis. However, the mechanisms underlying these effects remained unclear. To address this issue, we studied intracellular signaling in MDM and astrocytes. The effects of the different viral strains on signal transduction were investigated through measurements of cAMP. The viral strains tested (as shown above) included Lai, 89.6, ADA, and SF162. In MDM, the inhibition of FSK-stimulated cAMP was observed with the T-tropic (Lai), dual-tropic (89.6), and M-tropic (ADA and SF162) strains tested (Fig. 4A). A small, but clearly significant, response for inhibition of FSK-stimulated cAMP by T-tropic strains was also observed in astrocytes (Fig. 4B). However, a reduced effect on cAMP accumulation, compared to that seen with T-tropic strains, was seen following exposure of astrocytes to M-tropic strains (Fig. 4B).
FIG. 4.
Virion-induced alterations in chemokine receptor-mediated signal transduction in MDM and astrocytes. Human MDM (A) and astrocytes (B) were stimulated with FSK (30 μM) with or without the panel of progeny virions (outlined in Fig. 5 and normalized via equivalent RT values). After a 10-min incubation, intracellular cAMP production was measured as described in the text. FSK alone induced a 10- to 20-fold increase in cAMP production in these cells compared to the controls. Data are expressed in terms of the percent change in comparison to cells stimulated with FSK alone. These experiments represent the average of three replicate assays, performed three times, using MDM and astrocytes from three different donors. Data are expressed as means ± SD. ∗, P < 0.01 for the difference between virion-treated and control cells (FSK alone).
HIV-1 virions induce neuronal apoptosis.
Our initial studies demonstrated that SDF-1α could induce alterations in neuronal signaling and apoptosis and that the effect is both cell and chemokine specific (see above). The ability of each of the progeny virions to induce neuronal apoptosis was determined. With regard to viral strain differences, neuronal apoptotic responses were highest with the T-tropic (IIIB, MN, and Lai) and the dual-tropic (89.6) strains (Fig. 5A). Lesser degrees of apoptosis were observed following neuronal culture inoculation with ADA, JR-FL, MS-CSF, BAL, or DJV (Fig. 5A). The macrophage tropism (12) correlated inversely with the ability to induce neuronal apoptosis (R = 0.934) (Fig. 5B). The specificity of chemokine receptor utilization in these responses was next investigated. The CXCR4 antibody, 12G5 (10 μg/ml, 1 h prior to virion treatment), significantly blocked neuronal (Fig. 5C) and astrocyte (data not shown) apoptosis by the T-tropic HIV-1 strains. 12G5 only partially blocked the effects of M-tropic isolates (Fig. 5C). Mouse IgG (10 μg/ml) showed no inhibition of apoptosis (data not shown). In contrast, MDM apoptosis induced by ADA and JR-FL was not significantly changed with 12G5 (data not shown). Importantly, neuronal apoptosis was induced at low levels by 12G5. In this regard, the baseline value reported in Fig. 5 was normalized by subtracting the apoptotic effects caused by 12G5 alone (data not shown). Induction of neuronal apoptosis by T-tropic strains was also blocked by antibodies to gp120 and gp41 (2 μg/ml each; 1-h pretreatment prior to virion treatment) (Fig. 5C). To substantiate these results, the effect of purified gp120 on neuronal apoptosis was next tested. Not surprisingly, gp120MN (20 nM) also induced neuronal apoptosis, which can be blocked by 12G5 and antibody to gp120 and gp41 (Fig. 5C). Interestingly, the neuronal response induced by intact virions was much higher (129% increase) than that induced by purified gp120 (35% increase). Different forms of gp120, such as gp120SF-2, gp120MN, gp120CM, and gp120IIIB, were tested at various concentrations (from 0.05 to 50 nM), and similar results were obtained (data not shown). These results, taken together, demonstrate the importance of strain variation in neural cell apoptosis. Such observations underscore the importance of T-tropic strains in HIV-1 neurovirulence.
FIG. 5.
Virion-induced apoptosis in neurons. (A) A panel of progeny virions of T-tropic (MN, Lai, or IIB), dual-tropic (89.6), or M-tropic (JR-FL, Bal, MS-CSF, ADA, or DJV) strains was placed on neuron-enriched cultures for 4 days. Apoptosis was measured by ELISA utilizing antihistone and anti-DNA antibodies (see Materials and Methods). The amount of virus was normalized by performing RT assays, and the data were expressed in terms of the percent change in comparison to control cells treated with culture medium alone; the control value is 0%. (B) A correlation exists between neuronal apoptosis and the macrophage tropism indexes (TI) (12) of the panel of HIV-1 strains used. (C) Apoptosis induced by virions in the presence of the CXCR4 antibody 12G5 (1-h pretreatment at 10 μg/ml) or the anti-gp120 antibody 41 (1-h pretreatment at 2 μg/ml) was also analyzed. In this assay, gp120MN (20 nM) was used as a positive control. These experiments represent the average of three replicate assays, performed three times, using neurons from three different donors. Data are expressed as means ± SD. ∗, P < 0.01 for differences between virions-treated and control cells; †, P < 0.01 for differences between cells treated with virions in the presence and in the absence of antibody (Ab) 41; #, P < 0.01 for differences between virions treated in the presence and in the absence of 12G5.
Virion-chemokine receptor signaling in neurons.
The mechanism for CXCR4-mediated neuronal apoptosis remains in question. Such work could provide evidence of the intracellular events that may lead to the induction of neuronal apoptosis by progeny virions. This might also provide evidence that HIV-1 could influence neuronal function independent of binding to CD4 and infection of neurons. The effects of the different viral strains on signal transduction were investigated through measurements of cAMP and IP3 production. The inhibition of FSK-stimulated cAMP production was observed for many individual viral strains in neurons. The relative levels of inhibition, as a function of the viral strain, were as follows: Lai > 89.6 > ADA ≥ SF162 (Fig. 6A). Importantly, the effects of Lai and 89.6 on neurons were blocked by 12G5 (10 μg/ml) while the effects of ADA were only partially blocked (Fig. 6A). In all three cell types (MDM, astrocytes, and neurons), a small, but not significant, inhibition was observed with gp120CM, gp120SF-2, and gp120MN (administered at concentrations of from 0.2 to 20 nM) (data not shown). In subsequent experiments, we investigated the effects on IP3 signal transduction mediated by the panel of progeny virions. Lai, 89.6, ADA, MS-CSF, DJV, Bal, and JR-FL were tested. Interestingly, all of the viral strains tested activated IP3 in neurons. Lai and 89.6 induced higher levels of IP3 in neurons. Lower levels were observed with the M-tropic strains of virus (Fig. 6B). The effect induced by Lai was blocked by 12G5 and PTX pretreatment, while the effect induced by ADA was only partially blocked (Fig. 6C). SDF-1α, in conjunction with the CXCR4 antibody, served as the positive control for these assays (Fig. 6C).
FIG. 6.
Virion-induced alterations in chemokine receptor-mediated signal transduction in neurons. Neurons were pretreated with or without 12G5 (10 μg/ml) for 1 h and stimulated with FSK (30 μM) with or without the panel of progeny virions (outlined in Fig. 7 and normalized via equivalent RT values). After a 10-min incubation, intracellular cAMP was measured as described in the text. FSK alone induced a 10- to 20-fold increase in cAMP production in these cells compared to the control. (A) Data are expressed in terms of the percent change in comparison to cells stimulated with FSK alone. (B and C) Data are expressed in terms of the percent change in comparison to control cells treated with culture medium alone; the control value is 0%. For the IP3 assay, neurons were pretreated with or without 12G5 (10 μg/ml, 1 h), or with or without PTX (100 ng/ml, 12 h), and stimulated with different virions. (C) SDF-1α (100 nM) in conjunction with the CXCR4 antibody (Ab) served as the positive control for the assays shown. Intracellular IP3 production was measured as stated in Materials and Methods. (B and C) Data are expressed in terms of the percent change in comparison to control cells. The experiments are representative of three replicate assays. Data are expressed as means ± SD. ∗, P < 0.01 for differences between virion-treated and control cells (treated with culture medium); †, P < 0.01 for differences between cells treated with virions in the presence and in the absence of 12G5; #, P < 0.01 for differences between virion-treated cells with and without PTX pretreatment.
Linkages between virion-induced signal transduction and neuronal apoptosis.
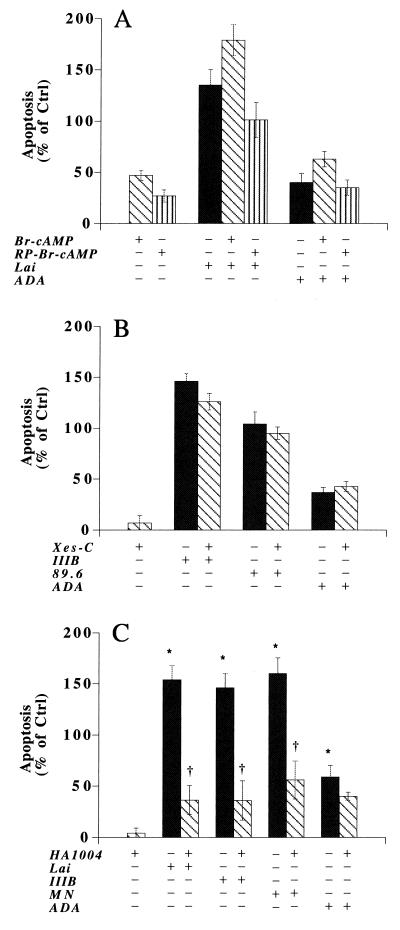
Our previous work demonstrated that progeny HIV-1 virions could inhibit FSK-stimulated cAMP production and increase the level of IP3, which in turn could induce functional changes in PKA, PKC, and/or CaMK, leading to alterations in calcium homeostasis and neuronal apoptosis. Certainly, any or all of these kinases may affect neuronal function. Therefore, to explore the relationships between virus-induced neuronal signal transduction and apoptosis, we employed a panel of kinase activators and inhibitors. First, a pair of cAMP analogs, RP-8-Br-cAMP and 8-bromo-cAMP, was tested. Both drugs are cell permeating and have greater resistance to phosphodiesterases than cAMP. RP-8-Br-cAMP is a potent inhibitor of PKA, and 8-bromo-cAMP activates PKA. After treatment of human neurons with RP-8-Br-cAMP (0.1 μM to 0.1 mM), a 15 to 30% increase in apoptosis was demonstrated (Fig. 7A). Surprisingly, 8-bromo-cAMP also increased neuronal apoptosis (40 to 55%) when administered at the same dose as RP-8-Br-cAMP (Fig. 7A). An additional cAMP analog, dibutyryl cAMP (a PKA activator), also induced similar levels of neuronal apoptosis (data not shown). Moreover, both PKA activators failed to block Lai- or ADA-induced neuronal death. However, 1 μM RP-8-Br-cAMP modestly inhibited virion- and SDF-1α-induced apoptosis. These data, taken together, suggested that the activation, more than the inhibition, of PKA induced neuronal apoptosis. However, both mechanisms were operative.
FIG. 7.
Effects of signal transduction inhibitors and activators on virion-induced neuronal apoptosis. Neuronal apoptosis was measured as stated in Materials and Methods. Cells were treated with different drugs alone or with virions in the presence or absence of 1 μM RP-8-Br-cAMP, 8-Br-cAMP, or X-C or 3 μM HA1004. Data are expressed in terms of the percent change in comparison to control (Ctrl) cells treated with culture medium alone; the control value is 0%. The experiments are representative of three replicate assays. Data are expressed as means ± SD. ∗, P < 0.01 for differences between virion-treated and control cells; †, P < 0.01 for differences between cellular responses with and without drug.
Second, we employed an inhibitor of IP3-mediated Ca2+ release, X-C, to test its role in the apoptotic process. X-C is a potent, reversible, membrane-permeating inhibitor of IP3-mediated Ca2+ release. Nonetheless, X-C does not directly interact with the IP3-binding site. X-C at 0.1 to 10 μM did not induce neuronal apoptosis. At 1 μM, X-C minimally inhibited HIV-1IIIB-induced neuronal apoptosis (Fig. 7B). This suggested that inhibiting IP3-induced calcium release was not sufficient to block virion-induced neuronal apoptosis.
Third, we tested the PKC inhibitor bisindolylmaleimide I. Bisindolylmaleimide I is a highly selective cell-permeating PKC inhibitor. It competitively inhibits the ATP-binding site of PKC and shows a high level of selectivity for the PKC isozymes α, βI, βII, γ, δ, and ɛ. Bisindolylmaleimide I at 1 μM induced a 40 to 70% increase in neuronal apoptosis. The drug failed to inhibit virion- or SDF-1α-induced neuronal apoptosis. RO-31-8425, a calcium-independent PKC inhibitor, also did not inhibit the virus-induced neuronal apoptosis (data not shown).
Fourth, we tested inhibitors of MAP kinase. This was based on our previous work suggesting that virions could act through P42/P44 MAP kinase phosphorylation (74). Since three major types of MAP kinases have been reported in mammalian cells (ERK1/ERK2 [p42/p44], c-Jun kinase/stress-activated protein kinase, and p38-reactivating kinase), three inhibitors of MAP kinase were tested. These included PD169316 (a potent cell-permeating and selective p38 MAP kinase inhibitor), PD98059 (which acts by inhibiting the activation of MAP kinase and subsequent phosphorylation of the MAP kinase substrate), and SKF-86002 (a bicyclic imidazole cytokine-suppressive anti-inflammatory drug that inhibits osmotic stress- and UV-induced apoptosis by blocking p38 MAP kinase activation). All of these drugs, when administered to neurons at a concentration of 1 μM, induced a 30 to 50% increase in neuronal apoptosis (data not shown). None of these drugs inhibited virion-induced neuronal apoptosis at a 1 μM concentration (data not shown). These data suggested that the intracellular signaling pathways involved in neuronal apoptosis are complex and likely involve multiple mechanisms.
To investigate the possibility that HIV-1 virions affect multiple neuronal signal transduction pathways, we utilized a drug that inhibits multiple signal transduction pathways. For this reason, HA1004 was selected as our next candidate for testing. HA1004 is a cell-permeating inhibitor of CaMK-II (Ki = 13 μM), PKC (Ki = 40 μM), PKA (Ki = 2.3 μM), protein kinase G (Ki = 1.3 μM), and myosin light chain kinase (Ki = 150 μM). It is also an intracellular Ca2+ antagonist. HA1004, when administered at a concentration of 0.1 to 10 μM, did not induce neuronal apoptosis. Importantly, it blocked neuronal apoptosis induced by all lymphotropic viruses tested, Lai, MN, and IIIB, at a 3 μM concentration (Fig. 7C). It also partially blocked ADA-induced apoptosis (Fig. 7C).
Role of CD4 in virion-induced neuronal apoptosis.
Previous work demonstrated that CD4 might potentiate gp120 binding to chemokine receptors such as CCR5 (42, 47, 69, 70). Therefore, we explored whether virion-induced neuronal apoptosis could be altered by the binding of virion-associated gp120 to CD4. Since neurons do not express CD4, soluble CD4 was utilized for these experiments. Neuronal apoptosis induced by purified gp120 and individual virions of strains such as Lai, 89.6, ADA, JR-FL, and MS-CSF (with or without soluble CD4) was tested. CD4 alone (5 μg/ml) did not affect neuronal apoptosis. The neuronal apoptosis induced by strains Lai, 89.6, ADA, JR-FL, and MS-CSF was not potentiated by CD4, while some inhibitory effect was observed with CD4 (5 μg/ml) (data not shown). A dose escalation of CD4 (from 1 to 10 μg/ml) was utilized but did not change the experimental outcome (data not shown). To confirm these results, we performed signal transduction assays using the panel of virions (with or without soluble CD4). The assay for cAMP was performed in neurons. As predicted, Lai, 89.6, and ADA inhibited FSK-stimulated cAMP production. However, this inhibition was not significantly altered in the presence of CD4 (data not shown). Similar results were obtained for IP3 induction by Lai, 89.6, and ADA (data not shown). These results suggested that virions signal through CXCR4 by binding to CXCR4, independent of binding of CD4.
DISCUSSION
In this study, we investigated the relationships between virus, chemokines and their receptors, and neural apoptosis. Our data demonstrate that apoptosis is induced by progeny HIV-1 virions interacting with chemokine receptors. Importantly, this can occur not only in neurons but also in astrocytes and macrophages. The importance of these results rests in the finding that HAD-related apoptosis involves all three of the cell types investigated. This also implies that neuronal apoptosis in HAD may occur both as a consequence of accessory cell loss in the brain and by damage mediated more directly through macrophage and glial secretory factors. Importantly, the work underscores the roles of both T-tropic and M-tropic strains in HIV-1 neuropathogenesis. Clearly, virus in the peripheral blood may traverse the BBB and affect neuronal function by binding to CXCR4 and eliciting subsequent neuronal damage. M-tropic viruses may be important in inducing the large numbers of cellular neurotoxic factors, produced by virus-infected and immune-competent macrophages, that lead to significant neuronal damage (72). This hypothesis for neuronal loss (which bridges the importance of both viral and cellular factors in HAD pathogenesis) is supported by the results presented in this paper and those to be described elsewhere (72).
Interestingly, CXCR4 can mediate virion-induced apoptosis independently of CD4 binding. The other chemokine receptors used as receptors for M-tropic HIV-1 strains (including CCR5 and CCR3) may not be involved in inducing astrocyte or MDM apoptosis. Indeed, virions recovered from T-tropic viral strains (MN, IIIB, and Lai), which produced the most significant effects in neuronal and astrocyte signaling and apoptosis, occurred through CXCR4. Moreover, the M-tropic strains (ADA, JR-FL, Bal, MS-CSF, DJV, and SF-162) produced the least neural cell damage while 89.6, a dual-tropic HIV-1 strain, elicited an intermediate level of neural damage. Antibodies to CXCR4 blocked the effects mediated by T-tropic strains in neurons and astrocytes. Virion-induced alterations in cell signaling events through CXCR4 included inhibition of cAMP, activation of PI hydrolysis, and apoptosis. Neuronal apoptosis induced by progeny HIV-1 virions was also blocked by antibodies to gp120 and gp41. Virion-induced neuronal apoptosis can be blocked by a less selective CaMK-II, PKA, and PKC inhibitor, HA1004. Finally, neuronal apoptosis was confirmed in rat cerebellar granule neurons, a cell system of nearly 95% purity. These data, taken together, demonstrate the importance of CXCR4 in mediating neural cell apoptosis by virions and highlight the likely role of T-tropic virus strains in disease pathogenesis.
Neuronal, astrocyte, and MP apoptosis is a major feature of HAD (3, 5, 19, 29, 49, 59, 62). The major cause of this cellular loss now appears, at least in part, to be mediated by virion binding to chemokine receptors present on the cell membrane surface. Alternatively, and perhaps more importantly, cell destruction may be mediated through cellular inflammatory products produced as a consequence of viral infection and immune system activation of MPs (19, 20, 45, 72). Clearly, virus-infected and immune system-activated macrophages secrete both viral and cellular factors that play pivotal roles in disease (72). Past studies have supported the notion that multiple viral and cellular factors are involved in disease pathogenesis, perhaps through overlapping mechanisms. Indeed, brain cell apoptosis can potentially occur through TNF-α (6), gp120 (8), and tat (49), as well as via c-kit activation (30). Apoptosis in brain cells may also be induced by activation of the transcription factor NF-κB (6). However, the actual signal transduction pathways involved in HIV-1-associated neural cell apoptosis remain undefined.
Previous work demonstrated that overactivation of the GTP-binding protein (G protein)-linked signaling pathways leads to aberrant neuronal function. Second messengers, including cAMP, diacylglycerol, IP3, and calcium, may mediate such events. The production of cAMP, in turn, activates PKA. Following activation, PKA phosphorylates proteins within the cell, leading to modification of enzymes, ion channels, and transcriptional regulators (33). Diacylglycerol activates PKC, an enzyme involved in regulating cell growth, differentiation, learning, and memory (51). IP3 can bind to IP3 receptors on the endoplasmic reticulum and trigger the release of intracellular calcium, which may act as a third messenger, exerting its own biochemical effects on the cell. Increased intracellular calcium levels activate CaMK-II, and CaMK-IV increases calcium influx and, hence, cell death. Although a linkage between intracellular signaling and neuronal apoptosis was observed in our work, the specific mechanisms of such events were not uncovered. First, our work demonstrated that the interaction of chemokines and progeny virions with a neuronal receptor(s) could lead to both cell signaling and apoptosis. Second, since chemokine receptors are G protein coupled and affect cAMP levels, the activation of phospholipase C, and/or the production of IP3, they can effect alterations in intracellular calcium levels, a prelude to cellular dysfunction. Excess levels of calcium can disrupt mitochondrial function or activate lipases, proteases, and endonucleases, which can lead to neuronal death (2, 15, 17, 46). Third, previous reports demonstrated that activation of IP3, PKA, and/or intracellular calcium can induce apoptosis (9, 18, 24, 35, 39, 44, 52). In our study, RP-8-Br-cAMP (1 μM) modestly inhibited virion-induced neuronal apoptosis. Chronic engagement of the G-protein-coupled α2 adrenergic, m2/m4 muscarinic, or opioid receptors can lead to an increase in cAMP, while acute activation leads to inhibition of cAMP production (4). This phenomenon is referred to as adenylate cyclase superactivation. If virions or SDF-1α induces such superactivation, this could explain why virion-mediated neuronal apoptosis was partially blocked by inhibitors, but not activators, of PKA. Fourth, virion-induced neuronal apoptosis was blocked by the broad CaMK-II, PKC, and PKA inhibitor HA1004. This demonstrated, at some level, a correlation between neuronal apoptosis and signal transduction.
CXCR4 is expressed on neurons, astrocytes, and MDM. Why SDF-1α selectively mediates apoptosis, through CXCR4, in neurons but not in astrocytes or MDM is unclear. Although CCR3 has been reported to be expressed on neurons (61), it has not been shown to be functionally important in this work. Moreover, the virion-induced apoptosis occurs principally in neurons, even though virions could induce signal transduction in both astrocytes and MDM. The observation that T-tropic strains of HIV-1 may play an important role in viral neuropathogenesis has also recently been documented by others. Indeed, similar results were shown by Ohagen et al. (50). These experiments demonstrate that T-tropic viral strains effect neuronal apoptosis to a greater degree than do M-tropic variants. HAD occurs late in the course of HIV-1 infection, during the development of significant immunosuppression and the emergence of predominantly T-tropic strains.
Interestingly, CXCR4 may not be the only functional neuronal receptor intimately involved in HAD pathogenesis. Indeed, other chemokine receptors appear to be operatively expressed on neurons. Recent work in our laboratory (74) demonstrated that CX3CR1 and CXCR2 are functional in neurons. This could explain not only the complexity of the signal transduction pathways but also why the CXCR4 antibody could not completely abrogate virion-induced apoptosis or neuronal signal transduction.
It is now well accepted that the MP plays a pivotal role in HIV-1 neuropathogenesis. However, what is not known is the composition of the viral strain or strains that are neurovirulent. A pivotal question in this regard is whether M-tropic virus is sufficient to cause central nervous system (CNS) injury (60, 64). HIV-1 infection (seeding) of the CNS likely occurs early in the course of disease with M-tropic viruses. These same viruses are present throughout much of what is considered subclinical disease. T-tropic viruses or dual-tropic viruses that use CXCR4 emerge later in the course of infection, at the time that HAD becomes prevalent (7, 13, 65). T-tropic HIV-1 strains may play a role in HAD for several reasons. First, the V3 region of the HIV-1 envelope, characteristic of T-tropic strains, is detected in brain tissue of patients with HAD (11, 40). Second, T cells are detected in the brains of demented patients with HIV infection (68). Third, infected T cells can gain access to the CNS through a disrupted BBB (56, 58), through the choroid plexus, or by direct infection of brain microvascular endothelial cells (10, 25, 48). In addition, M-tropic virions can also induce alterations in neuronal signal transduction and apoptosis through the CXCR4 receptor (14, 17). Taken together, our data suggest the following: (i) that HIV-1 virions can bind to and signal through chemokine receptors (principally CXCR4) in neurons, thereby inducing alterations in neuronal function which lead to apoptosis; (ii) the process is independent of binding to CD4 and productive viral replication in neurons; (iii) whole progeny virions induce increased levels of cell injury compared to purified gp120; and (iv) signal transduction pathways induced by virions, such as inhibition of cAMP and activation of IP3, may be linked to apoptosis. These data support the notion that highly active antiretroviral medicines with high-level BBB penetration, combined with anti-inflammatory drugs and neuroprotective compounds, can effectively treat HAD. Clearly, the data presented in this report demonstrate the interplay between the immune system, virus replication, and neurodegeneration in the neuropathogenesis of HIV-1 brain infection.
ACKNOWLEDGMENTS
We thank James A. Hoxie for kindly providing the CXCR4 monoclonal antibody, 12G5; Harris Gelbard, Pamela Carmines, Myron L. Toews, and Yuri Persidsky for scientific discussions and critical reading of the manuscript; Julie Ditter, Robin Taylor, Lesley B. Gendelman, and Alicia Lopez for administrative and secretarial support; and Walt Zink III and Winai Ratanasuwan for technical assistance.
This work was supported in part by research grants (to H.E.G.) from the National Institutes of Health (P01 NS31492-01, R01 NS34239-01, R01 NS34239-02, R01 NS36126-01, and P01MH57556-01), the University of Nebraska Biotechnology Start-Up Funds, and Carter-Wallace, Inc., Cranbury, N.J. Anuja Ghorpade is an Elizabeth Glaser Pediatric AIDS Foundation Scholar.
REFERENCES
- 1.Adamson D C, Wildmann B, Sasaki M, Glass J D, McArthur J C, Christov V I, Dawson T M, Dawson V L. Immunologic NO synthase: elevation in severe AIDS and induction by HIV-1 gp41. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.An S F, Giometto B, Scaravilli T, Tavolato B, Gray F, Scaravilli F. Programmed cell death in brains of HIV-1-positive AIDS and pre-AIDS patients. Acta Neuropathol. 1996;91:169–173. doi: 10.1007/s004010050409. [DOI] [PubMed] [Google Scholar]
- 4.Avidor-Reiss T, Nevo I, Levy R, Pfeuffer T, Vogel Z. Chronic opioid treatment induces adenylyl cyclase V superactivation: involvement of Gβγ. J Biol Chem. 1996;271:21309–21315. doi: 10.1074/jbc.271.35.21309. [DOI] [PubMed] [Google Scholar]
- 5.Bagetta G, Corasaniti M, Berliocchi L, Navarra M, Finazzi-Agro A, Nistico G. HIV-1 gp120 produces DNA fragmentation in the cerebral cortex of rat. Biochem Biophys Res Commun. 1995;211:130–136. doi: 10.1006/bbrc.1995.1787. [DOI] [PubMed] [Google Scholar]
- 6.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–787. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 7.Björndal Å, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenneman D E, Westbrook G L, Fitzgerald S P, Ennist D L, Elkins K L, Ruff M R, Pert C B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- 9.Bruno V, Copani A, Knopfel T, Kuhn R, Casabona G, Dell’Albani P, Condorelli D F, Nicoletti F. Activation of metabotropic glutamate receptors coupled to inositol phospholipid hydrolysis amplifies NMDA-induced neuronal degeneration in cultured cortical cells. Neuropharmacology. 1995;34:1089–1098. doi: 10.1016/0028-3908(95)00077-j. [DOI] [PubMed] [Google Scholar]
- 10.Carlos T, Kovach N, Schwartz B, Rosa M, Newman B, Wayner E, Benjamin C, Osborn L, Lobb R, Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular adhesion molecule-1. Blood. 1991;77:2266–2271. [PubMed] [Google Scholar]
- 11.Chang J, Jozwiak R, Wang B, Ng T, Ge Y C, Bolton W, Dwyer D E, Handle C, Osborn R, Cunningham A C, Saksena N D. Unique HIV type 1 V3 region sequences derived from six different regions of brain: region-specific evolution within host-determined quasispecies. AIDS Res Hum Retroviruses. 1998;14:25–30. doi: 10.1089/aid.1998.14.25. [DOI] [PubMed] [Google Scholar]
- 12.Collin M, Illei P, James W, Gordon S. Definition of the range and distribution of human immunodeficiency virus macrophage tropism using PCR-based infectivity measurements. J Gen Virol. 1994;75:1597–1603. doi: 10.1099/0022-1317-75-7-1597. [DOI] [PubMed] [Google Scholar]
- 13.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Kurkhart M, Marzio P D, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson Y K, Bell J E, Holmes E C, Hughes E S, Brown H K, Simmonds P. In vivo distribution and cytopathology of variants of human immunodeficiency virus type 1 showing restricted sequence variability in the V3 loop. J Virol. 1994;68:5991–6005. doi: 10.1128/jvi.68.9.5991-6005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Broder C, Kennedy P, Berger E. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 18.Fladmark K E, Gjertsen B T, Doskeland S O, Vintermyr O K. FAS/APO-1 (CD95)-induced apoptosis of primary hepatocytes is inhibited by cAMP. Biochem Biophys Res Commun. 1997;232:20–25. doi: 10.1006/bbrc.1997.6214. [DOI] [PubMed] [Google Scholar]
- 19.Gabuzda D, He J, Ohagen A, Vallat A. Chemokine receptors in HIV-1 infection of the central nervous system. Immunology. 1998;10:203–213. doi: 10.1006/smim.1998.0133. [DOI] [PubMed] [Google Scholar]
- 20.Gendelman H E, Eiden L, Epstein L, Grant I, Lipton S, McArthur J, Pomerantz R, Price R, Swindells S. The neuropathogenesis of HIV-1-dementia: a panel discussion. In: Gendelman H E, Lipton S A, Epstein L G, Swindells S, editors. The neurology of AIDS. 1st ed. New York, N.Y: Chapman and Hall; 1998. pp. 1–10. [Google Scholar]
- 21.Gendelman H E, Orenstein J M, Martin M A, Ferrua C, Mitra R, Phipps T, Wahl L A, Lane H C, Fauci A S, Burke D S, Meltzer M S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gendelman H E, Zheng J, Coulter C L, Ghorpade A, Che M, Thylin M, Rubocki R, Persidsky Y, Halhn F, Reinhard J, Jr, Swindells S. Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in human immunodeficiency virus-associated dementia. J Infect Dis. 1998;178:1000–1007. doi: 10.1086/515693. [DOI] [PubMed] [Google Scholar]
- 23.Ghorpade A, Xia M Q, Hyman B T, Persidsky Y, Nukuna A, Bock P, Che M, Limoges J, Gendelman H E, Mackay C R. Role of the β-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J Virol. 1998;72:3351–3361. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A, Greenberg M. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 25.Gilles P N, Lathey J L, Spector S A. Replication of macrophage-tropic and T-cell-tropic strains of human immunodeficiency virus type 1 is augmented by macrophage-endothelial cell contact. J Virol. 1995;69:2133–2139. doi: 10.1128/jvi.69.4.2133-2139.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glass J D, Fedor H, Wesselingh S L, McArthur J C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlation with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 27.Harouse J M, Kunsch C, Hartle H T, Laughlin M A, Hoxie J A, Wigdahl B, Gonzalez-Scarano F. CD-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989;63:2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatten M E. Neuronal regulation of astroglial morphology and proliferation in vitro. J Cell Biol. 1985;100:384–396. doi: 10.1083/jcb.100.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 30.He J, DeCastro C M, Vandenbark G R, Busciglio J, Gabuzda D. Astrocyte apoptosis induced by HIV-1 transactivation of the c-kit protooncogene. Proc Natl Acad Sci USA. 1997;94:3954–3959. doi: 10.1073/pnas.94.8.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper S C, Hoxie J, Kolson D L, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 32.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson D L, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1α mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 33.Iismaa T, Biden T, Shine J. G protein-coupled receptors. Austin, Tex: RG Landes Company; 1995. [Google Scholar]
- 34.Iyengar S, Schwartz D H, Hildreth J E K. T cell-tropic HIV gp120 mediates CD4 and CD8 cell chemotaxis through CXCR4 independent of CD4: implications for HIV pathogenesis. J Immunol. 1999;162:6263–6267. [PubMed] [Google Scholar]
- 35.Jayaraman T, Marks A R. T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis. Mol Cell Biol. 1997;17:3005–3012. doi: 10.1128/mcb.17.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson R A, Toews M L. Protein kinase C activations sensitize cyclic AMP accumulation by intact 1321N1 human astrocytoma cells. Mol Pharmacol. 1989;37:296–303. [PubMed] [Google Scholar]
- 37.Kalter D C, Gendelman H E, Meltzer M S. Monocytes, dendritic cells, and Langerhans cells in human immunodeficiency virus infection. Dermatol Clin. 1991;9:415–428. [PubMed] [Google Scholar]
- 38.Kelder W, McArthur J C, Nance-Sproson T, McClernon D, Griffin D E. β-Chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 39.Khan A, Soloski M, Sharp A, Schilling G, Sabatini D, Li S, Ross C, Snyder S. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-triphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- 40.Korber B T M, Kunstman K J, Patterson B K, Furtado M, McEvilly M M, Levy R, Wolinsky S M. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kornbluth R S, Kee K, Richman D D. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive beta-chemokines. Proc Natl Acad Sci USA. 1998;95:5205–5210. doi: 10.1073/pnas.95.9.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwong P D, Wyatt R, Robinson J, Sweets R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavi E, Strizki J M, Ulrich A M, Zhang W, Fu L, Wang Q, O’Connor M, Hoxie J A, Gonzalez-Scarano F. CXCR-4 (fusin), a coreceptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 44.Lipton S. Models of neuronal injury in AIDS: another role for the NMDA receptor? Trends Neurosci. 1992;15:75–79. doi: 10.1016/0166-2236(92)90013-x. [DOI] [PubMed] [Google Scholar]
- 45.Lipton S A, Gendelman H E. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;16:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 46.Mattson M, Rydel R, Lieberburg I, Smith-Swintosky V. Altered calcium signaling and neuronal injury: stroke and Alzheimer’s disease as examples. Ann N Y Acad Sci. 1993;679:1–21. doi: 10.1111/j.1749-6632.1993.tb18285.x. [DOI] [PubMed] [Google Scholar]
- 47.Mondor I, Moulard M, Ugolini S, Klasse P-J, Hoxie J, Amara A, Delaunay T, Wyatt R, Sodroski J, Sattentau Q J. Interactions among HIV gp120, CD4, and CXCR4: dependence on CD4 expression level, gp120 viral origin, conservation of the gp120 COOH- and NH2-termini and V1/V2 and V3 loops, and sensitivity to neutralizing antibodies. Virology. 1998;248:394–405. doi: 10.1006/viro.1998.9282. [DOI] [PubMed] [Google Scholar]
- 48.Moses A V, Bloom F E, Pauza C D, Nelson J A. HIV infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proc Natl Acad Sci USA. 1993;90:10474–10478. doi: 10.1073/pnas.90.22.10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.New D R, Ma M, Epstein L G, Nath A, Gelbard H A. Human immunodeficiency virus type-1 tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- 50.Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J Virol. 1999;73:897–906. doi: 10.1128/jvi.73.2.897-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olds J, Alkon D. A role for protein kinase C in associative learning. New Biol. 1991;3:27–35. [PubMed] [Google Scholar]
- 52.Parvathenani L A, Buescher E S, Chacon-Cruz E, Beebe S J. Type I cAMP-dependent protein kinase delays apoptosis in human neutrophils at a site upstream of caspase-3. J Biol Chem. 1998;273:6736–6743. doi: 10.1074/jbc.273.12.6736. [DOI] [PubMed] [Google Scholar]
- 53.Patton H, Benveniste E, Benos D. Astrocytes and the AIDS dementia complex. J Neuro-AIDS. 1996;1:111–113. doi: 10.1300/j128v01n01_06. [DOI] [PubMed] [Google Scholar]
- 54.Perry S W, Epstein L G, Gelbard H A. Simultaneous in situ detection of apoptosis and necrosis in monolayer cultures by TUNEL and trypan blue staining. BioTechniques. 1997;22:1102–1106. doi: 10.2144/97226st01. [DOI] [PubMed] [Google Scholar]
- 55.Persidsky, Y., A. Ghorpade, J. Rasmussen, J. Limoges, X. Liu, M. Stins, M. Fiala, D. Way, K. S. Kim, M. H. Witte, M. Weinand, L. Carhart, and H. E. Gendelman. Microglial chemokines regulate monocyte migration through the blood-brain barrier in HIV-1 encephalitis. Am. J. Pathol., in press. [DOI] [PMC free article] [PubMed]
- 56.Persidsky Y, Stins M, Way D, et al. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:499–510. [PubMed] [Google Scholar]
- 57.Peterson P K, Gekker G, Hu S, Anderson W R, Kravitz F, Portoghese P S, Balfour H H, Jr, Chao C C. Morphine amplifies HIV-1 expression in human brain cell cultures. J Neuroimmunol. 1994;20:83–90. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 58.Petito C K, Cash K S. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32:658–666. doi: 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- 59.Petito C K, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- 60.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders V J, Pittman C A, White M G, Wang G, Wiley C A, Achim C L. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- 62.Shi B, Girolami U D, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Investig. 1996;98:1979–1990. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shieh J T C, Albright A V, Sharron M, Gartner S, Strizki J, Doms R W, González-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simmonds P. Neurotropism of HIV type 1? AIDS Res Hum Retroviruses. 1996;12:469–470. [Google Scholar]
- 65.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stefano M D, Wilt S, Gray F, Dubois-Dalcq M, Chiodi F. HIV type 1 V3 sequences and the development of dementia during AIDS. AIDS Res Hum Retroviruses. 1996;12:471–482. doi: 10.1089/aid.1996.12.471. [DOI] [PubMed] [Google Scholar]
- 67.Vallat A-V, Girolami U D, He J, Mhashikar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith T W, Gabuzda D. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- 68.Weidenheim K M, Epshteyn T, Lyman W D. Immunocytochemical identification of T-cells in HIV-1 encephalitis: implication for pathogenesis of CNS disease. Mod Pathol. 1993;6:167–174. [PubMed] [Google Scholar]
- 69.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 70.Wyatt R, Kwong P, Desjardins E, Sweet R, Robinson J, Hendrickson W, Sodroski J. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 71.Zheng J, Thylin M R, Ghorpade A, Xiong H, Peridsky Y, Cotter R, Niemann D, Che M, Zeng Y, Shepard R B, Swartz J M, Gendelman H E. Intracellular CXCR4 signaling, neuronal apoptosis, and the neuropathogenic mechanisms for HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 72.Zheng, J., M. R. Thylin, Y. Persidsky, H. Xiong, A. Ghorpade, D. Niemann, M. H. Che, G. B. Leisman, R. L. Cotter, K. Lewis, and H. E. Gendelman. Neuronal destruction in HIV-1-associated dementia: secretory products from HIV-1 infected macrophages affect neuronal function, differentiation, long term potentiation viability. Submitted for publication.
- 73.Zheng J, Zhang P, Toews M, Hexum T D. Neuropeptide Y enhances ATP-induced formation of inositol phosphates in chromaffin cells. Biochem Biophys Res Commun. 1997;239:287–290. doi: 10.1006/bbrc.1997.7456. [DOI] [PubMed] [Google Scholar]
- 74.Zheng, J., et al. Unpublished data.