Abstract
Being commonly diagnosed in elderly women and associated with comorbidities as well as ageing-related cardio-vascular changes, heart failure with preserved ejection fraction (HFpEF) has been recently considered as a distinct cardiogeriatric syndrome. Frailty is another frequent geriatric syndrome. HFpEF and frailty share common underlying mechanisms, often co-exist, and represent each other’s risk factors. A threshold of 65 years old is usually used to screen patients for both frailty and HFpEF in research and clinical settings. However, both HFpEF and frailty are very heterogenous conditions that may develop at younger ages. In this review we aim to provide a broader overview on the coexistence of HFpEF and frailty throughout the lifetime. We hypothesize that HFpEF and frailty patients’ profiles (young, elderly, superaged) represent a continuum of the common ageing process modified by cumulative exposure to risk factors resulting to a presentation of HFpEF and frailty at different ages. We believe, that suggested approach might stimulate assessment of frailty in HFpEF assessment and vice versa regardless of age and early implementation of targeted interventions. Future studies of pathophysiology, clinical features, and outcomes of frailty in HFpEF by age are needed.
Keywords: Frailty, Diagnosis, Heart failure, Middle aged, Frail elderly, Young adult
INTRODUCTION
Both heart failure with preserved ejection fraction (HFpEF) and frailty are heterogeneous conditions that often coexist. Epidemiological studies have shown that frailty is highly prevalent in patients with heart failure (HF), particularly in the elderly individuals, affecting up to 90% of HFpEF patients.1,2) HF patients are 6-times more likely to have frailty, and frail patients exhibit a 30% increase in risk of new-onset HF.3) Frailty in HF is associated with a range of adverse outcomes such as more frequent hospitalizations, longer hospital stays, higher healthcare costs, and higher mortality rates.4) Frailty also impacts the quality of life of individuals with HF, leading to functional decline, decreased independence, and reduced overall well-being.2)
Although the incidence of frailty dramatically increases with age, it is important to understand that chronological age by default does not presuppose increased vulnerability, and vice versa younger age does not exclude frailty in the presence on chronic debilitating condition like HF. Several cluster-based analyses have revealed distinct features of young compared to elderly HFpEF patients.5) Similarly, over the last years, there has been an emerging interest to the translation of the frailty concept to a younger population who similarly to elderly patients might have a decrease in physiological reserve and higher vulnerability to stress due to long-term health problems.6) Compared to prior decades, current studies show an increase in the prevalence and severity of frailty at different ages starting from early adulthood.7) Differences in characteristics of frailty by age were also noted.8) The significant influence of risk factors and their prolonged exposure since childhood or early adulthood may contribute to a greater burden of frailty occurring earlier in life. This parallels the well-documented association between comorbidities and poorer psychosocial health, which is linked to a heightened trajectory of frailty as individuals transition into older age, particularly among middle-aged adults. In HFpEF, in addition to the clinical expression of frailty at any age in severe HF, the accumulation of deficits is also relevant, given the increasing prevalence of cardiovascular (CV) risk factors in young adults.5,9)
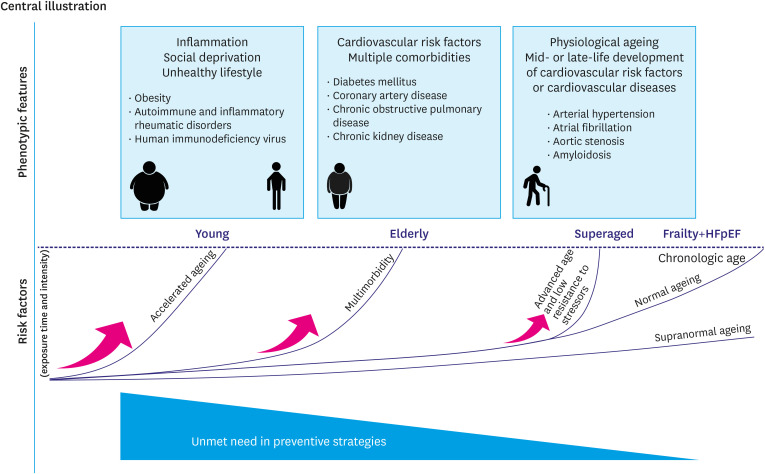
Thus, considering age-related heterogeneity of both HFpEF and frailty, we hypothesize that there might be distinct HFpEF and frailty patients’ profiles (young, elderly, superaged) which represent a continuum of the common ageing process modified by cumulative exposure to risk factors of different intensity (Figure 1). A cut-off of 65 years was used to delineate young from the older phenotype, considering its highest discrimination value to stratify the sample into younger and older adults with frailty8) and markedly raising incidence of HFpEF and frailty in senior adults 65 years and older. Though, this cut-off is applicable to HFpEF and frailty cohort only and is purely conditional as might shifts depending on demographic, social and healthcare system-related factors.10)
Figure 1. Progression of HFpEF and frailty across different age groups.
HFpEF = heart failure with preserved ejection fraction.
We believe that such a profiling might: 1) help better understanding the concept of frailty in a diverse cohort of HFpEF patients, 2) stimulate assessment and future studies of frailty in HFpEF independently of age, especially, when long-term conditions are present11) and lead to earlier targeted interventions that might improve patients’ outcomes.
PATHOPHYSIOLOGY OF FRAILTY IN HFpEF
Despite data showing a strong association between HF and frailty, the pathophysiologic mechanisms linking both conditions are not completely understood. It is even debated whether frailty is an independent syndrome, or just a result of chronic medical conditions or multimorbidity and polypharmacy related to ageing.12)
Common pathophysiological mechanisms include chronic low-grade inflammation, immune and hormonal dysregulation, oxidative stress, and impaired cellular repair contributing to a vicious cycle of functional decline and increased vulnerability in affected individuals.13,14,15) The etiology of systemic inflammation causing frailty may be related or independent to HF.3) Both aging and inadequate nutritional intake are associated with increased levels of pro-inflammatory factors like interleukin (IL)-6, C-reactive protein (CRP), tumor necrosis factor (TNF)-α.16) In a pilot study including elderly patients with stable HF elevated level of IL-6 was associated with a higher burden of comorbidities including atrial fibrillation (AF), type 2 diabetes mellitus (T2DM), hyperlipidemia, anemia, and renal failure.17) Increased white blood count (WBC) with impaired differentiation of T cells is not uncommon with frailty.3) A post-hoc analysis of the PARAGON-HF trial showed association of elevated neutrophil/lymphocyte ratio with worsening frailty in HFpEF.18) In addition, frail HFpEF patients demonstrated increased levels of biomarkers reflecting collagen turnover and higher concentrations of troponin T, pointing to one of the mechanisms how frailty leads to progressive worsening of HF over time.18) Urinary cyclic guanosine monophosphate (cGMP)/creatinine ratio that reflects responsiveness to natriuretic peptides was lower with increasing frailty.18) Stimulated by pro-inflammatory cytokines macrophages differentiate into so-called inflammaging or macrophage aging promoting chronic sterile inflammation.16) Similarly, imperfect DNA repair, oxidative stress and increased muscle protein turnover observed both in aging and HF lead eventually to cellular necrosis that initiates inflammatory response. In mice studies low level of STAT3 protein promoting cell response to signals coming from interleukins and growth factors was associated with increased pro-inflammatory cytokines and fibrosis in aging heart.3) On the contrary, aged mice with low levels of NOD-like receptor protein 3 (NLRP3) inflammasome that triggers maturation and secretion of IL-1β and IL-18 demonstrate better exercise tolerance compared to the wild-type controls.3) Interestingly NLRP3 inflammasome plays an important role in cardiac remodeling, fibroblast differentiation and pyroptosis, an inflammatory form of programmed cell death contributing to myocardial damage.19) Suppression of tumorigenicity 2 interleukin protein, a surrogate marker of inflammation and potential mediator of myocardial hypertrophy and fibrosis exhibits prognostic value both in frailty and HF.20)
Systemic inflammation in HFpEF may be partly attributed to eicosanoids, small bioactive substances derived from fatty acids. In a recent study certain eicosanoids and eicosanoid-related metabolites were associated with HFpEF-related exercise intolerance.21)
The up-regulation of pro-inflammatory substances promotes progression of atherosclerosis and insulin resistance, impairs hormones, such as cortisol, testosterone, insulin-like growth factor-1/growth hormone and growth-differentiating factor-15 resulting in imbalance between anabolic and catabolic state.13,14,22,23) This may result in sarcopenia defined as a decrease in lean body mass and cachexia defined as a generalized muscle wasting. Sarcopenia is often influenced by age-related changes in hormones, decreased physical activity and nutritional deficits. The latter is also often impaired in HF secondary to dietary limitations, early satiety and abdominal pain related to congestion and intestinal oedema, dementia, depression and social ill-being.22) HF patients demonstrate multiple skeletal muscle abnormalities including reduced mitochondrial function and capillary density, reduced oxidative type I muscle fibers, adipose intramuscular infiltration.13) Limited nitric oxide bioavailability aggravates impairment in oxygen transport and peripheral oxygen extraction contributing to reduced exercise tolerance.24) Skeletal myopathy in HF is not necessarily related to deconditioning and can be present regardless of physical activity level. It may be further worsened with frequent re-admissions by hospital environment and prolonged immobility.13,24) Impaired skeletal muscle function contributes to osteopenia and osteoporosis potentially resulting in pathological fractures.25)
Although characterized by decreased physiological reserve and increased vulnerability to stressors, frailty is not just limited to physical impairment. Cognitive, social, and psychological issues also play an important role. The concept of multidimensional approach has been recently proposed by Heart Failure Association Frailty Score incorporating 4 domains of interest: the clinical domain (co-morbidities, weight loss and falls), the psycho-cognitive domain (cognitive impairment, dementia, depression), the functional domain (impaired activities of daily living, low/non mobility and balance) and the social domain (living alone, institutionalization, no social support).14,26) Most of the HF patients have problems in each domain, and a huge overlap between those may be expected.26) According to FRAGILE-HF and OPERA-HF trials cognitive and psycho-social limitations were associated with re-admissions and HF-related mortality.22) Identifying those HF patients who are not meeting criteria for frailty but are at risk of frailty (“pre-frail”), may be beneficial in terms of early therapeutic interventions and prognostic improvement.23)
Frailty can vary depending on both chronological and biological age. For some individuals, chronological age may closely correlate with biological age, resulting in frailty appearing more consistent with advancing years. However, other individuals may exhibit signs and symptoms of frailty at a younger chronological age due to certain factors like chronic illness, sedentary lifestyle, bad habits, poor nutrition, or genetic predispositions, which accelerate biological aging. Conversely individuals with favorable lifestyle habits, appropriate management of chronic conditions, better social engagement and mentally stimulating activities may experience delayed onset and reduced severity of frailty even at older chronological ages. Understanding discrepancy between chronological and biological age is crucial for identifying patients at higher risk for frailty and implementing well-timed interventions.
YOUNG HFpEF AND FRAILTY PROFILE
Despite the absence of a consensus on what defines frailty and how to measure it in young adults,27) particularly with HFpEF, a small study showed that children with established cardiovascular disease (CVD) such as Fontan palliation, pulmonary hypertension, and HF performed significantly worse in all five domains of frailty such as slowness, weakness, exhaustion, body composition and physical activity compared to healthy controls.28) Composite frailty score developed for children and adolescents with CVDs correlated significantly with signs of HF severity.29) This data signifies the feasibility of translation of frailty phenotypes into the pediatric population with HF. Indeed, among young patients with established CVDs a particular attention has been raised to accelerated ageing and frailty in congenital heart diseases, pulmonary hypertension and HF.30,31,32,33)
HFpEF accounts for around 14–17% of HF cases among patients younger than 60 years old.34) In a large registry-based study of Asian HFpEF population 37% of patients were younger than 65 years, including 13% patients younger than 55 years.35) Data regarding HFpEF and frailty coexistence in younger than 65 years old population are lacking, as in line with recommendations for routine frailty screening36) many studies have investigated frailty among HFpEF patients older than 65 years.37) Sub-analyses of two randomized studies used Fried frailty phenotype and frailty index definitions showed that 20–23% of pre-frail and 15–20% of frail HFpEF patients were younger than 65 years.38,39) In real-world setting this proportion is expected to be larger given that patients with significant health conditions are usually excluded from clinical trials. Indeed, analyses of large population-based cohorts from the US, UK and Sweden, Canada, and China underscore the problem of growing prevalence of frailty among young and middle-age participants.7,8,40,41,42) In a study of the US National Health and Nutrition Examination Survey (NHANES) database (n=49,004) the proportion of young individuals with at least very mild frailty (frailty index score >0,1 to ≤0,2) was 15–20% in 20–35 years and step wisely increased in prevalence and severity with increasing age.7) Similar data were reported in the Chinese population of 30–79 years old40) and the Canadian population of 18–79 years old.28) When the frailty phenotype definition was used, the proportion of pre-frail and frail adults aged 18–64 years old was even higher.40) While pre-frailty and frailty conferred a higher risk of mortality regardless of age,42) they were more prognostically important in individuals <50 years compared to those 50 years old or above. Life expectancy was shorter by 2 and 6 years in adults having pre-frailty and frailty status at age 45 years.43) The significant impact of frailty on longevity might be partly related to strong associations between frailty and incident CVDs, which also seem to be stronger for younger adults compared to older adults.43)
CVDs disease and risk factors aside, in young adults, frailty is primarily related to unhealthy habits and social deprivation. A recent integrative review showed, that of physical factors human immunodeficiency virus (HIV) infection, pain, diabetes, both higher and lower body mass index, and higher rheumatoid arthritis (RA) disease activity and longer duration were associated with frailty in young.6) Depressive symptoms, illicit drug use, and smoking were the most common psychological factors, and unemployment and lower education were the most common social factors associated with frailty.6) Similar factors, including socioeconomic, might be associated with HFpEF in younger adults.44,45,46,47)
Traditional CV risk factors
Traditional HF risk factors such as obesity, hypertension, diabetes, and smoking history conferred a greater population attributable risk in young compared to older adults and accounted for 75% of total attributable risk for HF.33) Data on associations between obesity and younger age in HFpEF is consistent across multiple clustering-based studies.35,48,49) Subgroups of HFpEF patients 55 years or younger had higher body mass index and despite having fewer comorbidities, had worse quality of life compared with older patients (age ≥85 years).49) Obesity promotes inflammation, insulin resistance, cardiac and skeletal muscle dysfunction, arterial stiffness, and is frequently accompanied by multimorbidity and complications, such as joint problems, sleep apnea, sedentary lifestyle, and low cardiorespiratory fitness. These disorders predispose obese patients to develop both HFpEF and frailty.50) Reflecting significant morbidity associated with obesity, a specific phenotype of ‘sarcopenic obesity’ was postulated that can replace weight loss in frailty assessment.50) Importantly, recent consensus signifies, that sarcopenic obesity is not uniquely a geriatric syndrome and may occur in individuals at any age with HF representing one of the suspicious factors for the screening of sarcopenic obesity.51) Indeed, a systematic review showed a variable incidence of sarcopenic obesity in children and adolescents, that was significantly associated with cardiometabolic outcomes, non-alcoholic fatty liver disease severity, inflammation, and mental health.52)
Chronic inflammatory conditions
A prospective analysis of a very large UK Biobank dataset showed that self-reported exhaustion and weight loss were more prevalent frailty features in younger compared to older participants.53) In another research there were higher scores in immunological, mental well-being, and pain-related domains in younger participants.8) Chronic inflammatory conditions such as RA or other autoimmune and inflammatory rheumatic disorders or HIV might predispose to the development of both HFpEF and frailty.54,55) Autoimmune disorders are increasingly prevalent, with the highest risk among the most deprived patients.56) A higher risk of HF had been shown across multiple studies of RA patients, including a recent Mendelian randomization study.57) Multiple CV derangements might predispose autoimmune and inflammatory rheumatic conditions to HF, such as myocarditis, pericarditis, coronary vasculitis, thrombosis, microvascular dysfunction, valvular dysfunction as well as arrhythmias.58) Of HF phenotypes, inflammation in patients with RA was associated with HFpEF but not HFrEF.59,60) Autoimmune disorders might also alter CV performance by inducing excessive water retention, driving the development and progression of HF.61,62) HFpEF development in patients with RA is related to disease activity and the risk is highest within the first year after RA diagnosis, which is independent of incident CV or other comorbidities and medications that may have side effects and promote frailty.63) Accumulation of multiple other issues, including osteoporosis, and mental health problems, diminishes physiologic reserve and poses patients with autoimmune disorders to a higher risk of HFpEF-frailty development in a younger age. Indeed, in a systematic review of 16 studies of 8,556 RA patients with a mean age of 63 years, the pooled prevalence of pre-frailty and frailty was 40% and 33.5%.64) Similarly, to HFpEF in RA, frailty was also mainly influenced by the disease activity, as well as female sex, but not chronological age.64)
People living with HIV (PLWH) have a higher risk of developing HF in and in the modern era of anti-retrovirus therapy HFpEF is a predominant HF phenotype.65,66) Cardiac imaging studies suggest that there is a complex pattern of heart involvement in PLWH, including systolic and diastolic dysfunction, reduced myocardial strain, and increased left ventricular (LV) mass and remodeling.65,66) Cardiac biomarkers increase, diffuse myocardial fibrosis and remodeling and LV mass were independently predictive of CV events in PLWH.67) Of note, besides traditional CV risk factors that might play a role, several other mechanisms were assumed to be implicated in the pathophysiology of HFpEF in PLWH, such as inflammation, persistent monocyte/macrophage activation, chronic immune dysfunction, altered energy metabolism, activation of renin-angiotensin-aldosterone system.65) All these factors are involved in the pathophysiology of frailty as well. Frailty was found to be highly prevalent (5–19%) in middle-aged adults living with HIV.65) Compared to uninfected persons, HIV patients with frailty have an over 7-fold increased risk of death, independent of comorbidity and HIV disease stage.68)
Overall, accumulated data suggest that in young adults, HFpEF and frailty share pathophysiology and associated factors. Young HFpEF-frailty profile should be considered as a dynamic state starting from an incident risk factor which leads to cardiac involvement and deficit accumulation and further translates into a clear phenotypic expression of accumulated morbidity. Early intervention aiming to improve populational health and reduce the burden of HFpEF-frailty risk factors since childhood, as well as earlier geriatric patients’ assessment, might confer a greater impact on populational health compared to later intervention.69,70) Efforts should be made to promote lifestyle changes, especially among the most deprived groups, and implementation of antihypertensive medications and emerging novel therapies against diabetes and obesity with proved CV efficacy and safety (such as sodium-glucose cotransporter-2 inhibitor [iSGLT2] and glucagon-like peptide-1 receptor agonists [GLP1RA]) into routine CV care.
ELDERLY HFpEF AND FRAILTY PROFILE
Prevalence and incidence of both frailty and HFpEF are increasing with age. According to UK Biobank data, midlife frailty occurs in about 3-4% of individuals and accounts for 4–5% among those aged 65 and older.53) The proportion of frailty in the older population in European countries is even higher, about 17%.71) Screening for frailty is recommended in individuals over 65 years.36) The prevalence of HFpEF in those 65 years and older is about 8–9%. An increasing prevalence of both conditions with age could be partially explained by cumulative exposure of CV risk factors, as well as manifestation of a variety of CV and non-CVDs. The positive association between the burden of comorbidities and an incidence of frailty and HFpEF has been previously shown.71) Among comorbidities pathophysiologically linked to both frailty and HFpEF are CKD, coronary artery disease (CAD), T2DM and chronic obstructive pulmonary disease (COPD). Of note, these pathologies are characterized by the presence of low-grade systemic inflammation, oxidative stress, dysregulation of the neuro-hormonal system and metabolic dysfunction settling the basis for maladaptive cardiac remodeling and impaired function, and/or resulting in individual’s multisystem dysregulations, depletion of physical, cognitive reserve, and poor tolerance to stress, substantially leading to the development of frailty and HFpEF.72,73,74)
T2DM
The estimated global prevalence of T2DM is 9.3% and continues to rise annually.75) At midlife it accounts for 16% reaching 20% in those aged 65 and older.75) Metabolic disorders in diabetes include insulin resistance, lipid toxicity, glycation end product deposition, and microvascular disfunction contribute to development of both frailty and HFpEF.76) Prevalence of frailty in diabetic patients is about 13% wand associated with a greater risk of death, hospital admissions, as well as micro- and macrovascular complications.77) There are a few studies that demonstrated association between T2DM and with frailty.78,79) Therefore, T2DM considered as a risk factors for frailty, but sarcopenia, weight loss in response to longstanding hyperglycemia in diabetic patens can contribute to frailty as well.80) Clinical significance of frailty in T2DM patients was acknowledged by international diabetes guidelines. However, the approaches to frailty assessment and management in diabetic patients are yet to be developed. Nutritional status support and exercise have demonstrated a potential in frailty prevention in T2DM.81)
The pivotal role of diabetes in HFpEF is increasingly recognized and reported elsewhere.82) The prevalence of T2DM is expected to lie between 33% and 43%.83) The prognosis of having diabetes in HFpEF is unfavorable.83) Cardiometabolic HFpEF is the most frequent HFpEF phenotype, which is commonly developed by the end of midlife.84) Of note, a cardiac remodeling and dysfunction in diabetic patients can precede to HFpEF,84,85) and can be classified as pre-HF indicating that those patients who are at risk of developing symptomatic HF. Recent guidelines on diabetes mellitus recommend iSGLT2 as a first line therapy in high-risk patients.86,87) In 2023 focused update of HF guidelines update treatment with iSGLT2 and finerenone are recommended for prevention of HF hospitalization and CV death in diabetic patients with chronic kidney disease (CKD).88)
Therefore, along with routine screening for HFpEF and frailty in T2DM patient is required at mid-age and older adults, as well as early intervention including pharmacological therapy and exercise-based rehabilitation program to prevent both frailty and HFpEF at mid-and late life.
CKD
CKD is a highly prevalent condition that affects about 13.5% of individuals globally89) and increases with age affecting about 16% at 50s, while the proportion of those in 60s and 70s is 27.6% and 34.3%, respectively.90) Impaired kidney function results in volume overload, worsening hypertension, metabolic disorders, increased arterial stiffness and myocardial fibrosis, microvasculature disfunction, neurohormonal activation and inflammation all of which contribute to both HFpEF and frailty. CKD is independent risk factor of HF as well as adverse outcomes including HF hospitalization, all-cause and СV mortality in HFpEF individuals.91,92) The incidence rate of newly diagnosed HF in CKD subjects is 1.9 (95% confidence interval [CI], 1.7–2.1) events per 100 person-year.93) Half of HFpEF have a concomitant CKD.92)
Metabolic disorders and hemodynamic disturbances, as well as CKD accompanied conditions including sarcopenia, anemia, and vitamin D deficiency result in an overall poor state of CKD patients. The prevalence of frailty in CKD patients ranges from 7% in community settings reaching to 73% in those on hemodialysis.94) CKD patients have increased risks of frailty regardless of the CKD stage and independently of age, gender, race, co-morbidities, sarcopenia, anemia, acidosis, inflammation, vitamin D deficiency, hypertension, and CVD.95) Of note, both frailty and CKD are independently associated with mortality.95) The identification and assessment of frailty should become a routine aspect of CKD care, as well as screening for HFpEF. Although, pathophysiological mechanisms underlying HFpEF and frailty in CKD need to be better understood in order to identify therapeutic targets for intervention. Early rehabilitation programs or/and iSGLT2 administration can be disclosed earlier in CKD patients, however further studies are needed.96)
CAD
Mean age of individuals at diagnosis of CAD is 60 years old.97) About one fourth of CAD patients of all ages are frail. Pooled analysis of 22 studies reported 17% prevalence of frailty in those with stable CAD.98) Frail CAD patients have a higher mortality rate compared to non-frail CAD individuals and are less likely to receive revascularization, either PCI or bypass surgery.99) Presence of frailty in independent predictor of in-hospital, 30-day mortality and mid-term mortality in individuals who undergone bypass surgery.100) Frail patients admitted with acute myocardial infarction had a higher in-hospital mortality rate.101) On the other hand, those with CAD are at risk of developing and progression of frailty.102) The pathobiology mechanisms of frailty in CAD patients remain largely unclear. The role of oxidative stress and inflammation and impaired cellular metabolism is discussed elsewhere.103) The relationship between CAD and frailty appears to be bidirectional. Frailty screening in CAD patients can help to identify high risk patients and guide revascularization strategies. Frailty prevention should be timely addressed in patients impaired coronary arteries.
CAD is an independent risk factor for HFpEF and highly prevalent in those with an established diagnosis of HFpEF.104,105) Potential mechanisms leading to HFpEF in CAD attributed to low-grade inflammation, as well as and microvascular dysfunction of coronary vessels. Systemic low-grade inflammation plays a crucial role in CAD progression and outcome which was confirmed in resent clinical trials COLCOT and LoDoCo paving the way for anti-inflammatory therapy in CAD. Microvascular dysfunction of coronary micro vessels presents in majority of CAD patients and is acknowledged as one of the main contributors of HFpEF.105) Up to 70–91% of HFpEF patients have impaired coronary macro- or micro-vessels.105) According to the international HF guidance, examination for CAD is a part of HFpEF diagnostic work up, while there is no routing screening for HFpEF in CAD patients.88)
The preventive strategies in CAD need to be developed with non-pharmacological and pharmacological interventions targeting systemic inflammation and microvascular disfunction to prevent both frailty and HFPEF, however, further studies are needed.
COPD
COPD is a strong and independent predictor of frailty. Hanlon et al.106) using the data of 3,132 participants with COPD aged 40–70 years from UK Biobank showed that 17% of individuals were frail. The presence of frailty in COPD patients was associated with a greater risk of all-cause mortality, all-cause hospitalizations, and hospitalization with COPD exacerbation.106) A meta-analysis of 27 observational studies reported the risk of frailty increased in COPD patients a 2-fold. The prevalence of pre-frail in COPD individuals accounts for 56%.107) Additionally, COPD is associated with a range of extrapulmonary conditions which also may contribute to frailty including osteoporosis, malnutrition and muscle weakness.108,109,110)
The associations between COPD and HFpEF have been extensively reported previously. In COPD, the risk of having HFpEF is 2-fold higher. The prevalence of COPD in patients with HFpEF trials is about 10–19%.111,112,113) COPD has a major impact on outcomes and quality of life in HFpEF patients.114,115,116) Pathophysiological links between these 2 conditions are mainly attributed to systemic low-grade inflammation, endothelial dysfunction, oxidative stress and hypoxia, which is prominent in COPD patients. Altogether, the forementioned mechanisms contribute to airways remodeling, as well as cardiac impairment eventually leading to organ failure.117) The prognosis of having COPD in HFpEF is unfavorable and associated with increased mortality rates and risks of HF hospitalization.116)
Recently published results of prospective cluster-randomized diagnostic study RED-CVD confirmed the importance of screening COPD or T2DM patients for HFpEF in routine clinical practice. In this study, active screening, which included 24 points clinical questionnaire, electrocardiogram and N-terminal prohormone of brain natriuretic peptide, allowed for detection of 3.2% of HFpEF cases in the intervention arm compared to 0.7% in the usual care arm.118) Pulmonary rehabilitation in COPD patients can lead to improvement of frailty status.119) These findings emphasize COPD as an important factor in HFpEF and frailty progression. A development of preventive and treatment strategies is urgently required in the COPD population.
SUPERAGED HFpEF AND FRAILTY PROFILE
Age constitutes a significant risk factor for both HF and frailty. In the PURSUIT‐HFpEF study, which aimed to examine the predictive value of the Clinical Frailty Scale (CFS) in real‐world, median age of patients with HFpEF was 82 (n=842; interquartile range, 77–87).120) The prevalence of HF escalates with age, with around 1% of individuals aged over 50 years affected by HF. Moreover, this prevalence doubles with each successive decade of life. Consequently, HF emerges as a primary cause of mortality among the elderly population.121,122,123,124)
Typically, as individuals age, there is a thickening and stiffening tendency of the LV walls, especially the interventricular septum. Furthermore, there is an augmentation in the left atrium dilation, along with an overall escalation in cardiac fibrosis.125) In aged heart, cardiac reserve decline is the most observed functional change besides subclinical diastolic and systolic dysfunction.126) This decline is also a significant pathophysiological feature of HFpEF. Asymptomatic CV remodeling in aged persons might manifest as HFpEF under some triggers, such as infection, anemia, volume challenge, medications and others. Similar factors might lead to frailty manifestation. Prevalence of frailty defined as frailty index ≥0.21 among HFpEF patients older than 75 years reaches 75–80%.38,53) Patients 75 years and older represent 30–40% of overall HFpEF cohort35,38,39,49) and compared to younger HFpEF subgroups is characterized by the highest proportion of AF, CKD, valvular disease. Additionally, ageing is an independent risk factor for arterial stiffness and hypertension and hypertension is the most prevalent CV condition among superaged individuals, perhaps due to survival bias
Arterial hypertension
Hypertension and HF frequently coexist with aging and are closely linked with frailty, which worsens prognosis and increases hospitalizations, dependency, and mortality. Despite the common occurrence of these conditions, data on the prevalence and impact of frailty in older hypertensive patients with HF are limited.127)
Aprahamian’s cross-sectional study on 619 older outpatients revealed a higher prevalence of hypertension among pre-frail (72.5%) and frail (83%) individuals compared to controls (51.7%), as assessed by the FRAIL scale.128) Similarly, Kang et al.’s analysis of data129) from the 5th Korean National Health and Nutrition Examination Survey, involving 4,352 older adults aged 65 years and above, found hypertension to be more prevalent in frail individuals (67.8%) compared to pre-frail (60.8%) or robust individuals (49.2%). Frail individuals were also more likely to receive treatment for hypertension, but fewer achieved blood pressure control (<150/90 mm Hg) (p=0.005).
Vetrano et al.’s meta-analysis130) presented conflicting data on the relationship between frailty incidence and baseline hypertension, suggesting that specific subgroups of older hypertensive patients may exhibit increased fragility, warranting cautious monitoring during antihypertensive treatment.130)
In terms of intensive blood pressure lowering, an exploratory subgroup analysis of the SPRINT trial showed higher event rates with increasing frailty, but significantly lower rates in the intensive treatment group.131) Similarly, safety concerns were addressed in the Predictive Values of Blood Pressure and Arterial Stiffness in Institutionalized Very Aged Population (PARTAGE) study, which observed a higher risk of mortality in frail octogenarians with lower systolic blood pressure who were on multiple antihypertensive agents.132)
AF
AF is the most common cardiac arrhythmia encountered in the geriatric population and is associated with significant morbidity and mortality.133,134,135) It’s prevalence increases with age, ranging from 4.2% of those aged 60–70 years to 17% of those aged ≥80 years.136) Considering that patients with AF have higher risk of incident acute HF hospitalizations, ischemic and hemorrhagic stroke and dementia, it is undeniable that their quality of life can be affected accordingly.137,138,139,140) This aspect is particularly important in the elderly, in whom it seems to be inversely correlated to frailty and functional decline.141) A recent study confirmed that AF was strongly associated with age, CV history, congestive HF, as well as with multiple geriatric syndromes such as vascular dementia, malnutrition, functional decline in activities of daily living, mobility impairment and chronic ulcerous disease. Furthermore, there was a tight relationship between AF and Rockwoods' frailty phenotypes. This association was maintained throughout multivariable modelling including age (odds ratio [OR], 1.06; 95% CI, 1.03–1.14; p=0.042), sex (OR, 2.30; 95% CI, 1.11–4.84; p=0.026), congestive HF (OR, 3.70; 95% CI, 1.77–7.91; p<0.001) and a CFS more than 4 (OR, 2.68; 95% CI, 1.18–6.43; p=0.021).142) It was also found that the influence of AF on frailty status, especially among the elderly population, may be associated with the higher prevalence of falls, compared with those without AF.142)
Generally, the epidemiology and treatment of AF in elderly has not yet been deeply investigated, since this part of the population is usually under-represented in the majority of randomized clinical trial.143) This statement may apply to a specific group of elderly patients with HFpEF and AF.
Regarding frailty in AF and HFpEF, according to recent study of Meng et al.,143) patient characteristics and outcomes of death from any cause or readmission showed that among older patients with HFpEF and frailty, 14.6% had AF or atrial flutter.144)
Modern advances in pharmacology have led to a gradual aging of the population as a whole and, consequently, an increase in the number of patients with AF. Because many AF and HFpEF patients have concurrent frailty and vice versa, the management of AF and frailty has become a central concern for this aging society and careful considerations should be applied in older patients living with frailty.145)
Aortic stenosis (AS)
AS, the most common valvular heart disease in developed countries, is more prevalent in older people, with the prevalence increasing with age.146) AS has demographic, clinical features and impaired response to exercise similar to those present in HFpEF patients, and is considered as a distinct phenotype of HFpEF.147,148) A study found that 38.4% of older people (mean age 84.6±4.4 years) with severe AS were also frail.149) Another study showed that among patients aged ≥70 years who had asymptomatic severe AS, the prevalence of frailty was 59.6%, and frailty was independently associated with mortality. The overall 1-year survival rate for this population was only 76%.150) As for symptomatic severe AS, a prospective study revealed that the prevalence of frailty among patients aged ≥75 years was 49.3% and that frailty in these patients was also associated with increased mortality.151) Thus, these findings call our attention to the fact that frailty is highly complicated in patients with AS, regardless of whether they are symptomatic or asymptomatic.135)
Decisions regarding intervention for frail populations can also be difficult. Older adults undergoing surgical aortic valve replacement experience increased operative complications with a 30-day mortality rate of 10%,151) therefore, transcatheter aortic valve implantation is a viable option for patients with symptomatic AS.
LIMITATIONS
The prevalence of frailty similar to HFpEF increases with age and is mostly investigated in elderly patients and most diagnostic models for measuring frailty were validated in older adults. Conventional approaches for frailty assessment designed for older populations may prove inadequate in younger adults and thus future studies of diagnostic approaches for frailty assessment across all age groups are needed. Albeit, frailty is age-independent syndrome, there is a lack of evidence demonstrating clinical or prognostic benefits of frailty assessment in the younger population. Risk profile in younger adults may be different compared to geriatric population. Frailty domains demonstrate variations depending on co-morbidities and socioeconomic status potentially requiring specific approaches to assessment in different clinical settings. Additionally, the presence of frailty in individuals with HF, particularly in those with HFpEF could become a discriminatory in the care of these patients.
Therefore, we hypothesized the presence of different profiles, highlighting that future studies of pathophysiology, clinical features, and outcomes of frailty in HFpEF by age are needed.
CONCLUSION
Traditionally considered as mainly conditions of elderly population, both HFpEF and frailty are becoming more prevalent among younger adults. This poses unique medical and social challenges due to associated poor quality of life and increased mortality. Sedentary lifestyles, unhealthy dietary habits, low socioeconomic status, and psychological stress along with chronic inflammatory conditions are significant contributors to the early onset of HFpEF and frailty. Multiple comorbidities developed in older age are characteristic of the older HFpEF-frailty profile. Superaged HFpEF-frailty profile is associated with aging-associated CV conditions, such as arterial hypertension, AF, AS. Preventive strategies are paramount in mitigating the progression of HF and frailty. Through targeted medical interventions with novel agents like iSGLT2, glucose-dependent insulinotropic polypeptide, GLP1RA, and non-steroidal mineralocorticoid receptor antagonists, exercise training, nutritional support, social awareness, and psychologic counseling attempts can be made to alleviate the impact of frailty, provide better quality of life and potentially improve prognosis in HFpEF patients.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Rakisheva A, Soloveva A.
- Supervision: Rakisheva A.
- Writing - original draft: Rakisheva A, Soloveva A, Shchendrygina A, Giverts I.
- Writing - review & editing: Rakisheva A, Soloveva A, Shchendrygina A, Giverts I.
References
- 1.Ferrucci L, Giallauria F, Guralnik JM. Epidemiology of aging. Radiol Clin North Am. 2008;46:643–652. doi: 10.1016/j.rcl.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Sanders NA, Supiano MA, Lewis EF, et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail. 2018;20:1570–1577. doi: 10.1002/ejhf.1308. [DOI] [PubMed] [Google Scholar]
- 4.Goyal P, Yum B, Navid P, et al. Frailty and post-hospitalization outcomes in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2021;148:84–93. doi: 10.1016/j.amjcard.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J, Qiao Y, Zhao M, Magnussen CG, Xi B. Global, regional, and national burden of cardiovascular diseases in youths and young adults aged 15-39 years in 204 countries/territories, 1990-2019: a systematic analysis of Global Burden of Disease Study 2019. BMC Med. 2023;21:222. doi: 10.1186/s12916-023-02925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loecker C, Schmaderer M, Zimmerman L. Frailty in young and middle-aged adults: an integrative review. J Frailty Aging. 2021;10:327–333. doi: 10.14283/jfa.2021.14. [DOI] [PubMed] [Google Scholar]
- 7.Blodgett JM, Rockwood K, Theou O. Changes in the severity and lethality of age-related health deficit accumulation in the USA between 1999 and 2018: a population-based cohort study. Lancet Healthy Longev. 2021;2:e96–104. doi: 10.1016/S2666-7568(20)30059-3. [DOI] [PubMed] [Google Scholar]
- 8.Bai G, Wang Y, Mak JK, Ericsson M, Hägg S, Jylhävä J. Is frailty different in younger adults compared to old? Prevalence, characteristics and risk factors of early-life and late-life frailty in samples from Sweden and UK. Gerontology. 2023;69:1385–1393. doi: 10.1159/000534131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal R, Yeh RW, Joynt Maddox KE, Wadhera RK. Cardiovascular risk factor prevalence, treatment, and control in US adults aged 20 to 44 years, 2009 to March 2020. JAMA. 2023;329:899–909. doi: 10.1001/jama.2023.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinclair DR, Maharani A, Chandola T, et al. Frailty among older adults and its distribution in England. J Frailty Aging. 2022;11:163–168. doi: 10.14283/jfa.2021.55. [DOI] [PubMed] [Google Scholar]
- 11.Villani ER, Tummolo AM, Palmer K, et al. Frailty and atrial fibrillation: a systematic review. Eur J Intern Med. 2018;56:33–38. doi: 10.1016/j.ejim.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Bellumkonda L, Tyrrell D, Hummel SL, Goldstein DR. Pathophysiology of heart failure and frailty: a common inflammatory origin? Aging Cell. 2017;16:444–450. doi: 10.1111/acel.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey A, Kitzman D, Reeves G. Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment, and management. JACC Heart Fail. 2019;7:1001–1011. doi: 10.1016/j.jchf.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitale C, Jankowska E, Hill L, et al. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail. 2019;21:1299–1305. doi: 10.1002/ejhf.1611. [DOI] [PubMed] [Google Scholar]
- 15.Uchmanowicz I, Nessler J, Gobbens R, et al. Coexisting frailty with heart failure. Front Physiol. 2019;10:791. doi: 10.3389/fphys.2019.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Zeng X, He F, Huang X. Inflammatory biomarkers of frailty: a review. Exp Gerontol. 2023;179:112253. doi: 10.1016/j.exger.2023.112253. [DOI] [PubMed] [Google Scholar]
- 17.Povar-Echeverría M, Auquilla-Clavijo PE, Andrès E, et al. Interleukin-6 could be a potential prognostic factor in ambulatory elderly patients with stable heart failure: results from a pilot study. J Clin Med. 2021;10:1–11. doi: 10.3390/jcm10030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butt JH, Dewan P, Jhund PS, et al. Sacubitril/valsartan and frailty in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2022;80:1130–1143. doi: 10.1016/j.jacc.2022.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Butts B, Gary RA, Dunbar SB, Butler J. The importance of NLRP3 inflammasome in heart failure. J Card Fail. 2015;21:586–593. doi: 10.1016/j.cardfail.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato R, Vatic M, da Fonseca GW, von Haehling S. Sarcopenia and frailty in heart failure: is there a biomarker signature? Curr Heart Fail Rep. 2022;19:400–411. doi: 10.1007/s11897-022-00575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau ES, Roshandelpoor A, Zarbafian S, et al. Eicosanoid and eicosanoid-related inflammatory mediators and exercise intolerance in heart failure with preserved ejection fraction. Nat Commun. 2023;14:7557. doi: 10.1038/s41467-023-43363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talha KM, Pandey A, Fudim M, Butler J, Anker SD, Khan MS. Frailty and heart failure: state-of-the-art review. J Cachexia Sarcopenia Muscle. 2023;14:1959–1972. doi: 10.1002/jcsm.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitale C, Uchmanowicz I. Frailty in patients with heart failure. Eur Heart J Suppl. 2019;21:L12–L16. doi: 10.1093/eurheartj/suz238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson M, Parrott CF, Haykowsky MJ, Brubaker PH, Ye F, Upadhya B. Skeletal muscle abnormalities in heart failure with preserved ejection fraction. Heart Fail Rev. 2023;28:157–168. doi: 10.1007/s10741-022-10219-9. [DOI] [PubMed] [Google Scholar]
- 25.Farmakis D, Thodi M, Elpidoforou M, Filippatos G. Assessing frailty in heart failure. Eur J Heart Fail. 2020;22:2134–2137. doi: 10.1002/ejhf.1905. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka S, Yamashita M, Saito H, et al. Multidomain frailty in heart failure: current status and future perspectives. Curr Heart Fail Rep. 2021;18:107–120. doi: 10.1007/s11897-021-00513-2. [DOI] [PubMed] [Google Scholar]
- 27.Spiers GF, Kunonga TP, Hall A, et al. Measuring frailty in younger populations: a rapid review of evidence. BMJ Open. 2021;11:e047051. doi: 10.1136/bmjopen-2020-047051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panchangam C, White DA, Goudar S, et al. Translation of the frailty paradigm from older adults to children with cardiac disease. Pediatr Cardiol. 2020;41:1031–1041. doi: 10.1007/s00246-020-02354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Studyvin S, Birnbaum BF, Staggs VS, et al. Development and initial validation of a frailty score for pediatric patients with congenital and acquired heart disease. Pediatr Cardiol. 2024;45:888–900. doi: 10.1007/s00246-022-03045-1. [DOI] [PubMed] [Google Scholar]
- 30.Moons P, Marelli A. Born to age: when adult congenital heart disease converges with geroscience. JACC Adv. 2022;1:100012. doi: 10.1016/j.jacadv.2022.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naessen T, Einarsson G, Henrohn D, Wikström G. Peripheral vascular ageing in pulmonary arterial hypertension as assessed by common carotid artery intima thickness and intima/media thickness ratio: an investigation using non-invasive high-resolution ultrasound. Heart Lung Circ. 2023;32:338–347. doi: 10.1016/j.hlc.2022.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Culley MK, Chan SY. Endothelial senescence: a new age in pulmonary hypertension. Circ Res. 2022;130:928–941. doi: 10.1161/CIRCRESAHA.121.319815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tromp J, Paniagua SM, Lau ES, et al. Age dependent associations of risk factors with heart failure: pooled population based cohort study. BMJ. 2021;372:n461. doi: 10.1136/bmj.n461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong CM, Hawkins NM, Petrie MC, et al. Heart failure in younger patients: the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) Eur Heart J. 2014;35:2714–2721. doi: 10.1093/eurheartj/ehu216. [DOI] [PubMed] [Google Scholar]
- 35.Tromp J, MacDonald MR, Tay WT, et al. Heart failure with preserved ejection fraction in the young. Circulation. 2018;138:2763–2773. doi: 10.1161/CIRCULATIONAHA.118.034720. [DOI] [PubMed] [Google Scholar]
- 36.Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23:771–787. doi: 10.1007/s12603-019-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leong DP, Joseph P, McMurray JJ, et al. Frailty and outcomes in heart failure patients from high-, middle-, and low-income countries. Eur Heart J. 2023;44:4435–4444. doi: 10.1093/eurheartj/ehad595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butt JH, Jhund PS, Belohlávek J, et al. Efficacy and safety of dapagliflozin according to frailty in patients with heart failure: a prespecified analysis of the DELIVER trial. Circulation. 2022;146:1210–1224. doi: 10.1161/CIRCULATIONAHA.122.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaul P, Rathwell S, Lam CSP, et al. Patient-reported frailty and functional status in heart failure with preserved ejection fraction: insights from VITALITY-HFpEF. JACC Heart Fail. 2023;11:392–403. doi: 10.1016/j.jchf.2022.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Kehler DS, Ferguson T, Stammers AN, et al. Prevalence of frailty in Canadians 18-79 years old in the Canadian Health Measures Survey. BMC Geriatr. 2017;17:28. doi: 10.1186/s12877-017-0423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183:E487–E494. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan J, Yu C, Guo Y, et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. 2020;5:e650–e660. doi: 10.1016/S2468-2667(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou Y, Xu C, Lu Q, et al. Associations of frailty with cardiovascular disease and life expectancy: a prospective cohort study. Arch Gerontol Geriatr. 2022;99:104598. doi: 10.1016/j.archger.2021.104598. [DOI] [PubMed] [Google Scholar]
- 44.Zhao L, Zierath R, John JE, et al. Subclinical risk factors for heart failure with preserved and reduced ejection fraction among black adults. JAMA Netw Open. 2022;5:e2231878. doi: 10.1001/jamanetworkopen.2022.31878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai A, Chen C, Wang J, Ou Y, Nie Z, Feng Y. Social determinants of health, cardiovascular health, and outcomes in community-dwelling adults without cardiovascular disease. JACC Asia. 2023;4:44–54. doi: 10.1016/j.jacasi.2023.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teshale AB, Htun HL, Owen A, et al. The role of social determinants of health in cardiovascular diseases: an umbrella review. J Am Heart Assoc. 2023;12:e029765. doi: 10.1161/JAHA.123.029765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White-Williams C, Rossi LP, Bittner VA, et al. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141:e841–e863. doi: 10.1161/CIR.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 48.Woolley RJ, Ceelen D, Ouwerkerk W, et al. Machine learning based on biomarker profiles identifies distinct subgroups of heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:983–991. doi: 10.1002/ejhf.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tromp J, Shen L, Jhund PS, et al. Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74:601–612. doi: 10.1016/j.jacc.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 50.Buch A, Keinan-Boker L, Kis O, et al. Severe central obesity or diabetes can replace weight loss in the detection of frailty in obese younger elderly - a preliminary study. Clin Interv Aging. 2018;13:1907–1918. doi: 10.2147/CIA.S176446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donini LM, Busetto L, Bischoff SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022;15:321–335. doi: 10.1159/000521241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zembura M, Matusik P. Sarcopenic obesity in children and adolescents: a systematic review. Front Endocrinol (Lausanne) 2022;13:914740. doi: 10.3389/fendo.2022.914740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3:e323–e332. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Packer M, Synovial LB, Inflammation M. Conceptual framework to explain the pathogenesis of heart failure with preserved ejection fraction in patients with systemic rheumatic diseases. Cardiac Failure Review. 2020;6:e10 [Google Scholar]
- 55.Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–546. doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conrad N, Misra S, Verbakel JY, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401:1878–1890. doi: 10.1016/S0140-6736(23)00457-9. [DOI] [PubMed] [Google Scholar]
- 57.Wang M, Mei K, Chao C, et al. Rheumatoid arthritis increases the risk of heart failure-current evidence from genome-wide association studies. Front Endocrinol (Lausanne) 2023;14:1154271. doi: 10.3389/fendo.2023.1154271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sierra-Galan LM, Bhatia M, Alberto-Delgado AL, et al. Cardiac magnetic resonance in rheumatology to detect cardiac involvement since early and pre-clinical stages of the autoimmune diseases: a narrative review. Front Cardiovasc Med. 2022;9:870200. doi: 10.3389/fcvm.2022.870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayashi H, Kobayashi Y, Yokoe I, Akashi Y, Takei M, Giles JT. Magnetic resonance imaging-detected myocardial inflammation and fibrosis in rheumatoid arthritis: associations with disease characteristics and N-terminal pro-brain natriuretic peptide levels. Arthritis Care Res (Hoboken) 2017;69:1304–1311. doi: 10.1002/acr.23138. [DOI] [PubMed] [Google Scholar]
- 60.Huang S, Cai T, Weber BN, et al. Association between inflammation, incident heart failure, and heart failure subtypes in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2023;75:1036–1045. doi: 10.1002/acr.24804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Günther F, Ehrenstein B, Hartung W, Boschiero D, Fleck M, Straub RH. Increased extracellular water measured by bioimpedance analysis and increased serum levels of atrial natriuretic peptide in polymyalgia rheumatica patients : signs of volume overload. Z Rheumatol. 2021;80:140–148. doi: 10.1007/s00393-020-00845-9. [DOI] [PubMed] [Google Scholar]
- 62.Straub RH, Ehrenstein B, Günther F, et al. Increased extracellular water measured by bioimpedance and by increased serum levels of atrial natriuretic peptide in RA patients-signs of volume overload. Clin Rheumatol. 2017;36:1041–1051. doi: 10.1007/s10067-016-3286-x. [DOI] [PubMed] [Google Scholar]
- 63.Mantel Ä, Holmqvist M, Andersson DC, Lund LH, Askling J. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275–1285. doi: 10.1016/j.jacc.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 64.Gao RC, Wu ZG, Wu ZZ, Hao M, Wu GC. Frailty in rheumatoid arthritis: a systematic review and meta-analysis. Joint Bone Spine. 2022;89:105343. doi: 10.1016/j.jbspin.2022.105343. [DOI] [PubMed] [Google Scholar]
- 65.Sinha A, Feinstein M. Epidemiology, pathophysiology, and prevention of heart failure in people with HIV. Prog Cardiovasc Dis. 2020;63:134–141. doi: 10.1016/j.pcad.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erqou S, Lodebo BT, Masri A, et al. Cardiac dysfunction among people living with HIV: a systematic review and meta-analysis. JACC Heart Fail. 2019;7:98–108. doi: 10.1016/j.jchf.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 67.de Leuw P, Arendt CT, Haberl AE, et al. Myocardial fibrosis and inflammation by CMR predict cardiovascular outcome in people living with HIV. JACC Cardiovasc Imaging. 2021;14:1548–1557. doi: 10.1016/j.jcmg.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 68.Piggott DA, Muzaale AD, Mehta SH, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One. 2013;8:e54910. doi: 10.1371/journal.pone.0054910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rockwood K, Blodgett JM, Theou O, et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep. 2017;7:43068. doi: 10.1038/srep43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rockwood K, Howlett SE. Fifteen years of progress in understanding frailty and health in aging. BMC Med. 2018;16:220. doi: 10.1186/s12916-018-1223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santos-Eggimann B, Cuénoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64:675–681. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Junius-Walker U, Onder G, Soleymani D, et al. The essence of frailty: a systematic review and qualitative synthesis on frailty concepts and definitions. Eur J Intern Med. 2018;56:3–10. doi: 10.1016/j.ejim.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 73.Camafort M, Park SM, Kang SM. Lifestyle modification in heart failure management: are we using evidence-based recommendations in real world practice? Int J Heart Fail. 2023;5:21–33. doi: 10.36628/ijhf.2022.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim SR, Cho DH, Kim MN, Park SM. Rationale and study design of differences in cardiopulmonary exercise capacity according to coronary microvascular dysfunction and body composition in patients with suspected heart failure with preserved ejection fraction. Int J Heart Fail. 2021;3:237–243. doi: 10.36628/ijhf.2021.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 76.Abudureyimu M, Luo X, Wang X, et al. Heart failure with preserved ejection fraction (HFpEF) in type 2 diabetes mellitus: from pathophysiology to therapeutics. J Mol Cell Biol. 2022;14:mjac028. doi: 10.1093/jmcb/mjac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanlon P, Fauré I, Corcoran N, et al. Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: a systematic review and study-level meta-analysis. Lancet Healthy Longev. 2020;1:e106–e116. doi: 10.1016/S2666-7568(20)30014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouillon K, Kivimäki M, Hamer M, et al. Diabetes risk factors, diabetes risk algorithms, and the prediction of future frailty: the Whitehall II prospective cohort study. J Am Med Dir Assoc. 2013;14:851.e1–851.e6. doi: 10.1016/j.jamda.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aguayo GA, Hulman A, Vaillant MT, et al. Prospective association among diabetes diagnosis, HbA1c, glycemia, and frailty trajectories in an elderly population. Diabetes Care. 2019;42:1903–1911. doi: 10.2337/dc19-0497. [DOI] [PubMed] [Google Scholar]
- 80.Sinclair AJ, Abdelhafiz AH, Rodríguez-Mañas L. Frailty and sarcopenia - newly emerging and high impact complications of diabetes. J Diabetes Complications. 2017;31:1465–1473. doi: 10.1016/j.jdiacomp.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69:e61–e69. doi: 10.3399/bjgp18X700241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seferović PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853–872. doi: 10.1002/ejhf.1170. [DOI] [PubMed] [Google Scholar]
- 83.Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schiattarella GG, Rodolico D, Hill JA. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc Res. 2021;117:423–434. doi: 10.1093/cvr/cvaa217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yap J, Tay WT, Teng TK, et al. Association of diabetes mellitus on cardiac remodeling, quality of life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. J Am Heart Assoc. 2019;8:e013114. doi: 10.1161/JAHA.119.013114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Writing Committee Members; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail. 2022;28:e1–e167. doi: 10.1016/j.cardfail.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 87.Marx N, Federici M, Schütt K, et al. 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44:4043–4140. doi: 10.1093/eurheartj/ehad192. [DOI] [PubMed] [Google Scholar]
- 88.McDonagh TA, Metra M, Adamo M, et al. 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627–3639. doi: 10.1093/eurheartj/ehad195. [DOI] [PubMed] [Google Scholar]
- 89.Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 2022;12:7–11. doi: 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease -a systematic review and meta-analysis. PLoS One. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Löfman I, Szummer K, Dahlström U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail. 2017;19:1606–1614. doi: 10.1002/ejhf.821. [DOI] [PubMed] [Google Scholar]
- 92.Smith DH, Thorp ML, Gurwitz JH, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013;6:333–342. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zelnick LR, Shlipak MG, Soliman EZ, et al. Prediction of incident heart failure in CKD: the CRIC study. Kidney Int Rep. 2022;7:708–719. doi: 10.1016/j.ekir.2022.01.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr. 2017;68:135–142. doi: 10.1016/j.archger.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 95.Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–71.e2. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. 2016;17:426–433. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 97.Hosseini K, Mortazavi SH, Sadeghian S, et al. Prevalence and trends of coronary artery disease risk factors and their effect on age of diagnosis in patients with established coronary artery disease: Tehran Heart Center (2005-2015) BMC Cardiovasc Disord. 2021;21:477. doi: 10.1186/s12872-021-02293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liperoti R, Vetrano DL, Palmer K, et al. Association between frailty and ischemic heart disease: a systematic review and meta-analysis. BMC Geriatr. 2021;21:357. doi: 10.1186/s12877-021-02304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Damluji AA, Huang J, Bandeen-Roche K, et al. Frailty among older adults with acute myocardial infarction and outcomes from percutaneous coronary interventions. J Am Heart Assoc. 2019;8:e013686. doi: 10.1161/JAHA.119.013686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reichart D, Rosato S, Nammas W, et al. Clinical frailty scale and outcome after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2018;54:1102–1109. doi: 10.1093/ejcts/ezy222. [DOI] [PubMed] [Google Scholar]
- 101.Udell JA, Lu D, Bagai A, et al. Preexisting frailty and outcomes in older patients with acute myocardial infarction. Am Heart J. 2022;249:34–44. doi: 10.1016/j.ahj.2022.03.007. [DOI] [PubMed] [Google Scholar]
- 102.Gharacholou SM, Slusser JP, Lennon RJ, et al. Factors associated with change in frailty scores and long-term outcomes in older adults with coronary artery disease. J Geriatr Cardiol. 2021;18:196–203. doi: 10.11909/j.issn.1671-5411.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uchmanowicz I. Oxidative stress, frailty and cardiovascular diseases: current evidence. Adv Exp Med Biol. 2020;1216:65–77. doi: 10.1007/978-3-030-33330-0_8. [DOI] [PubMed] [Google Scholar]
- 104.John JE, Claggett B, Skali H, et al. Coronary artery disease and heart failure with preserved ejection fraction: the ARIC study. J Am Heart Assoc. 2022;11:e021660. doi: 10.1161/JAHA.121.021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rush CJ, Berry C, Oldroyd KG, et al. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6:1130–1143. doi: 10.1001/jamacardio.2021.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hanlon P, Lewsey J, Quint JK, et al. Frailty in COPD: an analysis of prevalence and clinical impact using UK Biobank. BMJ Open Respir Res. 2022;9:e001314. doi: 10.1136/bmjresp-2022-001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest. 2018;154:21–40. doi: 10.1016/j.chest.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 108.Romme EA, Smeenk FW, Rutten EP, Wouters EF. Osteoporosis in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2013;7:397–410. doi: 10.1586/17476348.2013.814402. [DOI] [PubMed] [Google Scholar]
- 109.Inoue D, Watanabe R, Okazaki R. COPD and osteoporosis: links, risks, and treatment challenges. Int J Chron Obstruct Pulmon Dis. 2016;11:637–648. doi: 10.2147/COPD.S79638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bernard S, LeBlanc P, Whittom F, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:629–634. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 111.Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Solomon SD, Rizkala AR, Lefkowitz MP, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail. 2018;11:e004962. doi: 10.1161/CIRCHEARTFAILURE.118.004962. [DOI] [PubMed] [Google Scholar]
- 113.McMurray JJ, Carson PE, Komajda M, et al. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008;10:149–156. doi: 10.1016/j.ejheart.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 114.Streng KW, Nauta JF, Hillege HL, et al. Non-cardiac comorbidities in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol. 2018;271:132–139. doi: 10.1016/j.ijcard.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Mooney L, Hawkins NM, Jhund PS, et al. Impact of chronic obstructive pulmonary disease in patients with heart failure with preserved ejection fraction: insights from PARAGON-HF. J Am Heart Assoc. 2021;10:e021494. doi: 10.1161/JAHA.121.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cuthbert JJ, Kearsley JW, Kazmi S, et al. The impact of heart failure and chronic obstructive pulmonary disease on mortality in patients presenting with breathlessness. Clin Res Cardiol. 2019;108:185–193. doi: 10.1007/s00392-018-1342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Trinkmann F, Saur J, Borggrefe M, Akin I. Cardiovascular comorbidities in chronic obstructive pulmonary disease (COPD)-current considerations for clinical practice. J Clin Med. 2019;8:69. doi: 10.3390/jcm8010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Groenewegen A, Zwartkruis VW, Rienstra M, et al. Improving early diagnosis of cardiovascular disease in patients with type 2 diabetes and COPD: protocol of the RED-CVD cluster randomised diagnostic trial. BMJ Open. 2021;11:e046330. doi: 10.1136/bmjopen-2020-046330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71:988–995. doi: 10.1136/thoraxjnl-2016-208460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosa VE, Lopes AS, Accorsi TA, et al. EuroSCORE II and STS as mortality predictors in patients undergoing TAVI. Rev Assoc Med Bras. 2016;62:32–37. doi: 10.1590/1806-9282.62.01.32. [DOI] [PubMed] [Google Scholar]
- 121.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 122.Speechley M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc. 1991;39:46–52. doi: 10.1111/j.1532-5415.1991.tb05905.x. [DOI] [PubMed] [Google Scholar]
- 123.Winograd CH. Targeting strategies: an overview of criteria and outcomes. J Am Geriatr Soc. 1991;39:25S–35S. doi: 10.1111/j.1532-5415.1991.tb05930.x. [DOI] [PubMed] [Google Scholar]
- 124.Li H, Hastings MH, Rhee J, Trager LE, Roh JD, Rosenzweig A. Targeting age-related pathways in heart failure. Circ Res. 2020;126:533–551. doi: 10.1161/CIRCRESAHA.119.315889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 126.Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Camafort M, Kario K. Hypertension, heart failure, and frailty in older people: a common but unclear situation. J Clin Hypertens (Greenwich) 2020;22:1763–1768. doi: 10.1111/jch.14004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aprahamian I, Sassaki E, Dos Santos MF, et al. Hypertension and frailty in older adults. J Clin Hypertens (Greenwich) 2018;20:186–192. doi: 10.1111/jch.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang MG, Kim SW, Yoon SJ, Choi JY, Kim KI, Kim CH. Association between frailty and hypertension prevalence, treatment, and control in the elderly Korean population. Sci Rep. 2017;7:7542. doi: 10.1038/s41598-017-07449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vetrano DL, Palmer KM, Galluzzo L, et al. Hypertension and frailty: a systematic review and meta-analysis. BMJ Open. 2018;8:e024406. doi: 10.1136/bmjopen-2018-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Benetos A, Labat C, Rossignol P, et al. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents: the PARTAGE study. JAMA Intern Med. 2015;175:989–995. doi: 10.1001/jamainternmed.2014.8012. [DOI] [PubMed] [Google Scholar]
- 133.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 134.Chung SC, Sofat R, Acosta-Mena D, et al. Atrial fibrillation epidemiology, disparity and healthcare contacts: a population-wide study of 5.6 million individuals. Lancet Reg Health Eur. 2021;7:100157. doi: 10.1016/j.lanepe.2021.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 136.Uchikado Y, Ikeda Y, Ohishi M. Current understanding of the role of frailty in cardiovascular disease. Circ J. 2020;84:1903–1908. doi: 10.1253/circj.CJ-20-0594. [DOI] [PubMed] [Google Scholar]
- 137.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 138.Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath-Ordoubadi F, Neyses L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676–683. doi: 10.1093/eurjhf/hfp085. [DOI] [PubMed] [Google Scholar]
- 139.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 140.Santangeli P, Di Biase L, Bai R, et al. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 2012;9:1761–1768. doi: 10.1016/j.hrthm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 141.Crocker TF, Brown L, Clegg A, et al. Quality of life is substantially worse for community-dwelling older people living with frailty: systematic review and meta-analysis. Qual Life Res. 2019;28:2041–2056. doi: 10.1007/s11136-019-02149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.de Terwangne C, Sorgente A, Macovei S, et al. Association between atrial fibrillation, frailty, and geriatric syndromes in the late elderly in a south Belgian outpatient and inpatient setting. Am Heart J Plus. 2022;13:100106. doi: 10.1016/j.ahjo.2022.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meng C, Chai K, Li YY, Luo Y, Wang H, Yang JF. Prevalence and prognosis of frailty in older patients with stage B heart failure with preserved ejection fraction. ESC Heart Fail. 2023;10:1133–1143. doi: 10.1002/ehf2.14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Joosten LP, van Doorn S, van de Ven PM, et al. Safety of switching from a vitamin K antagonist to a non-vitamin K antagonist oral anticoagulant in frail older patients with atrial fibrillation: results of the FRAIL-AF randomized controlled trial. Circulation. 2024;149:279–289. doi: 10.1161/CIRCULATIONAHA.123.066485. [DOI] [PubMed] [Google Scholar]
- 145.Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 146.De Biase N, Mazzola M, Del Punta L, et al. Haemodynamic and metabolic phenotyping of patients with aortic stenosis and preserved ejection fraction: a specific phenotype of heart failure with preserved ejection fraction? Eur J Heart Fail. 2023;25:1947–1958. doi: 10.1002/ejhf.3018. [DOI] [PubMed] [Google Scholar]
- 147.Anker SD, Usman MS, Anker MS, et al. Patient phenotype profiling in heart failure with preserved ejection fraction to guide therapeutic decision making. A scientific statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, and the European Society of Hypertension. Eur J Heart Fail. 2023;25:936–955. doi: 10.1002/ejhf.2894. [DOI] [PubMed] [Google Scholar]
- 148.Fukui S, Kawakami M, Otaka Y, et al. Physical frailty in older people with severe aortic stenosis. Aging Clin Exp Res. 2016;28:1081–1087. doi: 10.1007/s40520-015-0507-0. [DOI] [PubMed] [Google Scholar]
- 149.Ramos M, Quezada M, Ayala R, et al. Asymptomatic aortic stenosis in a geriatric population. The role of frailty and comorbidity in mortality. Rev Esp Cardiol (Engl Ed) 2021;74:167–174. doi: 10.1016/j.rec.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 150.Rodríguez-Pascual C, Paredes-Galán E, Ferrero-Martínez AI, et al. The frailty syndrome and mortality among very old patients with symptomatic severe aortic stenosis under different treatments. Int J Cardiol. 2016;224:125–131. doi: 10.1016/j.ijcard.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 151.Vasques F, Lucenteforte E, Paone R, Mugelli A, Biancari F. Outcome of patients aged ≥80 years undergoing combined aortic valve replacement and coronary artery bypass grafting: a systematic review and meta-analysis of 40 studies. Am Heart J. 2012;164:410–418.e1. doi: 10.1016/j.ahj.2012.06.019. [DOI] [PubMed] [Google Scholar]