The English-Speaking Caribbean comprises of 8 million persons predominantly of African descent, unified by a shared history of European (mainly British) colonization spanning centuries. Although most individual countries are now designated as high-income and middle-income sociodemographic index, lack of human and infrastructural resources, geopolitical stasis, and emerging climate stressors contribute to inequities in health care. Data on the epidemiology, causes, and outcomes of kidney disease in the region are sparse, limited largely to single-center studies and a voluntary registry effort undermined in its reach and sustainability by lack of centralized support (Table 1).
Table 1.
Summary of ascribed causes of end-stage kidney diseasea by country
| Country | Hypertension (%) | Diabetes mellitus (%) |
|---|---|---|
| Bahamas | 26 | 28 |
| Barbados | 56 | 27 |
| British Virgin Islands | 70 | 26 |
| Cayman Islands | 42 | 29 |
| Jamaica | 50 | 21 |
| Trinidad and Tobago | 29 | 25 |
Unpublished Caribbean Renal Registry data from AS.
Absent of any systematic data, we integrate historical context with available population ancestry data to posit that the recent advances in APOL1 high-risk genotype and emerging therapeutics could be highly applicable to the population living in the region. We also highlight environmental exposures enriched in this region, investigations into which could offer new insights into the development and progression of both APOL1 and non-APOL1 related kidney disease.
Historical Marginalization Ties to Present Inequities
Although there were indigenous populations present before the start of the transatlantic slave trade in the English-speaking Caribbean, most of the population from several islands, including Jamaica now has West African roots. The Caribbean received about one-half of all Africans brought to the Americas during the transatlantic slave trade1; almost 4-fold as many enslaved people disembarked in the Caribbean compared to the rest of North America (Figure 1). About 2.6 million enslaved African persons were brought to the British Caribbean (about 23% of the transatlantic slave trade).1 Since the latter part of the seventeenth century, the Caribbean has been mainly populated by people of African descent (Supplementary Table S1).1,S1–S3 A majority of the indigenous inhabitants of the Caribbean suffered from infections, violence, and slavery during the colonial period, and their numbers have since dwindled to a minority across the Caribbean.2 There is some heterogeneity among the islands: parts of the Caribbean (e.g., Trinidad and Tobago) received relatively fewer numbers of enslaved persons from Africa, and host a larger proportion of persons trafficked from the Indian subcontinent.
Figure 1.
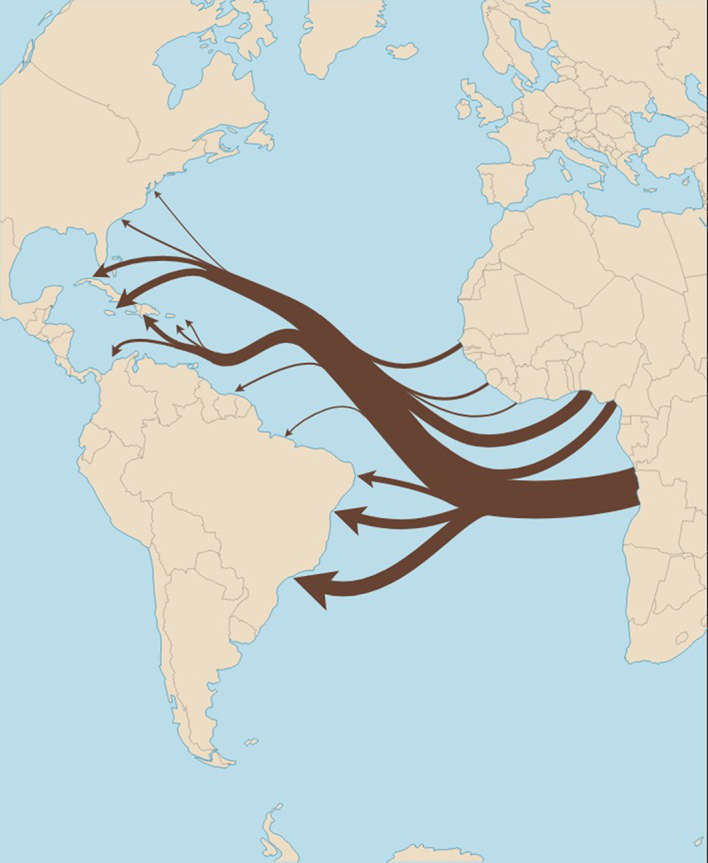
Direction of the transatlantic slave trade from West Africa to the Caribbean. Map Volume and direction transatlantic slave trade. Although the Caribbean had an existing native population, large numbers of enslaved persons from West Africa were disembarked in the early part of the 18th century; larger than any other country in North America. In total, about 2.6 million enslaved persons were brought to the British Caribbean (about 23% of the transatlantic slave trade).
The emancipation of enslaved persons in 1838 within the British Colonial Empire counterintuitively led to disenfranchisement of the Black majority, increasing poverty, and social injustices. Despite no longer being under colonial rule, the emancipated Caribbean still operated under the old imperial system with a small minority of White Caribbeans continuing to retain control. Black Caribbeans from different social strata were forced to build new connections in an attempt to form a new collective consciousness. In many ways, the struggle to create independent states was far ahead of the forging of national identities.2 High rates of infectious diseases and infant mortality occurred. Health care policies generated were from the purview of the anti-Black biases of the then leadership; it was therefore “ad hoc” or piece-meal while not systematically addressing underlying the social and economic inequities. During the postcolonial era, newly formed governments were now faced with generating policy responses after centuries of neglect, in addition to dealing with an uncertain economic and political future in the absence of British rule. Although health care policy gains have been made in the decades following independence, particularly in the control of infectious disease and maternal health and nutrition, a legacy of reactionary policy making persists. Stagnation of economic growth, social, and political unrest in the years after independence, led to lack of funding and investment in health care systems. Further compounding these issues are loss of skilled medical personnel due to migration, continued debt to international bodies, and small island environmental vulnerabilities.
These factors have influence on noncommunicable disease burden. In the case of kidney disease, both lack of specific policy and health care resources may underlie the inequities in kidney care, which are evident to nephrologists practicing in the region, many of whom have trained in the USA, UK, or Canada. For example, although the prevalence of the major risk factor for kidney disease (type 2 diabetes) is similar in the Caribbean, USA, and Canada, the prevalence of treated kidney failure is lower in the Caribbean, implying lack of access.3 These inequities remain unquantified; and therefore, could be argued are easier to neglect.
Unlike in many high-income and even in some comparably middle-income countries (e.g., Thailand), where national surveillance studies integrate kidney function evaluations and/or maintain a separate national registry mandated to capture data on all patients receiving renal replacement therapy, English-speaking Caribbean lacks any systematic data collection efforts related to kidney disease. Previous efforts for a Caribbean renal registry have faced challenges due to lack of public funding to sustain these efforts; the registry last reported data in 2011 (Table 1). The voluntary and decentralized nature of this effort has undermined its sustainability, especially during the pressures of the COVID-19 pandemic.
Sparse Epidemiological Data May Preclude Participation in Therapeutic Advances
The lack of investment in kidney health epidemiological surveillance may have important implications for kidney health in the Caribbean. The historical colonization of the region implies that the Caribbean region will host among the highest prevalence of people with APOL1 high-risk genotype in the Western hemisphere. However, sparse population-based data exist to estimate the prevalence of APOL1 high-risk alleles and associated kidney disease in the Caribbean. A single genomic investigation drawing on data from the Population Architecture using Genomic Study and the Consortia on Asthma on African-Ancestry populations of the Americas assessed high-risk alleles in 111 global populations.4 This study consisted of 58,297 individuals in total (the numbers sampled from the Caribbean are unknown), and estimated prevalence of APOL1 high-risk alleles of 10% to 22% in populations from Jamaica and Barbados.4 From a small study which evaluated APOL1 high-risk alleles in 33 potential kidney donors from Antigua and Barbuda versus Nigeria, the prevalence of homozygous G1, G2, or compound homozygous genotype was 42% in those from Antigua and Barbuda, and 43% in Nigeria. This likely indicates a high but severely underrecognized contribution of APOL1 high-risk alleles to kidney disease.
Currently, there are 3 active (phase I–II) clinical trials investigating medications to treat APOL1 nephropathy. A phase 1 study is examining the pharmacokinetics of drug AZD2373–designed to reduce APOL1 production-among healthy, male participants of West African ancestry (NCT05351047). A second phase 2 study aims to determine the safety and efficacy of the repurposing the drug, baricitinib (a JAK1 and JAK2 inhibitor) for reducing albuminuria in individuals with APOL1-associated focal segmental glomerulosclerosis and chronic kidney disease (CKD) due to hypertension (NCT05237388). Recently, Egbuna et al.5 detailed results of a phase 2a clinical trial of a small molecule drug, Inaxaplin, for biopsy-proven focal segmental glomerulosclerosis in patients with the high-risk genotype. The analysis found a 44% reduction in proteinuria after 13 weeks of treatment.
These promising treatments are potentially highly relevant to the residents of the Caribbean region. For example, in Jamaica, 79% of the 2.82 million population is aged >15 years and 78% in turn are of African descent.6 From estimates in the USA and West Africa-13% of African Americans have 2 APOL1 high-risk alleles7,S1 and in certain ethnic groups in Nigeria (Yoruba ethnic group) the population prevalence exceeds 25% we can estimate that 225,898 to 434,421 persons aged >15 years may carry 2 high-risk APOL1 alleles in Jamaica. This type of estimation provides support for, but does not replace, the critical need to assess the prevalence of APOL1 high-risk alleles in large population-based studies in the Caribbean; some priority approaches are outlined in Supplementary Table 2. Also critical is to study the association of these alleles with incident kidney disease, because this may differ from the risk experienced by African Americans. A large proportion of end-stage kidney disease in the English-speaking Caribbean is attributed to hypertension as of the registry effort (Table 1).
Unexplored Environmental Exposures
The Caribbean hosts a vulnerable population with increasing rates of heat stress, air pollution, and viral exposures, all of which are potentially de novo causes of kidney disease but may also mediate APOL1 kidney disease.8,S4 These environmental pollutants could play a role as second hits in APOL1 nephropathy because not every person with the APOL1 high-risk genotype develops kidney disease. Of the APOL1-associated viral risk factors, HIV appears to be the most prevalent, with the Caribbean being second to Africa with an adult prevalence rate between 1.9% to 3.1%, and an estimated 29,000 (1.6%) of persons living with HIV in Jamaica.9 Mosquito-borne viruses such as dengue and zika viruses have been associated with acute kidney disease and glomerulonephritis, with case reports of collapsing focal segmental glomerulosclerosis.S5 Heavy rainfall in various Caribbean islands have been linked to increased rates of Dengue fever.S6 The kidney health effects of these viruses endemic to the Caribbean are understudied, and may be a direction for further study (Supplementary Table 2).
A range of heavy metals inclusive of cadmium, lead, mercury are common water and soil contaminants in the Caribbean. These elements have been linked with incident CKD and kidney failure in worldwide observational studies.S7,S8 Jamaica has a high concentration of cadmium in the island’s soil reserves, with concentrations as high as 409 mg/kg, and mean concentration of 20 mg/kg of soil, approximately 100 times the world average (0.2 mg/kg). Furthermore, high levels of cadmium were found in the livers and kidneys of Jamaican adults in an autopsy series and in locally grown fruits and vegetables.S9 The role of cadmium in CKD in the Caribbean is unclear. In a cross-sectional analysis using data from 5426 participants from the National Health and Nutrition Examination Surveys, elevated serum cadmium levels (>1 mcg/g) were associated with an ∼50% increased risk of reduced estimated glomerular filtration rate (<60 ml/min per 1.73 m2) and ∼40% increased risk of albuminuria.S7 In a small cross-sectional study of 96 Jamaican residents attending outpatient medical clinics with and without CKD, high measured levels of the trace elements strontium, lead, and arsenic were negatively correlated with estimated glomerular filtration rate; however, there was no association with cadmium and estimated glomerular filtration rate.S8 The potential role of distinctly enriched elements and their potential causative role in CKD provides another opportunity for novel investigations in the Caribbean.
Conclusion
The story of APOL1 nephropathy evinces the benefits of careful elucidation of epidemiology and cause of kidney disease in diverse populations. Promising pathways to targeted treatments now exist. Although the current data on kidney disease prevalence, cause, and outcomes are sparse in English-speaking Caribbean, kidney disease is commonly attributed to hypertension in this region. Based on historical migration patterns, persons self-identifying as Black living in the English-Speaking Caribbean are likely to have significant West African biological ancestry.1 The distinctive environmental and infectious exposures of this island-living population can potentially offer new insights into the expression of APOL1 disease specifically and kidney disease broadly. Studying these exposures and genetic frequency of APOL1 high-risk alleles in the Caribbean can build rationale for ethical inclusion of persons living in the Caribbean, a region with potentially the highest absolute numbers of affected persons in North America, in treatment programs aimed at APOL1 nephropathy.
Acknowledgments
JM is funded by T32 Grant T32 HD 105176-1 A1. SA is funded by NIDDK R01DK127138, Doris Duke Charitable Fund, and Stanford University’s Center for Innovation in Global Health.
Footnotes
Supplem entary References.
Table S1. Link between past and present: proportion of enslaved persons, current Black population, and estimated West African ancestry.
Table S2. Priority areas for understanding the contribution of APOL1 polymorphisms to kidney disease in the Caribbean.
Supplementary Material
Supplementary References. Table S1. Link between past and present: proportion of enslaved persons, current Black population, and estimated West African ancestry. Table S2. Priority areas for understanding the contribution of APOL1 polymorphisms to kidney disease in the Caribbean.
References
- 1.Laurence K.O., Cuesta Jorge Ibarra. UNESCO; 2011. General history of the Caribbean, v. IV: The Long nineteenth century: nineteenth century transformations. [Google Scholar]
- 2.Knight F.W. Oxford University Press; New York: 2012. The Caribbean: the Genesis of a Fragmented Nationalism. [Google Scholar]
- 3.Bello A.K., McIsaac M., Okpechi I.G., et al. International society of nephrology global kidney health atlas: structures, organization, and services for the management of kidney failure in North America and the Caribbean. Kidney Int Suppl (2011) 2021;(11):e66–e76. doi: 10.1016/j.kisu.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadkarni G.N., Gignoux C.R., Sorokin E.P., et al. Worldwide frequencies of APOL1 renal risk variants. N Engl J Med. 2018;379:2571–2572. doi: 10.1056/NEJMc1800748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egbuna O., Zimmerman B., Manos G., et al. Inaxaplin for proteinuric kidney disease in persons with two APOL1 variants. N Engl J Med. 2023;388:969–979. doi: 10.1056/NEJMoa2202396. [DOI] [PubMed] [Google Scholar]
- 6.World Bank Group International development, poverty, & sustainability: World Bank. https://www.worldbank.org/en/home
- 7.Friedman D.J., Pollak M.R. APOL1 nephropathy: from genetics to clinical applications. Clin J Am Soc Nephrol. 2021;16:294–303. doi: 10.2215/CJN.15161219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kshirsagar A.V., Zeitler E.M., Weaver A., Franceschini N., Engel L.S. Environmental exposures and kidney disease. Kidney360. 2022;3:2174–2182. doi: 10.34067/KID.0007962021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Health and Wellness Jamaica. HIV epidemiological Profile 2015, facts & figures. Accessed June 19, 2023. HIV Epidemiological Profile 2015, Facts & Figures – Ministry of Health & Wellness, Jamaica (moh.gov.jm)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary References. Table S1. Link between past and present: proportion of enslaved persons, current Black population, and estimated West African ancestry. Table S2. Priority areas for understanding the contribution of APOL1 polymorphisms to kidney disease in the Caribbean.


