Abstract
Introduction
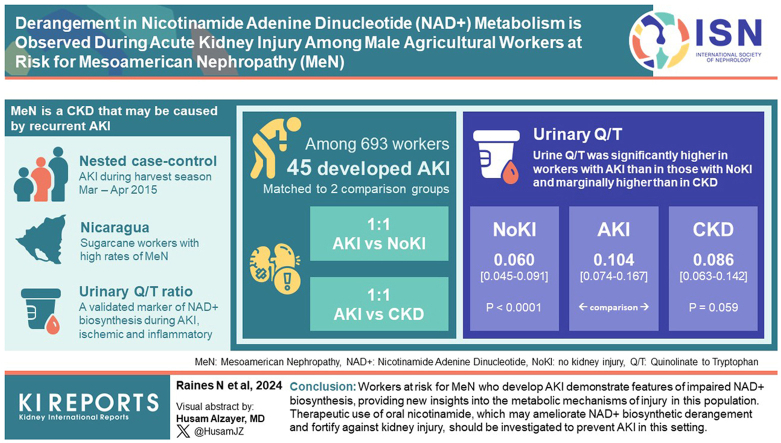
Mesoamerican nephropathy (MeN) is a chronic kidney disease (CKD) which may be caused by recurrent acute kidney injury (AKI). We investigated urinary quinolinate-to-tryptophan ratio (Q/T), a validated marker of nicotinamide adenine dinucleotide (NAD+) biosynthesis that is elevated during ischemic and inflammatory AKI, in a sugarcane worker population in Nicaragua with high rates of MeN.
Methods
Among 693 male sugarcane workers studied, we identified 45 who developed AKI during the harvest season. We matched them 1:1 based on age and job category with 2 comparison groups: (i) “no kidney injury,” active sugarcane workers with serum creatinine (sCr) <1.1 mg/dl; and (ii) “CKD,” individuals no longer working in sugarcane due to their CKD, who had additional 1:1 matching for sCr. We measured urine metabolites using liquid chromatography coupled tandem mass spectrometry (LC-MS/MS) and compared Q/T and other metabolic features between the AKI and comparison groups.
Results
Urine Q/T was significantly higher in workers with AKI than in those with no kidney injury (median interquartile Range [IQR]: 0.104 [0.074–0.167] vs. 0.060 [0.045–0.091], P < 0.0001) and marginally higher than in workers with CKD (0.086 [0.063–0.142], P = 0.059). Urine levels of the NAD+ precursor nicotinamide were lower in the AKI group than in comparison groups.
Conclusion
Workers at risk for MeN who develop AKI demonstrate features of impaired NAD+ biosynthesis, thereby providing new insights into the metabolic mechanisms of injury in this population. Therapeutic use of oral nicotinamide, which may ameliorate NAD+ biosynthetic derangement and fortify against kidney injury, should be investigated to prevent AKI in this setting.
Keywords: acute kidney injury (AKI), chronic kidney disease of unknown etiology (CKDu), chronic kidney disease of nontraditional cause (CKDnt), Mesoamerican nephropathy, metabolomics, nicotinamide adenine dinucleotide (NAD+)
Graphical abstract
MeN is a nondiabetic nonhypertensive syndrome of CKD characterized by high familial concordance, minimal proteinuria, hyperuricemia, and nonspecific biopsy findings demonstrating tubular injury with ischemic features and mononuclear cellular inflammation.1, 2, 3 MeN is a subset of a broader category of CKD epidemics in other regions around the world variably termed CKD of uncertain cause or CKD of nontraditional cause which share many epidemiologic and histopathologic features. It is a devastating epidemic in Mesoamerica, with studies showing a prevalence of disease as high as 40% in certain high risk areas.4,5 Those at highest risk for MeN are typically men, aged 20 to 50 years, who do strenuous agricultural work or other outdoor labor along the Pacific coast of Mesoamerica; sugarcane workers appear to be at particular risk for disease.5, 6, 7, 8, 9 Affected people are frequently vulnerable: low income and from areas with limited health care infrastructure.10
Research to date suggests that recurrent heat stress contributes to the development and progression of MeN.1,5,11, 12, 13 Heat stress may not be the only important risk factor, and may potentiate other harmful exposures such as agrichemicals, heavy metals, or environmental nephrotoxins.4 The natural history of the disease may involve repeated AKI episodes leading to eventual development of chronic damage.14, 15, 16, 17 Studies have demonstrated that a dose response relationship may exist between heat stress, AKI, and progressive chronic disease development, with interventions to reduce heat stress functioning to protect against both AKI and subsequent MeN development.11,18
Our current understanding of injury mechanisms in MeN development derives from biopsy data, animal models, biochemical analysis, and limited metabolomic data. Biopsies show features consistent with ischemic injury and interstitial inflammation, which may represent either a primary or reactive process.2,14 Animal models of heat stress induced kidney injury show similar evidence of ischemic changes and kidney interstitial inflammation.19,20 Increased markers of systemic inflammation have been linked with kidney injury in a population at high risk for MeN.21,22 Urine metabolomics and serum proteomics of individuals engaged in high risk sugarcane harvest and seed cutting have identified features consistent with inflammation and altered energy metabolism, possibly in the context of increased gut permeability.23,24
Ischemic and/or inflammatory injury to the kidney is characterized by deranged biosynthesis of NAD+, which may play an important role in the pathophysiology of injury.25, 26, 27 NAD+ biosynthetic derangement is also present in chemotoxic and crystalline AKI, and in pathophysiologic processes related to CKD development.28, 29, 30 NAD+ is an essential coenzyme linking glycolysis and the tricarboxylic acid cycle with the mitochondrial electron transport chain. It participates in cellular repair and immunomodulation. NAD+ can be rate limiting for normal oxidative metabolism, and deficiency may impair organ function.27,31
Biosynthesis of NAD+ occurs along 3 primary pathways: the de novo, salvage, and Preiss-Handler pathways. Flux along the de novo pathway of NAD+ biosynthesis may be particularly susceptible to stressors that trigger AKI.26,32 Previous work has shown that the ratio of quinolinate, a de novo pathway intermediary, to its precursor tryptophan may serve as a useful biomarker of NAD+ biosynthetic derangement. Because the only established metabolic fate for quinolinate is subsequent conversion to NAD+, urinary spillage of quinolinate constitutes a 1:1 molar loss of NAD+ forming equivalents from the body. Under conditions of kidney stress, the ratio of Q/T increases, initially as tryptophan is converted to quinolinate to accelerate NAD+ generation through the de novo pathway, and then subsequently as quinolinate phosphoribosyltransferase activity is impaired due to ischemic, inflammatory, or toxic signals.25,26,33 Altered urinary Q/T has been validated as a reliable biomarker of NAD+ biosynthetic derangement and AKI in multiple contexts, including cardiac bypass surgery, coronavirus-19 infection, and vascular surgery.26,34, 35, 36
In this study, we used targeted metabolomics to evaluate the presence of NAD+ biosynthetic derangement among Nicaraguan sugarcane workers who developed AKI during the harvest season. We hypothesized as follows: that (i) urine Q/T would be higher in workers who developed AKI than in those who maintained intact kidney function, reflecting NAD+ biosynthetic derangement characteristic of ischemic and/or inflammatory kidney tubular injury; (ii) levels of other urine metabolites associated with altered NAD+ metabolism would differ, in a manner complementary to Q/T findings, between individuals developing or not developing AKI; and (iii) comparison between workers with AKI and former workers with established CKD matched on age and sCr would demonstrate that differential metabolic features associated with AKI were not solely a reflection of differences in kidney filtration.
Methods
Ethics
This study was approved by the institutional review boards at the Nicaraguan Ministry of Health and Boston University Medical Center (approval numbers NIC-MINSA/Centro Nacional de Diagnóstico y Referencia CIRE-17/02/14-048.ver2 and H-32414, respectively). The work described here was carried out in accordance with the Declaration of Helsinki. Individual informed consent was obtained from all participants.
Study Population
This is a matched case control study nested within an ongoing multiyear parent case control study designed to investigate genetic factors driving the development of MeN. The parent case control study recruited men working in sugarcane agriculture in Nicaragua who either had already developed (“cases,” n = 366) or had not developed (“controls,” n = 327) CKD consistent with MeN as of October 2014, the beginning of the 2014 to 2015 sugarcane harvest season. Briefly, these were all men working in sugarcane in a high-MeN-prevalence region of Nicaragua who did not have diabetes, essential hypertension, or other alternative potential causes of CKD. Cases had sCr values ≥ 1.3 mg/dl on repeated measures at least 6 months apart, whereas controls had sCr values < 1.3 mg/dl. Case and control status was further adjudicated by the parent study principal investigator based on a review of the entire medical record. Additional details of the parent study from which this study was derived have been described previously in the literature.17,23
Among the participants recruited as controls for the original study who were visited between March and April 2015 for laboratory value measurement, 45 individuals had developed a rise in sCr by >0.3 mg/dl from their October 2014 value to a value ≥ 1.3 mg/dl, which is consistent with a change in sCr meeting Kidney Disease Improving Global Outcomes AKI criteria.37 For this study, all 45 individuals were designated as the “AKI” group. We next selected a primary comparison group for this study, the “no kidney injury (NoKI)” group, from among the controls in the original study with sCr ≤ 1.1 and no rise in sCr sufficient to meet the criteria for the AKI group. Individuals comprising the NoKI group were matched 1:1 with the AKI group on age and most recent sugarcane industry job function during the 2014 to 2015 harvest season, with matching for number of years worked in sugarcane agriculture used as a tiebreaker when necessary. We then selected a secondary comparison group for this study, the “CKD” group, from among the cases in the parent study; these individuals are former sugarcane workers who were no longer working at the time of data collection because of their CKD status. Individuals comprising the CKD group were matched 1:1 with the AKI group on age and sCr, with matching for the most recent type of job worked within sugarcane used as a tiebreaker when necessary. This group was chosen specifically to assess reverse causality by providing a comparator with similar kidney function but a different disease state, in order to determine whether the association between metabolite levels and kidney disease state was driven by altered clearance of metabolites or by actual metabolic differences. Individuals with diabetes or other known causes of CKD were excluded. A flow diagram depicting study participant selection is shown in Supplementary Figure S1.
Data and Specimen Collection
SCr values from October 2014 were obtained from the sugarcane company which employed and routinely screened participating workers at the beginning of the harvest season. These values were measured using a modified alkaline picrate method (traceable to isotope-dilution mass spectrometry reference standards; Dialab, Austria). Subsequent sCr values used to determine “AKI group” status were measured from blood specimens collected from participants by our team between March and April 2015. sCr and urine creatinine analyses for these specimens were conducted at the Centro Nacional de Diagnóstico y Referencia, the central laboratory of the Nicaraguan Ministry of Health, using a rate blanked compensated kinetic alkaline picrate method (traceable to isotope-dilution mass spectrometry reference standards). Serum sodium and potassium were measured using the Roche AVL 9180 electrolyte analyzer. Additional laboratory testing, including other serum electrolytes and uric acid, was done using the Cobas Integra 400 Plus (Roche Diagnostics International Ltd). Demographic, medical, and occupational history data were obtained from questionnaires. Diabetes was defined by self-report or based on a nonfasting serum glucose level > 200 mg/dl during baseline laboratory assessment. Portions of blood and urine specimens not analyzed immediately following collection were designated for further biochemical analysis and were stored at −80 oC at Beth Israel Deaconess Medical Center until analysis for this study.
Targeted Mass Spectrometry
We analyzed the metabolite content of urine specimens using LC-MS/MS at the mass spectrometry core at Beth Israel Deaconess Medical Center. To prepare each urine specimen for analysis,38 we added 800 μl methanol to 200 μl urine and incubated the resulting solution for 6 to 8 hours at −80 oC. Samples were then dried using a SVC100H SpeedVac Concentrator (Savant) and then resuspended in 20 μl HPLC grade water. Five to 7 μl were injected and analyzed using a hybrid 6500 QTRAP triple quadrupole mass spectrometer (AB/SCIEX) coupled to a Prominence UFLC HPLC system (Shimadzu) via selected reaction monitoring of a total of 300 endogenous water soluble metabolites for steady state analyses of samples. Some metabolites were targeted in both positive and negative ion mode for a total of 311 selected reaction monitoring transitions using positive/negative ion polarity switching. ESI voltage was +4950 V in positive ion mode and −4500 V in negative ion mode. The dwell time was 3 ms per selected reaction monitoring transition and the total cycle time was 1.55 seconds. Approximately 9 to 12 data points were acquired per detected metabolite. Samples were delivered to the mass spectrometer via hydrophilic interaction chromatography using a 4.6 mm i.d × 10 cm Amide XBridge column (Waters) at 400 μl/min. Gradients were run starting from 85% buffer B (HPLC grade acetonitrile) to 42% B from 0 to 5 minutes, 42% B to 0% B from 5 to 16 minutes, 0% B was held from 16 to 24 minutes, 0% B to 85% B from 24 to 25 minutes, and 85% B was held for 7 minutes to re-equilibrate the column. Buffer A was composed of 20 mm ammonium hydroxide/20 mm ammonium acetate (pH = 9.0) in 95:5 water:acetonitrile. Peak areas from the total ion current for each metabolite selected reaction monitoring transition were integrated using MultiQuant v3.0.2 software (AB/SCIEX). All paired specimens were analyzed in the same batch. Levels of each metabolite obtained were LC-MS/MS peak area under curve values rather than absolute concentrations.
Data Processing and Statistical Analysis
Values for each metabolite were divided by the urine creatinine value measured at Centro Nacional de Diagnóstico y Referencia to obtain a urine creatinine corrected level. Two participants were missing Centro Nacional de Diagnóstico y Referencia measured urine creatinine values, so we imputed their urine creatinine from their LC-MS/MS-based urine creatinine value using the equation: log (measured creatinine in mg/dl) = 8.043 × 10−10 × (LC-MS/MS creatinine in area under curve) + 0.1885, which in turn was derived from the relationship between LC-MS/MS creatinine and measured creatinine in the rest of the data set. Missing values for individual metabolites were assumed to be below the threshold of detection using our LC-MS/MS protocol and imputed as one-fifth the minimum value, after creatinine correction, for that feature across all groups.39,40 The number of individuals in each group with missing values for each metabolite studied is presented in Supplementary Table S1; no individuals were missing quinolinate or tryptophan.
Primary and secondary outcome variables, along with specific hypotheses for each comparison, are shown in Table 1. For primary and secondary analyses, metabolite levels were compared using the nonparametric Wilcoxon Signed Rank Test due to nonnormal data distributions; and 95% confidence intervals (CIs) calculated reflect the median difference between a sample from the AKI group and a sample from the comparison (either NoKI or CKD) group. In order to identify broad patterns of change in NAD+ biosynthetic pathways, we subsequently compared levels of all measured metabolites involved in the salvage, de novo, and Preiss-Handler pathways using the Wilcoxon Signed Rank Test, with statistical significance threshold set at P < 0.05. For Table 2 analyses, we used a paired t test to compare continuous variables between groups, and McNemar’s test to compare job discordance between pairs with job categorized as either sugarcane harvest cutter or any other job. Analyses were conducted using R v. 4.3.0 (the R Foundation for Statistical Computing, version 4.3.0, https://www.r-project.org/).
Table 1.
Primary and secondary hypotheses
| Outcome | AKI vs. NoKI | AKI vs. CKD |
|---|---|---|
| Primary | ||
| Urine quinolinate-to-tryptophan ratio | ↑ | |
| Secondary | ||
| Urine quinolinate-to-tryptophan ratio | ↑ | |
| Urine nicotinamide | ↓ | ↓ |
| Urine methylnicotinamide | ↓ | ↓ |
| Urine kynurenic acid-to-tryptophan ratio | ↑ | ↑ |
Arrows indicate when values are hypothesized to be higher (↑) or lower (↓) in the acute kidney injury (AKI) group than in either the no kidney injury (NoKI) or chronic kidney disease (CKD) comparison group.
Table 2.
Demographic and baseline laboratory values of comparison groups
| Variable | AKI (n = 45) | NoKI (n = 45) | AKI – NoKI | P | CKD (n = 45) | AKI – CKD | P |
|---|---|---|---|---|---|---|---|
| Age in yr, mean (SD) | 37.0 (7.3) | 37.0 (7.3) | 0.0 (0.5) | 0.74 | 36.1 (5.7) | 0.9 (2.8) | 0.044 |
| Most recent job within sugarcane, n (%) | |||||||
| Harvest cutter | 37 (82) | 34 (76) | 0.37 | 11 (24) | < 0.0001 | ||
| Seed cutter | 5 (11) | 6 (13) | 6 (13) | ||||
| Weeder | 2 (4) | 0 (0) | 8 (18) | ||||
| Pesticide applicator | 0 (0) | 1 (2) | 1 (2) | ||||
| Irrigator | 1 (2) | 4 (9) | 11 (24) | ||||
| Other | 0 (0) | 0 (0) | 8 (18) | ||||
| Years worked in sugar cane, mean (SD) | 12.8 (6.7) | 13.2 (5.1) | −0.4 (5.5) | 0.59 | 12.0 (4.9) | 0.8 (5.9) | 0.37 |
| Serum creatinine in mg/dl, mean (SD) | 1.68 (0.38) | 0.87 (0.13) | 0.81 (0.41) | < 0.0001 | 1.68 (0.38) | 0.00 (0.022) | 0.95 |
| Serum urea nitrogen in mg/dl, mean (SD) | 35.6 (7.1) | 22.1 (6.2) | 13.5 (9.4) | < 0.0001 | 36.0 (9.5) | −0.3 (1.3) | 0.81 |
| Serum uric acid in mg/dl, mean (SD) | 7.84 (1.41) | 5.68 (1.11) | 2.15 (1.70) | < 0.0001 | 8.65 (1.78) | −0.81 (2.26) | 0.02 |
| Serum sodium in mEq/l, mean (SD) | 134.7 (3.7) | 135.2 (2.8) | −0.5 (4.1) | 0.43 | 135.3 (2.5) | −0.7 (4.4) | 0.30 |
| Serum potassium in mEq/l, mean (SD) | 4.3 (0.5) | 4.1 (0.5) | 0.2 (0.7) | 0.035 | 4.6 (1.0) | −0.3 (1.3) | 0.10 |
| Serum chloride in mEq/l, mean (SD) | 99.9 (3.7) | 100.5 (2.7) | −0.6 (4.6) | 0.39 | 103.3 (2.8) | −3.4 (4.4) | < 0.0001 |
| Serum calcium in mg/dl, mean (SD) | 9.15 (0.46) | 9.17 (0.47) | −0.03 (0.52) | 0.72 | 9.46 (0.53) | −0.31 (0.74) | 0.007 |
| Serum magnesium in mg/dl, mean (SD) | 1.88 (0.24) | 1.96 (0.20) | −0.08 (0.33) | 0.09 | 1.80 (0.27) | 0.08 (0.38) | 0.18 |
| Serum phosphate in mg/dl, mean (SD) | 3.35 (0.55) | 3.29 (0.46) | 0.06 (0.75) | 0.59 | 3.57 (0.59) | −0.21 (0.90) | 0.12 |
| Urine protein-to-creatinine ratio in g/g, mean (SD) | 0.087 (0.071) | 0.061 (0.048) | 0.026 (0.074) | 0.02 | 0.30 (0.55) | −0.21 (0.56) | 0.02 |
Data shown are values for each of the acute kidney injury (AKI), no kidney injury (NoKI), and chronic kidney disease (CKD) groups. The “AKI – NoKI and “AKI – CKD” columns represent the mean difference in values for continuous variables or the number (and percentage) of discordant pairs for categorical variables between AKI and NoKI or CKD groups, respectively. P values describe the comparison between the AKI and each of the NoKI and CKD groups.
Results
Demographic and basic laboratory features of participants in each group are shown in Table 2. The difference in age between AKI and CKD groups was statistically significant, but with a magnitude of less than 1 year. Sugarcane harvest cutting was the most recent occupation for a higher proportion of participants in the AKI group than the CKD group. The number of years worked in sugarcane were similar across groups. sCr and urea nitrogen levels were higher in the AKI group than in the NoKI group and were similar to levels in the CKD group. Serum uric acid levels and urine protein-to-creatinine ratio were higher in the AKI group than in the NoKI group, but lower than in the CKD group.
Primary Outcome: Urine Q/T Between AKI and NoKI Groups
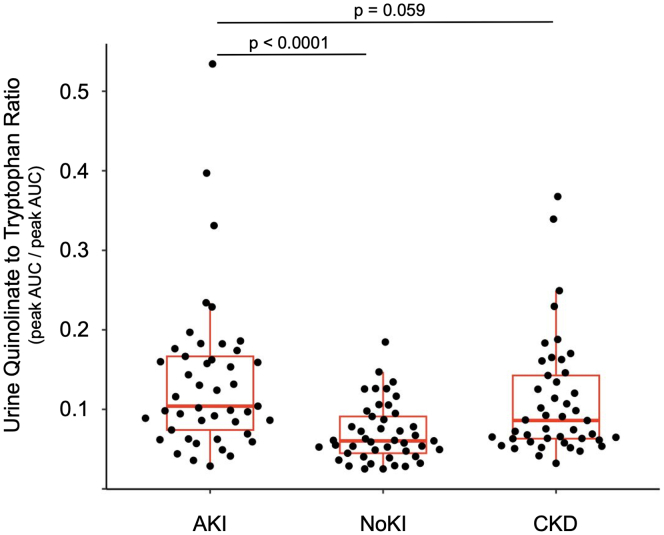
Urine Q/T ratio was significantly higher in the AKI than in the NoKI group (Median [IQR] = 0.104 [0.074–0.167] vs. 0.060 [0.045–0.091], P < 0.0001, 95% CI of difference = 0.030–0.082; Figure 1).
Figure 1.
Urine quinolinate to tryptophan (Q/T) ratio among kidney injury groups. Q and T are expressed in units of liquid chromatography - tandem mass spectrometry peak area under curve (AUC)
AKI, acute kidney injury group; CKD, chronic kidney disease; NoKI, no kidney injury.
Secondary Outcome: Comparison of Preselected Urine Metabolites Between AKI and NoKI or CKD Groups
Urine Q/T was marginally higher in the AKI group than in the CKD group (Median [IQR] = 0.104 [0.074–0.167] vs. 0.086 [0.063–0.142], P = 0.059, 95% CI of difference = −0.0005 to 0.054; Figure 1).
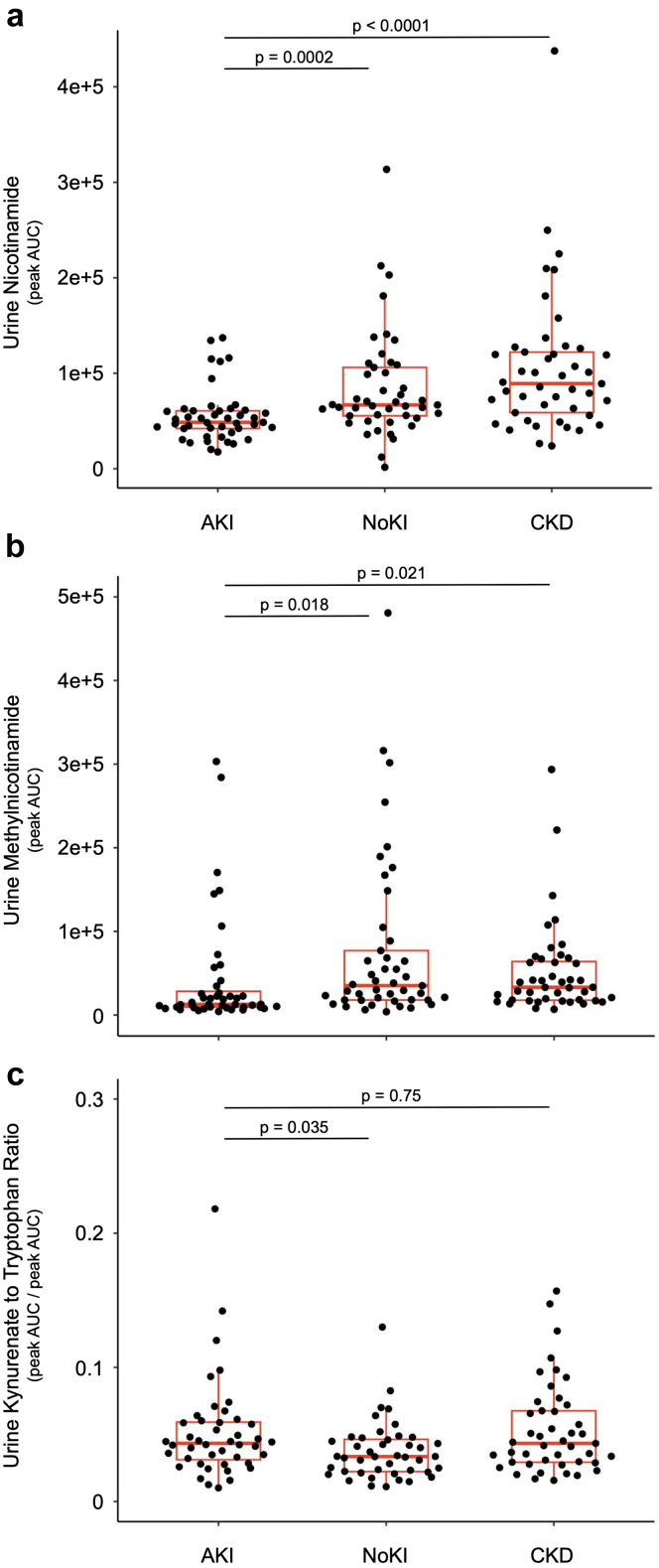
Comparison of other prespecified urine metabolite levels between AKI and each of NoKI and CKD groups is shown in Figure 2. Urine nicotinamide was lower in the AKI group (median [IQR] = 4.9 × 104 [4.2 × 104 – 6.1 × 104]) than in either of the NoKI (median [IQR] = 6.7 × 104 [5.5 × 104 – 10.6 × 104], P = 0.0002, 95% CI of difference = −3.6 × 104 to −1.0 × 104) and CKD (median [IQR] = 8.9 × 104 (5.9 × 104–12.2 × 104), P < 0.0001, 95% CI of difference = −6.0 × 104 to −2.1 × 104) groups. Urine methylnicotinamide was lower in the AKI group (median [IQR] = 12.8 × 103 [9.6 × 103–34.6 × 103]) than either of the NoKI (median [IQR] = 35.1 × 103 [18.0 × 103–77.1 × 103], P = 0.018, 95% CI of difference = −41.2 × 103 to −3.8 × 103) and CKD (median [IQR] = 33.2 × 103 [17.9 × 103–67.0 × 103], P = 0.021, 95% CI of difference = −28.2 × 103 to −2.8 × 103) groups. Urine kynurenic acid-to-tryptophan ratio (KynA/T) was higher in the AKI group than the NoKI group (Median [IQR] = 0.044 [0.032–0.060] vs. 0.034 [0.022–0.047], P = 0.035, 95% CI of difference = 0.0007–0.022). Urine KynA/T did not differ between AKI and CKD groups (median [IQR] = 0.044 [0.032–0.060] vs. 0.043 [0.029–0.068], P = 0.75, 95% CI of difference = −0.018 to 0.012).
Figure 2.
Selected urine metabolites (a–c) among kidney injury groups. Metabolites are expressed in units of liquid chromatography tandem mass spectrometry peak area under curve (AUC). AKI, acute kidney injury group; CKD, chronic kidney disease; NoKI, no kidney injury.
Secondary Outcome: Comparison of Other NAD+ Related Metabolites
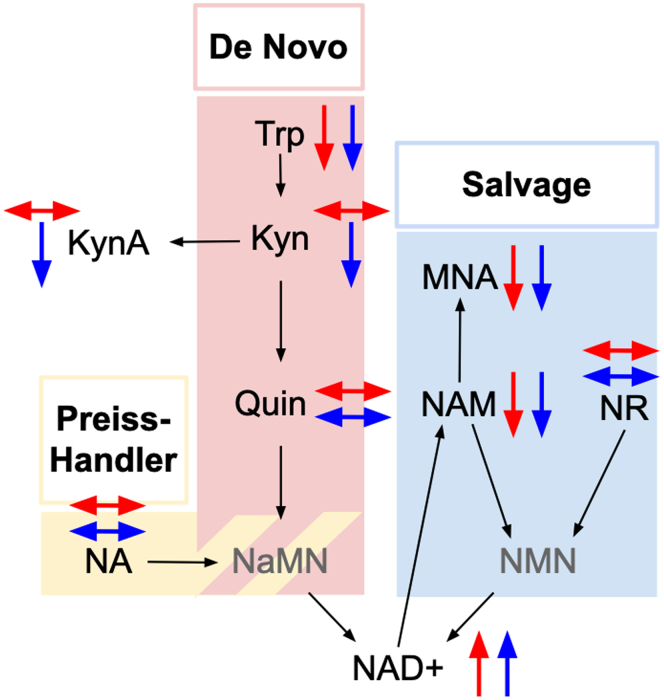
In Figure 3 and Supplementary Table S1, we show relative differences in metabolites related to NAD+ biosynthesis along the de novo, salvage, and Preiss-Handler pathways between the AKI group and each matched comparison group. Levels of the precursor metabolites, tryptophan and nicotinamide were lower in the AKI group, and levels of NAD+ were higher in the AKI group, than in each comparison group. Levels of the nicotinamide degradation product methylnicotinamide were also lower in the AKI group than in comparison groups. Levels of kynurenine and kynurenic acid were lower in the AKI group than the CKD group, but did not differ between the AKI and NoKI groups. Levels of quinolinate, nicotinamide riboside, and nicotinic acid did not differ between AKI and comparison groups (P > 0.05).
Figure 3.
Differences among kidney injury groups in urine metabolites related to nicotinamide adenine dinucleotide (NAD+) biosynthetic pathways. Upward and downward arrows respectively indicate a significant increase or decrease in mass spectroscopy-based area under curve (AUC) values in the acute kidney injury (AKI) group compared with the no kidney injury (NoKI, red) and chronic kidney disease (CKD, blue) groups. Grayed-out metabolites were not measured. Kyn, kynurenine; KynA, kynurenic acid; MNA, methylnicotinamide; NA, nicotinic acid; NaMN, nicotinate mononucleotide; NMN, nicotinamide mononucleotide; NR, nicotinate riboside; Quin, quinolinate; Trp, tryptophan;
Discussion
Nicaraguan sugarcane workers who developed AKI during the harvest season had higher urine Q/T ratios, a validated marker of NAD+ biosynthetic derangement, than those who did not develop AKI. These findings are consistent with, although not specific to, ischemic and inflammatory kidney injury mechanisms driving AKI in this population. Recurrent AKI leading to interstitial scarring and ultimately CKD is a leading hypothesis for how MeN develops.14, 15, 16, 17 Our observation of NAD+ biosynthetic derangement as a characteristic of AKI in individuals at high risk for MeN indicates the presence of tissue injury occurring during these AKI events. That these NAD+ biosynthetic derangements may arise among individuals who are otherwise active and healthy with few comorbidities other than kidney injury is a novel discovery. Our findings therefore represent an important step in further understanding the role and mechanisms of AKI in the pathophysiology of MeN.
Urine nicotinamide and 1-methylnicotinamide were lower in the AKI cohort than in the cohort without kidney injury, further supporting the presence of NAD+ biosynthetic derangement during AKI in this population. Nicotinamide is the main circulating NAD+ precursor in the body.41 The majority of dietary NAD+ precursors are converted in the liver to nicotinamide for systemic use, although the kidneys also use circulating tryptophan and nicotinic acid for NAD+ synthesis.42 Under conditions of increased NAD+ demand, nicotinamide stores are depleted.41 Lower urine levels of 1-methylnicotinamide complement the finding of lower nicotinamide in the AKI group. 1-methylnicotinamide is generated by an irreversible methylation reaction that removes nicotinamide from the cellular pool of precursors that can be converted to NAD+, and may be excreted unchanged in the urine or as carboxamide breakdown products.41,43 1-methylnicotinamide may also play a role in kidney fibrogenesis.44
The lack of difference in nicotinic acid or nicotinamide riboside among groups is also likely related to the specific role of nicotinamide, and not these other compounds, as the primary circulating NAD+ precursor. Our previous work identified increased urinary KynA/T, consistent with inflammation and kidney dysfunction,45,46 among sugarcane harvest and seed cutters compared to occupational groups with lower risk for MeN development.23 In this study, KynA/T was higher in the AKI group than the NoKI group and did not differ between AKI and CKD groups.
Urine Q/T and levels of other metabolites related to NAD+ synthesis showed similar directional differences when compared between AKI and NoKI groups as well as AKI and CKD groups. This similar directional relationship was present despite the fact that the CKD group was no longer working in sugarcane, whereas both the AKI and NoKI groups were sampled while actively exposed to the stressors attending sugarcane work. Previous work has established that NAD+ biosynthetic deficiency is characteristic of both AKI and CKD states.34 That Q/T was elevated to a greater extent in the AKI group than in the CKD group suggests that the degree of NAD+ biosynthetic derangement was greater during the acute injury phase than during chronic disease. Equally important, the comparison between AKI and CKD cohorts reduces the likelihood that reverse causality may be occurring. Because the CKD cohort was selected specifically to have similar age, biological sex, and sCr to the AKI cohort to ensure similar kidney function, our finding that the directionality of differences in key NAD+-related metabolites between the AKI and NoKI groups persists between AKI and CKD groups is evidence that these metabolites likely reflect metabolic features of AKI, and not just differences in clearance of these metabolites driven by decreased kidney function.
NAD+ biosynthetic derangement in AKI is of particular interest because it may be mitigated by the administration of nutritional precursors such as nicotinamide or nicotinic acid. Therapy with these precursors to mitigate NAD+ biosynthetic derangement has in turn been shown to ameliorate the degree of kidney injury and speed recovery in both preclinical and clinical settings.25,26,28,33,47,48 Fibrosis after an acute insult to the kidneys may be reduced through NAD+ precursor therapy.28 Our findings of increased urine Q/T consistent with NAD+ biosynthetic derangement, coupled with reduced urine nicotinamide levels, therefore suggest an exciting future direction: a clinical trial of NAD+ precursors as renoprotective agents during high risk activities such as sugarcane cutting. Oral Nicotinamide in particular may be an excellent candidate because of its good safety profile, low cost, and wide availability.49
This study has some important limitations. Data reflect a single cross-sectional measurement of urine metabolites. Further study should employ repeated metabolomics measures over the course of a harvest season. Because assessment of kidney function occurred at only 2 time points approximately 5 months apart, the precise acuity of kidney injury in the AKI group is not known. Based on the Kidney Disease Improving Global Outcomes criteria, these individuals fit most closely with the classification “acute kidney disease.”37,50 Metabolic findings in this population may therefore not be representative of processes occurring during Kidney Disease Improving Global Outcomes-standard AKI. Our population was a select group of male sugarcane cutters in Nicaragua and may not represent AKI in other populations at risk for MeN or CKD of nontraditional cause more broadly. Metabolites were analyzed in a well-established LC-MS/MS core using standard targeted techniques, and paired specimens were analyzed in the same batch. However, we did not use spiked samples. Therefore, although our area under curve values have good internal comparison validity, they cannot be reliably compared to other metabolite data sources. Statistical significance thresholds were set at P < 0.05 for all analyses; if applying a Bonferroni adjustment for secondary analyses, then the adjusted P value of < 0.007 would place analyses of methylnicotinamide and KynA/T above the statistical significance threshold. Despite our paired design, unmeasured confounding may have still existed and introduced bias.
Urine Q/T is a validated and well-studied biomarker, and our use of the CKD group as a second comparison group allowed us to determine that differences in the AKI and NoKI populations were unlikely to be driven by differential clearance of metabolites alone. Nevertheless, urine levels of some of our other metabolites related to NAD+ biosynthesis including NAD+ itself are less well-validated, and may reflect both intracellular and extracellular metabolic processes, movement between intracellular and extracellular spaces, and movement between blood and urine.51 Our findings would be further strengthened by complementary tissue metabolomics, as well as multiomics approaches.
NAD+ biosynthetic derangement, as evidenced by increased urine Q/T ratio and alterations in other NAD+ precursor metabolites, is present during AKI in male sugarcane workers at risk for MeN. Its presence complements preexisting findings suggesting ischemic and/or inflammatory processes are the underlying mechanism for injury development in MeN. Nicotinamide stores are also depleted during AKI in this population. Given that the administration of nicotinamide mitigates kidney damage associated with NAD+ metabolic derangement, our findings suggest a novel therapeutic approach to prevent kidney injury in populations at high risk for MeN.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Sinead Keogh provided additional support with specimen management. This work was supported by the Doris Duke Foundation Physician Scientist Fellowship Award #2021082 (NHR) and Clinical Scientist Development Award (DJF), as well as a Satellite Healthcare Foundation Coplon Award, the Comité Nacional de Productores de Azúcar, and funds from Beth Israel Deaconess Medical Center. DRB and DJF are further supported by NIDDK R01DK116021. NHR is further supported through The Harvard Catalyst / The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR00254) with financial contributions from Harvard University and its affiliated academic healthcare centers. The mass spectrometry work was partially funded by NIH grants 5P01CA120964 (J.M.A.) and 5P30CA006516 (J.M.A.) and the Beth Israel Deaconess Medical Center Capital Equipment Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. The funders had no role in study design, collection, analysis, and interpretation of data, or in the preparation or writing of this manuscript.
Data Availability Statement
Data supporting the findings of this study along with a data dictionary are available in the supplementary material as Supplementary Data File S1 and Supplementary Table S2.
Author Contributions
Research idea and study design was by NHR, DRB, SMP, and DJF. Data acquisition was by NHR, JMA, LF, JJA, DLP, and ISD. Data analysis/interpretation was by NHR, DAL, JMA, JL, MKS, SMP, DRB, and DJF. Statistical analysis was by NHR. Supervision and mentorship was by MKS, DRB, SMP, and DJF. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Figure S1. Study flow diagram. AKI, acute kidney injury; CKD, chronic kidney disease; NoKI, no kidney injury; sCr, serum creatinine.
Table S1. Levels of metabolites in nicotinamide adenine dinucleotide (NAD+) biosynthetic pathways among kidney injury groups. Data shown are values for each of the acute kidney injury (AKI), no kidney injury (NoKI), and chronic kidney disease (CKD) groups. P-values describe the comparison between the AKI and each of the NoKI and CKD groups.
Table S2. Supplementary data file dictionary. LC-MS/MS, liquid chromatography-coupled tandem mass spectrometry.
STROBE Statement.
Supplementary Material
Figure S1. Study flow diagram. Table S1. Levels of metabolites in nicotinamide adenine dinucleotide (NAD+) biosynthetic pathways among kidney injury groups. Table S2. Supplementary data file dictionary. STROBE Statement.
References
- 1.Correa-Rotter R., Wesseling C., Johnson R.J. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis. 2014;63:506–520. doi: 10.1053/j.ajkd.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijkström J., González-Quiroz M., Hernandez M., et al. Renal morphology, clinical findings, and progression rate in Mesoamerican nephropathy. Am J Kidney Dis. 2017;69:626–636. doi: 10.1053/j.ajkd.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Kupferman J., Amador J.J., Lynch K.E., et al. Characterization of Mesoamerican nephropathy in a kidney failure hotspot in Nicaragua. Am J Kidney Dis. 2016;68:716–725. doi: 10.1053/j.ajkd.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Johnson R.J., Wesseling C., Newman L.S. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med. 2019;380:1843–1852. doi: 10.1056/NEJMra1813869. [DOI] [PubMed] [Google Scholar]
- 5.Raines N., González M., Wyatt C., et al. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican nephropathy. MEDICC Rev. 2014;16:16–22. doi: 10.37757/MR2014.V16.N2.4. [DOI] [PubMed] [Google Scholar]
- 6.Wesseling C., Aragon A., González Quiroz M., et al. Kidney function in sugarcane cutters in Nicaragua - a longitudinal study of workers at risk of Mesoamerican nephropathy. Environ Res. 2016;147:125–132. doi: 10.1016/j.envres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Petropoulos Z.E., Keogh S.A., Jarquín E., et al. Heat stress and heat strain among outdoor workers in el Salvador and Nicaragua. J Expo Sci Environ Epidemiol. 2023;33:622–630. doi: 10.1038/s41370-023-00537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres C., Aragón A., González M., et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Peraza S., Wesseling C., Aragon A., et al. Decreased kidney function among agricultural workers in el Salvador. Am J Kidney Dis. 2012;59:531–540. doi: 10.1053/j.ajkd.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez Polo V., Garcia-Trabanino R., Rodriguez G., Madero M. Mesoamerican Nephropathy (MeN): what we know so far. Int J Nephrol Renov Dis. 2020;13:261–272. doi: 10.2147/ijnrd.S270709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson E., Glaser J., Weiss I., et al. Workload and cross-harvest kidney injury in a Nicaraguan sugarcane worker cohort. Occup Environ Med. 2019;76:818–826. doi: 10.1136/oemed-2019-105986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Quiroz M., Pearce N., Caplin B., Nitsch D. What do epidemiological studies tell us about chronic kidney disease of undetermined cause in Meso-America? A systematic review and meta-analysis. Clin Kidney J. 2018;11:496–506. doi: 10.1093/ckj/sfx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser J., Lemery J., Rajagopalan B., et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin J Am Soc Nephrol. 2016;11:1472–1483. doi: 10.2215/CJN.13841215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer R.S.B., Vangala C., Truong L., et al. Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int. 2018;93:681–690. doi: 10.1016/j.kint.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Fischer R.S.B., Mandayam S., Chavarria D., et al. Clinical evidence of acute Mesoamerican nephropathy. Am J Trop Med Hyg. 2017;97:1247–1256. doi: 10.4269/ajtmh.17-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paula Santos U., Zanetta D.M.T., Terra-Filho M., Burdmann E.A. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015;87:792–799. doi: 10.1038/ki.2014.306. [DOI] [PubMed] [Google Scholar]
- 17.Kupferman J., Ramírez-Rubio O., Amador J.J., et al. Acute kidney injury in sugarcane workers at risk for Mesoamerican nephropathy. Am J Kidney Dis. 2018;72:475–482. doi: 10.1053/j.ajkd.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Glaser J., Hansson E., Weiss I., et al. Preventing kidney injury among sugarcane workers: promising evidence from enhanced workplace interventions. Occup Environ Med. 2020;77:527–534. doi: 10.1136/oemed-2020-106406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato Y., Roncal-Jimenez C.A., Andres-Hernando A., et al. Increase of core temperature affected the progression of kidney injury by repeated heat stress exposure. Am J Physiol Ren Physiol. 2019;317:F1111–F1121. doi: 10.1152/ajprenal.00259.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roncal Jimenez C.A., Ishimoto T., Lanaspa M.A., et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int. 2014;86:294–302. doi: 10.1038/ki.2013.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson E., Glaser J., Jakobsson K., et al. Pathophysiological mechanisms by which heat stress potentially induces kidney inflammation and chronic kidney disease in sugarcane workers. Nutrients. 2020;12 doi: 10.3390/nu12061639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall S.M., Raines N.H., Ramirez-Rubio O., et al. Urinary metabolomic profile of youth at risk for chronic kidney disease in Nicaragua. Kidney360. 2023;4(7):899–908. doi: 10.34067/KID.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raines N.H., Leone D.A., O’Callaghan-Gordo C., et al. Metabolic features of increased gut permeability, inflammation, and altered energy metabolism distinguish agricultural workers at risk for Mesoamerican nephropathy. Metabolites. 2023;13:325. doi: 10.3390/metabo13030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson E., Broberg K., Wijkström J., et al. An explorative study of inflammation-related proteins associated with kidney injury in male heat-stressed workers. J Therm Biol. 2023;112 doi: 10.1016/j.jtherbio.2022.103433. [DOI] [PubMed] [Google Scholar]
- 25.Tran M.T., Zsengeller Z.K., Berg A.H., et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poyan Mehr A., Tran M.T., Ralto K.M., et al. De novo NAD + biosynthetic impairment in acute kidney injury in humans. Nat Med. 2018;24:1351–1359. doi: 10.1038/s41591-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralto K.M., Rhee E.P., Parikh S.M. NAD(+) homeostasis in renal health and disease. Nat Rev Nephrol. 2020;16:99–111. doi: 10.1038/s41581-019-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng M., Cai J., Liu Z., et al. Nicotinamide reduces renal interstitial fibrosis by suppressing tubular injury and inflammation. J Cell Mol Med. 2019;23:3995–4004. doi: 10.1111/jcmm.14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y., Liang Y., Hu T., et al. Endogenous Nampt upregulation is associated with diabetic nephropathy inflammatory-fibrosis through the NF-κB p65 and Sirt1 pathway; NMN alleviates diabetic nephropathy inflammatory-fibrosis by inhibiting endogenous Nampt. Exp Ther Med. 2017;14:4181–4193. doi: 10.3892/etm.2017.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallan S., Afkarian M., Zelnick L.R., et al. Metabolomics and gene expression analysis reveal down-regulation of the citric acid (TCA) cycle in non-diabetic CKD patients. EBiomedicine. 2017;26:68–77. doi: 10.1016/j.ebiom.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai P., Canto C., Oudart H., et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Späth M.R., Hoyer-Allo K.J.R., Seufert L., et al. Organ protection by caloric restriction depends on activation of the de novo NAD+ synthesis pathway. J Am Soc Nephrol. 2023;34:772–792. doi: 10.1681/ASN.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch M.R., Tran M.T., Ralto K.M., et al. TFEB-driven lysosomal biogenesis is pivotal for PGC1α-dependent renal stress resistance. JCI Insight. 2019;5 doi: 10.1172/jci.insight.126749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bignon Y., Rinaldi A., Nadour Z., et al. Cell stress response impairs de novo NAD+ biosynthesis in the kidney. JCI Insight. 2022;7 doi: 10.1172/jci.insight.153019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raines N.H., Cheung M.D., Wilson L.S., et al. NAD+ biosynthetic impairment and urinary metabolomic alterations observed in hospitalized adults with COVID-19-related acute kidney injury. Kidney Int Rep. 2021;0:3002–3013. doi: 10.1016/j.ekir.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mede A.I., Milne G.L., Wei D., Smith D.K., Smith L.E. NAD+ biosynthesis impairment and acute kidney injury after major vascular surgery. Antioxidants (Basel) 2023;12:821. doi: 10.3390/antiox12040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellum J.A., Lameire N., Aspelin P., et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 38.Yuan M., Breitkopf S.B., Yang X., Asara J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhold D., Pielke-Lombardo H., Jacobson S., Ghosh D., Kechris K. Pre-analytic considerations for mass spectrometry-based untargeted metabolomics data. Methods Mol Biol (Clifton NJ) 2019;1978:323–340. doi: 10.1007/978-1-4939-9236-2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia J., Psychogios N., Young N., Wishart D.S. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37:W652–W660. doi: 10.1093/nar/gkp356. W652-W660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark A.J., Saade M.C., Parikh S.M. The significance of NAD+ biosynthesis alterations in acute kidney injury. Semin Nephrol. 2022;42 doi: 10.1016/j.semnephrol.2022.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L., Su X., Quinn W.J., 3rd, et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018;27:1067–1080 e5. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bender D.A., Magboul B.I., Wynick D. Probable mechanisms of regulation of the utilization of dietary tryptophan, nicotinamide and nicotinic acid as precursors of nicotinamide nucleotides in the rat. Br J Nutr. 1982;48:119–127. doi: 10.1079/bjn19820094. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W., Rong G., Gu J., et al. Nicotinamide N-methyltransferase ameliorates renal fibrosis by its metabolite 1-methylnicotinamide inhibiting the TGF-β1/Smad3 pathway. FASEB J. 2022;36 doi: 10.1096/fj.202100913RRR. [DOI] [PubMed] [Google Scholar]
- 45.Cervenka I., Agudelo L.Z., Ruas J.L. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357 doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 46.Aregger F., Uehlinger D.E., Fusch G., et al. Increased urinary excretion of kynurenic acid is associated with non-recovery from acute kidney injury in critically ill patients. BMC Nephrol. 2018;19:44. doi: 10.1186/s12882-018-0841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan Y., Wang S.R., Huang X.Z., et al. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1- dependent manner. J Am Soc Nephrol. 2017;28:2337–2352. doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsyuba E., Mottis A., Zietak M., et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature. 2018;563:354–359. doi: 10.1038/s41586-018-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panel CIRE Final report of the safety assessment of niacinamide and niacin. Int J Toxicol. 2005;24:1–31. doi: 10.1080/10915810500434183. [DOI] [PubMed] [Google Scholar]
- 50.Levey A.S. Defining AKD: The Spectrum of AKI, AKD, and CKD. Nephron. 2021;146:302–305. doi: 10.1159/000516647. [DOI] [PubMed] [Google Scholar]
- 51.Rhee E.P. How omics data can be used in nephrology. Am J Kidney Dis Off. 2018;72:129–135. doi: 10.1053/j.ajkd.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flow diagram. Table S1. Levels of metabolites in nicotinamide adenine dinucleotide (NAD+) biosynthetic pathways among kidney injury groups. Table S2. Supplementary data file dictionary. STROBE Statement.
Data Availability Statement
Data supporting the findings of this study along with a data dictionary are available in the supplementary material as Supplementary Data File S1 and Supplementary Table S2.