Abstract
Introduction
Patients with primary hyperoxaluria type 1 (PH1), a genetic disorder associated with hepatic oxalate overproduction, frequently experience recurrent kidney stones and worsening kidney function. Lumasiran is indicated for the treatment of PH1 to lower urinary and plasma oxalate (POx).
Methods
ILLUMINATE-A (NCT03681184) is a phase III trial in patients aged ≥6 years with PH1 and estimated glomerular filtration rate (eGFR) ≥30 ml/min per 1.73 m2. A 6-month double-blind placebo-controlled period is followed by an extension period (≤54 months; all patients receive lumasiran). We report interim data through month 36.
Results
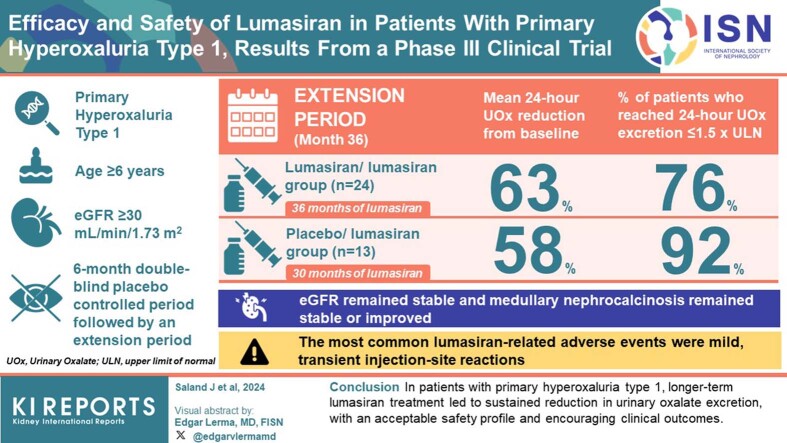
Of 39 patients enrolled, 24 of 26 (lumasiran/lumasiran group) and 13 of 13 (placebo/lumasiran group) entered and continue in the extension period. At month 36, in the lumasiran/lumasiran group (36 months of lumasiran treatment) and placebo/lumasiran group (30 months of lumasiran treatment), mean 24-hour urinary oxalate (UOx) reductions from baseline were 63% and 58%, respectively; 76% and 92% of patients reached a 24-hour UOx excretion ≤1.5× the upper limit of normal (ULN). eGFR remained stable. Kidney stone event rates decreased from 2.31 (95% confidence interval: 1.88–2.84) per person-year (PY) during the 12 months before consent to 0.60 (0.46–0.77) per PY during lumasiran treatment. Medullary nephrocalcinosis generally remained stable or improved; approximately one-third of patients (both groups) improved to complete resolution. The most common lumasiran-related adverse events (AEs) were mild, transient injection-site reactions.
Conclusion
In patients with PH1, longer-term lumasiran treatment led to sustained reduction in UOx excretion, with an acceptable safety profile and encouraging clinical outcomes.
See Supplemental Material for Video Abstract
Keywords: lumasiran, oxalate, primary hyperoxaluria type 1, rare disease, renal, RNA interference
Graphical abstract
Primary hyperoxaluria is a genetic disorder associated with hepatic oxalate overproduction, leading to increased renal oxalate excretion.1 PH1 the most commonly diagnosed subtype, is caused by AGXT gene mutations and absence or dysfunction of alanine-glyoxylate aminotransferase.1 Excess oxalate leads to calcium oxalate crystal formation in the kidneys and urinary tract, resulting in recurrent kidney stones and/or nephrocalcinosis.1,2 Many patients develop kidney damage, kidney failure, and systemic oxalosis.1,2
Conventional supportive care measures (e.g., hyperhydration and crystallization inhibitors) can improve prognosis and delay progression to kidney failure and/or organ transplantation but do not address the pathophysiology.1,3,4 Approximately 20% of patients, mainly those with specific AGXT genotypes, can benefit from treatment with pyridoxine (vitamin B6), a cofactor for alanine-glyoxylate aminotransferase; in those cases, the response ranges from partial to complete.5, 6, 7, 8 Lumasiran, a small interfering RNA approved for treatment of PH1,9,10 targets and promotes degradation of the mRNA of glycolate oxidase, thereby decreasing glyoxylate levels and reducing hepatic oxalate production.9,11
ILLUMINATE-A is an ongoing phase III trial evaluating the efficacy and safety of lumasiran in children aged ≥6 years and adults with PH1 and eGFR ≥30 ml/min per 1.73 m2. ILLUMINATE-A consists of a 6-month, randomized, placebo-controlled, double-blind period (DBP) and a 54-month extension period, in which all patients received lumasiran. In the primary analysis (encompassing the 6-month DBP), the placebo-adjusted treatment difference in the percentage change in 24-hour UOx excretion was −54%; lumasiran was well tolerated. Most patients who received lumasiran had normal or near-normal UOx levels at month 6,12 and the reduction in 24-hour UOx levels was sustained through month 12.13 Here, we report interim data through month 36 of ILLUMINATE-A.
Methods
Study Design and Patients
The study methodology (ClinicalTrials.gov: NCT03681184; EudraCT: 2018-001981-40) was reported previously.12 Briefly, eligible patients were aged ≥6 years with genetically confirmed PH1, eGFR ≥30 ml/min per 1.73 m2, and 24-hour UOx excretion ≥0.70 mmol/24h per 1.73 m2. During the DBP, patients received subcutaneous lumasiran or placebo for 6 months. When initiating lumasiran, patients received a loading dose of 3 mg/kg once monthly for 3 doses, followed by a maintenance dose of 3 mg/kg every 3 months beginning 1 month after the last loading dose. During the extension period, all patients received lumasiran.13 The study was conducted at 16 study centers in 8 countries in North America, Europe, and the Middle East.
The study was approved by central and local institutional review boards or ethics committees and conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. All patients or their legal guardians provided written informed consent.
Outcome Measures and Safety Assessments in the Extension Period
Secondary efficacy end points assessed during the extension period included change from baseline in 24-hour UOx excretion, 24-hour UOx-to-creatinine ratio (UOx:Cr), eGFR, and percentage of time in which 24-hour UOx was ≤1.5× the ULN (0.514 mmol/24h per 1.73 m2). Exploratory end points included changes in kidney stone event rates, changes in nephrocalcinosis as assessed by ultrasound, changes in urinary and plasma glycolate, changes in UOx:Cr as assessed by random spot urine collections, and frequency of antidrug antibodies (ADAs). Changes from baseline in POx, proportion of patients with 24-hour UOx ≤1.5 × ULN, and ratios of 24-hour UOx excretion to the ULN for UOx in the extension period were evaluated in post hoc analyses.
Urine and blood samples were collected for measurement of oxalate and glycolate using validated liquid chromatography-tandem mass spectrometry assays.14 The kidney stone event rate was calculated as the total number of kidney stone events divided by the total patient exposure time (events per PY). A kidney stone event included a visit to a health care provider (e.g., outpatient clinic, urgent care, emergency department, or procedure) because of a kidney stone, medication for renal colic, stone passage, and/or macroscopic hematuria due to a kidney stone. The degree of medullary nephrocalcinosis on renal ultrasound was graded on a 0 to 3 scale.15 Overall changes in nephrocalcinosis grade were grouped into 4 categories, accounting for both kidneys as follows: no change, improving, worsening, and indeterminate (defined as 1 kidney improving and 1 worsening).
Safety assessments included frequency and seriousness of AEs during the extension period. AEs were classified according to the Medical Dictionary for Regulatory Activities (version 25.0).
Statistical Analyses
This analysis was completed using data from January 2019 (start of enrollment) through February 26, 2022, when all active study patients had completed their month 36 visit. End points were summarized using descriptive statistics. Unless otherwise noted, the 24-hour urine pharmacodynamic parameters, plasma glycolate, kidney stone event rates, spot UOx:Cr, and eGFR analyses in this manuscript used the full analysis set. Other efficacy and safety analyses were conducted in the all-lumasiran-treated set (all patients who received any amount of lumasiran) and analyzed according to whether patients received lumasiran in the DBP and extension period (lumasiran/lumasiran group) or received placebo in the DBP and lumasiran during the extension period (placebo/lumasiran group). The POx analysis set (all patients who received any amount of study drug and had a baseline POx level ≥1.5 × the lower limit of quantitation [5.55 μmol/l]) was used to evaluate POx end points.
For analyses of nonpharmacodynamic end points during the extension period, baseline values for the lumasiran/lumasiran and placebo/lumasiran groups corresponded to value(s) collected before the first dose of lumasiran. For analyses of 24-hour urine pharmacodynamic parameters, baseline corresponded to the median of the respective pharmacodynamic measurements collected prior to the first dose of study drug (lumasiran or placebo). For other pharmacodynamic analyses, the baseline corresponded to the mean of the respective pharmacodynamic measurements collected before the first dose of study drug.
Cumulative safety data (first dose of lumasiran through February 26, 2022) are reported.
Results
Patients
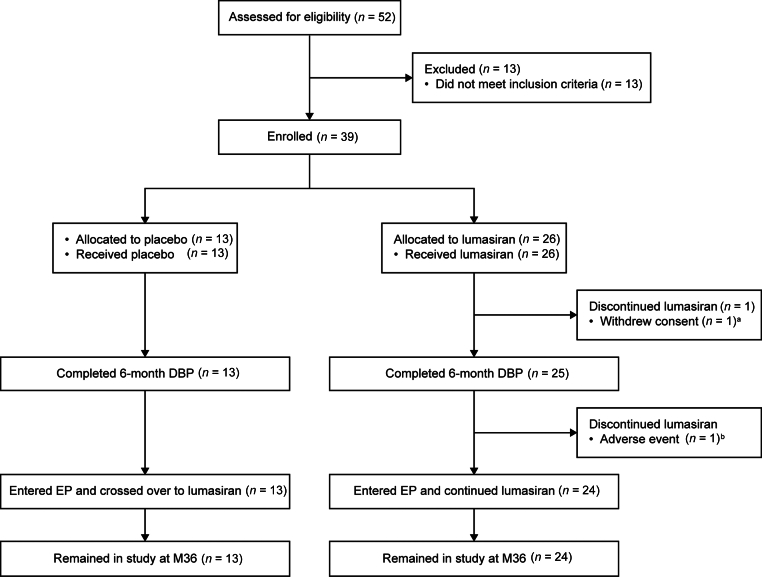
Of 39 patients enrolled, 37 (95%) entered the extension period and remained in the study at month 36 (Figure 1). Twenty-four of the 26 patients initially randomized to lumasiran during the DBP received lumasiran during the extension period (lumasiran/lumasiran group); all 13 patients initially randomized to placebo received lumasiran during the extension period (placebo/lumasiran group). Baseline characteristics are summarized in Supplementary Table S1.
Figure 1.
Patient disposition.
aParticipation stopped by the parent/guardian owing to the patient’s inability to comply with protocol-specific testing; the patient did not complete the 6-month DBP.
bDiscontinued treatment for adverse events (unrelated to treatment) of fatigue and disturbance in attention; completed 6-month DBP but did not enter the EP.
DBP, double-blind period; EP, extension period; M, month.
Efficacy
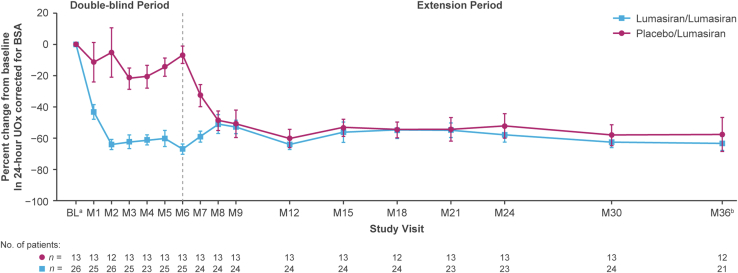
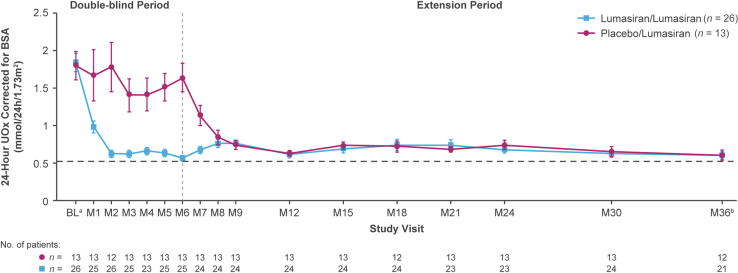
Sustained reductions in 24-hour UOx levels were observed during long-term lumasiran treatment. At month 36, mean SEM percentage reduction from baseline (before the first dose date or time of lumasiran or placebo) in 24-hour UOx was 63.5% (4.3) in the lumasiran/lumasiran group (after 36 months of lumasiran treatment) and 57.5% (10.7) in the placebo/lumasiran group (after 30 months of lumasiran treatment) (Figure 2). Mean (SEM) 24-hour UOx decreased from 1.84 (0.12) mmol/24h per 1.73 m2 at baseline to 0.60 (0.05) at month 36 in the lumasiran/lumasiran group, and from 1.79 (0.19) mmol/24h per 1.73 m2 at baseline to 0.60 (0.07) at month 36 in the placebo/lumasiran group (Figure 3). Similarly, rapid and sustained reductions in 24-hour UOx over time (Supplementary Figure S1A and B) were observed among patients either taking or not taking pyridoxine at baseline and likewise for those with or without a pyridoxine-responsive genotype. More specifically, patients with a pyridoxine-responsive genotype who were using pyridoxine at baseline achieved similarly rapid and sustained reductions in 24-hour UOx compared with the patients without pyridoxine-responsive genotypes, as well as those with pyridoxine-responsive genotypes who were not taking pyridoxine at baseline (Supplementary Figure S1C).
Figure 2.
Mean (SEM) percent change in 24-hour UOx over time.
aBaseline is the median of all valid 24-hour urine assessments collected prior to the first dose of the study drug (lumasiran or placebo).
bAt month 36, the lumasiran/lumasiran group had received lumasiran treatment for 36 months, and the placebo/lumasiran group had received lumasiran treatment for 30 months.
BL, baseline; BSA, body surface area; M, month; UOx, urinary oxalate.
Figure 3.
Mean (SEM) 24-hour UOx over time. Dotted line represents the ULN of 0.514 mmol/24h per 1.73 m2 for 24-hour UOx excretion.
aBaseline is the median of all valid 24-hour urine assessments collected prior to the first dose of the study drug (lumasiran or placebo).
bAt month 36, the lumasiran/lumasiran group had received lumasiran treatment for 36 months, and the placebo/lumasiran group had received lumasiran treatment for 30 months.
BL, baseline; BSA, body surface area; M, month; UOx, urinary oxalate.
Ratios of 24-hour UOx excretion to the ULN for UOx also decreased (Supplementary Figure S2). 24-hour UOx excretion was ≤1.5× ULN in 16 of 21 (76%) patients in the lumasiran/lumasiran group and 11 of 12 patients (92%) in the placebo/lumasiran group at month 36. Reductions in 24-hour UOx:Cr from baseline were sustained; mean SEM reduction from baseline was 63.6% (4.7) at month 36 in the lumasiran/lumasiran group and 62.6% (9.0) in the placebo/lumasiran group (Supplementary Figure S3). Similar reductions were observed for spot UOx:Cr (Supplementary Figure S4).
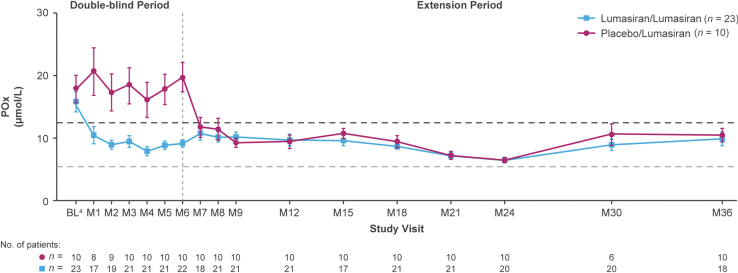
Mean POx concentrations decreased to within normal limits (ULN, 12.11 μmol/l) in both groups (Figure 4). The mean SEM POx concentration at month 36 was 9.9 (1.1) μmol/l in the lumasiran/lumasiran group (36% reduction from baseline) and 10.5 (1.1) μmol/l in the placebo/lumasiran group (35% reduction).
Figure 4.
Mean SEM POx concentration over time. The dark gray horizontal dotted line represents the ULN of 12.11 μmol/l for POx. The light-gray horizontal dotted line represents the LLOQ of the POx assay at 5.55 μmol/l; values below the LLOQ were assigned a value of 5.55 μmol/l.
aBaseline is the mean of all measurements prior to the first dose of the study drug (lumasiran or placebo).
bAt month 36, the lumasiran/lumasiran group had received lumasiran treatment for 36 months, and the placebo/lumasiran group had received lumasiran treatment for 30 months.
LLOQ, lower limit of quantitation; POx, plasma oxalate; ULN, upper limit of normal.
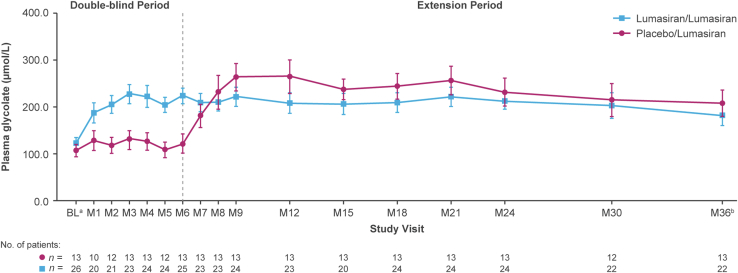
After an initial increase (first 3 months of lumasiran treatment), mean plasma glycolate levels plateaued and remained stable (Figure 5). Changes in 24-hour urinary glycolate-to-creatinine ratio were consistent with those in plasma glycolate (Supplementary Figure S5).
Figure 5.
Mean (SEM) plasma glycolate concentration over time.
aBaseline for plasma glycolate measurements during the extension period corresponded to the median of plasma glycolate measurements collected prior to the first dose of the study drug (lumasiran or placebo).
bAt month 36, the lumasiran/lumasiran group had received lumasiran treatment for 36 months, and the placebo/lumasiran group had received lumasiran treatment for 30 months.
BL, baseline, M, month.
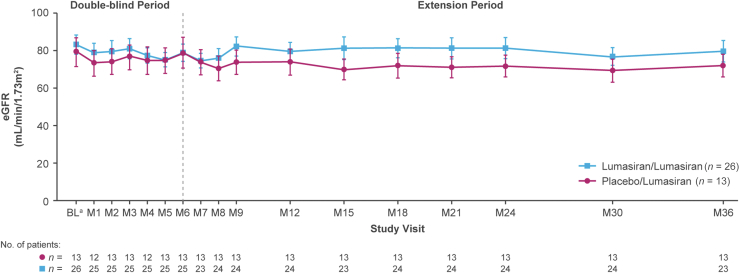
Mean (SEM) eGFR values over time are shown in Figure 6. Mean (SEM) baseline eGFR values were 83.0 (5.0) and 78.9 (7.4) ml/min per 1.73 m2 in the lumasiran/lumasiran and placebo/lumasiran groups, respectively; at month 36, mean (SEM) changes from baseline were −0.7 (1.8) ml/min per 1.73 m2 in the lumasiran/lumasiran group (n = 23) and −7.3 (2.1) in the placebo/lumasiran group (n = 13) after 30 months of lumasiran treatment. The decrease was largely driven by patients with a baseline eGFR ≥90 ml/min per 1.73 m2 (i.e., by changes within the normal range) (Supplementary Figure S6).
Figure 6.
Mean (SEM) eGFR over time.
aBaseline is the last assessment prior to the first dose of the study drug (lumasiran or placebo).
bAt month 36, the lumasiran/lumasiran group had received lumasiran treatment for 36 months, and the placebo/lumasiran group had received lumasiran treatment for 30 months.
BL, baseline; eGFR, estimated glomerular filtration rate; M, month.
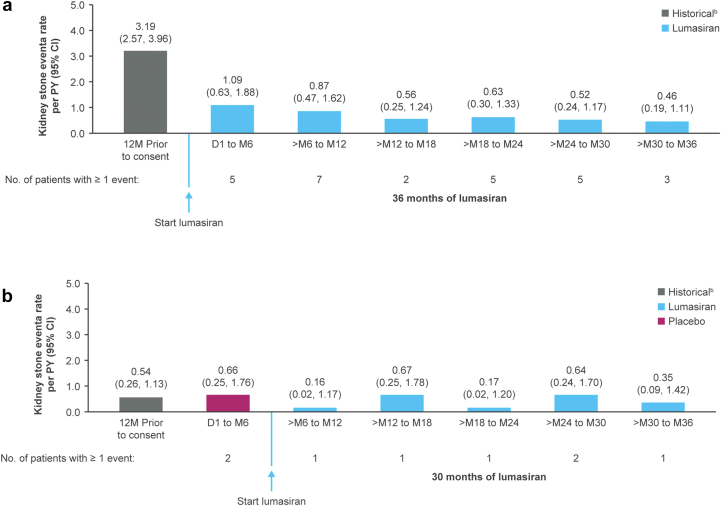
Kidney stone event rates decreased in the all-lumasiran-treated set (i.e., all patients who received any amount of lumasiran) from 2.31 (95% confidence interval: 1.88–2.84) per PY during the 12 months before study entry to 0.60 (0.46–0.77) per PY overall during lumasiran treatment (Supplementary Figure S7). Kidney stone event rates in both treatment groups are shown in Figure 7.
Figure 7.
Kidney stone event ratesa in the (a) lumasiran/lumasiran group and (b) placebo/lumasiran group.
aKidney stone event is defined as an event that includes at least 1 of the following: a visit to a health care provider because of a kidney stone, medication for renal colic, stone passage, or macroscopic hematuria due to a kidney stone.
bPatient-reported history of kidney stone events.
D, day; M, month; PY, person-year.
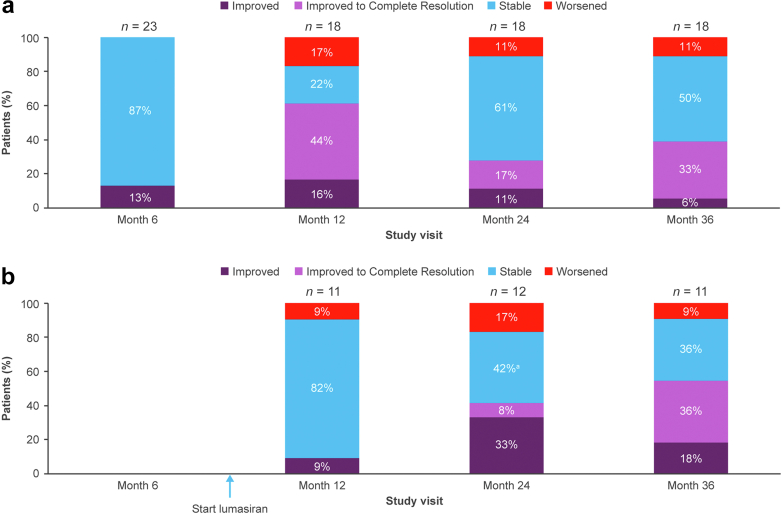
Medullary nephrocalcinosis generally remained stable or improved at month 36 (Figure 8). In the lumasiran/lumasiran group, 50% of patients were stable, 39% improved, and 11% worsened; in the placebo/lumasiran group, 36% were stable, 55% improved and 9% worsened. Approximately one-third of patients improved to complete resolution (33% in the lumasiran/lumasiran group; 36% in the placebo/lumasiran group). The majority (28/36) had medullary nephrocalcinosis at baseline (last assessment before the first lumasiran dose). Among the 13 patients in the lumasiran/lumasiran group with nephrocalcinosis at baseline and an assessment at month 36, 46% improved to complete resolution. A single patient (8%) in the lumasiran/lumasiran group with worsening nephrocalcinosis had nephrocalcinosis at baseline. Among the 10 patients in the placebo/lumasiran group with nephrocalcinosis at baseline and an assessment at month 36, 40% improved to complete resolution, and none worsened (Supplementary Figure S8). Most patients with complete resolution had grade 1 nephrocalcinosis in 1 or both kidneys at baseline.
Figure 8.
Change from baseline in medullary nephrocalcinosis in the (a) lumasiran/lumasiran group and (b) placebo/lumasiran group.
aIncludes 2 patients whose status was “indeterminate.”
No patients had ADAs at baseline. As of the cutoff date, 2 patients tested positive for ADAs as follows: in 1 patient (lumasiran/lumasiran group), ADAs were confirmed to be present at month 6 only (titer 50); in the other patient (placebo/lumasiran group), low-titer ADAs were confirmed to be present from month 18 to month 36 (titer range 50–100). The presence of ADAs was not associated with a decrease in the efficacy of lumasiran in either patient, as assessed by UOx levels.
Safety
Safety outcomes in the all-lumasiran-treated group are summarized in Table 1. Thirty-six patients (92%) experienced AEs. The most common AEs (reported in ≥15% of patients during lumasiran treatment) were injection-site reactions (49%), abdominal pain (21%), headache (18%), and COVID-19 (15%). All injection-site reactions were mild and transient. The injection-site reaction noted by the highest percentage of patients was injection-site erythema (36%), followed by injection-site pain and injection-site swelling, both in 15% (patients may have experienced ≥1 sign/symptom for any given injection). The percentage of patients reporting any injection-site reaction at a given visit decreased over time, from 21% at the baseline visit (first dose of lumasiran) to 8% at the month 30 visit (all-lumasiran-treated set; the last visit in which all patients were evaluable).
Table 1.
Safety profile of lumasiran
| Event, N (%) (Rate)a | Placebo/lumasiran (n =13) | Lumasiran/lumasiran (n = 26) | All lumasiran (n = 39) |
|---|---|---|---|
| Any AE | 12 (92) (100/323.3) | 24 (92) (255/369.5) | 36 (92) (355/355.2) |
| AE related to study drug | 6 (46) (29/93.8) | 13 (50) (59/85.5) | 19 (49) (88/88.1) |
| Serious AEb | 1 (8) (2/6.5) | 3 (12) (5/7.2) | 4 (10) (7/7.0) |
| Severe AEc | 0 | 2 (8) (2/2.9) | 2 (5) (2/2.0) |
| AE leading to discontinuation of study treatmentd | 0 | 1 (4) (2/2.9) | 1 (3) (2/2.0) |
| AEs occurring in ≥15% of patients in the all-lumasiran-treated sete | |||
| Injection-site reactionsf | 6 (46) (26/84.1) | 13 (50) (49/71.0) | 19 (49) (75/75.0) |
| Abdominal pain | 1 (8) (7/22.6) | 7 (27) (14/20.3) | 8 (21) (21/21.0) |
| Headache | 2 (15) (2/6.5) | 5 (19) (9/13.0) | 7 (18) (11/11.0) |
| COVID-19 | 3 (23) (4/12.9) | 3 (12) (3/4.3) | 6 (15) (7/7.0) |
| Death | 0 | 0 | 0 |
AE, adverse event; PY, patient-years.
Number of events/exposure-adjusted event rate per 100 PY.
Abdominal pain (n = 2 patients), dysuria (n = 1 patient), nephrectomy (n = 1 patient), postprocedural complications (n = 1 patient), and urosepsis (n = 1 patient); none of the serious AEs were considered related to lumasiran by the investigator.
Postprocedural complications (n = 1 patient) and urosepsis (n = 1 patient), considered not related to lumasiran by the investigator.
Fatigue and disturbance in attention, considered not related to lumasiran by the investigator.
All terms are Medical Dictionary for Regulatory Activities (version 25) preferred terms except injection-site reactions.
Defined as AEs that were mapped to the high-level term “Injection Site Reactions” or events reported by the sites as injection-site reactions.
Four patients (10%) had serious AEs, 2 (5%) had severe AEs, and 3 (8%) had AEs leading to treatment interruption; none were considered related to study drug. One patient (3%) had AEs leading to treatment discontinuation during the DBP (fatigue and difficulty concentrating, not considered related to study drug). One patient in the placebo/lumasiran group had an AE of acidosis with a bicarbonate level of 16 mmol/l immediately before the first dose of lumasiran at month 6 (not considered related to study drug); the acidosis was treated with sodium bicarbonate. No patients experienced any AE leading to study discontinuation. There were no deaths.
Discussion
In this 36-month analysis of ILLUMINATE-A, pediatric (aged ≥6 years) and adult patients with PH1 treated with lumasiran for up to 36 months showed substantial and sustained reductions in UOx excretion and POx, with an acceptable safety profile and encouraging clinical outcome data.
UOx decreased rapidly following initiation of lumasiran treatment,12,13 and this reduction was sustained through month 36 of the extension period. Consistent with this, 24-hour UOx excretion was ≥2× ULN in most patients at baseline, with some as high as ≥5× ULN; after initiation of lumasiran and through month 36, UOx was generally within the normal range or slightly above (≤1.5× ULN) in most patients. Parallel decreases were observed in the UOx:Cr ratio and POx concentration. These results suggest that lumasiran led to a rapid reduction in oxalate overproduction, which was maintained in the long-term with continued treatment.
Kidney function, measured by eGFR, remained stable during lumasiran treatment, even for patients with stage 3 chronic kidney disease (eGFR 30–<60 ml/min per 1.73 m2) at baseline. Patients who had a normal eGFR (≥90 ml/min per 1.73 m2) at baseline had a relatively stable eGFR after 30 months of lumasiran treatment (range 82.5–136.5 at month 30). Expected rates of eGFR decrease are higher in patients with PH1 with more severe chronic kidney disease, with reported mean annual changes (slopes) of eGFR ranging from −2.3 ml/min per 1.73 m2 per year in stage 2 chronic kidney disease to −16.6 ml/min per 1.73 m2 per year in stage 4 chronic kidney disease, over a mean follow-up time of 15.6 years.16
With lumasiran treatment, kidney stone event rates appeared to decrease compared with the 12 months before consent, although there was heterogeneity in baseline rates between treatment groups. Larger studies and/or longer follow-up periods are needed for more definitive conclusions.
Medullary nephrocalcinosis was generally either stable or improved. Variation in the percentage of patients in a category (improved, improved to complete resolution, stable, or worsened) between visits may reflect a combination of differential patient inclusion (e.g., 1 patient may have missed the assessment at 1 time point and a different patient may have missed it at a subsequent time point), scan characteristics, central reader variability, or other factors. However, the overall trend is relatively favorable in a disease in which the natural history is expected to worsen medullary nephrocalcinosis over time. Regression of nephrocalcinosis has been reported by others during lumasiran treatment.17
Plasma glycolate initially increased before plateauing,12,13,18, 19, 20 which is consistent with the reduction in hepatic glycolate oxidase activity per lumasiran’s mechanism of action.9 Glycolate oxidase suppression by lumasiran appears to be a safe strategy for PH1 treatment.13,21 The only acidosis-related AE observed in ILLUMINATE-A to date occurred in a patient from whom a low bicarbonate sample was drawn during placebo treatment, demonstrating the potential for lumasiran-naive patients with PH1 to develop metabolic acidosis.
The safety profile of lumasiran was consistent with previous observations. Mild, transient injection-site reactions were the most common AEs. None of the cases of injection-site reaction led to treatment discontinuation. The percentage of patients reporting injection-site reactions decreased over time. Two patients tested positive for low-titer ADAs, 1 at month 6 and the other from month 18 to month 36. With any medication, development of ADAs represents a potential barrier to long-term efficacy; however, in these patients, UOx levels remained low. To date, few patients participating in lumasiran clinical trials have tested positive for ADAs.19, 20, 21
This study has several strengths and weaknesses. The patient population is large for a rare disease, and the follow-up duration is relatively long. All patients who entered the extension period remained in the study at month 36. Pediatric patients aged <6 years were excluded; this younger population is being studied in another long-term clinical trial of lumasiran in PH1 (ILLUMINATE-B [NCT03905694]).18 Eligible patients had relatively preserved kidney function; patients with more severe dysfunction are being studied in a third long-term clinical trial of lumasiran in PH1 (ILLUMINATE-C [NCT04152200]).20 As is typical in an extension period, there was no placebo control group or blinding.
In conclusion, in ILLUMINATE-A, long-term treatment with lumasiran through month 36 resulted in sustained reductions in UOx and POx, stable eGFR, decreased kidney stone event rates, and improvements in medullary nephrocalcinosis. The most common AEs related to lumasiran were mild and transient injection-site reactions.
Disclosure
JMS reports grants, personal fees, and non-financial support from Alnylam Pharmaceuticals and consultancy fees from Dicerna Pharmaceuticals. JCL reports grants from Alnylam Pharmaceuticals, Dicerna Pharmaceuticals, Retrophin, OxThera, and Siemens; other support from Novobiome and Orfan-Bridgebio; and grants and other support from Allena and Synlogic. JWG reports grants from Alnylam Pharmaceuticals; and other and study grants from Alnylam Pharmaceuticals, Dicerna Pharmaceuticals, and UniQure Pharmaceuticals.
YF reports consultancy fees from membership in the safety review committee of Alnylam Pharmaceuticals. HS-L reports principal investigator, travel, and accommodation support from Alnylam Pharmaceuticals. DM reports research funding, consultancy fees, and nonfinancial support from Alnylam Pharmaceuticals. SHM reports consultancy fees from Allena Pharmaceuticals, Alnylam Pharmaceuticals, and Dicerna Pharmaceuticals; and principal investigator for research support from OxThera. ES reports principal investigator, travel, and accommodation expenses to attend international investigators’ meeting from Alnylam Pharmaceuticals. MC reports principal investigator support from Alnylam Pharmaceuticals. WH reports principal investigator, consultancy fees, travel, and accommodation from Alnylam Pharmaceuticals. JH reported consultant fees from Alnylam Pharmaceuticals and Traverse; travel fees from Biocodex; and research grant from CareDx. A-LS-L reports consultancy fees from Alnylam Pharmaceuticals and Dicerna Pharmaceuticals; and principal investigator support from OxThera. RW is an employee and a shareholder of Alnylam Pharmaceuticals. JMG is an employee and a shareholder of Alnylam Pharmaceuticals. S-AH reports travel expenses to participate in clinical research meetings, consultancy fee from Advisory Board, and consultancy fees paid to Birmingham Children’s Hospital Renal Research Fund from Alnylam Pharmaceuticals; and other support from Dicerna Pharmaceuticals and Chiesi Pharmaceuticals.
Acknowledgments
We thank the patients, their families, investigators, study staff, and collaborators for their participation in the lumasiran clinical studies. Medical writing and editorial assistance were provided by Karyn Liu, PhD; Jennifer Van Winckel, ELS; and Michael Morren, RPh, MBA, of Peloton Advantage, LLC, an OPEN Health company, in accordance with Good Publication Practice (GPP 2022) guidelines and funded by Alnylam Pharmaceuticals. This study was funded by Alnylam Pharmaceuticals.
Data Availability Statement
Access to anonymized individual participant data that support these results is made available 12 months after study completion and not less than 12 months after the product and indication have been approved in the US and/or the EU.
Data will be provided contingent upon the approval of a research proposal and the execution of a data sharing agreement. Requests for access to data can be submitted via the website www.vivli.org.
The protocol was developed by the sponsor, Alnylam Pharmaceuticals, and is available at NEJM.org (https://doi.org/10.1056/NEJMoa2021712).
Author Contributions
Study conception and design was done by JMG and YF. The acquisition of data was by JMS, JCL, JWG, YF, HS-L, DM, SHM, ES, MC, WH, JH, A-LS-L, and S-AH. Data analysis was done by JMS, JCL, JWG, YF, HS-L, DM, SHM, ES, MC, WH, JH, A-LS-L, JMG, RW, and S-AH. Drafting and revision of manuscript was done by JMS, JCL, JWG, YF, HS-L, DM, SHM, ES, MC, WH, JH, A-LS-L, JMG, RW, S-AH.
Footnotes
Figure S1. Mean (SEM) percent change in 24-hour UOx over time by (A) baseline pyridoxine use, (B) genotype, and (C) baseline pyridoxine use and genotype.
Figure S2. Ratio of 24-hour UOx to the ULNa over time.
Figure S3. Percent change in 24-hour UOx:Cr over time.
Figure S4. Percent change in spot UOx:Cr over time.
Figure S5. Mean (SEM) 24-hour urinary glycolate-to-Cr ratio over time.
Figure S6. Mean (SEM) eGFR over time in subgroups defined by baseline eGFR.
Figure S7. Change from baseline in medullary nephrocalcinosis in patients in the lumasiran/lumasiran group (after 36 months of lumasiran treatment) and placebo/lumasiran group (after 30 months of lumasiran treatment) with medullary nephrocalcinosis at baseline.
Figure S8. Kidney stone event ratesa in the (A) all-lumasiran-treated set overall during lumasiran treatment and (B) by 6-month intervals of lumasiran treatment.
Table S1. Baseline characteristics.
CONSORT.
Supplementary Material
Figure S1. Mean SEM percent change in 24-hour UOx over time by (A) baseline pyridoxine use, (B) genotype, and (C) baseline pyridoxine use and genotype. Figure S2. Ratio of 24-hour UOx to the ULNa over time. Figure S3. Percent change in 24-hour UOx:Cr over time. Figure S4. Percent change in spot UOx:Cr over time. Figure S5. Mean SEM 24-hour urinary glycolate-to-Cr ratio over time. Figure S6. Mean SEM eGFR over time in subgroups defined by baseline eGFR. Figure S7. Change from baseline in medullary nephrocalcinosis in patients in the lumasiran/lumasiran group (after 36 months of lumasiran treatment) and placebo/lumasiran group (after 30 months of lumasiran treatment) with medullary nephrocalcinosis at baseline. Figure S8. Kidney stone event ratesa in the (A) all-lumasiran-treated set overall during lumasiran treatment and (B) by 6-month intervals of lumasiran treatment. Table S1. Baseline characteristics. CONSORT.
References
- 1.Cochat P., Rumsby G. Primary hyperoxaluria. N Engl J Med. 2013;369:649–658. doi: 10.1056/NEJMra1301564. [DOI] [PubMed] [Google Scholar]
- 2.Hoppe B., Martin-Higueras C. Improving treatment options for primary hyperoxaluria. Drugs. 2022;82:1077–1094. doi: 10.1007/s40265-022-01735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandrile G., van Woerden C.S., Berchialla P., et al. Data from a large European study indicate that the outcome of primary hyperoxaluria type 1 correlates with the AGXT mutation type. Kidney Int. 2014;86:1197–1204. doi: 10.1038/ki.2014.222. [DOI] [PubMed] [Google Scholar]
- 4.Fargue S., Harambat J., Gagnadoux M.F., et al. Effect of conservative treatment on the renal outcome of children with primary hyperoxaluria type 1. Kidney Int. 2009;76:767–773. doi: 10.1038/ki.2009.237. [DOI] [PubMed] [Google Scholar]
- 5.Hoyer-Kuhn H., Kohbrok S., Volland R., et al. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin J Am Soc Nephrol. 2014;9:468–477. doi: 10.2215/cjn.06820613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Woerden C.S., Groothoff J.W., Wijburg F.A., Annink C., Wanders R.J., Waterham H.R. Clinical implications of mutation analysis in primary hyperoxaluria type 1. Kidney Int. 2004;66:746–752. doi: 10.1111/j.1523-1755.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- 7.Monico C.G., Rossetti S., Olson J.B., Milliner D.S. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 2005;67:1704–1709. doi: 10.1111/j.1523-1755.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 8.Toussaint C. Pyridoxine-responsive PH1: treatment. J Nephrol. 1998;11:49–50. [PubMed] [Google Scholar]
- 9.Oxlumo (lumasiran) [package insert] Alnylam Pharmaceuticals. https://www.oxlumo.com/
- 10.Oxlumo (lumasiran). [product fact sheet] Alnylam Netherlands. 2022. https://www.alnylam.com/sites/default/files/pdfs/OXLUMO-Product-Fact-Sheet.pdf
- 11.Liebow A., Li X., Racie T., et al. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J Am Soc Nephrol. 2017;28:494–503. doi: 10.1681/asn.2016030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrelfs S.F., Frishberg Y., Hulton S.A., et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N Engl J Med. 2021;384:1216–1226. doi: 10.1056/NEJMoa2021712. [DOI] [PubMed] [Google Scholar]
- 13.Hulton S.A., Groothoff J.W., Frishberg Y., et al. Randomized clinical trial on the long-term efficacy and safety of lumasiran in patients with primary hyperoxaluria type 1. Kidney Int Rep. 2021;7:494–506. doi: 10.1016/j.ekir.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clausen V.A., Cao K.H., Gansner J.M., Robbie G.J., Wu J.T. Quantification of oxalate by novel LC-MS/MS: assay development, validation, and application in lumasiran clinical trials. Bioanalysis. 2023;15:481–491. doi: 10.4155/bio-2022-0227. [DOI] [PubMed] [Google Scholar]
- 15.Dick P.T., Shuckett B.M., Tang B., Daneman A., Kooh S.W. Observer reliability in grading nephrocalcinosis on ultrasound examinations in children. Pediatr Radiol. 1999;29:68–72. doi: 10.1007/s002470050539. [DOI] [PubMed] [Google Scholar]
- 16.Singh P., Vaughan L.E., Schulte P.J., Sas D.J., Milliner D.S., Lieske J.C. Estimated GFR slope across CKD stages in primary hyperoxaluria type 1. Am J Kidney Dis. 2022;80:373–382. doi: 10.1053/j.ajkd.2022.01.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biebuyck N., Destombes C., Prakash R., Boyer O. Is withdrawal of nocturnal hyperhydration possible in children with primary hyperoxaluria treated with RNAi? J Nephrol. 2023;36:1473–1476. doi: 10.1007/s40620-023-01611-1. [DOI] [PubMed] [Google Scholar]
- 18.Sas D.J., Magen D., Hayes W., et al. Phase 3 trial of lumasiran for primary hyperoxaluria type 1: a new RNAi therapeutic in infants and young children. Genet Med. 2022;24:654–662. doi: 10.1016/j.gim.2021.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Hayes W., Sas D.J., Magen D., et al. Efficacy and safety of lumasiran for infants and young children with primary hyperoxaluria type 1: 12-month analysis of the phase 3 ILLUMINATE-B trial. Pediatr Nephrol. 2022;38:1075–1086. doi: 10.1007/s00467-022-05684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michael M., Groothoff J.W., Shasha-Lavsky H., et al. Lumasiran for advanced primary hyperoxaluria type 1: phase 3 ILLUMINATE-C trial. Am J Kidney Dis. 2023;81:145–155.e1. doi: 10.1053/j.ajkd.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Frishberg Y., Deschênes G., Groothoff J.W., et al. Phase 1/2 study of lumasiran for treatment of primary hyperoxaluria type 1: a placebo-controlled randomized clinical trial. Clin J Am Soc Nephrol. 2021;16:1025–1036. doi: 10.2215/cjn.14730920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mean SEM percent change in 24-hour UOx over time by (A) baseline pyridoxine use, (B) genotype, and (C) baseline pyridoxine use and genotype. Figure S2. Ratio of 24-hour UOx to the ULNa over time. Figure S3. Percent change in 24-hour UOx:Cr over time. Figure S4. Percent change in spot UOx:Cr over time. Figure S5. Mean SEM 24-hour urinary glycolate-to-Cr ratio over time. Figure S6. Mean SEM eGFR over time in subgroups defined by baseline eGFR. Figure S7. Change from baseline in medullary nephrocalcinosis in patients in the lumasiran/lumasiran group (after 36 months of lumasiran treatment) and placebo/lumasiran group (after 30 months of lumasiran treatment) with medullary nephrocalcinosis at baseline. Figure S8. Kidney stone event ratesa in the (A) all-lumasiran-treated set overall during lumasiran treatment and (B) by 6-month intervals of lumasiran treatment. Table S1. Baseline characteristics. CONSORT.
Data Availability Statement
Access to anonymized individual participant data that support these results is made available 12 months after study completion and not less than 12 months after the product and indication have been approved in the US and/or the EU.
Data will be provided contingent upon the approval of a research proposal and the execution of a data sharing agreement. Requests for access to data can be submitted via the website www.vivli.org.
The protocol was developed by the sponsor, Alnylam Pharmaceuticals, and is available at NEJM.org (https://doi.org/10.1056/NEJMoa2021712).