Abstract
Introduction
Sex/gender inequities persist in access to kidney transplantation. Whether differences in preemptive referral (i.e., referral before dialysis start) explain this inequity remains unknown.
Methods
All adults (aged 18–79 years; N = 44,204) initiating kidney replacement therapy (KRT; dialysis or transplant) in Georgia (GA), North Carolina (NC), or South Carolina (SC) between 2015 and 2019 were identified from the United States Renal Data System (USRDS). Individuals were linked to the Early Steps to Kidney Transplant Access Registry (E-STAR) to obtain data on preemptive referral and followed-up with through November 13, 2020, for outcomes of waitlisting and living donor transplant. Logistic regression assessed the association between sex/gender and likelihood of preemptive referral among all KRT patients. Cox-proportional hazards assessed the association between sex/gender and waitlisting or living donor among preemptively referred patients.
Results
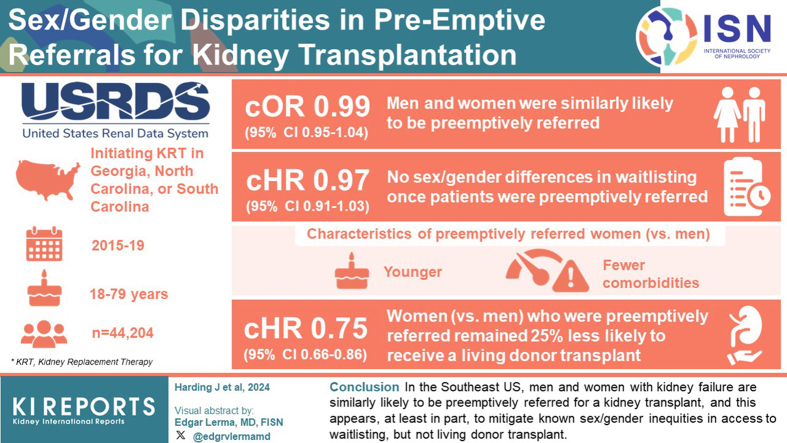
Overall, men and women were similarly likely to be preemptively referred (odds ratio [OR]: 0.99 [0.95–1.04]). Preemptively referred women (vs. men) were, on average, younger and with fewer comorbidities. There were no sex/gender differences in waitlisting once patients were preemptively referred (hazard ratio [HR]: 0.97 [0.91–1.03]); however, women (vs. men) who were preemptively referred remained 25% (HR: 0.75 [0.66–0.86]) less likely to receive a living donor transplant.
Conclusion
In the Southeast US, men and women initiating KRT are similarly likely to be preemptively referred for a kidney transplant, and this appears, at least in part, to mitigate known sex/gender inequities in access to waitlisting, but not living donor transplant. Despite this, preemptively referred women, on average, had a more favorable medical profile relative to preemptively referred men.
Keywords: epidemiology, gender disparities, health services research, kidney transplant, transplant referral
Graphical abstract
See Commentary on Page 1951
Transplantation remains the preferred treatment for the majority of people with kidney failure because it prolongs survival, improves quality of life, and provides cost saving relative to dialysis.1 Importantly, patients with kidney failure who receive a preemptive transplant (i.e., before initiating dialysis), experience better health outcomes following transplantation, including improved graft and patient survival.2 Unfortunately, access to transplantation is not equitable. National data show women are 10% to 20% less likely than men to be waitlisted and transplanted, including living donor transplant, despite similar or better posttransplant survival,3, 4, 5, 6, 7 and despite being more likely to be preemptively waitlisted (i.e., before dialysis initiation) compared to men.7
Whether the sex/gender inequity in access to overall transplant is caused by delayed referral for transplant evaluation among women is unknown due to a lack of national surveillance data collected on the necessary early transplant steps of referral and evaluation, including preemptive referral (i.e., referral before dialysis initiation). The E-STAR8 is the only regional registry with data on transplant referral and evaluation. Using E-STAR, we have previously shown that women with kidney failure are 14% less likely to be referred within 12 months of dialysis initiation than men after adjustment for several clinical and sociodemographic factors.9 We also found that the sex/gender disparity in referral was greatest in women with diabetes-attributed kidney failure, obesity, older age, and in White and Black women.10,11 Whether the rates of preemptive referral contribute to this sex/gender disparity remain unknown.
Therefore, the primary aim of this study was to examine sex/gender inequities in pre-emptive referral for kidney transplantation evaluation in the Southeast US, and to examine associations with subsequent rates of waitlisting and living donor transplant.
Methods
Study Population and Data Sources
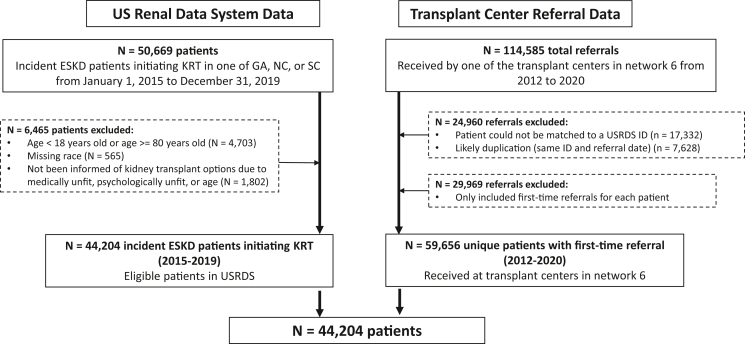
To create a cohort of all patients with kidney failure in the region who may have been preemptively referred for transplant, we identified all patients (aged 18 to <80 years) initiating KRT for the first time (i.e., dialysis or transplant) between January 1, 2015 and December 31, 2019 in End-Stage Renal Disease Network 6 (comprised the states of GA, NC, and SC) from the USRDS, a national registry of all patients in the United States initiating KRT.1 We excluded patients who were missing information on race (n = 565), and those who were listed as unsuitable transplant candidates on the USRDS Centers for Medicare and Medicaid Services (CMS) form 2728 (i.e., medically unfit, unsuitable due to age, or psychologically unfit; n = 1802). The final cohort included 44,204 people initiating KRT between 2015 and 2019 (Figure 1).
Figure 1.
Flow diagram of study inclusion and exclusion criteria for study population. GA, Georgia; ID, identification; NC, North Carolina; SC, South Carolina; USRDS, United States Renal Data System.
Sex/Gender
“Sex” is documented by dialysis center staff on CMS form 2728 completed within 45 days of dialysis initiation. In general, “sex” refers to biological characteristics linked to sexual reproduction in males and females, including genetics, gonads, and genitals. “Gende”’ refers to social and cultural norms, roles, behaviors, and interactions among women and men. Here, we use the variable of provider-perceived sex, which may actually capture provider-perceived gender, as a proxy for gender dimensions of interest that likely impact transplant access, including gender identity, expression, roles, expectations, and experiences of sexism, and thus opt for the term sex/gender throughout.12,13 As of October 2022, CMS form 2728 only contains “male” and “female” options for sex.
Outcomes
Outcome data were obtained by linking individuals in our USRDS cohort to patient-level referral data obtained from the E-STAR,8,14 a voluntary registry of transplant referral and evaluation forms collected from all 9 adult transplant centers in the End-Stage Renal Disease Network 6 (i.e., 100% coverage). With this unique E-STAR-linked-USRDS registry, we identified all referrals to transplant centers (from E-STAR) among individuals who initiate KRT (from USRDS) (Figure 1). The primary outcome examined was preemptive referral for kidney transplant evaluation. The date of referral is defined as the date the transplant center receives the referral (determined from E-STAR). Individuals who were preemptively waitlisted (i.e., waitlisted before KRT initiation), or where transplant was the first modality of KRT, were considered to be preemptively referred. Nonpreemptively referred individuals included those who were never referred and those who were referred after dialysis initiation. We chose to include patients not referred at all owing to the known sex/gender inequities in likelihood of referral after dialysis.9 Secondary outcomes, examined among preemptively referred patients included placement on the deceased donor waiting list or receipt of a living donor transplant (determined from USRDS), evaluated separately, with follow-up through November 13, 2020.
Covariates
“Patient-level characteristics,” as recorded in USRDS at time of KRT start, were ascertained from the CMS form 2728. Key variables of interest included attributed cause of kidney failure (diabetes, hypertension, glomerulonephritis, other), age (18–44, 45–64, and 65–80 years), race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and “other;” where other is made up of Middle Eastern, American Indian or Alaskan Native, Asian, Indian, Pacific Islander, and multiracial), and obesity as measured by body mass index (underweight: < 18.5 kg/m2, normal weight: 18.5–24 kg/m2, overweight: 25–29 kg/m2, obese class I: 30–34 kg/m2, obese class II: 35–40 kg/m2; and obese class III: >40 kg/m2). Other variables of interest collected on the CMS2728 form included access to pre-KRT nephrology care (yes, no), comorbidities (smoking status, congestive heart failure, diabetes, atherosclerotic heart disease, other cardiac disease, cerebrovascular disease, peripheral vascular disease, and cancer), transplant education (informed of transplant yes/no) and insurance status (no insurance, Medicaid, Medicare, private, or other). For insurance status, where patients indicated they had >1 insurance provider, we categorized them using a hierarchy of private, Medicaid, Medicare, and “other” to best reflect the highest level of socioeconomic status (private), followed by the lowest (Medicaid), the most common for KRT patients (Medicare) and all other insurance. For all nonprimary variables, excluding pre-KRT nephrology care, 5.2% of data were missing. For pre-KRT nephrology care, 13.1% of data were missing. Further, due to the high proportion of preemptively referred patients (i.e., > 95%) who reported having pre-KRT nephrology care, we did not adjust for this variable in models, as described below.
“Neighborhood-level characteristics” were determined from the 2014 to 2018 American Community Survey using patient 5-digit ZIP code linked to USRDS data and included poverty (≥ or < 20% of ZIP code living in poverty), percentage of Black individuals, and percentage of high school graduates. When summarizing this data, we report the average percentage of Black individuals or high school graduates across all ZIP codes.
Statistical Analysis
Baseline demographic and clinical characteristics among the incident KRT population, by sex/gender or preemptive referral status, were summarized using frequencies and proportions, or means and SDs; and compared using chi-square and t tests as appropriate.
Bivariable analyses were performed to determine the association between patient-level and neighborhood-level characteristics and preemptive referral. To assess the association between sex/gender and likelihood of preemptive referral, we used crude and multivariable-adjusted logistic regression. We included multivariable-adjusted models using a complete case analysis approach to explore if differences in transplant access were explained by underlying risk factors or comorbidities, including (when applicable) age, race and ethnicity, obesity, insurance, primary cause of kidney failure, transplant education, comorbidities, and neighborhood-level characteristics among individuals with complete data. We did this overall (i.e., all variables in the model), and using a forward stepwise approach to ascertain if any one variable was driving observed changes in OR estimates. Most of these factors likely lie on the causal pathway between sex/gender and transplant access and should therefore be considered as potential mediators of the associations under study rather than confounders as described by Swartling et al.15 in a prominent study examining sex/gender disparities in the recognition, monitoring, and management of chronic kidney disease (CKD). We therefore decided a priori to present crude risks as our primary results and consider adjusted results in our interpretations. We also performed interaction tests for potential effect modification of the association between sex/gender and preemptive referral by age, race, obesity, and attributed cause of kidney failure based on our prior work.9, 10, 11 Stratifications by race consider race as a proxy for social and structural forms of racism and/or bias. Given the lack of statistical power inherent in interaction tests, an interaction was deemed to be statistically significant using a P-value cut point of P < 0.2 in crude models,16 at which point stratification by that factor (e.g., age, race, or obesity) was performed.
To determine if preemptive referral was associated with placement on the deceased donor waitlist or receipt of living donor transplant, we used crude and adjusted Cox-proportional hazards models overall and stratified by sex/gender. Individuals were followed-up with from the date of first KRT (dialysis or transplant) until waitlisting, living donor transplant, death (a censored event), or end of follow-up (November 1, 2020), whichever occurred first. All patients were followed from the first KRT instead of referral date to ensure we did not artificially inflate time-to-event for those who were preemptively referred. For individuals who were preemptively waitlisted (i.e., before dialysis start), follow-up time to waitlisting was estimated as 1 day. For individuals whose first KRT was transplant, follow-up time to transplant was also estimated as 1 day. In sensitivity analyses, we restricted this analysis to all individuals who had been referred.
Finally, to examine if preemptive referral eliminates or reduces sex/gender disparities in downstream transplant steps, we used Cox-proportional hazards models to examine the association between sex/gender and waitlisting and living donor transplant among all patients who were preemptively referred. Follow-up time as well as crude and multivariable models were performed as described above. We performed interaction tests to determine if the relationship between sex/gender and waitlisting and living donor transplant among preemptively referred patients was modified by age, race, obesity, and attributed cause of kidney failure. Finally, in additional sensitivity analyses, we performed competing risk analyses using Fine-Gray models treating death as a competing risk for all outcomes. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC). Figures were created GraphPad Prism 9.5.0. for Windows (GraphPad Software, Boston, MA, www.graphpad.com).
This study adheres to the STROBE guidelines for observational studies (Supplementary Table S1), adheres to the Declaration of Helsinki, and was approved by the institutional review board at Emory University (IRB00113572). A waiver of informed consent was approved by Emory Institutional Review Board. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Results
Baseline Characteristics
We included 44,204 adult patients initiating KRT (mean [SD] age: 58.8 [13.2] years; 44.4% women; and 52.8% Black) in GA, SC, and NC between December 2015 and December 2019. Overall, 7841 (17.7%) were preemptively referred, of whom 2856 (36.4%) were preemptively waitlisted and 939 (12.0%) preemptively transplanted.
By sex/gender, women (vs. men) initiating KRT were more likely to have type 2 diabetes as the primary cause of kidney failure, administratively identify as Black race, have a higher body mass index, have Medicaid insurance, have pre-KRT care, and to live in a neighborhood with a higher poverty level and greater proportion of Black residents at time of KRT initiation (Table 1). Men and women were similarly likely to be informed of transplant as a treatment option at KRT initiation, and to have similar neighborhood-level education. In addition, comorbidities were similar between men and women, excluding diabetes, which was more common among women; and previous tobacco use, which was more common among men.
Table 1.
Baseline characteristics of patients initiating kidney replacement therapy from 2015 to 2019, overall and stratified by sex/gender, in the Southeast US
| Characteristics | Total | Women | Men |
|---|---|---|---|
| N | 44,204 | 19,619 | 24,585 |
| Patient-level characteristics | |||
| Age, yr | |||
| Mean ± SD | 58.8 ± 13.2 | 59.3 ± 13.3 | 58.5 ± 13.1 |
| 18–29, n (%) | 1297 (2.9) | 631 (3.2) | 666 (2.7) |
| 30–39, n (%) | 2953 (6.7) | 1278 (6.5) | 1675 (6.8) |
| 40–49, n (%) | 5953 (13.5) | 2396 (12.2) | 3557 (14.5) |
| 50–59, n (%) | 10,145 (23.0) | 4227 (21.6) | 5918 (24.1) |
| 60–69, n (%) | 13,487 (30.5) | 6253 (31.9) | 7234 (29.4) |
| 70–79, n (%) | 10,369 (23.5) | 4834 (24.6) | 5535 (22.5) |
| Race/ethnicity group, n (%) | |||
| Non-Hispanic White | 18,472 (41.8) | 7634 (38.9) | 10,838 (44.1) |
| Black | 23,348 (52.8) | 11,033 (56.2) | 12,315 (50.1) |
| Hispanic | 1355 (3.1) | 511 (2.6) | 844 (3.4) |
| Other | 1029 (2.3) | 441 (2.3) | 588 (2.4) |
| Insurance status, n (%) | |||
| Medicaid | 9603 (21.7) | 5435 (27.7) | 4168 (17.0) |
| Medicare | 17,659 (40.0) | 7853 (40.0) | 9806 (39.9) |
| Employer | 8976 (20.3) | 3617 (18.4) | 5,359 (21.8) |
| Other | 3709 (8.4) | 1166 (5.9) | 2543 (10.3) |
| None | 4257 (9.6) | 1548 (7.9) | 2709 (11.0) |
| Attributed cause of kidney failure, n (%) | |||
| Diabetes | 20,176 (46.3) | 9,300 (48.1) | 10,876 (44.9) |
| Hypertension | 15,452 (35.5) | 6478 (33.5) | 8974 (37.1) |
| Glomerulonephritis | 3261 (7.5) | 1606 (8.3) | 1655 (6.8) |
| Other | 4659 (10.7) | 1960 (10.1) | 2699 (11.2) |
| Obesity (BMI, kg/m2) | |||
| Mean BMI ± SD | 30.6 ± 8.3 | 31.6 ± 9.1 | 29.8 ± 7.6 |
| Underweight, n (%) | 1218 (2.8) | 609 (3.1) | 609 (2.5) |
| Normal, n (%) | 10,605 (24.1) | 4378 (22.4) | 6227 (25.4) |
| Overweight, n (%) | 11,918 (27.1) | 4533 (23.2) | 7385 (30.2) |
| Obese class I, n (%) | 9,261 (21.0) | 3997 (20.5) | 5264 (21.5) |
| Obese class II, n (%) | 5546 (12.6) | 2799 (14.3) | 2747 (11.2) |
| Obese class III, n (%) | 5467 (12.4) | 3202 (16.4) | 2265 (9.3) |
| Comorbidities, n (%) | |||
| Congestive heart failure | 11,623 (26.3) | 5329 (27.2) | 6294 (25.6) |
| Atherosclerotic heart disease | 3737 (8.5) | 1477 (7.5) | 2260 (9.2) |
| Other cardiac disease | 7483 (16.9) | 3097 (15.8) | 4386 (17.8) |
| Cerebrovascular disease | 4007 (9.1) | 1820 (9.3) | 2187 (8.9) |
| Peripheral vascular disease | 3307 (7.5) | 1271 (6.5) | 2036 (8.3) |
| Hypertension | 39,870 (90.2) | 17,744 (90.4) | 22,126 (90.0) |
| Diabetes | 26,838 (60.7) | 12,376 (63.1) | 14,462 (58.8) |
| COPD | 3696 (8.4) | 1792 (9.1) | 1904 (7.7) |
| Cancer | 2416 (5.5) | 952 (4.9) | 1464 (6.0) |
| Tobacco use | 3855 (8.7) | 1379 (7.0) | 2476 (10.1) |
| Pre-KRT nephrology care, n (%) | 30,496 (79.3) | 13,787 (80.8) | 16,709 (78.2) |
| Patient has been informed of kidney transplant options, n (%) | 39,861 (92.1) | 17,712 (92.4) | 22,149 (92.0) |
| Neighborhood-level factors | |||
| Neighborhood poverty level, n (%) | |||
| < 20% (low poverty) | 26,021 (59.7) | 11,175 (57.7) | 14,846 (61.3) |
| ≥ 20% (high poverty) | 17,570 (40.3) | 8187 (42.3) | 9383 (38.7) |
| Average % Black (mean ± SD) | 34.1 ± 23.7 | 35.4 ± 23.9 | 33.1 ± 23.5 |
| Average % high school graduates (mean ± SD) | 85.2 ± 6.8 | 85.0 ± 6.6 | 85.3 ± 6.9 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; ESKD, end-stage kidney disease; KRT, kidney replacement therapy.
Percent missing: 1.5% primary cause of ESKD, 0.4% BMI, 13.0% pre-KRT nephrology care, 2.1% patient informed of kidney transplant options, 1.4% neighborhood poverty level, 1.3% average percent Black, and 1.3% average percent high school graduates.
Compared to preemptively referred men, preemptively referred women were on average younger, more likely to be Black, more likely to be Medicaid insured, be underweight or of normal body mass index, and have fewer comorbidities (Supplementary Table S2).
Association Between Sex/Gender and Preemptive Referral
Overall, 17.7% of women and 17.8% of men initiating KRT were preemptively referred (Table 2 and Supplementary Table S2). In crude models, sex/gender was not associated with the likelihood of preemptive referral (OR: 0.99 [95% confidence interval: 0.95–1.04]) (Table 2). In fully adjusted models, women (vs. men) were more likely to be preemptively referred (1.09 [1.03–1.15]). Stepwise analysis indicated that adjustment for age and race was similar to crude models (1.04 [0.99–1.04]), whereas additional adjustment for insurance status and obesity (factors that lie on the causal pathway between sex/gender and transplant access and are unequally distributed across sex/gender) largely explained the change in OR between crude and adjusted models (Supplementary Table S3).
Table 2.
Association between patient and neighborhood-level characteristics, including sex/gender, and preemptive referral among patients initiating KRT from 2015 to 2019, with follow-up through 2020
| Characteristics | Preemptively referred | Not preemptively referred | Odds ratio (OR) reporting the association between each characteristic and preemptive referral |
|
|---|---|---|---|---|
| Crude OR (95%CI) | Adjusted OR (95% CI)a | |||
| N (%) | 7841 (17.7) | 36,363 (82.3) | ||
| Patient-level characteristics | ||||
| Sex/gender | ||||
| Men | 4371 (55.8) | 20,214 (55.6) | reference | reference |
| Women | 3470 (44.3) | 16,149 (44.4) | 0.99 (0.95–1.04) | 1.09 (1.03–1.15) |
| Age | ||||
| 18–29 | 316 (4.0) | 981 (2.7) | reference | reference |
| 30–39 | 721 (9.2) | 2232 (6.1) | 1.00 (0.86–1.17) | 1.19 (0.99–1.42) |
| 40–49 | 1383 (17.6) | 4570 (12.6) | 0.94 (0.82–1.08) | 1.10 (0.93–1.30) |
| 50–59 | 2053 (26.2) | 8092 (22.3) | 0.79 (0.69–0.90) | 0.93 (0.79–1.10) |
| 60–69 | 2431 (31.0) | 11,056 (30.4) | 0.68 (0.60–0.78) | 0.82 (0.69–0.96) |
| 70–79 | 937 (12.0) | 9432 (25.9) | 0.31 (0.27–0.36) | 0.36 (0.30–0.43) |
| Race/ethnicity group | ||||
| White | 3768 (48.1) | 14,704 (40.4) | reference | reference |
| Black | 3643 (46.5) | 19,705 (54.2) | 0.72 (0.69–0.76) | 0.84 (0.78–0.89) |
| Hispanic | 184 (2.4) | 1171 (3.2) | 0.61 (0.52–0.72) | 0.63 (0.53–0.76) |
| Other | 246 (3.1) | 783 (2.2) | 1.23 (1.06–1.42) | 1.08 (0.91–1.28) |
| Insurance status | ||||
| Medicaid | 1157 (14.8) | 8446 (23.2) | 0.25 (0.23–0.27) | 0.39 (0.36–0.43) |
| Medicare | 2546 (32.5) | 15,113 (41.6) | 0.31 (0.29–0.33) | 0.57 (0.53–0.62) |
| Employer | 3189 (40.7) | 5787 (15.9) | reference | reference |
| Other | 732 (9.3) | 2977 (8.2) | 0.45 (0.41–0.49) | 0.57 (0.53–0.62) |
| None | 217 (2.8) | 4040 (11.1) | 0.10 (0.08–0.11) | 0.12 (0.10–0.14) |
| Attributed cause of kidney failure | ||||
| Diabetes | 3174 (41.2) | 17,002 (47.4) | reference | reference |
| Hypertension | 2275 (29.6) | 13,177 (36.8) | 0.93 (0.87–0.98) | 0.89 (0.83–0.96) |
| Glomerulonephritis | 1045 (13.6) | 2216 (6.2) | 2.53 (2.33–2.74) | 1.47 (1.31–1.64) |
| Other | 1206 (15.7) | 3453 (9.6) | 1.87 (1.73–2.02) | 1.14 (1.02–1.26) |
| Obesity (BMI, kg/m2) | ||||
| Underweight | 126 (1.6) | 1092 (3.0) | 0.59 (0.49–0.72) | 0.58 (0.47–0.73) |
| Normal | 1,733 (22.2) | 8872 (24.5) | reference | reference |
| Overweight | 2374 (30.4) | 9544 (26.4) | 1.27 (1.19–1.36) | 1.22 (1.13–1.32) |
| Obese class I | 1928 (24.7) | 7333 (20.3) | 1.35 (1.25–1.45) | 1.27 (1.17–1.38) |
| Obese class II | 1067 (13.7) | 4479 (12.4) | 1.22 (1.12–1.33) | 1.14 (1.03–1.25) |
| Obese class III | 588 (7.5) | 4879 (13.5) | 0.62 (0.56–0.68) | 0.58 (0.52–0.65) |
| Comorbidities | ||||
| Congestive heart failure | 1019 (13.0) | 10,604 (29.2) | 0.36 (0.34–0.39) | 0.53 (0.49–0.57) |
| Atherosclerotic heart disease | 445 (5.7) | 3292 (9.1) | 0.60 (0.55–0.67) | 1.04 (0.93–1.17) |
| Other cardiac disease | 904 (11.5) | 6579 (18.1) | 0.59 (0.55–0.64) | 0.85 (0.78–0.92) |
| Cerebrovascular disease | 395 (5.0) | 3612 (9.9) | 0.48 (0.43–0.54) | 0.66 (0.59–0.74) |
| Peripheral vascular disease | 336 (4.3) | 2971 (8.2) | 0.50 (0.45–0.57) | 0.78 (0.69–0.89) |
| Hypertension | 7097 (90.5) | 32,773 (90.1) | 1.04 (0.96–1.14) | 1.35 (1.22–1.50) |
| Diabetes | 4129 (52.7) | 22,709 (62.5) | 0.67 (0.64–0.70) | 0.89 (0.83–0.96) |
| COPD | 246 (3.1) | 3450 (9.5) | 0.31 (0.27–0.35) | 0.56 (0.48–0.65) |
| Cancer | 299 (3.8) | 2117 (5.8) | 0.64 (0.57–0.73) | 0.70 (0.61–0.80) |
| Tobacco Use | 364 (4.6) | 3491 (9.6) | 0.46 (0.41–0.51) | 0.61 (0.54–0.69) |
| Pre-KRT nephrology care (reference = yes) | 6877 (95.1) | 23,619 (75.7) | 0.16 (0.15–0.18) | n/ab |
| Patient has been informed of kidney transplant options (reference = yes) | 6587 (95.4) | 33,274 (91.5) | 0.52 (0.46–0.58) | 0.62 (0.55–0.70) |
| Neighborhood-level factors | ||||
| Neighborhood poverty level | ||||
| < 20% (low poverty) | 5399 (69.9) | 20,622 (57.5) | reference | reference |
| ≥ 20% (high poverty) | 2326 (30.1) | 15,244 (42.5) | 0.58 (0.55–0.61) | 0.88 (0.81–0.94) |
| Average % Black (per 10% increase) | 30.9 ± 23.3 | 34.8 ± 23.7 | 0.93 (0.92–0.94) | 0.98 (0.97–1.00) |
| Average % high school graduates (per 10% increase) | 86.8 ± 6.7 | 84.8 ± 6.7 | 1.59 (1.53–1.66) | 1.29 (1.23–1.36) |
BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; KRT, kidney replacement therapy; OR, odds ratio.
Values are presented as n (%) and mean ± SD.
Multivariable model adjusted for all characteristics in Table 2, excluding pre-KRT nephrology care; 5.2% missing data and excluded from multivariable model.
Pre-KRT nephrology care not included in multivariable models.
Other factors associated with a reduced likelihood of preemptive referral included older (vs. younger) age, Black or Hispanic (vs. White) race, nonemployer (vs. employer) -based insurance, diabetes or hypertension as the primary cause of kidney failure (vs. other causes), most comorbidities (vs. no comorbidity), obese class III, and living in neighborhoods with high poverty, higher proportion of Black residents, and lower education (Table 2).
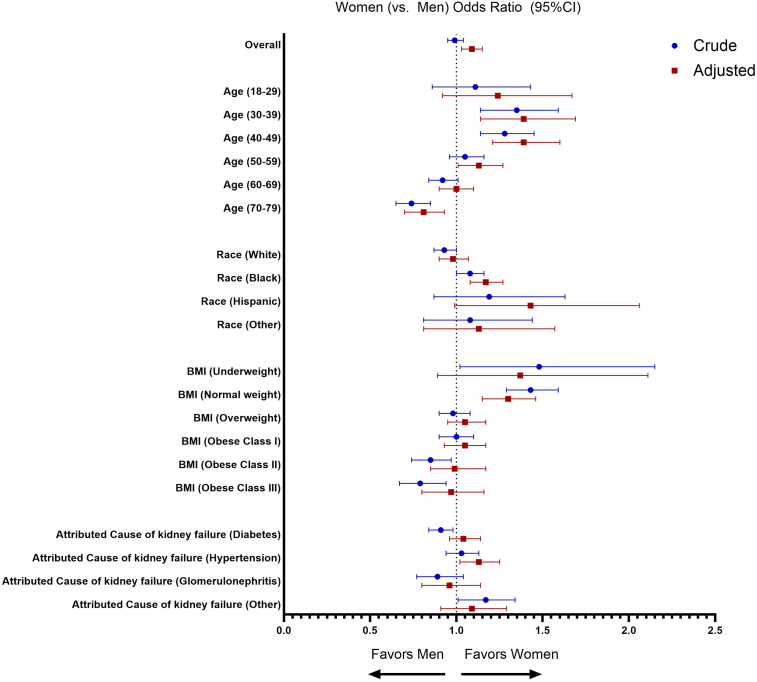
There were some differences among sex/gender subgroups. Younger women (aged 18–49 years) were 11% to 35% more likely to be preemptively referred, whereas older (aged 70–79 years) women were 26% less likely to be preemptively referred than men of the same age (Figure 2 and Supplementary Table S4). White women were 7% less likely to be preemptively referred than White men, whereas Black women were 8% more likely to be preemptively referred than Black men, with no sex/gender inequities observed among other race groups. Underweight or normal weight women were 43% to 48% more likely to be preemptively referred, whereas obese women were 15% to 21% less likely to be preemptively referred than men of the same weight. Finally, women (vs. men) with diabetes as attributed cause of kidney failure were 8% less likely to be preemptively referred, whereas women (vs. men) with kidney failure attributed to “other causes” were 18% more likely to be preemptively referred.
Figure 2.
Association between sex/gender and likelihood of preemptive referral overall and by subgroups of age, race, obesity, and attributed cause of kidney failure in crude (blue) and multivariable-adjusted (red) logistic regression models. CI, confidence interval.
Association Between Sex/Gender, Preemptive Referral, and Subsequent Waitlisting and Living Donor Receipt
Overall, patients who were preemptively referred were 8.2 times (HR: 8.23 [95% confidence interval: 7.88–8.59]) more likely to be waitlisted, and 14.7 times (14.72 [12.96–16.72]) more likely to receive a living donor transplant, compared to patients who were not preemptively referred (Table 3). This effect was similar in women and in men, and when accounting for the competing risk of death or deceased donor transplant (Supplementary Table S5). Patterns were similar but effect sizes reduced when the study population was restricted to only patients who had been referred (i.e., preemptively referred or referred after dialysis). Crude HR for waitlisting and living donor transplantation were 4.19 (4.01–4.40) and 8.20 (7.17–9.38), respectively (Supplementary Table S6).
Table 3.
Crude and adjusted hazards ratios for the association between preemptive referral and placement on the deceased donor waitlist or receipt of a living donor transplant, overall and by sex/gender, among patients initiating kidney replacement therapy between 2015 and 2019 with follow-up through 2020 in the Southeast US
| Outcome | Crude HR (95% CI) | Adjusted HR (95% CI)a |
|---|---|---|
| Waitlisting | HR examining association between preemptive referral and waitlisting | |
| Total population | 8.23 (7.88–8.59) | 5.06 (4.82–5.32) |
| Women | 9.49 (8.86–10.16) | 5.50 (5.10–5.92) |
| Men | 7.47 (7.06–7.90) | 4.78 (4.49–5.09) |
| Living donor transplantation | HR examining association between preemptive referral and receipt of living donor transplant | |
| Total population | 14.72 (12.96–16.72) | 4.10 (3.51–4.80) |
| Women | 18.33 (14.61–22.99) | 4.51 (3.46–5.89) |
| Men | 13.19 (11.31–15.40) | 3.92 (3.26–4.72) |
CI, confidence interval; HR, hazard ratio; KRT, kidney replacement therapy.
Multivariable model adjusted for all characteristics in Table 2, excluding pre-KRT nephrology care; 5.2% missing.
Among patients who were preemptively referred, there was no difference in likelihood of waitlisting in women (vs. men) in both crude (HR: 0.97 [0.91–1.03]) and adjusted (1.00 [0.93–1.07]) models (Table 4). However, preemptively referred women (vs. men) remained less likely to receive a living donor in crude (0.75 [0.66–0.86]) and adjusted (0.73 [0.60–0.89]) models (Table 5). Patterns were similar by age, race, obesity, and attributed cause of kidney failure subgroups, with some exceptions as follows: women (vs. men) with diabetes-attributed kidney failure were less likely to be waitlisted, whereas women with normal weight were more likely to be waitlisted compared with their normal weight male counterparts (Table 4).
Table 4.
Hazard ratios reporting the association between sex/gender (men as reference group) and access to the waitlist among patients preemptively referred for kidney transplant between 2015 and 2019, and followed-up until 2020, overall and stratified by age, race, obesity, and attributed cause of kidney failure
| Subgroup | N (%) with the outcome | Crude HR (95% CI) | P-value for interaction | Adjusted HR (95% CI)a | P-value for interaction |
|---|---|---|---|---|---|
| Waitlisting | HR examining association between sex/gender and waitlisting among patients preemptively referred | ||||
| Overall | 4575 (58.4) | 0.97 (0.91–1.03) | 1.00 (0.93–1.07) | ||
| Age, yr | |||||
| 18–29 | 219 (69.3) | 0.75 (0.58–0.98) | 0.16 | 0.81 (0.59–1.10) | 0.049 |
| 30–39 | 443 (61.4) | 1.01 (0.84–1.22) | 1.07 (0.87–1.33) | ||
| 40–49 | 907 (65.6) | 1.05 (0.92–1.20) | 1.18 (1.02–1.37) | ||
| 50–59 | 1250 (60.9) | 1.00 (0.90–1.12) | 1.02 (0.90–1.16) | ||
| 60–69 | 1349 (55.5) | 0.92 (0.82–1.02) | 0.93 (0.82–1.05) | ||
| 70–79 | 407 (43.4) | 0.85 (0.70–1.04) | 0.83 (0.66–1.04) | ||
| Race/ethnicity group | |||||
| White | 2351 (62.4) | 1.00 (0.92–1.08) | 0.44 | 0.97 (0.88–1.07) | 0.51 |
| Black | 1916 (52.6) | 0.96 (0.88–1.05) | 1.00 (0.91–1.11) | ||
| Hispanic | 133 (72.3) | 1.11 (0.79–1.56) | 1.20 (0.82–1.75) | ||
| Other | 175 (71.1) | 1.22 (0.90–1.64) | 1.18 (0.86–1.64) | ||
| Obesity (BMI, kg/m2) | |||||
| Underweight | 64 (50.8) | 0.79 (0.49–1.30) | 0.05 | 0.64 (0.35–1.20) | 0.45 |
| Normal | 1008 (58.2) | 1.18 (1.05–1.34) | 1.09 (0.95–1.26) | ||
| Overweight | 1466 (61.8) | 0.95 (0.85–1.06) | 0.98 (0.87–1.11) | ||
| Obese class I | 1225 (63.5) | 0.95 (0.85–1.06) | 0.95 (0.83–1.08) | ||
| Obese class II | 596 (55.9) | 0.97 (0.83–1.14) | 1.05 (0.88–1.25) | ||
| Obese class III | 206 (35.0) | 0.87 (0.66–1.14) | 0.93 (0.70–1.24) | ||
| Attributed cause of kidney failure | |||||
| Diabetes | 1434 (45.2) | 0.83 (0.75–0.92) | 0.01 | 0.88 (0.78–0.98) | 0.04 |
| Hypertension | 1263 (55.5) | 1.01 (0.90–1.12) | 1.08 (0.95–1.21) | ||
| Glomerulonephritis | 811 (77.6) | 0.97 (0.84–1.11) | 1.04 (0.88–1.22) | ||
| Other | 967 (80.2) | 1.09 (0.96–1.23) | 1.10 (0.94–1.30) | ||
BMI, body mass index; CI, confidence interval; ESKD, end-stage kidney disease; HR, hazard ratio; KRT, kidney replacement therapy.
Multivariable model adjusted for all characteristics in Table 2, excluding pre-ESKD nephrology care.
Table 5.
Hazard ratios reporting the association between sex/gender (men as reference group) and access to living donor transplantation among patients preemptively referred for kidney transplant between 2015 and 2019, and followed-up until 2020, overall and stratified by age, race, obesity, and attributed cause of kidney failure
| Subgroup | N (%) with the outcome | Crude HR (95% CI) | p-value for interaction | Adjusted HR (95% CI)a | P-value for interaction |
|---|---|---|---|---|---|
| Living donor transplantation | HR examining association between sex/gender and living donor transplantation among patients preemptively referred | ||||
| Overall | 940 (12.0) | 0.75 (0.66–0.86) | 0.73 (0.60–0.89) | ||
| Age, yr | |||||
| 18–29 | 86 (27.2) | 0.61 (0.39–0.94) | 0.18 | 0.64 (0.36–1.14) | 0.49 |
| 30–39 | 125 (17.3) | 0.76 (0.53–1.09) | 1.01 (0.61–1.66) | ||
| 40–49 | 221 (16.0) | 0.74 (0.57–0.98) | 0.67 (0.45–1.00) | ||
| 50–59 | 250 (12.2) | 0.89 (0.69–1.15) | 0.87 (0.60–1.26) | ||
| 60–69 | 198 (8.1) | 0.54 (0.40–0.73) | 0.56 (0.35–0.90) | ||
| 70–79 | 60 (6.4) | 0.92 (0.55–1.55) | 0.55 (0.24–1.30) | ||
| Race/ethnicity group | |||||
| White | 702 (18.6) | 0.89 (0.76–1.04) | 0.23 | 0.72 (0.57–0.91) | 0.50 |
| Black | 166 (4.6) | 0.71 (0.52–0.97) | 0.76 (0.51–1.14) | ||
| Hispanic | 31 (16.9) | 0.49 (0.22–1.10) | 0.39 (0.14–1.07) | ||
| Other | 41 (16.7) | 1.14 (0.62–2.11) | 1.00 (0.50–1.99) | ||
| Obesity (BMI, kg/m2) | |||||
| Underweight | 18 (14.3) | 1.43 (0.54–3.80) | 0.02 | 2.92 (0.34–25.03) | 0.64 |
| Normal | 255 (14.7) | 1.10 (0.86–1.41) | 0.83 (0.59–1.17) | ||
| Overweight | 320 (13.5) | 0.63 (0.49–0.80) | 0.65 (0.45–0.93) | ||
| Obese class I | 224 (11.6) | 0.68 (0.51–0.89) | 0.67 (0.44–1.02) | ||
| Obese class II | 97 (9.1) | 0.69 (0.46–1.04) | 0.78 (0.45–1.35) | ||
| Obese class III | 24 (4.1) | 0.63 (0.28–1.41) | 0.49 (0.17–1.38) | ||
| Attributed cause of kidney failure | |||||
| Diabetes | 191 (6.0) | 0.63 (0.47–0.85) | 0.02 | 0.73 (0.49–1.08) | 0.34 |
| Hypertension | 166 (7.3) | 0.56 (0.40–0.78) | 0.58 (0.39–0.88) | ||
| Glomerulonephritis | 249 (23.8) | 0.68 (0.53–0.88) | 0.68 (0.48–0.98) | ||
| Other | 298 (24.7) | 0.98 (0.78–1.23) | 0.97 (0.66–1.42) | ||
BMI, body mass index; CI, confidence interval; ESKD, end-stage kidney disease; HR, hazard ratio; KRT, kidney replacement therapy.
Multivariable model adjusted for all characteristics in Table 2, excluding pre-ESKD nephrology care.
Discussion
Among patients who initiated KRT (dialysis or transplantation) in the Southeast US, we show that similar proportions (∼18%) of men and women had been referred for transplant preemptively (before KRT), and this was associated with 8.2-fold and 14.7-fold increased likelihood of subsequent waitlisting or receipt of a living donor transplant relative to not being preemptively (or at all) referred, respectively. Among preemptively referred patients, we report no sex/gender inequities in access to waitlisting, although women remained 25% less likely to receive a living donor as compared with men. Importantly, women who were preemptively referred had, on average, more favorable characteristics than men (i.e., younger, fewer comorbidities and more likely to be under/normal weight), suggesting that though rates of preemptive referral may be similar, the eligibility standard may be higher for women. This was further iterated in our models adjusting for factors such as insurance and obesity, which demonstrated that if women had a similar distribution of these factors as men, they would have similar or greater likelihood of being preemptively referred. Understanding key drivers of these differential standards will be essential to understanding overall sex/gender inequities in access to all steps of the complex transplant process.
This study adds important information to a growing area of research documenting sex/gender disparities in prewaitlisting transplant steps. Our previous work has shown that women with kidney failure are less likely to be referred and evaluated for transplant as compared to men; however, rates of waitlisting and transplantation are similar if women complete the initial steps of referral and evaluation, highlighting that existing sex/gender inequities in prewaitlisting steps are the likely drivers of lower overall transplant rates in women.10,11 Indeed, we show here that if women are preemptively referred, their chances of waitlisting are similar to preemptively referred men. However, our findings appear to contrast with other literature examining rates of preemptive transplantation. A recent national US study using data from the Scientific Registry of Transplant Recipients reported that between 2015 and 2018, women were 38% more likely to be preemptively transplanted than men.17 Importantly, the discrepancy in reported sex/gender outcomes is likely explained by the fact that this study population consisted of patients who had already received a deceased donor transplant and did not include all patients initiating KRT. Schold et al.,7 using appropriate denominator data from USRDS, showed that women were 22% more likely to be preemptively waitlisted compared to men, but still had reduced access to waitlisting and living donor transplant, consistent with the findings in the current study specific to preemptive referral. Authors of this earlier study do not offer a specific hypothesis to support these sex/gender-specific findings, but do comment that, over time, the increasing number of patients being preemptively waitlisted, especially women, may reflect the growing burden of stage 4 CKD in the general population and thus, more patients qualifying for referral to transplant as opposed to an indication of improved access to care in the overall population.
The finding that preemptively referred women are younger, have fewer comorbidities, and more likely to be underweight or of normal weight than their male counterparts is a key contribution to the growing body of evidence, both within and outside the kidney disease community, demonstrating that for the same set of conditions, women receive poorer care compared to men.18 A recent study from Sweden showed that women with CKD were less likely to have a confirmed diagnosis of CKD, be referred to a nephrologist, undergo monitoring of creatinine or albuminuria, or be initiated on renin-angiotensin system inhibitors and statins, despite guideline-recommend indications, for the same level of estimated glomerular filtration rate as men.15 This suggests, therefore, that to receive the same level of access to a transplant (where a healthier and younger candidate is preferable), women may have to have a more favorable medical profile than men. Indeed, in our data, after we adjusted for baseline differences between men and women, women had a higher odds of preemptive referral (OR: 1.09 [1.03–1.15]). This change in direction of effect is perhaps what we would expect when adjusting for factors that lie on the causal pathway between sex/gender and preemptive referral; we bias the risk estimate to suggest that being a woman has a positive effect on transplant access, when the opposite is in fact true. This is highlighted when we adjust for 1 variable at a time (Supplementary Table S3). For example, when we adjust for age and race, which do not lie on the causal pathway (i.e., sex/gender cannot influence age and race), we see no association between sex/gender and preemptive referral as in crude results. However, once we start adjusting for additional variables, such as insurance status (where sex/gender influence insurance status, and insurance status in turn influences the likelihood of preemptive referral), you see the estimates “flip” from a null association to a positive one, where women have greater likelihood of preemptive referral, suggesting that insurance status serves as a mediator of access to transplant for women. This highlights why examining crude data is essential when reporting sex/gender inequities because it reflects the real-life access to transplant in men versus women.
Understanding the underlying mechanisms that create this sex/gender inequity is complex and requires information beyond what is captured in routine medical records. For example, sex/gender inequities likely relate to conscious or unconscious provider biases, possible differences in candidate self-selection (i.e., women are less likely to be self-advocating19), or a combination of these factors. Studies demonstrate that women with kidney failure, in particular older women, are often perceived as frailer than men which could lead providers to incorrectly assume women will not be able to tolerate major surgery,20 and this may impact referral practices. Obese men and women also do not appear to be viewed the same, with obese women up to 34% less likely to be referred, evaluated, and waitlisted even after adjusting for several clinical and sociodemographic factors, though mechanisms remain unknown.4,10 Importantly, both frailty and obesity are modifiable factors, and life expectancy improvements have been noted following transplant even in individuals with a body mass index up to 41 kg/m2.21 Finally, calculation of estimated glomerular filtration rate (which is used to guide initiation of dialysis) currently adjusts for sex, with unknown implications for subsequent access to transplantation. In liver transplant, previous studies have shown that the Model for End-stage Liver Disease score inaccurately estimates disease severity in women and subsequently reduces access to waitlisting.22 Whether this is true in kidney disease is unknown, but warrants investigation, particularly in light of recent changes to the removal of race from estimated glomerular filtration rate.23 Recognition of these biases, coupled with the development of policies to improve referral rates, and in particular preemptive referral rates, will be necessary to reduce provider-related variability and ensure equitable access to this limited resource for women with kidney failure.
The final important finding from this work is that women who are preemptively referred are still 25% less likely to receive a living donor transplant than preemptively referred men. Sex/gender inequities in living donor transplantation have long been documented, with women comprising more than 60% of living donors, whereas men constitute more than 60% of the living donor recipients.24 Although there is no conclusive evidence why women donate more than they receive, this inequality likely stems from factors linked to sex, gender, and their interaction.25,26 For example, previous work by Rota-Musoll et al.27 has shown that a woman’s decision to donate a kidney is driven by their desire to improve the life and health of recipients, likely stemming from the traditional caregiving roles that women have typically occupied in society. In terms of receipt of living donor transplant, pregnancy-induced sensitization is often considered a barrier for women.28 Other studies have suggested that sex/gender-based differences in living donor transplant receipt may instead be explained by differences in race, with Black patients with end-stage kidney disease less likely to seek living donor transplants.29 In our study, among those preemptively referred, we saw no difference in sex/gender disparities by race, though we may have been underpowered to detect this. However, another study confirmed that even among Black patients, sex/gender disparities in access to living donor transplant persist.30 The reasons for sex/gender disparities in seeking living donor transplant may be uniquely different from those for deceased donor transplant, highlighted by the greater magnitude of this disparity, and future interventions should be tailored accordingly. Importantly, a 2017 study showed that participation in a kidney paired exchange program eliminated sex-based differences in living donor kidney transplant, highlighting that despite any biological differences, sex/gender equity in access to living donor transplant can be achieved with the right policies in place.28
The key strength of this study includes the use of novel referral data across all 9 transplant centers in GA, NC, and SC, through the E-STAR database8 linked to the national USRDS registry allowing us to examine each step of the transplant process among an appropriate denominator population (i.e., all patients initiating KRT). However, there are some limitations to be considered. First, our results are generalizable only to the Southeastern US, which has a larger Black population, higher burden of chronic disease, and lower transplant rates compared with other regions in the US.31,32 Second, a small proportion (i.e., <10%)14 of patients who may have initiated dialysis in the region but were referred to transplant centers outside of GA, NC, and SC were excluded from the study population. Third, USRDS captures all patients initiating KRT (either dialysis or transplant). It therefore does not include patients with late-stage CKD who do not yet need dialysis, but who may be eligible for preemptive referral for transplant. Findings of this work are therefore limited to individuals with kidney disease who are initiating KRT. However, we believe this represents the majority of individuals being referred for a transplant. For example, in Figure 1 we report that ∼15% of individuals who were referred to a transplant center could not be linked to USRDS. We believe this represents the smaller proportion of referred patients who are late-stage CKD. Fourth, this study is limited to data routinely captured in transplant centers and therefore we are unable to examine the impact of several potentially important factors, such as income, education status, pregnancy, frailty, or antibodies such as calculated panel reactive antibody that may impact donor match among waitlisted patients. Finally, sex/gender, as determined from CMS2728, is assigned by the provider at KRT initiation and does not necessarily reflect patient self-identified sex/gender. Therefore, findings of this study will be influenced by provider perceptions of sex/gender.
Conclusion
In the Southeast US, a similar proportion of men and women with end-stage kidney disease initiating KRT are preemptively referred for a kidney transplant with substantial benefits for increased access to waitlisting and receipt of a living donor transplant. Nonetheless, the standard for preemptive referral appears higher for women (i.e., preemptively referred women have a more favorable medical profile relative to men) and understanding key mechanisms driving this differential will be essential to mitigating sex/gender inequities in access to transplant.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The data reported here has been supplied by the United States Data Renal System and the Southeastern Kidney Transplant Coalition. The conclusions presented are solely those of the authors and do not represent those of the Southeastern Kidney Coalition or USRDS Centers for Medicare and Medicaid Services. The content of this publication does not necessarily reflect the policies or positions of the Department of Health and Human Services, and mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. The authors assume responsibility for the accuracy and completeness of the ideas presented.
This project and The Reducing Disparities in Access to kidNey Transplantation Regional Study was funded in part by a National Institute on Minority Health and Health Disparities award U01MD010611, a National Institute of Diabetes and Digestive and Kidney Diseases award R01DK122701, an Emory University Health Services Center Pilot Award, and an American Society of Transplantation Career Development Award. Support for the preparation of this document was funded by Centers for Medicare and Medicaid Services (an agency of the US Department of Health and Human Services) ESRD Network 6 contract HHSM-500-2013-NW006C.
Author Contributions
JLH conceived the study, contributed to study design, oversaw analysis, and wrote the manuscript. AG conducted all analyses and reviewed/edited the manuscript. SOP contributed to funding and data acquisition, provided intellectual input, and reviewed/edited the manuscript. MD and KD assisted in analysis and reviewed/edited the manuscript. DD and AR contributed to study design, provided intellectual input, and reviewed/edited the manuscript. REP contributed to data acquisition, study conceptualization, provided intellectual input, and reviewed/edited the manuscript. All the authors approved the final version of this manuscript. JLH is the guarantor of this work and takes responsibility for final responsibility for the decision to submit for publication.
Footnotes
Table S1. STROBE Statement—Checklist of items that should be included in reports of cohort studies.
Table S2. Characteristics of patients initiating kidney replacement therapy from 2015 to 2019, stratified by sex/gender and referral status.
Table S3. Association between sex/gender and preemptive referral among patients initiating kidney replacement therapy from 2015 to 2019 using multivariable logistic regression and adding variables 1 at a time in a forward stepwise fashion.
Table S4. Odds ratio for the association between sex/gender and preemptive referral by age, race, obesity, and attributed cause of kidney failure among all patients initiating kidney replacement therapy 2015 to 2019 with follow-up through 2020.
Table S5. Crude and adjusted hazards ratios for the association between preemptive referral and (A) placement on the deceased donor waitlist or (B) receipt of a living donor transplant, overall and by gender, among all patients initiating kidney replacement therapy between 2015 and 2019 with follow-up through 2020 in the Southeast US, accounting for competing risk of death and deceased donor transplant.
Table S6. Crude and adjusted hazards ratios for the association between preemptive referral and (A) placement on the deceased donor waitlist or (B) receipt of a living donor transplant, overall and by sex/gender, among patients referred for kidney transplantation (n = 24,114) between 2015 and 2019 with follow-up through 2020 in the Southeast US.
Supplementary Material
Table S1. STROBE Statement—Checklist of items that should be included in reports of cohort studies. Table S2. Characteristics of patients initiating kidney replacement therapy from 2015 to 2019, stratified by sex/gender and referral status. Table S3. Association between sex/gender and preemptive referral among patients initiating kidney replacement therapy from 2015 to 2019 using multivariable logistic regression and adding variables 1 at a time in a forward stepwise fashion. Table S4. Odds ratio for the association between sex/gender and preemptive referral by age, race, obesity, and attributed cause of kidney failure among all patients initiating kidney replacement therapy 2015 to 2019 with follow-up through 2020. Table S5. Crude and adjusted hazards ratios for the association between preemptive referral and (A) placement on the deceased donor waitlist or (B) receipt of a living donor transplant, overall and by gender, among all patients initiating kidney replacement therapy between 2015 and 2019 with follow-up through 2020 in the Southeast US, accounting for competing risk of death and deceased donor transplant. Table S6. Crude and adjusted hazards ratios for the association between preemptive referral and (A) placement on the deceased donor waitlist or (B) receipt of a living donor transplant, overall and by sex/gender, among patients referred for kidney transplantation (n = 24,114) between 2015 and 2019 with follow-up through 2020 in the Southeast US.
References
- 1.Johansen K.L., Chertow G.M., Foley R.N., et al. US renal data system 2020 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2021;77(suppl 1):A7–A8. doi: 10.1053/j.ajkd.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azegami T., Kounoue N., Sofue T., et al. Efficacy of pre-emptive kidney transplantation for adults with end-stage kidney disease: a systematic review and meta-analysis. Ren Fail. 2023;45 doi: 10.1080/0886022X.2023.2169618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander G.C., Sehgal A.R. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998;280:1148–1152. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 4.Ladhani M., Craig J.C., Wong G. Obesity and gender-biased access to deceased donor kidney transplantation. Nephrol Dial Transplant. 2019;35:184–189. doi: 10.1093/ndt/gfz100. [DOI] [PubMed] [Google Scholar]
- 5.Meier-Kriesche H.U., Ojo A.O., Leavey S.F., et al. Gender differences in the risk for chronic renal allograft failure. Transplantation. 2001;71:429–432. doi: 10.1097/00007890-200102150-00016. [DOI] [PubMed] [Google Scholar]
- 6.Segev D.L., Kucirka L.M., Oberai P.C., et al. Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol. 2009;20:621–628. doi: 10.1681/ASN.2008060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schold J.D., Mohhan S., Huml A., et al. Failure to advance access to kidney transplantation over two decades in the United States. J Am Soc Nephrol. 2021;32:913–926. doi: 10.1681/ASN.2020060888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patzer R.E., Retzloff S., Buford J., et al. Community engagement to improve equity in kidney transplantation from the ground up: the southeastern kidney transplant coalition. Curr Transplant Rep. 2021;8:324–332. doi: 10.1007/s40472-021-00346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smothers L., Patzer R.E., Pastan S.O., DuBay D., Harding J.L. Gender disparities in kidney transplantation referral vary by age and race: a multiregional cohort study in the Southeast United States. Kidney Int Rep. 2022;7:1248–1257. doi: 10.1016/j.ekir.2022.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding J.L., Di M., Pastan S.O., et al. Examination of sex/gender disparities across the continuum of kidney transplant steps. Nephrol Dial Transplant. 2023;39:717–719. doi: 10.1093/ndt/gfad242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding J.L., Di M., Pastan S.O., et al. Sex/gender-based disparities in early transplant access by attributed cause of kidney disease – evidence from a multi-regional cohort in the Southeast United States. Kidney Int Rep. 2023;8:2580–2591. doi: 10.1016/j.ekir.2023.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer G.R. Sex and gender multidimensionality in epidemiologic research. Am J Epidemiol. 2023;192:122–132. doi: 10.1093/aje/kwac173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rioux C., Pare A., London-Nadeau K., et al. Sex and gender terminology: a glossary for gender-inclusive epidemiology. J Epidemiol Community Health. 2022 doi: 10.1136/jech-2022-219171. [DOI] [PubMed] [Google Scholar]
- 14.Patzer R.E., McPherson L., Wang Z., et al. Dialysis facility referral and start of evaluation for kidney transplantation among patients treated with dialysis in the Southeastern United States. Am J Transplant. 2020;20:2113–2125. doi: 10.1111/ajt.15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swartling O., Yang Y., Clase C.M., et al. Sex differences in the recognition, monitoring, and management of CKD in health care: an observational cohort study. J Am Soc Nephrol. 2022;33:1903–1914. doi: 10.1681/ASN.2022030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvin S. Oxford University Press; 1996. Statistical Analysis of Epidemiologic Data. [Google Scholar]
- 17.King K.L., Husain S.A., Jin Z., Brennan C., Mohan S. Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol. 2019;14:1500–1511. doi: 10.2215/CJN.03140319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauvais-Jarvis F., Bairey Merz N., Barnes P.J., et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396:565–582. doi: 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janoff-Bulman R., Wade M.B. Viewpoint: the dilemma of self-advocacy for women: another case of blaming the victim? J Soc Clin Psychol. 1996;14:143–152. doi: 10.1521/jscp.1996.15.2.143. [DOI] [Google Scholar]
- 20.Salter M.L., Gupta N., Massie A.B., et al. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatr. 2015;15:52. doi: 10.1186/s12877-015-0051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams-DeMarco M.A., Isaacs K., Darko L., et al. Changes in frailty after kidney transplantation. J Am Geriatr Soc. 2015;63:2152–2157. doi: 10.1111/jgs.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke J.E., Shelton B.A., Olthoff K.M., et al. Quantifying sex-based disparities in liver allocation. JAMA Surg. 2020;155 doi: 10.1001/jamasurg.2020.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado C., Baweja M., Crews D., et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol. 2021;32:2994–3015. doi: 10.1681/ASN.2021070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domínguez-Gil B., RM Newsletter Transplant International figures on donation and transplantation 2016. Eur: Eur Dir Qual Med Healthc Counc. 2017 https://freepub.edqm.eu/publications/42/detail [Google Scholar]
- 25.Katz-Greenberg G., Shah S. Sex and gender differences in kidney transplantation. Semin Nephrol. 2022;42:219–229. doi: 10.1016/j.semnephrol.2022.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salas M.A.P., Chua E., Rossi A., et al. Sex and gender disparity in kidney transplantation: historical and future perspectives. Clin Transpl. 2022;36 doi: 10.1111/ctr.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rota-Musoll L., Brigidi S., Molina-Robles E., Oriol-Vila E., Perez-Oller L., Subirana-Casacuberta M. An intersectional gender analysis in kidney transplantation: women who donate a kidney. BMC Nephrol. 2021;22:59. doi: 10.1186/s12882-021-02262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bromberger B., Spragan D., Hashmi S., et al. Pregnancy-induced sensitization promotes sex disparity in living donor kidney transplantation. J Am Soc Nephrol. 2017;28:3025–3033. doi: 10.1681/ASN.2016101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng F.L., Dhillon N., Lin Y., Mulgaonkar S., Patel A.M. Racial differences in outcomes of the evaluation of potential live kidney donors: a retrospective cohort study. Am J Nephrol. 2012;35:409–415. doi: 10.1159/000337949. [DOI] [PubMed] [Google Scholar]
- 30.Gillespie A., Hammer H., Kolenikov S., et al. Sex differences and attitudes toward living donor kidney transplantation among urban black patients on hemodialysis. Clin J Am Soc Nephrol. 2014;9:1764–1772. doi: 10.2215/CJN.12531213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.2017 American community survey single-year estimates. https://www.census.gov/newsroom/press-kits/2018/acs-1year.html US Census Bureau.
- 32.Ward B.W., Black L.I. State and regional prevalence of diagnosed multiple chronic conditions among adults aged ≥18 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:735–738. doi: 10.15585/mmwr.mm6529a3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. STROBE Statement—Checklist of items that should be included in reports of cohort studies. Table S2. Characteristics of patients initiating kidney replacement therapy from 2015 to 2019, stratified by sex/gender and referral status. Table S3. Association between sex/gender and preemptive referral among patients initiating kidney replacement therapy from 2015 to 2019 using multivariable logistic regression and adding variables 1 at a time in a forward stepwise fashion. Table S4. Odds ratio for the association between sex/gender and preemptive referral by age, race, obesity, and attributed cause of kidney failure among all patients initiating kidney replacement therapy 2015 to 2019 with follow-up through 2020. Table S5. Crude and adjusted hazards ratios for the association between preemptive referral and (A) placement on the deceased donor waitlist or (B) receipt of a living donor transplant, overall and by gender, among all patients initiating kidney replacement therapy between 2015 and 2019 with follow-up through 2020 in the Southeast US, accounting for competing risk of death and deceased donor transplant. Table S6. Crude and adjusted hazards ratios for the association between preemptive referral and (A) placement on the deceased donor waitlist or (B) receipt of a living donor transplant, overall and by sex/gender, among patients referred for kidney transplantation (n = 24,114) between 2015 and 2019 with follow-up through 2020 in the Southeast US.