Original statement in the Section 2:
2. Literature search
Our primary screening of 180 articles yielded the relevant data for this study.
2.1. Study selection
A total of 180 articles spanning from 1960 to the present day, including original research, reviews, case reports and studies reporting nitrosamine impurities above the no-observed-adverse-effect levels (NOAEL) established by regulatory agencies, were initially screened. During the primary screening, we considered factors such as relevance, publication date, access to the full article text, and content. Subsequently, in the secondary screening phase, we excluded the 33 reports including 15 with improper formats (such as case reports, letters, and short communications), 3 with incomplete content, and 15 with unavailable full texts. This process resulted in the selection of 147 articles for further evaluation. Of these, 2 articles were excluded from the study and 2 articles did not describe nitrosamine-induced toxicity or its pharmaceutical or analytical implications. In the end, 143 articles were chosen for systematic review.
Corrected statement in the Section 2:
2. Literature search
Our primary screening of 190 articles yielded the relevant data for this study.
2.1. Study selection
A total of 190 articles spanning from 1960 to the present day, including original research, reviews, case reports and studies reporting nitrosamine impurities above the no-observed-adverse-effect levels (NOAEL) established by regulatory agencies, were initially screened. During the primary screening, we considered factors such as relevance, publication date, access to the full article text, and content. Subsequently, in the secondary screening phase, we excluded the 33 reports including 15 with improper formats (such as case reports, letters, and short communications), 3 with incomplete content, and 15 with unavailable full texts. This process resulted in the selection of 157 articles for further evaluation. Of these, 2 articles were excluded from the study and 2 articles did not describe nitrosamine-induced toxicity or its pharmaceutical or analytical implications. In the end, 153 articles were chosen for systematic review.
The authors regret to find that there is a typographical error in Section 2. The initial and final numbers of articles were misrepresented. The errors did not affect the results and conclusion of the whole study.
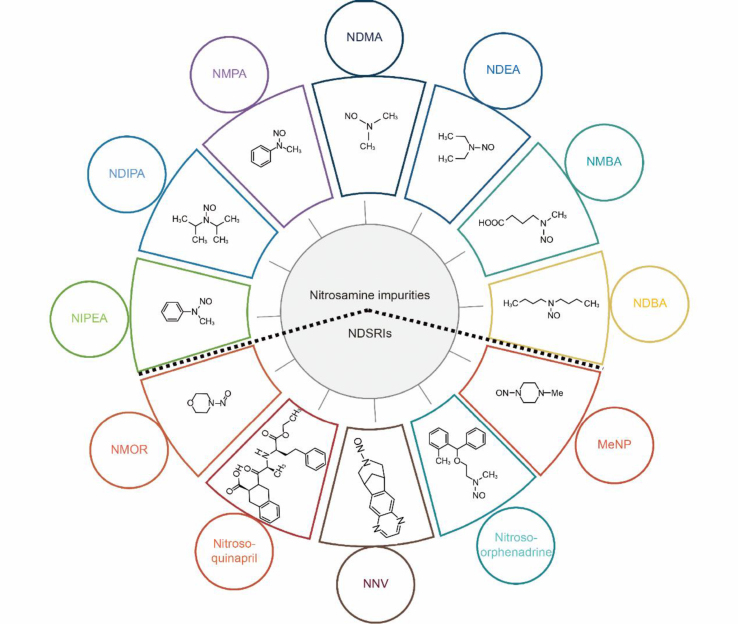
Original Fig. 1:
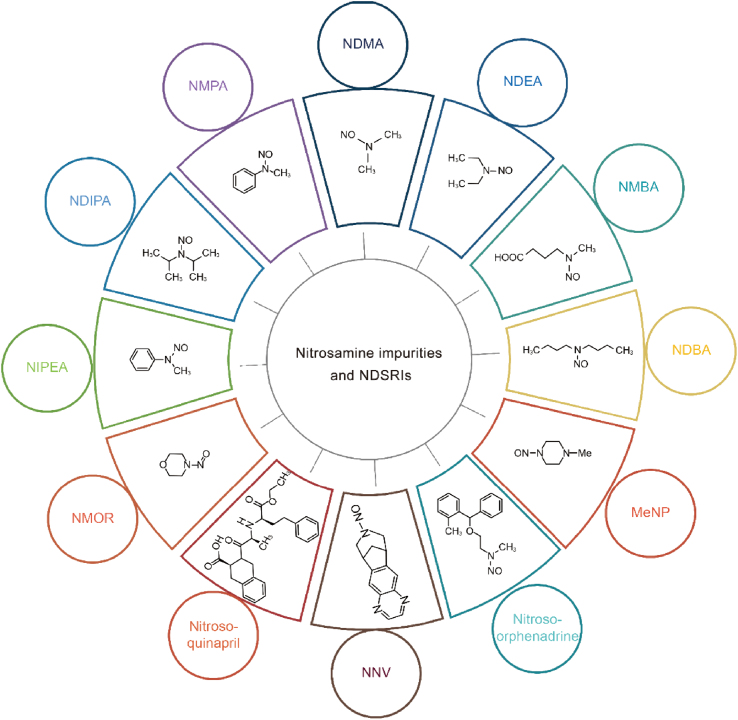
Corrected Fig. 1:
In the originally published Fig. 1, the dotted line is an artifact of the figure template used and does not represent any demarcation within the figure itself. The corrected Fig. 1, with the dotted line removed, avoids confusion for readers. The errors did not affect the results and conclusion of the whole study.
The authors would like to apologize for any inconvenience caused.