Abstract
Astrocytes are target cells for human immunodeficiency virus type 1 (HIV-1) in the central nervous system with attenuated virus replication in vivo and in vitro. In infected astrocytes, viral gene expression is restricted mainly to nonstructural (early) viral components like Nef, suggesting inhibition of Rev-dependent posttranscriptional processes in these cells. Because of the heterogeneity of astrocytic cells, the objective of this study was to determine whether restriction of HIV-1 Rev-associated activities is a common property of human astrocytes. To this end, we compared the trans activation capacity and intracellular distribution of Rev in four astrocytoma cell lines previously shown to be infectible by HIV-1 and in primary human fetal astrocytes from different sources with Rev-permissive nonglial control cell lines. In all astrocytic cell cultures, the Rev response was reduced to about 10% of that of Rev-permissive control cells. Rev was apparent both in cytoplasmic and in nuclear compartments of living astrocytes, in contrast to the typical nuclear and/or nucleolar localization of Rev in permissive control cells. Nuclear accumulation of Rev in astrocytes was restored by blocking export of Rev. The trans activation capacity and nuclear localization of Tat were not affected in astrocytes. These results demonstrate that inhibition of Rev-dependent posttranscriptional regulation of HIV-1 is a hallmark of human astrocytes and may contribute to suppression of HIV-1 production in these HIV-1 reservoirs. Astrocytes constitute the first example of a human cell type showing an impaired Rev response, indicating that posttranscriptional control of HIV-1 gene expression can be modulated in a cell-dependent manner.
Human immunodeficiency virus (HIV) invades the central nervous system and can cause progressive cognitive and motor dysfunction. Structural components of the virus are detected mainly in macrophage/microglial cells of the brain, which in the past often has led to the assumption that these are the predominant target cells for HIV in the brain (2). However, occurrence of HIV in astroglial cells has been repeatedly demonstrated in tissue sections from HIV-infected individuals by in situ techniques for detection of HIV nucleic acids or early viral gene products (3, 34, 38, 42, 54, 55). In contrast, virion structural components, indicative of productive infection, are observed only very rarely in astrocytes in vivo (reviewed in reference 7). These observations suggest that astrocytes are target cells for HIV in the central nervous system in vivo and harbor the virus in a nonproductive manner. HIV type 1 (HIV-1) infection of astrocytes in vitro has been shown with numerous human glioblastoma/astrocytoma cell lines and primary astrocyte cell cultures, by using a wide variety of HIV-1 isolates (reviewed in reference 7). Such infections are generally of very low and often only transient productivity, compared to infection of HIV-susceptible hematopoietic cells. These observations support the concept of astrocyte-directed restriction of HIV replication.
We have previously reported that diminished HIV production in the persistently HIV-infected astrocytoma cell line TH4-7-5 is associated with selective depletion of viral mRNAs for structural proteins (8, 32). These observations have been confirmed for other persistently HIV-infected primary and tumor-derived astrocytic cells (29, 56), suggesting that regulatory mechanisms required for efficient synthesis of viral structural proteins are inhibited in astrocytes. In the HIV-1 replication cycle, HIV-1 structural proteins are produced from incompletely spliced and unspliced mRNAs, which retain a specific recognition element for the HIV-1 Rev regulatory factor (Rev-responsive element [RRE]). Rev binds to the RRE, subsequently mediating the nuclear export of these mRNAs and promoting their translation (for detailed reviews, see references 9, 14, 17 and 35). In the absence of Rev, only very low level production of viral structural proteins takes place. Rev is a small nuclear protein which accumulates in nucleoli and trafficks between the nucleus and cytoplasm (39). The numerous activities required for the trans-activating function of Rev are directed by two domains, the amino-terminal domain being responsible for RNA binding, multimerization, and nuclear import and the carboxy-terminal activation domain being responsible for nuclear export.
Several mechanisms apart from inhibited Rev-dependent posttranscriptional control of HIV gene expression have been proposed to be responsible for suppression of HIV production in astrocytes. These include suppression of HIV transcription (24, 33), diminished translation of viral proteins (19), and aberrant processing of viral proteins (45). Since these conclusions are based on studies including only one or a few astrocytic cell lines or primary cell isolates, it is unclear whether they are representative of human astrocytes in general. Therefore, the objective of the present study was to determine whether human astrocytes in general share the capacity to inhibit posttranscriptional regulation of HIV-1 structural protein synthesis. To this end, we compared the Rev responses of several astrocytoma cell lines and primary astrocytes from different donors with those of nonglial Rev-permissive control cells. Our results show that the diminished capacity of Rev to trans-activate synthesis of HIV-1 structural proteins is a hallmark of astrocytes. Altered properties of Rev in astrocytes are also reflected by distinct cytoplasmic accumulation of Rev not observed in Rev-permissive cell lines.
MATERIALS AND METHODS
Cell lines.
All cells used in this study were of human origin. Glioblastoma/astrocytoma cell lines U87MG (36), U138MG (5), and U373MG (60) were obtained from the American Type Culture Collection (ATCC HTB-14, -16, and -17, respectively). U251MG (5) was kindly provided by M. Brenner, National Institutes of Health. The cell line 85HG66 has been described elsewhere (8, 52). The nonglial control cell lines used were embryonic lung-derived fibroblastoid cell line LC5 (26), the epitheloid cervix carcinoma cell line HeLa (obtained from either ATCC [ATCC-CCL-2] or kindly provided by Barbara Felber, National Cancer Institute-Advanced Bioscience Laboratories, Frederick, Md.), and the embryonal kidney cell line HEK293 (ATCC CRL-1573).
Cells were maintained under standard cell culture conditions in Dulbecco’s modified Eagle medium with Glutamax I, without Na-pyruvate, and with 4.5 g of glucose (Life Technologies, Karlsruhe, Germany) per liter, containing 10% fetal calf serum (Seromed, Berlin, Germany). Media were supplemented with 1% antibiotic-antimycotic solution (Life Technologies) during and after transfection.
Primary fetal astrocyte cultures.
Primary fetal astrocyte cultures prepared from myencephalon-mesencephalon (H5/95 and H3/95) or spinal cord (H1/96) of three 8- to 9-week-old embryos were kindly provided by Francesca Aloisi (Istituto Superiore di Sanità, Rome, Italy) (1). Third-passage cultures contained between 75 and 90% glial fibrillary acidic protein (GFAP)-positive cells and less than 2% fibronectin-positive fibroblasts. Cultures were maintained under standard cell culture conditions in minimal essential medium containing d-valine (Life Technologies), 10 μg of gentamicin per ml, 2 mM glutamine (Sigma, Deisenhofen, Germany), and 10% fetal calf serum (Life Technologies). For transfection experiments, fourth-passage cells were seeded on poly-l-lysine-coated culture dishes at a density of 7 × 105 cells/60-mm-diameter dish and grown at 37°C in a 95% air–5% CO2 humidified atmosphere.
Antigen profiling by fluorescence microscopy.
The following primary-secondary antibody pairs were used for antigen profiling of cells: mouse monoclonal antibody (MAb) against GFAP, immunoglobulin G1 (IgG1) (Sigma), and fluorescein isothiocyanate-conjugated sheep anti-mouse IgG (Dianova, Hamburg, Germany), and mouse MAb against human fibronectin, IgG (Sigma), and fluorescein isothiocyanate- or Texas Red-conjugated sheep anti-mouse IgG (Dianova). The following mouse MAbs were detected with cyanine 3-conjugated goat anti-mouse secondary antibodies (Dianova): MAb against S100-β, IgG1 (Sigma); MAb against CD56 (neural cell adhesion molecule [NCAM]), IgG (Sigma); and MAb against cytokeratin, IgG1 (Dako A/S, Hamburg, Germany). Vimentin was detected with a cyanine 3-conjugated MAb against vimentin, IgG (Sigma). Actin expression was detected with Bodipy TR-X-labelled phallacidin (Molecular Probes, Leiden, The Netherlands) diluted 1:40 and used according to the manufacturer’s instructions.
The indirect immunofluorescence assay was essentially performed as described elsewhere (16). Briefly, cells were fixed for 10 min at room temperature with 3.7% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized with NP-40 (0.1% in PBS) for 7 to 15 min. Alternatively, permeabilization was performed with ethanol-acetic acid (20:1) for 10 to 15 min at −20°C (GFAP and fibronectin). Primary antibodies were used in concentrations ranging between 1:20 and 1:100; secondary antibodies were diluted 1:50 to 1:200. Images were taken as described below. Negative control plates were treated identically, except that they were not incubated with primary antibody. Images were taken at subsaturating conditions as described below (“Fluorescence microscopy and imaging of living cells”). Care was taken to use identical exposure settings, normalization, and contrast settings to allow direct comparisons of images.
Plasmid constructs.
The following expression plasmids were used for analysis of Rev and Tat responses. (a) Of Rev response plasmids, pB37R (53) contains Gag-encoding sequences (p17 and p24) and the complete RRE (330-bp StyI fragment) and lacks the major splice donor site of HIV-1 and pHXB2fB (16) is a Rev-defective derivative of HIV-1 molecular clone pHXB2 with a frameshift mutation in rev (exon 2). (b) Tat response plasmid pHIVΔnps-LTR-CAT (24) directs expression of the chloramphenicol acetyltransferase (CAT) gene from the HIV long terminal repeat (LTR) (nef sequences upstream of the LTR were deleted). (c) Of plasmids for expression of Rev and Tat, Rev was expressed from pBsRev (10) and Tat was expressed from pL3Tat (15) or pcTat (25) (kindly provided by Bryan Cullen, Duke University, Durham, N.C.). Transcription of HIV-1 genes is directed by either the HIV LTR (pBsRev and pL3Tat) or the cytomegalovirus immediate-early promoter (pcTat) in these plasmids. (d) In control plasmids for non-HIV-related expression, production of the luciferase reporter is directed by C-type retroviral LTRs from either Rous sarcoma virus (pRSVluc [1]) or the murine leukemia virus-related Reilly-Finkel-Biskis virus (pGL3-RFB [18]).
Localization studies and functional assays with living cells were carried out with plasmids directing expression of HIV-1 genes fused with the open reading frame (ORF) of the green fluorescent protein (GFP), by using the pFred line of vectors described in reference 50, which contain a mutant of GFP with enhanced fluorescence under the control of the cytomegalovirus immediate-early promoter. For pCsRevsg143, the Rev ORF was amplified from pBsRev and inserted into an NheI restriction site immediately downstream of the ATG start codon of GFP in pFred143 (kindly provided by Kyoji Horie and George N. Pavlakis, National Cancer Institute-Advanced Bioscience Laboratories). This resulted in fusion of the Met-Ala-Ser-encoding sequence of GFP to codon 2 of the rev ORF (Ala). A Gly-Ala-Gly-Ala hinge region was inserted between the last Rev codon and the following GFP ORF to allow correct folding of both protein domains. In order to obtain pCsRevM10BLsg143, a 247-nucleotide HindIII/BstEII fragment containing the M10BL mutant nuclear export signal region of Rev was taken from pBsRevM10BL (48) and inserted in the corresponding sites of pCsRevsg143. The plasmid pTat-GFP is described in reference 51.
The plasmid pBSPL (44) was used as fill-up DNA for transfections. Plasmids were purified with Qiagen DNA purification columns according to the manufacturer’s instructions.
Transfections.
Cells were seeded at densities between 4 × 105 and 8 × 105 cells per 60-mm-diameter dish depending on the cell line and left to attach for 24 h. Cells were then transfected by calcium phosphate coprecipitation with a commercially available kit (CellPhect; Pharmacia, Freiburg, Germany). Rev response assays were typically performed with 5 μg of reporter plasmid (pB37R or pHXB2fB), 1 μg of effector plasmid (pBsRev or pCsRevsg143), and 1 μg of expression control plasmid (pRSVluc or pGL3-RFB). In transfections with pB37R, 1 μg of Tat expression plasmid (pL3Tat) was added. Tat response assays were carried out with 1 μg of reporter plasmid (pHIVΔnpsLTR-CAT), 1 μg of effector plasmid (pcTat), and 1 μg of expression control plasmid (pRSVluc or pGL3-RFB). Plasmid mixtures were adjusted to 17 μg of total DNA per 60-mm-diameter culture dish with pBSPL. In each experiment, the same plasmid mixture was added to at least two dishes of cells from the same line or primary culture. DNA precipitates were left on the cells for 4 to 6 h depending on cell type, after which cells were washed twice with prewarmed Dulbecco’s modified Eagle medium and refed with complete medium. Cells were harvested 48 h after transfection.
To assay Rev response (see also reference 32), cells were harvested in 1 ml of 1× TNE (40 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl, pH 7.4) per plate. Two hundred microliters of this cell suspension was used to measure luciferase activity with the luciferase assay system (Promega). The remaining cells were lysed by three freeze-thaw cycles in 0.5% Triton X-100 in PBS and cleared by centrifugation, and the total protein content was determined (bicinchoninic acid kit; Pierce, Rockford, Ill.). Levels of HIV-1 Gag were determined by p24 antigen capture enzyme-linked immunosorbent assay (ELISA) with cell lysates (Du Pont, Bad Homburg, Germany, and Coulter, Krefeld, Germany) and standardized to 100 μg of total protein. Rev-mediated increase of p24 production represents the ratio of Gag protein produced with Rev to that produced without Rev.
For analysis of Tat response, CAT reporter gene expression was quantitated either on the protein level by ELISA or by assay of CAT function. CAT ELISA (Boehringer, Mannheim, Germany) was performed with cell lysates prepared as described above, and CAT protein was standardized to 50 μg of total protein. To measure CAT activity, cells were lysed by freeze-thawing in detergent-free buffer (25 mM Tris-HCl, pH 7.4). Lysate volumes yielding conversion rates within the linear range of the assay (5 to 30 μg of total protein, depending on cell line) were incubated overnight with 5 μl of fluorescently labelled chloramphenicol substrate (FAST-CAT kit; Molecular Probes) and 9 mM acetyl coenzyme A. Substrate was extracted and subjected to thin-layer-chromatography. Acetylated and nonacetylated chloramphenicol were quantified with a STORM fluorescence scanner (Molecular Dynamics, Krefeld, Germany). Inductions reflect the ratio of CAT expressed with Tat to that without Tat. Luciferase activity was analyzed as described above.
Transfection efficiencies were monitored by cotransfections with GFP-expressing plasmids (pFred143 and pCsRevsg143) and by visual estimation of the percentage of GFP-expressing cells or, alternatively, fluorescence-activated cell sorting (FACS) analysis of GFP fluorescence with a FACScalibur cytometer (Becton Dickinson).
The influence of transfection on the metabolic activities of cells was determined by measuring mitochondrial dehydrogenase activity by a colorimetric assay (30).
Fluorescence microscopy and imaging of living cells.
Microscopy of fixed and living cells expressing GFP fusion proteins was performed as described extensively in reference 23. For GFP analysis in living cells, cells were seeded in glass-bottomed petri dishes (Mattek Corp., Ashland, Mass.) transfected as described above and observed 24 to 48 h later. In some experiments, cells were treated for 10 min with 2 μg of the dye Hoechst 33342 (Molecular Probes) per ml to allow easy identification of nuclei. Medium was exchanged against complete medium without phenol red, and dishes were put on an inverted Axiovert 135TV research microscope (Zeiss, Oberkochen, Germany) with a mounted environmental chamber. Images were taken at a 320-fold or a 400-fold magnification with a cooled 12-bit charge-coupled device camera (Quantix; Photometrics, Tucson, Ariz.). Filters, shutters, and camera were controlled by a Macintosh PowerPC computer with IPLab Spectrum 3.2 software. Care was taken to ensure that images used for comparative purposes (e.g., immunofluorescence [see above]) were taken under identical lighting conditions, exposures, and gain settings. Images were all taken at subsaturating conditions to allow quantitation of signals. Images were prepared for presentation by using IPLab Spectrum 3.2, Adobe Photoshop 4.0, and Adobe Illustrator 7.0.
Statistical analysis.
Statistical analysis of data was carried out with GraphPadPRISM, version 2.0 (GraphPad Software, Inc., San Diego, Calif.). Significance between data sets was determined by calculating two-tailed P values by the Mann-Whitney U test. Data sets consisting of ≥12 values were evaluated for Gaussian distribution by calculating the Kolmogorov-Smirnov distance and the corresponding P value.
RESULTS
Characterization of astrocytic tumor-derived cell lines. (i) Expression of cellular antigens.
For this study, we chose astrocytic tumor-derived cell lines established from human grade III glioblastomas/astrocytomas (for references, see Materials and Methods) and widely used to study infection of astrocytic cells by HIV-1 in vitro (summarized in reference 7). In addition, primary fetal astrocyte cultures were obtained from three different sources. As Rev-permissive control cells, we chose human nonglial cell lines previously shown to support high-level virus production and/or HIV-1 Rev function in transient-transfection assays.
To identify antigen markers for these cell lines, we investigated the expression of a panel of cellular proteins by immunohistochemical analysis (data not shown). These included GFAP, the most specific marker antigen for astrocytes (41); CD56/NCAM and S100-β, which are both highly enriched in nervous tissue (CD56/NCAM [4] and S100-β [43]); fibronectin, vimentin, and actin, which are produced by a wide range of cells; and cytokeratins reported to be specific for human epithelial cells.
All cell lines were positive for S100-β, vimentin, and actin. GFAP expression was detected in primary fetal astrocyte cultures and only in astrocytoma cell line U251MG, presumably reflecting the previously described loss of GFAP expression during long-term culture in the other astrocytoma cell lines (5). Staining with anti-CD56 antibodies was observed in all astrocytoma cell lines and primary fetal astrocyte cultures but in only one nonglial cell line (HEK293). Expression of fibronectin was observed in three astrocytoma cell lines (U138MG, U87MG, and 85HG66) but only very rarely in primary fetal astrocyte cultures (≤2% positive cells) and in none of the nonglial control cell lines. Reactivity with the human cytokeratin antibody was observed exclusively in the nonglial control cells and not in the astrocytoma cell lines.
These results show varied expression of cellular antigens in cultured astrocytic cells, with differences occurring both between individual astrocytic tumor-derived cell lines and between cell lines and primary fetal astrocytes. Failure of all astrocytoma cell lines to react with antibodies against human cytokeratin indicates that cytokeratin is a useful marker to distinguish the astrocytic tumor-derived cell lines from the nonglial control cell lines used in this study.
(ii) Transfectability of cells.
To determine whether astrocytoma cells are suitable for transfection experiments, a colorimetric cell viability test (30) was used to assay the effect of transfection on metabolic activities of cells. Transfected cells retained ≥70% of metabolic activities of untransfected cells for all cell lines except astrocytoma cell line U138MG (≥55%). The metabolic activities of cells transfected with the Rev expression plasmid were similar to those transfected without rev (data not shown). Transfection efficiencies were assayed by FACS quantitation of the percentage of fluorescent cells in cultures cotransfected with a plasmid directing expression of the GFP (pFred143) and HIV-1 expression plasmids. Transfection efficiencies fell into two broad categories, ranging from 15 to 20% (astrocytoma cell lines U138MG, U87MG, and U373MG and Rev-permissive cell line HeLa) and from 45 to 90% (astrocytoma cell line U251MG, 45%; control cell line HEK293, 90%), both categories including astrocytoma and nonastrocytic cell lines.
These results show that the transfection procedure did not significantly influence viability of astrocytic cells. Furthermore, astrocytoma cell lines are transfected with efficiencies comparable with those of Rev-permissive nonastrocytic control cell lines.
Diminished Rev-dependent stimulation of HIV-1 protein production in astrocytic cells. (i) Rev response assays.
To compare the trans-activating effects of Rev on viral protein synthesis in astrocytoma and control cell lines, we carried out transient-transfection assays with HIV-1-derived plasmids which direct synthesis of Gag in a Rev-responsive manner. Rev reporter plasmids contained the gag gene either in the context of a Rev-defective HIV-1 provirus (pHXB2fB) or fused to the RRE as a subgenomic Gag expression plasmid (pB37R). Cells were transfected with the respective Rev response plasmid with and without rev expression plasmid pBsRev. Tat was supplemented in transfections with the subgenomic Gag expression plasmid pB37R by including the pL3Tat plasmid. All transfection reaction mixtures included an HIV-independent expression plasmid containing the luciferase gene under the control of a C-type retroviral LTR (Reilly-Finkel-Biskis virus or Rous sarcoma virus). Lysates of transfected cells were analyzed for levels of total protein, Gag antigen, and luciferase activity.
(ii) Astrocytoma cell lines.
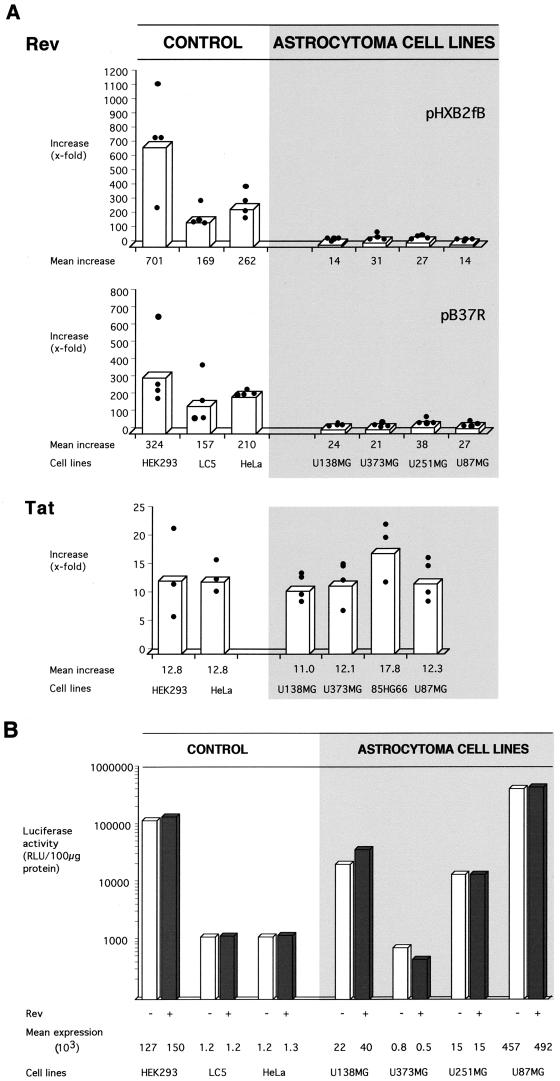
Four astrocytoma and three Rev-permissive control cell lines were analyzed in four independent transfection experiments with each Rev response plasmid. At least one astrocytoma cell line and one control cell line were analyzed in parallel in each experiment. All cell lines showed low-level Gag synthesis without Rev, which was increased in the presence of Rev. However, the extent of the Rev-mediated increase was clearly different between astrocytoma and nonglial cells (Fig. 1A). Whereas a less-than-40-fold Rev-mediated increase of Gag synthesis was observed for astrocytoma cell lines, an up-to-700-fold stimulatory effect of Rev was observed for the Rev-permissive control cells. Similar results were obtained with both Rev response plasmids.
FIG. 1.
Stimulatory capacity of HIV-1 trans-activating factors Rev and Tat in human astrocytoma and Rev-permissive control cell lines. Astrocytoma cell lines show a strongly diminished Rev response, whereas the activity of Tat is unchanged. (A) Stimulatory capacity of HIV-1 trans-activating factors, or function of HIV-1 regulatory proteins. Rev response was assayed by determining the effect of Rev on production of HIV-1 Gag structural protein. Rev response plasmid pHXB2fB or pB37R (5 μg) was transfected into cells without or in combination with Rev expression plasmid pBsRev (1 μg). Transfection mixtures included pRSVluc (1 μg) as control for HIV-independent gene expression. Tat expression plasmid pL3Tat (1 μg) was added to transfection mixtures with Rev response plasmid pB37R. Gag protein levels were quantitated in transfected cells by ELISA, and the ratio of Gag produced with Rev to that without Rev was determined (y axis). In each experiment, the Rev response of a cell line was assayed in triplicate and the results were averaged. The Tat response was assayed by determining the effect of Tat on expression of a reporter gene (CAT) under the control of the HIV-1 LTR. Cells were cotransfected with Tat response plasmid pHIVΔnps-LTR-CAT (1 μg) alone or in combination with Tat expression plasmid pcTat (1 μg) and with pGL3-RFB (1 μg) to control for HIV-independent expression. Expression of CAT was quantitated in transfected cells by ELISA or functional CAT assay, and the ratio of CAT produced with Tat to that without Tat was determined (y axis). In each experiment, the Tat response of a cell line was assayed in duplicate and the results were averaged. Filled circles represent stimulatory values of individual experiments, and bars represent the resulting mean values. (B) HIV-independent gene expression in astrocytoma and control cells in Rev response assays with pB37R. The graph shows mean levels of pRSVluc-directed luciferase expression in each cell line in the presence (+) and absence (−) of Rev (Rev responses shown in panel A). Luciferase activity (in relative light units [RLU]) was normalized to protein levels in lysates of transfected cells. Various cell lines showed different levels of luciferase expression. Rev did not influence luciferase expression in cultures of the same cell line.
The cell lines used in this study produced various levels of the luciferase reporter for HIV-independent expression (Fig. 1B), indicating variations in levels of overall gene expression between cell lines. However, luciferase expression varied less than twofold between cultures of the same cell line in the presence or absence of Rev. In agreement, calculated levels of the Rev response did not change significantly after normalization of Gag expression to luciferase activity (data not shown). These controls indicate similar transfection efficiencies with and without Rev in each cell line and rule out toxic effects of HIV-1 Rev as a cause of the diminished Rev response on astrocytoma cells.
Experiments were carried out to study the effect of varying amounts of reporter and effector plasmids on the diminished Rev response in astrocytoma cell lines. Transfected genes were expressed to the highest levels in astrocytoma cell line U87MG and nonastrocytic control cell line HEK293 (Fig. 1B), suggesting highly active gene expression in both cell lines. A dose-response experiment with different amounts of the Gag expression plasmid pB37R (0.5 to 15 μg) showed that the difference in Rev response between the cell lines was maintained for various levels of Gag expression (data not shown). These results indicate that the decreased Rev response in U87MG cells was not due to a saturation of Gag synthesis in this highly active cell line.
The influence of various amounts of Rev expression plasmid pBsRev (0.001 to 10 μg) on the Rev response was assessed in the U373MG astrocytoma cell line and the Rev-permissive HeLa cell line, which both showed comparably low-level expression of the HIV-independent reporter gene (Fig. 1B). A dose-dependent increase of the Rev response was observed with up to 1 μg of pBsRev, yielding peak Rev-mediated increases of 10-fold for U373MG cells and 250-fold for HeLa cells (data not shown). No increase in Rev response was observed with amounts of pBsRev exceeding 1 μg. Similar results were obtained for astrocytoma cell line 85HG66 (data not shown). These results indicate that diminished trans activation of Rev is not overcome by increasing expression of Rev.
HIV gene expression involves the activity of another viral RNA binding regulatory factor, Tat, which acts to stimulate transcription of viral genes from the HIV LTR. To compare the trans-activating capacities of Tat in astrocytoma and in control cell lines, we determined Tat-dependent stimulation of expression of a reporter gene (CAT) under the control of the HIV LTR. Astrocytoma cell lines did not display diminished trans activation function of Tat (Fig. 1A). These results indicate that diminished stimulation of Gag synthesis in astrocytes does not involve impaired functionality of Tat.
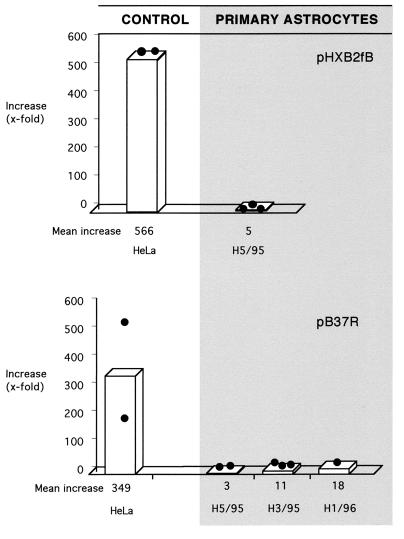
(iii) Primary fetal astrocytic cells.
To determine whether primary astrocytes also show limited Rev-dependent trans activation, Rev response assays were carried out with different passages of primary brain-derived explant culture (H5/95) and with two additional primary isolates of fetal astrocytes, one of which was derived from spinal cord (H1/96). In all experiments, the Rev response was dramatically diminished in primary human astrocytes, compared with that in Rev-permissive HeLa control cells (Fig. 2). Similar results were obtained when Rev was expressed in fusion with GFP (Rev expression plasmid pCsRevsg143 [data not shown]). The diminished Rev response of primary astrocytes was not overcome by varying amounts of Rev reporter or Rev expression plasmids (data not shown), in agreement with results obtained with astrocytoma cell lines.
FIG. 2.
Diminished Rev-dependent stimulation of Gag production in human primary fetal astrocytes. Rev response was assessed in cell isolates from myelencephalon-mesencephalon (H5/95 and H3/95) and spinal cord (H1/96) of three donors. Transfections were done in duplicate plates. Filled circles represent stimulatory values of individual experiments, and bars represent the resulting mean values. HeLa, Rev-permissive control cells. For further details, see the legend to Fig. 1.
Primary fetal astrocytes produced much higher levels of both HIV-1 Gag protein in the absence of Rev and the luciferase reporter for HIV-independent expression than did HeLa cells (about 100-fold). This suggests highly active overall gene expression in the primary fetal astrocytes. Relative basal Gag synthesis was similar in both cell types after normalization of Gag to luciferase expression (data not shown), arguing against specific elevation of Rev-independent Gag synthesis in the primary astrocyte cultures.
(iv) Quantitative comparison of HIV-1 trans activation functions in astrocytic and control cells.
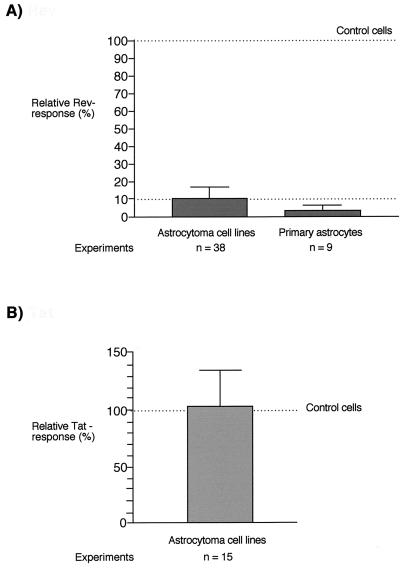
To quantitate the Rev responses in astrocytes relative to those in control cells, data from all experiments carried out under standard assay conditions were divided into separate data sets for Rev-permissive control cells (n = 36), astrocytoma cell lines (n = 38), and primary astrocytes (n = 9). Each data set consisted of Rev response values for both Rev reporter plasmids (pB37R and pHXB2fB), because they did not differ significantly for each cell type (P > 0.08). The Rev response in control cells (shown as median/mean) was 219.5/295.9, compared with 22.8/25.0 for astrocytoma cell lines and 5.9/8.1 for primary astrocytes. The data set for Rev-permissive control cells did not pass the test for Gaussian distribution, whereas that for astrocytoma cell lines did. Use of a nonparametric test for statistical significance (Mann-Whitney U test) revealed that differences in Rev response between control cells and astrocytic cells were extremely significant (P < 0.0001). The Rev responsiveness of astrocytes relative to that of Rev-permissive cells was calculated by normalizing values for astrocytic cells to the median Rev induction in control cells (219.5). This procedure revealed that the mean Rev response in astrocytes is about 10% of that in Rev-permissive control cells (Fig. 3A).
FIG. 3.
Quantification of Rev (A) and Tat (B) responses in astrocytes relative to those in HIV-permissive control cells. The graph shows mean values and standard deviations of Rev and Tat responses in astrocytes normalized to nonglial control cells (100%). (A) Relative Rev response. Three separate data sets, consisting of compiled values for Rev responses determined with both Rev reporter plasmids under standard assay conditions for nonastrocytic Rev-permissive control cell lines (HEK293, LC5, and HeLa; 36 values), astrocytoma cell lines (U138MG, U373MG, U251MG, and U87MG; 38 values), and primary astrocytes (three primary cell isolates; 9 values), were generated. Values for Rev response in astrocytes were normalized to the median Rev response in control cells (219.5). (B) Relative Tat response. Two data sets consisting of compiled values for Tat responses in control cell lines (HEK293 and HeLa; 9 values) and astrocytoma cell lines (U138MG, U373MG, 85HG66, and U87MG; 13 values) were generated. Values for Tat response in astrocytes were normalized to the mean Tat response in control cells (12.8).
The Tat responses in control cell lines and astrocytoma cell lines were compared in a similar manner (n = 6 for control cell lines and n = 15 for astrocytoma cell lines). The data set for astrocytoma cell lines passed the test for Gaussian distribution. The Tat activity in control cells (shown as median/mean) was 11.9/12.8 and in astrocytoma cells was 12.7/13.0. Tat responses did not differ significantly between control and astrocytoma cells (P > 0.5). Normalization of Tat activity in astrocytes to the mean Tat activity in control cells revealed that astrocytoma cells display >100% of the Tat activity of control cells (Fig. 3B).
Altered intracellular distribution of Rev in astrocytes.
Rev has been reported to be a nuclear protein which accumulates predominantly in the nucleoli of Rev-expressing cells. Nuclear localization of Rev has been shown both in fixed cells analyzed by indirect immunofluorescence staining with antibodies against Rev (16, 28, 39) and in living cells expressing Rev fused to GFP (48).
To examine the intracellular localization of Rev in astrocytes and Rev-permissive control cells, cells were transfected with a plasmid containing the rev gene fused to GFP-encoding sequences (pCsRevsg143). The Rev-GFP ORF in this fusion construct does not contain an internal translation initiation site downstream of the rev sequences to rule out synthesis of unfused GFP. Expression of a single Rev-GFP fusion protein was confirmed by Western blot analysis of lysates of HEK293 cells transfected with pCsRevsg143 by using antibodies against GFP and Rev (data not shown). The Rev-GFP protein synthesized from pCsRevsg143 retained high-level trans activation function in HeLa cells (about 500-fold stimulation of Gag synthesis).
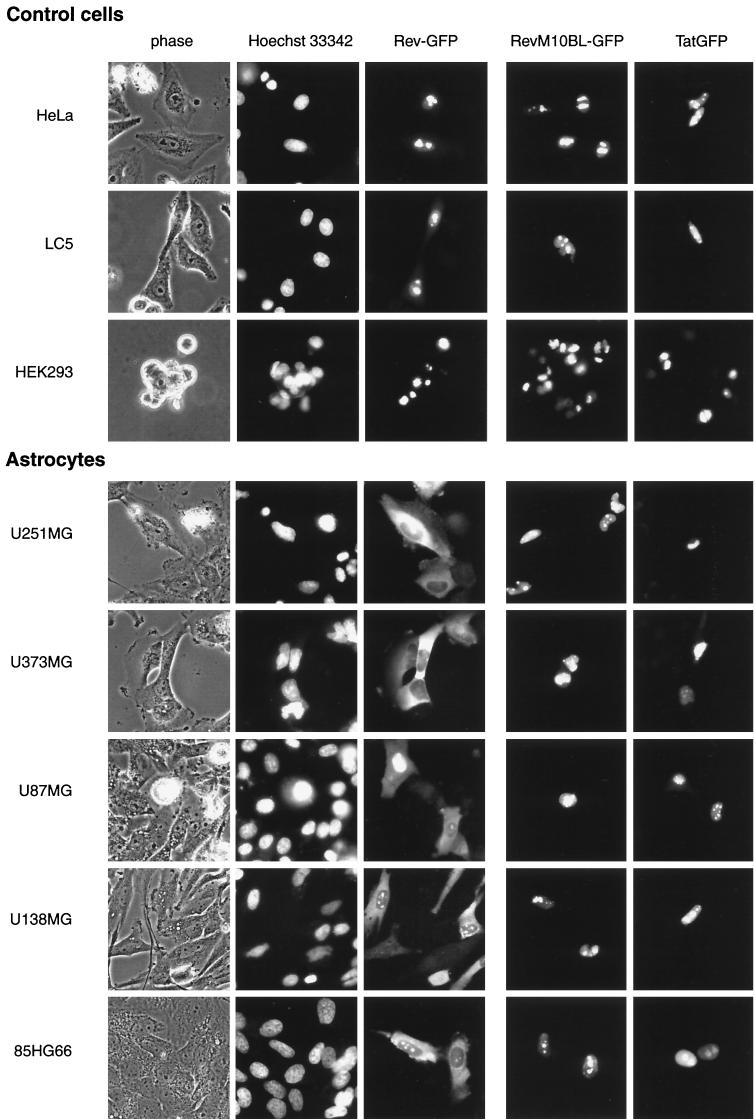
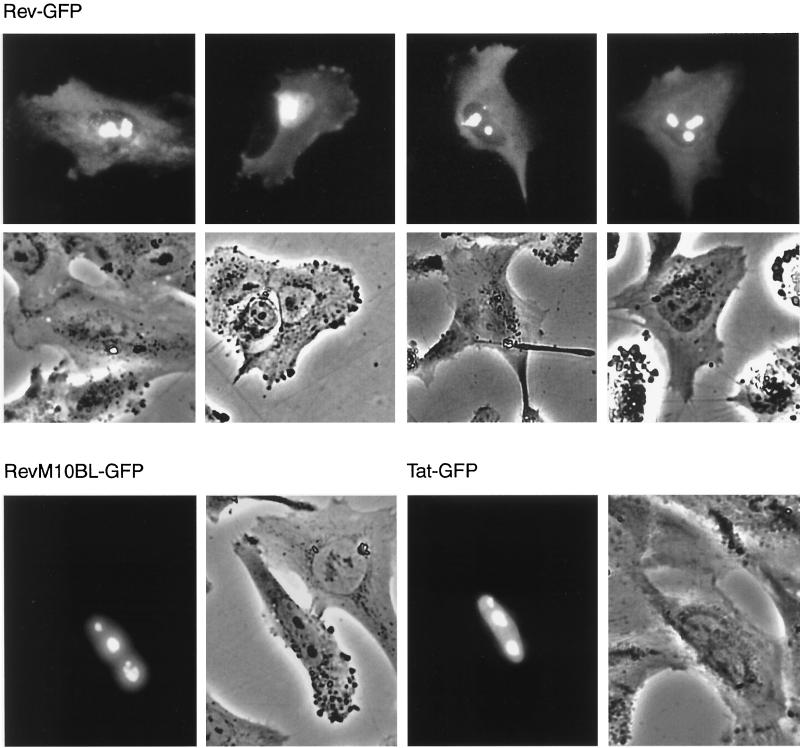
As expected, Rev-permissive control cells transfected with pCsRevsg143 showed a predominantly nuclear and/or nucleolar distribution of Rev-GFP (Fig. 4). In contrast, astrocytoma cells expressing Rev-GFP revealed strong cytoplasmic fluorescence with high frequency (≥80% of that of Rev-expressing cells). Cytoplasmic localization of Rev was also clearly visible in primary astrocytes in all fetal cultures analyzed (≥60% of that of Rev-expressing cells) (Fig. 5). Cytoplasmic localization of Rev was confirmed by indirect immunofluorescence staining of fixed cells with antibodies against Rev for all astrocytoma cell lines and primary astrocytes (data not shown). Cotransfection with Rev response plasmid pB37R or pHXB2fB did not alter intracellular distribution of Rev-GFP in astrocytes (compare localization of Rev in U87MG cells in Fig. 4 [transfected with pCsRevsg143] and in the image for 0 nM leptomycin B in Fig. 6 [cotransfected with pCsRevsg143 and pHXB2fB]).
FIG. 4.
Altered intracellular distribution of Rev in astrocytic tumor-derived cell lines. Rev is visible in the cytoplasm of astrocytoma cells but not in control cells. Cytoplasmic localization is not apparent for an export-deficient mutant of Rev (M10BL) or Tat. Cells were transfected with plasmids (1 μg) directing expression of the indicated viral proteins fused to GFP (plasmid pCsRevsg143 for expression of wild-type Rev-GFP, pCsRevM10BLsg143 for export-deficient Rev mutant M10BL-GFP, and pTat-GFP for Tat-GFP). Images of living cells were taken 24 h after transfection as described in Materials and Methods. Nuclei of cells were identified by counterstaining with 2 μg of Hoechst dye 33342 per ml for 10 min. Phase, phase-contrast image.
FIG. 5.
Detection of Rev in the cytoplasm of primary fetal astrocytes. For details, see the legend to Fig. 4.
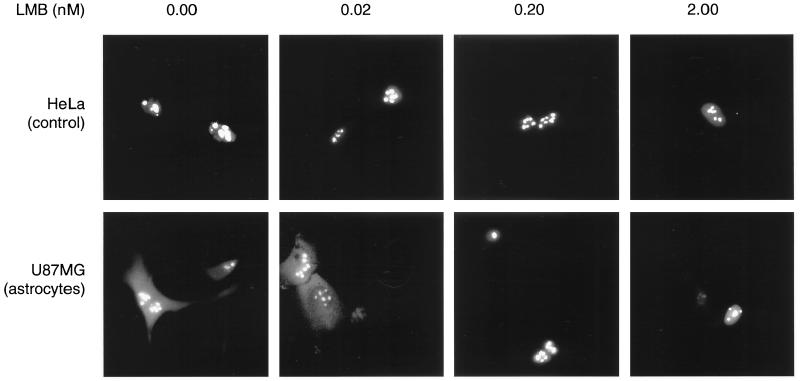
FIG. 6.
Blockage of nuclear export leads to nuclear accumulation of Rev in astrocytoma cells. Control cells (HeLa) and astrocytoma cells (U87MG) were transfected with Rev-GFP expression plasmid pCsRevsg143 and pHXB2fB under standard conditions. Leptomycin B (LMB) was added at the indicated concentrations 4 h after transfection, and images of living cells were taken 24 h after transfection.
Transfection of astrocytic and control cells with a Tat-GFP expression plasmid (pTat-GFP) revealed localization of Tat-GFP predominantly in the nuclear and/or nucleolar compartments of both cell types (Fig. 4 and 5). In contrast to Rev, localization of Tat was not different in astrocytes and control cells.
Cytoplasmic accumulation of Rev in astrocytoma cells requires nuclear export.
To assess whether cytoplasmic accumulation of Rev is associated with nuclear export of Rev, we analyzed intracellular distribution of the export-deficient Rev mutant M10BL (16, 27), fused to GFP (pCsRevM10BLsg143). As expected, this mutant of Rev was not functional in either astrocytic or control cells (data not shown). In contrast to wild-type Rev, RevM10BL did not accumulate in the cytoplasm of astrocytic cells (Fig. 4 and 5). Export of Rev can also be blocked by treatment of Rev-expressing cells with leptomycin B (2 to 10 nM [63]), a drug that blocks binding of the Crm1 export factor to Rev (6). Treatment of Rev-expressing astrocytoma cells (U87MG) with leptomycin B abolished cytoplasmic accumulation of Rev at concentrations ≥0.2 nM (Fig. 6). Similar results were obtained for other astrocytoma cell lines (U138MG and 85HG66 [data not shown]). These results clearly demonstrate that nuclear export is required for cytoplasmic accumulation of Rev in astrocytes.
DISCUSSION
Astrocytes are target cells for HIV-1 in the nervous system and permit only very limited replication of HIV-1 (reviewed in reference 7). Astrocytes are essential for neuronal performance and protection of the brain from harmful compounds in the blood and cerebrospinal fluid. Up to 5% of astrocytes have been demonstrated previously to be infected with HIV in individuals with AIDS (3, 54). Because of the abundance of astrocytes in the brain (total number, 0.4 × 1012 to 2 × 1012 [41]), the pool of infected astrocytes in vivo can reach the proportion of infected lymphocytes in peripheral lymphoid tissues of HIV-infected individuals (7). Thus, astrocytes may contribute to HIV-1-associated neuropathogenesis and establishment of virus reservoirs in infected individuals.
As a cell population, astrocytes are quite heterogeneous, capable of expressing numerous different cellular proteins and fulfilling many different functions. They exist in different morphological forms in the brain, and it has been proposed elsewhere that different classes of astrocytes are responsible for different functions in the brain (37). In culture, astrocytes display an enormous heterogeneity with respect to profiles of enzymes, antigenic markers, transporters, receptors, and ion channels (reviewed in reference 61). Heterogeneity of astrocytes is further increased by the capacity of astrocytes to be activated by external stimuli, resulting in upregulated expression of a variety of different molecules, including the astrocyte-specific marker GFAP (reviewed in references 13 and 40). This heterogeneity is presumably responsible for the lack of a common specific cellular marker for all astrocytes.
In this study, we used a panel of human astrocytoma cell lines previously demonstrated to be infectible by HIV-1, in addition to primary human fetal astrocytes from different sources, to study the influence of Rev on HIV-1 protein synthesis in astrocytes. These astrocytic cells showed different expression profiles of cellular antigens and different levels of expression of HIV-independent reporter gene expression. We demonstrate here that, despite this heterogeneity, astrocytic cells from all origins showed distinctly suppressed Rev-dependent stimulation of HIV-1 Gag production and abnormal intracellular distribution of Rev. These results indicate that suppression of Rev-dependent posttranscriptional regulation is a hallmark of human astrocytes and relies on specific features of astrocyte biology not found in replication-competent nonglial target cells of HIV-1. Cell-directed negative modulation of posttranscriptional control of HIV-1 gene expression may contribute to establishment and/or maintenance of the low-producer phenotype typical of infected astrocytes.
Recently, it was suggested that diminished production of HIV-1 proteins in astrocytes results from inefficient translation of HIV mRNAs (19). In this report, Rev was shown to stimulate expression of a heterologous (CAT) reporter gene linked to the RRE. Since the data presented in this study concerning Rev were obtained with a single cell line (U251MG), it cannot be ruled out that these observations reflect, at least in part, specific features of this cell line. Furthermore, we have found variations in Rev responses of U251MG cells during extended culture (24a), suggesting that proliferation of these cells may influence their capacity to support Rev function.
Astrocytes constitute the first example of a human cell type showing an impaired Rev response. The only other cell types reported not to support Rev-dependent posttranscriptional regulation of HIV-1 are murine fibroblasts (57), which also do not support Tat function (62). Failures of Rev and Tat functions were ascribed to lack of essential human factors in murine cells, and both defects could be complemented by fusion with human cells or addition of selected human chromosomes (46, 47, 57, 62). Whereas human cyclin T was identified as a candidate host cell factor for Tat activity (59), human host cell factors capable of restoring Rev activity in rodent cells have not yet been identified. Specificity of HIV-1 regulatory factors for primate cells has been a major obstacle in developing murine animal models for HIV-1 infection. Preliminary results from our group indicate expression of several known human Rev-interacting factors in astrocytoma cell lines (8a). Synthesis of HIV-1 structural proteins was not elevated by fusion of persistently HIV-1-infected astrocytoma cell line TH4-7-5 with Rev-permissive human cells (31a). Finally, diminished Rev response in astrocytes does not appear to be caused by absence of individual human chromosomes, since it occurs in primary astrocytes as well as in different astrocytoma cell lines. These observations indicate that the mechanisms underlying the diminished Rev response in human astrocytes are different from those in murine cells.
Rev function is tightly linked to nuclear localization of Rev. Thus, mutants carrying mutations of the amino-terminal domain of Rev which show cytoplasmic localization are generally not functional (22, 49). Rev fused to the steroid-binding domain of the glucocorticoid receptor shows cytoplasmic localization and lack of function in the absence of dexamethasone, whereas exposure to hormone induces both function and nuclear localization of the Rev-glucocorticoid receptor protein (20). In a therapeutic approach to inhibiting Rev, cytoplasmic sequestration of Rev is achieved by coexpression of Rev-specific single-chain antibodies (21, 64). In Rev-permissive cells, maintenance of nuclear localization of Rev appears to be dependent on cellular transcription, since inhibitors of transcription, like 5,6 dichlorobenzimidazol, actinomycin D, and alpha-amanitin induce cytoplasmic localization of Rev (10, 12, 28).
In astrocytes, diminished Rev response is associated with cytoplasmic localization of Rev. Cytoplasmic localization of Rev is overcome by blocking nuclear export of Rev, resulting in accumulation of Rev in the nuclei of astrocytes. These results show that Rev retains shuttling properties in astrocytes and indicate that Rev is not irreversibly trapped in the cytoplasm of astrocytes. Rather, the changed balance of Rev between nucleus and cytoplasm may reflect alterations in the relative rates of import or export of Rev, e.g., in the dynamics of trafficking. Furthermore, cytoplasmic accumulation of Rev may be augmented by transient interaction of Rev with cytoplasmic components in astrocytes, reducing the size of the pool of Rev molecules available for active shuttling. These possibilities are not mutually exclusive and are currently under investigation (31a).
In analogy to the cytoplasmic localization of Rev in astrocytes shown here, altered intracellular localization of the influenza virus shuttling nucleoprotein NP has been reported to occur in cells which do not support replication of the influenza virus (31, 58). These two examples, linking lack of virus production with changes in the localization of viral shuttle proteins, suggest that disruption of the nuclear-cytoplasmic distribution of viral shuttle proteins may be a more general mechanism by which cells can turn off virus replication.
In summary, we have shown in this report that, despite the heterogeneity of human astrocytes, they share the capacity to downmodulate Rev-dependent posttranscriptional regulation of HIV-1. This restriction is associated with a shift in the balance of Rev in the nuclear and cytoplasmic compartments in astrocytes, which can be overcome by inhibiting nuclear export of Rev. Cytoplasmic localization of Rev may be due to alterations in the dynamics of trafficking of Rev and/or reversible cytoplasmic retention of Rev. These results show that negative modulation of HIV-1 regulation occurs in cells constituting an important reservoir for HIV-1 in humans and can contribute to cell-directed attenuation of virus production.
ACKNOWLEDGMENTS
E.L. and F.C.S. contributed equally to this work.
We thank Petra Willnauer for excellent assistance with cell culture and Elena Shumay for FACS analysis of transfection efficiencies. We thank Claudia Gerhartz and Thomas Werner for valuable discussions. We are grateful to Kyoji Horie, Roland Stauber, George N. Pavlakis, and Bryan Cullen for providing expression plasmids. We especially thank Francesca Aloisi for the gift of primary fetal astrocytes.
This work was supported by the Deutsche Forschungsgemeinschaft (F.C.S. and J.V.; SFB 464) and the AIDS Stipendienprogramm des Bundesministeriums für Bildung und Forschung (M.N.).
REFERENCES
- 1.Aloisi F, Borsellino G, Samoggia P, Testa U, Chelucci C, Russo G, Peschle C, Levi G. Astrocyte cultures from human embryonic brain: characterization and modulation of surface molecules by inflammatory cytokines. J Neurosci Res. 1992;32:494–506. doi: 10.1002/jnr.490320405. [DOI] [PubMed] [Google Scholar]
- 2.Atwood W J, Berger J R, Kaderman R, Tornatore C S, Major E O. Human immunodeficiency virus type 1 infection of the brain. Clin Microbiol Rev. 1993;6:339–366. doi: 10.1128/cmr.6.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner J P, Tawadros R, Pomerantz R J. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin T J, Fazeli M S, Doherty P, Walsh F S. Elucidation of the molecular actions of NCAM and structurally related cell adhesion molecules. J Cell Biochem. 1996;61:502–513. doi: 10.1002/(sici)1097-4644(19960616)61:4<502::aid-jcb3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Bigner D D, Bigner S H, Ponten J, Westermark B, Mahaley M S, Ruoslahti E, Herschman H, Eng L F, Wikstrand C J. Heterogeneity of genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981;40:201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bogerd H P, Echarri A, Ross T M, Cullen B R. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J Virol. 1998;72:8627–8635. doi: 10.1128/jvi.72.11.8627-8635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 8.Brack-Werner R, Kleinschmidt A, Ludvigsen A, Mellert W, Neumann M, Herrmann R, Khim M C, Burny A, Muller-Lantzsch N, Stavrou D, et al. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS. 1992;6:273–285. [PubMed] [Google Scholar]
- 8a.Ceccherini Silberstein, F. Unpublished observations.
- 9.Cullen B R. Posttranscriptional regulation by the HIV-1 Rev protein. Semin Virol. 1998;8:327–334. [Google Scholar]
- 10.D’Agostino D M, Ciminale V, Pavlakis G N, Chieco-Bianchi L. Intracellular trafficking of the human immunodeficiency virus type 1 Rev protein: involvement of continued rRNA synthesis in nuclear retention. AIDS Res Hum Retroviruses. 1995;11:1063–1071. doi: 10.1089/aid.1995.11.1063. [DOI] [PubMed] [Google Scholar]
- 11.de Wet J R, Wood K V, DeLuca M, Helinski D R, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dundr M, Leno G H, Hammarskjold M L, Rekosh D, Helga-Maria C, Olson M O. The roles of nucleolar structure and function in the subcellular location of the HIV-1 Rev protein. J Cell Sci. 1995;108:2811–2823. doi: 10.1242/jcs.108.8.2811. [DOI] [PubMed] [Google Scholar]
- 13.Eddleston M, Mucke L. Molecular profile of reactive astrocytes: implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emerman M, Malim M H. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 15.Felber B K, Drysdale C M, Pavlakis G N. Feedback regulation of human immunodeficiency virus type 1 expression by the Rev protein. J Virol. 1990;64:3734–3741. doi: 10.1128/jvi.64.8.3734-3741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felber B K, Hadzopoulou-Cladaras M, Cladaras C, Copeland T, Pavlakis G N. Rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci USA. 1989;86:1495–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel A D, Young J A. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Frech K, Danescu-Mayer J, Werner T. A novel method to develop highly specific models for regulatory units detects a new LTR in GenBank which contains a functional promoter. J Mol Biol. 1997;270:674–687. doi: 10.1006/jmbi.1997.1140. [DOI] [PubMed] [Google Scholar]
- 19.Gorry P R, Howard J L, Churchill M J, Anderson J L, Cunningham A, Adrian D, McPhee D A, Purcell D F. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J Virol. 1999;73:352–361. doi: 10.1128/jvi.73.1.352-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hope T J, Huang X J, McDonald D, Parslow T G. Steroid-receptor fusion of the human immunodeficiency virus type 1 Rev transactivator: mapping cryptic functions of the arginine-rich motif. Proc Natl Acad Sci USA. 1990;87:7787–7791. doi: 10.1073/pnas.87.19.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubota S, Duan L, Furuta R A, Hatanaka M, Pomerantz R J. Nuclear preservation and cytoplasmic degradation of human immunodeficiency virus type 1 Rev protein. J Virol. 1996;70:1282–1287. doi: 10.1128/jvi.70.2.1282-1287.1996. . (Authors’ correction, 72:3505–3506, 1998.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota S, Furuta R, Maki M, Hatanaka M. Inhibition of human immunodeficiency virus type 1 Rev function by a Rev mutant which interferes with nuclear/nucleolar localization of Rev. J Virol. 1992;66:2510–2513. doi: 10.1128/jvi.66.4.2510-2513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Neumann M, Stearman R, Stauber R, Pause A, Pavlakis G N, Klausner R D. Transcription-dependent nuclear-cytoplasmic trafficking is required for the function of the von Hippel-Lindau tumor suppressor protein. Mol Cell Biol. 1999;19:1486–1497. doi: 10.1128/mcb.19.2.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludvigsen A, Werner T, Gimbel W, Erfle V, Brack-Werner R. Down-modulation of HIV-1 LTR activity by an extra-LTR nef gene fragment. Virology. 1996;216:245–251. doi: 10.1006/viro.1996.0056. [DOI] [PubMed] [Google Scholar]
- 24a.Ludwig, E. Unpublished observations.
- 25.Malim M H, Hauber J, Fenrick R, Cullen B R. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988;335:181–183. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- 26.Mellert W, Kleinschmidt A, Schmidt J, Festl H, Emler S, Roth W K, Erfle V. Infection of human fibroblasts and osteoblast-like cells with HIV-1. AIDS. 1990;4:527–535. doi: 10.1097/00002030-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Mermer B, Felber B K, Campbell M, Pavlakis G N. Identification of trans-dominant HIV-1 rev protein mutants by direct transfer of bacterially produced proteins into human cells. Nucleic Acids Res. 1990;18:2037–2044. doi: 10.1093/nar/18.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer B E, Malim M H. The HIV-1 Rev trans-activator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- 29.Moreno T N, Fortunato E A, Hsia K, Spector S A, Spector D H. A model system for human cytomegalovirus-mediated modulation of human immunodeficiency virus type 1 long terminal repeat activity in brain cells. J Virol. 1997;71:3693–3701. doi: 10.1128/jvi.71.5.3693-3701.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 31.Neumann G, Castrucci M R, Kawaoka Y. Nuclear import and export of influenza virus nucleoprotein. J Virol. 1997;71:9690–9700. doi: 10.1128/jvi.71.12.9690-9700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Neumann, M. Unpublished observations.
- 32.Neumann M, Felber B K, Kleinschmidt A, Froese B, Erfle V, Pavlakis G N, Brack-Werner R. Restriction of human immunodeficiency virus type 1 production in a human astrocytoma cell line is associated with a cellular block in Rev function. J Virol. 1995;69:2159–2167. doi: 10.1128/jvi.69.4.2159-2167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niikura M, Dornadula G, Zhang H, Mukhtar M, Lingxun D, Khalili K, Bagasra O, Pomerantz R J. Mechanisms of transcriptional transactivation and restriction of human immunodeficiency virus type I replication in an astrocytic glial cell. Oncogene. 1996;13:313–322. [PubMed] [Google Scholar]
- 34.Nuovo G J, Gallery F, MacConnell P, Braun A. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. Am J Pathol. 1994;144:659–666. [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlakis G N. The molecular biology of human immunodeficiency virus type 1. In: DeVita T V, Hellmann S, Rosenberg A S, editors. AIDS: biology, diagnosis, treatment and prevention. Vol. 4. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 45–74. [Google Scholar]
- 36.Ponten J, Macintyre E H. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74:465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- 37.Raff M C. Glial cell diversification in the rat optic nerve. Science. 1989;243:1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- 38.Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Richard N, Iacampo S, Cochrane A. HIV-1 Rev is capable of shuttling between the nucleus and cytoplasm. Virology. 1994;204:123–131. doi: 10.1006/viro.1994.1516. [DOI] [PubMed] [Google Scholar]
- 40.Ridet J L, Malhotra S K, Privat A, Gage F H. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. . (Erratum, 21:80, 1998.) [DOI] [PubMed] [Google Scholar]
- 41.Rutka J T, Murakami M, Dirks P B, Hubbard S L, Becker L E, Fukuyama K, Jung S, Tsugu A, Matsuzawa K. Role of glial filaments in cells and tumors of glial origin: a review. J Neurosurg. 1997;87:420–430. doi: 10.3171/jns.1997.87.3.0420. [DOI] [PubMed] [Google Scholar]
- 42.Saito Y, Sharer L R, Epstein L G, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich T A, Blumberg B M. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- 43.Schäfer B W, Heizmann C W. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz S, Felber B K, Benko D M, Fenyo E M, Pavlakis G N. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J Virol. 1990;64:2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shahabuddin M, Bentsman G, Volsky B, Rodriguez I, Volsky D J. A mechanism of restricted human immunodeficiency virus type 1 expression in human glial cells. J Virol. 1996;70:7992–8002. doi: 10.1128/jvi.70.11.7992-8002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shukla R R, Kimmel P L, Kumar A. Human immunodeficiency virus type 1 Rev-responsive element RNA binds to host cell-specific proteins. J Virol. 1994;68:2224–2229. doi: 10.1128/jvi.68.4.2224-2229.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shukla R R, Marques S M, Kimmel P L, Kumar A. Human chromosome 6- and 11-encoded factors support human immunodeficiency virus type 1 Rev function in A9 cells. J Virol. 1996;70:9064–9068. doi: 10.1128/jvi.70.12.9064-9068.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stauber R, Gaitanaris G A, Pavlakis G N. Analysis of trafficking of Rev and transdominant Rev proteins in living cells using green fluorescent protein fusions: transdominant Rev blocks the export of Rev from the nucleus to the cytoplasm. Virology. 1995;213:439–449. doi: 10.1006/viro.1995.0016. [DOI] [PubMed] [Google Scholar]
- 49.Stauber R H, Afonina E, Gulnik S, Erickson J, Pavlakis G N. Analysis of intracellular trafficking and interactions of cytoplasmic HIV-1 Rev mutants in living cells. Virology. 1998;251:38–48. doi: 10.1006/viro.1998.9295. [DOI] [PubMed] [Google Scholar]
- 50.Stauber R H, Horie K, Carney P, Hudson E A, Tarasova N I, Gaitanaris G A, Pavlakis G N. Development and applications of enhanced green fluorescent protein mutants. BioTechniques. 1998;24:462–466. doi: 10.2144/98243rr01. , 468–471. [DOI] [PubMed] [Google Scholar]
- 51.Stauber R H, Pavlakis G N. Intracellular trafficking and interactions of the HIV-1 Tat protein. Virology. 1998;252:126–136. doi: 10.1006/viro.1998.9400. [DOI] [PubMed] [Google Scholar]
- 52.Stavrou D, Keiditsch E, Schmidberger F, Bise K, Funke I, Eisenmenger W, Kurrle R, Martin B, Stocker U. Monoclonal antibodies against human astrocytomas and their reactivity pattern. J Neurol Sci. 1987;80:205–220. doi: 10.1016/0022-510x(87)90155-9. [DOI] [PubMed] [Google Scholar]
- 53.Tabernero C, Zolotukhin A S, Valentin A, Pavlakis G N, Felber B K. The posttranscriptional control element of the simian retrovirus type 1 forms an extensive RNA secondary structure necessary for its function. J Virol. 1996;70:5998–6011. doi: 10.1128/jvi.70.9.5998-6011.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi K, Wesselingh S L, Griffin D E, McArthur J C, Johnson R T, Glass J D. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 55.Tornatore C, Chandra R, Berger J R, Major E O. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- 56.Tornatore C, Meyers K, Atwood W, Conant K, Major E. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J Virol. 1994;68:93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trono D, Baltimore D. A human cell factor is essential for HIV-1 Rev action. EMBO J. 1990;9:4155–4160. doi: 10.1002/j.1460-2075.1990.tb07638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang P, Palese P, O’Neill R E. The NPI-1/NPI-3 (karyopherin α) binding site on the influenza A virus nucleoprotein NP is a nonconventional nuclear localization signal. J Virol. 1997;71:1850–1856. doi: 10.1128/jvi.71.3.1850-1856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 60.Westermark B, Ponten J, Hugosson R. Determinants for the establishment of permanent tissue culture lines from human gliomas. Acta Pathol Microbiol Scand Sect A. 1973;81:791–805. doi: 10.1111/j.1699-0463.1973.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 61.Wilkin G P, Marriott D R, Cholewinski A J. Astrocyte heterogeneity. Trends Neurosci. 1990;13:43–46. doi: 10.1016/0166-2236(90)90065-i. [DOI] [PubMed] [Google Scholar]
- 62.Winslow B J, Trono D. The blocks to human immunodeficiency virus type 1 Tat and Rev functions in mouse cell lines are independent. J Virol. 1993;67:2349–2354. doi: 10.1128/jvi.67.4.2349-2354.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolff B, Sanglier J J, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- 64.Wu Y, Duan L, Zhu M, Hu B, Kubota S, Bagasra O, Pomerantz R J. Binding of intracellular anti-Rev single chain variable fragments to different epitopes of human immunodeficiency virus type 1 Rev: variations in viral inhibition. J Virol. 1996;70:3290–3297. doi: 10.1128/jvi.70.5.3290-3297.1996. . (Authors’ correction, 72:3505–3506, 1998.) [DOI] [PMC free article] [PubMed] [Google Scholar]