Highlights
-
•
Malignant struma ovarii is rare and without established criteria regarding management and surveillance.
-
•
Many authors have supported stratification of malignant struma ovarii into low vs high-risk disease to guide management.
-
•
Case: recurrent metastatic malignant struma ovarii after surveillance in the setting of initially low-risk disease.
-
•
Serial thyroglobulin levels in addition to imaging, may serve as a monitoring tool in patients with functioning thyroid.
Keywords: Malignant struma ovarii, Germ cell tumors, Serial thyroglobulin
Abstract
Malignant struma ovarii is an exceedingly rare pathology with a paucity of established criteria regarding management and surveillance with recommendations largely based on case reports and retrospective data. Many authors have supported stratification of malignant struma ovarii into low vs high-risk disease with more conservative management reserved for those deemed low-risk. Here we present a unique case of recurrent metastatic malignant struma ovarii after surveillance was undertaken in the setting of initially low-risk disease.
1. Introduction
Struma ovarii is a highly specialized monodermal mature teratoma that is composed solely or predominantly (>50 %) of thyroid tissue (Shaco-levy et al., 2020). Struma ovarii constitutes 0.3–1 % of all ovarian tumors and 2–5 % of ovarian teratomas (Oudoux et al., 2016). While most struma ovarii are benign, up to 23 % have been reported to contain malignant thyroid tissue (Oudoux et al., 2016, Addley et al., 2021). Given the rarity of malignant struma ovarii, recommendations for diagnosis and management are largely based on retrospective case reports and series; there is even less data to support consensus for surveillance and treatment of recurrent disease (Addley et al., 2021, Kraemer et al., 2011, Marti et al., 2012, Chandran et al., 2022). Here, we present a unique case of malignant struma ovarii with recurrence identified after surveillance with serial thyroglobulin levels in a patient with an intact thyroid.
2. Case presentation
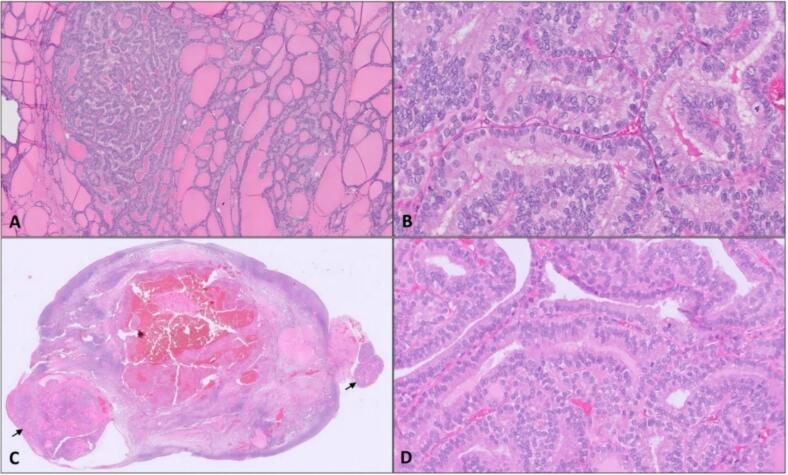
A 49-year-old with no pertinent past medical history presented in 2021 with pelvic pain and abnormal uterine bleeding. A pelvic ultrasound demonstrated a 6.5 cm complex left adnexal mass with features consistent with a dermoid cyst. An endometrial biopsy showed normal proliferative endometrium, and Thyroid-stimulating hormone (TSH)/Free T4 were normal. Tumor markers, including CA-125, CEA, CA 19–9, bHCG, and inhibin A/B were within normal limits. A pelvic magnetic resonance imaging (MRI) was obtained, which reported concern for malignancy in the setting of a large, complex, and predominantly solid mass with heterogeneous enhancement. The patient underwent a robotic bilateral salpingectomy and left oophorectomy, where the 9 cm left adnexal mass grossly consistent with a dermoid cyst was removed through the midline port site in a bag with contained rupture. Intra-operative frozen section was obtained and reported as struma ovarii. Contralateral oophorectomy and hysterectomy were not performed as the patient had elected for minimal surgical intervention at time of pre-operative counseling. Final surgical pathology demonstrated struma ovarii predominantly composed of mature thyroid tissue with variable-sized follicles and three microscopic foci of papillary thyroid carcinoma (PTC), the largest measuring 1.6 mm, characterized by papillary architecture and lined by cells showing classic nuclear features of enlarged and overlapping nuclei with cleared chromatin and frequent nuclear grooves (Fig. 1A and 1B). No lymphovascular space invasion or ovarian capsule involvement was appreciated. Pelvic washings were also performed with no evidence of malignant cells on final cytology.
Fig. 1.
Representative section of the left ovarian mass showing features of struma ovarii with variable-sized thyroid (1A, 50X) and focal areas with cytologic features of papillary thyroid carcinoma (1B, 400X). Right ovarian surface with foci of strumosis (black arrows, 1B 5X) with areas demonstrating cytologic features of papillary thyroid carcinoma (1D, 400X).
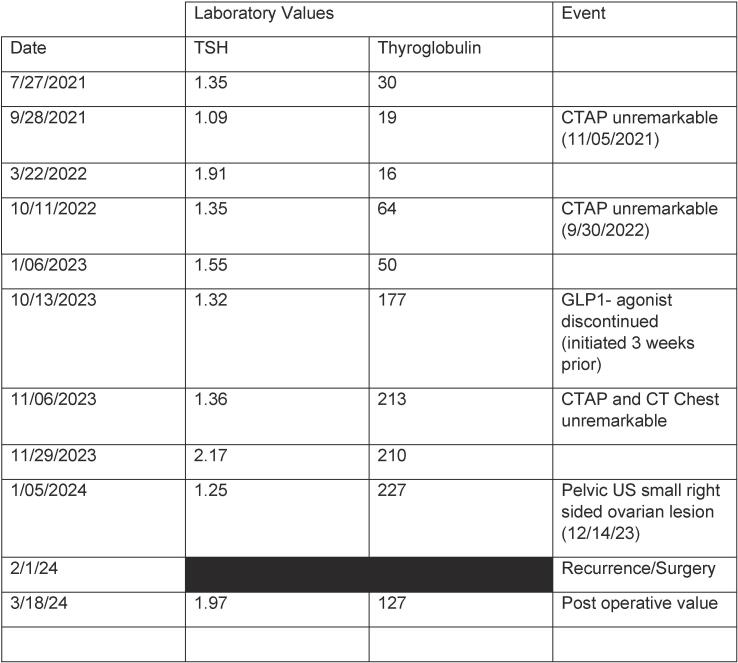
The patient was referred to endocrinology. Further workup including thyroid and neck ultrasounds, thyroglobulin level and thyroid function tests were normal. Following extensive discussion regarding the options of up-front thyroidectomy vs monitoring, in the setting of very small microscopic disease with a R0 resection, the absence of lymphovascular invasion, and negative margins, the patient elected for monitoring. Thyroglobulin monitoring was performed (every 2–4 months) and abdominal imaging (yearly). Thyroglobulin levels were normal for 2 years, at which time an increase was noted (see Fig. 2) in the setting of normal thyroid function tests. The patient had been started on a GLP-1 agonist several weeks prior to the elevated thyroglobulin level; however, thyroglobulin remained elevated off this medication for two months. A computed tomography scan (CT) chest/abdomen/pelvis and neck ultrasound were obtained and remained reassuring without evidence of disease. A pelvic ultrasound was additionally obtained which demonstrated a small hyperechoic right ovarian lesion measuring 6.4x9 x7.5 mm with increased vascularity. The patient was re-evaluated by gynecologic oncology and the decision was made to proceed with removal of remaining ovary and possible hysterectomy.
Fig. 2.
TSH/Thyroglobulin level trend.
At the time of surgery, thyroglobulin level was 227 ng/ml with a TSH of 1.26. Diagnostic laparoscopy revealed peritoneal carcinomatosis where diffuse red, cystic, nodular lesions were appreciated to cover the omentum, peritoneal surfaces, sigmoid mesentery, and uterine serosa. The right ovary was enlarged with a complex cystic appearance. These findings were not appreciated on the CT performed less than 3 months prior. The intraoperative frozen section of the omentum was consistent with thyroid neoplasm. A decision was made to proceed with cytoreduction, and she underwent a robotic-assisted total laparoscopic hysterectomy, right salpingo-oophorectomy, extensive peritoneal resection, and omentectomy to no gross residual disease. The pathology of the resected specimen from right ovary and fallopian tube, omentum, peritoneum, sigmoid mesentery, and anterior abdominal wall demonstrated foci ranging from similar histologic features as primary with variable-sized mature thyroid follicles consistent with strumosis (Fig. 1C). Similar to the primary lesion, the metastatic lesions showed foci with cytologic features consistent with PTC as characterized by enlarged, elongated nuclei with chromatin clearing and frequent nuclear grooves (Fig. 1D). PD-L1 testing was positive (CPS score 4), comprehensive molecular tumor testing (Tempus XT; 648 genes, fusions) demonstrated a pathogenic NRAS Q61R mutation, a tumor mutational burden was moderate at 4.2 m/MB, and there was no evidence of microsatellite stability.
Inter-disciplinary discussion was had with gynecologic oncology, pathology, endocrinology, and surgical oncology. Six weeks after surgery, thyroglobulin was lower but still was elevated versus the prior baseline at 127 ng/ml with a TSH of 1.97. Due to this level and the metastases on laparoscopy, the plan was made to proceed with total thyroidectomy followed by radioactive iodine therapy. The patient subsequently underwent an uncomplicated thyroidectomy and final pathology was benign. The patient is set to start I-131 therapy in the coming months with plan for post treatment whole body I-131 scan and subsequent follow up in 3–4 months.
3. Discussion
Our case highlights the unpredictability of disease transformation in the setting of malignant struma ovarii. There is a paucity of established criteria regarding the management and surveillance of this pathology given its rarity. Here, we present a case of recurrent malignant struma ovarii after surveillance was undertaken in the setting of initially very low-risk disease based on pathology.
Criteria have been suggested to categorize malignant struma ovarii as being low versus high risk of disease recurrence, to assist with prognostic counseling of patients. High risk of recurrence has been associated with larger lesion size (>4cm), extraovarian extension, presence of BRAF and RAS mutations, and/or non-papillary histology (Wolff et al., 2010, Ayhan et al., 2021, Gobitti et al., 2017, Coyne and Nikiforov, 2010). However, the frequency of mutations in very small malignant struma ovarii or their independent predictive value in smaller tumors had not been determined. The recommendation for adjuvant treatment is often dependent upon recurrence risk. Patients with no metastatic disease at low risk of recurrence may be managed conservatively after oophorectomy without adjuvant therapy and enter surveillance with serial thyroglobulin assessment and pharmacologic thyroid suppression (Addley et al., 2021, Chandran et al., 2022, Rose et al., 1998). Patients with metastatic disease or at high risk of recurrence are generally recommended to undergo adjuvant treatment with radioactive iodine therapy after thyroidectomy (Addley et al., 2021, Kraemer et al., 2011, Marti et al., 2012, Chandran et al., 2022). In our case, molecular testing was not performed on the primary specimen as it was not indicated based on the initial reassuring pathology and the 1.5 mm size precluded such testing, however, our patient’s recurrence (specifically posterior cul-de-sac biopsy) was noted to have an NRAS Q61R mutation. Although the frequency of this mutation in benign struma ovarii is not known, it is important to note that mutations in RAS oncogenes are found in both benign and malignant primary thyroid tumors. This further supports consideration of molecular testing, to categorize disease risk and guide treatment recommendations.
A review of 24 cases of malignant struma ovarii performed by DeSimone et al found that the most were diagnosed from a left-sided pelvic mass (63 %). Pelvic pain and abnormal uterine bleeding were the most common presenting symptoms; rarely did patients demonstrate clinical hyperthyroidism (8 %). All patients underwent surgical management, most commonly with a total abdominal hysterectomy and bilateral salpingo-oophorectomy (42 %), versus unilateral salpingo-oophorectomy (37 %). Many patients (45.8 %) were found to have metastatic disease at the time of surgery, but most did not receive adjuvant treatment and the recurrence rate was noted to be 35 %. At the time of recurrence, most patients underwent radioactive iodine treatment with or without thyroidectomy, with a 100 % complete response rate (DeSimone et al., 2003).
Although fewer than 5 % of malignant struma ovarii cases present with metastatic disease, recurrence rate is reported to be as high as 35 % (Chandran et al., 2022, Makani et al., 2004). In addition, the majority of malignant struma ovarii are diagnosed post-operatively on final pathology (Makani et al., 2004) with intra-operative frozen rarely detecting malignancy (Oudoux et al., 2016); thus, very few patients undergo complete staging on initial surgery.
A single-institution review of 11 patients with malignant struma ovarii by Addley et al commented on the lack of preoperative diagnostics for these tumors, highlighting the lack of a role for tumor markers as well as limited utility of initial imaging studies with most cases appearing as benign dermoid cysts. All cases in their study underwent surgery with a benign gynecologist, and the majority of patients underwent cystectomy versus unilateral salpingo-oophorectomy. All patients with final pathology confirming malignant struma ovarii were discussed with a multi-disciplinary team including gynecology oncology, endocrinology, radiology and head and neck surgery. All patients underwent post-operative imaging, and none had findings suggestive of metastatic disease. The authors argue that unilateral salpingo-oophorectomy may be appropriate in apparent early-stage disease and recommended reserving the use of radioactive iodine therapy with or without thyroidectomy to patients with metastatic disease or those with high risk of recurrence (tumor size > 10 mm, close margins, or aggressive histology). The authors did recommend surveillance with annual thyroglobulin levels regardless of projected recurrence risk (Addley et al., 2021).
Here, however, we highlight a unique case of recurrent malignant struma ovarii in the setting of initial diagnosis of low-risk disease. As previously mentioned, the literature suggests management of malignant struma ovarii with oophorectomy followed by surveillance utilizing thyroglobulin as a “tumor marker” is a reasonable and often recommended option for low-risk disease (Oudoux et al., 2016, Addley et al., 2021, Rose et al., 1998).
Cases that meet “low risk” criteria for recurrence are not exempt from surveillance, as the potential for recurrent/metastatic disease certainly exists as exemplified in our case. Our case strongly supports the importance of monitoring patients, even with the smallest of malignant struma ovarii, using serum thyroglobulin levels even in the presence of a normal thyroid gland. Further studies are needed to define the role of up-front molecular testing in all cases to fully assess risk as this testing has not been uniformly applied to all patients with struma ovarii. Finally, although there was no evidence of residual struma ovarii at the primary surgery in this case, the predilection of malignant struma ovarii points to the importance of care during primary surgery to avoid potential seeding at the primary procedure. Overall, the use of an interdisciplinary approach to surveillance with the collaboration of gynecology oncology, endocrinology and radiology can facilitate timely identification of recurrent disease which in turn will expedite treatment, and results in overall good prognosis for these patients.
4. Conclusion
This case demonstrates the importance of monitoring for all patients with malignant struma ovarii even in the setting of early-stage, completely resected, microscopic disease. Serial thyroglobulin levels in addition to imaging modalities, may serve as an appropriate monitoring tool even in patients with intact and functioning thyroid.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
CRediT authorship contribution statement
Morgan Brown: Writing – original draft, Resources, Conceptualization. Paulina Haight: Writing – review & editing. Matthew Ringel: Writing – review & editing, Resources. John Phay: Writing – review & editing, Resources. Ashwini Esnakula: Writing – review & editing, Visualization, Resources. Jennifer Vazzano: Visualization, Resources. Casey Cosgrove: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Addley S., Mihai R., Alazzam M., et al. Malignant struma ovarii: surgical, histopathological and survival outcomes for thyroid-type carcinoma of struma ovarii with recommendations for standardising multi-modal management. A retrospective case series sharing the experience of a single institution over 10 years. Arch. Gynecol. Obstet. 2021;303:863–870. doi: 10.1007/s00404-021-05969-0. [DOI] [PubMed] [Google Scholar]
- Ayhan S., Kilic F., Ersak B., Aytekin O., Akar S., Turkmen O., Akgul G., Toyran A., Turan T., Kimyon C.G. Malignant struma ovarii: from case to analysis. J. Obstet. Gynaecol. Res. 2021;47(9):3339–3351. doi: 10.1111/jog.14902. [DOI] [PubMed] [Google Scholar]
- Chandran C., Lekprasert P., Minimo C., Win K. Papillary thyroid carcinoma in struma ovarii: management after surgery. BMJ Case Rep. 2022;15(11):e252129. doi: 10.1136/bcr-2022-252129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne C., Nikiforov Y.E. RAS mutation-positive follicular variant of papillary thyroid carcinoma arising in a struma ovarii. Endocr. Pathol. 2010;21(2):144–147. doi: 10.1007/s12022-009-9097-8. [DOI] [PubMed] [Google Scholar]
- DeSimone C.P., Lele S.M., Modesitt S.C. Malignant struma ovarii: a case report and analysis of cases reported in the literature with focus on survival and I131 therapy. Gynecol. Oncol. 2003;89(3):543–548. doi: 10.1016/s0090-8258(03)00141-0. [DOI] [PubMed] [Google Scholar]
- Gobitti C., Sindoni A., Bampo C., Baresic T., Giorda G., Alessandrini L., Canzonieri V., Franchin G., Borsatti E. Malignant struma ovarii harboring a unique NRAS mutation: case report and review of the literature. Hormones (Athens). 2017;16(3):322–327. doi: 10.14310/horm.2002.1750. [DOI] [PubMed] [Google Scholar]
- Kraemer B., Grischke E.M., Staebler A., et al. Laparoscopic excision of malignant struma ovarii and 1 year follow-up without further treatment. Fertil. Steril. 2011;95 doi: 10.1016/j.fertnstert.2010.12.047. 2124.e9. [DOI] [PubMed] [Google Scholar]
- Makani S., Kim W., Gaba A.R. Struma Ovarii with a focus of papillary thyroid cancer: a case report and review of the literature. Gynecol. Oncol. 2004;94(3):835–839. doi: 10.1016/j.ygyno.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Marti J.L., Clark V.E., Harper H., Chhieng D.C., Sosa J.A., Roman S.A. Optimal surgical management of well-differentiated thyroid cancer arising in struma ovarii: a series of 4 patients and a review of 53 reported cases. Thyroid. 2012;22(4):400–406. doi: 10.1089/thy.2011.0162. Epub 2011 Dec 19. [DOI] [PubMed] [Google Scholar]
- Oudoux, A., Leblanc, E., Beaujot, J., et al., 2016. Treatment and follow-up of malignant struma ovarii: regarding two cases, Gynecol. Oncol. Rep.;17:569. [DOI] [PMC free article] [PubMed]
- Rose P.G., Arafah B., Abdul-Karim F.W. Malignant struma ovarii: recurrence and response to treatment monitored by thyroglobulin levels. Gynecol. Oncol. 1998;70(3):425–427. doi: 10.1006/gyno.1998.5056. [DOI] [PubMed] [Google Scholar]
- Shaco-levy R., Stewart C.J.R., Struma Ovarii F.M. International Agency for Research on Cancer; Lyon (France): 2020. In: WHO Classification of Tumours Editorial Board. Female genital tumours. [Google Scholar]
- Wolff E.F., Hughes M., Merino M.J., Reynolds J.C., Davis J.L., Cochran C.S., Celi F.S. Expression of benign and malignant thyroid tissue in ovarian teratomas and the importance of multimodal management as illustrated by a BRAF-positive follicular variant of papillary thyroid cancer. Thyroid. 2010;20(9):981–987. doi: 10.1089/thy.2009.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]