Summary
There is increasing evidence of the clinical utility of genetic and genomic testing (GT); however, factors influencing personal utility of GT, especially in diverse, multilingual populations, remain unclear. We explored these factors in a diverse cohort of parents/guardians (participants) whose children received clinical GT through the NYCKidSeq program. A total of 847 participants completed surveys at baseline, post-results disclosure, and 6 months (6m) post-results. The largest population groups were Hispanic/Latino(a) (48%), White/European American (24%), and Black/African American (16%). Personal utility was assessed using the Personal Utility (PrU) scale, adapted for pediatric populations and included on the surveys. Three PrU subscales were identified using factor analysis: practical, educational, and parental psychological utility. Overall personal utility summary score and the three subscales significantly decreased after receiving results and over time. Hispanic/Latino(a) participants identified greater overall personal utility than European American and African American participants at all time points (p < 0.001) as did participants whose children received positive/likely positive results compared with those with negative and uncertain results (post-results: p < 0.001 and p < 0.001; 6m post-results: p = 0.002 and p < 0.001, respectively). Post-results, higher subscale scores were associated with lower education levels (practical, parental psychological: p ≤ 0.02) and higher levels of trust in the healthcare system (practical, parental psychological: p ≤ 0.04). These findings help to understand the perspectives of diverse parents/guardians, which is critical to tailoring pre- and post-test counseling across a variety of populations and clinical settings.
Keywords: genomic testing, pediatric genetic testing, personal utility, parents, diversity, genetic testing, genome sequencing, targeted gene panel
This study evaluated personal utility of pediatric genomic testing among a diverse cohort, identifying three domains: practical, educational, and parental psychological. Personal utility decreased over time. Sociodemographic factors including education, health literacy, and healthcare distrust significantly influenced personal utility. Understanding these factors is crucial for tailored counseling across diverse populations.
Introduction
Genetic and genomic testing (GT) has revolutionized our understanding of the genetic basis of disease and has emerged as a valuable tool in pediatric clinical settings. Advances in sequencing technology have increased access to genetic testing within a clinically useful time frame, providing families and healthcare providers with the opportunity for a molecular diagnosis, tailored treatment, screening recommendations, and cascade testing.1 There is increasing evidence of the utility of GT in clinical care, or clinical utility, with previous studies demonstrating how GT helps provide a diagnosis, identify disease risk, and guide medical interventions.2,3 However, the value of GT beyond, or independent from, clinical utility, is not as well understood. Personal utility is a concept that takes into account the non-medical value of GT information to the patient or the patients’ family members.4 Reported elements of personal utility associated with genetic testing encompass personal knowledge, understanding of a health condition (i.e., diagnosis, prognosis, recurrence risk), altruism, and coping ability.4,5,6,7,8,9
Unlike clinical utility, there is still limited information on the factors that contribute to the personal utility of GT. The few studies that have explored patients’ and parents’ perspectives on the impact of GT, suggest that patients and parents experience utilities and disutilities across clinical, psychological, and pragmatic domains. For example, for both patients’ and parents’, utilities may include benefits of early disease detection, satisfaction of curiosity, and future planning. On the other hand, disutilities can include blame from family members for passing on a health condition and discrimination from employers, schools, or insurance.10,11,12,13 In the context of a diagnostic (positive) genetic result, personal utility has been linked to ending the diagnostic odyssey, reducing emotional and financial burdens, and yielding information relevant to making reproductive decisions and accessing appropriate services and support.4,7,8,14,15,16 For non-diagnostic (uncertain and/or negative) genetic results, personal utility can be related to ruling out certain conditions, giving parents confidence that they have exhausted diagnosis testing for their child, and allowing families to pause the diagnostic odyssey to focus on symptom management.7,17 Although there is overlap, it is important to note the differences in motivations and utilities between adult patients and parents whose children undergo GT. One qualitative study of parents whose child received genetic testing reported that many parents worried about the possibility of a lethal diagnosis, suggesting heightened anxiety around GT for parents when compared with adult patients.17 Overall, however, the extent to which previously published findings on personal utility of GT apply to a diversity of patients and families remains unclear, since the majority of studies primarily involved highly educated, non-Hispanic, White parents.7,8,17,18,19,20
Sociodemographic factors, including education level, income, primary language, and healthcare system factors related to racial bias and access to insurance, all have the potential to impact the personal utility of GT.6,21,22,23 It is therefore critical to evaluate parents’ personal utility of GT in a diverse pediatric patient population to gain a more thorough and nuanced understanding of the value parents place on GT for their child. The NYCKidSeq research program enrolled diverse and medically underserved children with suspected genetic conditions who underwent GT and evaluated the clinical and personal utility of the genetic testing.24,25 NYCKidSeq was jointly funded by the National Human Genome Research Institute and the National Institute on Minority Health and Health Disparities and was one of seven national clinical sites that were part of the Clinical Sequencing Evidence-Generating Research consortium.26 We previously reported on the diagnostic yield of GT3 as well as the impact of a digital application on parents’ understanding of their child’s GT results.27 In the present study, we describe participants’ assessment of the personal utility of the testing at baseline, post-results disclosure, and 6-months (6m) post-results disclosure, which highlights the factors that contribute to personal utility in a diverse pediatric patient population.
Materials and methods
Overview
The NYCKidSeq program comprised two research studies, the NYCKidSeq randomized control trial (NCT03738098), which evaluated the use of a digital platform designed to facilitate results disclosure, and the TeleKidSeq pilot study, which assessed the benefits screensharing during results disclosure. These studies have been described previously by Odgis et al., in 2021, and Sebastin et al., in 2023, respectively.24,25 Recruitment for the NYCKidSeq and TeleKidSeq studies occurred at two New York City health systems, Mount Sinai Medical Center and Albert Einstein/Montefiore Medical Center. This study includes children and their parents/legal guardians from both study cohorts. The institutional review boards at the Icahn School of Medicine at Mount Sinai and the Albert Einstein College of Medicine approved the studies, and all participants provided written informed consent.
Study population
Study participants are parents and legal guardians of the children (<21 years) who received GT through the NYCKidSeq program. These children had a suspected genetic etiology for their neurologic, cardiac, or immunologic condition, had not had genetic testing or had a previously uninformative genetic test, and had not undergone genetic counseling in the 3 months preceding enrollment. They underwent clinical GT, which included genome sequencing (GS) and targeted gene panels (TGPs) for a subset (n = 642). For those who did receive a TGP, one or multiple panel(s) were run based on the child’s phenotype. GT was performed by Clinical Laboratory Improvement Amendments (CLIA)-certified and New York State approved genetic testing laboratories.3 Study genetic counselors (GCs) provided parents/legal guardians (participants) with pre-test genetic counseling to discuss the risks, benefits, and limitations of GT and post-test counseling to review their child’s results and care recommendations approved by their referring provider. Participants were required to be fluent in English and/or Spanish to participate in the study. Pre- and post-test genetic counseling was conducted with the use of a Spanish interpreter per participant preference. The NYCKidSeq study developed a digital platform designed to facilitate the communication of GT results, called GUÍA (Genomic Understanding, Information, and Awareness application). This platform was designed to facilitate results disclosure of GT results in English and Spanish with the use of text and images.28 Participants enrolled in NYCKidSeq were randomized to post-test counseling with or without the use of GUÍA. Participants either received the results of the GT in person (n = 155, 16.4%) or via telehealth (n = 789, 83.6%). Participants who received the results via telehealth either received their results with (n = 192, 20.3%) or without (n = 597, 63.2%) screen sharing capabilities. The results of GT were entered into the child’s electronic medical record. Participants completed surveys before genetic testing (baseline), post-results disclosure (typically immediately after results disclosure, with a maximum of 1 month post-results disclosure), and 6m post-results disclosure. Bilingual research coordinators administered surveys in English or Spanish per participant preference, and recorded responses in REDCap (Research Electronic Data Capture), a secure, web-based software platform.29,30 Participants received an incentive (up to $80) for completion of all components of the study.
Personal utility measure
We assessed participants’ personal utility of GT across the three time points using the 19-item Personal Utility (PrU) scale9 adapted for parents/legal guardians of pediatric populations (see Table S1 for PrU questions).31 The measure evaluated expected personal utility at baseline, and then actual personal utility post-results disclosure. Participants who indicated a preference to complete the surveys in Spanish received a Spanish translation of the same PrU scale. Participants responded to each item using a seven-point scale ranging from “not useful at all” (1) to “extremely useful” (7) (see Table S2).
Analytic sample
Participants enrolled in the NYCKidSeq program were eligible for this study unless they were enrolled in the lead-in phase (n = 37), did not complete all three surveys (n = 44), completed the post-result survey >1 month from results disclosure (n = 1), or their child’s case-level clinical interpretation was amended between the two post-results disclosure surveys (n = 1). After exclusion of participants who were lost to follow-up (n = 79), the final analytic sample consisted of 944 individuals’ post-results disclosure and 847 6m post-results disclosure.
Sociodemographic and clinical variables
We collected participants’ sociodemographic data at baseline, which included self-reported race and ethnicity, education level, and health insurance type. In addition, we captured the following clinical information on enrolled children: primary phenotype (neurologic, cardiac, immunologic), sex assigned at birth, and case-level interpretation of GT. At baseline, participants completed the validated four-item Brief Health Literacy Screening Tool32 and the validated nine-item Health Care Distrust scale, comprising two subscales (competence and values).33 See Table S3 for all population characteristics that were collected.
Participants were asked to select one or more of nine population descriptors that applied to them, and these were transformed into population groups used for analysis. Due to the wording of the question, the survey instrument failed to differentiate between legal guardians and biological parents when asking for population descriptors. Therefore, data were collected for biological parents only (n = 904), legal guardians were not assigned population groups (n = 40). Participants who selected Hispanic/Latino(a) (H/L) were assigned as H/L regardless of any other population designation made and collapsed into one category (n = 435). Participants who selected more than one population descriptor, not including H/L, were collapsed and assigned as “More than one population” (n = 17). Participants who selected just one population descriptor, not including H/L, were assigned as those population groups; American Indian (n = 3), Asian (n = 55), Black or African American (AA) (n = 141), Middle Eastern or North African/Mediterranean (n = 10), and White or European American (EA) (n = 217). Participants also had the option to select a population descriptor of other (n = 9), prefer not to answer (n = 11), or unknown/none of these fully describe the child’s mother/father (n = 6).
Education level was collapsed into four categories: less than a high school degree (HS) (n = 188), HS/General Education Development (GED)/post-HS training (n = 422) college graduate (n = 190), and greater than a college degree (N = 161). Case-level clinical interpretation of genetic results was determined by GCs as positive, likely positive, uncertain, or negative following criteria described previously.3 Positive and likely positive categories were combined in the present study due to similarities in results disclosure. Mode of result delivery was collapsed into either in-person (n = 197) or telehealth, which consisted of those who were met over teleconference (n = 740) and over the phone (n = 7).
We assessed participants’ understanding of their child’s GT results using two novel measures developed for the NYCKidSeq study.27 Perceived understanding of the results was measured on a Likert scale of 1 (“very little or none of it”) to 5 (“almost all or all of it”). For our analyses, survey response 1 was eliminated due to a low number of responses (N = 11) and the survey responses of 2 (N = 13) and 3 (N = 81) were collapsed into a single category designated as level 1. Survey response 4 (N = 261) was categorized as level 2. Finally, survey response 5 (N = 578) was categorized as level 3. Objective understanding was assessed through four questions (Table S2) asked to participants and GCs following the return of results. An objective understanding summary score was calculated by totaling the agreement of participant responses with the GCs. The objective understanding summary score ranged from 0 as the lowest possible score to 4 as the highest.
Statistical analyses
A previously published factor analysis of the adult version of the PrU identified three subscales: self-knowledge (6 items), reproductive planning (2 items), and practical benefits (6 items).31 We conducted a factor analysis for the adapted pediatric PrU, removing two items (“Use for testing a future pregnancy, if appropriate” and “Inform my child’s decisions about having children”) a priori since these items were not applicable to the entire study cohort (e.g., the question about future pregnancy was not relevant to legal guardians). The remaining 15 items loaded on to 3 subscales, which we described as: “practical utility” (8 items, possible subscale score range: 8–56), “educational utility” (5 items, possible subscale score range: 5–35), and “parental psychological utility” (2 items, possible subscale score range: 2–14; Table S1) based on the utilities that comprised each category. Internal consistency was high among the analytical study sample for each subscale (“practical utility” Cronbach’s α = 0.94, “educational utility” Cronbach’s α = 0.87, and “parental psychological” Cronbach’s α = 0.97). For each time point, we calculated individual subscale scores and constructed an overall personal utility score by adding responses for all 15 items. Participants with missing responses were not included in the subscale and overall score calculations.
We employed descriptive statistics to characterize all study variables within the analytic sample. One-way analysis of variance (ANOVA) was used to evaluate the mean of PrU subscale scores and summary score separately within the three time points. Bivariate analysis was used to assess the association of sociodemographic and clinical characteristics with the PrU summary score at post-results disclosure and 6m post-results disclosure time points (see Table S4). Of these, we identified sociodemographic and clinical factors to use as covariates in the analyses, including: survey language, education level, insurance type, case-level clinical interpretation of genetic results, history of previous genetic testing, health literacy score, and healthcare distrust scores (values subscale and competence subscale). Population group was not included as a covariate in our models due to several categories having small sample sizes. Baseline surveys were completed pre-test, therefore case-level clinical interpretation was not known and not included in baseline models.
Using multivariate linear regression, we evaluated the association of the covariates with the three PrU subscale scores and the PrU summary score. Separate analyses were run for each time point: baseline, post-results disclosure, and 6m post-results disclosure. To further analyze the association between population group and personal utility, we conducted ANOVAs and post-hoc Tukey tests using the overall PrU summary score across all three time points and the three largest population groups (EA, AA, and H/L). Stata version 17 was used for all analyses (2021, StataCorp LP).
To further investigate the factors that may impact personal utility among different population groups, an additional multivariate linear regression was conducted, including stratification by the three largest population groups. Models were conducted at two time points, post-results disclosure, and 6m post-results disclosure, and included mode of result delivery (in person vs. telehealth), perceived understanding, objective understanding, and genetic counselor as additional covariates. Stata version 17 was used for all analyses (2021, StataCorp LP).
Results
Participants and children characteristics
The mean age of study participants was 40.9 years and the majority were mothers (87.2%) (Table 1). The three largest population groups were H/L (48.1%), EA (24.0%), and AA (15.6%), followed by Asian (6.1%), Middle Eastern or North African/Mediterranean (1.1%), American Indian (0.3%), more than one population (1.9%), prefer not to answer (1.2%), other (1.0%), and unknown/none of these (0.7%). Baseline surveys were conducted in Spanish for 22.4% of participants. Over half had less than a college degree (62.7%), 43.5% had a household income reported that was at or below 200% of the New York City (NYC) Federal poverty level, and 51.5% resided within a Health Resources and Services Administration (HRSA)-defined medically underserved area (https://data.hrsa.gov).34 The mean age of enrolled children was 9.6 years (range 1 month–21 years) and 62.6% were male. The majority had a primary neurological phenotype (90.6%). Of the children, 16.5% received positive/likely positive results, 57.3% received uncertain results, and 26.0% received negative results. Population characteristics of participants enrolled in the NYCKidSeq and TeleKidSeq studies are also displayed in Table 1.
Table 1.
Participant characteristics for NYCKidSeq, TeleKidSeq, and the total sample
| Participant characteristic, N (%) | NYCKidSeq (n = 551) | TeleKidSeq (n = 393) | Total sample (n = 944) |
|---|---|---|---|
| Age of parent at baseline (mean, SD, range)a | 41.1 (9.0) 20.9–81.5 |
40.5 (9.2) 21.3–71.7 |
40.89 (9.1) 20.9–81.5 |
| Relationship to child | |||
| Mother | 488 (88.6) | 335 (85.2) | 823 (87.2) |
| Father | 43 (7.8) | 38 (9.7) | 81 (8.6) |
| Legal guardian | 20 (3.6) | 20 (5.1) | 40 (4.2) |
| Recruitment site | |||
| Montefiore Medical Center | 187 (34.0) | 134 (34.1) | 321 (34.0) |
| Mount Sinai Health System | 364 (66.0) | 259 (66.0) | 623 (66.0) |
| Previous genetic testingb | 222 (40.3) | 146 (37.2) | 368 (39.0) |
| Self-reported race and ethnicityc | |||
| American Indian | 2 (0.4) | 1 (0.3) | 3 (0.3) |
| Asian | 38 (7.2) | 17 (4.6) | 55 (6.1) |
| Black or African American | 84 (15.8) | 57 (15.3) | 141 (15.6) |
| Hispanic/Latino(a) | 245 (46.1) | 190 (51.0) | 435 (48.1) |
| Middle Eastern or North African/Mediterranean | 8 (1.5) | 2 (0.5) | 10 (1.1) |
| White or European American | 129 (24.3) | 88 (23.6) | 217 (24.0) |
| More than one population | 9 (1.7) | 8 (2.1) | 17 (1.9) |
| Other | 4 (0.8) | 5 (1.3) | 9 (1.0) |
| Prefer not to answer | 6 (1.1) | 5 (1.3) | 11 (1.2) |
| Unknown/None of these fully describe my child | 6 (1.1) | 0 (0.00) | 6 (0.7) |
| Survey conducted in Spanish | 121 (22.0) | 90 (23.0) | 211 (22.4) |
| Interpreter present at post-test | 107 (19.4) | 85 (21.6) | 192 (20.3) |
| How was the survey administered?d | |||
| Phone | 128 (23.2) | 157 (40.1) | 285 (30.2) |
| In-person | 148 (26.9) | 5 (1.3) | 153 (16.2) |
| Videoconference | 275 (49.9) | 230 (58.7) | 505 (53.6) |
| Education levele | |||
| < HS | 100 (18.2) | 68 (17.4) | 168 (17.9) |
| HS/GED/post-HS training | 242 (44.1) | 180 (45.9) | 422 (44.9) |
| College graduate | 114 (20.8) | 76 (19.4) | 190 (20.2) |
| > College graduate | 93 (17.0) | 68 (17.4) | 161 (17.1) |
| Public insurance (Medicaid/Medicare) | 357 (64.8) | 249 (63.4) | 606 (64.2) |
| MUAP (residence in an HRSA defined “medically underserved area”) | 297 (53.9) | 189 (48.1) | 486 (51.5) |
| 200% below NYC federal poverty levelf | 230 (41.7) | 181 (46.1) | 411 (43.5) |
| Brief Health Literacy Score (mean, SD, range) | 16.8 (3.6) 4.0–20.0 |
17.1 (3.6) 4.0–20.0 |
17.0 (3.6) 4.0–20.0 |
| Healthcare Distrust Values Subscale Score (mean, SD, range)d | 15.6 (3.9) 5.0–25.0 |
15.8 (3.7) 5.0–25.0 |
15.7 (3.8) 5.0–25.0 |
| Healthcare Distrust Competence Subscale Score (mean, SD, range)d | 10.91 (2.9) 4.0–20.0 |
10.97 (3.0) 4.0–20.0 |
10.94 (2.9) 4.0–20.0 |
| Child characteristic, N (%) | |||
| Age of child at baseline in years (mean, SD, range) | 9.8 (5.8) 0.1–22.0 |
9.2 (5.7) 0.1–22.0 |
9.6 (5.7) 0.1–22.0 |
| Child sex assigned at birth | |||
| Female | 202 (36.7) | 151 (38.4) | 353 (37.4) |
| Male | 349 (63.3) | 242 (61.6) | 591 (62.6) |
| Primary phenotype | |||
| Cardiac | 21 (3.8) | 20 (5.1) | 41 (4.3) |
| Immunologic | 29 (5.3) | 19 (4.8) | 48 (5.1) |
| Neurologic | 501 (90.9) | 354 (90.1) | 855 (90.6) |
| Case-level clinical interpretation of genetic test result | |||
| Positive/likely positive | 91 (16.5) | 73 (18.6) | 164 (17.3) |
| Uncertain | 317 (57.5) | 165 (42.0) | 482 (51.1) |
| Negative | 143 (26.0) | 155 (39.4) | 298 (31.6) |
SD, standard deviation; HS, high school; GED, General Education Development Test; NYC, New York City; HRSA, Health Resources & Services Administration.
Due to missing data, NYCKidSeq n = 549, TeleKidSeq n = 391, total sample n = 940.
Eleven participants answered, “Don’t Know”: NYCKidSeq, n = 6, TeleKidseq, n = 5.
Race and ethnicity was not collected for legal guardians. Therefore, NYCKidSeq n = 531, TeleKidSeq n = 373, total sample n = 904.
Due to missing data, NYCKidSeq n = 551, TeleKidSeq n = 392, total sample n = 943.
Due to missing data, NYCKidSeq n = 549, TeleKidSeq n = 392, total sample n = 941.
Missing data due to incomplete information regarding household size and approximate household income.
Three domains of personal utility of pediatric genomic testing
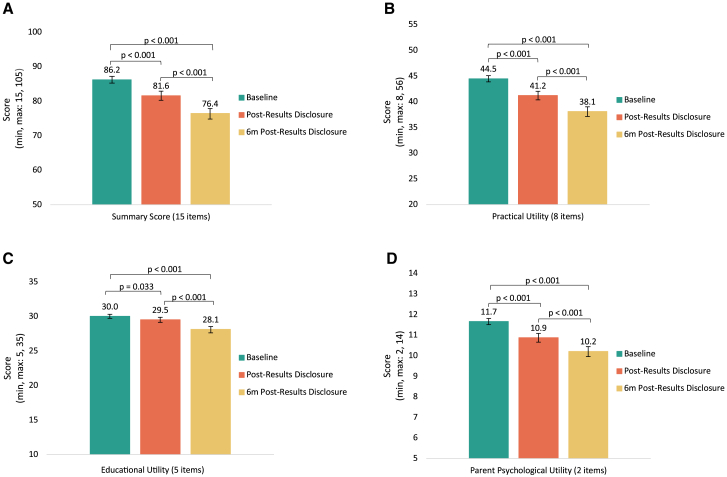
We assessed participants’ overall perceptions of the personal utility of the GT at baseline, post-results disclosure, and 6m post-results disclosure (with an average completion time of 6.4 months) using the summary score of the PrU measure. The possible range for the summary score was 15–105, with higher scores indicating greater personal utility. Overall, PrU summary scores were high across the three time points. However, PrU scores significantly decreased over time, from an average summary score of 86.2 (standard deviation [SD] = 15.1) at baseline to 81.6 (SD = 20.7) post-results disclosure to 76.4 (SD = 22.7) 6m post-results disclosure (p < 0.001; Figure 2A).
Figure 2.
Comparison of personal utility of genomic results assessed at baseline, post-results disclosure, and 6m post-results disclosure
Error bars represent 95% confidence intervals. One-way ANOVAs analyzed significant differences in PrU mean scores at baseline, post-results disclosure, and 6m post-results disclosure using (A) PrU summary scores, (B) the practical utility subscale, (C) the educational utility subscale, and (D) the parental psychological utility subscale. p values were calculated from post-hoc Tukey HSD analyses.
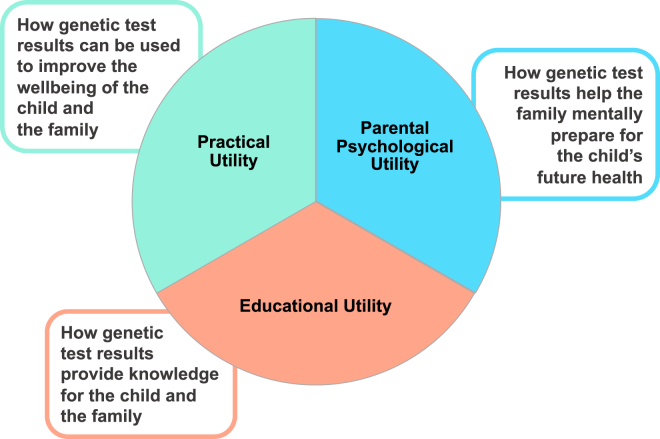
We conducted a factor analysis of the PrU measure, identifying three subscales, or domains, of personal utility, which we describe as practical utility, educational utility, and parental psychological utility (Figure 1). Practical utility encompasses how GT results can be helpful in supporting the child’s well-being and development. Educational utility captures GT’s usefulness in providing families with sought-after information and knowledge about their child’s condition. Lastly, parental psychological utility involves how the GT may help families mentally prepare for the future, mainly in relation to their child’s health. For practical utility, the mean subscale score was 44.5 (SD = 9.4; possible range 8–56) at baseline, 41.2 (SD = 13.1) at post-results disclosure, and 38.1 (SD = 14.0) at 6m post-results disclosure. The educational utility mean subscale score was 30.0 (SD = 4.6; possible range 5–35) at baseline, 29.5 (SD = 5.7) at post-results disclosure, and 28.1 (SD = 6.8) at 6m post-results disclosure. The mean parental psychological utility subscale score was 11.7 (SD = 2.3; possible subscale range 2–14) at baseline, 10.9 (SD = 3.3) at post-results disclosure, and 10.2 (SD = 3.6) at 6m post-results disclosure. We also evaluated the impact of time on the individual subscales and observed that, like the summary score, the average subscale scores significantly decreased over time (practical utility p ≤ 0.001, educational utility p ≤ 0.001, parental psychological utility p ≤ 0.001; Figures 2B–2D).
Figure 1.
Domains of personal utility of genetic test results
The results of the factor analysis of the Personal Utility (PrU) scale adapted for parents/legal guardians of pediatric populations identified three subscales: practical utility, educational utility, and parental psychological utility.
Socioeconomic and clinical factors associated with personal utility of pediatric genomic testing
To explore how socioeconomic and clinical factors impact participants’ views on the personal utility of GT, we performed multivariate linear regression models across the three study time points. This analysis included the PrU summary score as well as the three PrU subscales, evaluating six participant characteristics: survey language, education level, insurance type, health literacy, healthcare distrust (values and competence), case-level clinical interpretation of GT results, and previous genetic testing. We also conducted a comparison of PrU summary scores among the three largest AA, H/L, and EA population groups in our study. We excluded other populations from this analysis due to their limited sample size.
Personal utility of pediatric genomic testing: Summary score
We observed that the PrU summary score was associated with participants’ education level, health literacy score, healthcare distrust, and the case-level interpretation of the GT results (Table 2). At baseline, participants with HS/GED/post-HS training expected significantly greater personal utility than those with less than an HS education (b = 3.57, p = 0.016); however, there were no differences between those with less than HS education and those with a college degree or greater. Once participants received their results, the association between education level and personal utility abated. At post-results disclosure, higher health literacy levels were associated with lower personal utility summary scores (b = −0.47, p = 0.024). We also observed that higher levels of distrust in the competence of the healthcare system were associated with lower personal utility at baseline and post-results disclosure (b = −0.67, p = 0.003 and b = −0.65, p = 0.030, respectively), with similarly trending results at 6m post-results disclosure (b = −0.68, p = 0.052). Furthermore, the classification of the clinical results significantly impacted participants’ views on the personal utility of the GT. Those receiving a positive/likely positive result had higher PrU summary scores post-results disclosure compared with those receiving a negative result (b = 13.36, p < 0.001) and to those receiving an uncertain result (b = 14.04, p < 0.001). This trend persisted 6m post-results disclosure (b = 7.11, p = 0.002 and b = 10.56, p < 0.001, respectively). Conversely, at 6m post-results disclosure, those with uncertain results had lower personal utility summary scores when compared with those receiving a negative result (b = −3.85, p = 0.025). Insurance type, previous genetic testing, and the healthcare distrust values subscale were not associated with the PrU summary score.
Table 2.
Multivariate linear regression of the personal utility summary score assessed at baseline, post-results disclosure, and 6m post-results disclosure
| Baseline (n = 900) |
Post-results disclosure (n = 923) |
6m Post-results disclosure (n = 828) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| Socioeconomic factors | ||||||
| Education level | ||||||
| HS/GED/post-HS training vs. < HS | 3.57 (0.67, 6.47) | 0.016 | 2.72 (−1.05, 6.50) | 0.157 | 3.17 (−1.20, 7.53) | 0.155 |
| College graduate vs. < HS | 0.35 (−3.15, 3.86) | 0.843 | −3.83 (−8.41, 0.75) | 0.101 | −0.22 (−5.56, 5.12) | 0.936 |
| > College+ vs. < HS | −1.67 (−5.48, 2.15) | 0.392 | −4.71 (−9.70, 0.29) | 0.065 | −4.19 (−10.02, 1.64) | 0.159 |
| Survey language | ||||||
| Spanish vs. English | 0.51 (−2.30, 3.32) | 0.722 | 1.28 (−2.32, 4.88) | 0.486 | 2.65 (−1.48, 6.79) | 0.208 |
| Insurance type | ||||||
| Private vs. public | −1.85 (−4.17, 0.48) | 0.119 | −2.41 (−5.45, 0.64) | 0.121 | −2.51 (−6.08, 1.06) | 0.168 |
| Healthcare distrust | ||||||
| Values Subscale | 0.09 (−0.26, 0.43) | 0.631 | 0.21 (−0.25, 0.67) | 0.370 | −0.17 (−0.70, 0.36) | 0.529 |
| Competence Subscale | −0.67 (−1.12, −0.23) | 0.003 | −0.65 (−1.24, −0.06) | 0.030 | −0.68 (−1.37, 0.01) | 0.052 |
| Health Literacy Score | −0.17 (−0.48, 0.14) | 0.288 | −0.47 (−0.87, −0.06) | 0.024 | −0.22 (−0.70, 0.25) | 0.354 |
| Clinical factors | ||||||
| Case-level clinical interpretation | ||||||
| Positive/likely positive vs. negative | – | 13.36 (9.55, 17.18) | <0.001 | 7.11 (2.69, 11.52) | 0.002 | |
| Uncertain vs. negative | – | −0.68 (−3.54, 2.18) | 0.642 | −3.85 (−7.22, −0.49) | 0.025 | |
| Positive/likely positive vs. uncertaina | – | 14.04 (10.49, 17.58) | <0.001 | 10.56 (6.86, 15.06) | <0.001 | |
| Previous genetic testing | ||||||
| Yes vs. No | −1.46 (−3.46, 0.55) | 0.154 | −1.83 (−4.46, 0.80) | 0.173 | −2.70 (−5.78, 0.37) | 0.085 |
HS, high school; GED, General Education Development Test.
Bold enteries signify significant results.
Post-hoc analyses were conducted using uncertain result as the reference variable for case-level clinical interpretation.
To gain a deeper understanding of how different populations perceive the personal utility of GT, we compared PrU summary scores between populations and identified significant differences between the groups using ANOVA and post-hoc Tukey tests (baseline: p < 0.001; post-results disclosure: p < 0.001, 6m post-results disclosure: p < 0.001; Figure S2). Overall, H/L participants identified greater personal utility of the GT than EA and AA participants, a consistent finding across the three study time points (Figure S2). The PrU summary scores were not significantly different between AA and EA groups at either post-results disclosure time points.
To further explore what factors affecting personal utility might be driving differences among the three largest population groups in our study, we conducted a multivariate linear regression. We stratified by population groups and included a number of covariates (Tables S5 and S6). At post-results disclosure, perceived understanding, objective understanding, insurance type, healthcare distrust, and case-level clinical interpretation were all found to have associations with overall personal utility at the population level. In the full sample, higher perceived understanding was associated with greater personal utility summary scores (level 2 vs. level 1: b = 6.8, p = 0.004; level 3 vs. level 1: b = 10.2, p < 0.001), which persisted across the three population groups. Conversely, higher objective understanding was associated with lower personal utility summary scores (b = −2.3, p < 0.001). This association was also found for AA (b = −5.0, p = 0.008) and H/L (b = −1.7, p = 0.038) participants, but not for EA participants. Participants who received positive/likely positive results reported higher personal utility than those who received negative results across the three population groups (EA: b = 15.09, p < 0.001; AA: b = 20.479, p = 0.001; H/L: b = 13.95, p < 0.001). Only EA participants demonstrated a negative association between personal utility and distrust in the competence of the healthcare system (b = −1.53, p = 0.014). H/L parents with private insurance reported lower personal utility than those with public (b = −4.95, p = 0.045), as did those with previous genetic testing (b = −4.26, p = 0.026). There was no association with personal utility and mode of results disclosure in the full sample at either time point. In addition, within the population groups, there was no association between personal utility and mode of results disclosure, education level, survey language, or health literacy score. At the 6m post-results disclosure time point, although some significant associations seen post-results disclosure persisted, most of the associations were no longer observed.
Personal utility of pediatric genomic testing: Practical utility subscale
Several socioeconomic and clinical factors were associated with the practical utility subscale, including participants’ education level, healthcare distrust, insurance type, health literacy level, history of previous genetic testing, and case-level interpretation of the results (Table 3). We found that participants’ level of education impacted their views on the practical utility of the testing, which varied depending on the study time point. Those with HS/GED/post-HS training reported higher expected practical utility at baseline compared with those with less than HS education (b = 1.83, p = 0.042), but this difference did not persist after results were disclosed. We also observed that those with higher education levels, including college graduates and those with higher than a college degree, had significantly lower practical utility scores post results disclosure compared with those with less than HS education (b = −3.24, p = 0.027 and b = −4.09, p < 0.001, respectively). At the 6m post-results time point, those with an education level higher than a college degree continued to report significantly lower practical utility scores (b = −3.62, p = 0.046). Across the three study time points, higher levels of distrust in the competence of the healthcare system were negatively associated with practical utility (baseline: b = −0.49, p = 0.001; post results disclosure: b = −0.46, p = 0.014; 6m post-results disclosure b = 0.43, p = 0.044). In addition, those with private insurance had lower practical utility scores at baseline (b = −1.47, p = 0.042) and 6m post-results disclosure (b = −2.30, p = 0.039) compared with those with public insurance. Lastly, health literacy levels were negatively associated with practical utility at the post-results disclosure time point (b = −0.28, p = 0.028). In the context of the clinical factors, prior genetic testing was associated with lower practical utility scores at both post-results disclosure (b = −1.69, p = 0.044) and 6m post-results disclosure (b = −2.11, p = 0.027) time points. In addition, participants whose child received a positive/likely positive test result identified significantly higher practical utility of the testing compared with a negative result and with an uncertain result at post-results disclosure (b = 8.33, p < 0.001 and b = 8.67, p < 0.001, respectively) and 6m post-results disclosure (b = 5.27, p < 0.001 and b = 7.62, p < 0.001, respectively). Uncertain results were associated with lower practical utility scores compared with negative results only at 6m post-results disclosure (b = −2.35, p = 0.025). Survey language and the healthcare distrust values subscale were not associated with practical utility.
Table 3.
Multivariate linear regression of the practical utility subscale assessed at baseline, post-results disclosure, and 6m post-results disclosure
| Baseline (n = 913) |
Post-results disclosure (n = 927) |
6m Post-results disclosure (n = 831) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| Socioeconomic factors | ||||||
| Education level | ||||||
| HS/GED/post-HS training vs. <HS | 1.83 (0.06, 3.61) | 0.042 | 1.29 (−1.08, 3.65) | 0.286 | 0.95 (−1.71, 3.61) | 0.485 |
| College graduate vs. <HS | −0.42 (−2.56, 1.72) | 0.702 | −3.24 (−6.10, −0.38) | 0.027 | −1.20 (−4.46, 2.05) | 0.469 |
| College+ vs. <HS | −1.73 (−4.07, 0.60) | 0.145 | −4.09 (−7.22, −0.97) | <0.001 | −3.62 (−7.17, −0.06) | 0.046 |
| Survey language | ||||||
| Spanish vs. English | −0.03 (−1.74, 1.67) | 0.971 | 0.78 (−1.47, 3.03) | 0.497 | 0.95 (−1.57, 3.47) | 0.458 |
| Insurance type | ||||||
| Private vs. Public | −1.47 (−2.88, −0.05) | 0.042 | −1.66 (−3.56, 0.24) | 0.086 | −2.30 (−4.48, −0.12) | 0.039 |
| Healthcare distrust | ||||||
| Values subscale | 0.07 (−0.15, 0.28) | 0.538 | 0.13 (−0.16, 0.41) | 0.382 | −0.14 (−0.46, 0.18) | 0.397 |
| Competence subscale | −0.49 (−0.76, −0.21) | 0.001 | −0.46 (−0.83, −0.10) | 0.014 | −0.43 (−0.84, −0.01) | 0.044 |
| Health Literacy Score | −0.14 (−0.33, 0.05) | 0.161 | −0.28 (−0.54, −0.03) | 0.028 | −0.20 (−0.49, 0.09) | 0.176 |
| Clinical factors | ||||||
| Case-level clinical interpretation | ||||||
| Positive/likely positive vs. negative | – | 8.33 (5.95, 10.71) | <0.001 | 5.27 (2.57, 7.96) | <0.001 | |
| Uncertain vs. negative | – | −0.34 (−2.13, 1.45) | 0.708 | −2.35 (−4.40, −0.30) | 0.025 | |
| Positive/likely positive vs. uncertaina | – | 8.67 (6.95, 10.89) | <0.001 | 7.62 (5.12, 10.12) | <0.001 | |
| Previous genetic testing | ||||||
| Yes vs. no | −1.21 (−2.44, 0.01) | 0.052 | −1.69 (−3.34, −0.04) | 0.044 | −2.11 (−3.98, −0.24) | 0.027 |
HS, high school; GED, General Education Development Test.
Bold enteries signify significant results.
Post-hoc analyses were conducted using uncertain result as the reference variable for case-level clinical interpretation.
Personal utility of pediatric genomic testing: Educational utility subscale
Educational utility subscale scores were associated with participants’ education level, healthcare distrust, and the case-level interpretation of the results (Table 4). Across the three study time points, having an HS/GED/post-HS training was associated with higher educational utility scores compared with those with less than HS education (baseline: b = 1.08, p = 0.018; post-results disclosure: b = 1.18, p = 0.032; 6m post-results disclosure: b = 1.60, p = 0.020). A higher level of distrust in the competence of the healthcare system was negatively associated with educational utility at baseline (b = −0.15, p = 0.029). Positive/likely positive results were more likely to have higher educational utility scores at post-result disclosure compared with negative and uncertain results (b = 3.02, p < 0.001 and b = 3.61, p < 0.001, respectively). Uncertain results were more likely to have lower educational utility scores than negative results at 6m post-results disclosure (b = −1.19, p = 0.024). Survey language, insurance type, health literacy score, healthcare distrust values subscale, and previous genetic testing were not associated with educational utility.
Table 4.
Multivariate linear regression of the educational utility subscale assessed at baseline, post-results disclosure, and 6m-post results disclosure
| Baseline (n = 908) |
Post-results disclosure (n = 924) |
6m Post-results disclosure (n = 828) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| Socioeconomic factors | ||||||
| Education level | ||||||
| HS/GED/post-HS training vs. <HS | 1.08 (0.18, 1.97) | 0.018 | 1.18 (0.10, 2.27) | 0.032 | 1.60 (0.25, 2.94) | 0.020 |
| College graduate vs. <HS | 0.43 (−0.64, 1.51) | 0.430 | 0.23 (−1.08, 1.54) | 0.726 | 0.85 (−0.80, 2.49) | 0.311 |
| College+ vs. <HS | 0.18 (−1.00, 1.35) | 0.769 | 0.39 (−1.05, 1.82) | 0.598 | 0.16 (−1.64, 1.96) | 0.862 |
| Survey language | ||||||
| Spanish vs. English | 0.19 (−0.68, 1.06) | 0.663 | 0.25 (−0.78, 1.28) | 0.631 | 1.17 (−0.10, 2.45) | 0.071 |
| Insurance type | ||||||
| Private vs. public | −0.28 (−0.99, 0.44) | 0.447 | −0.49 (−1.36, 0.38) | 0.267 | 0.22 (−0.88, 1.32) | 0.695 |
| Healthcare distrust | ||||||
| Values subscale | 0.04 (−0.07, 0.14) | 0.508 | 0.05 (−0.08, 0.19) | 0.418 | −0.01 (−0.17, 0.16) | 0.965 |
| Competence subscale | −0.15 (−0.29, −0.02) | 0.029 | −0.09 (−0.26, 0.08) | 0.303 | −0.14 (−0.35, 0.08) | 0.206 |
| Health Literacy Score | −0.01 (−0.11, 0.08) | 0.779 | −0.11 (−0.22, 0.01) | 0.070 | 0.03 (−0.12, 0.17) | 0.722 |
| Clinical factors | ||||||
| Case-level clinical interpretation | ||||||
| Positive/likely positive vs. negative | – | 3.02 (1.93, 4.11) | <0.001 | 0.62 (−0.74, 1.98) | 0.372 | |
| Uncertain vs. negative | – | −0.59 (−1.41, 0.23) | 0.159 | −1.19 (−2.23, −0.16) | 0.024 | |
| Positive/likely positive vs. uncertaina | – | 3.61 (2.59, 4.62) | <0.001 | 1.81 (0.55, 3.07) | 0.005 | |
| Previous genetic testing | ||||||
| Yes vs. no | −0.01 (−0.62, 0.61) | 0.982 | 0.12 (−0.63, 0.87) | 0.757 | −0.30 (−1.24, 0.65) | 0.539 |
HS, high school; GED, General Education Development Test.
Bold enteries signify significant results.
Post-hoc analyses were conducted using uncertain result as the reference variable for case-level clinical interpretation.
Personal utility of pediatric genomic testing: Parental psychological utility subscale
A number of socioeconomic and clinical factors were associated with parental psychological utility scores, including education level, health literacy score, healthcare distrust, survey language, and case-level interpretation of the results (Table 5). At baseline, those with HS/GED/post-HS training had higher expected parental psychological utility scores than those with less than HS education (b = 0.56, p = 0.014). However, this trend did not persist post-results. In fact, after results disclosure, college graduates and those with more than a college education were more likely to report lower parental psychological utility of the GT (b = −0.74, p = 0.048 and b = −0.93, p = 0.024, respectively). In addition, we observed that those with higher health literacy levels and greater distrust in the competence of the healthcare system had lower parental psychological utility scores post-results disclosure (b = −0.06, p = 0.033 and b = −0.10, p = 0.037, respectively). Parental psychological utility is the only subscale impacted by the language the survey was completed in (English or Spanish). We found that, after results disclosure, those who completed the survey in Spanish had higher parental psychological utility compared with those completing the English version of the survey (b = 2.00, p < 0.001). As with the summary score and other subscales, positive/likely positive results were positively associated with parental psychological utility when compared with negative and uncertain results, but only at the 6m post-results disclosure time point (b = 1.17, p = 0.001 and b = 1.53, p < 0.001, respectively). At post-results disclosure, there was no association seen for uncertain results when compared with negative results however, positive/likely positive results had higher parental psychological utility than uncertain results (b = 1.84, p < 0.001). We did not find an association between parental psychological utility and insurance type, healthcare distrust values subscale, and previous genetic testing.
Table 5.
Multivariate linear regression of the parental psychological subscale assessed at baseline, post-results disclosure, and 6m post-results disclosure
| Baseline (n = 917) |
Post-results disclosure (n = 926) |
6m Post-results disclosure (n = 831) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| Socioeconomic factors | ||||||
| Education level | ||||||
| HS/GED/post-HS training vs. <HS | 0.56 (0.11, 1.00) | 0.014 | 0.29 (−0.32, 0.89) | 0.352 | 0.64 (−0.06, 1.34) | 0.074 |
| College graduate vs. <HS | 0.19 (−0.35, 0.72) | 0.497 | −0.74 (−1.48, −0.01) | 0.048 | 0.13 (−0.72, 0.99) | 0.763 |
| College+ vs. <HS | −0.33 (−0.92, 0.25) | 0.261 | −0.93 (−1.73, −0.12) | 0.024 | −0.61 (−1.54, 0.32) | 0.199 |
| Survey language | ||||||
| Spanish vs. English | 0.08 (−0.35, 0.50) | 0.725 | 0.36 (−0.22, 0.93) | 0.225 | 0.52 (−0.14, 1.18) | 0.125 |
| Insurance type | ||||||
| Private vs. Public | −0.04 (−0.40, 0.31) | 0.808 | −0.34 (−0.82, 0.15) | 0.175 | −0.41 (−0.98, 0.16) | 0.161 |
| Health Literacy Score | −0.03 (−0.08, 0.02) | 0.177 | −0.07 (−0.14, −0.01) | 0.033 | −0.06 (−0.13, 0.02) | 0.139 |
| Healthcare distrust | ||||||
| Values subscale | 0.01 (−0.05, 0.06) | 0.898 | 0.03 (−0.04, 0.11) | 0.401 | −0.08 (−0.12, 0.05) | 0.397 |
| Competence subscale | −0.06 (−0.13, 0.01) | 0.029 | −0.10 (−0.19, −0.01) | 0.037 | −0.08 (−0.19, 0.03) | 0.139 |
| Clinical factors | ||||||
| Case-level clinical interpretation | ||||||
| Positive/likely positive vs. negative | – | 2.00 (1.39, 2.61) | <0.001 | 1.17 (0.47, 1.88) | 0.001 | |
| Uncertain vs. negative | – | −0.16 (−0.30, 0.62) | 0.491 | −0.36 (−0.89, 0.18) | 0.195 | |
| Positive/likely positive vs. uncertaina | – | 1.84 (1.27, 2.41) | <0.001 | 1.52 (0.87, 2.19) | <0.001 | |
| Previous genetic testing | ||||||
| Yes vs. no | −0.07 (−0.37, 0.24) | 0.675 | −0.28 (−0.70, 0.14) | 0.191 | −0.34 (−0.83, 0.15) | 0.172 |
HS, high school; GED, General Education Development Test.
Bold enteries signify significant results.
Post-hoc analyses were conducted using uncertain result as the reference variable for case-level clinical interpretation.
Discussion
In this study, we explored perceptions of the personal utility of GT in a diverse cohort of participants whose children underwent GT through the NYCKidSeq program. Overall, parents/guardians identified high levels of personal utility for genetic testing. Parents’/guardians’ views on personal utility were highest prior to undergoing testing and declined over time after learning the results, suggesting that the actual utility of the genetic test results did not align with their initial expectations. Notably higher levels of personal utility were reported by those whose children received positive or likely positive results compared with negative and uncertain results, which is unsurprising given the potential clinical actionability and emotional relief of diagnostic results. In contrast to previous research, which has primarily comprised qualitative studies with small sample sizes and/or was typically conducted with highly educated, non-Hispanic White women, we examined personal utility in a socioeconomically, racially, ethnically, and linguistically diverse cohort.7,8,17,18,19,20,35 There were significant differences observed between H/L participants, who reported the highest levels of overall personal utility, compared with EA and AA participants. In addition, sociodemographic factors, including education, health literacy, and healthcare distrust, emerged as having a significant impact on personal utility. Understanding the differences in how population groups may perceive the utility of GT and the socioeconomic factors driving these differences provides insights to inform provider communication and counseling strategies.
Identifying the various facets of personal utility (educational, psychological, and practical) allows for a deeper understanding of the motivations and values participants place on GT for their child. The high educational utility scores demonstrate that participants believe that a benefit of GT is its ability to provide information that may otherwise be unavailable. This finding is consistent with previous literature describing similar utilities, including providing families with sought-after information and satisfying their curiosity.5,11 Yet, educational utility identified through this work extends past the patient to include utility for other family members and the medical community as a whole. Likewise, identification of parent psychological utility allows us to expand on the previously defined “affective” domain of personal utility, which includes utilities of enhancing coping and mental preparation,5 to include enabling families to mentally prepare for their child’s future health. Furthermore, participants found value in the practical utility of GT, which is centered on how GT can be used to improve the well-being of the child as well as the family. Although similar domains have been described previously as “pragmatic” or “behavioral” utilities, these primarily include actionable measures, such as informing long-term care, discussion with family about health conditions, and reproductive considerations.5,11,36 The practical utility we describe here also encompasses informing future plans for school or the child’s career and improving communication with family members. We also found that parents’ perceptions on the practical utility of GT may involve fewer tangible concepts, such as feeling more in control of the child’s life and health. As such, when contemplating GT, parents may not exclusively consider the impact of actionable recommendations on the child’s physical health but may also place importance on its emotional impact. Gaining these insights into the various aspects of personal utility helps to appreciate how diverse families make decisions to undergo GT and how they adapt to their child’s results. This deeper understanding is essential for healthcare professionals discussing genetic testing in pediatric settings.
We found that personal utility significantly declined from pre-test to post-test, and this decline continued even 6 months after results disclosure. This finding highlights the discrepancy between the anticipated and actualized personal utility of GT. It is consistent with existing qualitative research demonstrating parents’ unmet expectations of GT, where parents often anticipated that the GT would conclusively diagnose their child’s condition.13,18,19,37 Our study not only validates these findings in a diverse population, but underscores the importance of utilizing pre-test counseling strategies, as discussed in the previous literature, that foster realistic expectations. Moreover, our study enhances our appreciation of the necessity to address parental expectations during post-test counseling to effectively manage expectations throughout and beyond the GT process. In addition, our research builds on previous studies13,38,39 by delineating personal utility into practical, educational, and parental psychological domains. While there is often an emphasis on managing expectations relating to clinical utility, our findings offer insights for providers to tailor their pre- and post-test counseling to address these three domains of personal utility.
A noteworthy finding from this study is the interplay between trust, or distrust, in the competence of the healthcare system and perceptions of the personal utility of genetic testing. Low levels of trust were associated with low levels of personal utility prior to testing and after receiving results. This finding remained consistent for the overall summary score and for the parent psychological and practical utility domains. Trust has been suggested as an important factor related to attitudes toward receiving genetic and genomic results, specifically patients’ trust in the ability of healthcare professionals to interpret and translate GT results into meaningful medical care.20,40 Notably, it was found that participants with less than a high school education reported higher levels of trust in the healthcare system and reported higher practical and parental psychological utility compared with those who were college educated. Perhaps participants with higher education levels perceived less utility in GT due to lower levels of trust and skepticism of the ability of the healthcare system to provide constructive and effective guidance based on the results. As this skepticism may not apply to the accuracy of the genetic test result itself, but rather its successful applicability,20 this may explain why there was no association with educational utility and education level nor healthcare distrust. Studies have reported conflicting results on the relationship between educational attainment and healthcare distrust.41,42,43 Further research is needed to disentangle the potential contributors to personal utility of different levels of education and its relationship to distrust of the healthcare system.
When considering how diverse populations experience personal utility of pediatric GT, it is notable that H/L parents had higher personal utility scores than EA and AA parents. Two studies exploring attitudes of women toward prenatal genetic screening revealed that Spanish-speaking H/L women tended to express more trust in the advice given by their healthcare provider, were less inclined to question recommendations, and were more likely to favor a provider-driven approach to decision-making compared with non-H/L women.44,45 Therefore, to further explore factors that might be driving differences in personal utility across the three population groups, we performed a post-hoc stratified analysis that included additional sociodemographic factors, including parents’ perceived and objective understanding of GT results, trust in the healthcare system, and mode of results. Although no differences in trust or mode of delivery were observed, interestingly, this analysis demonstrated across populations that higher perceived understanding was associated with greater personal utility, and higher levels of objective understanding were associated with lower personal utility scores. This suggests that perceptions of the understanding of GT results are a greater indicator of personal utility than actual understanding, suggesting that how individuals feel about their comprehension of the results may be more important to their sense of utility. However, no consistent pattern explained the initial observation of higher personal utility in H/L parents, indicating that personal utility from GT may be not solely dependent on the clinical result, but also shaped by perceptions of understanding in combination with other psychological, social, and systemic factors that vary among different populations which may not have been measured in this study.
Limitations of the study
There are limitations to this study that should be considered. The method in which participants received GT results depended on whether they were enrolled in the NYCKidSeq or the TeleKidSeq studies, which differed by use of telemedicine and/or screen sharing capabilities and by the use GUÍA, a digital platform designed to facilitate the communication of GT results. Although post-hoc analyses did not find a significant difference between personal utility levels in individuals who received results in-person vs. via telehealth, it is possible that these factors impacted personal utility. The PrU measure was adapted for participants of pediatric populations and has not been validated in this study population. Generalizability of the study findings may be limited as we recruited from one urban area, participants were predominantly mothers (potentially overshadowing the perspectives of fathers or legal guardians), and the majority of participants’ children who underwent GT had a neurological phenotype.
Conclusions
Understanding the role of patient-centric factors, including personal utility, is critical for implementing genetic testing in an evidence-based manner. This study, involving a diverse cohort of parents of children undergoing genetic testing, personal utility encompassed three key domains: practical, educational, and psychological utility. A range of factors influenced participant’s views on personal utility across these domains. Most notable were time and the type of genetic result, as well as participants' education level, health literacy, and trust in the healthcare system. Additional research involving diverse patient populations in other clinical contexts may further elucidate factors impacting perceptions of the utility of GT to further inform pre- and post-test counseling for GT across a variety of populations and clinical settings. As the diversity of patients receiving GT expands, it is critical to understand the perspectives of those underrepresented populations who have been historically excluded from genomics research, and who may experience the utility of GT differently.
Data and code availability
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Acknowledgments
Research reported in this article was supported by the National Human Genome Research Institute (NHGRI) and the National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health under award no. U01HG009610. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank all of the children, parents, and families who participated in this study. The authors also thank the New York Genome Center, Rady Children’s Institute for Genomic Medicine, and the Sema4 laboratory; the members of the Mount Sinai Hospital Genomics Stakeholder Board; and referring physicians in the Mount Sinai and Montefiore Health Systems.
This work was supported in part through the computational and data resources and staff expertise provided by Scientific Computing and Data at the Icahn School of Medicine at Mount Sinai and supported by the Clinical and Translational Science Awards (CTSA) grant UL1TR004419 from the National Center for Advancing Translational Sciences.
Author contributions
L.J.B., B.D.G., C.R.H., G.A.D., J.M.G., M.P.W., N.S.A.-H., R.E.Z., and E.E.K. contributed to the conception and design of the study. P.N.M., S.A.S., J.A.O., K.B., K.M.G., M.S., M.A.R., M.D.B., and J.E.R. contributed to the acquisition of data. L.S. and B.J.I. performed the analysis. P.N.M., S.A.S., L.S., B.J.I., M.A.R., N.R.K., E.E.K., and C.R.H. contributed to the interpretation of data. P.N.M., S.A.S., L.S., B.J.I., K.B., J.A.O., E.E.K., and C.R.H. drafted the manuscript. All authors read and approved the final manuscript.
Declaration of interests
N.S.A.-H. is currently employed by 23andMe, was previously employed by Regeneron Pharmaceuticals, received personal fees from Genentech, Allelica, and 23andMe, received research funding from Akcea, and serves as a scientific advisory board member for Allelica. E.E.K. received personal fees from Illumina, 23andMe, Allelica, and Regeneron Pharmaceuticals, received research funding from Allelica, and serves as a scientific advisory board member for Encompass, Bio, Overtone, and Galateo Bio.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xhgg.2024.100321.
Contributor Information
Eimear E. Kenny, Email: eimear.kenny@mssm.edu.
Carol R. Horowitz, Email: carol.horowitz@mountsinai.org.
Web resources
Find shortage areas by address: https://data.hrsa.gov/tools/shortage-area/by-address.
Supplemental information
References
- 1.Horton R.H., Lucassen A.M. Recent developments in genetic/genomic medicine. Clin. Sci. 2019;133:697–708. doi: 10.1042/CS20180436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark M.M., Stark Z., Farnaes L., Tan T.Y., White S.M., Dimmock D., Kingsmore S.F. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom. Med. 2018;3:16. doi: 10.1038/s41525-018-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abul-Husn N.S., Marathe P.N., Kelly N.R., Bonini K.E., Sebastin M., Odgis J.A., Abhyankar A., Brown K., Di Biase M., Gallagher K.M., et al. Molecular diagnostic yield of genome sequencing versus targeted gene panel testing in racially and ethnically diverse pediatric patients. medRxiv. 2023 doi: 10.1101/2023.03.18.23286992. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayeems R.Z., Luca S., Assamad D., Bhatt A., Ungar W.J. Utility of Genetic Testing from the Perspective of Parents/Caregivers: A Scoping Review. Children. 2021;8 doi: 10.3390/children8040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler J.N., Turbitt E., Biesecker B.B. Personal utility in genomic testing: a systematic literature review. Eur. J. Hum. Genet. 2017;25:662–668. doi: 10.1038/ejhg.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halley M.C., Young J.L., Fernandez L., Kohler J.N., Undiagnosed Diseases Network. Bernstein J.A., Wheeler M.T., Tabor H.K. Perceived utility and disutility of genomic sequencing for pediatric patients: Perspectives from parents with diverse sociodemographic characteristics. Am. J. Med. Genet. 2022;188:1088–1101. doi: 10.1002/ajmg.a.62619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollison L., O’Daniel J.M., Henderson G.E., Berg J.S., Skinner D. Parents’ perceptions of personal utility of exome sequencing results. Genet. Med. 2020;22:752–757. doi: 10.1038/s41436-019-0730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tutty E., Amor D.J., Jarmolowicz A., Paton K., Downie L. Personal utility of genomic sequencing for infants with congenital deafness. Am. J. Med. Genet. 2021;185:3634–3643. doi: 10.1002/ajmg.a.62411. [DOI] [PubMed] [Google Scholar]
- 9.Kohler J.N., Turbitt E., Lewis K.L., Wilfond B.S., Jamal L., Peay H.L., Biesecker L.G., Biesecker B.B. Defining personal utility in genomics: A Delphi study. Clin. Genet. 2017;92:290–297. doi: 10.1111/cge.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith H.S., Morain S.R., Robinson J.O., Canfield I., Malek J., Rubanovich C.K., Bloss C.S., Ackerman S.L., Biesecker B., Brothers K.B., et al. Perceived Utility of Genomic Sequencing: Qualitative Analysis and Synthesis of a Conceptual Model to Inform Patient-Centered Instrument Development. Patient. 2022;15:317–328. doi: 10.1007/s40271-021-00558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malek J., Slashinski M.J., Robinson J.O., Gutierrez A.M., Parsons D.W., Plon S.E., McCullough L.B., McGuire A.L. Parental Perspectives on Whole Exome Sequencing in Pediatric Cancer: A Typology of Perceived Utility. JCO Precis. Oncol. 2017;1:1–10. doi: 10.1200/PO.17.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wynn J., Ottman R., Duong J., Wilson A.L., Ahimaz P., Martinez J., Rabin R., Rosen E., Webster R., Au C., et al. Diagnostic exome sequencing in children: A survey of parental understanding, experience and psychological impact. Clin. Genet. 2018;93:1039–1048. doi: 10.1111/cge.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donohue K.E., Dolan S.M., Watnick D., Gallagher K.M., Odgis J.A., Suckiel S.A., Teitelman N., Gelb B.D., Kenny E.E., Wasserstein M.P., et al. Hope versus reality: Parent expectations of genomic testing. Patient Educ. Couns. 2021;104:2073–2079. doi: 10.1016/j.pec.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer S.L., Hartley T., Dyment D.A., Beaulieu C.L., Schwartzentruber J., Smith A., Bedford H.M., Bernard G., Bernier F.P., Brais B., et al. Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin. Genet. 2016;89:275–284. doi: 10.1111/cge.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Normand E.A., Alaimo J.T., Van den Veyver I.B. Exome and genome sequencing in reproductive medicine. Fertil. Steril. 2018;109:213–220. doi: 10.1016/j.fertnstert.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Sapp J.C., Dong D., Stark C., Ivey L.E., Hooker G., Biesecker L.G., Biesecker B.B. Parental attitudes, values, and beliefs toward the return of results from exome sequencing in children. Clin. Genet. 2014;85:120–126. doi: 10.1111/cge.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosell A.M., Pena L.D.M., Schoch K., Spillmann R., Sullivan J., Hooper S.R., Jiang Y.-H., Mathey-Andrews N., Goldstein D.B., Shashi V. Not the End of the Odyssey: Parental Perceptions of Whole Exome Sequencing (WES) in Pediatric Undiagnosed Disorders. J. Genet. Couns. 2016;25:1019–1031. doi: 10.1007/s10897-016-9933-1. [DOI] [PubMed] [Google Scholar]
- 18.Roberts J.S., Robinson J.O., Diamond P.M., Bharadwaj A., Christensen K.D., Lee K.B., Green R.C., McGuire A.L., MedSeq Project team Patient understanding of, satisfaction with, and perceived utility of whole-genome sequencing: findings from the MedSeq Project. Genet. Med. 2018;20:1069–1076. doi: 10.1038/gim.2017.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner-Lin A., Zaspel L., Carlson M., Mueller R., Walser S.A., Desai R., Bernhardt B.A. Gratitude, protective buffering, and cognitive dissonance: How families respond to pediatric whole exome sequencing in the absence of actionable results. Am. J. Med. Genet. 2018;176:578–588. doi: 10.1002/ajmg.a.38613. [DOI] [PubMed] [Google Scholar]
- 20.Lupo P.J., Robinson J.O., Diamond P.M., Jamal L., Danysh H.E., Blumenthal-Barby J., Lehmann L.S., Vassy J.L., Christensen K.D., Green R.C., et al. Patients’ perceived utility of whole-genome sequencing for their healthcare: findings from the MedSeq project. Per. Med. 2016;13:13–20. doi: 10.2217/pme.15.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canedo J.R., Miller S.T., Myers H.F., Sanderson M. Racial and ethnic differences in knowledge and attitudes about genetic testing in the US: Systematic review. J. Genet. Couns. 2019;28:587–601. doi: 10.1002/jgc4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall W.J., Chapman M.V., Lee K.M., Merino Y.M., Thomas T.W., Payne B.K., Eng E., Day S.H., Coyne-Beasley T. Implicit Racial/Ethnic Bias Among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. Am. J. Public Health. 2015;105:e60–e76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson E.B., Chou W.-Y.S., Gaysynsky A., Krakow M., Elrick A., Khoury M.J., Kaphingst K.A. Communication of cancer-related genetic and genomic information: A landscape analysis of reviews. Transl. Behav. Med. 2018;8:59–70. doi: 10.1093/tbm/ibx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odgis J.A., Gallagher K.M., Suckiel S.A., Donohue K.E., Ramos M.A., Kelly N.R., Bertier G., Blackburn C., Brown K., Fielding L., et al. The NYCKidSeq project: study protocol for a randomized controlled trial incorporating genomics into the clinical care of diverse New York City children. Trials. 2021;22:56. doi: 10.1186/s13063-020-04953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastin M., Odgis J.A., Suckiel S.A., Bonini K.E., Di Biase M., Brown K., Marathe P., Kelly N.R., Ramos M.A., Rodriguez J.E., et al. The TeleKidSeq pilot study: incorporating telehealth into clinical care of children from diverse backgrounds undergoing whole genome sequencing. Pilot Feasibility Stud. 2023;9:47. doi: 10.1186/s40814-023-01259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amendola L.M., Berg J.S., Horowitz C.R., Angelo F., Bensen J.T., Biesecker B.B., Biesecker L.G., Cooper G.M., East K., Filipski K., et al. The Clinical Sequencing Evidence-Generating Research Consortium: Integrating Genomic Sequencing in Diverse and Medically Underserved Populations. Am. J. Hum. Genet. 2018;103:319–327. doi: 10.1016/j.ajhg.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suckiel S.A., Kelly N.R., Odgis J.A., Gallagher K.M., Sebastin M., Bonini K.E., Marathe P.N., Brown K., Di Biase M., Ramos M.A., et al. The NYCKidSeq randomized controlled trial: Impact of GUÍA digitally enhanced genetic results disclosure in diverse families. Am. J. Hum. Genet. 2023;110:2029–2041. doi: 10.1016/j.ajhg.2023.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suckiel S.A., Odgis J.A., Gallagher K.M., Rodriguez J.E., Watnick D., Bertier G., Sebastin M., Yelton N., Maria E., Lopez J., et al. GUÍA: a digital platform to facilitate result disclosure in genetic counseling. Genet. Med. 2021;23:942–949. doi: 10.1038/s41436-020-01063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turbitt E., Kohler J.N., Angelo F., Miller I.M., Lewis K.L., Goddard K.A.B., Wilfond B.S., Biesecker B.B., Leo M.C. The PrU: Development and validation of a measure to assess personal utility of genomic results. Genet. Med. 2023;25 doi: 10.1016/j.gim.2022.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Haun J., Luther S., Dodd V., Donaldson P. Measurement variation across health literacy assessments: implications for assessment selection in research and practice. J. Health Commun. 2012;17:141–159. doi: 10.1080/10810730.2012.712615. [DOI] [PubMed] [Google Scholar]
- 33.Shea J.A., Micco E., Dean L.T., McMurphy S., Schwartz J.S., Armstrong K. Development of a revised Health Care System Distrust scale. J. Gen. Intern. Med. 2008;23:727–732. doi: 10.1007/s11606-008-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.(2024). Find shortage areas by address [Internet]. https://data.hrsa.gov/tools/shortage-area/by-address.
- 35.Miller E.G., Young J.L., Rao A., Ward-Lev E., Halley M.C. Demographic Characteristics Associated With Perceptions of Personal Utility in Genetic and Genomic Testing: A Systematic Review. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J.-H., Crouch J., Jamal S.M., Tabor H.K., Bamshad M.J. Attitudes of African Americans toward return of results from exome and whole genome sequencing. Am. J. Med. Genet. 2013;161A:1064–1072. doi: 10.1002/ajmg.a.35914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris E.D., Ziniel S.I., Amatruda J.G., Clinton C.M., Savage S.K., Taylor P.L., Huntington N.L., Green R.C., Holm I.A. The beliefs, motivations, and expectations of parents who have enrolled their children in a genetic biorepository. Genet. Med. 2012;14:330–337. doi: 10.1038/gim.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biesecker B.B., Klein W., Lewis K.L., Fisher T.C., Wright M.F., Biesecker L.G., Han P.K. How do research participants perceive “uncertainty” in genome sequencing? Genet. Med. 2014;16:977–980. doi: 10.1038/gim.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makhnoon S., Shirts B.H., Bowen D.J. Patients’ perspectives of variants of uncertain significance and strategies for uncertainty management. J. Genet. Couns. 2019;28:313–325. doi: 10.1002/jgc4.1075. [DOI] [PubMed] [Google Scholar]
- 40.Hobbs A., Starkbaum J., Gottweis U., Wichmann H.E., Gottweis H. The privacy-reciprocity connection in biobanking: comparing German with UK strategies. Public Health Genomics. 2012;15:272–284. doi: 10.1159/000336671. [DOI] [PubMed] [Google Scholar]
- 41.Özer Ö., Budak F., Alp S. Is Vaccine Hesitancy Affected by Distrust in the Healthcare System? A Study in Turkish Population. Soc. Work Public Health. 2023;38:323–333. doi: 10.1080/19371918.2022.2160855. [DOI] [PubMed] [Google Scholar]
- 42.Alijanzadeh M., Ahorsu D.K., Alimoradi Z., Mahmoudi N., Griffiths M.D., Lin C.-Y., Liu H.-K., Pakpour A.H. Fear of COVID-19 and Trust in the Healthcare System Mediates the Association between Individual’s Risk Perception and Preventive COVID-19 Behaviours among Iranians. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph182212146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong K., Ravenell K.L., McMurphy S., Putt M. Racial/ethnic differences in physician distrust in the United States. Am. J. Public Health. 2007;97:1283–1289. doi: 10.2105/AJPH.2005.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Floyd E., Allyse M.A., Michie M. Spanish- and English-speaking pregnant women’s views on cfDNA and other prenatal screening: Practical and ethical reflections. J. Genet. Couns. 2016;25:965–977. doi: 10.1007/s10897-015-9928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molina F., Dehlendorf C., Gregorich S.E., Kuppermann M. Women’s preferences for and experiences with prenatal genetic testing decision making: Sociodemographic disparities in preference-concordant decision making. Patient Educ. Couns. 2019;102:595–601. doi: 10.1016/j.pec.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.