Abstract
BACKGROUND
Effective management of patients with aneurysmal subarachnoid hemorrhage (aSAH) demands vigilant monitoring and treatment, given the risks of complications such as cerebral vasospasm and delayed ischemic neurological deficits (DINDs). Transcranial transmission ultrasound (TTUS) is a well-established technique for assessing brain pulsatility. This pilot study aims to explore the utility of TTUS in detecting impaired intracerebral blood flow associated with DINDs.
OBSERVATIONS
The authors examined 2 male patients, ages 45 and 52 years, with aSAH Hunt and Hess grades 4 and 2, respectively, who developed DINDs during their clinical course. Simultaneous recordings of arterial blood pressure, heart rate, and TTUS measurements were obtained in the intensive care unit. TTUS analysis revealed abnormal arrhythmic wave patterns during DIND episodes, whereas baseline measurements on DIND-free days showed no abnormalities. Following endovascular spasmolysis, TTUS demonstrated a normalization of abnormal waves, returning to baseline levels, alongside the resolution of neurological symptoms.
LESSONS
TTUS, a noninvasive method for assessing brain pulsatility, shows promise as a novel tool for monitoring aSAH patients, potentially aiding in prompt diagnostics and additional therapeutic interventions. Its capacity to provide further insights for individuals at risk of delayed cerebral ischemia warrants further investigation in clinical studies.
Keywords: aneurysmal subarachnoid hemorrhage, vasospasm, noninvasive, ultrasound
ABBREVIATIONS: aSAH = aneurysmal subarachnoid hemorrhage, CT = computed tomography, CVS = cerebral vasospasm, DCI = delayed cerebral ischemia, DIND = delayed ischemic neurological deficit, DSA = digital subtraction angiography, GCS = Glasgow Coma Scale, ICP = intracranial pressure, MCA = middle cerebral artery, MRI = magnetic resonance imaging, TCD = transcranial Doppler, TTUS = transcranial transmission ultrasound, Vmean = mean velocity
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating disease because of its high mortality rate of over 30% and its high incidence of severe disability or cognitive impairment in surviving patients.1, 2 Five percent of all strokes result from ruptured intracranial aneurysms and the subsequent subarachnoid hemorrhage.1
Cerebral vasospasm (CVS) is the most frequent complication after aSAH and occurs between days 3 and 14 after intracranial aneurysm rupture, with maximal expression between days 6 and 8. Angiographically apparent CVS occurs in more than half of all patients, but the relationship between angiographic CVS and clinical symptoms due to impaired cerebral perfusion is not fully understood.3–5 The feared clinical sequelae of CVS is delayed ischemic neurological deficit (DIND) and delayed cerebral ischemia (DCI), occurring in roughly 30% of aSAH patients with CVS.6, 7
Although CVS after aSAH has been the subject of several studies, the exact mechanisms of its development remain largely unclear to date. The pathogenesis is obviously multifactorial and highly complex.8 Recently, in addition to a radiographically evident macrovasospasm, microvasospasm and cortical spreading depression, as well as inflammatory processes, have been discussed.9 Concurrently with vasospasm, electrolyte imbalances, notably hyponatremia, natriuresis, and hypovolemia, frequently emerge, contributing to the development of DINDs.10, 11
Reducing the incidence of DINDs and DCI resulting from CVS can be accomplished in certain cases through measures such as administering oral nimodipine, elevating arterial blood pressure, and, as salvage therapy, conducting endovascular vasospasmolysis.12 Still, a prerequisite of therapeutic success remains the early detection of symptomatic CVS. Transcranial Doppler (TCD) ultrasonography, digital subtraction angiography (DSA), computed tomography (CT) angiography, CT perfusion, and perfusion magnetic resonance imaging (MRI) can document changes in vessel caliber either directly or indirectly. However, each technique has its own disadvantages.13, 14 Imaging techniques like DSA, CT, and MRI pose logistical challenges and provide only a snapshot of vascular status. Additionally, CT and DSA involve significant exposure to ionizing radiation, making them unsuitable for daily monitoring diagnostics.
TCD ultrasonography is a portable examination technique that is free of ionizing radiation, enabling assessment of the large vessels in the circle of Willis. However, several studies have demonstrated reliable identification of CVS sufficient for clinical application, primarily in the middle cerebral artery (MCA).15, 16 Additionally, correct diagnosis is even more difficult in unconscious or sedated patients, in whom DCI is often detected only after irreversible ischemic changes have already occurred. Therefore, there is great clinical interest in a noninvasive, portable method that allows for the assessment of clinically relevant CVS.
Transcranial transmission ultrasound (TTUS) is a noninvasive technology that acquires changes in the density and volume of brain tissue arising from cerebral blood flow via ultrasound pulses propagating along the human brain.17 For selected frequencies, a dispersive character of the brain tissue has been demonstrated. Dispersion is an effect in which the medium's nonlinear, frequency-dependent compression modulus results in different propagation velocities for different ultrasound frequencies.17
In this study, we report on 2 male patients with aSAH who underwent TTUS measurements as an additional diagnostic tool during DINDs. To our knowledge, this is the first report on the application of TTUS to evaluate DINDs in aSAH.
Illustrative Cases
Data Acquisition
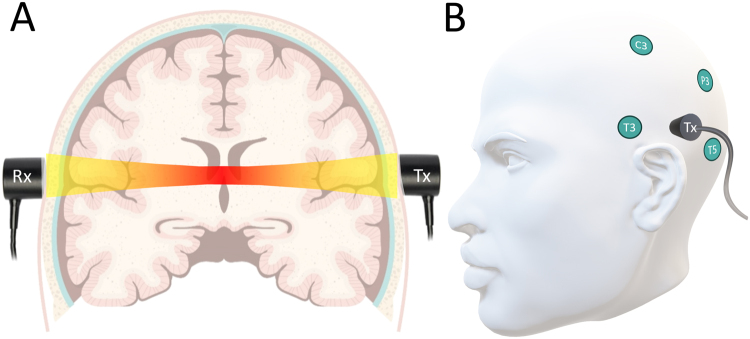
For the data analysis, only patients experiencing DINDs were included. Arterial blood pressure, heart rate, and TTUS measurements were simultaneously recorded in conditions with and without DIND. A DIND was defined as the occurrence of a new focal neurological deficit and/or a decrease of at least 2 points on the Glasgow Coma Scale (GCS) after excluding other potential causes such as postoperative hematoma or seizures.18 TTUS measurements (UltraEasy 3ACG, Sonovum GmbH) were performed daily within a short interval after the TCD measurements were acquired in the clinical routine. The setup to perform TTUS measurements consisted of a transmitting and a receiving ultrasound probe set up perpendicular to the head surface (Fig. 1). Ultrasound gel was applied to achieve sufficient coupling of the ultrasound. A predefined ultrasound signal in the low-MHz spectrum was transmitted during the measurement sequences between the transmitting and receiving probe. A series of 80 ultrasound waveform packages were accumulated to a single data point to increase the signal-to-noise ratio, leading to the accumulated time-of-flight signal. The accumulated time of flight was then registered. TCD measurements were recorded as the mean velocity (Vmean) of the flow in the anterior communicating artery, MCA, and posterior communicating artery.
FIG. 1.
The receiving (Rx) and transmitting (Tx) ultrasound probes are placed behind the ears (A). Placement of the ultrasound probes according to the electroencephalography 10–20 system (B).
Pathological TTUS measurements, TCD measurements showing an increased Vmean, and the occurrence of DIND events were recorded. The timeline illustration provides a combined overview of these parameters (Fig. 2). The preprocessing of TTUS measurements involved the identification and exclusion of episodes with high noise based on a frequency analysis of pattern > 10 Hz using fast Fourier transformation. Further data analyses were done using Python 3.19.14. After preprocessing, 2 data scientists manually evaluated the TTUS measurements in a blinded manner for characteristic patterns associated with the DIND events.
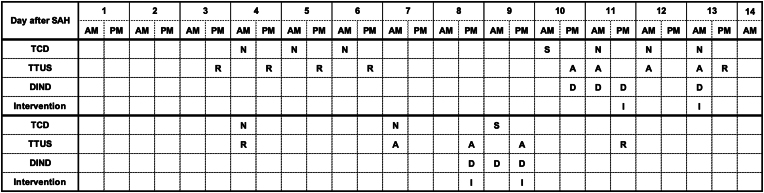
FIG. 2.
Data on the mean flow velocity (MFV) in TCD measurements, TTUS measurements, and DIND events on a timeline for each patient experiencing a DIND. A = arrhythmic; D = DIND present; I = intervention performed; N = MFV not severely increased; R = rhythmic; S = MFV severely increased > 200 cm/sec.
Findings
Two male patients, aged 52 and 45 years, were examined between January 2022 and April 2023. Initial aSAH grades according to the Hunt and Hess classification were 2 and 4, respectively. The aneurysms were in the pericallosal artery and anterior communicating artery, and both aneurysms were occluded via an endovascular approach. In the first patient, an external ventricular drain was placed for monitoring intracranial pressure (ICP) and drainage of cerebrospinal fluid, which did not indicate elevated ICP throughout all TTUS measurements performed. Nine TTUS measurements were performed in the first patient, and 5 were performed in the second patient. No adverse events were reported regarding TTUS measurements.
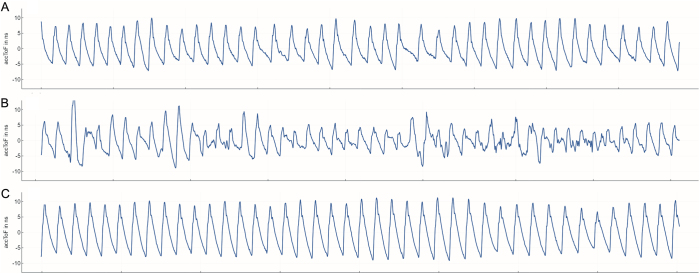
DINDs occurred twice in the first patient on days 10 and 13 after aSAH onset, respectively, and once in the second patient on day 8. Symptoms included a decrease in the GCS score and new motor deficits, respectively. Endovascular spasmolysis was performed in all cases of DIND according to the internal standard-of-care protocol. TTUS measurements were available for both patients before, during the onset of, and after the DIND events. During the days without neurological decline, typical rhythmic waves were recorded in these patients, as shown in Fig. 3A. Data analysis did not reveal any apparent changes in TTUS signals preceding the onset of a DIND. However, a distinct signal displaying arrhythmic waveforms was consistently detected whenever TTUS was performed concurrently with the DIND episodes (Fig. 3B). Notably, the TTUS waveforms promptly normalized following resolution of the DIND after effective spasmolysis interventions (Fig. 3C).
FIG. 3.
TTUS measurements performed in the first patient before the onset of a DIND on day 5 (A), during a DIND on day 11 (B), and after successful interventional spasmolysis on day 13 (C).
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Apart from urgent occlusion of the ruptured aneurysm, intensive care unit treatment and extensive neurological monitoring are crucial in the treatment of aSAH. Early detection of neurological deterioration is essential to prevent irreversible damage resulting in persisting disabilities or death. Symptomatic CVS requires immediate diagnostics and additional treatment. However, especially in patients with a compromised neurological status and impaired consciousness, performing detailed neurological monitoring is notably challenging. Further diagnostic tools to allow for early identification of patients at risk and tools to enable a more thorough examination of patients with an impaired neurological status are warranted.
TCD ultrasonography, as a standard cerebrovascular monitoring tool, often falls short of reliably identifying clinically relevant CVSs.15, 16 Additionally, the routine assessment of smaller vessels is challenging, leading to the underdiagnosis of microvasospasms.
In the present study, we evaluated the diagnostic value of TTUS in assessing CVS in patients with aSAH. The application of TTUS was straightforward and easily applicable in an intensive care unit setting, as previously described.19 During multiple measurements, no adverse events or technical issues were observed.
Although no linear change in the TTUS measurements allowing for direct correlation or automated analysis was observed, specific arrhythmic pulse waveforms occurred in both patients during DINDs as compared to baseline wave patterns and resolved with improved DINDs (Fig. 3). In these cases, TTUS was able both to identify DIND and to determine treatment success by resolving neurological symptoms. This suggests that TTUS may serve as a tool for identifying patients experiencing neurological decline due to the perfusion deficit associated with CVS beyond merely measuring the apparatus-detectable CVS.
No clear early changes in TTUS were identified before the onset of a DIND in this study. However, the measurements were conducted intermittently, potentially introducing gaps in data acquisition. Larger-scale investigations are needed to explore the potential prognostic value of TTUS, where alterations in wave patterns may indicate the onset of a DIND at a later stage.
A significant question lingers regarding the physiological explanation of the phenomenon of how TTUS can detect the onset of neurological deficits associated with perfusion deficits. In our opinion, the most plausible theory is related to changes in brain compliance: CVSs induce blood-brain barrier opening, cytotoxic edema, ischemic components, and altered cerebrovascular autoregulation, resulting in changes in blood flow, a shift in volume, and by this, alterations in compliance.20 These changes probably result in wave pattern alterations as assessed by TTUS.
Lessons
To our knowledge, the present case study is the first to explore the potential of TTUS as a noninvasive method for assessing clinically relevant CVS. TTUS demonstrated promise in identifying patients with DINDs associated with CVS. The dynamic changes in TTUS waveforms correlated with the occurrence of DINDs and reflected treatment success following interventional spasmolysis. These findings suggest that TTUS may serve as an additional novel tool for monitoring neurological decline following CVS. Further investigations with standardized evaluation protocols are warranted to explore the potential prognostic value of TTUS.
Disclosures
Dr. Schwendner reported that the transcranial transmission ultrasound device was provided by Sonovum GmbH. Dr. Meyer reported being a shareholder in Sonovum GmbH during the conduct of the study; grants from Brainlab and Zeiss; royalties from Spineart and Medacta; and personal fees from CompanionSpine outside the submitted work. Dr. Wostrack reported that the ACG device for ACG measurements was provided free of charge by Sonovum GmbH during the study period.
Author Contributions
Conception and design: Meyer, Wostrack. Acquisition of data: Schwendner, Kram, Zhang, Liang, Negwer, Wostrack. Analysis and interpretation of data: Schwendner, Kram, Meyer, Wostrack. Drafting the article: Schwendner, Wostrack. Critically revising the article: Schwendner, Kram, Joerger, Liang, Negwer, Meyer, Wostrack. Reviewed submitted version of manuscript: Schwendner, Joerger, Liang, Meyer, Wostrack. Approved the final version of the manuscript on behalf of all authors: Schwendner. Statistical analysis: Schwendner. Administrative/technical/material support: Meyer. Study supervision: Negwer, Wostrack.
Correspondence
Maximilian Schwendner: Heidelberg University Hospital, Ruprecht-Karls-University Heidelberg, Germany. maximilian.schwendner@med.uni-heidelberg.de.
References
- 1.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635-642. [DOI] [PubMed] [Google Scholar]
- 2.Korja M, Silventoinen K, Laatikainen T, Jousilahti P, Salomaa V, Kaprio J. Cause-specific mortality of 1-year survivors of subarachnoid hemorrhage. Neurology. 2013;80(5):481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40(6):1963-1968. [DOI] [PubMed] [Google Scholar]
- 4.Chalet FX, Briasoulis O, Manalastas EJ, Talbot DA, Thompson JC, Macdonald RL. Clinical burden of angiographic vasospasm and its complications after aneurysmal subarachnoid hemorrhage: a systematic review. Neurol Ther. 2023;12(2):371-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16(4):562-572. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44-58. [DOI] [PubMed] [Google Scholar]
- 7.Hijdra A, Van Gijn J, Stefanko S, Van Dongen KJ, Vermeulen M, Van Crevel H. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: clinicoanatomic correlations. Neurology. 1986;36(3):329-333. [DOI] [PubMed] [Google Scholar]
- 8.Adamczyk P, He S, Amar AP, Mack WJ. Medical management of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: a review of current and emerging therapeutic interventions. Neurol Res Int. 2013;2013:462491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care. 2016;20(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rooij NK, Rinkel GJ, Dankbaar JW, Frijns CJ. Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke. 2013;44(1):43-54. [DOI] [PubMed] [Google Scholar]
- 11.Kieninger M, Kerscher C, Bründl E, et al. Acute hyponatremia after aneurysmal subarachnoid hemorrhage: frequency, treatment, and outcome. J Clin Neurosci. 2021;88:237-242. [DOI] [PubMed] [Google Scholar]
- 12.Diringer MN, Bleck TP, Claude Hemphill J, III, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15(2):211-240. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg ED, Gold R, Reichman M, et al. Diagnostic accuracy of CT angiography and CT perfusion for cerebral vasospasm: a meta-analysis. AJNR Am J Neuroradiol. 2010;31(10):1853-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck J, Raabe A, Lanfermann H, et al. Effects of balloon angioplasty on perfusion- and diffusion-weighted magnetic resonance imaging results and outcome in patients with cerebral vasospasm. J Neurosurg. 2006;105(2):220-227. [DOI] [PubMed] [Google Scholar]
- 15.Kumar G, Shahripour RB, Harrigan MR. Vasospasm on transcranial Doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg. 2016;124(5):1257-1264. [DOI] [PubMed] [Google Scholar]
- 16.Mascia L, Fedorko L, terBrugge K, et al. The accuracy of transcranial Doppler to detect vasospasm in patients with aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2003;29(7):1088-1094. [DOI] [PubMed] [Google Scholar]
- 17.Dobkowska-Chudon W, Wrobel M, Karlowicz P, et al. Detecting cerebrovascular changes in the brain caused by hypertension in atrial fibrillation group using acoustocerebrography. PLOS ONE. 2018;13(7):e0199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391-2395. [DOI] [PubMed] [Google Scholar]
- 19.Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012;71(4):853-861. [DOI] [PubMed] [Google Scholar]
- 20.Dodd WS, Laurent D, Dumont AS, et al. Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: a review. J Am Heart Assoc. 2021;10(15):e021845. [DOI] [PMC free article] [PubMed] [Google Scholar]