Abstract
BACKGROUND
Space-occupying tumor bed cysts may exceptionally happen after the resection of diffuse low-grade glioma. Their mechanism and management remain debated. The authors report two cases of tumor bed cysts occurring after the resection of a left temporal diffuse low-grade glioma with two different evolutions.
OBSERVATIONS
The first patient showed a spontaneous decrease in the cyst volume and did not report any symptoms. In contrast, the second patient showed a progressive increase in the cyst volume and reported headaches and difficulties in finding words. Endoscopic cyst fenestration was performed and led to symptom relief and normalization of the surgical cavity.
LESSONS
A tumor bed cyst is a rare complication of temporal low-grade glioma resection. Its formation is due to entrapment of the choroid plexus in the temporal horn widely opened into the surgical cavity. Endoscopic cyst fenestration should be offered only in symptomatic cases.
Keywords: glioma resection, temporomesial, CSF entrapment, astrocytoma
ABBREVIATIONS: CSF = cerebrospinal fluid, DLGG = diffuse low-grade glioma, MRI = magnetic resonance imaging
Surgery plays an essential role in the modern management of IDH-mutated glioma (also known as “diffuse low-grade glioma” [DLGG]). Ample evidence indicates that the greater the extent of resection, the better the survival.1 While the formation of space-occupying tumor bed cysts has been reported after high-grade glioma surgery,2–5 such a complication has been exceptionally encountered after DLGG resection; a single study reported one case out of a series of 346 patients.6 Moreover, the mechanism underlying cerebrospinal fluid (CSF) entrapment within the surgical cavity remains debated, as does the optimal management of this rare event. In the present study, we sought to address these two questions by reporting on the management of this complication in two cases with resection of a DLGG of the mesial temporal lobe. Our observations suggest a mechanism different from the usual “valve-like” mechanism. Moreover, the natural history toward spontaneous shrinking in one of the two patients argues for a proactive stance only in cases with clinical symptoms attributable to the mass effect on surrounding structures and/or increased intracranial pressure.
Illustrative Cases
Case 1
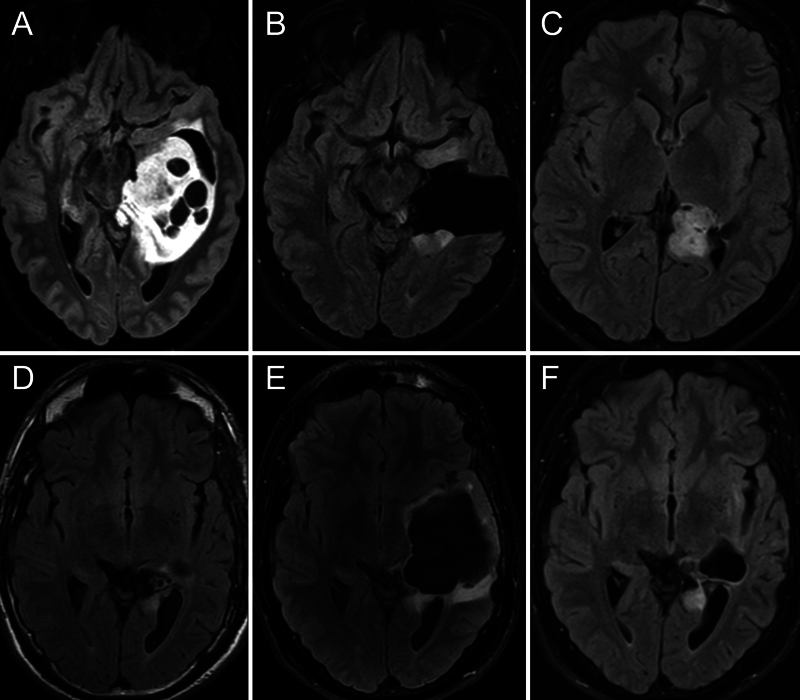
A 27-year-old male was referred to Lariboisière Hospital in November 2016 for a second opinion regarding a left glioma of the mesial temporal lobe (Fig. 1A). This lesion was discovered on magnetic resonance imaging (MRI) performed to investigate episodes of speech arrest. Awake surgery was offered, and the patient was operated on in December via a basal transcorticosubcortical approach.7, 8 Postoperative MRI demonstrated subtotal resection, with two residual nodules located anteroinferiorly and posterosuperiorly to the surgical cavity (Fig. 1B). The patient presented with a slight deterioration in lexical access but no other deficits. A wait-and-watch approach was recommended, and successive MRI studies demonstrated the slow growth of both residuals (Fig. 1C). Thus, reoperation was proposed in early 2018. As the patient was Spanish, this second surgery was performed under awake conditions at the Hospital Universitario Marques de Valdecilla in Santander. Gross-total resection was achieved, as demonstrated on postoperative MRI (Fig. 1D), without any change in neurological and cognitive status. Follow-up MRI 6 months after surgery showed progressive enlargement of the surgical cavity with mass effect on surrounding structures and edema on its borders (Fig. 1E). The patient did not report any symptoms. As it was unclear whether it was a tumor recurrence, another MRI study was performed 6 months later, showing a decrease in the volume of the cavity together with relief of the mass effect and edema (Fig. 1F). Ultimately, tumor progression was detected in late 2019 and treated by radiation therapy concomitantly with temozolomide and 6 cycles of adjuvant temozolomide. Since then, the patient has been clinically and radiologically stable.
FIG. 1.
Case 1. Images obtained in a 27-year-old right-handed man with a left temporal mesial World Health Organization (WHO) grade 2 astrocytoma. A: Preoperative axial fluid-attenuated inversion recovery (FLAIR) MRI demonstrating a left temporomesial tumor. B: Postoperative MRI demonstrated subtotal resection, with two residual nodules located anteroinferiorly and posterosuperiorly to the surgical cavity. C: Postoperative MRI performed 12 months after surgery demonstrated the slow growth of both residuals. D: Postoperative MRI after the second surgery. Gross-total resection was achieved. E: Postoperative MRI 6 months after surgery demonstrated progressive enlargement of the surgical cavity with mass effect on surrounding structures and edema on its borders. F: MRI 6 months later showed a decrease in the volume of the cavity together with relief of the mass effect and edema.
Case 2
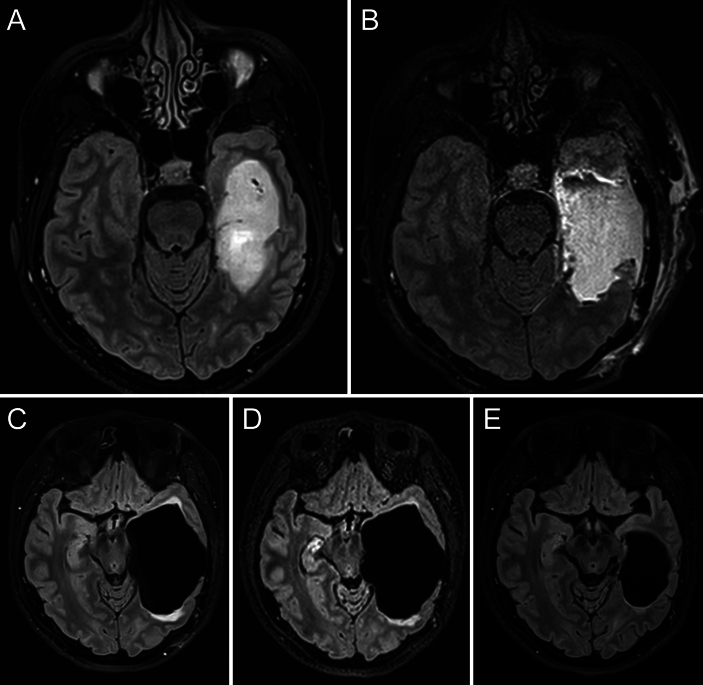
A 36-year-old female patient was referred to Lariboisière Hospital in January 2022, seeking a second opinion regarding a left glioma of the mesial temporal lobe (Fig. 2A). She had presented to an outside institution in September 2021 with a partial seizure (speech arrest) with secondary generalization. Awake surgery was offered and performed in March 2022 via a temporobasal transcortical approach.7, 8 Complete resection was achieved (Fig. 2B), with no other deficits other than mild impairment in lexical access. At the 4-month follow-up, the patient reported headaches, and MRI demonstrated an enlargement of the surgical cavity with mass effect on surrounding structures and edema on its borders (Fig. 2C). Based on the spontaneous favorable evolution of case 1, a watch-and-wait approach was offered, with a close radiological follow-up. However, her symptoms worsened. In addition to an increase in headache intensity, the patient reported more difficulties in finding words. Another MRI study performed 1 month later showed an increase in the volume of the cyst and mass effect (Fig. 2D). Endoscopic fenestration of the neomembrane was offered and performed in August 2022 (Video 1). Immediate postoperative computed tomography (CT) showed clear improvement. The last MRI of September 2023 demonstrated a fully normal surgical cavity (Fig. 2E), with complete disappearance of the mass effect and edema. The patient reported relief of her headaches, and her lexical access progressively improved.
FIG. 2.
Case 2. Images obtained in a 36-year-old right-handed woman with a left temporal mesial WHO grade 2 astrocytoma. A: Preoperative axial FLAIR MRI with a left temporomesial tumor. B: Postoperative MRI demonstrated gross-total resection. C: Postoperative MRI 4 months after surgery demonstrated an enlargement of the surgical cavity with mass effect on surrounding structures and edema on its borders. D: Follow-up MRI performed 1 month later demonstrated progressive enlargement, in agreement with a neurological deterioration. E: MRI 12 months after endoscopic fenestration, showing a decrease in the volume of the cavity together with the relief of mass effect and edema.
VIDEO 1. Clip showing the endoscopic fenestration of neo-membrane of the left atrium in case 2. Click here to view.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
To the best of our knowledge, this is the first report describing dilatation of the surgical cavity after resection of a DLGG of the mesial temporal lobe. In the literature, this complication, termed “space-occupying tumor bed cyst,” has been rarely reported in the context of malignant tumor: its occurrence after malignant glioma resection has been estimated to range between 2.5% and 4%.2, 3 Ventricle opening has been established as a strong risk factor, even when it is attempted to restore ventricle wall integrity by using fibrinogen-coated collagen.9 The pathogenesis is thought to be a slit valve mechanism,4, 9 and several types of treatment have been proposed, including cysto- or ventriculoperitoneal shunts and open or endoscopic fenestration.2, 3
In the present cases, as observed in the intraoperative video, the mechanism was instead a trapping of the temporal horn at the level of the atrium. It is supposed that the large surgical cavity caused a downward shift of the superolateral walls of the atrium, resulting in an abnormal sealing of the ependyma between the lateral wall of the atrium and the pulvinar. The formation of this neomembrane may have been favored by inflammatory healing after Surgicel application over the pulvinar and brainstem at the end of the surgery.
Lessons
Interestingly, the asymptomatic case spontaneously evolved favorably, indicating that reoperation should be proposed only in symptomatic cases. It can be hypothesized that progressive enlargement of the cyst ultimately tore the occlusive membrane, thus restoring normal CSF communication between the temporal surgical cavity and the lateral ventricle. However, we were not able to prove such a phenomenon, as no three-dimensional T2 or kinetic sequences were available in the longitudinal MRI follow-up of this patient.
On the contrary, symptomatic cases should be proactively treated. Our case demonstrated that endoscopic fenestration is efficient, with no recurrence after a follow-up of 12 months and rapid clinical improvement after reoperation.
In conclusion, valve-like mechanisms may not be at stake in space-occupying tumor bed cysts of the temporomesial region. A simple trapping of the choroid plexus in the temporal horn, widely opened in the cavity, may explain the progressive enlargement of the surgical cavity. Endoscopic fenestration should be considered as the first treatment option, but only in symptomatic cases.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Mandonnet. Acquisition of data: Mandonnet, Caudron, Martino. Analysis and interpretation of data: Mandonnet, Martino. Drafting the article: Mandonnet, Caudron, Martino. Critically revising the article: Mandonnet, Caudron, Martino. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Mandonnet. Administrative/technical/material support: Froelich. Study supervision: Mandonnet.
Supplemental Information
Videos
Video 1. https://vimeo.com/892642676.
Correspondence
Emmanuel Mandonnet: Hôpital Lariboisière, Paris, France. emmanuel.mandonnet@aphp.fr.
References
- 1.Albuquerque LAF, Almeida JP, de Macêdo Filho LJM, Joaquim AF, Duffau H. Extent of resection in diffuse low-grade gliomas and the role of tumor molecular signature—a systematic review of the literature. Neurosurg Rev. 2021;44(3):1371-1389. [DOI] [PubMed] [Google Scholar]
- 2.Korinth MC, Weinzierl MR, Krings T, Gilsbach JM. Occurrence and therapy of space-occupying cystic lesions after brain tumor surgery. Zentralbl Neurochir. 2001;62(3):87-92. [DOI] [PubMed] [Google Scholar]
- 3.Beez T, Burgula S, Kamp M, Rapp M, Steiger HJ, Sabel M. Space-occupying tumor bed cysts as a complication of modern treatment for high-grade glioma. World Neurosurg. 2017;104:509-515. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Xiong W, Qu L, Huang H. Reoperation as a result of raised intracranial pressure associated with cyst formation in tumor cavity after intracranial tumor resection: a report of two cases. Case Rep Med. 2010;2010:634839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong B, Polemikos M, Heissler HE, Hartmann C, Nakamura M, Krauss JK. Challenges in cerebrospinal fluid shunting in patients with glioblastoma. Fluids Barriers CNS. 2018;15(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hönikl LS, Lange N, Meyer B, Gempt J, Meyer HS. Postoperative communicating hydrocephalus following grade 2/3 glioma resection: incidence, timing and risk factors. Cancers (Basel). 2023;15(14):3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DA, Hanalioglu S, Chaichana K, Duffau H. Transcorticosubcortical approach for left posterior mediobasal temporal region gliomas: a case series and anatomic review of relevant white matter tracts. World Neurosurg. 2020;139:e737-e747. [DOI] [PubMed] [Google Scholar]
- 8.Morshed RA, Young JS, Han SJ, Hervey-Jumper SL, Berger MS. The transcortical equatorial approach for gliomas of the mesial temporal lobe: techniques and functional outcomes. J Neurosurg. 2019;130(3):822-830. [DOI] [PubMed] [Google Scholar]
- 9.Beez T, Remmel D, Steiger HJ. Endoscopic visualization of an iatrogenic valve mechanism: elucidating the pathogenesis of postoperative tumor bed cysts. World Neurosurg. 2018;115:213-215. [DOI] [PubMed] [Google Scholar]