Abstract
Background:
Individual risk assessment allows donors to be evaluated based on their own behaviors. Study objectives were to assess HIV risk behaviors in men who have sex with men (MSM) and estimate the proportion of the study population who would not be deferred for higher risk HIV sexual behaviors.
Study Design and Methods:
A cross-sectional survey and biomarker assessment was conducted in eight U.S. cities. Participants were sexually active MSM interested in blood donation aged 18 – 39 years, assigned male sex at birth. Participants completed surveys during two study visits to define eligibility, self-reported sexual and HIV prevention behaviors. Blood was drawn at study visit one and tested for HIV and the presence of tenofovir, one of the drugs in oral HIV pre-exposure prophylaxis (PrEP). Associations were assessed between HIV infection status or HIV PrEP use and behaviors, including sex partners, new partners, and anal sex.
Results:
A total of 1566 MSM completed the visit one questionnaire and blood draw and 1197 completed the visit two questionnaire. Among 1562 persons without HIV, 789 (50.4%) were not taking PrEP. Of those not taking PrEP, 66.2% reported one sexual partner or no anal sex and 69% reported no new sexual partners or no anal sex with a new partner in the past three months.
Conclusion:
The study found that questions were able to identify sexually active, HIV-negative MSM who report lower-risk sexual behaviors. About a quarter of enrolled study participants would be potentially eligible blood donors using individual risk assessment questions.
Introduction
Blood donor deferral related to HIV began in 1983 when men who have sex with men (MSM) along with other groups with higher risk of HIV infection1 were deferred indefinitely from donation. In the subsequent 40 years, blood donation testing has evolved. Now, all blood donations are screened using sensitive HIV serology (since 1985) and nucleic acid tests (NAT, since 1999).2 In 2015, given performance of testing technology and results from studies, such as the Retrovirus Epidemiology Donor Studies,3–5 the U.S. Food and Drug Administration (FDA) revised guidance on blood donor eligibility allowing donation by MSM following a 12-month deferral period since the last oral or anal sex with a man.6 In 2020, given results from the Transfusion Transmissible Infections Monitoring System (TTIMS) following the change from a lifetime deferral to a time-based deferral for MSM7,8 and the negative impact of the SARS-CoV-2/COVID-19 pandemic on the U.S. blood supply, FDA again revised blood donor eligibility for MSM to three months since last sexual encounter.9 Most U.S. blood collection organizations implemented the new deferral criteria in mid-2020. Three months amply covers the NAT window period during which the virus cannot be detected by the test but may be present and could infect a transfusion recipient. For minipool HIV NAT in pools of 6 to 16 donations the window period is around 10 days from infection to detection,10 assuming no use of pre-exposure or post-exposure prophylaxis.
Compared to other groups, MSM are at higher risk for HIV and account for more than 70% of new HIV diagnoses in the U.S. and about 80% of those among men.11 HIV risk in the MSM population is associated with condomless anal sex and the per sex act risk of infection increases with the number of sexual partners.12 Unprotected receptive anal sex (i.e., without condoms or other HIV prevention strategies) remains the highest per act risk of HIV transmission for both males and females.12,13 As part of Ending the HIV Epidemic (EHE) by 2030, the U.S. seeks to reduce inequities in access to HIV treatment and prevention and to increase access to pre-exposure prophylaxis (PrEP).14 PrEP, in combination with safer sex practices, is indicated to reduce the risk of sexually-acquired HIV in adults at high risk.15,16 PrEP use, the behavioral characteristics of PrEP users, and differences in oral and injected PrEP17,18 have implications for the safety of the blood supply due to the possibility of breakthrough HIV infection and window period donations that could infect a transfusion recipient.19,20 PrEP use is a blood donation deferral criterion because of the concern that a PrEP breakthrough infection would be undetected by screening assays.21 Consequently, complex U.S. blood donation policy changes must be evaluated within the context of contemporary HIV prevention strategies and donor selection procedures.
In May 2023, FDA released final guidance moving from time-based deferrals to individual risk assessments for all prospective blood donors.22 The United Kingdom and Canada previously transitioned from 3-month deferral since last sex for MSM to individual risk questions, focusing on the use of PrEP and post-exposure prophylaxis, the number of sexual partners, and specific sexual behaviors.23–26 The FDA recommendations approximately mirror these donor selection approaches. The Assessing Donor Variability and New Concepts in Eligibility (ADVANCE) study was designed to assess among U.S. MSM interested in donating blood whether simple questions differentiate those with lower and higher risk of HIV exposure. We report findings from the ADVANCE study, focused on differences in self-reported behaviors among MSM.
Methods
Study Design
The ADVANCE study is a cross-sectional behavioral and biomarker assessment of sexually active MSM conducted in eight U.S. metropolitan areas (Atlanta, GA; Los Angeles, CA; Memphis, TN; Miami, FL; New Orleans/Baton Rouge, LA; Orlando, FL; San Francisco, CA; and Washington DC) between December 2020 and November 2022. This study was conducted by three large blood collection organizations (Vitalant, OneBlood, and the American Red Cross) with assistance from Stanford Blood Center, LGBTQ+ organizations in each city, the University of California San Francisco Center for AIDS Prevention Studies, and FDA. The study protocol was approved by the Institutional Review Board, Advarra (Columbia, MD), under protocol number 00043278. A Certificate of Confidentiality was obtained from the Division of Inspections and Surveillance, Office of Compliance and Biologics Quality, Center for Biologics Evaluation and Research, FDA, protecting the participants from release of any study information except when required by law.
Study Population
Sexually active MSM (defined as having had oral or anal sex with a male in the past three months) were recruited to participate in the study. Individuals were required to be biologically male at birth, reside within the designated geographic area (by zip code), and between 18 to 30 years old to include the MSM demographic at highest risk of new HIV infection. Age eligibility was expanded on May 10, 2021, to 18 to 39 years to help increase enrollment. Individuals were excluded who: were ever diagnosed with HIV; reported injection drug use, exchanging money, drugs or goods for sex, or had been diagnosed with a sexually transmitted infection (syphilis, gonorrhea, or chlamydia) in the past three months; and/or were not able to complete the study documents in English or Spanish. When data collection began, follow-up questionnaire eligibility was restricted to participants who tested HIV-positive or tested HIV-negative and PrEP-reactive. In May 2021, follow-up questionnaire eligibility was expanded to include participants who tested HIV-negative and PrEP non-reactive or inconclusive but who reported PrEP use on the HIV risk questionnaire, and in July 2021 to all participants, due to the relevance of follow-up survey responses for study objectives.
Participant Recruitment
Participant recruitment was supported using multiple outreach methods. In partnership with LGBTQ+ community organizations, the ADVANCE study was promoted using printed material, by direct email invitations, and via social media. Recruitment was also conducted at in-person events (e.g., Pride events, concerts) after relaxation of COVID-19 restrictions. All interested persons were referred to the study website to learn more and to schedule an in-person eligibility visit. By design, the exclusion criterion of being a person living with HIV as well as other exclusion criteria were not explicitly stated in recruitment materials. Participants were compensated a maximum of $85.
Study Visits
Participants completed eligibility screening at their first study visit. Self-reported responses to survey questions were entered in an electronic informed consent and data capture system (Medrio, Inc. San Francisco, CA) using tablet computers provided by the study. Eligible and consented participants completed a set of HIV risk questions focused on different time intervals (past 1, 3, and 12 months) inquiring about number of sex partners, sexual behaviors, HIV-status of sex partners, and use of PrEP. PrEP use was ascertained by questions covering three intervals, i.e., Have you taken pre-exposure prophylaxis (PrEP) during the past month, [3 months], [12 months]? Participants then provided a whole blood phlebotomy sample totaling <30mL for testing for HIV and tenofovir.
During the second study visit, a median of 22 days after the first, participants were informed of their HIV and PrEP testing results and participants with HIV (hereafter referred to as HIV-positive) were referred to local HIV treatment clinics. Participants were consented for additional study activities and then completed the follow-up questionnaire that included questions from the National HIV Behavioral Surveillance (NHBS) survey, 2021–2022 MSM cycle.27 Content was designed to elicit additional behavior information (e.g., number and sex/gender of sexual partners, sexual behaviors, HIV prevention including types of PrEP and condom use) as well as motivations and interest in blood donation.
HIV RNA and Antibody Testing
Creative Testing Solutions (Tempe, AZ) conducted NAT to detect HIV RNA using the Ultrio Elite assay on the Panther System (Grifols Diagnostics, San Diego, CA) and for antibodies using the HIV-1/HIV-2 PLUS O enzyme immunoassay (Bio-Rad, Hercules, CA).
HIV Limiting Antigen and Tenofovir Testing
Limiting Antigen (LAg) Avidity enzyme immunoassay testing (Sedia Biosciences®, Portland, OR) was conducted at Vitalant Research Institute (VRI) and was used to define the infection as recently acquired or long-standing among those who tested HIV NAT and serology reactive.28
Tenofovir is one of the two antiretroviral drugs in oral PrEP medications. We used a plate-based enzyme linked immunosorbent assay (ELISA, from OraSure®, Bethlehem, PA) with whole blood as the specimen input type to assess biomarkers of PrEP use. Additional information on the study testing is provided in the supplement.
Data Analysis
To compare behavioral profiles, primary analysis focused on frequencies of HIV risk questionnaire responses stratified by HIV-status and self-reported PrEP use. Chi-square statistics tested for significant statistical association (p ≤ 0.05). Secondary analyses assessed the proportion of participants eligible for blood donation as recommended in the FDA Final Guidance22 using a combination of five questions beginning with PrEP use in the past three months followed by the number of sex partners in the past three months and a question about anal sex if there has been more than one partner, and any new sex partners in the past three months and a question about anal sex if there has been a new partner. We used HIV risk questionnaire responses to estimate the number of donation-eligible participants for the “number of sex partners” questions and responses from the HIV risk and the follow-up questionnaires to estimate the number of donation-eligible participants for the “new sex partner” questions.
Results
Participant enrollment and follow-up study visits occurred from December 7, 2020, to November 9, 2022. Of 1781 participants screened for eligibility, 1588 (89.2%) were eligible and consented to participate (Table 1). Common reasons for ineligibility were age or reporting a recent sexually transmitted infection. The demographic characteristics of eligible and ineligible participants differed significantly in several respects, including their site of enrollment, gender identity and employment status (Table 2). Of those eligible, 1566 (98.6%) completed the HIV risk questionnaire and blood draw of whom 1561 (99.7%) were notified of their test results. Accounting for shifting inclusion criteria over time, 1473 (94.4%) were eligible for the follow-up survey, 1200 (81.5%) consented, and 1197 (99.83%) completed the survey.
Table 1.
ADVANCE Study Eligibility and Completion Rates
| Study Participation Category | N | Percent (of preceding row) |
|---|---|---|
|
| ||
| Total Screened | 1781 | |
|
| ||
| Eligible for ADVANCE Study | 1593 | 89.4 |
|
| ||
| Consented for ADVANCE Study | 1588 | 99.7 |
|
| ||
| Completed HIV Risk Questionnaire | 1588 | 100 |
|
| ||
| Successful Blood Draw | 1566 | 98.6 |
|
| ||
| Notified of Blood Test Results | 1561 | 99.7 |
|
| ||
| Eligible for Follow-up Questionnaire* | 1473 | 94.4 |
|
| ||
| Consented for Follow-up Questionnaire | 1200 | 81.5 |
|
| ||
| Completed Follow-up Questionnaire | 1197 | 99.8 |
|
| ||
|
| ||
| Ineligible | 188 | 10.6† |
|
| ||
| Ineligibility reason‡ | (% of ineligible) | |
| Not biological male | 15§ | 7.9 |
| <18 or >39 years of age | 48|| | 25.5 |
| Zip code ineligible (out of area) | 27 | 14.4 |
| No oral or anal sex with a male past 3 months | 19 | 10.1 |
| Exchanging money or goods for sex past 3 months | 11 | 5.6 |
| IDU past 3 months | 1 | <1 |
| Self-reported HIV-positive | 15 | 8.0 |
| Positive for syphilis past 3 months | 4 | 2.1 |
| Positive for gonorrhea past 3 months | 47 | 25.0 |
| Positive for chlamydia past 3 months | 30 | 16.0 |
| Discontinued eligibility survey | 2¶ | 1.1 |
When data collection started on December 7, 2020, follow-up eligibility was restricted to participants who tested HIV-positive or tested HIV-negative and PrEP-reactive. Starting on May 10, 2021, participants who tested HIV-negative and PrEP negative/inconclusive but who reported any PrEP use in the HIV risk questionnaire became eligible for a follow-up survey. Starting on July 6, 2021, participants who tested HIV-negative and PrEP-negative/-inconclusive and did not report PrEP use in the HIV risk questionnaire also became eligible.
Of all presenting participants.
Respondents may have reported more than one reason for ineligibility; 159 (84.6%) were ineligible for 1 reason, 27 (14.4%) for 2 reasons, and 2 (1.1%) for 3 reasons.
Includes 1 case who declined to answer the question (and was otherwise eligible).
The number of age-ineligible participants (n=48) exceeds what is recorded in Table 2 for <18 and 40+ (n=39), because the upper limit of age eligibility expanded from 30 to 39 on May 10, 2021.
Both participants were males within the eligible age range.
Table 2.
Demographic and Social Characteristics of Eligible and Ineligible Respondents
| Variable | Category | Total | Eligible | Ineligible | p-value* | |
|---|---|---|---|---|---|---|
|
| ||||||
| N=1781 (%) | N=1593 (%) | N=188 (%) | ||||
|
| ||||||
| Site† | Atlanta | 179 (100) | 169 (94.4) | 10 (5.6) | <0.001 | |
| Los Angeles | 345 (100) | 327 (94.8) | 18 (5.2) | |||
| Memphis | 59 (100) | 53 (89.8) | 6 (10.2) | |||
| Miami | 131 (100) | 117 (89.3) | 14 (10.7) | |||
| New Orleans/Baton Rouge | 108 (100) | 94 (87.0) | 14 (13.0) | |||
| Orlando | 193 (100) | 164 (85.0) | 29 (15.0) | |||
| San Francisco | 289 (100) | 234 (81.0) | 55 (19.0) | |||
| Washington, DC | 477 (100) | 435 (91.2) | 42 (8.8) | |||
|
| ||||||
| Gender Identity | Male | 1745 (98.0) | 1571 (98.6) | 174 (92.6) | <0.001 | |
| Female‡ | 1 (0.1) | 0 | 1 (0.5) | |||
| Transgender‡ | 23 (1.3) | 11 (0.7) | 12 (6.4) | |||
| Prefer not to answer§ | 12 (0.7) | 11 (0.7) | 1 (0.5) | |||
|
| ||||||
| Age | <18§ | 1 (0.1) | 0 | 1 (0.5) | 0.724 | |
| 18–24 | 250 (14.0) | 224 (14.1) | 26 (13.8) | |||
| 25–29 | 552 (31.0) | 506 (31.8) | 46 (24.5) | |||
| 30–34 | 527 (29.6) | 483 (30.3) | 44 (22.4) | |||
| 35–39 | 413 (23.2) | 380 (23.9) | 33 (17.6) | |||
| 40+§ | 38 (2.1) | 0 | 38 (20.2) | |||
|
| ||||||
| Race | White/Caucasian | 1272 (71.4) | 1139 (71.5) | 133 (70.7) | 0.015 | |
| Black/African American | 120 (6.7) | 99 (6.2) | 21 (11.2) | |||
| Asian/Pacific Islander | 127 (7.1) | 120 (7.5) | 7 (3.7) | |||
| Native American¶ | 11 (0.6) | 8 (0.5) | 3(1.6) | |||
| More than 1 race | 149 (8.4) | 138 (8.7) | 11 (5.9) | |||
| Other | 91 (5.1) | 80 (5.0) | 11 (5.9) | |||
| Prefer not to answer§ | 11 (0.6) | 9 (0.6) | 2 (1.1) | |||
|
| ||||||
| Ethnicity | Hispanic | 336 (18.9) | 298 (18.7) | 38 (20.2) | 0.621 | |
| Non-Hispanic | 1442 (81.0) | 1293 (81.2) | 149 (79.3) | |||
| Prefer not to answer§ | 3 (0.2) | 2 (0.1) | 1 (0.5) | |||
|
| ||||||
| Marital Status | Married/Domestic partner | 461 (25.9) | 409 (25.7) | 52 (27.7) | 0.859 | |
| Separated/Divorced | 66 (3.7) | 58 (3.6) | 8 (4.3) | |||
| Widowed** | 3 (0.2) | 1 (0.1) | 2 (1.1) | |||
| Never married | 1204 (67.6) | 1080 (67.8) | 124 (66.0) | |||
| Other | 45 (2.5) | 43 (2.7) | 2 (1.1) | |||
| Prefer not to answer§ | 2 (0.1) | 2 (0.1) | 0 | |||
|
| ||||||
| Education | Never been to school†† | 0 | 0 | 0 | 0.029 | |
| Completed grade school†† | 2 (0.1) | 1 (0.1) | 1 (0.5) | |||
| Some high school†† | 3 (0.1) | 2 (0.1) | 1 (0.5) | |||
| Completed high school | 49 (2.8) | 43 (2.7) | 6 (3.2) | |||
| Some college/tech school | 272 (15.3) | 231 (14.5) | 41 (21.8) | |||
| Completed college | 832 (46.7) | 755 (47.4) | 77 (41.0) | |||
| Completed graduate school | 621 (34.9) | 561 (35.2) | 60 (31.9) | |||
| Prefer not to answer§ | 2 (0.1) | 0 | 2 (1.1) | |||
|
| ||||||
| Employment Status | Full-time employment | 1355 (76.1) | 1234 (77.5) | 121 (64.4) | <0.001 | |
| Part-time employment | 147 (8.3) | 124 (7.8) | 23 (12.2) | |||
| Unemployed | 111 (6.2) | 87 (5.5) | 24 (12.8) | |||
| Currently in school | 151 (8.5) | 136 (8.5) | 15 (8.0) | |||
| Prefer not to answer§ | 17 (1.0) | 12 (0.8) | 5 (2.7) | |||
|
| ||||||
| Annual Income | $12,760 or less | 69 (3.9) | 53 (3.3) | 16 (8.5) | 0.032 | |
| $12,761 – $19,999 | 41 (2.3) | 35 (2.2) | 6 (3.2) | |||
| $20,000 – $29,999 | 80 (4.5) | 75 (4.7) | 5 (2.7) | |||
| $30,000 – $39,999 | 121 (6.8) | 106 (6.7) | 15 (8.0) | |||
| $40,000 – $49,999 | 136 (7.6) | 124 (7.8) | 12 (6.4) | |||
| $50,000 – $74,999 | 305 (17.1) | 271 (17.0) | 34 (18.1) | |||
| $75,000 – $99,999 | 244 (13.7) | 223 (14.0) | 21 (11.2) | |||
| $100,000 or more | 698 (39.2) | 630 (39.5) | 68 (36.2) | |||
| Don’t know‡ | 43 (2.4) | 36 (2.3) | 7 (3.7) | |||
| Prefer not to answer‡ | 44 (2.5) | 40 (2.5) | 4 (2.1) | |||
|
| ||||||
| Last Donation | Never donated blood | 666 (37.4) | 599 (37.6) | 67 (35.6) | 0.971 | |
| Over 5 years ago | 779 (43.7) | 696 (43.7) | 83 (44.1) | |||
| 1–5 years ago | 240 (13.5) | 214 (13.4) | 26 (13.8) | |||
| 3–12 months ago | 50 (2.8) | 47 (3.0) | 3 (1.6) | |||
| Within past 3 months‡‡ | 13 (0.7) | 10 (0.6) | 3 (1.6) | |||
| Prefer not to answer when§ | 12 (0.7) | 10 (0.6) | 2 (1.1) | |||
| Prefer not to answer if ever§ | 21 (1.2) | 17 (1.1) | 4 (2.1) | |||
Note: Two San Francisco participants discontinued the eligibility survey. They are included in the “Prefer not to answer” category for Education, Employment Status, and Annual Income and in the “Prefer not to answer if ever” for Last Donation In the “Ineligible” column.
Chi-square statistic comparing eligible respondents to ineligible respondents
Cell entries are row N and row percentage, all others are column N and column percentage
Combined into “Other” category for calculation of chi-square statistic
Excluded from calculation of chi-square statistic
Combined with “Other” category for calculation of chi-square statistic
Combined with “Separated/Divorced” category for calculation of chi-square statistic
Combined with “Completed high school” category for calculation of chi-square statistic
Combined into “3–12 months ago” category for calculation of chi-square statistic
The study eligibility questionnaire included a question asking if potential participants knew their HIV status. Among those screened for eligibility, 15 (0.84%) disclosed their HIV-positive status and were not eligible for the study. Four (0.25%) of the enrolled participants tested HIV-positive. The four had detectable HIV RNA and antibodies and were classified as not having recently acquired HIV infection by LAg avidity testing (Supplement Table 1). Tenofovir was detected for one of these participants. One participant who was unwilling to return was notified by certified mail; the others returned for in-person results notification. At that time, one participant disclosed previous knowledge of his HIV infection, likely explaining the tenofovir result as part of antiretroviral therapy. Two of the HIV-positive participants completed the follow-up interview. Due to their small number, no demographic characteristics are reported for HIV-positive participants.
In the primary analysis of behavior and exposures that may be associated with HIV risk, we compared self-reported PrEP users to non-users. Among HIV-negative participants, 803/1553 (51.7%) reported not taking PrEP in the past month and 789/1552 (50.8%) reported not taking PrEP in the past three months. In both periods, the total number of sex partners was significantly different when comparing PrEP users and non-users, with 225 (30.0%) PrEP-users reporting one or no sex partner in the past month and 103 (13.5%) reporting one sex partner in the past three months, whereas 601 (74.8%) of non-PrEP users reported one or no sex partner in the past month and 492 (62.4%) reported one sex partner in the past three months (Table 3).
Table 3.
Sexual Behavior with Male Sex Partners and PrEP Use Self-reported by MSM in the HIV Risk Questionnaire
| Behavior | Category | Behavior in Past Month* |
Behavior in Past 3 Months† |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tested HIV-negative |
HIV-positive | Tested HIV-negative |
HIV-positive | |||||||
| Used PrEP | No PrEP Use | p-value‡ | Used PrEP | No PrEP Use | p-value‡ | |||||
|
|
|
|
|
|||||||
| N=750 (%) | N=803 (%) | N=4 (%) | N=763 (%) | N=789 (%) | N=4 (%) | |||||
|
| ||||||||||
| # male partners | 0 | 21 (2.8) | 30 (3.7) | <0.001 | - | - | - | <0.001 | - | |
| 1 | 204 (27.2) | 571 (71.1) | 2 (50) | 103 (13.5) | 492 (62.4) | 2 (50) | ||||
| 2 | 147 (19.6) | 96 (12.0) | 1 (25) | 103 (13.5) | 105 (13.3) | - | ||||
| 3–5 | 284 (37.9) | 84 (10.5) | - | 240 (31.5) | 136 (17.2) | 1 (25) | ||||
| 6–10 | 74 (9.9) | 19 (2.4) | - | 208 (27.3) | 38 (4.8) | - | ||||
| >10 | 20 (2.7) | 3 (0.4) | 1 (25) | 109 (14.3) | 17 (2.2) | 1 (25) | ||||
| Missing data§ | 0 | 0 | - | 0 | 1 (0.1) | - | ||||
|
| ||||||||||
| Type of sex | No male sex partners¶ | 21 (2.8) | 30 (3.7) | <0.001 | - | - | - | <0.001 | - | |
| No anal sex | 43 (5.7) | 122 (15.2) | - | 28 (3.7) | 83 (10.5) | - | ||||
| Always used condoms | 57 (7.6) | 82 (10.2) | - | 49 (6.4) | 91 (11.5) | - | ||||
| Sometimes used condoms | 224 (29.9) | 124 (15.4) | 3 (75) | 321 (42.1) | 166 (21.0) | 3 (75) | ||||
| Never used condoms | 400 (53.3) | 442 (55.0) | 1 (25) | 363 (47.6) | 444 (56.3) | 1 (25) | ||||
| Missing data§ | 5 (0.7) | 3 (0.4) | - | 2 (0.3) | 5 (0.6) | - | ||||
|
| ||||||||||
| Sex with HIV-positive partner | Yes | 70 (9.3) | 18 (2.2) | <0.001 | 1 (25) | 86 (11.3) | 22 (2.8) | <0.001 | 1 (25) | |
| No | 659 (87.9) | 755 (94.0) | 3 (75) | 675 (88.5) | 766 (97.1) | 3 (75) | ||||
| No male sex partners** | 21 (2.8) | 30 (3.7) | - | - | - | - | ||||
| Missing data§ | 0 | 0 | - | 2 (0.3) | 1 (0.1) | - | ||||
4 HIV-negative participants declined to answer whether they took PrEP in the past month
5 HIV-negative participants declined to answer whether they took PrEP in the past 3 months
Chi-square statistic comparing PrEP-users to non-users
Excluded from calculation of chi-square statistic
Combined with “No anal sex” category for calculation of chi-square statistic
Combined with “No” category for calculation of chi-square statistic
Among those who completed the HIV risk questionnaire, 744 (96.4%) PrEP users had oral or anal sex in the past month and 772 (100%) in the past three months. For non-PrEP users 756 (96.8%) had oral or anal sex in the past month and 781 (100%) in the past three months (Table 4). Anal sex was reported by most HIV-negative respondents regardless of PrEP use or time interval, but the proportion was significantly higher among PrEP users compared to non-PrEP users. In each time period, both insertive and receptive anal sex were more common among PrEP users compared to non-PrEP users.
Table 4.
Sexual Behavior and PrEP Use in Past 3 Months (Self-reported in HIV Risk Questionnaire) and Types of Sexual Partners and PrEP Use in Past 3 Months (Self-reported in Follow-up Questionnaire)
| Behavior | Most Recent Occurrence | Tested HIV-negative* |
HIV-positive | ||
|---|---|---|---|---|---|
| Used PrEP Past 3 Months | No PrEP Use Past 3 Months | p-value† | |||
|
| |||||
| HIV risk questionnaire responses | N=772 (%) | N=781 (%) | N=4 (%) | ||
|
| |||||
| Oral/Anal Sex | Past month | 744 (96.4) | 758 (96.8) | 0.644 | 4 (100) |
| Past 3 months | 772 (100) | 781 (100) | -- | 4 (100) | |
| Past 12 months | 772 (100) | 781 (100) | -- | 4 (100) | |
|
| |||||
| 2+ Partners | Past month | 534 (69.2) | 193 (24.7) | <0.001 | 2 (50) |
| Past 3 months | 668 (86.5) | 292 (37.4)‡ | <0.001 | 2 (50) | |
| Past 12 months | 738 (95.6) | 401 (51.3) | <0.001 | 3 (75) | |
|
| |||||
| Anal Sex | Past month | 727 (94.2)‡ | 660 (84.5) | <0.001 | 4 (100) |
| Past 3 months | 751 (97.3) | 705 (90.3)‡ | <0.001 | 4 (100) | |
| Past 12 months | 760 (98.4) | 740 (94.8)‡ | <0.001 | 4 (100) | |
|
| |||||
| Insertive Anal Sex | Past month | 543 (70.3)‡ | 467 (59.8) | <0.001 | 4 (100) |
| Past 3 months | 608 (78.8) | 538 (68.9) ‡ | <0.001 | 4 (100) | |
| Past 12 months | 671 (86.9) | 608 (77.8) ‡ | <0.001 | 4 (100) | |
|
| |||||
| Receptive Anal Sex | Past month | 494 (64.0)‡ | 431 (55.2) | <0.001 | 3 (75) |
| Past 3 months | 559 (72.4)‡ | 508 (65.0) § | 0.002 | 3 (75) | |
| Past 12 months | 632 (81.9) ‡ | 578 (74.0) § | <0.001 | 4 (100) | |
|
| |||||
| Anal Sex Without Condoms | Past month | 636 (82.4) †† | 552 (70.7) ¶ | <0.001 | 4 (100) |
| Past 3 months | 694 (89.9) ‡ | 617 (79.0) § | <0.001 | 4 (100) | |
| Past 12 months | 718 (93.0) | 663 (84.9) ‡ | <0.001 | 4 (100) | |
|
| |||||
| HIV-positive Partner | Past month | 71 (9.2) | 17 (2.2) | <0.001 | 1 (25) |
| Past 3 months | 92 (11.9)** | 22 (2.8) ‡ | <0.001 | 1 (25) | |
| Past 12 months | 151 (19.6)** | 27 (3.5) ‡ | <0.001 | 1 (25) | |
|
| |||||
|
| |||||
| Follow-up questionnaire responses | N=690 (%) | N=502 (%) | N=2 (%) | ||
|
| |||||
| More Than 1 Partner Past 3 Months | Male | 569 (82.3) ‡‡ | 178 (35.5)** | <0.001 | 1 (50) |
| Female | 6 (0.9) ¶ | 13 (2.6) ‡ | 0.020 | 0 | |
| Transgender | 3 (0.4)††† | 2 (0.4)‡‡ | 1.000 | 0 | |
|
| |||||
| New Partner Past 3 Months | Male | 541 (78.4)*** | 167 (33.3)‡‡ | <0.001 | 1 (50) |
| Female | 7 (1.0) ¶ | 14 (2.8) ‡ | 0.022 | 0 | |
| Transgender | 17 (2.5)‡‡‡ | 6 (1.2%)§§ | 0.115 | 0 | |
Notes for HIV Risk Questionnaire Responses:
12-month data are adjusted for 3-month and 1-month responses, and 3-month data are adjusted for 1-month responses (including PrEP use)
4 HIV-negative participants declined to answer whether they took PrEP in the past 3 months
For chi-square statistic comparing PrEP-users to non-users
1 case missing data
2 participants missing data
3 participants missing data
4 participants missing data
5 participants missing data
6 participants missing data
7 participants missing data
9 participants missing data
10 participants missing data
11 participants missing data
The follow-up questionnaire provides more specific information on sex in the past month and 3-month periods stratified by self-reported PrEP use in the past three months. Among PrEP users 569 (82.5%) reported more than one male sex partner in the past three months compared with 178 (35.5%) non-PrEP users (Table 4). The proportions reporting multiple female sex partners were small and significantly higher for non-PrEP users. Similarly, 541 (78.4%) PrEP users reported a new male sex partner in the past three months compared to 167 (33.3%) non-PrEP users. Few reported a new female sex partner, but they were significantly more common for non-PrEP users. No difference was evident regarding multiple or new transgender sex partners.
We estimated the proportion of the study population who would meet the proposed donor selection criteria in the FDA draft guidance for individual risk assessment questions. Each hierarchical analysis was restricted to HIV-negative participants. Among those who were not taking PrEP, 522 of 789 respondents (66.2%) reported fewer than two sex partners or not having anal sex with any partner in the past three months (Figure 1a). A similar analysis of new sex partners in the past 3-months showed that among HIV-negative, non-PrEP users 352 of 510 (69.0%) did not have a new partner or did not have anal sex with a new partner in the past three months (Figure 1b). If we consider all eligible study participants to estimate the proportion who would meet the “number of sex partners” questions, 522/1557 (33.5%) might qualify for donation. For the “new sex partner” questions 352/1278 (27.5%), based on 1557 minus the 279 participants who did not complete the follow-up questionnaire, might qualify for donation. By combining the “number of sex partner” and “new sex partner” which are restricted to those who completed the follow-up questionnaire, 313/1278 (24.5%) might qualify for donation.
Figure 1.
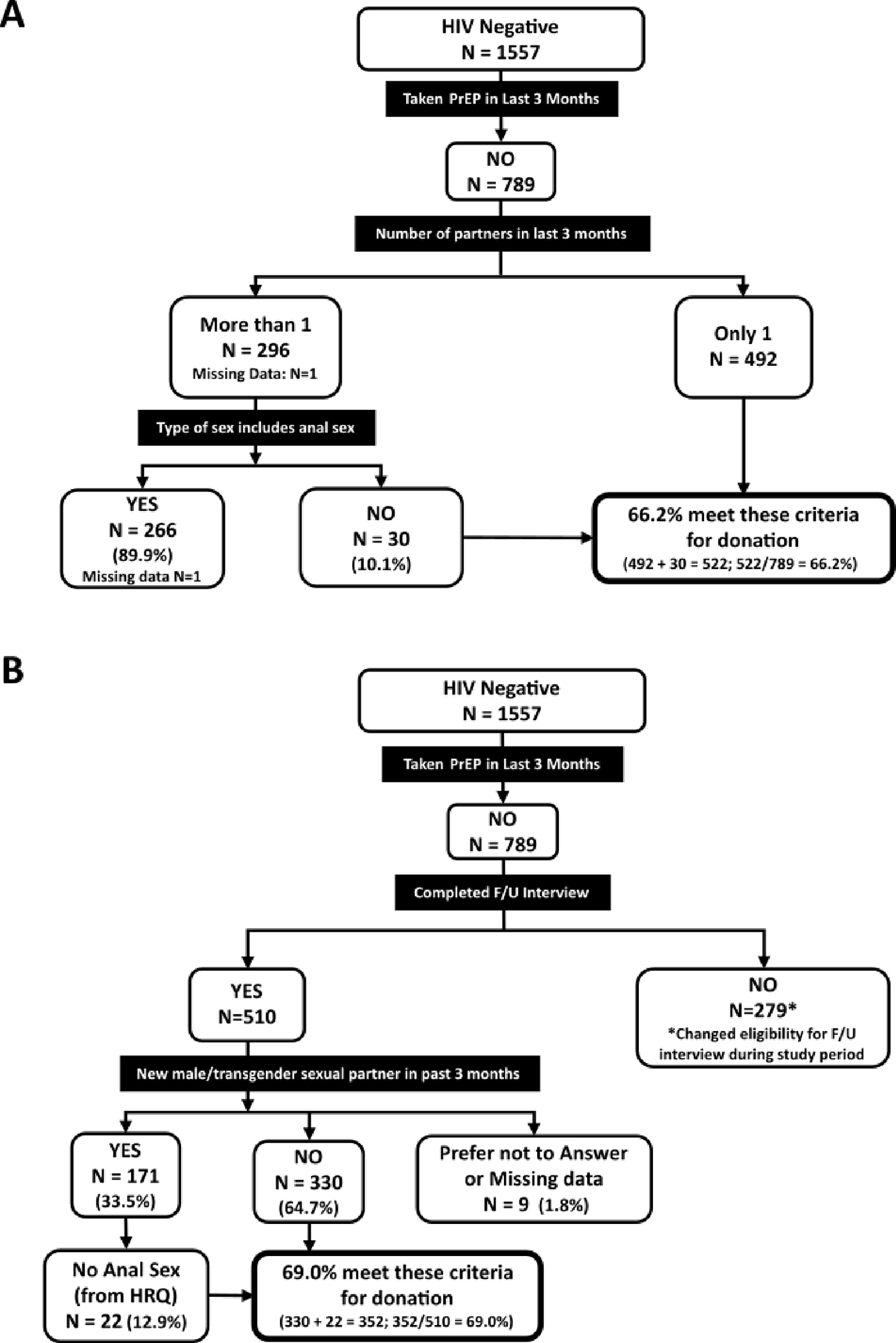
Projected donor eligibility using ADVANCE study results under 2023 draft FDA guidance, based on screening questions regarding (A) number of sexual partners, and (B) change in sexual partners in the past 3 months. F/U – follow-up, HRQ – HIV risk questionnaire.
Discussion
To assess behaviors associated with HIV risk, we enrolled sexually active MSM living in eight metropolitan areas in the U.S. who indicated they were interested in blood donation. Testing revealed four HIV-positive participants, none with an incident HIV infection. Among HIV-negative participants, the HIV risk questions we evaluated demonstrated differences in the risk exposure profiles of the participants, with those not taking PrEP having lower numbers of sexual partners and a lower prevalence of anal sex.
The proportion of HIV-positive MSM in this study was about 1%, consisting of four persons with HIV enrolled in this study and 15 individuals reported having HIV during eligibility screening. This percentage is notably lower than surveillance estimates for the overall MSM population in the U.S.29 The lower proportion of HIV-positive MSM in this study could reflect knowledge among the participants that HIV-positive persons are not eligible to be blood donors. It could also represent self-selection based on communications among potential participants regarding the study eligibility criteria.
In the present study we focused on self-reported sexual and HIV-prevention behaviors. The reason for this is the answers reported by participants are the best available data to understand the proportion of the sexually active MSM population who would meet the individual risk assessment criteria. [Note, results comparing self-disclosed PrEP use and PrEP testing of participant blood samples will be reported separately.]
Nearly half of the study population reported recent PrEP use, a proportion higher than reported for other studies of MSM in the U.S.14,30 In addition, high levels of educational achievement and income among study participants may indicate higher levels of access to PrEP than for the overall population of MSM. The substantial frequency of PrEP use in the study population may have directly contributed to the observed low prevalence of HIV infection. The availability of PrEP and the success of public health initiatives to expand access are critical components to reduce the burden of HIV infection as part of EHE. Those with higher numbers of sexual partners were far more likely to take PrEP than those with fewer partners.
Our study has limitations. First, ADVANCE was not powered to determine if an individual risk-based assessment was as effective at reducing HIV risk as the time-based MSM deferral in donor selection. Second, the study was designed to assess behaviors among sexually active MSM. Therefore, the results of the study are not inclusive of MSM who have not had sex with a male in the past three months. If these MSM are not taking PrEP, are HIV-negative and have no other risks, they are eligible to donate under the policy adopted in April 2020. Third, the study was not designed to assess the potential impact of the new FDA guidance on non-MSM donor risk, eligibility, and rates of deferral among those donors for the behaviors we studied in ADVANCE.
The number of sexual partners and types of sex reported by study participants are likely generalizable to the MSM population in other areas of the country. However, access to PrEP varies by geography and is likely to differ even within metropolitan areas, thereby precluding analytical adjustment for these differences. The study cities were selected because surveillance data indicated elevated HIV incidence among MSM31 allowing ADVANCE to assess behaviors associated with HIV in areas with increased HIV risk.
The COVID-19 pandemic may have led to changes in sexual risk behaviors among MSM32 as well as reductions in HIV-testing.33 At the start of the study, pandemic-associated reduction in close contacts, including sexual contacts, may have occurred. Later in the study enrollment period, when the largest proportion of participants were enrolled, close contacts probably increased with wide availability of COVID-19 vaccination but may have also decreased compared to previous periods because of the mpox virus outbreak.34
Blood Donation Policy Next Steps
The FDA has now finalized the blood donor eligibility guidance document implementing individual risk assessment, and blood centers are working to update procedures, modify computer systems, and train staff before the new recommendations can be implemented. The FDA guidance recommends implementation of question hierarchies to be asked of all potential donors. The questions adopted in Canada are similar, with a minor difference that in Canada the use of oral PrEP is a 4-month deferral. Questions on PrEP use, number of sexual partners, and anal sex among those with multiple partners or new sex partners in the past three months will be asked in sequence and will assess these risks in all donors. Donors who are not taking PrEP and have had no sex partners or one sex partner who is not a new partner, regardless of sex at birth or gender, will not be asked about anal sex.
The process for the implementation of changes to the donor history questionnaire requires time. Changes to the approach to donor selection are carefully monitored to assess whether the risk to blood recipients has changed using programs such as TTIMS7,8, where FDA will continue to track the latest data relevant to blood donation and deferral criteria.
The results from ADVANCE indicate that, among sexually active MSM interested in blood donation, there are subgroups who test HIV-negative, have had no new sexual partners and only one sexual partner within the past three months; studies in MSM cohorts suggest that these men are likely at lower risk of HIV infection than those with new or multiple sexual partners. ADVANCE results indicate questions can distinguish different groups of MSM, some of whom will meet the new individual risk assessment blood donation criteria, allowing persons previously not permitted to become blood donors.
Supplementary Material
Acknowledgements
We acknowledge and thank all persons who were screened for participation in the study and especially those who completed study visits. This study would not have occurred without the dedication and commitment of all study staff at Vitalant, Stanford Blood Center, OneBlood, and the American Red Cross, and the assistance of many different partnering organizations; please see the study website. Specifically, we thank all the LGBTQ+ community organizations who partnered with the study investigators: Whitman-Walker Health; The Center Orlando; Project More; Los Angeles LGBT Center; City of Hope/Pride in the City; OutMemphis; Friends For Life; CrescentCare; PFLAG New Orleans; New Orleans LGBT Center; Baton Rouge Pride; Ray Rico Freelance; Atlanta Pride, Pridelines; Sunserve, and the Pride Center at Equality Park. In addition, we acknowledge Dr. Marisabel Rodriguez Messan for graphic design and medical writer, Kelly Stimpert, for editorial support.
Funding:
ADVANCE was funded by the U.S. Food and Drug Administration contract 75F40119P10697
Abbreviations
- ADVANCE
Assessing Donor Variability And New Concepts in Eligibility
- COVID-19
Coronavirus Disease 19
- EHE
Ending the HIV Epidemic
- ELISA
Enzyme Linked Immunosorbent Assay
- HIV
Human Immunodeficiency Virus
- LGBTQ+
Lesbian, Gay, Bisexual, Transgender, Queer, plus
- LAg
Limiting Antigen
- MSM
Men who have Sex with Men
- NHBS
National HIV Behavioral Surveillance
- NAT
Nucleic Acid Test
- PrEP
Pre-exposure Prophylaxis
- PEP
Post-exposure Prophylaxis
- RNA
Ribonucleic Acid
- TTIMS
Transfusion Transmissible Infections Monitoring Systems
- FDA
U.S. Food and Drug Administration
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosures: Several authors are employees of the US FDA or blood collection organizations. No authors report conflicts of interests related to this manuscript.
References
- 1.Institute of Medicine, Committee to Study HIV Transmission Through Blood and Blood Products. Editors: Leveton LB, Sox HC Jr., Stoto MA. HIV and the Blood Supply: An Analysis of Crisis Decisionmaking. National Academies Press (US), Washington, DC; 1995. [PubMed] [Google Scholar]
- 2.Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion 2010;50: 2080–99. [DOI] [PubMed] [Google Scholar]
- 3.Custer B, Kessler D, Vahidnia F, Leparc G, Krysztof DE, Shaz B, Kamel H, Glynn S, Dodd RY, Stramer SL, Nhlbi Retrovirus Epidemiology Donor Study I. Risk factors for retrovirus and hepatitis virus infections in accepted blood donors. Transfusion 2015;55: 1098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Custer B, Sheon N, Siedle-Khan B, Pollack L, Spencer B, Bialkowski W, D’Andrea P, Sullivan M, Glynn S, Williams A. Blood donor deferral for men who have sex with men: the Blood Donation Rules Opinion Study (Blood DROPS). Transfusion 2015;55: 2826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd RY, Notari EP, Nelson D, Foster GA, Krysztof DE, Kaidarova Z, Milan-Benson L, Kessler DA, Shaz BH, Vahidnia F, Custer B, Stramer SL. Development of a multisystem surveillance database for transfusion-transmitted infections among blood donors in the United States. Transfusion 2016;56: 2781–9. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration, Center for Biologics Evaluation and Research. Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products - Guidance for Industry. Food and Drug Administration. Accessed 02/16/2023, 2023. https://www.federalregister.gov/d/2015-32250 [Google Scholar]
- 7.Grebe E, Busch MP, Notari EP, Bruhn R, Quiner C, Hindes D, Stone M, Bakkour S, Yang H, Williamson P, Kessler D, Reik R, Stramer SL, Glynn SA, Anderson SA, Williams AE, Custer B. HIV incidence in US first-time blood donors and transfusion risk with a 12-month deferral for men who have sex with men. Blood 2020;136: 1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steele WR, Dodd RY, Notari EP, Haynes J, Anderson SA, Williams AE, Reik R, Kessler D, Custer B, Stramer SL. HIV, HCV, and HBV incidence and residual risk in US blood donors before and after implementation of the 12-month deferral policy for men who have sex with men. Transfusion 2021;61: 839–50. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Provides Updated Guidance to Address the Urgent Need for Blood During the Pandemic (2020). Accessed 04/09/2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-updated-guidance-address-urgent-need-blood-during-pandemic.
- 10.Busch MP, Glynn SA, Stramer SL, Strong DM, Caglioti S, Wright DJ, Pappalardo B, Kleinman SH. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion 2005;45: 254–64. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019. U.S. Department of Health and Human Services. Accessed 03/23/2023, 2023. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Google Scholar]
- 12.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. Aids 2014;28: 1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baggaley RF, Owen BN, Silhol R, Elmes J, Anton P, McGowan I, van der Straten A, Shacklett B, Dang Q, Swann EM, Bolton DL, Boily MC. Does per-act HIV-1 transmission risk through anal sex vary by gender? An updated systematic review and meta-analysis. Am J Reprod Immunol 2018;80: e13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Mitchell JW, Zhang C, Liu Y. Evidence and implication of interventions across various socioecological levels to address pre-exposure prophylaxis uptake and adherence among men who have sex with men in the United States: a systematic review. AIDS Res Ther 2022;19: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truvada® Package Insert. 2020. Accessed 04/03/2023. https://www.gilead.com/~/media/Files/pdfs/medicines/hiv/truvada/truvada_pi.pdf
- 16.Descovy® Package Insert. 2022. Accessed 04/03/2023. https://www.gilead.com/~/media/Files/pdfs/medicines/hiv/descovy/descovy_pi.pdf
- 17.Eshleman SH, Fogel JM, Piwowar-Manning E, Chau G, Cummings V, Agyei Y, Richardson P, Sullivan P, Haines CD, Bushman LR, Petropoulos C, Persaud D, Kofron R, Hendrix CW, Anderson PL, Farrior J, Mellors J, Adeyeye A, Rinehart A, St Clair M, Ford S, Rooney JF, Mathew CA, Hunidzarira P, Spooner E, Mpendo J, Nair G, Cohen MS, Hughes JP, Hosseinipour M, Hanscom B, Delany-Moretlwe S, Marzinke MA. Characterization of Human Immunodeficiency Virus (HIV) Infections in Women Who Received Injectable Cabotegravir or Tenofovir Disoproxil Fumarate/Emtricitabine for HIV Prevention: HPTN 084. J Infect Dis 2022;225: 1741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neilan AM, Landovitz RJ, Le MH, Grinsztejn B, Freedberg KA, McCauley M, Wattananimitgul N, Cohen MS, Ciaranello AL, Clement ME, Reddy KP, Hyle EP, Paltiel AD, Walensky RP. Cost-Effectiveness of Long-Acting Injectable HIV Preexposure Prophylaxis in the United States : A Cost-Effectiveness Analysis. Ann Intern Med 2022;175: 479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Custer B, Quiner C, Haaland R, Martin A, Stone M, Reik R, Steele WR, Kessler D, Williamson PC, Anderson SA, Williams AE, Raymond HF, McFarland W, Robinson WT, Glick S, Sey K, Melton CD, Glynn SA, Stramer SL, Busch MP. HIV antiretroviral therapy and prevention use in US blood donors: a new blood safety concern. Blood 2020;136: 1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvala H, Reynolds C, Ijaz S, Maddox V, Penchala SD, Amara A, Else L, Brailsford S, Khoo S. Evidence of HIV pre-exposure or post-exposure prophylaxis (PrEP/PEP) among blood donors: a pilot study, England June 2018 to July 2019. Sex Transm Infect 2022;98: 132–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seed CR, Styles CE, Hoad VC, Yang H, Thomas MJ, Gosbell IB. Effect of HIV pre-exposure prophylaxis (PrEP) on detection of early infection and its impact on the appropriate post-PrEP deferral period. Vox Sang 2021;116: 379–87. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration, Center for Biologics Evaluation and Research. Recommendations for Evaluating Donor Eligibility Using Individual Risk-Based Questions to Reduce the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products, Office of Communication, Outreach and Development. Accessed 06/08/2023. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/recommendations-evaluating-donor-eligibility-using-individual-risk-based-questions-reduce-risk-human. [Google Scholar]
- 23.Davison KL, Reynolds CA, Andrews N, Brailsford SR. Blood donation by men who have sex with men: using evidence to change policy. Vox Sang 2021;116: 260–72. [DOI] [PubMed] [Google Scholar]
- 24.Lambert G, Cox J, Fourmigue A, Dvorakova M, Apelian H, Moodie EEM, Grace D, Skakoon-Sparling S, Moore DM, Lachowsky N, Jollimore J, Lal A, Parlette A, Hart TA. HIV incidence and related risks among gay, bisexual, and other men who have sex with men in Montreal, Toronto, and Vancouver: Informing blood donor selection criteria in Canada. Transfusion 2022;62: 2555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canadian Blood Services. Sexual Behaviour-based Screening. Accessed 03/14/2023, 2023. https://www.blood.ca/en/blood/am-i-eligible-donate-blood/sexual-behaviour-based-screening
- 26.U.K. National Health Service Blood and Transplant. Blood Safety. Accessed 03/14/2023, 2023. https://www.blood.co.uk/the-donation-process/further-information/your-safety/
- 27.U.S. Centers for Disease Control and Prevention. HIV Infection Risk, Prevention, and Testing Behaviors Among Men Who Have Sex With Men—National HIV Behavioral Surveillance, 13 U.S. Cities, 2021. Department of Health and Human Services. Accessed 03/23/2023, 2023. https://www.cdc.gov/hiv/library/reports/hiv-surveillance-special-reports/no-31/index.html [Google Scholar]
- 28.Sedia Biosciences® HIV-1 LAg-Avidity EIA. Accessed 02/20/2020, 2020. http://www.sediabio.com/LiteratureRetrieve.aspx?ID=139682
- 29.Crepaz N, Hess KL, Purcell DW, Hall HI. Estimating national rates of HIV infection among MSM, persons who inject drugs, and heterosexuals in the United States. Aids 2019;33: 701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones J, Pampati S, Emrick K, Siegler AJ. Demographic and behavioral characteristics of urban and non-urban PrEP-using MSM in the South. AIDS Care 2022;34: 1461–4. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan PS, Satcher Johnson A, Pembleton ES, Stephenson R, Justice AC, Althoff KN, Bradley H, Castel AD, Oster AM, Rosenberg ES, Mayer KH, Beyrer C. Epidemiology of HIV in the USA: epidemic burden, inequities, contexts, and responses. Lancet 2021;397: 1095–106. [DOI] [PubMed] [Google Scholar]
- 32.Pampati S, Emrick K, Siegler AJ, Jones J. Changes in Sexual Behavior, PrEP Adherence, and Access to Sexual Health Services Because of the COVID-19 Pandemic Among a Cohort of PrEP-Using MSM in the South. J Acquir Immune Defic Syndr 2021;87: 639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiNenno EA, Delaney KP, Pitasi MA, MacGowan R, Miles G, Dailey A, Courtenay-Quirk C, Byrd K, Thomas D, Brooks JT, Daskalakis D, Collins N. HIV Testing Before and During the COVID-19 Pandemic - United States, 2019–2020. MMWR Morb Mortal Wkly Rep 2022;71: 820–4. [DOI] [PubMed] [Google Scholar]
- 34.Chitwood MH, Kwon J, Savinkina A, Walker J, Bilinski A, Gonsalves G. Estimated Testing, Tracing, and Vaccination Targets for Containment of the US Mpox Outbreak. JAMA Netw Open 2023;6: e2250984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaney KP, Hanson DL, Masciotra S, Ethridge SF, Wesolowski L, Owen SM. Time Until Emergence of HIV Test Reactivity Following Infection With HIV-1: Implications for Interpreting Test Results and Retesting After Exposure. Clin Infect Dis 2017;64: 53–9. [DOI] [PubMed] [Google Scholar]
- 36.Lee HY, Giorgi EE, Keele BF, Gaschen B, Athreya GS, Salazar-Gonzalez JF, Pham KT, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Hahn BH, Shaw GM, Korber BT, Bhattacharya T, Perelson AS. Modeling sequence evolution in acute HIV-1 infection. J Theor Biol 2009;261: 341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duong YT, Kassanjee R, Welte A, Morgan M, De A, Dobbs T, Rottinghaus E, Nkengasong J, Curlin ME, Kittinunvorakoon C, Raengsakulrach B, Martin M, Choopanya K, Vanichseni S, Jiang Y, Qiu M, Yu H, Hao Y, Shah N, Le LV, Kim AA, Nguyen TA, Ampofo W, Parekh BS. Recalibration of the limiting antigen avidity EIA to determine mean duration of recent infection in divergent HIV-1 subtypes. PLoS One 2015;10: e0114947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sempa JB, Welte A, Busch MP, Hall J, Hampton D, Facente SN, Keating SM, Marson K, Parkin N, Pilcher CD, Murphy G, Grebe E, Consortium for the E, Performance of HIVIA. Performance comparison of the Maxim and Sedia Limiting Antigen Avidity assays for HIV incidence surveillance. PLoS One 2019;14: e0220345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


