Summary
Background
In May, 2018, Children’s Hospital Colorado noted an outbreak of enterovirus A71 (EV-A71) neurological disease. We aimed to characterise the clinical features of EV-A71 neurological disease during this outbreak.
Methods
In this retrospective observational cohort study, children (younger than 18 years) who presented to Children’s Hospital Colorado (Aurora, CO, USA) between March 1 and November 30, 2018, with neurological disease (defined by non-mutually exclusive criteria, including meningitis, encephalitis, acute flaccid myelitis, and seizures) and enterovirus detected from any biological specimen were eligible for study inclusion. The clinical characteristics of children with neurological disease associated with EV-A71 were compared with those of children with neurological disease associated with other enteroviruses during the same period. To explore the differences in clinical presentation of acute flaccid myelitis, we also used a subgroup analysis to compare clinical findings in children with EV-A71-associated acute flaccid myelitis during the study period with these findings in those with enterovirus D68 (EV-D68)-associated acute flaccid myelitis at the same hospital between 2013 and 2018.
Findings
Between March 10 and Nov 10, 2018, 74 children presenting to Children’s Hospital Colorado were found to have enterovirus neurological disease; EV-A71 was identified in 43 (58%) of these children. The median age of the children with EV-A71 neurological disease was 22·7 months (IQR 4·0–31·9), and most of these children were male (34 [79%] children). 40 (93%) children with EV-A71 neurological disease had findings suggestive of meningitis, 31 (72%) children showed evidence of encephalitis, and ten (23%) children met our case definition of acute flaccid myelitis. All children with EV-A71 disease had fever and 18 (42%) children had hand, foot, or mouth lesions at or before neurological onset. Children with EV-A71 disease were best differentiated from those with other enteroviruses (n=31) by the neurological findings of myoclonus, ataxia, weakness, and autonomic instability. Of the specimens collected from children with EV-A71, this enterovirus was detected in 94% of rectal, 79% of oropharyngeal, 56% of nasopharyngeal, and 20% of cerebrospinal fluid specimens. 39 (93%) of 42 children with EV-A71 neurological disease who could be followed up showed complete recovery by 1–2 months. Compared with children with EV-D68-associated acute flaccid myelitis, children with EV-A71-associated acute flaccid myelitis were younger, showed neurological onset earlier after prodromal symptom onset, had milder weakness, showed more rapid improvement, and were more likely to completely recover.
Interpretation
This outbreak of EV-A71 neurological disease, the largest reported in the Americas, was characterised by fever, myoclonus, ataxia, weakness, autonomic instability, and full recovery in most patients. Because EV-A71 epidemiology outside of Asia remains difficult to predict, identification of future outbreaks will be aided by prompt recognition of these distinct clinical findings, testing of non-sterile and sterile site specimens, and enhanced enterovirus surveillance.
Funding
None.
Introduction
Enterovirus A71 (EV-A71) is a non-polio enterovirus that causes hand, foot, and mouth disease and neurological disease, particularly in young children.1 In the Asia–Pacific region, EV-A71 has caused large-scale, cyclical epidemics (every 1–3 years) since the late 1990s. This virus has been associated with brainstem encephalitis, leading to fatal cardiopulmonary collapse, and severe cases of acute flaccid myelitis, leading to long-term paralysis.2
Outside of Asia, EV-A71 circulation is poorly understood and therefore difficult to predict; however, outbreaks have been described in Australia (in 2013),3 Spain (in 2016),4 France (in 2016–17 and 2019),5 and Germany (in 2015–16 and 2019).6 Although small-scale, sporadic, regional outbreaks have occurred in the USA (in 2003 and 2005),7 EV-A71 is not commonly reported, accounting for less than 1% of typed enteroviruses that have been reported to the US Centers for Disease Control and Prevention (CDC) National Enterovirus Surveillance System since 1970.8-10 In our study, we aimed to describe the clinical characteristics of an outbreak of EV-A71 neurological disease amongst children in Colorado, USA in 2018.
Methods
Study design and participants
In this retrospective observational cohort study, children (younger than 18 years) with enterovirus-associated neurological disease (which included meningitis, encephalitis, acute flaccid myelitis, or seizures) presenting to Children’s Hospital Colorado (Aurora, CO, USA) between March 1 and November 30, 2018, were eligible for study inclusion. Enteroviruses were detected by testing biological specimens, including cerebrospinal fluid, nasopharyngeal, oropharyngeal and rectal specimens. We compared the clinical characteristics of children with neurological disease associated with EV-A71 with those of children presenting with neurological disease and another enterovirus during the same period. To explore the differences in clinical presentation of acute flaccid myelitis cases, we used a subgroup analysis to compare clinical findings in children with EV-A71-associated acute flaccid myelitis during the study period with these findings in children with acute flaccid myelitis associated with enterovirus D68 (EV-D68) at the same hospital between 2013 and 2018.
Neurological involvement, including meningitis, encephalitis, and acute flaccid myelitis, was defined with non-mutually exclusive criteria. Meningitis was defined as the presence of cerebrospinal fluid pleocytosis (>10 white blood cells per μL), identification of enterovirus in cerebrospinal fluid by RT-PCR or, in the absence of cerebrospinal fluid available for testing, documented meningeal signs on physical examination. Encephalitis was defined as the presence of encephalopathy, focal neurological findings (including myoclonus and ataxia, but excluding weakness attributable to myelitis), or MRI or electroencephalography (EEG) abnormalities suggestive of encephalitis. Cases of acute flaccid myelitis were confirmed by the CDC using the 2017 Council of State and Territorial Epidemiologists criteria11 of acute onset of flaccid limb weakness with longitudinal grey-matter-predominant spinal cord lesion noted on MRI. Summative limb strength scores (SLSS) were calculated in children with acute flaccid myelitis by adding together documented Medical Research Council strength scores (1–5) for all four limbs at initial examination, at the time of maximal weakness (nadir), and at 1–2 months of follow-up, as previously described.12
Research and data collection protocols were approved by the Colorado Multiple Institutional Review Board, with a waiver of informed consent.
Procedures
Diagnostic and therapeutic interventions were selected at the discretion of the treating provider for clinical purposes, and we collected data retrospectively through review of chart data that we entered into a standardised REDCap database. Oropharyngeal and rectal specimens were initially tested at Children’s Hospital Colorado with Xpert EV (Cepheid; Sunnyvale, CA, USA), which is FDA-approved for RT-PCR detection of enteroviruses in cerebrospinal fluid and modified and validated by the Children’s Hospital Colorado laboratory for these specimen types.13 Cerebrospinal fluid was initially tested at Children’s Hospital Colorado with Xpert EV or the FilmArray Meningitis Encephalitis Panel (BioFire Diagnostics; Salt Lake City, UT, USA), which are both FDA-approved for RT-PCR detection of enteroviruses in cerebrospinal fluid.14 Nasopharyngeal specimens were initially tested at Children’s Hospital Colorado with the FilmArray Respiratory Pathogen Panel (BioFire Diagnostics), which is FDA-approved for RT-PCR detection of rhinoviruses and enteroviruses, but cannot distinguish between these viruses.15 Specimens from children with neurological disease that were positive for enterovirus or rhinovirus were sent to the CDC Picornavirus Laboratory for typing using enterovirus viral protein 1 (VP1)-specific RT-PCR and sequencing as previously described.16
Statistical analysis
Descriptive statistics were used to characterise the cohort of children with enteroviral neurological disease, with denominators reflecting the number of patients tested. Categorical data were compared using Fisher’s exact test or a χ2 test. Continuous variables were analysed using a one-way analysis of variance, a Wilcoxon rank-sum test, or a Kruskal-Wallis test. Statistical significance was defined as p<0·05. Data were statistically analysed with SAS software version 9.4.
Role of the funding source
There was no study funder. The corresponding author had full access to all the data in the study and had final responsibility for decision to submit for publication.
Results
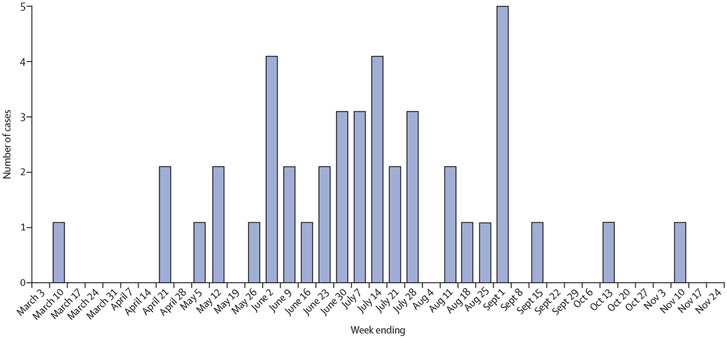
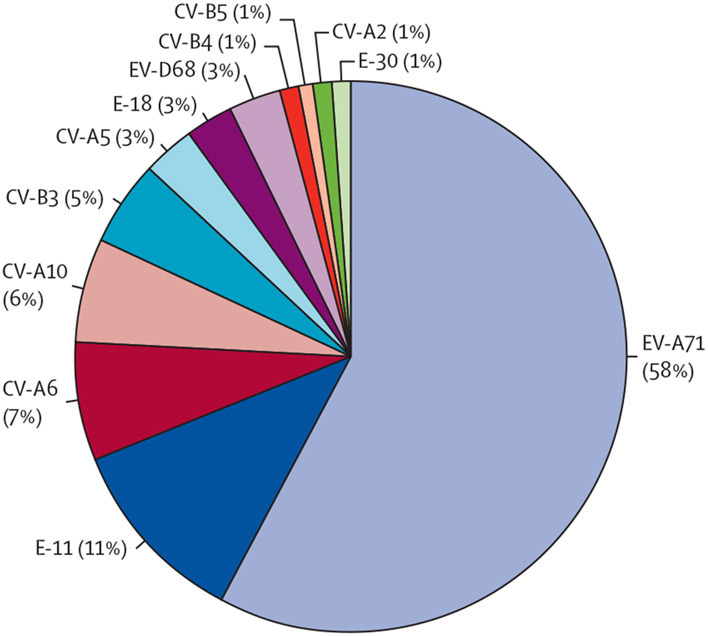
Between March 10, and Nov 10, 2018, 74 children presenting to Children’s Hospital Colorado met our case definition for enterovirus neurological disease. EV-A71 was the most commonly identified enterovirus serotype, which was identified in 43 (58%) of 74 children (figure 1; figure 2).
Figure 1: Number of children with enterovirus A71-associated neurological disease presenting to Children’s Hospital Colorado between March and November, 2018, by date of illness onset.
Figure 2: Proportion of each enteroviral serotype identified amongst children with enterovirus-associated neurological disease at Children’s Hospital Colorado between March and November, 2018.
Data are of 12 different serotypes identified in 74 children. EV=enterovirus. E=echovirus. CV=coxsackievirus.
The median age of the 43 children with EV-A71 neurological disease was 22·7 months (IQR 4·0–31·9); three (7%) children were aged 4 years or older at presentation (table 1). Most children had previously been healthy (39 [91%] children) and were male (34 [79%] children), white (33 [77%] children), and non-Hispanic (36 [84%] children). All children with EV-A71 neurological disease resided within the greater Denver metropolitan area, which has a population of approximately 3 million people. 20 (47%) children attended daycare, including a cluster of three (7%) children with acute flaccid myelitis from the same daycare classroom. 12 (28%) children had household contacts who were unwell, including one (2%) child with EV-A71-associated meningitis with a sibling from the same household who presented concurrently to a different hospital with untyped enteroviral meningitis.
Table 1:
Demographic and clinical characteristics of children presenting to Children’s Hospital Colorado with enterovirus-associated neurological disease between March and November, 2018
| Children with enterovirus A71 neurological disease (n=43) |
Children with other enterovirus neurological disease (n=31) |
p value | |
|---|---|---|---|
| Demographics or exposures | |||
| Age, months | 22·7 (4·0–31·9) | 14·4 (0·9–53·3) | 0·66 |
| Sex | ·· | ·· | 0·041 |
| Female | 9 (21%) | 14 (45%) | ·· |
| Male | 34 (79%) | 17 (55%) | ·· |
| Underlying medical condition | 4 (9%) | 3 (10%) | 0·49 |
| Attend daycare | 20 (47%) | 3 (10%) | 0·0007 |
| Have household contact with someone unwell | 12 (28%) | 11 (35%) | 0·49 |
| Associated signs and symptoms | |||
| Fever | 43 (100%) | 28 (90%) | 0·069 |
| Duration, days | 2·5 (2·0–3·0) | 1·0 (0–2·5) | 0·0066 |
| Maximum temperature, °C | 39·1 (38·7–39·8) | 38·8 (38·7–39·7) | 0·35 |
| Irritability | 37 (86%) | 9 (29%) | <0·0001 |
| Hand, foot, or mouth lesions | 18 (42%) | 6 (19%) | 0·041 |
| Respiratory illness | 20 (47%) | 12 (39%) | 0·50 |
| Vomiting | 21 (49%) | 10 (32%) | 0·15 |
| Diarrhoea | 8 (19%) | 1 (3%) | 0·046 |
| Neurological signs and symptoms | |||
| Time from symptom onset to neurological onset, days | 1 (1–3) | 1 (0–2) | 0·29 |
| Nuchal rigidity | 10 (23%) | 5 (16%) | 0·45 |
| Myoclonus | 26 (60%) | 5 (16%) | <0·0001 |
| Ataxia | 25 (58%) | 5 (16%) | 0·0003 |
| Weakness | 13 (30%) | 2 (6%) | 0·012 |
| Cranial nerve dysfunction | 4 (9%)* | 1 (3%) | 0·39 |
| Autonomic instability | 20 (47%) | 3 (10%) | <0·0001 |
| Mottled skin | 6 (14%) | 1 (3%) | 0·12 |
| Irregular breathing pattern | 12 (28%) | 1 (3%) | 0·006 |
| Tachycardia | 10 (23%) | 1 (3%) | 0·017 |
| Hypertension | 2 (5%) | 0 (0%) | 0·22 |
| Neurological involvement | |||
| Meningitis | 40 (93%) | 21 (68%) | 0·0048 |
| Encephalitis | 31 (72%) | 8 (26%) | <0·0001 |
| Acute flaccid myelitis | 10 (23%) | 2 (6%) | 0·053 |
| Seizures | 1 (2%)† | 5 (16%)‡ | 0·074 |
Data are median (IQR) or n (%). *Three children had oculomotor weakness (two with cranial nerve III and VI dysfunction, one with cranial nerve VI dysfunction), and one child had bulbar weakness. †Child had meningitis and encephalitis with a witnessed generalised tonic-clonic seizure during hospital admission. ‡One child had a simple febrile seizure, two children had complex febrile seizures, and two children had febrile status epilepticus.
Neurological symptoms were commonly preceded by fever (in 43 [100%] children; median duration 2·5 days [IQR 2·0–3·0]; median maximum temperature 39·1°C), irritability (in 37 [86%] children), vomiting (21 [49%] children), and respiratory symptoms (20 [47%] children; table 1). Prodromal symptom onset preceded neurological symptom onset by a median time of 1 day (IQR 1–3). 18 (42%) children with EV-A71 neurological disease had hand, foot, or mouth lesions (or a combination of the three) at or before neurological onset.
EV-A71 was most frequently detected in rectal specimens (in 32 [94%] of 34 specimens) and least frequently detected in cerebrospinal fluid (in eight [20%] of 40 specimens; table 2). Of the cases of EV-A71 with specimens from nasopharyngeal and oropharyngeal sites tested, 14 (54%) of 26 children had enterovirus detected at only one site. Amino-terminal VP1 sequences (297 nucleotides, including the B-C and D-E variable regions) of all EV-A71 strains from this outbreak belonged to clade C1 genotype, except for a single C2 genotype; however, all strains showed 100% consistency in amino acid (capsid) identity from translated nucleic acid sequences. VP1 nucleotide sequences from all enteroviruses identified in our study have been deposited in the GenBank sequence database (accession numbers MK836112–81 and MK491180).
Table 2:
Laboratory and imaging findings in children presenting to Children’s Hospital Colorado with enterovirus-associated neurological disease between March and November, 2018
| Children with enterovirus A71 neurological disease (n=43) |
Children with other enterovirus neurological disease (n=31) |
p value | |
|---|---|---|---|
| Cerebrospinal fluid findings | |||
| Pleocytosis | 34/39 (87%) | 14/25 (56%) | 0·0076 |
| White blood cell count, cells per μL | 135 (50–292) | 41 (3–179) | 0·030 |
| Increased protein | 17/37 (46%) | 15/22 (68%) | 0·10 |
| Protein concentration | 40 (32–59) | 50 (30–80) | 0·31 |
| Magnetic resonance neuroimaging findings | |||
| Leptomeningeal enhancement | 8/24 (33%) | 1/10 (10%) | 0·23 |
| Supratentorial lesion or lesions | 3/24 (13%) | 2/10 (20%) | 0·62 |
| Cranial nerve enhancement | 6/24 (25%) | 1/7 (14%) | 1·00 |
| Cerebellar lesion or lesions | 12/24 (50%) | 0 | 0·0058 |
| Brainstem lesion or lesions | 16/24 (67%) | 2/10 (20%) | 0·023 |
| Pons | 15/24 (63%) | 1/10 (10%) | 0·0079 |
| Midbrain | 4/24 (17%) | 1/10 (10%) | 1·00 |
| Medulla | 12/24 (50%) | 2/10 (20%) | 0·14 |
| Spinal cord lesion or lesions | 14/19 (74%) | 2/7 (29%) | 0·069 |
| Cervical spine lesion | 14/19 (74%) | 2/7 (29%) | 0·069 |
| Thoracic spine lesion | 8/18 (44%) | 2/7 (29%) | 0·66 |
| Lumbar spine lesion | 7/15 (47%) | 2/4 (50%) | 1·00 |
| Spinal cord nerve root enhancement | 6/19 (32%) | 2/7 (29%) | 1·00 |
| Sample containing enterovirus | |||
| Cerebrospinal fluid | 8/40 (20%)* | 20/26 (77%) | <0·0001 |
| Nasopharyngeal specimen | 19/34 (56%) | 10/15 (67%) | 0·48 |
| Oropharyngeal specimen | 27/34 (79%) | 10/15 (67%) | 0·34 |
| Rectal specimen | 32/34 (94%) | 14/16 (88%)† | 0·42 |
Data are n with finding/N tested (%) or median (IQR). Pleocytosis was defined as a white blood cell count of more than 10 white blood cells per μL. Increased protein was defined as concentrations of more than 40 mg/dL. *Of these eight specimens positive for enterovirus A71, six (75%) patients were younger than 2 months, six (75%) patients presented within 2 days of symptom onset, and six (75%) patients only had meningitis (no encephalitis or myelitis). †Two patients with negative enterovirus tests in rectal specimens had acute flaccid myelitis, in whom enterovirus D68 was detected in the nasopharynx.
40 (93%) children with EV-A71 neurological disease had findings suggestive of meningitis (table 1). Of 39 children with EV-A71 who had cerebrospinal fluid collected and analysed, 34 (87%) children had a pleocytosis (median white blood cell count, 135 cells per μL [IQR 50–292]) with a neutrophil predominance in 16 (47%) of 34 children and lymphocyte predominance in 13 (38%) of 34 children (table 2). Six (75%) of the eight children who had EV-A71 that was identified in cerebrospinal fluid had these samples collected within 2 days of symptom onset, six (75%) of eight samples were from infants younger than 2 months, and six (75%) of eight children presented with fever alone without evidence of encephalitis or myelitis. Eight (33%) of 24 children who were examined by MRI showed leptomeningeal enhancement.
31 (72%) children with EV-A71 neurological disease showed evidence of encephalitis, which included neurological findings of myoclonus in 26 (60%) children, ataxia in 25 (58%) children, and autonomic instability in 20 (47%) children, often in association with irritability (table 1). Myoclonus that worsened during sleep and truncal ataxia interfering with normal gait were commonly cited reasons for caregivers seeking medical attention (appendix; video). None of the six children with jerking movements captured while receiving EEG monitoring showed electrographic seizure activity. 12 (28%) children showed irregular breathing patterns, ten (23%) children showed persistent tachycardia (without evidence of myocarditis in any of the five children assessed by cardiology evaluation), six (14%) children showed mottling of the skin, and two (5%) children showed hypertension, all of which were features consistent with autonomic instability. MRI abnormalities consistent with encephalitis included dorsal brainstem lesions (in 16 [66%] of 24 children imaged) and lesions in the dentate nuclei of the cerebellum (in 12 [50%] of 24 children imaged); however, supratentorial lesions were uncommon (in three [13%] of 24 children imaged; table 2).
14 (33%) children with EV-A71 neurological disease were treated with intravenous immunoglobulin (1 g/kg per day for 2 days) to treat brainstem encephalitis with autonomic instability or acute flaccid myelitis (or both; table 3), and two children with brainstem encephalitis and acute flaccid myelitis refractory to intravenous immunoglobulin subsequently received intravenous methylprednisolone (30 mg/kg per day for 3–5 days). Children with EV-A71 neurological disease were treated in hospital for a median duration of 3 days (IQR 2–5) with ten (23%) children receiving intensive care unit support for autonomic instability (n=7), bulbar paralysis (n=1), altered mental status (n=1), or seizures (n=1). Three (7%) children subsequently required acute inpatient rehabilitation for acute flaccid myelitis. 20 (47%) children had fully recovered by discharge and 17 (41%) children received outpatient rehabilitation therapy. 39 (93%) of 42 children with EV-A71 neurological disease showed complete recovery by 1–2 months of follow-up, including nine (90%) of ten children with acute flaccid myelitis. Persistent deficits included residual, but improved, tremor and ataxia in two (5%) children at 1–2 months of follow-up, which subsequently resolved, and complete paralysis of one arm in a child with acute flaccid myelitis, which persisted for 1 year after disease onset. One child with residual tremor at discharge was lost to follow-up.
Table 3:
Hospital stay, treatments, and outcomes in children presenting to Children’s Hospital Colorado with enterovirus-associated neurological disease between March and November, 2018
| Children with enterovirus A71 neurological disease (n=43) |
Children with other enterovirus neurological disease (n=31) |
p value | |
|---|---|---|---|
| Hospital stay | |||
| Admitted to hospital | 43 (100%) | 26 (84%) | 0·0060 |
| Duration of stay, days | 3 (2–5) | 2 (2–3) | 0·020 |
| Received critical care | 10 (23%) | 4 (13%) | 0·26 |
| Duration of stay in the ICU, days | 2 (1–2) | 2 (1–7) | 0·77 |
| Admitted to inpatient rehabilitation | 3 (7%) | 2 (6%) | 1·00 |
| Received outpatient therapies | 17 (40%) | 3 (10%) | 0·0035 |
| Treatments | |||
| Intravenous immunoglobulin | 14 (33%) | 4 (13%) | 0·052 |
| Steroids | 2 (5%) | 1 (3%) | 0·76 |
| Anti-epileptics | 3 (7%) | 4 (13%) | 0·36 |
| Intubation | 0 | 2 (6%) | 0·091 |
| Supplemental feeding | 4 (9%) | 2 (6%) | 0·66 |
| Outcomes | |||
| Full recovery at discharge | 20 (47%) | 19 (61%) | 0·21 |
| Full recovery at 1–2 months of follow-up | 39 (93%)* | 24 (77%) | 0·26 |
| Death | 0 | 1 (3%)† | 1·00 |
Data are n (%) or median (IQR). ICU=intensive care unit. *One child with a residual tremor at discharge was lost to follow-up, so percentage is of 42 patients. †A neonate with echovirus 11 sepsis with multiorgan system involvement, who died at age 6 days.
During the study period, an additional 31 cases of neurological disease associated with 11 enteroviruses other than EV-A71 were reported (tables 1-3). EV-A71 and other enteroviruses were most commonly identified in rectal specimens, but EV-A71 was less likely to be identified in cerebrospinal fluid than other enteroviruses (in eight [20%] of 40 specimens vs 20 [77%] of 26 specimens; p<0·0001). Fever was common in all children, but EV-A71 showed a longer median duration of fever (2·5 vs 1·0 days; p=0·0066), irritability (in 37 [86%] of 43 children vs nine [29%] of 31 children; p<0·0001), and presence of hand, foot, or mouth lesions (in 18 [42%] children vs six [19%] children; p=0·041) than other enteroviruses. Neurological signs (myoclonus, ataxia, and autonomic instability) and imaging findings, suggestive of encephalitis with brainstem and cerebellar involvement, best distinguished EV-A71 cases from other enterovirus-associated cases, and weakness was also more common in children with EV-A71. No children with EV-A71 developed non-cardiogenic pulmonary oedema or cardiopulmonary collapse, and there were no deaths of children with EV-A71 in our cohort; however, one neonate with echovirus 11 sepsis with multiorgan system involvement died at age 6 days.
Ten (23%) children with EV-A71 neurological disease met the Council of State and Territorial Epidemiologists case definition of acute flaccid myelitis (table 4). All were male and had cerebrospinal fluid pleocytosis suggestive of meningitis, and all but one had concomitant signs of encephalitis. Three of these ten children with EV-A71-associated acute flaccid myelitis were hypotonic at initial presentation, with most developing more generalised and symmetric hypotonia and weakness, including the trunk and extremities, later in the disease course. Urinary retention was observed in six of these children.
Table 4:
Subgroup analysis comparing children with enterovirus A71-associated vs enterovirus D68-associated acute flaccid myelitis presenting to Children’s Hospital Colorado, 2013–18
| Children with enterovirus A71-associated acute flaccid myelitis (n=10) |
Children with enterovirus D68-associated acute flaccid myelitis, 2013–18 (n=8) |
p value | |
|---|---|---|---|
| Demographics | |||
| Age, months | 19·3 (12·9–22·9) | 100·2 (41·6–152·6) | 0·034 |
| Sex | ·· | ·· | 0·18 |
| Female | 0 | 2 (25%) | ·· |
| Male | 10 (100%) | 6 (75%) | ·· |
| Prodromal illness | |||
| Fever | 10 (100%) | 7 (88%) | 0·44 |
| Irritability | 8 (80%) | 1 (13%) | 0·015 |
| Hand, foot, or mouth lesions | 6 (60%) | 0 | 0·013 |
| Respiratory illness | 3 (30%) | 8 (100%) | 0·0040 |
| Vomiting | 8 (80%) | 3 (38%) | 0·14 |
| Time from symptom onset to neurological onset, days | 1 (0–3) | 6 (3–6) | 0·011 |
| Associated neurological signs and symptoms | |||
| Cranial nerve dysfunction | 1 (10%) | 3 (38%) | 0·27 |
| Myoclonus | 9 (90%) | 0 | 0·00040 |
| Ataxia | 9 (90%) | 1 (13%) | 0·0029 |
| Autonomic instability | 3 (30%) | 1 (13%) | 0·59 |
| Urinary retention | 6 (60%) | 5 (63%) | 1·00 |
| Limb findings | |||
| Hyporeflexia or hypotonia at presentation | 3 (30%) | 6 (75%) | 0·15 |
| Number of limbs with weakness | 3 (1–4) | 3 (2–4) | 0·71 |
| Sensory findings | 0 | 1 (13%) | 0·44 |
| Laboratory results | |||
| Pleocytosis | 10 (100%) | 8 (100%) | 1·00 |
| White blood cell count, cells per μL | 125 (84–430) | 60 (49–87) | 0·093 |
| Enterovirus detected | |||
| Cerebrospinal fluid | 1/10 (10%) | 0/5 | 1·00 |
| Oropharyngeal specimen | 9/9 (100%) | 1/6 (17%) | 0·0020 |
| Nasopharyngeal specimen | 4/9 (44%) | 8/8 (100%) | 0·029 |
| Rectal specimen | 10/10 (100%) | 0/6 | <0·0001 |
| Magnetic resonance imaging | |||
| Supratentorial lesion | 3/10 (30%) | 1/7 (14%) | 0·60 |
| Cranial nerve enhancement | 4/10 (40%) | 1/7 (14%) | 0·34 |
| Cerebellar lesion | 8/10 (80%) | 3/7 (43%) | 0·16 |
| Brainstem lesion | 10/10 (100%) | 7/7 (100%) | 1·00 |
| Cervical spinal cord lesion | 10/10 (100%) | 7/8 (88%) | 0·44 |
| Thoracic spinal cord lesion | 5/9 (56%) | 7/8 (88%) | 0·29 |
| Lumbar spinal cord lesion | 4/9 (44%) | 6/8 (75%) | 0·33 |
| Spinal cord nerve root enhancement | 6/10 (60%) | 4/8 (50%) | 1·00 |
| Hospital treatment and outcomes | |||
| Intubation | 0 | 2 (25%) | 0·18 |
| Supplemental feeding support | 3 (30%) | 4 (50%) | 0·63 |
| Duration of stay, days | 6 (5–12) | 32 (11–59) | 0·023 |
| Full recovery of limb strength at 1–2 months of follow-up | 9 (90%) | 1 (13%) | 0·0029 |
Data are median (IQR), n (%), or n/N tested (%). Pleocytosis was defined as a white blood cell count of more than 10 white blood cells per μL. Increased protein was defined as concentrations of more than 40 mg/dL. There were no deaths in either group.
Longitudinal grey matter-predominant spinal cord lesions were present on MRI in 14 (74%) of 19 children with EV-A71 neurological disease who received imaging, including all children with acute flaccid myelitis, which affected the cervical (in 14 [74%] of 19 children imaged), thoracic (in eight [44%] of 18 children imaged), and lumbar regions (in seven [47%] of 15 children imaged), and these lesions were accompanied by nerve root enhancement in six (32%) of 19 children imaged. Notably, four (29%) of 14 children with abnormalities in the central grey matter of the cervical cord had no clinically documented weakness. An additional three children with EV-A71 neurological disease had weakness but did not receive imaging; therefore, these cases were not classified as acute flaccid myelitis.
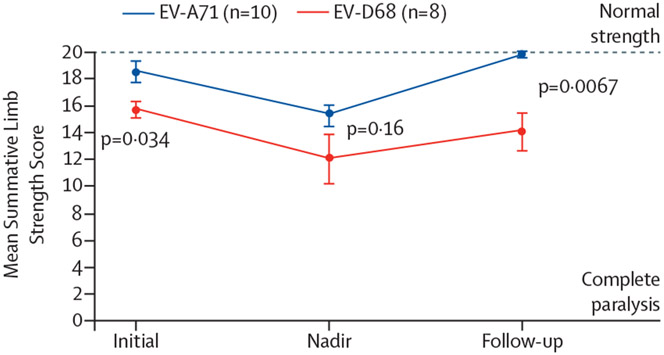
A comparison of children with EV-A71-associated acute flaccid myelitis during the study period (n=10) and children with EV-D68-associated acute flaccid myelitis presenting to Children’s Hospital Colorado between 2013 and 2018 (n=8) is shown in table 4. Enteroviruses were detected more frequently in rectal and oropharyngeal specimens in children with EV-A71-associated acute flaccid myelitis (in our cohort) and in nasopharyngeal specimens in children with EV-D68-associated acute flaccid myelitis (in the children presenting to Children’s Hospital Colorado between 2013 and 2018). Both groups showed low detection of enterovirus in cerebrospinal fluid. Compared with children with EV-D68-associated acute flaccid myelitis, children with EV-A71-associated acute flaccid myelitis were younger (aged 19 vs 100 months; p=0·034) and had neurological onset earlier after prodromal symptom onset (median 1 vs 6 days; p=0·011). The presence of hand, foot, or mouth lesions (in six of ten children with EV-A71 disease vs zero of eight children with EV-D68 disease; p=0·013) and irritability distinguished EV-A71-associated acute flaccid myelitis; whereas, in children with EV-D68-associated acute flaccid myelitis, prodromal respiratory symptoms were more uniformly noted across the groups (in three of ten children with EV-A71 vs eight of eight children with EV-D68; p=0·04). Notably, patterns of brainstem and spinal cord involvement on MRI were comparable in both groups. Compared with children with EV-D68-associated acute flaccid myelitis, those with EV-A71-associated acute flaccid myelitis had milder weakness at onset and improved strength at 1–2 months of follow-up (figure 3). Those with EV-A71-associated acute flaccid myelitis also had a shorter duration of hospital stay (median 6 vs 32 days; p=0·023) and greater proportion of these children showed full neurological recovery (nine [90%] of ten children vs one [13%] of eight children; p=0·0029).
Figure 3: Limb strength in children with acute flaccid myelitis associated with enterovirus A71 vs in those with acute flaccid myelitis associated with enterovirus D68.
Discussion
To our knowledge, this is the largest reported outbreak to date of EV-A71 neurological disease in the Americas. Children with EV-A71 in this outbreak were predominantly boys, with the clinically distinguishing findings of myoclonus, ataxia, weakness, and autonomic instability. Approximately half of this cohort had the clinical hallmark of hand, foot, or mouth lesions. Characteristic imaging findings included changes in the dorsal brainstem, dentate nuclei of the cerebellum, and grey matter of the spinal cord that were similar to previous outbreaks of EV-A71.17 We found no cases of non-cardiogenic pulmonary oedema, cardiopulmonary collapse, or death, as has been reported with previous EV-A71 outbreaks, and most children showed full recovery, despite most having evidence of encephalitis or myelitis.
Temporal and geographic clustering of young children presenting with a febrile illness associated with myoclonus, ataxia, and autonomic instability indicated a possible EV-A71 outbreak in our community. One factor provoking the initial outbreak investigation was an increase in enterovirus-associated neurological cases in the spring, before the expected late summer to early autumn circulation of enteroviruses in Colorado. The reasons for the geographically isolated outbreak and prolonged period of circulation, extending from spring into the late autumn, are unclear. Other notable epidemiological features of this outbreak included clustering of cases amongst children aged 4 years and younger in the Denver metropolitan area, with evidence of intraclassroom and intrahousehold spread. Outside of the paediatric cases included in this report, EV-A71 neurological disease was identified in two adults in the Denver metropolitan area during the outbreak period (unpublished data). Both cases occurred in adults who were immunocompromised, one of whom met Council of State and Territorial Epidemiologists criteria for confirmed acute flaccid myelitis and the other had probable acute flaccid myelitis; both had EV-A71 identified in cerebrospinal fluid.
Early recognition of the EV-A71 outbreak enabled educational outreach to help frontline providers to recognise the unique clinical features differentiating neurological disease due to EV-A71 from other clinical syndromes, to reinforce the value of timely specimen collection and enterovirus testing, and to standardise management strategies. These steps were important because some of the neurological symptoms due to EV-A71, such as myoclonus, ataxia, or autonomic dysfunction, could be easily misattributed to other diagnoses. For instance, myoclonus could prompt evaluation for seizures or be mistaken for benign infantile myoclonus; ataxia can be misdiagnosed as post-infectious cerebellitis, acute cerebellar ataxia, or suspected toxic ingestion; and autonomic dysfunction can present as irregular breathing patterns, which can be mistaken for Kussmaul respirations, prompting evaluation for diabetic ketoacidosis or toxic ingestion, as persistent tachycardia, prompting cardiology evaluation for myocarditis, or as skin mottling, prompting evaluation and therapy for presumed bacterial sepsis. When these symptoms present in the setting of a febrile illness, a heightened index of suspicion for neurological disease associated with EV-A71 is warranted to avoid delayed diagnosis.
The Colorado Department of Public Health and Environment conducted public health outreach via an immediate electronic public health notification network (Epi-X) to alert health departments in other states and three statewide Health Alert Network messages to inform Colorado health-care providers. The CDC did an investigation of enterovirus-related cases of neurological disease throughout the USA, finding no additional clusters of EV-A71 neurological disease (unpublished data).
Identification of children with EV-A71 in this outbreak relied upon viral detection from non-sterile site specimens, particularly rectal specimens, where EV-A71 can be detected for weeks to months after an infection.18 The low frequency of EV-A71 detection in the cerebrospinal fluid of children with neurological disease is similar to that of other outbreaks;4,7 however, this outbreak additionally highlights that EV-A71 might be more likely to be found in cerebrospinal fluid early in the course of disease, as evidenced by increased detection in cerebrospinal fluid from infants undergoing lumbar puncture shortly after fever onset. Although identification of enterovirus in cerebrospinal fluid is specific, inclusion of non-sterile site specimen collection and testing is essential to the sensitivity of enterovirus surveillance and neurological case identification.19 Further, nasopharyngeal and oropharyngeal specimens are commonly considered interchangeable for enterovirus detection; however, we found a high discrepancy of enterovirus testing results between these two sites in EV-A71 cases. Optimal enterovirus detection in neurological cases should include enterovirus PCR testing of cerebrospinal fluid, the rectum or stool, skin and oral lesions (when present), and nasopharyngeal and oropharyngeal specimens. Although not done in our cohort, increasing evidence suggests that blood might also be a high-yield specimen for enterovirus detection, particularly in neonates.20 Novel serological assays have detected enterovirus antibodies in cerebrospinal fluid from patients with acute flaccid myelitis, including during this outbreak, which provides additional supportive evidence and could become the diagnostic standard for enterovirus neurological infection in the absence of detected virus in cerebrospinal fluid.21
The high number of children showing full neurological recovery in our cohort is similar to that reported in EV-A71 outbreaks in Spain (in 2016)4 and China (in 2013),22 but differs from the long-term outcomes found in Australia (in 2013),3 Taiwan (in 1998–2003),23 and Colorado (in 2003 and 2005),7 where a higher prevalence of long-term paralysis due to myelitis, neurodevelopmental delays due to encephalitis, and mortality due to cardiopulmonary collapse have been noted. More severe disease in some outbreaks of EV-A71 might reflect a combination of previously identified factors, including a genetic predisposition among certain ethnic groups,24,25 differences in viral strains,26 and different treatment protocols.27,28 Children with brainstem encephalitis were selectively administered intravenous immunoglobulin if they showed signs of autonomic instability or acute flaccid myelitis; methylprednisolone was administered to those refractory to intravenous immunoglobulin and at least 2 days from symptom onset, similar to protocols used in previous outbreaks, with good outcomes.4 However, our uncontrolled observational cohort study does not allow systematic evaluation of response to therapy, and most children recovered regardless of treatment.
This outbreak demonstrates that EV-A71 can cause clusters of acute flaccid myelitis that are clinically distinguishable from EV-D68-associated acute flaccid myelitis.29 Hand, foot, or mouth lesions were only found in children with EV-A71-associated acute flaccid myelitis and not those with EV-D68-associated acute flaccid myelitis, whereas preceding respiratory symptoms were more characteristic of EV-D68-associated acute flaccid myelitis. EV-A71-associated acute flaccid myelitis was initially difficult to diagnose because most children presented within a day of illness onset with myoclonus, ataxia, and irritability before developing weakness; whereas children with EV-D68-associated acute flaccid myelitis presented with limb weakness at the time of neurological onset without these associated findings. By contrast with EV-D68-associated acute flaccid myelitis, the weakness that ultimately developed with EV-A71 was more generalised and more symmetrical, often involving the trunk and proximal extremities, despite similar spinal cord imaging findings in patients in both groups.30 The weakness in EV-A71-associated acute flaccid myelitis fully resolved in all but one patient: an older child with persistent asymmetrical upper limb weakness similar to cases of EV-D68-associated acute flaccid myelitis. The frequency of full neurological recovery in EV-A71-associated acute flaccid myelitis cases from this outbreak was much higher than in some previously described cases of EV-A71-associated acute flaccid myelitis31 and of EV-D68-associated acute flaccid myelitis.32 Notably, during this outbreak, we detected EV-A71 in some children with acute flaccid myelitis and EV-D68 in others, underscoring that several non-polio enteroviruses that likely cause acute flaccid myelitis can circulate concurrently but have differentiating clinical presentations and outcomes.
Limitations of our outbreak investigation include that no standardised comprehensive laboratory and imaging evaluation approaches were used; testing was based on the clinical discretion of treating providers, likely biasing results towards more severe presentations. Only children meeting the confirmed acute flaccid myelitis case definition were classified as having acute flaccid myelitis, which excluded potential cases of acute flaccid myelitis with weakness that we speculate would have had characteristic lesions had MRI been performed. Follow-up was restricted to documented neurological examinations at follow-up visits after 1–2 months without standardised neurocognitive or functional assessments; therefore, subtle longer term neurological sequelae cannot be ruled out. Cases were presumed to be caused by enterovirus infection if an enterovirus was identified by VP1 sequencing in any specimen; however, enteroviruses are ubiquitous and can be shed from non-sterile sites without being the cause of disease. Additionally, the case definition for EV-A71 neurological disease included all children who had EV-A71 identified in any specimen, allowing the potential for including co-infections in this group, as evidenced by one enterovirus coinfection (EV-A71 detected in an oropharyngeal specimen with EV-D68 detected in nasopharyngeal and rectal specimens). Because we did several statistical comparisons between findings in the different groups, p values should be interpreted accordingly.
In conclusion, despite low-level seasonal endemic EV-A71 circulation in the USA since the 1980s and previous small sporadic outbreaks, including in Colorado,7 EV-A71 has not previously been reported to cause an outbreak of this size or duration in the USA. However, since the 1990s in the Asia–Pacific region, EV-A71 has continued to cause large epidemics of hand, foot, and mouth disease, and neurological disease every 1–3 years, which has prompted the development of EV-A71 vaccines.33 These cyclical patterns of enterovirus circulation are driven by serotype-specific immunity and the growth of susceptible birth cohorts,34 with sudden shifts in dynamics due to changes in viral properties; however, why similar patterns have not been seen with EV-A71 to date in the USA is unclear. Enhanced clinical and laboratory surveillance for enterovirus neurological disease, including non-sterile site testing, is necessary to determine whether the 2018 Colorado EV-A71 outbreak is a sporadic, isolated incident or foreboding of impending cycles of neurological disease associated with EV-A71 in the USA.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for relevant articles published in English between Jan 1, 1950, and April 1, 2019, with the search terms “(‘enterovirus 71’ OR ‘enterovirus A71’) AND (‘neurologic’ OR ‘meningitis’ OR ‘encephalitis’ OR ‘myelitis’)”. Enterovirus A71 (EV-A71) is a non-polio enterovirus that has caused large-scale, cyclical epidemics of neurological disease (every 1–3 years) in the Asia–Pacific region. Outbreaks outside of this region are sporadic and difficult to predict, most recently occurring in Australia, Spain, France, and Germany. EV-A71 remains uncommon in the USA, with low-level endemic circulation and only small-scale, sporadic, geographically isolated outbreaks previously reported.
Added value of this study
To our knowledge, this is the largest reported outbreak of EV-A71 neurological disease in the Americas, affecting 43 children with meningitis, encephalitis, or acute flaccid myelitis (or a combination of these conditions) in Colorado, USA, between March and November, 2018. The clinical features that enabled identification of the outbreak and that distinguished neurological cases associated with EV-A71 versus other co-circulating enteroviruses included myoclonus, ataxia, weakness, and autonomic instability in the setting of a febrile illness. A greater proportion of children with EV-A71-associated acute flaccid myelitis had hand, foot, or mouth lesions, concomitant signs of brainstem encephalitis and cerebellitis, milder and more generalised weakness, and more rapid and complete recovery than those with enterovirus D68-associated acute flaccid myelitis.
Implications of all the available evidence
Whether this geographically and temporally isolated outbreak of enterovirus was a sporadic event or forebodes impending outbreaks of EV-A71 neurological disease in the USA is unclear. Surveillance for enterovirus-associated neurological disease should include timely collection and testing of non-sterile and sterile site specimens to enable early detection of EV-A71 neurological disease.
Acknowledgments
We thank Kristen Pretty and the Children’s Hospital Colorado clinical microbiology laboratory for their assistance with diagnostic testing, and Brian Emery and Shur-Wern Chern at the Centers for Disease Control and Prevention (CDC) Picornavirus Laboratory for enterovirus sequencing. We also thank Christina Castro at the CDC for sequence annotation and submission to the GenBank sequence database. The findings and conclusions in this report are solely those of the authors and do not necessarily represent the official position of the National Institutes of Health or the CDC.
Footnotes
Declaration of interests
KM reports a grant from the US National Institutes of Health and the National Institute of Allergy and Infectious Diseases, during the conduct of the study. ES-D reports grants from the US Centers for Disease Control and Prevention (CDC) during the conduct of the study. MSO and WAN hold a patent for nucleic acid molecules and kits (including VP1 and VP3) for detecting and identifying enteroviruses (US patent number 7,714,122) and a patent for detecting and identifying enteroviruses by semi-nested amplification of enterovirus VP1 protein (number 7,247,457). RH reports grants from the CDC during the conduct of the study. SRD reports grants from BioFire Diagnostics and from Pfizer, and personal fees from BioFire Diagnostics, outside the submitted work. All other authors declare no competing interests.
Contributor Information
Kevin Messacar, Section of Infectious Diseases, School of Medicine, University of Colorado, Aurora, CO, USA; Children’s Hospital Colorado, Aurora, CO, USA.
Emily Spence-Davizon, Colorado Department of Public Health and the Environment, Denver, CO, USA.
Christina Osborne, Section of Infectious Diseases, School of Medicine, University of Colorado, Aurora, CO, USA; Children’s Hospital Colorado, Aurora, CO, USA.
Craig Press, Section of Child Neurology, School of Medicine, University of Colorado, Aurora, CO, USA; Children’s Hospital Colorado, Aurora, CO, USA.
Teri L Schreiner, Section of Child Neurology, School of Medicine, University of Colorado, Aurora, CO, USA; Children’s Hospital Colorado, Aurora, CO, USA.
Jan Martin, Section of Child Neurology, School of Medicine, University of Colorado, Aurora, CO, USA; Children’s Hospital Colorado, Aurora, CO, USA.
Ricka Messer, Section of Child Neurology, School of Medicine, University of Colorado, Aurora, CO, USA; Children’s Hospital Colorado, Aurora, CO, USA.
John Maloney, Section of Radiology, School of Medicine, University of Colorado, Aurora, CO, USA; Children’s Hospital Colorado, Aurora, CO, USA.
Alexis Burakoff, Colorado Department of Public Health and the Environment, Denver, CO, USA; Centers for Disease Control and Prevention, Atlanta, GA, USA.
Meghan Barnes, Colorado Department of Public Health and the Environment, Denver, CO, USA.
Shannon Rogers, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Adriana S Lopez, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Janell Routh, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Susan I Gerber, Centers for Disease Control and Prevention, Atlanta, GA, USA.
M Steven Oberste, Centers for Disease Control and Prevention, Atlanta, GA, USA.
W Allan Nix, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Mark J Abzug, Section of Infectious Diseases, School of Medicine, University of Colorado, Aurora, CO, USA; Children’s Hospital Colorado, Aurora, CO, USA.
Kenneth L Tyler, Department of Pediatrics and Department of Neurology, School of Medicine, University of Colorado, Aurora, CO, USA.
Rachel Herlihy, Colorado Department of Public Health and the Environment, Denver, CO, USA.
Samuel R Dominguez, Section of Infectious Diseases, School of Medicine, University of Colorado, Aurora, CO, USA; Children’s Hospital Colorado, Aurora, CO, USA.
Data sharing
Individual participant data will not be made available.
References
- 1.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 2010; 9: 1097–105. [DOI] [PubMed] [Google Scholar]
- 2.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10: 778–90. [DOI] [PubMed] [Google Scholar]
- 3.Teoh HL, Mohammad SS, Britton PN, et al. Clinical characteristics and functional motor outcomes of enterovirus 71 neurological disease in children. JAMA Neurol 2016; 73: 300–07. [DOI] [PubMed] [Google Scholar]
- 4.Casas-Alba D, de Sevilla MF, Valero-Rello A, et al. Outbreak of brainstem encephalitis associated with enterovirus-A71 in Catalonia, Spain (2016): a clinical observational study in a children’s reference centre in Catalonia. Clin Microbiol Infect 2017; 23: 874–81. [DOI] [PubMed] [Google Scholar]
- 5.Ngangas ST, Lukashev A, Jugie G, et al. Multirecombinant enterovirus A71 subgenogroup C1 Isolates associated with neurologic disease, France, 2016–2017. Emerg Infect Dis 2019; 25: 1204–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Böttcher S, Diedrich S, Keeren K. Increased detection of enterovirus A71 infections, Germany, 2019. Euro Surveill 2019; 24: 1900556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Vélez CM, Anderson MS, Robinson CC, et al. Outbreak of neurologic enterovirus type 71 disease: a diagnostic challenge. Clin Infect Dis 2007; 45: 950–57. [DOI] [PubMed] [Google Scholar]
- 8.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 2006; 55: 1–20. [PubMed] [Google Scholar]
- 9.Abedi GR, Watson JT, Pham H, Nix WA, Oberste MS, Gerber SI. Enterovirus and human parechovirus surveillance—United States, 2009–2013. MMWR Morb Mortal Wkly Rep 2015; 64: 940–43. [DOI] [PubMed] [Google Scholar]
- 10.Abedi GR, Watson JT, Nix WA, Oberste MS, Gerber SI. Enterovirus and parechovirus surveillance—United States, 2014–2016. MMWR Morb Mortal Wkly Rep 2018; 67: 515–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Council of State and Territorial Epidemiologists. Standardized case definition for acute flaccid myelitis. 2015. http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/2015PS/2015PSFinal/15-ID-01.pdf (accessed Oct 30, 2015).
- 12.Messacar K, Sillau S, Hopkins SE, et al. Safety, tolerability, and efficacy of fluoxetine as an antiviral for acute flaccid myelitis. Neurology 2019; 92: e2118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kost CB, Rogers B, Oberste MS, et al. Multicenter beta trial of the GeneXpert enterovirus assay. J Clin Microbiol 2007; 45: 1081–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leber AL, Everhart K, Balada-Llasat JM, et al. Multicenter evaluation of BioFire FilmArray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol 2016; 54: 2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poritz MA, Blaschke AJ, Byington CL, et al. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS One 2011; 6: e26047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol 2006; 44: 2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Chen F, Liu T, Wang L. MRI findings of neurological complications in hand-foot-mouth disease by enterovirus 71 infection. Int J Neurosci 2012; 122: 338–44. [DOI] [PubMed] [Google Scholar]
- 18.Chung PW, Huang YC, Chang LY, Lin TY, Ning HC. Duration of enterovirus shedding in stool. J Microbiol Immunol Infect 2001; 34: 167–70. [PubMed] [Google Scholar]
- 19.Harvala H, Broberg E, Benschop K, et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J Clin Virol 2018; 101: 11–17. [DOI] [PubMed] [Google Scholar]
- 20.Messacar K, Dominguez SR. Blood PCR testing for enteroviruses in young children. Lancet Infect Dis 2018; 18: 1299–301. [DOI] [PubMed] [Google Scholar]
- 21.Schubert RD, Hawes I, Ramachandran PS, et al. Pan-viral serology implicates enteroviruses in acute flaccid myelitis. Nat Med 2019; 25: 1748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Jiang L, Peng HL. Clinical analysis of 134 children with nervous system damage caused by enterovirus 71 infection. Pediatr Infect Dis J 2015; 34: 718–23. [DOI] [PubMed] [Google Scholar]
- 23.Chang LY, Huang LM, Gau SS, et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med 2007; 356: 1226–34. [DOI] [PubMed] [Google Scholar]
- 24.Chang LY, Chang IS, Chen WJ, et al. HLA-A33 is associated with susceptibility to enterovirus 71 infection. Pediatrics 2008; 122: 1271–76. [DOI] [PubMed] [Google Scholar]
- 25.Yen TY, Shih WL, Huang YC, Lee JT, Huang LM, Chang LY. Polymorphisms in enterovirus 71 receptors associated with susceptibility and clinical severity. PLoS One 2018; 13: e0206769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Wu X, Huang K, et al. The variations of VP1 protein might be associated with nervous system symptoms caused by enterovirus 71 infection. BMC Infect Dis 2014; 14: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, MacDonald NE, Smith JC, et al. Severe enterovirus type 71 nervous system infections in children in the Shanghai region of China: clinical manifestations and implications for prevention and care. Pediatr Infect Dis J 2014; 33: 482–87. [DOI] [PubMed] [Google Scholar]
- 28.Zhang G, Wang J, Yao G, Shi B. Efficacy of high-dose methylprednisolone pulse therapy in the treatment of enterovirus 71 encephalitis. Pak J Pharm Sci 2016; 29 (suppl): 1421–27. [PubMed] [Google Scholar]
- 29.Messacar K, Asturias EJ, Hixon AM, et al. Enterovirus D68 and acute flaccid myelitis—evaluating the evidence for causality. Lancet Infect Dis 2018; 18: e239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maloney JA, Mirsky DM, Messacar K, Dominguez SR, Schreiner T, Stence NV. MRI findings in children with acute flaccid paralysis and cranial nerve dysfunction occurring during the 2014 enterovirus D68 outbreak. AJNR Am J Neuroradiol 2015; 36: 245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HF, Chi CS. Enterovirus 71 infection-associated acute flaccid paralysis: a case series of long-term neurologic follow-up. J Child Neurol 2014; 29: 1283–90. [DOI] [PubMed] [Google Scholar]
- 32.Martin JA, Messacar K, Yang ML, et al. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology 2017; 89: 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong P, Liu CC, Chow YH, Chou AH, Klein M. Review of enterovirus 71 vaccines. Clin Infect Dis 2015; 60: 797–803. [DOI] [PubMed] [Google Scholar]
- 34.Pons-Salort M, Grassly NC. Serotype-specific immunity explains the incidence of diseases caused by human enteroviruses. Science 2018; 361: 800–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data will not be made available.