Abstract
Objective
Increased visit-to-visit blood pressure variability (vvBPV) has negative effects on multiple organ systems. Prior research has suggested that dihydropyridine calcium channel blockers (CCB) may reduce vvBPV, which we attempted to verify in a high-quality dataset with robust statistical methodology.
Methods
We performed a post-hoc analysis of the SPRINT trial and included participants who were on a dihydropyridine CCB either 0% or 100% of follow-up study visits. The primary outcome was vvBPV, defined as residual standard deviation (rSD) of systolic blood pressure from month 6 until study completion. We estimated the average treatment effect of the treated (ATET) after augmented inverse-probability-weighting (AIPW) matching.
Results
Of the 9,361 participants enrolled in SPRINT, we included 5,020, of whom 1,959 were on a dihydropyridine CCB and 3,061 were not; mean age was 67.4±9.2 years, 34.5% were male, 65.9% were white, 49.4% were randomized to intensive blood pressure control, and the rSD was 10.1±4.0 mm Hg. Amlodipine represented >95% of dihydropyridine CCB use. After AIPW matching of demographics and other antihypertensive medications, the ATET estimation for participants on a dihydropyridine CCB was an rSD that was 2.05 mm Hg lower (95% CI −3.19, −0.91). We did not find that other antihypertensive medications classes decreased vvBPV, and several increased it.
Conclusions
In the SPRINT trial, consistent use of a dihydropyridine CCB was associated with a 2 mm Hg reduction in vvBPV. The implication of this hypothesis-generating finding in a high-quality dataset is that future trials to reduce vvBPV could consider using dihydropyridine CCBs.
Keywords: Blood pressure variability, antihypertensive medication, hypertension
Introduction
Increased blood pressure variability (BPV) has negative effects on multiple organ systems, independent of the presence or severity of hypertension.[1–5] Studies have shown that increased visit-to-visit BPV (vvBPV) is associated with a higher risk of cardiovascular disease, stroke, and worse outcome after both.[6–10] Prior research has also demonstrated that patients with increased vvBPV are at elevated risk of all-cause mortality in disease states ranging from coronary artery disease to diabetes.[11–16] However, apart from a small trial,[17] there has not been prospective clinical research that attempted to lower BPV, either as a visit-to-visit or short-term outcome. In part, this is due to uncertainty if increased BPV is causal of worse outcome, but also because there is no proven medication strategy to reduce BPV.[18]
Several post-hoc analyses have explored the effect of antihypertensive medication class on vvBPV,[19–24] but the evidence from these analyses has limitations. The antihypertensive exposure is determined by randomization or self-reported medication use at a single point in time, the outcome of BPV is often captured at infrequent intervals with different measurement methodology, and the research is largely from studies that predate the intensive blood pressure lowering that has followed the SPRINT trial.[18,23,25] However, prior research, including a small clinical trial, has consistently shown an association between lower vvBPV and calcium channel blocker (CCB) antihypertensive medications, specifically dihydropyridine CCBs such as amlodipine.[17,20,24,26–28] To validate this finding, we performed a post-hoc analysis of the SPRINT trial in which we leveraged the granularity of data on medications and the standardized blood pressure measurement and management to fit a sophisticated treatment effects model that estimates the causal impact of dihydropyridine CCBs on vvBPV.
Methods
Study Population
We performed a post-hoc analysis of participants enrolled in the SPRINT trial, using a publicly available deidentified dataset supplied by the NHLBI.[29] We included participants who were on a dihydropyridine CCB either 0% or 100% of our cohort’s 101,006 study visits during follow-up. The dihydropyridine CCBs allowed in SPRINT included amlodipine and nifedipine, but amlodipine accounted for >95% of medication use in this class. We used blood pressures measured from month 6 until study completion, which created stability in the participant blood pressure following the initial interventions in the SPRINT trial (Supplemental Figure 1). The approach of excluding blood pressure from before month 6 mirrors that of Rothwell et al. in their analysis of the ASCOT-BPLA trial and Muntner et al. in their analysis of ALLHAT.[6,9] We excluded participants with less than 4 blood pressure measurements, to improve the accuracy of BPV measurement,[30,31] and also outlier participants whose BPV was >4 standard deviations above the mean.
Study Exposure, Outcome, and Covariates
The study exposure was dihydropyridine CCB use, which was a binary variable that reflected a participant either being on a dihydropyridine CCB during the entirety of follow-up versus at no point during follow-up. In SPRINT, trained study personnel recorded data on participants’ complete medication profiles at each study visit. The primary outcome was vvBPV, defined as the residual standard deviation (rSD) of the seated systolic blood pressure. rSD is calculated using the formula: , where BPi is the seated blood pressure average for an individual study visit and BPest is the expected blood pressure from a linear regression of blood pressure from all the patient’s visits. Prior research has suggested rSD is the best methodology to measure vvBPV, mainly because rSD is less influenced by blood pressure change over time.[32] The methodology of blood pressure measurement in SPRINT has been previously described,[29] but in our study we used one blood pressure per visit that was an average of three seated measurements.
As a sensitivity analysis, we: 1) defined the vvBPV as the standard deviation (SD) and average real variability (ARV) of the systolic blood pressure during follow-up and 2) used diastolic blood pressure to calculate vvBPV. SD and ARV were calculated using the formulas: ; . Covariates included the mean systolic blood pressure during follow-up, age, race (white, Black, other), sex, randomization arm, diabetes, atrial fibrillation, congestive heart failure, prior myocardial infarction, current smoking, alcohol use, percentage of time on other antihypertensive medication classes, and the interactions between all terms. The percentage of time on other antihypertensive classes reflected the proportion of the 101,006 study visits that participants were on the following other classes: angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, nonselective beta blocker, selective beta blocker, non-dihydropyridine calcium channel blocker, thiazide diuretic, loop diuretic, alpha blocker, aldosterone blocker, and other medications (hydralazine, nitrates).
Statistical Analysis
A common characteristic of all observational data is that treatment status is not randomized, which creates a scenario where outcomes and treatment are not necessarily independent. The purpose of treatment effect estimators is to utilize covariates to make outcome and treatment independent, allowing an estimation of the efficacy of treatments in observational data.[33] This in turn raises concerns related to proper model specification and balance of covariates on the treatment levels. To combat these obstacles and concerns, we used augmented inverse probability weighted (AIPW) estimators and least absolute shrinkage and selection operator (LASSO) techniques to estimate the average treatment effect on the treated (ATET).
AIPW is a combination of regression adjustment and inverse probability weighted estimators. AIPW estimators are doubly robust, which means that only one of the models (treatment or outcome) needs to be correctly specified to obtain accurate treatment effect estimates.[34,35] With the large number of functional forms and interactions in our dataset, LASSO is used for the model building phase of both the treatment and outcome models to pick a best-fit combination for each model.[36,37] AIPW and LASSO work together to abide by the two assumptions of treatment effect estimation: conditional independence and overlap assumptions. As such, it has been recommended that model selection techniques such as LASSO be combined with doubly robust estimators such as AIPW to accurately estimate the ATET.[33] To obtain a more accurate estimate of the ATET, we resampled 10 times and ran 10-fold cross-fit repetitions on each sample. Therefore, we effectively average the treatment effects of 100 ATET estimates to reduce the randomness of sample splitting on the estimated treatment effects.
To explore the effect of all antihypertensive medication classes in the cohort, we also fit a linear regression model to rSD and used a restrictive LASSO to determine covariates, which included age, sex, congestive heart failure, atrial fibrillation, smoking, and mean systolic blood pressure. We added the percentage of time on antihypertensive classes into this model and confirmed that multicollinearity was not excessive by determining the variance inflation factor (VIF) was <2 for any single term in the model and that the mean VIF was <2 for the model. We attempted to add the per-patient mean total number of antihypertensive medications during follow-up into the model, but it was highly collinear with multiple terms, including mean blood pressure and several medication classes, so was not included.
In an exploratory analysis intended to estimate the impact of lower vvBPV in our cohort, we fit a multivariable Cox model to the outcome of major adverse cardiovascular events (MACE). In this model, the exposure was rSD from 6 to 18 months during SPRINT, followed by outcome ascertainment from month 18 to the end of follow-up. For this sensitivity analysis, patients were excluded if they had the MACE outcome before month 18. This model was a priori adjusted for age, atrial fibrillation, prior myocardial infarction, smoking, and randomization arm. We confirmed the proportional hazards assumption of the Cox model by testing the Schoenfeld residuals. We fit additional Cox models, using the same approach and adjustments, to the outcomes of stroke and myocardial infarction during follow-up. After obtaining the hazard ratio point estimate for a 1 mm Hg shift in rSD from these models, we estimated the impact of being on a dihydropyridine CCB as the exponentiation of the hazard ratio by the ATET treatment effect (HRÂTET). This approach overcomes the inherent difficulty of directly including the dihydropyridine CCB term in the Cox model, which would introduce confounding by the exposure/outcome in respect to the ATET model. All analysis was performed in Stata 16.1 (StataCorp, College Station, TX).
Results
Of the 9,361 participants enrolled in SPRINT, we included 5,020 in our study. Participants were excluded for not being on a dihydropyridine CCB 0% or 100% of follow-up (n=3,894), having less than 4 systolic pressure measurements (n=413), missing demographic data (n=26), and having outlier vvBPV that was >4 standard deviations above the mean (n=8). Patient characteristics are seen in Table 1. The mean (SD) follow-up was 3.5 (0.8) years, number of blood pressures from month 6 to the end of follow-up was 15.3 (4.9), mean systolic blood pressure was 127.3 (10.2) mm Hg, baseline age was 67.4 (9.2) years, 34.5% were male, 66.0% were white, and 49.4% were randomized to the intensive blood pressure control arm. In our cohort of 5,020, there were 1,959 participants on a dihydropyridine CCB at all follow-up visits and 3,061 participants not on a dihydropyridine CCB at any time during follow-up. Among patients prescribed a dihydropyridine CCB, 95.8% were prescribed amlodipine. The baseline differences between participants in the dihydropyridine CCB stratification are seen in Table 1. The percentage of time spent on the other classes of antihypertensive medications after stratification by dihydropyridine CCBs is seen in Figure 1.
Table 1.
Participant demographics, stratified by on versus not on a dihydropyridine calcium channel blocker (D-CCB) during follow-up.
| Variable | On a D-CCB* (n=1,959) |
Not on a D-CCB* (n=3,061) |
p value |
|---|---|---|---|
| Age (years) | 67.2±9.4 | 67.5±9.1 | 0.243 |
| Male sex | 671 (34.3%) | 1,061 (34.7%) | 0.766 |
| Other | 56 (2.9%) | 120 (3.9%) | |
| Intensive blood pressure reduction trial arm | 1,395 (66.6%) | 1,174 (38.4%) | <0.001 |
| Diabetes | 26 (1.3%) | 55 (1.8%) | 0.198 |
| Atrial fibrillation | 110 (5.6%) | 302 (9.9%) | <0.001 |
| Congestive heart failure | 64 (3.3%) | 99 (3.2%) | 0.952 |
| Prior myocardial infarction | 134 (6.8%) | 272 (8.9%) | 0.010 |
| Smoking | 295 (15.1%) | 360 (11.8%) | 0.001 |
| Alcohol use | 1,240 (63.3%) | 2,079 (67.9%) | 0.001 |
| Follow-up (years) | 3.5±0.8 | 3.5±0.8 | 0.746 |
| Mean systolic blood pressure (mm Hg) | 126.8±10.4 | 127.6±10.0 | 0.016 |
| Systolic residual standard deviation (mm Hg) | 9.9±3.9 | 10.3±4.1 | <0.001 |
| Systolic standard deviation (mm Hg) | 10.0±3.9 | 10.4±4.0 | <0.001 |
| Systolic average real variability (mm Hg) | 11.1±4.7 | 11.7±5.0 | <0.001 |
On a D-CCB: patient was on a D-CCB during 100% of follow-up, Not on a D-CCB: patient was not on a D-CCB at any point during follow-up. ACE: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, NS-BB: nonselective beta blocker, S-BB: selective BB, ND-CCB: non-dihydropyridine calcium channel blocker, THZ: thiazide diuretic, DIU: loop diuretic, ALP: alpha blocker, ALD: aldosterone blocker, OTH: other medications (hydralazine, nitrates)
Figure 1.
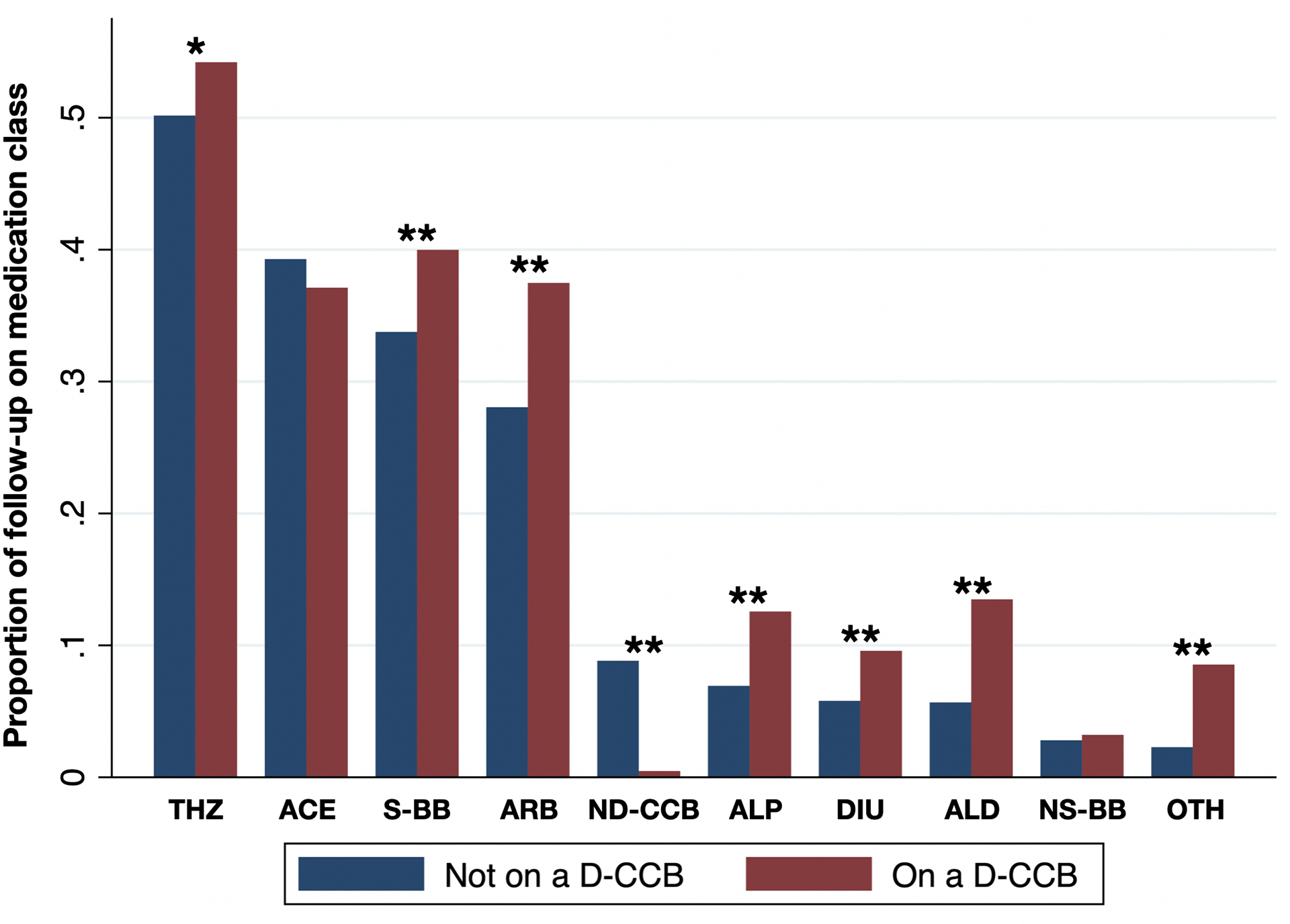
Proportion of time during follow-up on antihypertensive drug classes stratified by if patients were also on a dihydropyridine calcium channel blocker.
*: p<0.05, **: p<0.001, ACE: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, NS-BB: nonselective beta blocker, S-BB: selective BB, ND-CCB: non-dihydropyridine calcium channel blocker, THZ: thiazide diuretic, DIU: loop diuretic, ALP: alpha blocker, ALD: aldosterone blocker, OTH: other medications (hydralazine, nitrates)
The mean (SD) of rSD was 10.1 (4.0) mm Hg with a range of 0.6–26.6 mm Hg. We confirmed that the distribution of rSD was normal (Figure 2). In the linear regression model fit to rSD, with all classes of antihypertensive medication in the model (mean VIF=1.25), only dihydropyridine CCBs were associated with a reduction in vvBPV, and on average the other classes increased vvBPV (Table 2). The largest increases in rSD were for ACE inhibitors, alpha blockers, and nonselective beta-blockers. Being on those classes for the entirety of follow-up was associated with a 1.30, 1.23, and 2.51 mm Hg increase in rSD, respectively (all p<0.001), while being on a dihydropyridine CCB was associated with a 0.73 mm Hg reduction in rSD (95% CI −0.95, −0.51).
Figure 2.
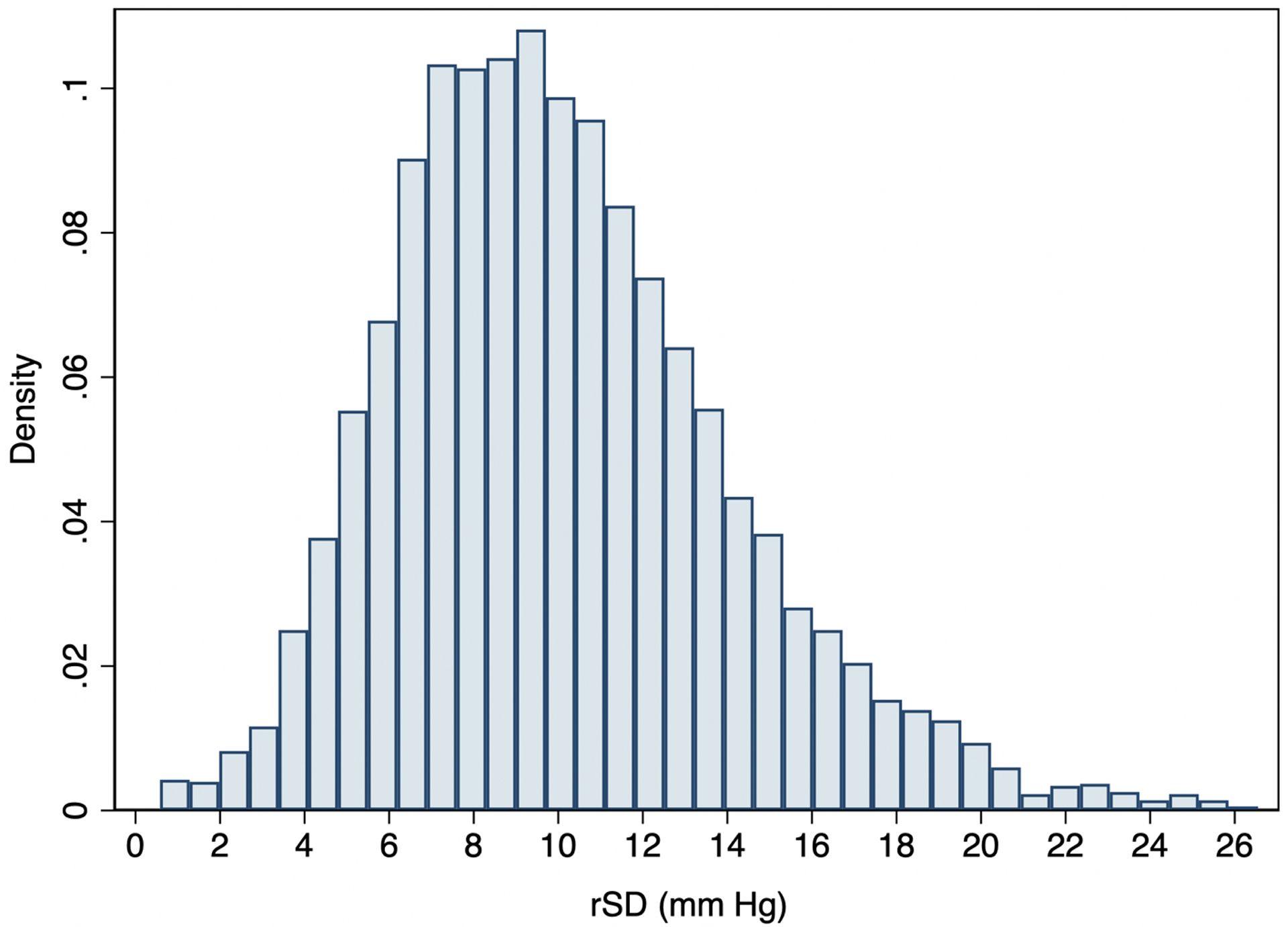
Distribution of residual standard deviation (rSD) values in our cohort.
*rSD is calculated with blood pressure from month 6 visit to the end of follow-up, which excluded the trends in blood pressure during the first 6 months of the study intervention.
Table 2.
Multivariable linear regression model of all antihypertensive classes showing the adjusted effect on vvBPV during follow-up.
| Variable | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Age | 0.06 | 0.04, 0.07 | <0.001 |
| Smoking | 1.28 | 0.96, 1.59 | <0.001 |
| Atrial fibrillation | 0.39 | 0.01, 0.78 | 0.046 |
| Congestive heart failure | 0.96 | 0.37, 1.56 | 0.001 |
| Male sex | 1.10 | 0.88, 1.32 | <0.001 |
| Mean systolic blood pressure | 0.10 | 0.09, 0.11 | <0.001 |
| ACE | 1.30 | 1.03, 1.58 | <0.001 |
| ARB | 1.01 | 0.72, 1.29 | <0.001 |
| NS-BB | 2.51 | 1.82, 3.19 | <0.001 |
| S-BB | 0.97 | 0.73, 1.21 | <0.001 |
| ND-CCB | 0.13 | −0.36, 0.61 | 0.62 |
| D-CCB | −0.73 | −0.95, −0.51 | <0.001 |
| THZ | 0.25 | 0.01, 0.50 | 0.045 |
| DIU | 0.73 | 0.25, 1.21 | 0.003 |
| ALP | 1.23 | 0.83, 1.64 | <0.001 |
| ALD | 0.42 | −0.00, 0.84 | 0.051 |
| OTH | 1.55 | 0.97, 2.13 | <0.001 |
ACE: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, NS-BB: nonselective beta blocker, S-BB: selective BB, ND-CCB: non-dihydropyridine calcium channel blocker, D-CCB: dihydropyridine CCB, THZ: thiazide diuretic, DIU: loop diuretic, ALP: alpha blocker, ALD: aldosterone blocker, OTH: other medications (hydralazine, nitrates)
For the treatment effects model, Table 3 shows the covariates selected by LASSO. After IPW matching, there were 2,295 participants on a dihydropyridine CCB and 2,725 not on a dihydropyridine CCB. The overlap assumption was met in our model, as seen in Supplemental Figure 2. By the ATET estimation, participants on a dihydropyridine CCB had an rSD that was 2.05mm Hg lower (95% CI −3.19, −0.91) than matched participants who were not on a dihydropyridine CCB. In the sensitivity analysis, we found that the outcome of vvBPV measured as SD or ARV had a similar ATET point estimate for the effect of being on a dihydropyridine CCB. For SD, the reduction was 1.91 mm Hg (95% CI −2.79, −1.02) and for ARV the reduction was 2.17 mm Hg (95% CI −3.25, −1.09). There was also a significant reduction in vvBPV when diastolic blood pressure was used in place of systolic, shown in Table 4.
Table 3.
Covariates selected by LASSO for the models in the treatment effect analysis.
| Term | rSD(0) | rSD(1) | D-CCB |
|---|---|---|---|
| Mean systolic blood pressure | x | x | x |
| NS-BB | x | ||
| S-BB | x | ||
| DIU | x | x | |
| ND-CCB | x | ||
| ARB | x | ||
| Male#Mean systolic blood pressure | x | ||
| Male#ACE | x | x | |
| Randomization#Mean systolic blood pressure | x | x | |
| Randomization#ARB | x | x | |
| Male#ARB | x | x | |
| Diabetes#NS-BB | x | ||
| Diabetes#S-BB | x | ||
| Race#S-BB | x | ||
| Male#S-BB | x | ||
| Randomization#ALP | x | ||
| Male#ALP | x | ||
| Atrial fibrillation#ARB | x | ||
| Race#ARB | x | ||
| Male#ARB | x | ||
| Race#Mean systolic blood pressure | x | ||
| Prior myocardial infarction#ND-CCB | x | ||
| Congestive heart failure#ND-CCB | x | ||
| Prior myocardial infarction#ALP | x | ||
| Atrial fibrillation#ALD | x | ||
| Randomization#ALD | x |
For interaction terms with categorical or ordinal variables, the specific level of the interaction that was selected is not shown. ACE: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, NS-BB: nonselective beta blocker, S-BB: selective BB, ND-CCB: non-dihydropyridine calcium channel blocker, D-CCB: dihydropyridine CCB, DIU: loop diuretic, ALP: alpha blocker, ALD: aldosterone blocker, OTH: other medications (hydralazine, nitrates)
Table 4.
Treatment effect model (ATET) showing the reduction in visit-to-visit blood pressure variability (vvBPV) in participants on dihydropyridine calcium channel blockers.
| vvBPV measure | ATET | 95% CI | p value |
|---|---|---|---|
| Average real variability | −2.17 | −3.25, −1.09 | <0.001 |
| Average real variability | −0.53 | −0.84, −0.22 | 0.001 |
In the exploratory analysis, which modelled the impact of lower vvBPV on cardiovascular outcomes, we excluded 188 participants who had a MACE outcome during the exposure period, leaving 4,832 participants of which 212 (4.4%) had a MACE outcome during follow-up. This model met the proportional hazards assumption (p=0.15). After adjustment, a 1 mm Hg shift in rSD had a hazard ratio of 1.08 (95% CI 1.05, 1.12) for the MACE outcome, of 1.09 (95% CI 1.03, 1.16) for the stroke outcome, and 1.06 (95% CI 1.01, 1.12) for the myocardial infarction outcome. After exponentiation, the estimated effect of a 2.05 mm Hg BPV reduction, equivalent to the effect of being on a dihydropyridine CCB, would thus be a 17.1% risk reduction in MACE, 19.3% risk reduction in stroke, and 12.7% risk reduction in myocardial infarction.
Discussion
While treatment effects estimations are not a substitute for randomized clinical trials, they provide an approximation of the causal effect.[38,39] Leveraging this methodology and the detailed data available in the SPRINT trial, we show an association between treatment with a dihydropyridine CCB and a 2.05 mm Hg reduction in systolic vvBPV. A reduction of this magnitude is comparable to what was reported in a post-hoc analysis of the Anglo-Scandinavian Cardiac Outcomes Trial Blood Pressure Lowering Arm (ASCOT-BPLA) trial by Rothwell et al.[19] In that study, 9,228 patients received an atenolol-based regimen and 9,302 an amlodipine-based regimen. The vvBPV, which was also measured with rSD, was 12.2±5.1 in the atenolol group versus 10.0±4.3 mm Hg in the amlodipine group (p<0.001). The resulting 2.18 mm Hg reduction in vvBPV seen in ASCOT-BPLA is thus similar to our cohort, but the mean systolic blood pressure in ASCOT-BPLA trial was 141 mm Hg compared to 127 mm Hg in our cohort. This difference reflects the more intensive blood pressure reduction in SPRINT, and represents the current standard of care.[25]
Additional studies and two meta-analyses have consistently shown a reduction in vvBPV with CCB antihypertensives,[6,20,22,24,26–28,40] but they do not use treatment effects modelling and predate the more intensive blood pressure reduction in the SPRINT trial. For example, the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) contributes the most patients to the meta-analysis by Webb et al.,[40] but the mean systolic blood pressure at year 3 in that trial was 136 mm Hg.[41] Because the SPRINT trial represents a more contemporary and guideline-based approach to blood pressure reduction,[25] our analysis provides a useful addition to the existing literature. In addition, because we attempted to minimize confounding from imbalances between our study groups through AIPW and LASSO, we may have reduced bias in our estimation of the impact of adding a dihydropyridine CCB to an intensive blood pressure lowering regimen.
The Comparison of Blood Pressure Variability Between Losartan and Amlodipine in Essential Hypertension (COMPAS-BPV) trial was the first prospective randomized trial that attempted to reduce vvBPV.[17] In 144 participants with essential hypertension, 73 received an increasing dose of amlodipine/hydrochlorothiazide and 71 received losartan/hydrochlorothiazide. The ARV of systolic blood pressure during 6 months of follow-up was reduced by 1.5 mm Hg in the amlodipine group (9.1±3.4 vs. 10.6±4.3, p=0.002), but the medication uptitration in COMPAS-BPV continued until the 3-month point, which would have impacted vvBPV because the blood pressure was still trending down during a majority of the study. In addition, the small sample size limits the conclusions that can be drawn from COMPAS-BPV.
The effect on cardiovascular outcomes of lowering vvBPV 2 mm Hg is difficult to anticipate. In the largest analysis of vvBPV and cardiovascular outcomes, Muntner et al. used ALLHAT to show that being in the highest, compared to the lowest, quintile of SD was associated with lower rates of myocardial infarction, stroke, and cardiovascular death.[6] However, it would be inappropriate to extrapolate a 2 mm Hg vvBPV reduction to that analysis, because the individual patient starting point, non-linear risk of vvBPV, and differences between the cohorts would introduce considerable bias. In our exploratory analysis, we estimated a risk reduction of 17.1% for MACE outcomes with a 2.05 mm Hg reduction in vvBPV, although this analysis is only hypothesis generating. The question of how much vvBPV would have to be reduced to effectively prevent cardiovascular disease is pertinent, and 2 mm Hg may not result in a large enough risk reduction, particularly in a primary prevention trial. For future trials, it is possible that a dihydropyridine CCB would have to be combined with an adjunctive therapy that can also reduce vvBPV.[42–44]
The mechanism of vvBPV reduction induced by dihydropyridine CCBs is not known.[45] As in other datasets, in the SPRINT trial, amlodipine accounted for the vast majority (>95%) of dihydropyridine CCB use. One potential explanation for our finding is that the half-life of oral amlodipine is 40–60 hours,[46] which would allow for a very stable steady state of antihypertensive medication, despite occasional missed or mistimed doses. Amlodipine is inexpensive, effective at reducing mean blood pressure, has few drug-drug interactions, and is well tolerated.[47,48] SPRINT participants were on a variety of other classes of antihypertensive treatment, as seen in Figure 1, which lends our analysis an element of generalizability because the results reflect the addition of amlodipine to the assortment of antihypertensive medications required to achieve intensive blood pressure reduction.
Nonetheless, our study has several important limitations. It is a post-hoc analysis of a dataset that was not designed to answer this research question. Although we attempted to minimize the confounding effect of baseline differences between the exposure groups (e.g. a significantly higher rate of prior myocardial infarction and atrial fibrillation in the non-CCB group), we cannot exclude the possibility that our findings reflect these baseline imbalances. We do not have data on patient adherence to prescribed antihypertensive therapy, which could confound our exposure in participants assigned to the dihydropyridine CCB group, but this would be expected to bias towards the null. There are variable lengths of follow-up among participants, but that did not differ significantly between those on versus not on a dihydropyridine CCB. Finally, although the ATET model with AIPW and LASSO is a robust methodology to estimate treatment effect, there is inevitably unmeasured confounding that can only be addressed with a clinical trial.
Conclusion
In the SPRINT trial, consistent use of a dihydropyridine CCB, primarily amlodipine, was associated with a 2 mm Hg reduction in vvBPV in a treatment effects model with matched participants. While similar results have been reported in other datasets, we provide a causal estimate of the effect of adding a dihydropyridine CCB to a variety of other antihypertensive medications. The implication of this hypothesis-generating finding in a high-quality dataset is that future trials to reduce vvBPV could consider using dihydropyridine CCBs.
Supplementary Material
Supplemental Figure 1. Schematic of data collection periods for the study outcome and exposure.
Supplemental Figure 2. Estimated densities of the probability of getting a dihydropyridine calcium channel blocker after augmented inverse-probability-weighting.
Acknowledgements
We acknowledge NHLBI and the SPRINT investigators for making the trial’s dataset publicly available. This article was prepared using SPRINT Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of SPRINT or the NHLBI.
Sources of support
Dr. de Havenon reports NIH/NINDS funding (K23NS105924). Dr. de Havenon has received investigator initiated clinical research funding from Regeneron and AMAG pharmaceuticals. Dr. Sheth reports NIH/NINDS funding: U01NS106513, R01NS11072, R01NR018335, R03NS112859, U24NS107215, U24NS107136, and American Heart Association 17CSA33550004. Dr. Sheth reports funding from Biogen, Novartis, Bard, Hyperfine, Astrocyte, Alva Health, NControl, and is DSMB Chair for Zoll.
Footnotes
Previous presentations or publication of the work: None
References
- 1.Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, et al. Association of Systolic Blood Pressure Variability With Mortality, Coronary Heart Disease, Stroke, and Renal Disease. J Am Coll Cardiol 2016; 68:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hata J, Arima H, Rothwell PM, Woodward M, Zoungas S, Anderson C, et al. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation 2013; 128:1325–1334. [DOI] [PubMed] [Google Scholar]
- 3.Dai L, Song L, Li X, Yang Y, Zheng X, Wu Y, et al. Association of visit-to-visit blood pressure variability with the risk of all-cause mortality and cardiovascular events in general population. J Clin Hypertens 2018; 20:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muntner P, Levitan EB. Visit-to-visit variability of blood pressure: current knowledge and future research directions. Blood Press Monit 2013; 18:232–238. [DOI] [PubMed] [Google Scholar]
- 5.Noshad S, Mousavizadeh M, Mozafari M, Nakhjavani M, Esteghamati A. Visit-to-visit blood pressure variability is related to albuminuria variability and progression in patients with type 2 diabetes. J Hum Hypertens 2014; 28:37–43. [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure and mortality: A cohort study. Ann Intern Med 2015; 163:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manning LS, Rothwell PM, Potter JF, Robinson TG. Prognostic Significance of Short-Term Blood Pressure Variability in Acute Stroke Systematic Review. Stroke 2015; 46:2482–2490. [DOI] [PubMed] [Google Scholar]
- 8.de Havenon A, Stoddard G, Saini M, Wong K-H, Tirschwell D, Bath P, et al. Increased blood pressure variability after acute ischemic stroke increases the risk of death: A secondary analysis of the Virtual International Stroke Trial Archive. JRSM Cardiovasc Dis 2019; 8:2048004019856496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. The Lancet 2010; 375:895–905. [DOI] [PubMed] [Google Scholar]
- 10.de Havenon A, Majersik JJ, Stoddard G, Wong K-H, McNally JS, Smith AG, et al. Increased Blood Pressure Variability Contributes to Worse Outcome After Intracerebral Hemorrhage. Stroke 2018; 49:1981–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yano Y, Reis JP, Lewis CE, Sidney S, Pletcher MJ, Bibbins-Domingo K, et al. Association of Blood Pressure Patterns in Young Adulthood With Cardiovascular Disease and Mortality in Middle Age. JAMA Cardiol Published Online First: 22 January 2020. doi: 10.1001/jamacardio.2019.5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehlum MH, Liestøl K, Kjeldsen SE, Julius S, Hua TA, Rothwell PM, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J 2018; 39:2243–2251. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury EK, Nelson MR, Wing LMH, Jennings GLR, Beilin LJ, Reid CM, et al. Change in Blood Pressure Variability Among Treated Elderly Hypertensive Patients and Its Association With Mortality. J Am Heart Assoc 2019; 8:e012630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilo G, Dolan E, O’Brien E, Facchetti R, Soranna D, Zambon A, et al. The impact of systolic and diastolic blood pressure variability on mortality is age dependent: Data from the Dublin Outcome Study. Eur J Prev Cardiol 2020; 27:355–364. [DOI] [PubMed] [Google Scholar]
- 15.Basson MD, Klug MG, Hostetter JE, Wynne J. Visit-to-Visit Variability of Blood Pressure Is Associated With Hospitalization and Mortality in an Unselected Adult Population. Am J Hypertens 2018; 31:1113–1119. [DOI] [PubMed] [Google Scholar]
- 16.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertens Dallas Tex 1979 2000; 36:901–906. [DOI] [PubMed] [Google Scholar]
- 17.Lee J-W, Choi E, Son J-W, Youn YJ, Ahn S-G, Ahn M-S, et al. Comparison of Blood Pressure Variability Between Losartan and Amlodipine in Essential Hypertension (COMPAS-BPV). Am J Hypertens 2020; 33:748–755. [DOI] [PubMed] [Google Scholar]
- 18.Anastasios Kollias, Stergiou George S., Kyriakoulis Konstantinos G., Bilo Grzegorz, Parati Gianfranco. Treating Visit-to-Visit Blood Pressure Variability to Improve Prognosis. Hypertension 2017; 70:862–866. [DOI] [PubMed] [Google Scholar]
- 19.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Effects of β blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010; 9:469–480. [DOI] [PubMed] [Google Scholar]
- 20.Webb AJ, Rothwell PM. Effect of Dose and Combination of Antihypertensives on Interindividual Blood Pressure Variability: A Systematic Review. Stroke 2011; 42:2860–2865. [DOI] [PubMed] [Google Scholar]
- 21.Morgan TO, Anderson A. Different drug classes have variable effects on blood pressure depending on the time of day*: Am J Hypertens 2003; 16:46–50. [DOI] [PubMed] [Google Scholar]
- 22.Levi-Marpillat N, Macquin-Mavier I, Tropeano A-I, Parati G, Maison P. Antihypertensive drug classes have different effects on short-term blood pressure variability in essential hypertension. Hypertens Res 2014; 37:585–590. [DOI] [PubMed] [Google Scholar]
- 23.Webb AJS, Fischer U, Rothwell PM. Effects of - blocker selectivity on blood pressure variability and stroke: A systematic review. Neurology 2011; 77:731–737. [DOI] [PubMed] [Google Scholar]
- 24.Wang J-G, Yan P, Jeffers BW. Effects of amlodipine and other classes of antihypertensive drugs on long-term blood pressure variability: Evidence from randomized controlled trials. J Am Soc Hypertens 2014; 8:340–349. [DOI] [PubMed] [Google Scholar]
- 25.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi R, Liu K, Shi D, Liu Q, Chen X. Effects of Amlodipine and Valsartan on Blood Pressure Variability and Pulse Wave Velocity in Hypertensive Patients. Am J Med Sci 2017; 353:6–11. [DOI] [PubMed] [Google Scholar]
- 27.Umemoto S, Ogihara T, Matsuzaki M, Rakugi H, Ohashi Y, Saruta T. Effects of calcium channel blocker-based combinations on intra-individual blood pressure variability: post hoc analysis of the COPE trial. Hypertens Res 2016; 39:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Agnoletti D, Safar ME, Blacher J. Effect of antihypertensive agents on blood pressure variability: the Natrilix SR versus candesartan and amlodipine in the reduction of systolic blood pressure in hypertensive patients (X-CELLENT) study. Hypertens Dallas Tex 1979 2011; 58:155–160. [DOI] [PubMed] [Google Scholar]
- 29.SPRINT Research Group Wright JT, Williamson JD Whelton PK, Snyder JK Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mena LJ, Maestre GE, Hansen TW, Thijs L, Liu Y, Boggia J, et al. How many measurements are needed to estimate blood pressure variability without loss of prognostic information? Am J Hypertens 2014; 27:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim HM, Chia YC, Ching SM, Chinna K. Number of blood pressure measurements needed to estimate long-term visit-to-visit systolic blood pressure variability for predicting cardiovascular risk: a 10-year retrospective cohort study in a primary care clinic in Malaysia. BMJ Open 2019; 9:e025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano Y Visit-to-Visit Blood Pressure Variability—What is the current challenge? Am J Hypertens 2017; 30:112–114. [DOI] [PubMed] [Google Scholar]
- 33.Farrell MH. Robust inference on average treatment effects with possibly more covariates than observations. J Econom 2015; 189:1–23. [Google Scholar]
- 34.Rosenbaum PR, Rubin DB. Reducing Bias in Observational Studies Using Subclassification on the Propensity Score. J Am Stat Assoc 1984; 79:516–524. [Google Scholar]
- 35.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 36.Koch B, Vock DM, Wolfson J. Covariate selection with group lasso and doubly robust estimation of causal effects. Biometrics 2018; 74:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tibshirani R Regression shrinkage and selection via the lasso: a retrospective. J R Stat Soc Ser B Stat Methodol 2011; 73:273–282. [Google Scholar]
- 38.John ER, Abrams KR, Brightling CE, Sheehan NA. Assessing causal treatment effect estimation when using large observational datasets. BMC Med Res Methodol 2019; 19:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevenson M, Gomersall T, Jones ML, Rawdin A, Hernández M, Dias S, et al. Review of observational studies: estimating mortality differences between treatment for vertebral compression fractures. NIHR Journals Library; 2014. https://www.ncbi.nlm.nih.gov/books/NBK261732/ (accessed 26 Apr2021). [Google Scholar]
- 40.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. The Lancet 2010; 375:906–915. [DOI] [PubMed] [Google Scholar]
- 41.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 42.Kazuomi Kario, Kim Byeong-Keuk Aoki Jiro, Wong Anthony Yiu-tung Lee Ying-Hsiang, Nattawut Wongpraparut, et al. Renal Denervation in Asia. Hypertension 2020; 75:590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persu A, Gordin D, Jacobs L, Thijs L, Bots ML, Spiering W, et al. Blood pressure response to renal denervation is correlated with baseline blood pressure variability: a patient-level meta-analysis. J Hypertens 2018; 36:221–229. [DOI] [PubMed] [Google Scholar]
- 44.Zuern CS, Rizas KD, Eick C, Stoleriu C, Bunk L, Barthel P, et al. Effects of Renal Sympathetic Denervation on 24-hour Blood Pressure Variability. Front Physiol 2012; 3. doi: 10.3389/fphys.2012.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nardin C, Rattazzi M, Pauletto P. Blood Pressure Variability and Therapeutic Implications in Hypertension and Cardiovascular Diseases. High Blood Press Cardiovasc Prev 2019; 26:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abernethy DR. Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology 1992; 80 Suppl 1:31–36. [DOI] [PubMed] [Google Scholar]
- 47.Taylor SH, Chen MF, Lee SJ, Koanantakul B, Zhu JR, Santoso T, et al. Efficacy and tolerability of amlodipine in the general practice treatment of essential hypertension in an asian multinational population. Clin Drug Investig 1998; 16:177–185. [DOI] [PubMed] [Google Scholar]
- 48.Perna GP, Stanislao M, De Luca G. Tolerability of Amlodipine. Clin Drug Investig 1997; 13:163–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Schematic of data collection periods for the study outcome and exposure.
Supplemental Figure 2. Estimated densities of the probability of getting a dihydropyridine calcium channel blocker after augmented inverse-probability-weighting.


