Abstract
Background/objectives:
Despite the pervasiveness of late effects in childhood cancer survivors, many parents feel inadequately informed about their child’s risks. We assessed early parental knowledge of risks of late effects and predictors of increased knowledge.
Design/methods:
Parents of children receiving cancer treatment at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center were surveyed about their knowledge of their child’s likelihood of eight late effects. Individual risk for each late effect (yes/no) was assessed using the Children’s Oncology Group’s Long-Term Follow-Up Guidelines v5 as a reference. Descriptive statistics were used to summarize knowledge scores; ordinal logistic regression was used to identify predictors of higher knowledge.
Results:
Of 96 parent participants, 11 (11.46%) correctly identified all of their child’s risks for the eight late effects. Five of eight was the median number of correctly identified late effect risks. Among 21 parents whose children were at risk for ototoxicity, 95% correctly identified this risk. Conversely, parents of at-risk children were less knowledgeable about risks of secondary malignancy (63% correct identification, of N = 94 at risk), cardiac toxicity (61%; N = 71), neurocognitive impairment (56%; N = 63), and infertility (28%; N = 61). Ordinal logistic regression analysis identified no significant differences in parental knowledge of late effect risks by any factors evaluated.
Conclusions:
Gaps in parental knowledge of potential late effects of childhood cancer treatment emerge early in a child’s care, and parents are more knowledgeable about some late effects, such as ototoxicity, than others, such as infertility. As no child- or parent-specific factors were associated with increased knowledge of late effect risks, interventions must be applied broadly.
Keywords: health care communication, late effects, parental knowledge, pediatric cancer, survivorship
1 |. INTRODUCTION
Pediatric cancer survival rates in the United States have improved significantly since the 1970s, resulting in a growing number of childhood cancer survivors.1 Unfortunately, the therapeutic advances that contributed to this improvement in survival are associated with long-term health problems. The vast majority of childhood cancer survivors experience at least one chronic medical condition, or late effect, as a result of their cancer or its treatment.2–4 Long-term follow-up studies have established clear links between specific cancer treatments and particular late effects, such as anthracyclines and cardiac toxicity.5 Awareness of these causal relationships has helped identify those at greatest risk of late effects, allowing for more tailored survivorship screening and preventive care.
However, communicating late effect risks with patients and families has proved challenging. Prior efforts to evaluate and enhance knowledge of late effects have largely focused on communication during survivorship, when many late effects occur. Yet childhood cancer survivors consistently underestimate their risks of late effects, and many survivors do not attend survivorship clinics where they can receive risk-based information, screening, and care.6–14 Importantly, parents rather than patients themselves, typically serve as their children’s treatment decision-makers and caretakers, and often as the repositories of information about their children’s diagnoses and treatment. Theoretically, increased parental knowledge of late effects may result in greater survivor knowledge, and thereby improve survivors’ engagement in risk-based screening. Additionally, parents express a desire for late effects information starting at diagnosis, in part as it informs their treatment decision-making.15 Yet parents also report feeling suboptimally prepared for long-term effects of treatment, starting during therapy and extending into survivorship.16 Parental knowledge of late effect risks is not well described, even though it may play a crucial role in survivors’ late effects knowledge and engagement in risk-based screening and care in survivorship.
In this study, we evaluated parental understanding of their children’s risks for late effects early in treatment as well as predictors of greater knowledge. We assessed overall knowledge of risks of late effects to determine parents’ understanding of their child’s overall risk profile. We also sought to understand parent knowledge of specific common late effects—infertility, neurocognitive impairment, cardiac toxicity, second malignancy, ototoxicity, pulmonary toxicity, osteonecrosis, and renal toxicity. We hypothesized that similar to survivors of childhood cancer, parents of children who are actively undergoing cancer therapy would underestimate their child’s overall risks of late effects but that this knowledge may vary by specific late effect.
2 |. METHODS
2.1 |. Study participants
Participants were parents of children with cancer who participated in a larger survey-based study investigating parent decision-making preferences and late effects awareness at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center between November 2016 and June 2018.17 Parents were eligible to participate if they spoke and read English and if their child was within a year of diagnosis, undergoing initial cancer-directed treatment, and ≤18 years old. Permission to approach each family was obtained from the child’s primary oncologist, and parents whose child was considered to have no realistic chance of cure were excluded in this study of long-term outcomes. Parents were approached in person at clinic visits or during hospital admissions, and one parent per child was invited to complete the survey on a computer tablet; $20 gift cards were provided as a thank you for participation. All parents who participated in this study had received and signed an informed consent document, which listed potential acute and long-term effects of their child’s chemotherapy per our institutional standard.
The institutional review board of the Dana-Farber Cancer Institute approved this study. A waiver of documentation of informed consent was obtained.
2.2 |. Survey instrument
Parents completed a survey that included previously utilized items or adapted items drawn from established questionnaires.17–23 Limited novel items were developed after literature review, employing general principles of survey design.24 The survey was pilot tested with 12 parents in the same setting as data collection to assess face and content validity and took approximately 15–20 minutes to complete.
The parent’s perception of their child’s risk of late effects was assessed using a modified checklist item,11 which asked parents to select from a comprehensive list, all late effects they believed their child was at risk of experiencing. Terms and descriptions for late effects were drawn from the Children’s Oncology Group’s Long-Term Follow-Up Guidelines (COG LTFU) parent information website and targeted toward an eighth-grade reading level.
The survey also assessed potential explanatory factors, which we hypothesized may be associated with parental knowledge such as the patient’s clinical characteristics, parent perceived prognosis,21 parent-reported experiences with late effects communication (receipt of late effects information, feelings of preparedness, worry, and distress),17,23 dispositional optimism (using the Life Orientation Test - revised25), and parent/child demographic information. Child attributes and household income were obtained on medical record review. Additional details of survey items, development, and pilot testing have been previously described elsewhere.23
2.3 |. Treatment data
Chart abstractions were conducted to collect comprehensive clinical information for each patient. Information regarding all planned treatment exposures at the time of survey completion was collected, including chemotherapy, radiation, surgery, and hematopoietic stem cell transplant (HSCT). Total planned doses of relevant chemotherapy, sites of radiation, and types of surgery were abstracted. The primary data source for treatment exposures was chemotherapy consent forms that had been signed prior to taking the survey. For surgery, radiation, and HSCT modalities, provider notes were used as a secondary data source to determine what had been communicated as an expected part of the treatment plan at the time of survey completion. Two research assistants (RAs) performed the abstractions, and training sets were completed to establish acceptable interrater reliability of three consecutive charts (97%; 326 fields in agreement/336 total fields) prior to data extraction.
A child was determined to be at risk (yes/no) for eight late effects (infertility, neurocognitive impairment, cardiac toxicity, second malignancy, ototoxicity, pulmonary toxicity, osteonecrosis, renal toxicity) by survivorship experts based on planned treatment exposures (chemotherapy, radiation, surgery) and using COG LTFU v5 as a reference. These late effects were selected as they are impactful, common across pediatric malignancies, and have well-characterized risks based on exposure.
2.4 |. Study outcomes
Parental knowledge of risks of late effects was assessed by comparing parents’ perceived risk for late effects against the late effect risk profile determined by treatment data. Parents were considered knowledgeable about a given late effect if they correctly identified their child as being either at risk or not at risk for the specified late effect. Caregivers were considered knowledgeable for a late effect if (a) their child was at risk for a specific late effect and they correctly recognized this risk, or (b) their child was not at risk and they did not consider their child at risk. Conversely, a parent was considered not knowledgeable if (a) they did not recognize a late effect that their child was at risk of experiencing, or (b) if they indicated their child was at risk for a late effect when, in fact, the child was not at risk. For the primary outcome, overall parental knowledge, one point was assigned for each correctly identified late effect risk, and an overall knowledge score was calculated by tabulating the percentage of the eight late effects about which the parent was knowledgeable, with possible scores ranging from 0% (zero of eight late effect risks correctly identified) to 100% (eight of eight late effect risks correctly identified). We also assessed parental knowledge of risks for each of the eight specific late effects individually.
2.5 |. Data analysis
Descriptive statistics were used to summarize knowledge scores by patient and parent characteristics. Ordinal logistic regression was used to identify predictors of greater knowledge; parents were categorized into three groups based on number of late effect risks correctly identified, low (0–4), moderate (5–6), and high (7–8) knowledge. These categorizations were deemed to be meaningful clinical cutoffs per literature review and expert opinion.7 This classification was utilized to account for both over- and under-estimation of late effect risk, and given the potential clinical impact of these specific late effects, knowledge or lack thereof of even two of eight of these late effects was felt to be clinically meaningful. Explanatory factors were dichotomized consistent with prior work, with prespecified cutoffs as shown in the tables and text.16 For each of the eight specific late effects assessed, Fisher’s exact test was used to evaluate the association between parental perceived risk of experiencing the late effect and accuracy of knowledge. Two-sided nominal p-values were reported. Tests with a false discovery rate (FDR) <5% were considered statistically significant after multiple testing adjustment using the Benjamini–Hochberg method.26 Descriptive and comparative analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
3 |. RESULTS
Out of 117 eligible parents who were approached, 96 completed the survey (82% response rate). Participating parents were predominantly female (74.0%), White (85.1%), and highly educated (76.05% with a college degree or higher) (Table 1).
TABLE 1.
Characteristics of participating parents and patients (N = 96)
| N (%) | |
|---|---|
| Parent age (years) | |
| ≤39 | 53 (55.2%) |
| >40 | 43 (44.8%) |
| Sex | |
| Female | 71 (74.0%) |
| Male | 25 (26.0%) |
| Race | |
| White | 80/94 (85.1%) |
| Black or African American | 3/94 (3.2%) |
| Asian American or Pacific Islander | 8/94 (8.5%) |
| Native American or other | 3/94 (3.2%) |
| Ethnicity | |
| Non-Hispanic | 86/95 (90.5%) |
| Hispanic | 9/95 (9.5%) |
| Language spoken at home | |
| English | 91 (94.8%) |
| Non-English | 5 (5.2%) |
| Household income | |
| Low income (≤$50,000) | 17/86 (19.8%) |
| High income (>$50,000) | 69/86 (80.2%) |
| Education | |
| Less than college graduate | 23 (14.6%) |
| College graduate | 45 (46.9%) |
| Graduate or professional school | 28 (29.2%) |
| Days from diagnosis to survey, median (range) | 139 (29,365) |
| Child sex | |
| Female | 53 (55.2%) |
| Male | 43 (44.8%) |
| Age at diagnosis | |
| 0–2 | 17 (17.7%) |
| 3–6 | 37 (38.5%) |
| 7–12 | 23 (24.0%) |
| 13–18 | 19 (19.8%) |
| Cancer diagnosis | |
| Hematologic malignancy | 53 (55.2%) |
| Extracranial solid tumor | 32 (33.3%) |
| Brain tumor | 11 (11.5%) |
| Treatment received a | |
| Chemotherapy | 95 (99.0%) |
| Radiation | 20 (20.8%) |
| Surgery | 37 (38.5%) |
| Hematopoietic stem cell transplant | 7 (7.3%) |
Categories are not mutually exclusive.
The mean overall knowledge score was 68.1% (SD = 18.8%) and the median was five of eight late effects correct. Eleven percent (11/96) of parents correctly identified their child’s risk for all eight late effects; no parents scored 0/8 or 1/8 (Figure 1).
FIGURE 1.
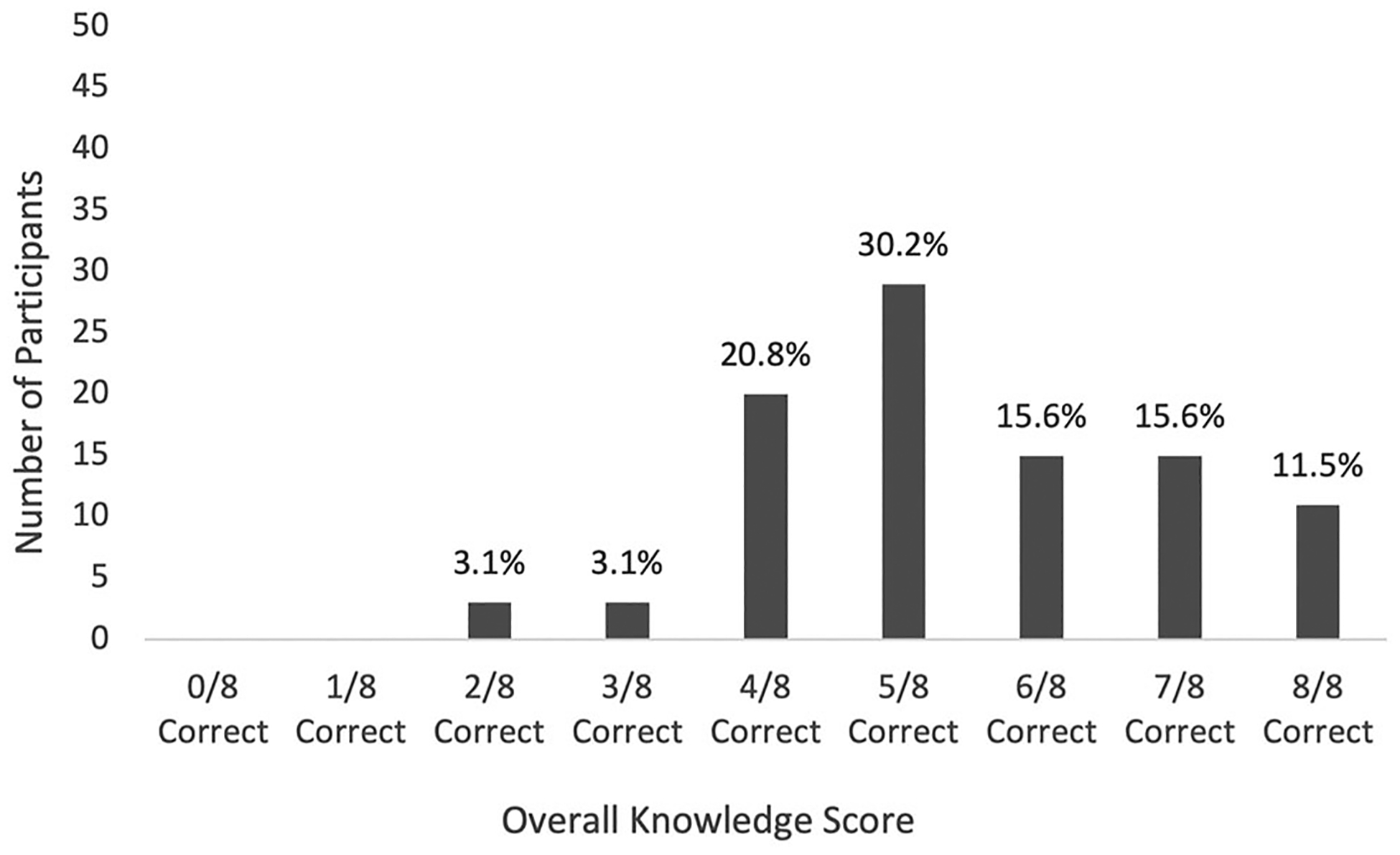
Overall parental knowledge scores
Univariate logistic regression was used to explore factors associated with greater overall parental knowledge of late effects. There was no statistically significant relationship between knowledge and understanding of prognosis, late effects communication or information preferences, or any other child or parent factors assessed (Table 2). Although child’s sex approached significance, this trend was not significant after adjusting for multiple testing.
TABLE 2.
Univariate ordinal logistic regression analysis of factors associated with parental knowledge score of late effects
| Parameter estimates | |||
|---|---|---|---|
| Parameter | % | Odds ratioa (95% confidence interval) | p-Value |
| Parental age (years) | Global p = .020 | ||
| ≤39 | 55.2 | 2.51 (1.16, 5.45) | |
| >40 | 44.8 | Ref | |
| Parental sex | Global p = .20 | ||
| Female | 74.0 | Ref | |
| Male | 26.0 | 0.56 (0.23, 1.35) | |
| Race | Global p = .92 | ||
| White | 85.1 | Ref | |
| Other | 14.9 | 1.06 (0.37, 3.05) | |
| Ethnicity | Global p = .48 | ||
| Non-Hispanic | 90.5 | Ref | |
| Hispanic | 9.5 | 1.57 (0.44, 5.59) | |
| Household income | Global p = .49 | ||
| ≤$50,000 | 19.8 | Ref | |
| >$50,000 | 80.2 | 0.71 (0.26, 1.88) | |
| College graduate | Global p = .27 | ||
| Yes | 76.1 | Ref | |
| No | 24.0 | 0.60 (0.25, 1.48) | |
| Time from diagnosis to survey | Global p = .60 | ||
| ≤100 days | 21.9 | Ref | |
| >100 days | 78.1 | 1.28 (0.51, 3.17) | |
| Child age at diagnosis, years | Global p = .25 | ||
| 0–6 | 56.3 | Ref | |
| 7–12 | 24.0 | 0.53 (0.21, 1.35) | .18 |
| 13–18 | 19.8 | 0.51 (0.19, 1.39) | .19 |
| Child sex | Global p = .0071 | ||
| Female | 44.8 | 2.88 (1.33, 6.24) | |
| Male | 55.2 | Ref | |
| Cancer diagnosis | Global p = .12 | ||
| Hematologic malig. | 55.2 | Ref | |
| Solid tumor | 33.3 | 2.33 (1.02, 5.32) | .045 |
| Brain tumor | 11.5 | 1.80 (0.54, 6.02) | .34 |
| Parent-reported worry about late effects | Global p = .26 | ||
| Extremely/very | 39.6 | Ref | |
| Somewhat/a little/none | 60.4 | 1.56 (0.72, 3.36) | |
| Parent-reported receipt of late effect information | Global p = .86 | ||
| Yes | 76.0 | Ref | |
| No | 24.0 | 0.93 (0.39, 2.22) | |
| Parent-reported preparation for late effects | Global p = .51 | ||
| Extremely/very | 49.5 | Ref | |
| Somewhat/a little/none | 50.5 | 1.29 (0.61, 2.72) | |
| Parent-reported level of upset by information about late effects | Global p = .54 | ||
| Extremely or very | 66.0 | Ref | |
| Other categories | 34.0 | 0.78 (0.35, 1.73) | |
| Parent-reported perception of prognosis | Global p = .040 | ||
| ≥75% chance of cure | 87.2 | Ref | |
| Less favorable | 12.8 | 3.34 (1.06, 10.54) | |
| Optimism | Global p = .65 | ||
| Optimistic | 51.6 | Ref | |
| Less optimistic | 48.4 | 0.84 (0.40, 1.78) | |
Note: None of these factors are considered statistically significant after adjusting for multiple testing (false discovery rate <5%).
The odds ratio in the ordinal logistic model describes the change in odds between those with higher knowledge of late effects versus a lower knowledge category.
Parents were more knowledgeable about some individual late effect risks than others (Table 3). Ninety-two percent (88/96) of parents correctly identified their child’s risks of ototoxicity. In contrast, parents were less knowledgeable about risk of secondary malignancy (63.5% correctly identified their child’s risk), cardiac toxicity (66.7% correct overall), neurocognitive impairment (63.5% correct overall), and infertility (38.5% correct overall). Some parents whose children were not at risk for specific late effects misunderstood their child to be at risk. This percentage was highest for infertility (42.9% [15/35] of those not at risk) and renal toxicity (38.9% [21/54] of those not at risk). Parents of children who were at risk for certain late effects, namely, pulmonary toxicity, osteonecrosis, or infertility, were significantly less likely to understand their child’s risk as compared to those parents whose children were not at risk for these late effects (p = .0003, .011, and .0084, respectively). There was no significant difference in knowledge by risk for secondary malignancy, cardiac toxicity, neurocognitive impairment, renal toxicity, or ototoxicity.
TABLE 3.
Number and proportion of parents knowledgeable and not knowledgeable of their child’s risk status for specific late effects
| Knowledge of LE N (%) |
|||||
|---|---|---|---|---|---|
| Knowledgeable | Not knowledgeable | Total | Fisher’s p-value* | ||
| Risk of secondary malignancy | At risk | 59 (62.8%) | 35 (37.2%) | 94 | .53 |
| Not at risk | 2 (100.0%) | 0 (0.0%) | 2 | ||
| Total | 61 (63.5%) | 35 (36.5%) | |||
| Risk of pulmonary dysfunction | At risk | 6 (40.0%) | 9 (60.0%) | 15 | .0003 |
| Not at risk | 70 (86.4%) | 11 (13.6%) | 81 | ||
| Total | 76 (79.2%) | 20 (20.8%) | |||
| Risk of cardiac toxicity | At risk | 43 (60.6%) | 28 (39.4%) | 71 | .047 |
| Not at risk | 21 (84.0%) | 4 (16.0%) | 25 | ||
| Total | 64 (66.7%) | 32 (33.3%) | |||
| Risk of neurocognitive effects | At risk | 35 (55.6%) | 28 (44.4%) | 63 | .028 |
| Not at risk | 26 (78.8%) | 7 (21.2%) | 33 | ||
| Total | 61 (63.5%) | 35 (36.5%) | |||
| Risk of osteonecrosis | At risk | 37 (56.1%) | 29 (43.9%) | 66 | .011 |
| Not at risk | 25 (83.3%) | 5 (16.7%) | 30 | ||
| Total | 62 (64.6%) | 34 (35.4%) | |||
| Risk of renal toxicity | At risk | 19 (45.2%) | 23 (54.8%) | 42 | .15 |
| Not at risk | 33 (61.1%) | 21 (38.9%) | 54 | ||
| Total | 52 (54.2%) | 44 (45.8%) | |||
| Risk of ototoxicity | At risk | 20 (95.2%) | 1 (4.8%) | 21 | .68 |
| Not at risk | 68 (90.7%) | 7 (9.3%) | 75 | ||
| Total | 88 (91.7%) | 8 (8.3%) | |||
| Risk of infertility | At risk | 17 (27.9%) | 44 (72.1%) | 61 | .0084 |
| Not at risk | 20 (57.1%) | 15 (42.9%) | 35 | ||
| Total | 37 (38.5%) | 59 (61.5%) |
Note: p-Values for factors with a calculated false discovery rate <5% are given in bold. These factors are considered statistically significant after adjusting for multiple testing.
Abbreviation: LE, late effect.
4 |. DISCUSSION
A majority of childhood cancer survivors experience late effects secondary to cancer treatment, yet survivors often underestimate their personal risks of experiencing late effects. As parents play a critical role in childhood cancer care from diagnosis through survivorship, and as parents express a desire for early information about their children’s risks of late effects, we evaluated parental knowledge of risks of late effects during initial cancer treatment. This study found that many parents of children actively undergoing cancer therapy have an incomplete understanding of their children’s risks of late effects. Parents in our study correctly recognized approximately two thirds of their children’s potential risks of late effects. Parents were more knowledgeable about some late effects, such as ototoxicity, than others, such as renal toxicity, pulmonary dysfunction, and infertility. No child or parent factors were associated with increased overall knowledge of late effect risks.
Our findings of limited early parental understanding of late effect risks are consistent with prior studies, which demonstrated suboptimal knowledge of late effect risks in survivors of childhood cancer.7,27 The cause of this underestimation of late effects among both parents of children undergoing treatment and survivors of childhood cancer is likely multifactorial and starts at diagnosis. Many topics must be covered in initial treatment conversations, including information about the diagnosis, prognosis, and anticipated acute effects of treatment. Given these competing needs, particularly at the emotionally overwhelming time of a new pediatric cancer diagnosis, the risks of late effects associated with treatment recommendations are often underemphasized.28 Long-term effects are not always discussed during treatment unless there is a need for screening studies, such as echocardiograms, or concerns about treatment side effects arise. This natural tendency to minimize conversations about late effects likely contributes to both parental and survivor knowledge gaps, but it also provides a window of opportunity to improve late effects communication.
Parents were significantly more knowledgeable about some late effect risks than others. Nearly all parents whose children were at risk of ototoxicity correctly identified their child’s risk. This may be because audiology screening begins early in treatment or because parents readily recognize a problem when their child begins to turn up the volume on devices or becomes less responsive to their parent’s voice. Conversely, there are some long-term effects, such as infertility, which are less visible, or which may not become a problem until years after treatment completion. Unfortunately, a greater number of children are at risk of experiencing some of these less noticeable or delayed late effects such as secondary malignancy, cardiac toxicity, and osteonecrosis, yet parents were less aware of these risks. Similar to childhood cancer survivors,8 parents were least aware of the risk of infertility, revealing a knowledge gap in this area and an important target for future interventions. In addition, a subset of parents whose children’s treatment did not pose a risk for certain late effects, incorrectly believed their child was at risk for experiencing them. This overestimation of risk was greatest for infertility and renal toxicity. Lack of knowledge in this direction may cause unnecessary anxiety, oversurveillance, and in the case of infertility, could increase risk of unintended pregnancy. Of note, we assessed risk of experiencing late effects as a binary at risk/not at risk. However, the likelihood of experiencing individual late effects may play a role in parental knowledge. For example, while many treatments carry a potential risk of second malignancies, the likelihood of experiencing a second malignant neoplasm is low. Conversely, the likelihood of experiencing ototoxicity when exposed to ototoxic chemotherapies is often considerably higher.
Despite the significant challenges in communicating late effect risks, parents want information about this topic starting at the time of diagnosis.15 The current study suggests typical information provision practices are inadequate at conveying what life may look like after cancer treatment is over. Improved early parental awareness of late effect risks has several important clinical implications. First, accurate parental knowledge of late effect risks can ensure parents have the information they desire and support them in feeling fully informed to make treatment decisions.17 Second, as much treatment communication and many care responsibilities fall on parents, particularly parents of young children, improved parental awareness of potential late effects may translate into increased survivor knowledge. Lastly, parents with a more complete understanding of late effect risks may help ensure that their children engage in risk-based screening and survivorship care. This is particularly important as survivors have been shown to have suboptimal rates of recommended late effect screening tests and attendance in survivorship clinics.29–31 Formal survivorship care can help reinforce knowledge of diagnosis/late effects, improve rates of recommended screenings, provide emotional support, and offer necessary counseling on risky behaviors.11–13
Given the challenges in discussing late effects information, tools to enhance communication of risks of late effects are needed, starting at diagnosis and extending through treatment and into survivorship. To date, most interventions to improve knowledge of late effects have targeted survivors. A key innovation has been survivorship care plans (SCPs): comprehensive yet simple summaries of diagnosis, treatment, and late effects given to survivors of childhood cancer with the goal of empowering them to take ownership of their care.32 However, these care plans are usually completed at the end of therapy, which may be too late to address the early parental desire for information and the knowledge gaps observed in this study. Adapting some of the information provided in SCPs to create a longitudinal intervention to support awareness about risks of late effects from the time of diagnosis may facilitate early conversations about late effect risks and assist parents in digesting and interpreting information. In the hopes of informing future late effects communication interventions, we aimed to identify child or parent factors that were associated with increased knowledge of late effects. However, none of the factors investigated including parent education, household income, worry about late effects, receipt of late effects information, or child’s cancer diagnosis were significant. This may be due, in part, to a lack of power after adjusting for multiple testing; however, it also suggests that nearly all parents are at risk for knowledge gaps and thus interventions must be applied broadly. Current mixed methods research is being performed to determine the ideal timing, content, and delivery of this information.
Our study should be interpreted in the context of limitations. First, our patient and parental sample had limited racial/ethnic, socioeconomic, and educational diversity, and this was a single-site study, both of which may impact the generalizability of our results. However, the rates of late effect risk knowledge are similar to those found in survivors at different institutions so we hypothesize that our findings and communication practices are not specific to our institution. These findings should be further investigated in multisite studies with more diverse participants. Furthermore, we chose a select number of late effects based on their prevalence and importance to families and clinicians, and those about which there are clear data on exposure-based risks, but we were unable to investigate all known late effects. We surveyed parents of children with a variety of malignancies, each with distinct risks of late effects, and we evaluated risk as binary (at risk or not at risk), but risk is far more nuanced. Future work should evaluate how degree of risk impacts parental knowledge and assess parental understanding of late effects in specific malignancies with standardized treatment approaches. Additionally, while we derived late effect risks from treatment information, we do not know what exactly was communicated to parents, nor do we know if all caregivers participated equally in all conversations. Changes to treatment that do not result in a new consent form (such as dose modifications) may not have been captured, given our use of chemotherapy consent forms as the primary data collection source. However, these small changes to treatment are unlikely to significantly impact binary risk (yes/no) for the late effects investigated. Finally, our relatively small sample size limited our ability to conduct subgroup analyses.
5 |. CONCLUSIONS
Parents of children with cancer play an integral role in both treatment and posttreatment decision-making and care, yet our findings suggest they often have an incomplete understanding of their children’s risks for late effects. Research regarding optimal strategies for early communication of late effects information is essential to empower both parents and children throughout all phases of cancer care.
Abbreviations:
- COG LTFU
Children’s Oncology Group’s Long-Term Follow-Up Guidelines
- HSCT
hematopoietic stem cell transplant
- SCP
survivorship care plan
Footnotes
CONFLICT OF INTEREST
The authors have no relevant financial or nonfinancial conflict of interests to disclose.
Abstract presented as a poster with title “Early parental knowledge of risks of late effects in children with cancer” at the virtual 2021 American Society of Pediatric Hemotology/Oncology (ASPHO) Conference; April 20–23, 2021. https://asphoconf.showcare.io/exhibitors1/poster-131-early-parental-knowledge-of-risks-of-late-effects-in-children-with-cancer/
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
REFERENCES
- 1.Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality: childhood & adolescent cancer mortality. Cancer. 2014;120(16):2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomark Prev. 2015;24(4):653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Kawashima T, Friedman DL, Kadan-Lottick NS, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 5.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27(14):2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson TM, Li C, Armstrong GT, et al. Perceptions of future health and cancer risk in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study: concern in childhood cancer survivors. Cancer. 2018;124(16):3436–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syed IA, Klassen AF, Barr R, et al. Factors associated with childhood cancer survivors’ knowledge about their diagnosis, treatment, and risk for late effects. J Cancer Surviv. 2016;10(2):363–374. [DOI] [PubMed] [Google Scholar]
- 8.Gilleland Marchak J, Elchuri SV, Vangile K, Wasilewski-Masker K, Mertens AC, Meacham LR. Perceptions of infertility risks among female pediatric cancer survivors following gonadotoxic therapy. J Pediatr Hematol Oncol. 2015;37(5):368–372. [DOI] [PubMed] [Google Scholar]
- 9.Hess SL, Jóhannsdóttir IM, Hamre H, Kiserud CE, Loge JH, Fosså SD. Adult survivors of childhood malignant lymphoma are not aware of their risk of late effects. Acta Oncol. 2011;50(5):653–659. [DOI] [PubMed] [Google Scholar]
- 10.Ruud E, Kanellopoulos A, Zeller B, Widing E, Tjønnfjord GE, Fosså SD. Patient knowledge of late effects of acute lymphoblastic leukaemia. Tidsskr Nor Laegeforen. 2012;132(18):2052–2055. [DOI] [PubMed] [Google Scholar]
- 11.Lindell RB, Koh SJ, Alvarez JM, et al. Knowledge of diagnosis, treatment history, and risk of late effects among childhood cancer survivors and parents: the impact of a survivorship clinic: knowledge among childhood cancer survivors. Pediatr Blood Cancer. 2015;62(8):1444–1451. [DOI] [PubMed] [Google Scholar]
- 12.Prasad PK, Bowles T, Friedman DL. Is there a role for a specialized follow-up clinic for survivors of pediatric cancer? Cancer Treat Rev. 2010;36(4):372–376. [DOI] [PubMed] [Google Scholar]
- 13.Friedman DL, Freyer DR, Levitt GA. Models of care for survivors of childhood cancer. Pediatr Blood Cancer. 2006;46(2):159–168. [DOI] [PubMed] [Google Scholar]
- 14.Zheng DJ, Sint K, Mitchell H-R, Kadan-Lottick NS. Patterns and predictors of survivorship clinic attendance in a population-based sample of pediatric and young adult childhood cancer survivors. J Cancer Surviv. 2016;10(3):505–513. [DOI] [PubMed] [Google Scholar]
- 15.Greenzang KA, Dauti A, Mack JW. Parent perspectives on information about late effects of childhood cancer treatment and their role in initial treatment decision making. Pediatr Blood Cancer. 2018;65(6):e26978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenzang KA, Cronin AM, Kang T, Mack JW. Parent understanding of the risk of future limitations secondary to pediatric cancer treatment. Pediatr Blood Cancer. 2018;65(7):e27020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenzang KA, Al-Sayegh H, Ma C, Najafzadeh M, Wittenberg E, Mack JW. Parental considerations regarding cure and late effects for children with cancer. Pediatrics. 2020;145(5):e20193552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenzang KA, Cronin AM, Mack JW. Parental preparedness for late effects and long-term quality of life in survivors of childhood cancer. Cancer. 2016;122(16):2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack JW, Wolfe J, Grier HE, Cleary PD, Weeks JC. Communication about prognosis between parents and physicians of children with cancer: parent preferences and the impact of prognostic information. J Clin Oncol. 2006;24(33):5265–5270. [DOI] [PubMed] [Google Scholar]
- 20.Mack JW, Cronin AM, Kang TI. Decisional regret among parents of children with cancer. J Clin Oncol. 2016;34(33):4023–4029. [DOI] [PubMed] [Google Scholar]
- 21.Mack JW, Cook EF, Wolfe J, Grier HE, Cleary PD, Weeks JC. Understanding of prognosis among parents of children with cancer: parental optimism and the parent-physician interaction. J Clin Oncol. 2007;25(11):1357–1362. [DOI] [PubMed] [Google Scholar]
- 22.Mack JW, Wolfe J, Cook EF, Grier HE, Cleary PD, Weeks JC. Hope and prognostic disclosure. J Clin Oncol. 2007;25(35):5636–5642. [DOI] [PubMed] [Google Scholar]
- 23.Greenzang KA, Kelly CA, Al-Sayegh H, Ma C, Mack JW. Thinking ahead: parents’ worries about late effects of childhood cancer treatment. Pediatr Blood Cancer. 2021;68(12):e29335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler FJ Jr. Survey Research Methods. SAGE Publications; 2013. [Google Scholar]
- 25.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the life orientation test. J Pers Soc Psychol. 1994;67(6):1063–1078. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 27.Lee JL, Gutierrez-Colina A, Williamson Lewis R, et al. Knowledge of late effects risks and healthcare responsibility in adolescents and young adults treated for childhood cancer. J Pediatr Psychol. 2019;44(5):557–566. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez LY, Huestis SE, Yap TY, Zyzanski S, Drotar D, Kodish E. Potential chemotherapy side effects: what do oncologists tell parents? Pediatr Blood Cancer. 2009;52(4):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(27):4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steele JR, Wall M, Salkowski N, et al. Predictors of risk-based medical follow-up: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2013;7(3):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zabih V, Kahane A, O’Neill NE, Ivers N, Nathan PC. Interventions to improve adherence to surveillance guidelines in survivors of childhood cancer: a systematic review. J Cancer Surviv. 2019;13(5):713–729. [DOI] [PubMed] [Google Scholar]
- 32.King-Dowling S, Psihogios AM, Hill-Kayser C, et al. Acceptability and feasibility of survivorship care plans and an accompanying mobile health intervention for adolescent and young adult survivors of childhood cancer. Pediatr Blood Cancer. 2021;68(3): e28884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.


